Abstract
Tumor expression of inducible nitric oxide synthase (iNOS) predicts poor outcomes for melanoma patients. We have reported the regulation of melanoma iNOS by the mitogen-activated protein kinase (MAPK) pathway. In the current study, we test the hypothesis that NF-κB mediates this regulation. Western blotting of melanoma cell lysates confirmed the constitutive expression of iNOS. Baseline levels of activated nuclear ERK and NF-κB were also detected by western blot. Indirect immunofluorescence confirmed the presence of NF-κB p50 and p65 in melanoma cell nuclei, with p50 being more prevalent. Electrophoretic mobility shift assay demonstrated baseline NF-κB activity, the findings confirmed by supershift analysis. Treatment of melanoma cells with the MEK inhibitor U0126 decreased NF-κB binding to its DNA recognition sequence, implicating the MAPK pathway in NF-κB activation. Two specific NF-κB inhibitors suppressed iNOS expression, demonstrating regulation of iNOS by NF-κB. Several experiments indicated the presence of p50 homodimers, which lack a transactivation domain and rely upon the transcriptional co-activator Bcl-3 to carry out this function. Bcl-3 was detected in melanoma cells and co-immunoprecipitated with p50. These data suggest that the constitutively activated melanoma MAPK pathway stimulates activation of NF-κB hetero- and homodimers, which, in turn, drive iNOS expression and support melanoma tumorigenesis.
Keywords: MAPK, NF-κB, iNOS, p50 homodimer, Bcl-3
INTRODUCTION
Malignant melanoma is currently the most virulent form of skin cancer, with incidence and mortality rates steadily rising in the United States population (Jemal et al., 2007). Although much improvement has been achieved regarding the treatment of early stage disease, many patients die each year from melanoma metastases as tumor cells become increasingly resistant to chemotherapy. As such, the elucidation of pathways supporting metastatic cell growth is needed to develop specific targeted therapies for advanced disease.
The p44/42 mitogen-activated protein kinase (MAPK) pathway is arguably the most critical signaling cascade supporting the uncontrolled growth of melanoma cells (Smalley, 2003). In normal cells, the MAPK pathway is cytokine inducible. Signal induced by ligand binding is transported to a series of protein kinases that eventually culminate in the phosphorylation and activation of the extracellular signal-regulated kinase (ERK). Activated ERK translocates to the nucleus and initiates the transcription of a variety of growth-related genes. In melanoma cells, the MAPK pathway is constitutively active, a condition attributed in part to somatic mutations of NRAS and BRAF, which occur in the majority of cases (Goydos et al., 2005). The aberrant activation of this pathway confers a protective benefit to melanoma cells by driving their proliferation and survival. The critical nature of the MAPK pathway has generated interest in melanoma-specific targets downstream of ERK which may participate in these oncogenic processes.
One such target of particular interest to our laboratory is inducible nitric oxide synthase (iNOS). The catalytic product of iNOS is nitric oxide (NO), a diatomic free radical that mediates processes such as neurotransmission, vasodilation, and host defense in normal cells. NO has been shown to contribute to the pathogenesis of a variety of cancers, as well. (Thomsen et al., 1994; Cobbs et al., 1995). Specifically, in melanoma, iNOS-generated NO has been shown to protect tumor cells from apoptosis (Salvucci et al., 2001; Tang and Grimm, 2004). We have recently reported that the MAPK pathway drives constitutive iNOS expression in melanoma cells (Ellerhorst et al., 2006). Furthermore, we have published data demonstrating an association of tumor iNOS expression with poor prognosis in patients diagnosed with Stage III melanoma (Ekmekcioglu et al., 2006).
The findings described above have prompted us to explore the molecular links between melanoma ERK and iNOS, to further clarify this tumor-promoting pathway. A particularly promising candidate protein is the transcription factor NF-κB, the activation of which is a hallmark of many cancers, including melanoma. (Wang et al., 1999; Huang et al.,2000; Dhawan and Richmond, 2002; Tian et al., 2006). NF-κB consists of a family of structurally related proteins, including Rel A (p65), Rel B, c-Rel, NF-κB1 (p50/p105), and NF-κB2 (p52/p100). In quiescent cells, NF-κB exists as cytoplasmic hetero- or homodimers associated with an inhibitory protein belonging to the IkB family. Upon appropriate cytokine stimulation, IκB kinase (IKK) induces the phosphorylation of IκB, promoting its subsequent ubiquitination and degradation in the proteasome (Baldwin, 1996). This process allows the translocation of NF-κB to the nucleus where it binds to target DNA elements and induces transcription of various genes involved in inflammation and survival, including iNOS (Taylor et al., 1998). Evidence for a role of MAPK in NF-κB activation has been reported in several non-melanoma systems, as well as in the HS294T human melanoma cell line (Kurland et al., 2003; Wang et al., 1999; Dhawan and Richmond, 2002; Jiang et al., 2004).
