Abstract
Introduction
Prenatal alcohol exposure via maternal liquid diet consumption by C57BL/6 (B6) mice causes conspicuous midline neural tube deficit (dysraphia) and disruption of genesis and development of serotonin (5-HT) neurons in the raphe nuclei, together with brain growth retardation. The current study tested the hypothesis that concurrent treatment with either an activity-dependent neurotrophic factor (ADNF) agonist peptide [SALLRSIPA, (SAL)] or an activity-dependent neurotrophic protein (ADNP) agonist peptide [NAPVSIPQ, (NAP)] would protect against these alcohol-induced deficits in brain development.
Methods
Timed-pregnant B6 dams consumed alcohol from embryonic day 7 (E7, before the onset of neurulation) until E15. Fetuses were obtained on E15 and brain sections processed for 5-HT immunocytochemistry, for evaluation of morphologic development of the brainstem raphe and its 5-HT neurons. Additional groups were treated either with SAL or NAP daily from E7 to E15 to assess the potential protective effects of these peptides. Measures of incomplete occlusion of the ventral canal and the frequency and extent of the openings in the rhombencephalon were obtained to assess fetal dysraphia. Counts of 5-HT-immunostained neurons were also obtained in the rostral and caudal raphe.
Results
Prenatal alcohol exposure resulted in abnormal openings along the midline and delayed closure of ventral canal in the brainstem. This dysraphia was associated with reductions in the number of 5-HT neurons both in the rostral raphe nuclei (that gives rise to ascending 5-HT projections) and in the caudal raphe (that gives rise to the descending 5-HT projections). Concurrent treatment of the alcohol-consuming dams with SAL prevented dysraphia and protected against the alcohol-induced reductions in 5-HT neurons in both the rostral and caudal raphe. NAP was less effective in protecting against dysraphia and did not protect against 5-HT loss in the rostral raphe, but did protect against loss in the caudal raphe.
Conclusions
These findings further support the potential usefulness of these peptides for therapeutic interventions in pregnancies at risk for alcohol-induced developmental deficits. Notably, the ascending 5-HT projections of the rostral raphe have profound effects in regulating forebrain development and function, and the descending 5-HT projections of the caudal raphe are critical for regulating respiration. Protection of the rostral 5-HT-system may help prevent structural and functional deficits linked to abnormal forebrain development, and protection of the caudal systems may also reduce the increased risk for sudden infant death syndrome associated with prenatal alcohol exposure.
Keywords: Fetal Alcohol Syndrome, Dysraphia, Neurotrophic Factor, Neural Tube Defect, Activity-Dependent Neuroprotective Peptide
Fetal alcohol syndrome (FAS) is the leading nongenetic cause of developmental disabilities in the Western world, with an estimated incidence in the United States between 0.5 and 2.0 cases per 1000 live births (Bertrand et al., 2005). FAS is diagnosed by the presence of craniofacial dysmorphology, growth retardation, and evidence of central nervous system dysfunction (Jones and Smith, 1973; Jones et al., 1973). However, it is estimated that there may be up to 10 times as many individuals with confirmed prenatal alcohol exposure who have significant brain damage and cognitive/behavioral deficits, but who do not have the facial dysmorphology or growth retardation needed to fulfill the diagnostic criteria for FAS (Aase et al., 1995; Barr and Streissguth, 2001; Mattson et al., 1998; Roebuck et al., 1998; Sampson et al., 1997; Stratton et al., 1996). The term fetal alcohol spectrum disorders (FASD) has been adopted as an umbrella classification to convey the wide range of phenotypic effects resulting from prenatal alcohol exposure, ranging from subtle effects on growth or neurobehavioral function to full-blown FAS at the severe end of the spectrum (Bertrand et al., 2005; Manning and Hoyme, 2007).
Abnormal development of midline structures of the face and brain is a hallmark of FAS. These include the characteristic features of facial dysmorphology (indistinct philtrum, narrow palpebral fissures, short nose, and flattened mid-face) and the disproportionate reductions in midline brain structures such as the corpus callosum, the anterior vermis of the cerebellum, and the caudate nucleus (Bookstein et al., 2002a,b; Sowell et al., 2001; Swayze et al., 1997; Zimmerberg and Scalzi, 1989). Profound structural abnormalities in midline brain regions have also been modeled by heavy-binge alcohol exposure on gestational day 7 in mice (Sulik and Johnston, 1983; Sulik et al., 1984). These outcomes suggest that alcohol may alter cellular processes associated with neural tube fusion and early morphogenesis of the midline neuroaxis. Under very high exposure circumstances, total dysraphia may result, i.e., the failure of the neural tube to close or rupture of a closed neural tube (Gardener, 1973), as has been reported in a mouse embryo culture model (Chen et al., 2005) and in human autopsy cases of fetuses from the first trimester that failed to survive heavy prenatal alcohol exposure (Kovetskii, 1991).
Using a mouse model of prenatal alcohol exposure in which inbred C57BL/6 (B6) dams drink alcohol in a chocolate-flavored liquid diet as their sole source of calories beginning on gestational day 7 (just before the onset of neurulation), we have identified a midline neural tube deficit that is evident microscopically in the fetal brain in either (or both) the floor or roof plates (Zhou et al., 2003). In the brainstem, a delayed occlusion of ventral canal (fusion of 2 sides of the brainstem) was evident. This effect on embryonic brain development may best be characterized as an incomplete dysraphia, because the neural tube and ventral canal eventually did close with this alcohol exposure paradigm. Yet, adverse consequences are likely to result from this midline abnormality, including mistimed formation and differentiation of midline neurons and altered growth of fiber processes that cross the midline. Consistent with that, we have shown a delayed formation of the rostral raphe nuclei in the brainstem and reduced numbers and dystrophic morphogenesis of serotonin (5-HT) neurons in the dorsal and median raphe in this model (Zhou et al., 2001, 2002). Deficits in development of the 5-HT system have also been shown in rat models of prenatal exposure using liquid diets (Druse et al., 1991).
Development of effective strategies of interventions (prevention and treatment) is an important goal of current fetal alcohol research. Prevention of FASD would be assured by abstinence from alcohol drinking during pregnancy. However, interventions intended to reduce at-risk drinking during pregnancy are often unsuccessful in alcohol-dependent individuals or are difficult to implement for individuals at greatest risk for an FASD outcome. Moreover, effective treatment of children with FASD has not yet been identified (Kalberg and Buckley, 2007). One potential approach to reduce the incidence and life-long adverse consequences of FASD is to develop a molecular intervention that could limit or prevent the effects of prenatal alcohol exposure on the developing brain. Towards that goal, several agents targeting various putative mechanisms of pathogenesis have been demonstrated to have some success in different experimental animal models [(Chen et al., 2001; Ieraci and Herrera, 2006; Kim and Druse, 1996; Thomas et al., 2000, 1997; Wilkemeyer et al., 2002; for review see Goodlett et al. (2005)].
One promising molecular intervention for alcohol-induced teratogenesis is a class of neurotrophic factors that are regulated by vasoactive intestinal peptide in an activity-dependent fashion. These trophic molecules known as activity-dependent neurotrophic factor (ADNF) (Gozes and Brenneman, 1996; Gressens et al., 1999; Guo et al., 1999) and the structural and immunological homologue, activity-dependent neuroprotective protein (ADNP) (Bassan et al., 1999; Gozes et al., 1999) have been shown to prevent alcohol-induced growth deficits and embryonic teratogenesis (Spong et al., 2001), including the total dysraphia produced in cultured mouse embryos by high alcohol concentrations (Chen et al., 2005). ADNF and ADNP have extremely potent neurotrophic (Brenneman and Gozes, 1996; Brenneman et al., 1997) or neuroprotective actions (Gressens et al., 1997), with an efficacy in the femtomolar range (Gozes and Brenneman, 1996). A functional peptide fragment of ADNF, SALLRSIPA (SAL), a potent neurotrophic agent (Brenneman et al., 1998), ameliorated microencephaly in B6 fetal mice induced by prenatal alcohol exposure via maternal liquid diet consumption (Zhou et al., 2004). A functional peptide fragment of ADNP, NAPVSIPQ (NAP), which shares many protective effects of SAL, has also been identified as a potent neuroprotectant (Gozes and Brenneman, 2000; Zamostiano et al., 1999).
