Abstract
Dimethacrylate polymers and composites are seeing increased usage in orthopedics. As these applications require the material to integrate with the surrounding tissues, direct contact cytotoxicity assays should be used to assess the biocompatibility. This study utilized a combinatorial testing platform to evaluate the cell response to dimethacrylate composites with a variety of properties on a single sample. MC3T3-E1 pre-osteoblasts were cultured directly on composites with varying filler content, filler type, degree of conversion (DC), and surface topography. Cell viability, density, and area depended on an interplay of the material properties, with low DC causing a reduction in cell area but having minimal effect on cell viability, high filler content causing an increase in cell density, and filler content/type altering the surface roughness as a function of DC. The combinatorial testing platform successfully quantified the effects of numerous material properties on several aspects of the osteoblast response.
Keywords: Composite, contact angle, cytotoxicity, osteoblast, photopolymerisation, surface topography
Introduction
Dimethacrylate polymers and composites that are found ubiquitously in dental restorative materials are being increasingly evaluated for orthopedic applications. For instance, several dimethacrylates have been used to reattach tooth fragments [1], and 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy)-phenyl] propane (BisGMA)-based materials have been evaluated for cortical bone void fillers [2], frontal bone defect repair [3], augmentation of orthopedic screws [4], augmentation of orthopedic pin fixation in clinical applications [5], biomimetic mineralization [6], vertebral augmentation [7], and 3D printing of composite scaffolds [8]. Other dimethacrylate-based composites have also been considered for bone defect repair [9] and temporary bone replacement [10]. With an increased number of dimethacrylate-based orthopedic therapies, a growing number of in vitro studies have examined the interactions of osteoblasts and osteoblast-like cells with dimethacrylates and their composites [8,11-13].
Dimethacrylate monomers are known to be cytotoxic [14] and to have deleterious effects on cells even at sub-lethal concentrations of monomer [15,16]; correspondingly, extracts from dimethacrylate polymers and composites [17] and eluates from materials with a reduced degree of conversion (DC) [18] have been found to be cytotoxic. Therefore, evaluating the cytotoxicity of resin-based composites with osteoblasts is a critical component of material testing that affects their applicability. Moreover, as these materials will be in direct contact with bone tissue for most orthopedic applications, direct contact cytotoxicity assays would be more appropriate.
In addition, surface chemistry [19,20] and surface topography/roughness [21,22] have been shown to affect cell response, including osteoblast function and osseointegration. For instance, materials with micro- and nano-scale topography have been used to enhance biocompatibility or integration into host tissue for applications such as bone implants [23], and roughness has been shown to affect proliferation and spreading [22,24]. Thus, the effects of surface roughness of dimethacrylate composites on osteoblast response are of great interest, especially considering the increasing number of orthopedic applications for these types of materials.
Polymerizable composites, such as those used for dental restoratives and in the orthopedic examples above, are complex and typically composed of at least two monomers, an initiator system, and one or more types of filler particles. Due to the number of potential variables in the formulation and the polymerization protocol, a combinatorial approach is highly advantageous for evaluating cell response to these materials. When properly designed, the combinatorial approach allows for the characterization of material properties and cell response on the same sample [25]. Multiple properties, such as chemical functionality, surface properties, and cell response, can be measured at each location, so properties can be better correlated with one another, rather than with an average ± standard deviation based upon a separate set of samples. An improved correlation can be particularly useful when sample-to-sample variations are large. Thus, in addition to evaluating the cell response over a large material parameter space, a combinatorial approach enables a thorough correlation of cell results with material properties.
We have developed a combinatorial platform for photopolymerized polymers and composites to quantify material properties, including mechanical, chemical, physical, and biological properties [26-28]. Our previous biocompatibility work utilized RAW 264.7 macrophage-like cells, a cell line relevant for the inflammatory response to biomaterials. For two-dimensional (2D) combinatorial arrays consisting of binary dimethacrylate blends with variations in monomer ratio and DC, macrophage viability was relatively unaffected by monomer composition but significantly reduced when DC was less than 52 % for all compositions [27,29]. 2D photopolymerized composite arrays have also been prepared with discrete variations in filler type and content in one direction and a continuous variation in irradiation in the orthogonal direction, resulting in a composition gradient and a DC/surface roughness gradient. On the composite samples, macrophage viability decreased significantly when filler content was below 50 % by mass and DC was less than 64 % [28]. This testing platform provided a simple way to simultaneously evaluate variations in composition and polymerization protocol on a single sample for both polymers and composites.
While some studies have used the gradient approach to evaluate the effects of roughness on osteoblast response [19,22,24,30,31], resin composites and the other variables they contribute, such as DC and the effect of filler type and loading, have yet to be evaluated. Therefore, the objective of this study was to quantify the osteoblast response to dimethacrylate-based composites using the aforementioned combinatorial platform, with the hypothesis that changes in filler content, DC, and roughness would affect osteoblast response. Combinatorial samples were prepared, and DC, contact angle, and surface roughness were evaluated. MC3T3-E1 osteoblast-like cells were cultured on the composites for 24 h, and cell viability, density, and area were quantified. Effects of material properties on the cell response are discussed and compared to previously published results for macrophages on similar combinatorial composite samples.
Materials and Methods
Materials1
BisGMA and triethylene glycol dimethacrylate (TEGDMA) were obtained from Esstech, Inc. Camphorquinone (CQ) and ethyl 4-N,N-dimethylaminobenzoate (4E) were purchased from Aldrich Corp. SP 345 silane glass filler (SG, average diameter = 0.7 μm) and fumed amorphous silica filler (OX50, average diameter = 0.04 μm) were provided by L.D. Caulk Company. Methacryloxypropyltrimethoxy-silane (MPTMS) was purchased from Gelest, Inc. Alpha minimum essential medium (αMEM) was purchased from Lonza, and all other cell culture reagents and fluorescent probes were purchased from Invitrogen Corp. All reagents were used as received.