We have thus hypothesized that NF-κB serves as the regulatory link between the MAPK pathway and iNOS expression in human melanoma cells. In the current study, we explore this proposed sequence of molecular signaling events and show that, indeed, the constitutively activated melanoma MAPK pathway drives NF-κB activation, which, in turn induces iNOS expression.
RESULTS
Human melanoma cells constitutively express activated ERK, activated NF-κB, and iNOS
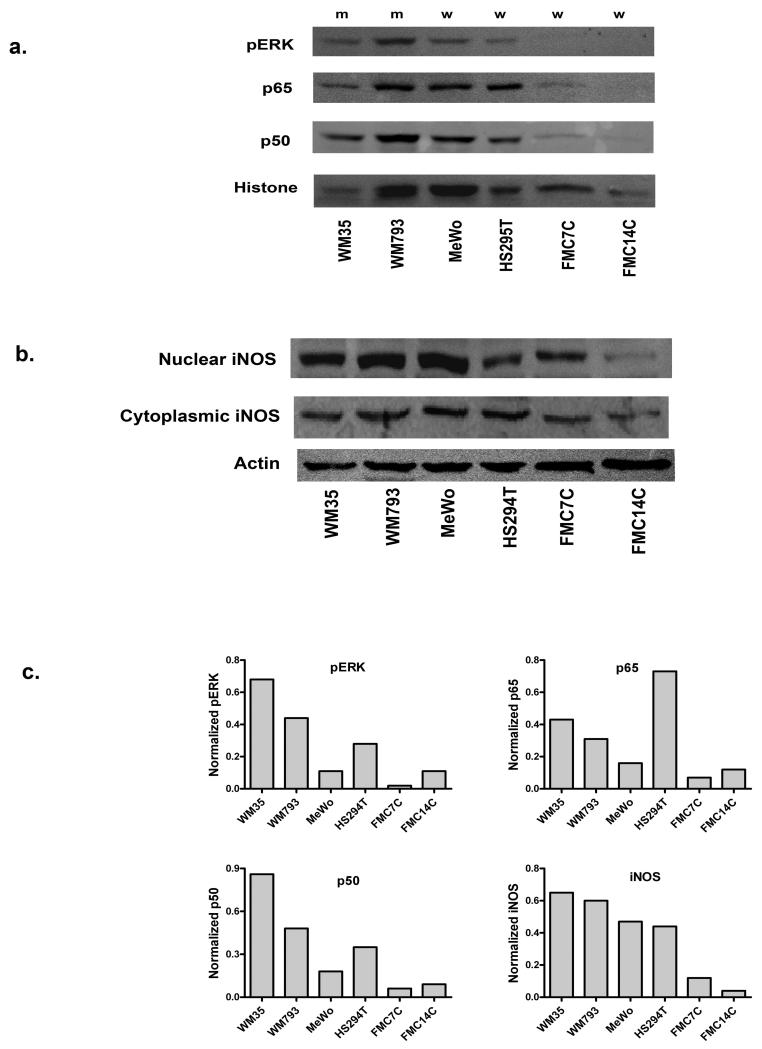
Initial experiments were performed to demonstrate the presence in melanoma cells of the three proteins included in our proposed pathway: ERK, NF-κB, and iNOS The human melanoma cell lines WM35, WM793, MeWo, and HS294T, as well as primary melanocytes (FMC7C and FMC14C), were used in these experiments. WM793 and WM35 carry BRAF T1799A mutations, whereas MeWo, HS294T, and both melanocyte lines are BRAF wild type. None of these lines carry NRAS mutations. Nuclear extracts prepared from these cells under basal growth conditions contained both NF-κB p50 and p65, and phosphorylated ERK (pERK), indicating the presence of these proteins in the activated form (Figure 1a). Nuclear expression varied from one cell line to the next, but tended to be higher in the melanoma cells when compared to the melanocytes (Figure 1c). A western blot of whole cell lysates from the same cells revealed the constitutive presence of iNOS protein and demonstrated the same pattern of higher levels in the melanoma cells (Figures 1b and 1c). An unanticipated observation was the presence of iNOS protein in the nuclear extracts of the melanoma cell lines and melanocytes (Figure 1b), a finding that has not been previously reported.
Figure 1.
Expression of activated NF-κB, activated ERK, and iNOS in melanoma cell lines and melanocytes. (a) Western blotting of nuclear extracts confirms the nuclear localization of pERK, and NF-κB p50 and p65 in these cell lines. Histone serves as a loading control. (b) Western blot of whole cell lysates demonstrates the constitutive presence of iNOS in melanoma cells and melanocytes. iNOS is present in nuclear extracts as well. (c) Normalization of the immunoblotting results in (a) and (b) to the histone or actin bands is shown, to control for differences in loading and transfer. The iNOS chart refers to cytoplasmic levels. m: BRAF mutant cell lines; w: BRAF wild type cell lines.