The goal of the current study was to test the hypothesis that the NAP and SAL peptides would prevent alcohol-induced deficits in embryonic midline development in the brainstem (dysraphia) and would prevent the alcohol-induced deficits in early development of 5-HT neurons in the rostral and caudal raphe. Notably, the current study is the first to test whether there is an alcohol-induced effect on development of 5-HT neurons in the caudal raphe.
MATERIALS AND METHODS
Testing NAP and SAL in C57BL/6 Mouse Liquid Diet Model
The protocols used in this study were reviewed and approved in advance by the Indiana University School of Medicine Institutional Animal Care and Use Committee, and the use of animals followed the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996). C57BL/6 mice were obtained from Harlan, Inc. (Indianapolis, IN) and were acclimated for at least 1 week prior to mating. The mice were maintained in the Indiana University Laboratory Animal Research Center vivarium with ad lib chow and water at 22°C room temperature, 30% humidity, on a reverse 12:12 light:dark cycle (lights off at 9:00 am). Only nulliparous females were used for mating. Females were placed with a male for 2 hours and checked for a sperm plug immediately after the mating session. Positive sperm plug detection was designated as embryonic day 0 (E0), hour 0. Within a breeding cohort, females were mated in subgroups separated by 2 to 3 days. This facilitated contemporaneous pair-feeding (below), which required a lag in gestation between the alcohol (ethanol)-consuming dam and its matched pair-fed (PF) dam.
Pregnant females were weighed daily and on the day before treatment were assigned pseudo randomly (matched for weight) to 1 of 3 dietary treatment groups that started on E7: (i) free access alcohol liquid diet (ALC) delivering 25% ethanol-derived calories (EDC) as the sole source of nutrients; (ii) PF control, yoked individually (with a 2 to 3 day lag) to an ALC dam and given free access to matched daily amounts of isocaloric liquid diet in which maltose–dextrin was isocalorically substituted for ethanol; or (iii) ad libitum chow and water (Chow) at all times during gestation.
The liquid diet was a fortified Sustacal formula adapted from the published protocols reported by Middaugh and Ayers (1988). The diet contained 237 ml of chocolate-flavored Sustacal (Mead Johnson, Evansville, IL), 1.44 g Vitamin Diet Fortification Mixture (ICN #904654), and 1.2 g Salt Mixture XIV (ICN #902850). For the ethanol diet, 15.3 ml of 95% ethanol was added to the fortified Sustacal formula, and water was then added to make 320 ml of diet containing 1 Cal/ml (25% EDC; ethanol 4.54% v/v). For the isocaloric control diet, 20.2 g maltose–dextrin (BioServ, San Diego, CA) was added to the fortified Sustacal formula with water to bring it to 1 Cal/ml. On E6, 1 day before the diet manipulations began, all dams of the ALC and PF groups were adapted to the liquid diet by giving the control diet (without ethanol) as their sole source of calories. Each day between 8:00 am and 9:00 am, the dams were weighed, the volume of liquid diet consumed during the previous 24 hours was recorded from 30-ml graduated screw-cap tubes, and freshly prepared diet was provided.
For the alcohol-consuming dams, subgroups of randomly assigned dams were given either saline vehicle injections (ALC; n = 9) or NAP (ALC + NAP; n = 9) or SAL (ALC + SAL; n = 10). The daily dose of either NAP or SAL, freshly prepared in sterile saline, was 20 µg/0.2 ml/dam, a dose adopted from our previous study. The l-form NAP and SAL were synthesized in the Molecular Biotechnology Facility, Indiana University School of Medicine (IN) and tested for efficacy against cultured cells by Dr. D. Brenneman prior to use. The drugs were administered once daily from E7 to E15 by intraperitoneal (IP) injection 30 minutes before the beginning of the dark cycle, to allow for sufficient time for absorption prior to the onset of the dark phase when the circadian patterns of feeding typically produced the highest blood alcohol concentration (BAC) levels of the day. To assess the potential effects of NAP or SAL on normal fetal brain development, some dams of the Chow group (n = 4) were randomly assigned to be given NAP or SAL as described above.
All treatments were carried from E7 (approx. 168 hours postcoitus, PC) until E15 (approx. 360 hours PC). On the morning of E15 (over a 4-hour range), the dams were killed by CO2 inhalation followed by cervical dislocation, and all fetuses were then removed from uteri for analysis. One fetus from each dam was used with 5 to 9 fetuses per group obtained for analysis of brain stem morphology and immunocytochemistry of 5-HT neurons.
Blood Alcohol Concentrations
Maternal BACs were tested with 2 sets of 5 C57BL/6 dams given the 25% EDC diet (not used for embryonic studies). In brief, pregnant mice were given the same feeding protocol as the other experimental dams (ethanol diet provided on E7 at 9:00 am) and 2 tail blood samples of 50-µl each were obtained (at 11:00 am and 13:00 pm) on E8, E11, and E14. The blood samples were collected in heparinized capillary tubes, centrifuged, and 5 µl plasma samples were analyzed for alcohol concentration using the Analox Alcohol Analyzer (Lunenberg, MA), calibrated using a 100 mg/dl ethanol standard.
Neural Tube Opening
An open neural tube was defined by incomplete formation of “neural tissue” (not non-neural membrane) occurring at the floor or dorsal folds. The openings (underneath the wrapping membrane) were observed in whole embryos under a stereomicroscope, followed by microscopic examination of tissue after sectioning as described previously (Zhou et al., 2003). The brains were embedded in gelatin to avoid tearing of the dorsal or ventral plates. The number of sections with openings (failure of ventral canal occlusion, designated “o”) was counted through the entire collection of forebrain sections evaluated (n) for each brain. The ratio (r) of sections containing openings along the neural tube axis was determined by the formula r = o/n. The length (l) of the opening along the neural tube axis was determined by multiplying the number of sections with openings (o) by the thickness of the section (average fresh section) (h), as determined by a high-magnification lens: l = o × h (Zhou et al., 2003).
Immunocytochemistry
The immunocytochemistry for 5-HT was performed as previously described (Zhou et al., 2001, 2002, 2005). Fetal brains obtained from the pregnant dams were immersed overnight in freshly prepared 4% paraformaldehyde. A parallel system of processing pairs of brains together was adopted, in which 2 fetal brains [an ALC, PF (or Chow), and ALC + NAP (or ALC + SAL] were embedded together in a single gelatin block with careful rostrocaudal and dorsoventral alignments. Using a vibratome, serial 50 µm coronal (or sagittal) sections were cut, each containing a section of each of the 2 embedded brains. The two-brain sections were then processed free-floating in the same vial and thus treated equally in all aspects of the immunocytochemical processing. This practice eliminates the potential difference in amounts of exposure time or concentration of antibodies available to the antigens, chromogen development, or color reaction time, because pairs of sections (experimental and control group) were processed together in the same conditions. The sections were incubated in Triton-X-100 in phosphate buffer overnight before incubation with anti-5-HT antibody (host rabbit; Incstar, Stillwater, MN) overnight. The Sternberger peroxidase–antiperoxidase (PAP) indirect enzyme method was used for staining. The PAP reaction was performed with 0.003% H202 and 0.05% 3′ 3-diaminobenzidine. The primary, secondary, and marker antibodies were diluted in phosphate-buffered saline (PBS) containing 0.3% Triton X-100 and 1.5% normal sheep serum. The primary antibody was incubated overnight; the second and third antibodies for 1 hour each. Between antibody incubations, the sections were washed 5-minute rinses, 3 times in PBS. All sections were Nissl-counterstained with methyl green to reveal background cells to identify brain structures.