Composite Preparation
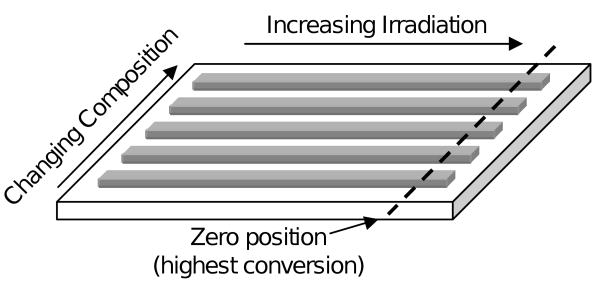
Each test sample was prepared on a single glass slide (75 mm × 50 mm) and consisted of five discrete composite formulations, each with a continuous variation in irradiation intensity (Fig. 1). Samples were fabricated as previously described [28], using composite pastes with varying filler type and filler mass ratio (Table 1). Briefly, BisGMA and TEGDMA (50:50 by mass) activated with 0.2 % CQ and 0.8 % 4E (by mass) were mixed with varying amounts of SG and OX50 fillers. The composite pastes were individually packed into a polydimethylsiloxane (PDMS) spacer placed on top of a glass slide pre-treated with MPTMS for enhanced adhesion to the composite. The composite pastes and spacer were then covered with a poly(ethylene terephthalate) (PET) release film and an additional glass slide. The assembly was clamped together in preparation for the two-step photopolymerization. For the first step, the assembly was cured for 15 s per side while placed 10 cm beneath a light source (Dentsply Triad 2000 replacement Tungsten halogen light bulb 250 W, 120 V) with the zero-position located under the center of the illumination field where the light intensity was the highest. For the second step, a majority of the sample was shielded, leaving only the area near the zero-position exposed, and the sample was further irradiated for 1 min per side. The second step was designed to increase the DC at the high DC end while having little or no effect on the low DC end. After polymerization, the top glass slide, the release film, and the PDMS spacer were removed. Samples were then stored in the dark for at least 24 h prior to characterization.
Figure 1.
Schematic of combinatorial sample. Glass slide dimensions are 75 mm by 50 mm. Each composite stripe is 5 mm wide, 50 mm long, and 2 mm thick.
Table 1.
Composite formulations used in the combinatorial sample
| Composition | Activated Resin (mass %) |
SG Filler (mass %) |
OX50 Filler (mass %) |
|---|---|---|---|
| C1 (highest filler) | 35 | 65 | - |
| C2 | 50 | 50 | - |
| C3 | 50 | 45 | 5 |
| C4 | 65 | 30 | 5 |
| C5 (lowest filler) | 80 | 15 | 5 |
NIR Spectroscopy
Degree of conversion was measured for each gradient sample prior to further sample analysis. A Nicolet Magna 550 Fourier transform infrared (FTIR) spectrometer (Madison, WI) configured with a white light source, a CaF2 beam splitter, and an InSb detector was used to collect the transmission near infrared (NIR) spectra of the composite pastes and the polymerized composite samples at 10 mm intervals. Spectra were acquired from 32 co-added scans over 7000 cm-1 to 4000 cm-1 with a resolution of 6 cm-1. The methacrylate peak height (4743 cm-1) was normalized to the aromatic peak height (4623 cm-1), and DC was calculated as the percent reduction in the normalized 4743 cm-1 peak height due to polymerization [26].
Sample Sterilization
Combinatorial samples were sterilized using ethylene oxide gas (Anprolene Sterilization System, Andersen Products, Inc.) and degassed for 3 d. The samples were then rinsed and aged in phosphate buffered saline (PBS) for 7 d at 37 °C to remove any highly toxic leachables. The PBS was changed on days 1, 3, 5, and 7. The aged samples were used for cell seeding and for characterization of surface properties.
Contact Angle
Surface hydrophobicity was evaluated on the aged samples using a water contact angle goniometer (Kruss DSA100) in sessile drop mode with 2 μL drops. Measurements were made on four separate combinatorial samples (n = 4).
Surface Topography
Images of the surface topography of the aged samples were collected using a field-emission scanning electron microscope (FE-SEM, Hitachi S-4700, Hitachi High Technology America, Inc., Schaumburg, IL). Samples were sputter-coated with gold, and images (10 000 × magnification) were collected at three locations on each composite stripe corresponding to 75 %, 65 %, and 55 % DC.
Cell Culture
Murine MC3T3-E1 pre-osteoblasts (Riken Cell Bank, Hirosaka, Japan) were cultured in αMEM supplemented with 10 % (volume fraction) fetal bovine serum, 5 mmol/L L-glutamine, and 60 mg/mL kanamycin sulfate. Cells were maintained in humidified incubators (5 % by volume CO2, 37 °C).
After aging in PBS for 7 d, each sample was transferred under sterile conditions to a new dish (OmniTray, Nalge Nunc International) and seeded using a two-step process modified from a previously published protocol [28]. First, 5 mL growth medium containing 3.9 × 104 cells/mL were added to cover each sample, and samples were left undisturbed for 15 min to allow initial cell attachment to the substrate. Then, 25 mL growth medium containing 1.7 × 104 cells/mL were added to fill the entire dish, and samples were transferred to the incubator. Control cells were seeded in 6-well tissue culture polystyrene (TCPS) plates (2 mL per well of 2.4 × 104 cells/mL). The final seeding density on the combinatorial samples and on the control TCPS wells was 5.0 × 103 cells/cm2.