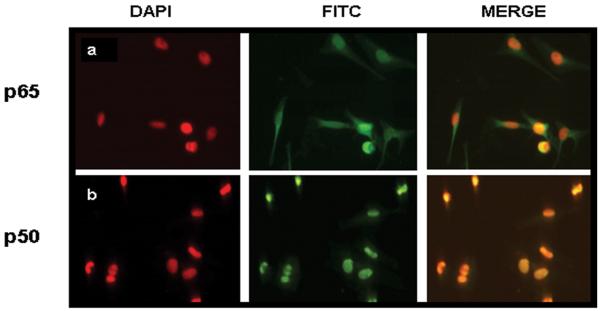
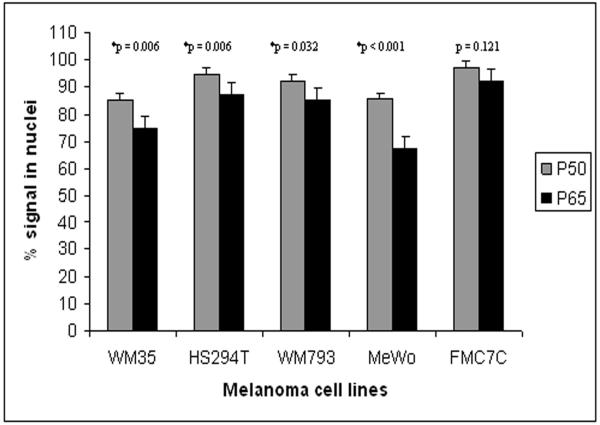
Indirect immunofluorescence studies were next carried out to confirm the nuclear localization of NF-κB in these cells. In keeping with the immunoblotting data, constitutive translocation of NF-κB p50 and p65 to the nuclei of melanoma cells was demonstrated (Figure 2). Because nuclear NF-κB can exist as p50/p65 heterodimers or p50 homodimers, the prevalence of each of these NF-κB subunits was examined in the immunofluorescently stained cells. Among the melanoma cell lines, there was a significant difference in the number of cells expressing nuclear p50 and p65, with some cells expressing only p50 (Figure 3). This finding was not observed in the melanocytes (FMC7C). These data suggest that melanoma cells utilize both heterodimeric and homodimeric NF-κB forms for transcriptional activation, and that the p50 homodimer activity may be tumor specific.
Figure 2.
Nuclear localization of NF-κB p50 and p65. Immunofluorescent staining reveals the presence of the NF-κB subunits in (a) MeWo and (b) HS294T cells.
Figure 3.
Comparison of the prevalences of NF-κB p50 and p65 signals in the nuclei of melanoma cells and melanocytes. A significantly higher number of melanoma cells express p50 relative to p65. Error bars represent the mean +/-SD. *P-values were found to be statistically significant by chi-square analysis.
The melanoma MAPK pathway regulates NF-κB
The first segment of the proposed MAPK/NF-κB/iNOS pathway involves the regulation of melanoma NF-κB by MAPK. An electrophoretic mobility shift assay (EMSA), carried out with nuclear extracts from WM793 cells at baseline, revealed NF-κB binding to its consensus oligonucleotide DNA target sequence (Figure 4a). Supershift experiments with antibodies to NF-κB p50 and p65 revealed even greater retardation of the DNA:protein:antibody complexes. The antibody to NF-κB p50 produced a more complete supershift than antibody to the p65 subunit, consistent with the retardation of both p50 homodimers and p50/p65 heterodimers by the anti-p50 antibody, whereas the p65 antibody recognizes only the heterodimers, leaving the homodimers unaffected (Figure 4b).
Figure 4.
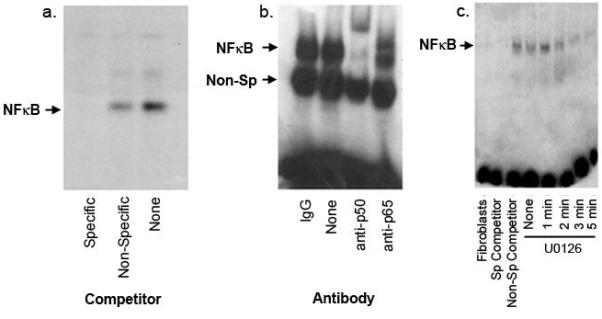
Melanoma NF-κB is regulated by the MAPK pathway. (A) EMSA was carried out with 32P-labeled NF-κB target oligonucleotide and 4μg of nuclear protein from unstimulated WM793 cells. Binding experiments were performed in the presence of a specific competitor (unlabeled NF-κB oligonucleotide), a non-specific competitor (SP1 target oligonucleotide), and in the absence of competitor. Results demonstrate NF-κB binding activity in these lysates. (B) Supershift experiments were conducted to confirm the specificity of NF-κB binding. Four micrograms of lysate were incubated with one microgram of antibody to p50 or p65, followed by EMSA analysis. (C) Nuclear extracts of WM793 cells treated with 10μm U0126 were prepared and examined for NF-κB activity. Reversal of NF-κB binding is observed. Sp: specific.