Counting the Number of 5-HT-Immunostained Neurons
The E15 fetal 5-HT neurons were counted through the rhombencephalon, which includes the rostral raphe (future dorsal and median raphe, including the B9 group) and the caudal raphe (descending) 5-HT neurons. The rostral and caudal raphe 5-HT neurons were clearly separated at E15 and were divided at the fourth ventricle. The range of the rostral 5-HT neurons begins at rostral border of the brainstem and ends in the upper border of the future fourth ventricle. We have previously adopted and systemically compared 2 independent methods of counting rostral 5-HT-immunostained (5-HT-im) neurons in the raphe nuclei (Sari and Zhou, 2004). One method used the optical fractionator stereological methods (West, 1993; West et al., 1991) analyzed with stereo-investigator software (MicroBright-Field Inc., Williston, VT). The second method was manual counting of the cells in serial sections under a 20× objective with a Leica Orthoplan II microscope (Bannockburn, IL), using a grid-marked eye piece overlay of the raphe region, with correction for overcounting bias because of split neurons in adjacent sections using Abercrombie’s formula (Zhou et al., 2004). The 2 methods yielded similar counts in the number of 5-HT-im neurons and had the same ratio of changes among treatment groups. The preliminary counting of the 5-HT-im neurons in the caudal raphe using the 2 methods also showed similar findings.
In the current study, the manual counting was used for the estimation of the 5-HT neurons because the landmark vessels that mark the boundaries of the raphe had not been fully established at this embryonic age. The tissue processing and counting were performed by 2 different persons. The identifying notation on the slides was masked prior to counting and the person performing the counting was blind to treatment condition. The tissue shrinkage had been estimated: the expected shrinkage of a 50 µm-thick section in the z-plane averages approximately 14 µm; shrinkage over x–y direction was negligible. The penetration of 5-HT immunostaining through the full thickness of the brain sections was verified using a 100× objective. Cell counts were performed in every section of the raphe nuclei, with final counts adjusted by the use of Abercrombie’s formula (Abercombie, 1946) using the fresh tissue thickness in the formula. We chose to count neurons instead of nuclei because 5-HT staining extends through the entire cells.
Statistical Analysis
For all the above measurements, 1 fetus (sex undetermined) was sampled from each dam for analysis, with the exception of brain weight, for which an average of brain weights was obtained for each whole litter. Treatment effects for parametric data were analyzed using One-way analyses of variance (anova) with treatment group as a between-subjects factor. Significant treatment effects were followed up by Fisher’s Protected Least Significant Difference (PLSD) tests for pair-wise comparisons. In all dependent measures of this study, there were no significant differences between PF and Chow groups, but alcohol treatment effects were still compared separately to the PF and the Chow groups in these follow-up comparisons. For the measures of neural tube openings, any opening that was greater than 2 SDs from the control value was considered significantly compromised for purposes of classifying affected cases. The frequency of cases with significant neural tube openings in the brainstem region was analyzed using Chi-squared analyses (ALC vs. each other group). The length (l) of the openings and the ratio of sections having openings (r) were analyzed with anova and follow-up PLSD tests.
RESULTS
BACs and E15 Brain Weights
There were no significant differences in daily alcohol intake between the groups given vehicle injections (mean ± SEM daily intake of 24.9 ± 1.4 g/kg/d) and those given SAL injections (22.2 ± 1.1 g/kg/d) or NAP injections (27.2 ± 0.7 g/kg/d). However, the NAP group did have significantly higher mean daily alcohol intake than the SAL group (p < 0.01). The BACs in the separate group of dams given the ALCs ranged from 40 to 140 mg/dl over E8 to E14 were consistent with our previous report (Zhou et al., 2003). Alcohol significantly reduced the brain weight of the alcohol treatment group (31.6 ± 2.1 mg) when compared with that of Chow (41.2 ± 1.3 mg) and PF (39.3 + 1.2 mg) (p < 0.01, respectively). NAP treatment protected against the alcohol-induced reduction of brain weight in ALC ± NAP group (39.6 ± 0.8 mg, p < 0.01); SAL treatment also protected against the alcohol-induced brain weight reductions, as observed in our previous report (Zhou et al., 2004).
Dysraphia
Prenatal alcohol exposure via the liquid diet produced dysraphia, i.e., the presence of unclosed segments of the rhombencephalic ventral canal at this embryonic age (E15). As shown in Fig 1 and Fig 2, there was clear evidence of opening in the midline of the ventral aspect of the brainstem in fetuses of the ALC group because of an incomplete or delayed occlusion of the ventral canal, perhaps best considered as a “partial dysraphia”. As shown in Table 1, the incidence of affected cases was significantly higher in the ALC group than in the Chow group (χ2 = 10.5, df = 1; p < 0.01) or the PF group (χ2 = 6.563, df = 1; p < 0.05); the significant reductions in the ALC + NAP group compared to the ALC group (χ2 = 7.778, df = 1; p < 0.01) confirms that NAP treatment significantly protected against this measure of partial dysraphia. Significant group differences were evident in the ratio of sections with ventral canal openings [F(3,25) = 8.56, p < 0.001], because of the significantly higher ratio of the ALC group compared with Chow, PF, and ALC + NAP groups. Significant group differences were also confirmed in the lengths of these midline openings [F(3,25) = 14.72, p < 0.001], again because of the significantly longer lengths in the ALC group compared with the other 3 groups (Table 1). This partial dysraphia evident at E15 in the ALC group was similar to the observations made in our previous report (Zhou et al., 2003), in which we also reported microencephaly and lateral ventricle enlargement. In confirmation with our previous findings (Zhou et al., 2004), SAL also effectively protected against the alcohol-induced dysraphia of the rhombencephalic ventral canal (Fig 1 and Fig 2).
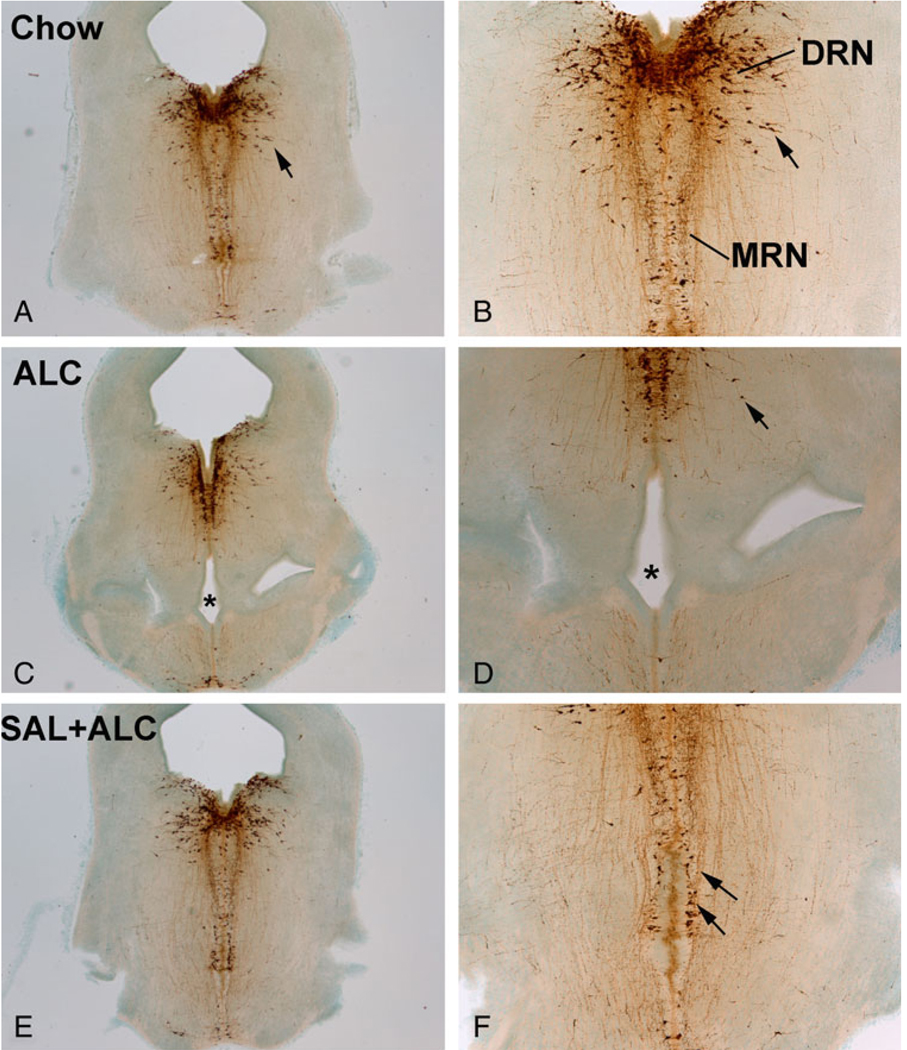
Fig. 1.