Quantitative Viability Assay
After 24 h, a viability assay based on calcein acetoxymethyl ester (calcein AM, live cells), ethidium homodimer-1 (EthD-1, dead cells), and Hoechst 33342 (H33342, all nuclei) was used as previously described [27]. Briefly, samples were incubated 15 min with growth medium containing 2 μmol/L calcein AM, 2 μmol/L EthD-1, and 10 μmol/L H33342. Cells were imaged in fresh growth medium using a Leica DMA upright microscope with epifluorescence capabilities, a Hamamatsu digital camera, and Image-Pro Plus software (Media Cybernetics, Inc.). Using the DC data specific for each sample, images were collected on each composition as a function of DC, covering the range of 52 % to 76 % DC at intervals of 4 %. At least three separate combinatorial samples were imaged, and at least five images were collected for each location using a 10 × objective and a 0.63 × camera adapter, resulting in a total imaged area greater than 4.3 mm2 for each location. Cell viability was quantified using custom macros written in Image-Pro Plus. The number of nuclei stained with EthD-1 represented the dead cells, while the number of nuclei stained with only H33342 represented the live cells. The calcein AM stain was used for visualization purposes.
Cell Density and Area
Combinatorial samples were fixed, permeabilized, and stained with 2 μg/mL 4′,6-diamidino-2-phenylindole,dihydrochloride (DAPI, nuclear stain) and 1 μg/mL Alexa Fluor 488-maleimide (cell body stain) for 1 h. Images were collected as described above. Cell density was determined by counting the total number of nuclei per image from both the viability images and the fixed cell images. Analysis of individual cell area was performed for the maleimide-stained cells using Image-Pro Plus custom macros [19]. Linear trendlines and their corresponding correlation coefficients were calculated for cell density and area data.
Statistical Analysis
All data were analyzed using one-way or two-way analysis of variance (ANOVA) and Tukey's test with a 95 % confidence interval to indicate significant differences. Area measurements were collected from individual cells and natural logarithm transformed prior to statistical analysis. Standard uncertainties associated with the cell measurements are estimated by the error bars on the corresponding figures. The significance of the correlation coefficients for cell density and area were evaluated with a 95 % confidence interval in VassarStats.
Results
Degree of Conversion
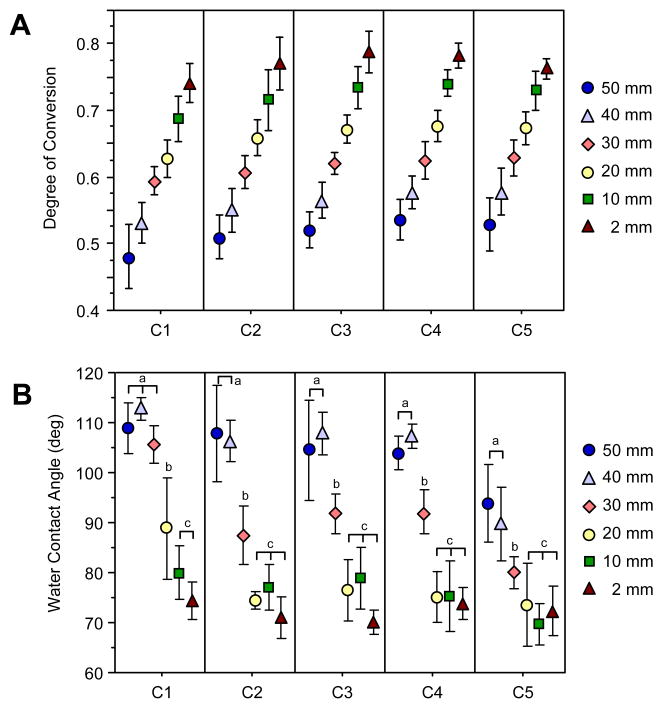
A DC gradient was measured for all compositions on each combinatorial sample using NIR spectroscopy (Fig. 2A). For each composition, the highest DC was located at the 2 mm position (the position centered under the light source), and the lowest DC was at the 50 mm position. Composition C1, which had the highest filler loading, had a slightly reduced DC profile overall as compared to the other compositions, with a lower maximum DC obtained. Statistically, the DC of C1 was significantly lower than that of C3 and C4 at all positions, C5 at all positions except 2 mm, and C2 at 2 mm and 20 mm. Compositions C2-C5 were statistically the same.
Figure 2.
Material properties as a function of position and composition. Data points represent the mean value, and error bars represent the standard deviation and serve as an estimate of the standard uncertainty. (A) DC gradient along the long axis for each composition, n = 10. (B) Water contact angle (sessile drop) data, n = 4. Lowercase letters (a, b, c) each represent a data point or set of data points that are statistically the same for a given composition.
Contact Angle
Surface properties were characterized on the aged samples, the same surface to which the cells were exposed. Water contact angle changed as a function of position (Fig. 2B), with the highest contact angles corresponding to the positions with the lowest DC values. ANOVA analysis confirmed that position, composition, and their interaction term (position × composition) all had significant effects on the contact angle. Overall, compositions C2-C4 were statistically the same (P > 0.05), while C1 and C5 were each different from all other compositions (P < 0.05). All compositions were the same at the 2 mm position (lowest contact angle), but C1 had a significantly higher contact angle than all other compositions at 20 mm and 30 mm. Contact angle on C5 was lower overall compared to the other compositions, with a significantly lower contact angle than all compositions at 30 mm and 40 mm and all compositions (except C2) at 50 mm.
When evaluating each composition individually, there were three significantly different levels for the contact angle measurements within each composition (indicated by the lowercase letters in Fig. 2B). For C2 through C5, the contact angle was high at 50 mm and 40 mm, decreased at the 30 mm position, and then further decreased at 2 mm to 20 mm, where DC was the highest. On the other hand, the contact angle for highly filled C1 remained high from 30 mm to 50 mm. It then decreased at the 20 mm position followed by another decrease for the 10 mm and 2 mm positions.