To test the hypothesis that NF-κB is regulated by the melanoma MAPK pathway, additional EMSA experiments were performed using WM793 cells to assess the effects of the MEK inhibitor U0126 on NF-κB nuclear binding activity. These experiments revealed diminished NF-κB binding as early as 2 minutes, with nearly complete abrogation of activity after 5 minutes of exposure to U0126 (Figure 4c). Experiments repeated with MeWo cells yielded similar results (data not shown). These data convincingly support the activation of NF-κB by the MAPK pathway in melanoma cells.
NF-κB regulates iNOS expression in melanoma cells
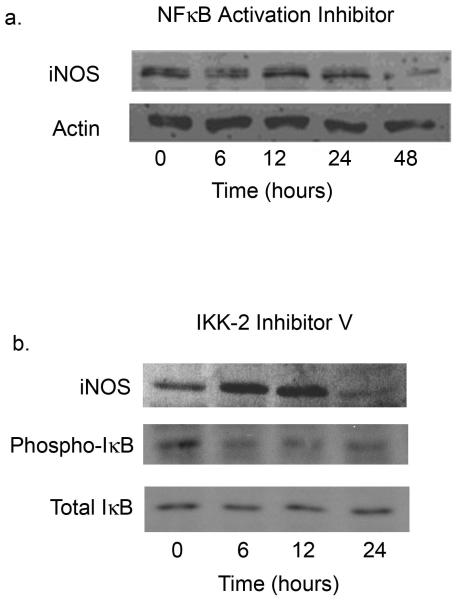
The second segment of the proposed pathway involves the induction of melanoma iNOS expression by NF-κB. To examine this regulatory event, melanoma cells were treated with each of two inhibitors which target different aspects of NF-κB activation. The first, NF-κB Activation Inhibitor, interferes with the transcriptional ability of NF-κB, whereas the second inhibitor, IKK-2 inhibitor V, selectively blocks IκBα phosphorylation, preventing the translocation of NF-κB to the nucleus.
Figure 5a shows the inhibition of iNOS protein expression in MeWo cells over the course of a 48-hour incubation with NF-κB Activation Inhibitor. Similar findings are demonstrated in WM793 cells treated with IKK-2 inhibitor V (Fig. 5b); in this experiment, the decline in iNOS levels is preceded by a reduction in phosphorylation of IκBα. With both inhibitors, the stable, dimeric iNOS functional unit persists for the first 12 hours, but is markedly diminished thereafter. Similar findings were seen with WM35 cells (data not shown). These results are consistent with the hypothesis that iNOS expression is induced by NF-κB in human melanoma.
Figure 5.
NF-κB regulates iNOS expression in melanoma cells. MeWo cells (a) were treated with the NF-κB Activation Inhibitor (10 μM) and WM793 cells (b) with IKK-2 inhibitor V (1 μM) over a time course of up to 48 hours. Western blotting was performed for iNOS protein and, in the case of IKK-2 Inhibitor V, for total and phosphorylated IkBa.
Melanoma cells express the transcriptional co-activator Bcl-3 which complexes with NF-κB p50
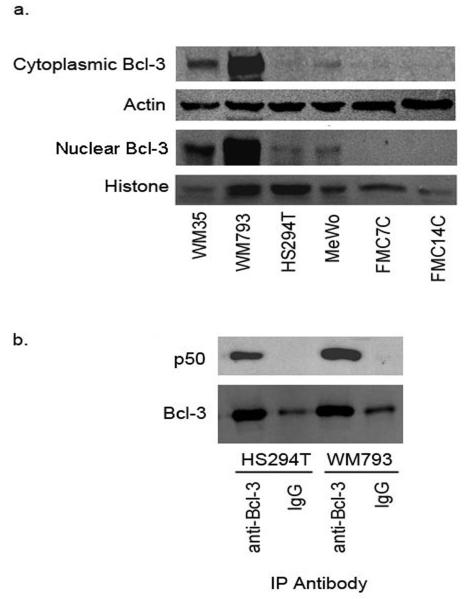
Data from several of the experiments described above indicated the presence of both NF-κB p50 homodimers and p50/p65 heterodimers in the nuclei of melanoma cells. The p50 homodimer binds DNA in vitro, but lacks a transcriptional activation domain and, consequently, initiates transcription weakly, if at all. In fact, this NF-κB homodimer appears to function as transcriptional inhibitor in some cell types by competing with p50/p65 for promoter binding (Kang et al., 1992; Plaksin et al., 1993). Countering this inhibitory effect in a variety of transformed cell types is the oncoprotein Bcl-3, which binds NF-κB p50 homodimers and robustly co-activates transcription (Fujita et al., 1993). Experiments were thus conducted to explore a potential role of Bcl-3 in melanoma NF-κB p50 transcriptional co-activation. Western blots of whole cell lysates and nuclear extracts from melanoma cells revealed the presence of the Bcl-3 protein in both the cytoplasmic and nuclear compartments (Figure 6a). Notably, Bcl-3 was virtually undetectable in melanocytes. Subsequently, the association of Bcl-3 and NF-κB p50 in melanoma cells was examined. Proteins from whole cell lysates were immunoprecipitated with rabbit IgG or anti-Bcl-3 antibody, followed by western blotting with antibody to p50. Figure 6b demonstrates the presence of NF-κB p50 in the Bcl-3 immunoprecipitates, indicating the presence of Bcl-3/p50 complexes in melanoma cells, with the potential for transcriptional activity.