Effects of alcohol exposure and neurotrophic peptide treatment on brainstem neural tube opening and on serotonin (5-HT) neurons. Alcohol exposure increased the incidence of the incomplete ventral canal occlusion (dysraphia) when compared with the Chow control (stars; C and D vs. A and B). Deficits in the formation of 5-HT neurons typically accompanied the dysraphia (C and D). Neurotrophic peptide, SAL, protected against the dysraphia and the reduction of 5-HT neurons (arrows; E and F). SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA).
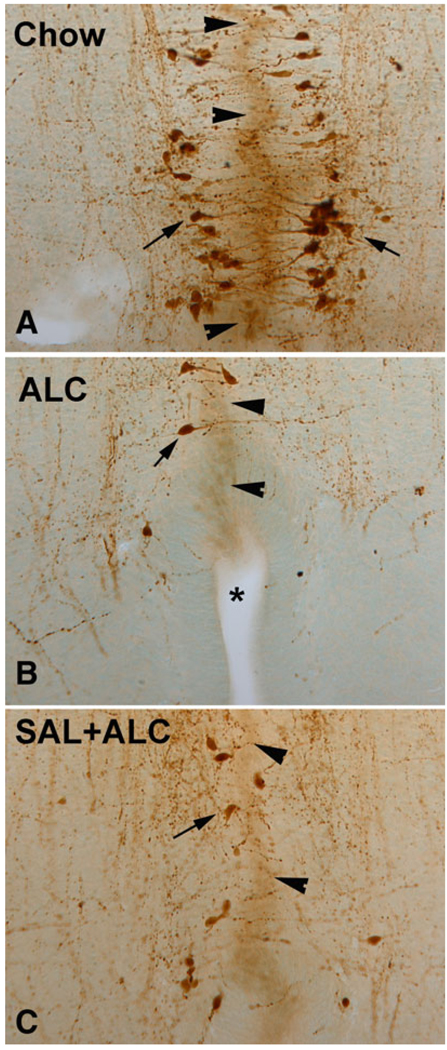
Fig. 2.
Alcohol exposure caused increased incidence of dysraphia (star; B) in the brainstem. The serotonin (5-HT) neurons (arrows) were born after the occlusion of the ventral canal (fusion of 2 rhombencephalon hemispheres, arrowheads) and formation of raphe. The newborn 5-HT neurons send processes toward the midline, probably seeking neurotrophic support (A). SAL treatment ameliorated the dysraphia (C). SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA).
Table 1.
Effect of Alcohol and NAP Treatments on Neural Tube Openings in Brainstem
| Prenatal treatments | ||||
|---|---|---|---|---|
| Measures of dysraphia | Chow | PF | ALC | ALC + NAP |
| Proportion of affected cases | 1/7 | 3/8 | 7/7* | 2/7 |
| Ratio of sections with openingsa | 0.03 ± 0.03 | 0.08 ± 0.03 | 0.29 ± 0.06** | 0.05 ± 0.04 |
| Length of openingsb | 14 ± 14 µm | 44 ± 15 µm | 143 ± 17 µm*** | 21 ± 15 µm |
PF, pair-fed; ALC, alcohol liquid diet; SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA); NAP, activity-dependent neurotrophic protein agonist peptide (NAPVSIPQ).
Significantly higher than Chow (p < 0.01), PF (p < 0.05) or ALC + NAP (p < 0.01) groups, Chi-squared test.
Mean ± SEM, ALC group significantly greater than all other groups
p < 0.001.
Mean ± SEM, ALC group significantly greater than all other groups
p < 0.001.
Serotonin Neurons
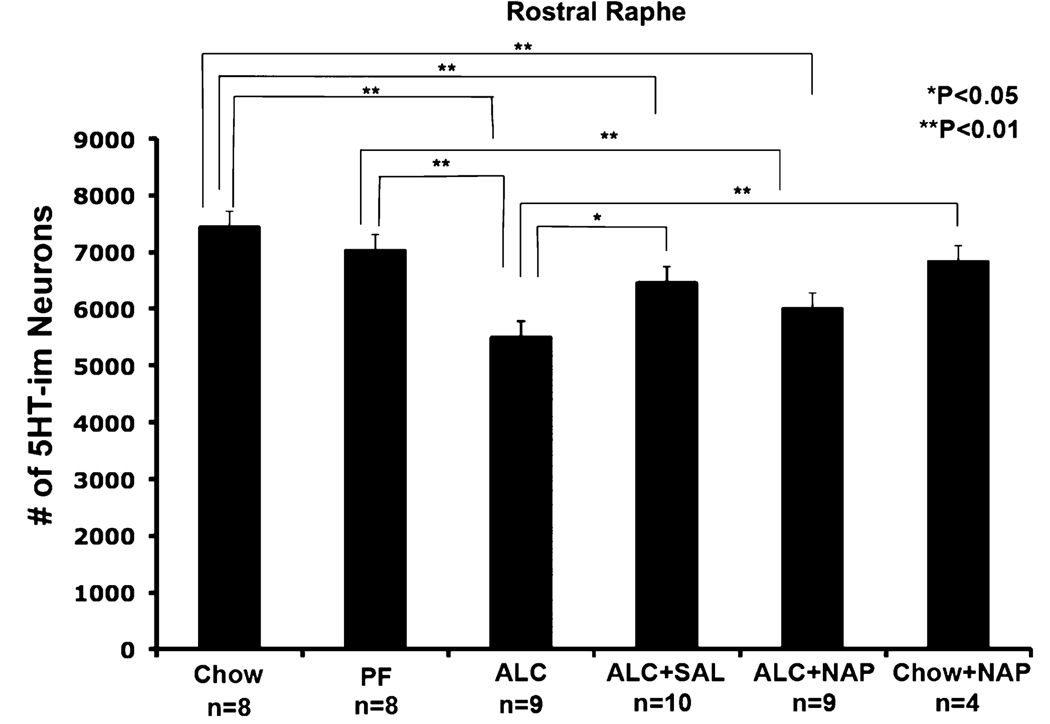
In the rostral raphe, the distribution pattern of 5-HT-im neurons was qualitatively similar across groups, but there were on average 26 and 22% fewer immunolabeled neurons in the rostral raphe (dorsal and median raphe) of ALC group compared to the PF or Chow control groups, respectively (Fig 1 and Fig 3). This alcohol-induced reduction was similar to that reported in our previous studies (Zhou et al., 2001, 2002). As shown in Fig. 3, the differences between the Chow and PF groups were not statistically significant. The SAL treatments (ALC + SAL) resulted in significant increases in the number of rostral raphe 5-HT-im neurons relative to the ALC group, to a level not statistically different from the PF group (though still significantly lower than Chow controls). The NAP treatment effect (ALC + NAP) was not significant for the rostral 5-HT-im neurons, i.e., the ALC + NAP group was not significantly different from the ALC group and was also significantly reduced relative to both the PF and the Chow controls. Separate analysis of dorsal and medial groups of the rostral raphe indicated that the effect of peptide treatment was most prominent in the dorsal raphe nucleus (Table 2).
Fig. 3.
Effect of alcohol exposure and NAP and SAL treatment on the number of serotonin (5-HT)-immunostained (5-HT-im) neurons in the rostral raphe. The 5-HT-im neurons were counted in dorsal raphe and median raphe, and B9 group. Alcohol reduced the number of 5-HT-im neurons in the rostral raphe; treatment with SAL significantly protected against the alcohol induced reduction of 5-HT-im neurons (*significantly different from comparison group, p < 0.05; **significantly different from comparison group, p<0.01). SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA); NAP, activity-dependent neurotrophic protein agonist peptide (NAPVSIPQ).
Table 2.