Surface Topography via SEM
SEM images of the aged surface revealed a change in surface topography as a function of DC (Fig. 3). A decrease in roughness with increasing DC was evident for all compositions, with the surface structure changing from a smooth surface to filler/matrix aggregates separated by gaps or open “pores,” similar to microporous materials. The presence of the nanofiller in C3 to C5 was also evident. Composition C1 had a rougher topography at 65 % DC as compared to the other compositions. In addition, 65 % DC on C1 had surface features slightly less than 1 μm, approximately the expected size of a single microfiller particle (0.7 μm), but at the low conversion of 55 % DC, filler aggregates on the surface were larger than 1 μm.
Figure 3.
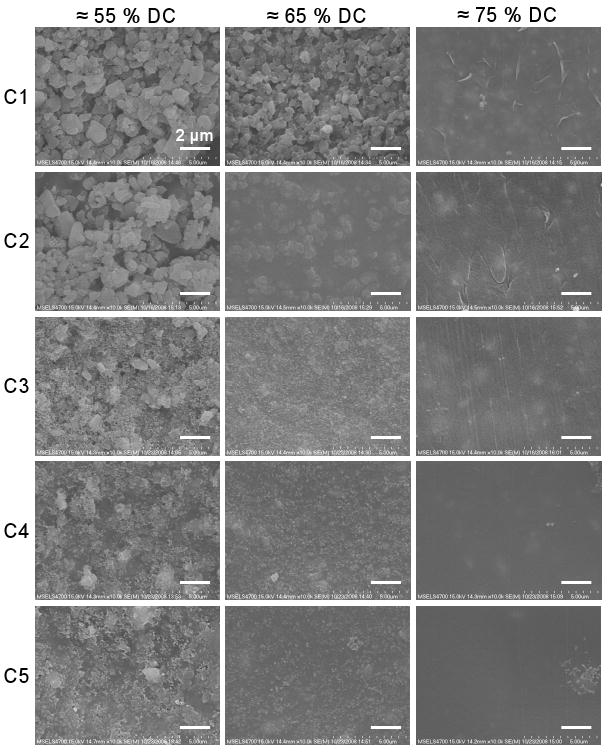
SEM images (10 000 × magnification) of surface topography as a function of composition and DC. Scale bars = 2 μm.
Cell Viability
All samples used for evaluating the cell response were first used for measuring the DC gradients. The DC data was then used to identify the locations for specific DC values (52 % to 76 % in 4 % intervals) for all compositions on each sample. Cell response parameters were evaluated at these locations, which differed slightly for each sample due to sample-to-sample variations.
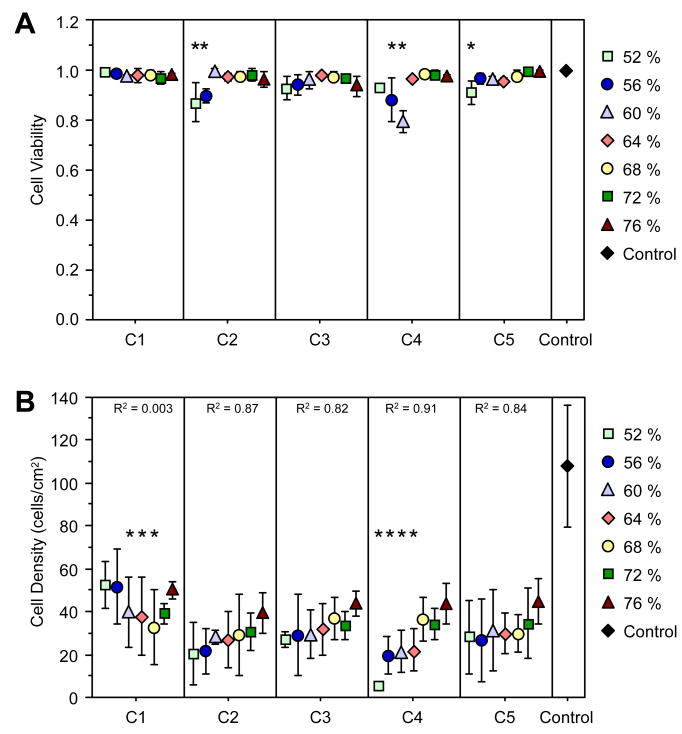
Imaging of cells stained with calcein AM, EthD-1, and H33342 revealed the viability of the cells, with green cells having intracellular esterase activity and intact membranes, red-stained nuclei indicating cells with compromised cell membranes, and blue-stained nuclei for all cells. From the image analysis, viability was nearly 100 % at the high DC end for all compositions (Fig. 4A). Significant decreases in viability were present for compositions C2, C4, and C5 at low DC values. Although these decreases were statistically significant, the viability dropped no more than 20 %.
Figure 4.
MC3T3-E1 cell parameters on combinatorial samples. Data points represent the average value (n ≥ 3), and error bars represent the standard deviation and are the estimate of standard uncertainty. (A) Fraction of viable cells plotted as a function of composition and DC. * P-value < 0.05 compared to unstarred DC levels in the same composition using ANOVA. (B) Cell density on combinatorial samples, with correlation coefficient (R) for the respective linear trendline for each composition. * P-value < 0.05 compared to the 76 % DC in the same composition using ANOVA. Control surfaces were TCPS.