Figure 6.
Melanoma cells express Bcl-3/p50 complexes. (a) Whole cell lysates and nuclear extracts from melanoma cells and melanocytes were used to carry out western blotting for Bcl-3. (b) Whole cell lysates from melanoma cells were used for immunoprecipitation with anti-Bcl-3 antibody followed by western blotting with anti-p50.
DISCUSSION
The Ras/Raf/MEK/ERK pathway is a major signaling pathway involved in tumor cell growth and survival. As human melanoma is driven by constitutive activation of this pathway, elucidation of downstream effector molecules is critical to the understanding of melanoma pathobiology. We have previously reported the positive regulation of melanoma iNOS expression by the MAPK pathway and the profoundly negative clinical implications of tumor iNOS expression in this malignancy (Ekmekcioglu et al., 2006; Ellerhorst et al., 2006). We now show that NF-κB mediates this regulatory event. Previous work in the literature has described the activation of NF-κB by the MAPK pathway in the HS294T human melanoma cell line (Dhawan and Richmond, 2002). Our data now confirm and extend this finding, as we demonstrate that the inhibition of MAPK signaling with the MEK inhibitor U0126 is accompanied by diminished nuclear NF-κB DNA binding. Furthermore, this is this first study to show that NF-κB regulates expression of iNOS in melanoma cells. Notably, the MAPK/NF-κB/iNOS pathway was demonstrated in both BRAF mutant and wild type cells, suggesting that the source of MAPK activation does not alter these downstream events.
An unexpected finding was the presence of iNOS in the nuclei of cultured melanoma cells and melanocytes. Although nuclear iNOS has been reported in neutrophils and adipocytes of rat origin (Giordano et al., 2002; Saini et al., 2006), this is the first description of iNOS in the nuclei of human cells. Of note, endothelial NOS (eNOS) has been detected in the nuclei of cultured human mast cells (Gilchrist et al., 2004). In those cell lines, eNOS phosphorylation preceded translocation from the cytoplasm to the nucleus. The specific role of nuclear NOS and, implicitly, nuclear NO, has yet to be explored. In melanoma cells, it could be hypothesized that nuclear compartmentalization of iNOS and NO may confer a strategic advantage in terms of efficient modification of DNA and nuclear proteins by nitric oxide, in such a way as to promote growth and survival. Although nuclear iNOS was detected in melanocytes as well, these normally quiescent cells are maintained in growth media containing potent mitogens such as cholera toxin and phorbol myristate acetate, providing growth pathway stimulation similar to that seen in tumor cells. It therefore remains to be determined whether nuclear translocation of iNOS is a tumor-specific event. Future research in our laboratory will address this issue as well as the question of iNOS phosphorylation, as has been described for eNOS.
The presence of NF-κB p50 homodimers in the nuclei of melanoma cells is an intriguing finding. This NF-κB subtype may play a role in the competitive transcriptional inhibition of p50/p65, a function frequently attributed to the p50 homodimer (Kang et al., 1992; Plaksin et al., 1993). However, the expression of the transcriptional co-activator Bcl-3 by the melanoma cell lines, and the complexing of Bcl-3 with p50, provide preliminary evidence to suggest that the p50 homodimers may be transcriptionally active. The oncoprotein Bcl-3 is a member of the IκB family of NF-κB -binding proteins. The BCL3 gene, located on chromosome 19, is translocated intact and is activated in cases of t(14;19)(q32.3;q13.2) B-cell chronic lymphocytic leukemia (Ohno et al., 1990). Bcl-3 has additionally been implicated in the pathogenesis of nasopharyngeal carcinoma, breast cancer, and a variety of lymphomas, all cases giving evidence for co-activation of NF-κB p50 by Bcl-3 as the transforming event (Fujita et al., 1993; Watanabe et al., 1997; Cogswell et al., 2000; Thornburg et al., 2003; Mathas et al., 2005). Bcl-3 expression and its interaction with NF-κB p50 have not been previously reported in melanoma. It is interesting to speculate that Bcl-3/p50 complexes may play an important role in the transcription of melanoma-promoting genes, such as INOS.