The Effect of Peptide Treatments on the Alcohol-Induced Reduction of 5-HT-im Neurons Is Effective in the Dorsal Than the Median Raphe
| Chow | PF | ALC | ALC + SAL | ALC + NAP | Chow + NAP | |
|---|---|---|---|---|---|---|
| Dorsal raphe | 4092 ± 241** | 3646 ± 158** | 2795 ± 216 | 3504 ± 186* | 3171 ± 176 | 3901 ± 412** |
| Median raphe | 3339 ± 155** | 3374 ± 113* | 2696 ± 124 | 2950 ± 118* | 2820 ± 81 | 2922 ± 207 |
ALC, alcohol liquid diet; PF, pair-fed; SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA); NAP, activity-dependent neurotrophic protein agonist peptide (NAPVSIPQ); 5-HT-im, 5-HT-immunostained.
The number of 5-HT neurons in the ALC group is significantly reduced as compared with Chow and PF control groups in the dorsal raphe; SAL protect against the alcohol-induced reduction of 5-HT-im neurons. The protection is less effective in the median raphe. After significant difference in anova, the post hoc comparisons listed below were shown between treatments against alcohol group.
Mean ± SEM
p < 0.05
p < 0.01 as compared with ALC.
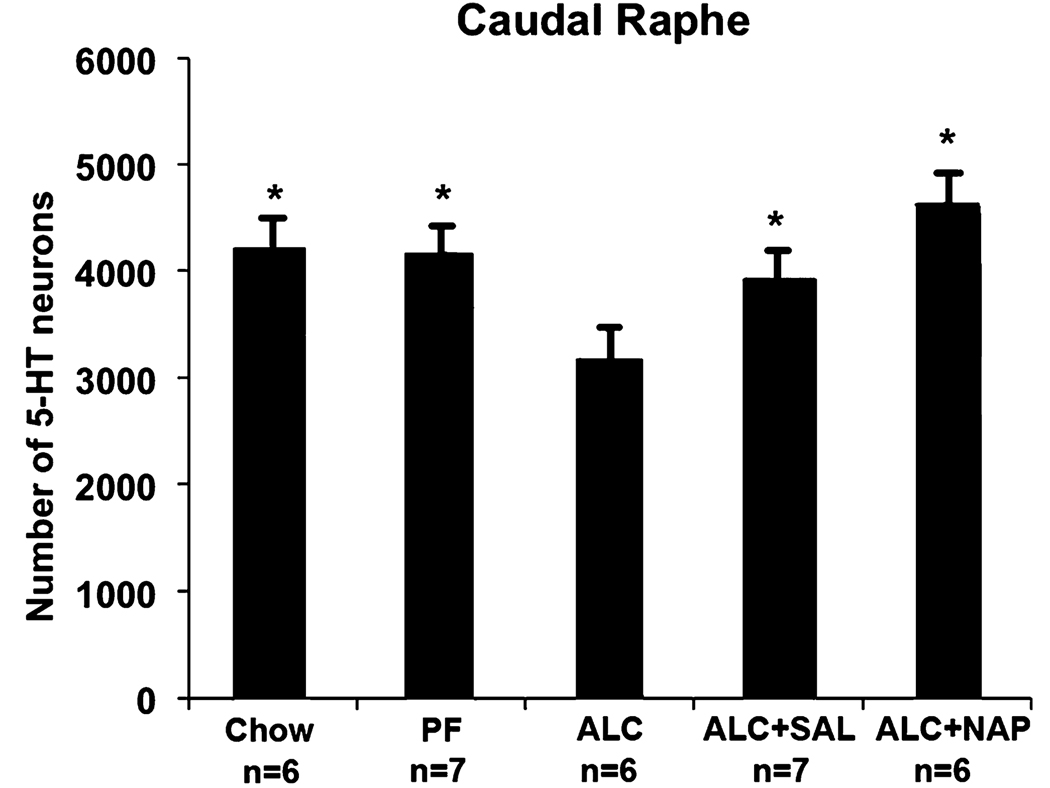
In the caudal raphe, there were on average 22 and 21% fewer 5-HT-im neurons in ALC groups compared to the Chow and PF control groups, respectively (Fig 4 and Fig 5). Both the SAL treatment (ALC + SAL) and the NAP treatment (ALC + NAP) significantly increased the number of caudal raphe 5-HT neurons relative to the ALC group to levels not statistically different from the Chow and PF control groups.
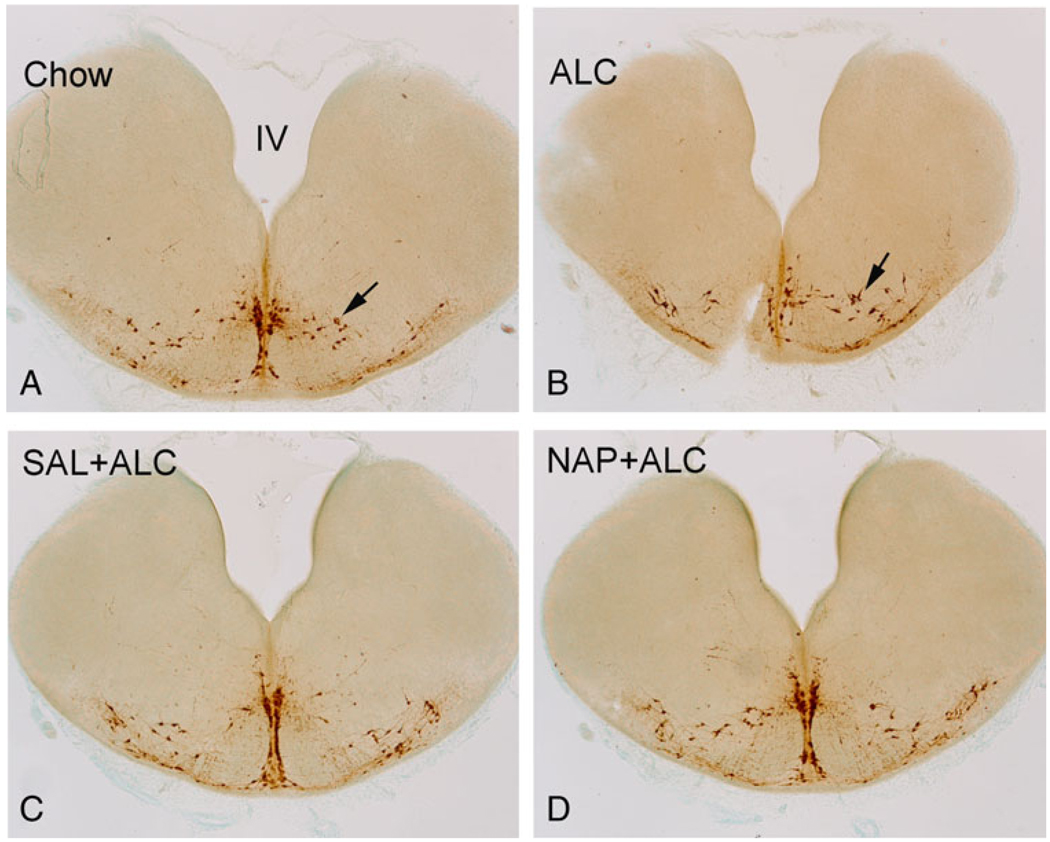
Fig. 4.
The effect of alcohol exposure and SAL and NAP treatment on the caudal raphe. Alcohol exposure (B) reduced the number of serotonin (5-HT)-immunostained (5-HT-im) neurons (arrows) in caudal raphe when compared with those of Chow control (A). The SAL (ALC + SAL, C) and NAP (ALC + NAP, D) protected against the alcohol-induced reduction of 5-HT-im neurons (see number of 5-HT-im neurons in Fig. 5). ALC, alcohol liquid diet; SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA); NAP, activity-dependent neurotrophic protein agonist peptide (NAPVSIPQ).
Fig. 5.