Cell Density
Cell density measurements revealed that the trend in density measured as a function of DC depended on the total filler loading (Fig. 4B). Compositions C2 to C5 all showed a significant (P < 0.05) directional decrease in cell density as DC decreased, as determined by statistical analysis of the correlation coefficient (R) for the linear trendlines. ANOVA analysis with post-hoc testing revealed that C4 had significant reductions in cell density at low DC values (≤ 64 %). C1, on the other hand, had a different trend relative to the other compositions. As DC decreased, cell density was reduced significantly at mid-range DC values of 60 % to 68 %; however, a recovery in cell density was observed as DC continued to decrease, such that the cell densities at 52 % and 56 % were the same (P > 0.05) as those observed at high DCs of 72 % and 76 %. Cell density on the test samples was significantly lower than that on the control TCPS surfaces.
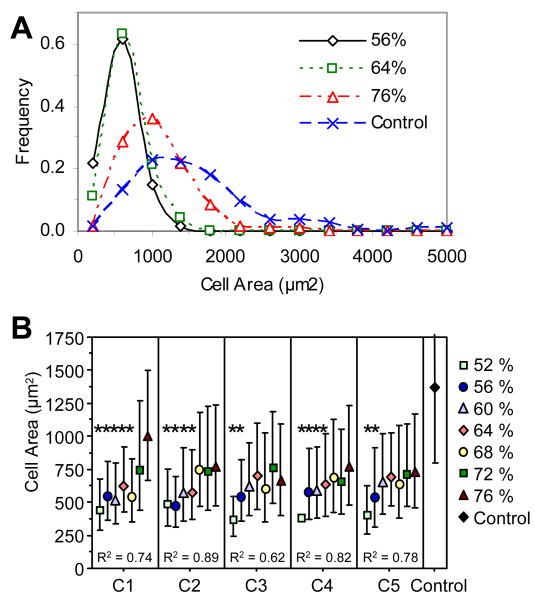
Cell Area
Figure 5 illustrates representative images of cells that were cultured on a combinatorial composite sample, fixed, and stained using fluorescent maleimide, which binds to sulfhydryl groups. Qualitative differences seen in the images were quantified using image analysis macros. Cell measurements were collected for individual cells – cells not touching the border of the image or any other cells (only one nucleus); 100 cells to 250 cells were measured for each location. A histogram of the area distribution for cells on composition C1 illustrates (1) variations in cell area distribution as a function of DC, and (2) a non-normal distribution of cell area (Fig. 6A). Cell area distributions for 56 % and 64 % DC were similar, but the area histograms for 76 % DC and the control TCPS were shifted to the right, indicating an increase in cell area on these samples. In order to analyze the data using parametric tests, the data were normalized using a natural logarithm transformation. The average values ± one standard deviation were then transformed back using an exponential transformation and plotted (Fig. 6B). Cell area was significantly reduced on all sample locations as compared to the control TCPS. As DC decreased, significant reductions in cell area began at (68, 64, 56, 64, and 56) % DC for compositions C1, C2, C3, C4, and C5, respectively. When a linear trendline was calculated for each composition, statistical analysis of the correlation coefficient (R) of that line revealed that all the trendlines were directional (P < 0.05), indicating that cell area decreased with decreasing DC for all compositions.
Figure 5.
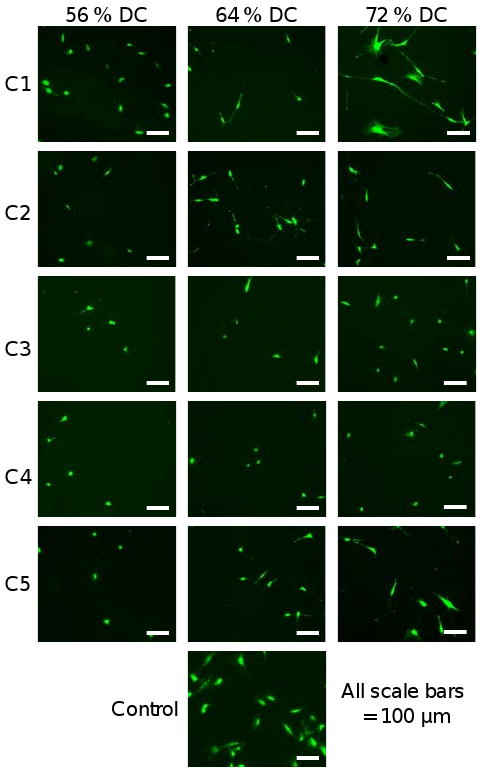
Representative images of MC3T3-E1 cells on a combinatorial sample and control TCPS surface. Cells were fixed and stained with Alexa Fluor-488 maleimide. Control surfaces were TCPS.
Figure 6.
MC3T3-E1 cell spreading. (A) Histograms of cell area for composition C1. Lines are drawn to aid the readers' eyes. (B) Cell area data where the mean and standard deviation of the natural log transformed data were untransformed and plotted as the data points and error bars, respectively (n ≥ 100). Correlation coefficients (R) are given for the linear trendlines. Error bars are the estimate of standard uncertainty. * P-value < 0.05 compared to unstarred DC values in the same composition using ANOVA. Control surfaces were TCPS.
Discussion
The use of a combinatorial approach to quantify the response of MC3T3-E1 pre-osteoblast cells to dimethacrylate composites varying in filler content, degree of conversion, and surface roughness is described. Methods to measure osteoblast response to composites are needed, as these materials are being increasingly used in orthopedic applications. In this study, combinatorial methods enabled the testing of a range of filler contents and material properties on a single sample. Significant changes in cell viability, density, and area were evident for the MC3T3-E1 cells and did not always match the results from RAW 264.7 macrophages, which were previously evaluated on similar combinatorial composite samples [28].
As DC decreased, roughness (via SEM) and water contact angle increased for all compositions. Surface topography depended on the composition, with a notable difference coming from the addition of the nanofiller in compositions C3 to C5. This finding is consistent with our previous studies using laser scanning confocal microscopy to measure surface roughness [28]. A change in surface roughness is likely due to the kinetics of polymerization (rate of polymer contraction vs. particle aggregation).