In summary, we have further defined the pathway leading from the constitutively active melanoma MAPK pathway to iNOS expression by demonstrating that NF-κB is an important intermediary protein. In the process, we have made the novel observations of the nuclear localization of iNOS and of Bcl-3/NF-κB p50 complexes in cultured melanoma cells. These findings will stimulate further exploration of these regulatory molecules and pathways, always with the ultimate goal of identifying targets for future therapy.
MATERIALS AND METHODS
Cell lines
The human melanoma cell lines WM793 and WM35 were generous gifts from Dr. Robert Kerbel (Sunnybrook Health Science Center, Toronto, ON, Canada) and the human metastatic melanoma MeWo cell line was provided by Dr. David Menter (M. D. Anderson Cancer Center). The HS294T melanoma cell line was kindly provided by Dr. Ann Richmond (Vanderbilt University School of Medicine, Nashville TN). BJ fibroblasts were purchased from the American Type Culture Collection (Manassas, VA). Cells were grown in RPMI 1640 medium, supplemented with 10% fetal bovine serum (FBS). Primary melanocytes FMC7C and FMC14C were derived from neonatal foreskin and were maintained in MCDB-153 media (Sigma, St. Louis MO), supplemented with 1% FBS, 10 ng/ml phorbal-12-myristate-13-acetate (Calbiochem, San Diego CA), 1 ng/ml basic fibroblast growth factor (Invitrogen, Carlsbad, CA), 5 ug/ml transferrin (Sigma), 10 nM cholera toxin (Calbiochem), 0.1 mM 3-isobutyl-1-methylxanthine (Calbiochem), 30 ug/ml bovine pituitary extract (Sigma), and 5ug/ml insulin (Sigma). All cells were maintained at 37°C with 5% CO2.
Reagents and antibodies
NF-κB Activation inhibitor and IKK-2 inhibitor V were purchased from Calbiochem and U0126 from Cell Signaling Technology (Beverly, MA). Protein A agarose was obtained from Sigma. Antibodies to ERK, phosphorylated ERK, IκBα, and phosphorylated IκBα were purchased from Cell Signaling Technology. Antibodies to histone, iNOS, and NF-κB p50 and p65 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti Bcl-3 antibody was purchased from Abcam (Cambridge, MA).
Whole cell extracts and western blotting
Cells were washed with cold PBS and harvested into PBS with 1 mM phenylmethylsulfonyl fluoride (PMSF). Cell pellets were then treated with lysis buffer (140 mM NaCl, 25 mM Tris HCl pH 7.4, and 1% NP-40) with freshly added protease inhibitor cocktail (BD Biosciences, San Jose, CA). The supernatants were then collected after rigorous agitation and protein concentration was measured. Proteins were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and blocked for 1 hour in 5% nonfat milk in PBS. Primary antibody was diluted in 5% nonfat dry milk/PBS/ 0.1% Tween and incubated overnight at 4°C, followed by 45 minutes incubation with horseradish peroxidase labeled secondary antibody, again diluted in 5% nonfat dry milk/PBS/ 0.1% Tween. Membrane development was achieved with enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ).
Nuclear extracts
The two extraction buffers used, A and B, comprised of the following reagents: Buffer A: 10 mM Hepes pH 7.9, 2 mM MgCl2, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 5 mM NaF, 1 mM Na3VO4; Buffer B: 20 mM Hepes, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 5 mM NaF, 1 mM Na3VO4. Cells were washed with cold PBS and harvested into PBS/1mM PMSF. Cells were then treated with cold buffer A, followed by the addition of protease inhibitor cocktail 1:50. After the addition of 10% NP-40 and centrifugation at 3500g, the cytoplasmic fraction was collected. The remaining pellet was washed 3 times with buffer A, followed by the addition of Buffer B and 1:50 protease cocktail inhibitor. After final centrifugation at 16,000g, the nuclear fraction was collected.
Indirect immunofluorescence
Cells grown on chamber slides were fixed with 2% paraformaldehyde on ice (30 minutes), blocked with 5% serum in PBS (30 minutes room temperature), and incubated with primary antibody diluted in blocking solution (2 hours, 4°C). This was followed by incubation with FITC-labeled secondary antibody (1 hour, room temperature). Staining was observed and imaged with a Nikon Eclipse TE 2000-U microscope equipped with a Nikon digital DXM 1200F camera.