Effect of alcohol exposure and NAP and SAL treatment on the number of serotonin (5-HT)-immunostained (5-HT-im) neurons in the caudal raphe. The alcohol treatment significantly reduced the number of 5-HT neurons relative to pair-fed (PF) and Chow controls. Administration of either peptide to the alcohol liquid diet (ALC) group prevented these alcohol-induced deficits, confirmed by the significant increase in the number of 5-HT neurons relative to the vehicle-injected ALC group to a level not different from PF and Chow controls. (*significantly greater than the ALC group, but not different from each other). ALC, alcohol liquid diet; SAL, activity-dependent neurotrophic factor agonist peptide (SALLRSIPA); NAP, activity-dependent neurotrophic protein agonist peptide (NAPVSIPQ).
DISCUSSION
Neuroprotective Effects of SAL and NAP
The current study is the first to demonstrate that prenatal alcohol exposure induces deficits in fetal 5-HT neurons in the caudal raphé, and that prenatal treatment with the neuroprotective peptides SAL or NAP protects against alcohol-induced deficits in 5-HT neurons. Both SAL and NAP fully protected against the reductions in 5-HT neurons in the caudal raphé; in the rostral raphé SAL, but not NAP, provided significant protection against 5-HT neuronal loss. Another novel finding of the current study is that NAP prevented the delayed closing of the rhombencephalic ventral canal (the partial dysraphia evident in the alcohol–saline group) and prevented the alcohol-induced reductions of fetal brain and body weights in this maternal liquid diet model of prenatal alcohol exposure in B6 mice.
Prenatal Alcohol and Midline Deficit: Dysraphia and Its Sequelae in FAS
Our past studies have reported that offspring of pregnant C57BL/6 mice exposed to alcohol via a liquid diet containing 25% EDCs present a profile of neurodevelopmental disorders characterized by microencephaly, hydroencephaly, and compromised neural tube midline development (Zhou et al., 2003, 2004). The current study further confirmed these previous observations, again demonstrating the alcohol-induced brain growth restriction and abnormal neural tube fusion in the ventral canal, floor, and roof plates, together with ventricular enlargement. Of particular interest, the maternal BACs produced in the B6 mouse dams ranged between 40 and 140 mg/dl, consistent with our prior reports in which we reported reductions in fetal brain and body weights, reduced volumes of specific brain regions, cortical thinning, and compromised early midline brain development (Zhou et al., 2003). This liquid diet model contrasts sharply with other experimental animal studies of fetal alcohol exposure that use high concentrations of alcohol, typically given by IP injection or intragastric intubation to produce very high peak BACs. Such binge models of heavy exposure during gastrulation or early neurulation produce severe defects in embryonic development of ventromedial brain structures derived from the anterior neural plate (Sulik and Johnston, 1983; Sulik et al., 1984). In addition, severe neural tube defects (dysraphia) and anencephaly have also been demonstrated in C57BL/6J mouse embryo cultures with binge-like exposure to high media concentrations of ethanol (100 mM, about 460 mg/dl) for 6 hours on gestational day 8 (Chen et al., 2005). In contrast, in this in utero model using liquid diet consumption, the midline abnormalities (partial dysraphia and 5-HT neuron deficits) occur with maternal drinking that generates BAC profiles that are much more clinically relevant.
Both SAL (Zhou et al., 2004) and NAP treatment (current study) attenuated alcohol-induced brain weight reductions and the abnormal opening of the ventral aspect of the brainstem on E15. SAL also protected against the deficits in 5-HT neuronal development; the alcohol-exposed group had fewer 5-HT-im neurons than controls and this effect was significantly attenuated by the concurrent SAL treatment. It is known that the floor plate/midline tissue receives the morphogen sonic hedgehog (shh) from the notochord, and it produces shh (Ericson et al., 1995). Shh triggers the production of fibroblast growth factor (FGF) in the floor plate and in midline tissue. FGF is essential for regulating dorsoventral pattern development and for specification of 5-HT and dopamine neuronal development (Hynes and Rosenthal, 1999; Rubenstein, 1998; Ye et al., 1998). It is possible that alcohol interferes with this Shh-FGF morphogenetic regulation.
The sequelae of effects of abnormal midline embryonic development is increasingly recognized as being related to the phenotypic features of FAS. The facial phenotype (indistinct philtrium and narrow nose) is consistent with facial midline deficits (Dociu et al., 1976; Francesconi and Fortunato, 1969), and midline brain structures are particularly vulnerable, e.g., the corpus callosum (Bookstein et al., 2002a,b; Sowell et al., 2001), cerebrum (Coulter et al., 1993), caudate (Mattson et al., 1996), and cavum septi pellucida/cavum vergae (Swayze et al., 1997). Frank neural tube defects and dysraphia have been reported from autopsy cases of first trimester fetuses, known to have heavy prenatal exposure (Kovetskii, 1991). Although the cells that contribute to the midline formation appear vulnerable to alcohol, the cause of the specific midline deficits is unclear. The midline tissue may be prone to suffering a combination of effects of alcohol exposure including neural apoptosis, abnormal migration, and delay in timely neurogenesis, which together may lead to thinning and rupture along the neural tube. In many of the cases, the alcohol-induced neural tube rupture or impaired fusion may be repaired by the inherent ongoing robust growth of the developing embryo. However, these transient effects on midline development are likely to have adverse consequences on the developmental cascade of early brain development, e.g., because of altered timing of morphogenetic processes regulating differentiation of specific neurons (e.g., raphe neurons) and growth of processes (e.g., crossing of commissural fibers).
Possible Mechanism of SAL and NAP Protection
The mechanisms by which these neurotrophic peptides protect against alcohol-induced dysraphia and reduction of 5-HT neurons are unknown. However, candidate mechanisms are suggested by the known actions of ADNP on embryonic development. ADNP is expressed at the time of neural tube closure in the mouse; it can be detected at beginning of neurulation and increases with active neural tube formation and pattern formation from E9.5 to E12; it is then sustained throughout embryonic development. In ADNP-knockout mice, the cranial neural tube fails to close, resulting in death at E8.5 to 9.0. The deduced protein structure of ADNP contains 9 zinc fingers, a proline-rich region, a nuclear bipartite localization signal, and a homeobox domain profile, consistent with a transcription factor function (Zamostiano et al., 2001). These suggest that ADNP has a key function in regulating morphogenesis during neurulation, and alcohol treatment may affect neurulation processes normally regulated by ADNP.
The expression of Oct4, a gene associated with germ-line maintenance, was augmented in the ADNP-knockout embryos. In contrast, the expression of Pax6, a gene crucial for neural tube patterning, was abolished in the brain primordial tissue (Pinhasov et al., 2003). Thus, ADNP may act through regulation of Pax6 and Oct4 during morphogenesis as part of the mechanism of action of ADNP on neural tube development. It has been reported that Pax6 is a major transcription factor that is reduced by alcohol exposure during early development in Xenopus, leading to growth retardation (Peng et al., 2004). It is likely that NAP helps to reinstate the development through maintaining crucial functions of homeodomain genes such as Pax6 and Oct4 in the rhombencephalon, where neural patterning was otherwise disrupted by the alcohol exposure. The alcohol-induced deficiency of the ventral canal closure in rhombencephalone was protected by NAP and SAL treatment. Whether NAP and SAL protection of 5-HT neurons reflects a direct protection of emerging 5-HT neurons or is a downstream consequence of protecting the earlier processes of neurulation is unknown and remains to be investigated.
Another potential mechanism of action of these peptides may be the attenuation of alcohol-induced excitotoxicity, which may contribute to the reduction of cells death frequently observed in the midline neural tube. Known cellular actions of ADNF/ADNP include increased expression of nuclear factor kappaB (Glazner et al., 1999, 2000; Gozes et al., 1997) and heat shock protein-60 (Glazner et al., 2000; Zamostiano et al., 1999). ADNF/ADNP is known to reduce the production of reactive oxygen species and diminish of oxidative stress (Glazner et al., 1999). The neurotrophic peptides have been found to protect the developing mouse brain against excitotoxicity through activation of protein kinase C and mitogen-associated protein kinase (Gressens, 1999). Cell death and growth abnormalities elicited by alcohol exposure during development are believed to be associated, in part, with severe oxidative damage and generation of reactive oxygen species. Also, a combination treatment of the 2 peptides together was found to attenuate alcohol-induced decreases in glutathione (Spong et al., 2001) which protects against neurotoxicity. In summary, the ADNF or ADNP is a potent neurotrophic as well as neuroprotective agent functionally, particularly in early development. The timing of the NAP and SAL treatment at neurulation would have the potential to affect neural tube formation, particularly the vulnerable process of neural tube closure at midline. As the birth and survival of 5-HT neurons depend on the fusion of 2 hemispheres of rhombencephalon (for gene expression and midline trophic factors), the timely closure of ventral canal in the rhombencephalon (altered by alcohol or NAP/SAL treatment) would contribute to the neurogenesis or maturation of the 5-HT neurons in the raphe.