Overall, the highly filled composition C1 differed from the other compositions in DC, contact angle, and surface topography. C1 exhibited a different transition point for the contact angle as compared to the other compositions, which all showed a transition point but at a lower DC than C1. In addition, C1 had a more drastic change in surface topography from a relatively smooth surface to a very rough surface (as evaluated using SEM). The increased contact angle seen in C1 at the 20 mm position (63 % DC) corresponded well with the increase in surface roughness, consistent with previous results that showed an increasing contact angle with increasing surface roughness [32] and increasing hole size for porous surfaces [33]. These properties in C1 may be due to a higher exposed filler content as compared to the other compositions.
Composition C5, which had the lowest filler content, was also different when compared to the other compositions. C5 was similar to C2 to C4 in DC profile but differed in water contact angle with a significantly lower contact angle at low DC. At low DC, the surface of C5 appeared smoother than that of the other compositions, likely due to its large polymer content and low macro-filler content. The lower contact angle is believed to result from the reduced surface roughness.
Regarding filler type, compositions C2 and C3 had the same total filler loading, but different filler types. C2 had 50 % (by mass) microfiller, while C3 had 45 % (by mass) microfiller and 5 % (by mass) nanofiller. The only notable difference between the surface properties of these two compositions was the visible effect of the nanofiller on the surface roughness in the SEM images. The DC and contact angle values were not affected by the presence of the nanofiller at this concentration.
After material characterization, MC3T3-E1 osteoblasts were cultured and evaluated on the composites. Viability remained high (80 % or greater) for all locations on all compositions (Fig. 4). The high viability at low DC, particularly for C5, was unexpected as previous results have shown that the under-cured polymers and composites can be cytotoxic. For instance, macrophage viability dropped below 50 % at low DC (60 % DC) and low filler content (20 % by mass) on similar combinatorial samples [28]. Thus, MC3T3-E1 cells appear to be more robust than RAW 264.7 macrophages in terms of viability on these dimethacrylate composites.
Highly filled C1 had a deviant behavior from the other compositions in terms of the osteoblast density. Cell density was reduced at low DC on compositions with a filler mass was ≤ 50 %, but cell density recovered at low DC when filler content was 65 % (C1) such that density at the lowest DC was statistically the same as density at the highest DC for C1. This trend was not seen for any of the other compositions. Similar results were previously seen for macrophages where cell density did not change significantly as a function of DC when filler content was ≥ 50 % (C1-C3) [28]. Therefore, two cell types independently confirmed that the combination of low DC with high filler content does not reduce cell density.
The high cell density at low DC on C1 may be due to a number of factors, one of which is the increased surface roughness, as effects of nanoscale and microscale surface features on osteoblast density have been previously demonstrated [22,24]. Since composition C2, which like C1 had a rough surface at low DC, did not have a high cell density at low DC, the recovery of cell density on C1 was not due to increased surface roughness alone. The decreased presence of under-cured polymer in the highly filled C1 formulation may also contribute to the high cell density. At low DC, the polymer is significantly under-cured and can affect cell response. Since C1 has less polymer than all other compositions, the potentially toxic portions of the under-cured polymer should have been minimally presented to the cells. The SEM images confirm an increased presentation of clustered filler particles on the surface of C1, which effectively reduces the surface polymer concentration and therefore reduces any detrimental effects on cell density.
Cell area measurements for the MC3T3-E1 cells revealed a significant trend of decreasing area with decreasing DC, even on composition C1 which had no decrease in cell density at the lowest DC values. Similar trends were seen, although not as clearly, for the RAW 264.7 macrophage cells [28]. The increased contact angle at low DC may have contributed to the decreased cell area, as studies using MC3T3-E1 cells have shown that cell area decreased as contact angle increased from 60° to 90° [19].
Overall, the cell response was found to depend upon multiple material properties. For instance, cell viability was highest at higher DC, less rough, more hydrophilic regions of the sample and was only mildly affected by filler type/content. These results are encouraging for the use of dimethacrylate-based materials in orthopedic applications, as only minimal toxicity was evident for the MC3T3-E1 cells. Cell density was greatly affected by high filler content, with high filler content dominating other material parameters, such as low DC and increased roughness/hydrophobicity. Cell area, on the other hand, was not affected by composition, as all compositions had a decrease in cell area at lower DC, higher hydrophobicity, and increased roughness. These responses measured for the MC3T3-E1 cells corresponded with the cell response from RAW 264.7 cells [28], with the MC3T3-E1 cells being more robust in terms of cell viability and having more consistent trends in cell area with respect to DC. Future studies to evaluate other compositions or polymerization techniques would be useful in determining the effects of these variations on osteoblast response.
The surfaces of titanium-based materials used in orthopedic applications are often roughened on the micrometer or nanometer scale in order to encourage cell and tissue growth as well osseointegration [21,23]. The results presented here for MC3T3-E1 cells on dimetharcylate-based composites suggest that an increased surface roughness from smooth (75 % DC) to that shown at 55 % DC may reduce cell spreading and potentially cell density on these materials (depending on the filler content). Others have also shown reductions in osteoblast cell area due to increased roughness [34]. Reductions in cell area may also be accompanied by a change in cell phenotype, as studies have demonstrated an increase in osteoblastic differentiation along with a change in cell morphology as surface roughness increases [35]. It should be noted that variations in several material properties, including DC, roughness, and hydrophobicity, were present and intercorrelated in the current study, making it difficult to single out roughness as the sole cause for reduced cell area. Additional studies are needed to further explore these possibilities.