Electrophoretic mobility shift assay
Nuclear protein extracts for EMSA were prepared as described above. The NF-κB, AP2, and SP1 target oligonucleotides and gel shift binding buffer were purchased from Promega (Madison, WI). Binding reactions were performed with 4 μg of nuclear extract and 1.75 pmol of NF-κB target oligonucleotide (unlabeled or end-labeled with 32P). AP2 or SP1 target DNA sequences were used as nonspecific competitor oligonucleotides. The sequence of the double-stranded NF-κB target oligonucleotide is:
5′-AGTTGAGGGGACTTTCCCAGGC-3′
3′-TCAACTCCCCTGAAAGGGTCCG-5′
DNA-protein complexes were resolved on a 6% non-denaturing gel. The gel was dried and exposed to film at -70 °C. For supershift experiments, 2-4 μg of nuclear extracts were incubated with antibodies to the different subunits of NF-κB for 20 minutes prior to the addition of the radiolabeled probe.
Immunoprecipitation
Whole cell lysates were prepared as described above. A mixture of Protein A agarose, ice-cold PBS and 1μg of anti-Bcl-3 or rabbit IgG was tumble incubated overnight at 4°C. Whole cell extracts were precleared with Protein A agarose for 30 minutes at 4°C followed by high speed centrifugation. Precleared lysates were added to the protein A agarose/antibody mixture and incubated for 2 hours at 4°C. Complexes were washed with wash buffer (0.1% Triton X-100, 50 mM Tris pH 7.4, 300 mM NaCl, and 5 mM EDTA), resuspended in SDS-PAGE loading buffer, and examined by western blotting.
Supplementary Material
Supplemental Figure 1. ChIP analysis for the binding of p65 to the -5.8 kb region of the iNOS promoter in the melanoma cell line WM793. The experiment was performed using the QuickChIP kit from Imgenex (San Diego, CA) according to the manufacturer’s directions. PCR results show bands of the appropriate size for the input DNA and the anti-p65 immunoprecipitation, and a weaker band for the IgG control. neg, negative PCR control.
Supplemental Figure 2. Specificity of the p50 and p65 antibodies in western blotting. Immunoblots of melanoma cell lysates for NFκB p50 and p65 are shown in their entirety. Bands of the correct size are demonstrated and no extraneous bands are seen.
Supplemental Figure 3. Specificity of the p65 and p50 antibodies in indirect immunofluorescence. Cells were plated on chamber slides and incubated with antibody to p50 or p65 in the absence or presence of specific blocking peptide (10-fold molar excess for p65, 100-fold molar excess for p50). The remainder of the procedure is the same as described in Materials and Methods. A complete loss of staining occurs in the presence of peptide, indicating that antibody binding is specific. Antibodies and peptides were purchased from Santa Cruz.
Supplemental Figure 4. BRAF exon 15 sequencing chromatograms for the six cell lines used in the manuscript. WM35 and WM793 carry the T1799A mutation. The remaining lines are wild type at this locus. Only the relevant portions of the chromatograms are shown.
ACKNOWLEDGMENTS
We are grateful to the following individuals for their valuable technical assistance in this project: Ms. Marilyn Johnson, Ms. Carolyn Cooke, Dr. Shyam Dang, and Dr. Eugene Walch. This work was supported by NIH P50 CA093459 (DGU, EAG, JAE) and NIH R01 CA90282 (EAG). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Baldwin AS. The NF-κB and IκB proteins: New discoveries and insights. Ann Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA. Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res. 1995;55:727–730. [PubMed] [Google Scholar]
- Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS. Selective activation of NF-κB subunits in human breast cancer: potential roles for NF-κB/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. A novel NF-κB-inducing kinase-MAPK signaling pathway up-regulates NF-κB activity in melanoma cells. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerhorst JA, Ekmekcioglu S, Johnson MK, Cooke CP, Johnson MM, Grimm EA. Regulation of iNOS by the p44/42 mitogen-activated protein kinase pathway in human melanoma. Oncogene. 2006;25:3956–3962. doi: 10.1038/sj.onc.1209419. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119:861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-κB p50 homodimers. Genes & Dev. 1993;7:1354–1363. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- Gilchrist M, McCauley SD, Befus AD. Expression, localization, and regulation of NOS in human mast cell lines: effects on leukotriene production. Blood. 2004;104:462–469. doi: 10.1182/blood-2003-08-2990. [DOI] [PubMed] [Google Scholar]
- Giordano A, Tonello C, Bulbarelli A, Cozzi V, Cinti S, Carruba MO, et al. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett. 2002;514:135–140. doi: 10.1016/s0014-5793(02)02245-7. [DOI] [PubMed] [Google Scholar]