In considering potential mechanisms of the neuroprotective effects of NAP and SAL, the important caveat noted was that the potential effects of peptide treatments on the daily profiles of BACs were not evaluated. Even though the average daily alcohol consumption did not differ significantly between saline and peptide-treated dams, it may still be possible that the peptides could have altered the pattern of liquid diet consumption or even the pharmocokinetics of alcohol in such a way that BAC profiles were not equivalent. Consequently, in the absence of a full empirical characterization of maternal BACs, the possibility that these peptides alter maternal BAC profiles in this model cannot yet be excluded.
Implications
The current study is the first to demonstrate protection against prenatal alcohol-induced deficits in 5-HT neuron development in the rostral raphe afforded by SAL, and confirmed that prevention of dysraphia by this peptide was accompanied by protection of the 5-HT neurons. The 5-HT neurons in the rostral raphe send widespread ascending projections to nearly the entire forebrain. During development, 5-HT from these early-arising 5-HT projections has been implicated as a trophic signal that regulates early growth and differentiation of the forebrain (Lauder, 1990; Whitaker-Azmitia et al., 1996). In the mature brain, 5-HT mediates wide range of function ranging from sleep, feeding, and sexual behavior. If these alcohol-induced deficits in the rostral 5-HT system persist to maturity, they may be associated with anxiety, disturbance of sleep and circadian regulation, aggression, depression, or drug abuse.
One important new finding was that alcohol also disrupted the development of the caudal raphe, which contains 5-HT neurons that give rise to descending 5-HT projections to the metencephalon, myelencephalon, and spinal cord that regulate respiration, heart rate, sleep, spinal segmental locomotion, and pain perception. A deficit in 5-HT signaling in pontine regions has been implicated in sudden infant death syndrome (SIDS) (Kinney et al., 2003, 2005; Panigrahy et al., 2000). Infants exposed to alcohol prenatally have an increased risk for SIDS that has been linked to altered 5-HT function during early development (Alm et al., 1999; Burd and Wilson, 2004; Iyasu et al., 2002; Mitic and Greschner, 2002).
In summary, alcohol-induced deficits in the early development of 5-HT neurons are implicated as an early event in the pathogenic cascade that leads to brain growth deficits and functional abnormalities, and possibly increased risk for SIDS. These serotonergic neuronal deficits in early brain development are likely targets for therapeutic interventions. The current study demonstrates that SAL and, to a lesser extent, NAP, effectively protect against the alcohol-induced dysraphia and reduction on 5-HT neurons. The protection against alcohol-induced deficits in development of the embryonic 5-HT system may either be independent of or correlated with protective actions of these peptides identified in other neural systems or cellular processes that are essential for normal embryonic brain development (Parnell et al., 2007; Pascual and Guerri, 2007; Spong et al., 2001; Toso et al., 2006). The protection against prenatal alcohol-induced damage to fetal development, now consistently reported in several studies, underscores the therapeutic potential of these peptides and encourages development of these peptides as potential interventions for pregnancies at risk for FASD.
ACKNOWLEDGMENTS
This study is supported by U01 AA014829 to F. Zhou and C. Goodlett. The animal treatments were performed by T. Powrozek and in part by Y. Sari and R. Tonade. The tissue processing and immunocytochemistry was performed by F. Yung and cell count by R. Tonade and F. Yuan.
REFERENCES
- Aase JM, Jones KL, Clarren SK. Do we need the term “FAE”? Pediatrics. 1995;95:428–430. [PubMed] [Google Scholar]
- Abercombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Alm B, Wennergren G, Norvenius G, Skjaerven R, Oyen N, Helweg-Larsen K, Lagercrantz H, Irgens LM. Caffeine and alcohol as risk factors for sudden infant death syndrome. Nordic Epidemiological SIDS Study. Arch Dis Child. 1999;81:107–111. doi: 10.1136/adc.81.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr HM, Streissguth AP. Identifying maternal self-reported alcohol use associated with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2001;25:283–287. [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney G, Glazner G, Brenneman DE, Gozes I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54:1–14. [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec. 2002a;269:162–174. doi: 10.1002/ar.10110. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002b;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Gozes I. A femtomolar-acting neuroprotective peptide. J Clin Invest. 1996;97:2299–2307. doi: 10.1172/JCI118672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Hauser J, Neale E, Rubinraut S, Fridkin M, Davidson A, Gozes I. Activity-dependent neurotrophic factor: structure activity relationships of femtomolar-acting peptides. J Pharmacol Exp Ther. 1998;285:619–627. [PubMed] [Google Scholar]
- Brenneman DE, Phillips TM, Festoff BW, Gozes I. Identity of neurotrophic molecules released from astroglia by vasoactive intestinal peptide. Ann N Y Acad Sci. 1997;814:167–173. doi: 10.1111/j.1749-6632.1997.tb46155.x. [DOI] [PubMed] [Google Scholar]
- Burd L, Wilson H. Fetal, infant, and child mortality in a context of alcohol use. Am J Med Genet C Semin Med Genet. 2004;127:51–58. doi: 10.1002/ajmg.c.30016. [DOI] [PubMed] [Google Scholar]
- Chen SY, Charness ME, Wilkemeyer MF, Sulik KK. Peptide-mediated protection from ethanol-induced neural tube defects. Develop Neurosci. 2005;27:13–19. doi: 10.1159/000084528. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wilkemeyer MF, Sulik KK, Charness ME. Octanol antagonism of ethanol teratogenesis. FASEB J. 2001;15:1649–1651. doi: 10.1096/fj.00-0862fje. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol. 1993;50:771–775. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- Dociu I, Galaction-Nitelea O, Sirjita N, Murgu V. Centrofacial lentiginosis. A survey of 40 cases. Br J Dermatol. 1976;94:39–43. doi: 10.1111/j.1365-2133.1976.tb04339.x. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Kuo A, Tajuddin N. Effects of in utero ethanol exposure on the developing serotonergic system. Alcohol Clin Exp Res. 1991;15:678–684. doi: 10.1111/j.1530-0277.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Jessell TM, Edlund T. Sonic hedgehog: a common signal for ventral patterning along the rostrocaudal axis of the neural tube. Int J Dev Biol. 1995;39:809–816. [PubMed] [Google Scholar]
- Francesconi G, Fortunato G. Median dysraphia of the face. Plast Reconstr Surg. 1969;43:481–491. doi: 10.1097/00006534-196905000-00005. [DOI] [PubMed] [Google Scholar]
- Gardener WJ. The Dysraphic States From Syringomyelia to Anencephaly. Amsterdam: Excerpta medica; 1973. [Google Scholar]
- Glazner GW, Boland A, Dresse AE, Brenneman DE, Gozes I, Mattson MP. Activity-dependent neurotrophic factor peptide (ADNF9) protects neurons against oxidative stress-induced death. J Neurochem. 1999;73:2341–2347. doi: 10.1046/j.1471-4159.1999.0732341.x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Camandola S, Mattson MP. Nuclear factor-kappaB mediates the cell survival-promoting action of activity-dependent neurotrophic factor peptide-9. J Neurochem. 2000;75:101–108. doi: 10.1046/j.1471-4159.2000.0750101.x. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Gozes I, Bardea A, Bechar M, Pearl O, Reshef A, Zamostiano R, Davidson A, Rubinraut S, Giladi E, Fridkin M, Brenneman DE. Neuropeptides and neuronal survival: neuroprotective strategy for Alzheimer’s disease. Ann N Y Acad Sci. 1997;814:161–166. doi: 10.1111/j.1749-6632.1997.tb46154.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Bassan M, Zamostiano R, Pinhasov A, Davidson A, Giladi E, Perl O, Glazner GW, Brenneman DE. A novel signaling molecule for neuropeptide action: activity-dependent neuroprotective protein. Ann N Y Acad Sci. 1999;897:125–135. doi: 10.1111/j.1749-6632.1999.tb07884.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. Activity-dependent neurotrophic factor (ADNF) An extracellular neuroprotective chaperonin?. J Mol Neurosci. 1996;7:235–244. doi: 10.1007/BF02737061. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. A new concept in the pharmacology of neuroprotection. J Mol Neurosci. 2000;14:61–68. doi: 10.1385/JMN:14:1-2:061. [DOI] [PubMed] [Google Scholar]