Conclusions
A combinatorial approach provided a simple method to quantify osteoblast response to a range of dimethacrylate-based composite formulations and bulk/surface properties in order to characterize the potential of these materials for orthopedic applications. A thorough characterization of the combinatorial samples allowed for an improved interpretation of the resulting cell response. Low DC values were only slightly cytotoxic, but no obvious effect of filler content on MC3T3-E1 viability was evident. While compositions with ≤ 50 % filler (by mass) showed decreasing cell density with decreasing DC/increasing roughness/increasing hydrophobicity, an increased filler content of 65 % (by mass) successfully overcame these effects and enabled the recovery of normal cell density at low DC for composition C1. Cell area decreased as a function of decreasing DC for all compositions, indicating that the cells could not spread as well on the rough, hydrophobic surfaces (seen at low DC values). Thus, the osteoblasts were affected by changes in the filler content, DC, roughness, and hydrophobicity. This study sheds light on what studies might be appropriate for future evaluation of photopolymerized composites for use in orthopedic applications.
Acknowledgments
The authors acknowledge EssTech, Inc. for providing the monomers and Denstply Caulk for providing the filler particles. The authors also thank Dr. Joseph M. Antonucci and Dr. Diana N. Zeiger for technical discussions. Financial support was provided through an Interagency Agreement (Y1-DE-7005-01) between the National Institute of Dental and Craniofacial Research and NIST.
Footnotes
Certain equipment, instruments or materials are identified in this paper to adequately specify the experimental details. Such identification does not imply recommendation by the National Institute of Standards and Technology, nor does it imply the materials are necessary the best available for the purpose.
Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nancy J. Lin, Email: nancy.lin@nist.gov, Polymers Division, National Institute of Standards and Technology, 100 Bureau Drive, MS 8543 Gaithersburg, MD 20899-9543, Phone: 301-975-4935, Fax: 301-975-4977.
Sheng Lin-Gibson, Email: slgibson@nist.gov, Polymers Division, National Institute of Standards and Technology, 100 Bureau Drive, MS 8543 Gaithersburg, MD 20899-9543, Phone: 301-975-6765, Fax: 301-975-4977.
References
- 1.Demarco FF, de Moura FR, Tarquinio SB, Lima FG. Reattachment using a fragment from an extracted tooth to treat complicated coronal fracture. Dent Traumatol. 2008;24(2):257–61. doi: 10.1111/j.1600-9657.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 2.Erbe EM, Clineff TD, Gualtieri G. Comparison of a new bisphenol-a-glycidyl dimethacrylate-based cortical bone void filler with polymethyl methacrylate. Eur Spine J. 2001;10(Suppl 2):S147–S152. doi: 10.1007/s005860100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuusa SM, Peltola MJ, Tirri T, Lassila LV, Vallittu PK. Frontal bone defect repair with experimental glass-fiber-reinforced composite with bioactive glass granule coating. J Biomed Mater Res B Appl Biomater. 2007;82(1):149–55. doi: 10.1002/jbm.b.30716. [DOI] [PubMed] [Google Scholar]
- 4.Frihagen F, Madsen JE, Reinholt FP, Nordsletten L. Screw augmentation in displaced femoral neck fractures. Clinical and histological results using a new composite. Injury. 2007;38(7):797–805. doi: 10.1016/j.injury.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Smit RS, van d V, Hegeman JH. Augmented pin fixation with Cortoss for an unstable AO-A3 type distal radius fracture in a patient with a manifest osteoporosis. Arch Orthop Trauma Surg. 2008;128(9):989–93. doi: 10.1007/s00402-008-0680-0. [DOI] [PubMed] [Google Scholar]
- 6.Vakiparta M, Forsback AP, Lassila LV, Jokinen M, Yli-Urpo AU, Vallittu PK. Biomimetic mineralization of partially bioresorbable glass fiber reinforced composite. J Mater Sci Mater Med. 2005;16(9):873–9. doi: 10.1007/s10856-005-3576-3. [DOI] [PubMed] [Google Scholar]
- 7.Palussiere J, Berge J, Gangi A, Cotten A, Pasco A, Bertagnoli R, et al. Clinical results of an open prospective study of a bis-GMA composite in percutaneous vertebral augmentation. Eur Spine J. 2005;14(10):982–91. doi: 10.1007/s00586-003-0664-2. [DOI] [PubMed] [Google Scholar]
- 8.Suwanprateeb J, Sanngam R, Suwanpreuk W. Fabrication of bioactive hydroxyapatite/bis-GMA based composite via three dimensional printing. J Mater Sci Mater Med. 2008;19(7):2637–45. doi: 10.1007/s10856-007-3362-5. [DOI] [PubMed] [Google Scholar]
- 9.Tuusa SM, Peltola MJ, Tirri T, Puska MA, Roytta M, Aho H, et al. Reconstruction of critical size calvarial bone defects in rabbits with glass-fiber-reinforced composite with bioactive glass granule coating. J Biomed Mater Res B Appl Biomater. 2008;84(2):510–9. doi: 10.1002/jbm.b.30898. [DOI] [PubMed] [Google Scholar]
- 10.Brauer DS, Russel C, Vogt S, Weisser J, Schnabelrauch M. Fabrication and in vitro characterization of porous biodegradable composites based on phosphate glasses and oligolactide-containing polymer networks. J Biomed Mater Res A. 2007;80(2):410–20. doi: 10.1002/jbm.a.30902. [DOI] [PubMed] [Google Scholar]
- 11.Imazato S, Horikawa D, Ogata K, Kinomoto Y, Ebisu S. Responses of MC3T3-E1 cells to three dental resin-based restorative materials. J Biomed Mater Res A. 2006;76(4):765–72. doi: 10.1002/jbm.a.30422. [DOI] [PubMed] [Google Scholar]
- 12.Imazato S, Horikawa D, Nishida M, Ebisu S. Effects of monomers eluted from dental resin restoratives on osteoblast-like cells. J Biomed Mater Res B Appl Biomater. 2008 doi: 10.1002/jbm.b.31067. [DOI] [PubMed] [Google Scholar]