- Goydos JS, Mann B, Kim HJ, Gabriel EM, Alsina J, Germino FJ, et al. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg. 2005;200:362–370. doi: 10.1016/j.jamcollsurg.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-κB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573–2581. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J Biol Chem. 2004;279:1323–1329. doi: 10.1074/jbc.M307521200. [DOI] [PubMed] [Google Scholar]
- Kang SM, Tran AC, Grilli M, Lenardo MJ. NF-κB subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992;256:1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Kurland JF, Voehringer DW, Meyn RE. The MEK/ERK pathway acts upstream of NF-κB1 (p50) homodimer activity and Bcl-2 expression in a murine B-cell lymphoma line. J Biol Chem. 2003;34:32465–32470. doi: 10.1074/jbc.M212919200. [DOI] [PubMed] [Google Scholar]
- Mathas S, Johrens K, Joos S, Lietz A, Hummel F, Janz M, et al. Elevated NF-κB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas. Blood. 2005;106:4287–4293. doi: 10.1182/blood-2004-09-3620. [DOI] [PubMed] [Google Scholar]
- Ohno H, Takimoto G, McKeithan TW. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990;60:991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Plaksin D, Baeuerle PA, Eisenbach L. KBF1 (p50 NF-κB homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. J Exp Med. 1993;177:1651–1662. doi: 10.1084/jem.177.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R, Patel S, Saluja R, Sahasrabuddhe AA, Singh MP, Habib S, et al. Nitric oxide synthase localization in the rat neutrophils: immunocytochemical, molecular, and biochemical studies. J Leukoc Biol. 2006;79:519–528. doi: 10.1189/jlb.0605320. [DOI] [PubMed] [Google Scholar]
- Salvucci O, Carsana M, Bersani I, Tragni G, Anichini A. Antiapoptotic role of endogenous nitric oxide in human melanoma cells. Cancer Res. 2001;61:318–326. [PubMed] [Google Scholar]
- Smalley KSM. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–532. doi: 10.1002/ijc.10978. [DOI] [PubMed] [Google Scholar]
- Tang CH, Grimm EA. Depletion of endogenous nitric oxide enhances cisplatin-induced apoptosis in a p53-dependent manner in melanoma cell lines. J Biol Chem. 2004;279:288–298. doi: 10.1074/jbc.M310821200. [DOI] [PubMed] [Google Scholar]
- Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, et al. Multiple NF-κB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Lawton FG, Knowles RG, Beesley JE, Riveros-Moreno V, Moncada S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994;54:1352–1354. [PubMed] [Google Scholar]
- Thornburg NJ, Pathmanathan R, Raab-Traub N. Activation of nuclear factor-κB p50 homodimer/Bcl-3 complexes in nasopharyngeal carcinoma. Cancer Res. 2003;63:8293–8301. [PubMed] [Google Scholar]
- Tian F, Zang WD, Hou WH, Liu HT, Xue LX. Nuclear factor-kB signaling pathway constitutively activated in esophageal squamous cell carcinoma cell lines and inhibition of growth of cells by small interfering RNA. Acta Biochim Biophys Sin. 2006;38:318–326. doi: 10.1111/j.1745-7270.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-κB relA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- Watanabe N, Iwamura T, Shinoda T, Fujita T. Regulation of NFKB1 proteins by the candidate oncoprotein BCL-3: generation of NF-κB homodimers from the cytoplasmic pool of p50-p105 and nuclear translocation. EMBO J. 1997;16:3609–3620. doi: 10.1093/emboj/16.12.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. ChIP analysis for the binding of p65 to the -5.8 kb region of the iNOS promoter in the melanoma cell line WM793. The experiment was performed using the QuickChIP kit from Imgenex (San Diego, CA) according to the manufacturer’s directions. PCR results show bands of the appropriate size for the input DNA and the anti-p65 immunoprecipitation, and a weaker band for the IgG control. neg, negative PCR control.
Supplemental Figure 2. Specificity of the p50 and p65 antibodies in western blotting. Immunoblots of melanoma cell lysates for NFκB p50 and p65 are shown in their entirety. Bands of the correct size are demonstrated and no extraneous bands are seen.
Supplemental Figure 3. Specificity of the p65 and p50 antibodies in indirect immunofluorescence. Cells were plated on chamber slides and incubated with antibody to p50 or p65 in the absence or presence of specific blocking peptide (10-fold molar excess for p65, 100-fold molar excess for p50). The remainder of the procedure is the same as described in Materials and Methods. A complete loss of staining occurs in the presence of peptide, indicating that antibody binding is specific. Antibodies and peptides were purchased from Santa Cruz.
Supplemental Figure 4. BRAF exon 15 sequencing chromatograms for the six cell lines used in the manuscript. WM35 and WM793 carry the T1799A mutation. The remaining lines are wild type at this locus. Only the relevant portions of the chromatograms are shown.