- Gressens P. VIP neuroprotection against excitotoxic lesions of the developing mouse brain. Ann N Y Acad Sci. 1999;897:109–124. doi: 10.1111/j.1749-6632.1999.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Gressens P, Marret S, Bodenant C, Schwendimann L, Evrard P. Activity-dependent neurotrophic factor-14 requires protein kinase C and mitogen-associated protein kinase kinase activation to protect the developing mouse brain against excitotoxicity. J Mol Neurosci. 1999;13:199–210. doi: 10.1385/JMN:13:1-2:199. [DOI] [PubMed] [Google Scholar]
- Gressens P, Paindaveine B, Hill JM, Brenneman DE, Evrard P. Growth factor properties of VIP during early brain development. Whole embryo culture and in vivo studies. Ann N Y Acad Sci. 1997;814:152–160. doi: 10.1111/j.1749-6632.1997.tb46153.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sebastian L, Sopher BL, Miller MW, Glazner GW, Ware CB, Martin GM, Mattson MP. Neurotrophic factors [activity-dependent neurotrophic factor (ADNF) and basic fibroblast growth factor (bFGF)] interrupt excitotoxic neurodegenerative cascades promoted by a PS1 mutation. Proc Natl Acad Sci USA. 1999;96:4125–4130. doi: 10.1073/pnas.96.7.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M, Rosenthal A. Specification of dopaminergic and serotonergic neurons in the vertebrate CNS. Curr Opin Neurobiol. 1999;9:26–36. doi: 10.1016/s0959-4388(99)80004-x. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Medicine. 2006;3:0548–0557. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyasu S, Randall LL, Welty TK, Hsia J, Kinney HC, Mandell F, McClain M, Randall B, Habbe D, Wilson H, Willinger M. Risk factors for sudden infant death syndrome among Northern Plains Indians. JAMA. 2002;288:2717–2723. doi: 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kalberg WO, Buckley D. FASD: What types of intervention and rehabilitation are useful? Neurosci Biobehav Rev. 2007;31:278–285. doi: 10.1016/j.neubiorev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Kim J-A, Druse MJ. Protective effects of maternal buspirone treatment on serotonin reuptake sites in ethanol-exposed offspring. Brain Res Dev Brain Res. 1996;92:190–198. doi: 10.1016/0165-3806(96)00015-6. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Fingers ST, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J Neuropathol Exp Neurol. 2005;64:689–694. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- Kovetskii NS. Neural tube dysraphia at the level of the midbrain in alcoholic embryopathy. Zh Nevropati Psikhiatr Im S S Korsakova. 1991;91:79–83. [PubMed] [Google Scholar]
- Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci. 1990;600:297–313. doi: 10.1111/j.1749-6632.1990.tb16891.x. discussion 314. [DOI] [PubMed] [Google Scholar]
- Manning MA, Hoyme EH. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Ayers KL. Effects of ethanol on mature offspring of mice given ethanol during pregnancy. Alcohol Clin Exp Res. 1988;12:388–393. doi: 10.1111/j.1530-0277.1988.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Mitic W, Greschner J. Alcohol’s role in the deaths of BC children and youth. Can J Public Health. 2002;93:173–175. doi: 10.1007/BF03404994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Parnell SE, Chen S-Y, Charness ME, Hodge CW, Dehart DB, Sulik KK. Concurrent dietary administration of D-SAL and ethanol diminishes ethanol’s teratogenesis. Alcohol Clin Exp Res. 2007;31:2059–2064. doi: 10.1111/j.1530-0277.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Guerri C. The peptide NAP promotes neuronal growth and differentiation through extracellular signal-regulated protein kinase and Akt pathways, and protects neurons co-cultured with astrocytes damaged by ethanol. J Neurochem. 2007;103:557–568. doi: 10.1111/j.1471-4159.2007.04761.x. [DOI] [PubMed] [Google Scholar]
- Peng Y, Yang PH, Ng SS, Wong OG, Liu J, He ML, Kung HF, Lin MC. A critical role of Pax6 in alcohol-induced fetal microcephaly. Neurobiol Dis. 2004;16:370–376. doi: 10.1016/j.nbd.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, Servoss SJ, Brenneman DE, Gozes I. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:339–344. doi: 10.1111/j.1530-0277.1998.tb03658.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL. Development of serotonergic neurons and their projections. Biol Psychiatry. 1998;44:145–150. doi: 10.1016/s0006-3223(98)00133-4. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Brookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol sydrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297:774–779. [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC, USA: National Academy Press; 1996. [Google Scholar]
- Sulik KK, Johnston MC. Sequence of developmental alterations following acute ethanol exposure in mice: craniofacial features of the fetal alcohol syndrome. Am J Anat. 1983;166:257–269. doi: 10.1002/aja.1001660303. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Lauder JM, Dehart D. Brain malformations in prenatal mice following acute maternal ethanol administration. Int J Dev Neurosci. 1984;2:203–214. doi: 10.1016/0736-5748(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Swayze VWn, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Thomas JD, La Fiette MH, Quinn VR, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicol Teratol. 2000;22:703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Weinert SP, Sharif S, Riley EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcohol Clin Exp Res. 1997;21:1218–1225. [PubMed] [Google Scholar]
- Toso L, Roberson R, Woodard J, Abebe D, Spong CY. Prenatal alcohol exposure alters GABAA[alpha]5 expression: a mechanism of alcohol-induced learning dysfunction. Am J Obstet Gynecol. 2006;195:522–527. doi: 10.1016/j.ajog.2006.01.098. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Wilkemeyer MF, Menkari CE, Charness ME. Novel antagonists of alcohol inhibition of l1-mediated cell adhesion: multiple mechanisms of action. Mol Pharmacol. 2002;62:1053–1060. doi: 10.1124/mol.62.5.1053. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Bassan M, Perl O, Steingart RA, Atlas R, Brenneman DE, Gozes I. A femtomolar-acting neuroprotective peptide induces increased levels of heat shock protein 60 in rat cortical neurons: a potential neuroprotective mechanism. Neurosci Lett. 1999;264:9–12. doi: 10.1016/s0304-3940(99)00168-8. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, Bassan M, Wollman Y, Eyre HJ, Mulley JC, Brenneman DE, Gozes I. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol Clin Exp Res. 2005;29:141–149. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li T-K. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res Dev Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek TA, Spong CY. A neuroprotective peptide antagonizes fetal alcohol exposure-compromised brain growth. J Mol Neurosci. 2004;24:189–199. doi: 10.1385/JMN:24:2:189. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Zhang JK, Goodlett CR, Li T. Prenatal alcohol exposure retards the migration and development of serotonin neurons in fetal C57BL mice. Brain Res Dev Brain Res. 2001;126:147–155. doi: 10.1016/s0165-3806(00)00144-9. [DOI] [PubMed] [Google Scholar]
- Zhou F, Sari Y, Zhang JK, Li T-K, Goodlett CR, Azmitia EC. Deviations in brain early serotonergic development as a result of fetal alcohol exposure. Neurotoxicity Res. 2002;4:337–342. doi: 10.1080/10298420290030532. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Scalzi LV. Commissural size in neonatal rats: effects of sex and prenatal alcohol exposure. Int J Dev Neurosci. 1989;7:81–86. doi: 10.1016/0736-5748(89)90046-4. [DOI] [PubMed] [Google Scholar]