- 13.Pinna L, Brackett MG, Lockwood PE, Huffman BP, Mai S, Cotti E, et al. In vitro cytotoxicity evaluation of a self-adhesive, methacrylate resin-based root canal sealer. J Endod. 2008;34(9):1085–8. doi: 10.1016/j.joen.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Heil TL, Volkmann KR, Wataha JC, Lockwood PE. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J Oral Rehabil. 2002;29(5):401–7. doi: 10.1046/j.1365-2842.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre CA, Wataha JC, Bouillaguet S, Lockwood PE. Effects of long-term sub-lethal concentrations of dental monomers on THP-1 human monocytes. J Biomater Sci Polym Ed. 1999;10(12):1265–74. doi: 10.1163/156856299x00063. [DOI] [PubMed] [Google Scholar]
- 16.Noda M, Wataha JC, Lockwood PE, Volkmann KR, Kaga M, Sano H. Sublethal, 2-week exposures of dental material components alter TNF-alpha secretion of THP-1 monocytes. Dent Mater. 2003;19(2):101–5. doi: 10.1016/s0109-5641(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 17.Schweikl H, Hiller KA, Bolay C, Kreissl M, Kreismann W, Nusser A, et al. Cytotoxic and mutagenic effects of dental composite materials. Biomaterials. 2005;26(14):1713–9. doi: 10.1016/j.biomaterials.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Caughman WF, Caughman GB, Shiflett RA, Rueggeberg F, Schuster GS. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials. 1991;12(8):737–40. doi: 10.1016/0142-9612(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy SB, Washburn NR, Simon CG, Jr, Amis EJ. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials. 2006;27(20):3817–24. doi: 10.1016/j.biomaterials.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 20.Schweikl H, Muller R, Englert C, Hiller KA, Kujat R, Nerlich M, et al. Proliferation of osteoblasts and fibroblasts on model surfaces of varying roughness and surface chemistry. J Mater Sci Mater Med. 2007;18(10):1895–905. doi: 10.1007/s10856-007-3092-8. [DOI] [PubMed] [Google Scholar]
- 21.Mendonca G, Mendonca DB, Aragao FJ, Cooper LF. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials. 2008;29(28):3822–35. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Washburn NR, Yamada KM, Simon CG, Jr, Kennedy SB, Amis EJ. High-throughput investigation of osteoblast response to polymer crystallinity: influence of nanometer-scale roughness on proliferation. Biomaterials. 2004;25(78):1215–24. doi: 10.1016/j.biomaterials.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NH. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006;85(6):496–500. doi: 10.1177/154405910608500603. [DOI] [PubMed] [Google Scholar]
- 24.Kunzler TP, Drobek T, Schuler M, Spencer ND. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials. 2007;28(13):2175–82. doi: 10.1016/j.biomaterials.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Amis EJ. Reaching beyond discovery. Nat Mater. 2004;3(2):83–5. doi: 10.1038/nmat1064. [DOI] [PubMed] [Google Scholar]
- 26.Lin-Gibson S, Landis FA, Drzal PL. Combinatorial investigation of the structure-properties characterization of photopolymerized dimethacrylate networks. Biomaterials. 2006;27(9):1711–7. doi: 10.1016/j.biomaterials.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Lin NJ, Drzal PL, Lin-Gibson S. Two-dimensional gradient platforms for rapid assessment of dental polymers: a chemical, mechanical and biological evaluation. Dent Mater. 2007;23(10):1211–20. doi: 10.1016/j.dental.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Lin NJ, Hu H, Sung L, Lin-Gibson S. Quantification of cell response to polymeric composites using a two-dimensional gradient platform Combinatorial Chemistry & High Throughput Screening. 2009;12 doi: 10.2174/138620709788681943. in press. [DOI] [PubMed] [Google Scholar]
- 29.Lin NJ, Bailey LO, Becker ML, Washburn NR, Henderson LA. Macrophage response to methacrylate conversion using a gradient approach. Acta Biomater. 2007;3(2):163–73. doi: 10.1016/j.actbio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Kunzler TP, Huwiler C, Drobek T, Voros J, Spencer ND. Systematic study of osteoblast response to nanotopography by means of nanoparticle-density gradients. Biomaterials. 2007;28(33):5000–6. doi: 10.1016/j.biomaterials.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Zapata P, Su J, Garcia AJ, Meredith JC. Quantitative high-throughput screening of osteoblast attachment, spreading, and proliferation on demixed polymer blend micropatterns. Biomacromolecules. 2007;8(6):1907–17. doi: 10.1021/bm061134t. [DOI] [PubMed] [Google Scholar]
- 32.Mendez-Vilas A, Donoso MG, Gonzalez-Carrasco JL, Gonzalez-Martin ML. Looking at the micro-topography of polished and blasted Ti-based biomaterials using atomic force microscopy and contact angle goniometry. Colloids Surf B Biointerfaces. 2006;52(2):157–66. doi: 10.1016/j.colsurfb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Ran C, Ding G, Liu W, Deng Y, Hou W. Wetting on nanoporous alumina surface: transition between Wenzel and Cassie states controlled by surface structure. Langmuir. 2008;24(18):9952–5. doi: 10.1021/la801461j. [DOI] [PubMed] [Google Scholar]
- 34.Schuler M, Kunzler TP, de WM, Sprecher CM, Trentin D, Brunette DM, et al. Fabrication of TiO2-coated epoxy replicas with identical dual-type surface topographies used in cell culture assays. J Biomed Mater Res A. 2009;88(1):12–22. doi: 10.1002/jbm.a.31720. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999:1338–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]