Abstract
Proprotein convertases (PC) are a family of proteases that cleave target proproteins at basic aminoacids, generating mature, biologically active polypeptides. Furin, the founding member of this family, is reported to have a number of potential substrates. However, germline deletion of fur is embryonically lethal1, and therefore the cell-type specific functions of furin remain poorly understood. Although furin is one of the predominant PC family members expressed by T cells, is induced by T cell activation and is a direct target of Stat family transcription factors2, the function of furin in T cells is also not clear. Herein, we show that conditional deletion of furin in T cells results in loss of peripheral tolerance characterized by activated T cells that overproduce both Th1 and Th2 type cytokines, circulating autoantibodies and development of inflammatory bowel disease. PCs are reportedly involved in the processing of several key immunoregulatory cytokines and we found that furin-deficient T cells have impaired production of the anti-inflammatory cytokine TGFβ-1. Like TGFβ-1-deficient T regulatory (Treg) cells, furin-deficient Treg cells, are less protective in a T cell transfer colitis model in vivo. Furthermore, furin-deficient effector cells were found to be resistant to suppressive activity of wild-type Tregs. Our results indicate that furin is indispensable in maintaining peripheral tolerance, which is due, at least in part, to its nonredundant, essential function in regulating TGFβ-1 production and controlling the levels of bioavailable TGFβ-1. These findings shed light on the specific function of furin as a T cell activation gene that modulates an important immunosuppressive cytokine. However, the results may also have broader implications, as targeting furin has emerged as a strategy in malignant and infectious disease3, 4. The current work suggests that inhibiting furin might activate immune responses, but may result in a breakdown in peripheral tolerance.
The seven proprotein convertase family members (furin, PC1/3, PC2, PC4, PACE, PC5/6, PC7) all promote proteolytic maturation of substrate proteins in the secretory pathway5. However, recombinant PCs can have similar specificities towards basic amino acid sequences, making the identification of bonafide enzyme-substrate pairs challenging. Whereas the lack of one family member, PC7, has minimal consequences, deficiency of furin is embryonically lethal1. Although, the latter phenotype argues for critical, non-redundant functions for furin, it has severely restricted the elucidation of furin’s physiological substrates.
Furin is expressed ubiquitously, but in T cells its expression is highly regulated upon T cell activation and is a direct target of the transcription factor, Stat42. In addition, recombinant furin has been reported to cleave several proteins known to be important for T cell biology, including Notch1 and TGFβ family cytokines6. These facts prompted us to investigate furin’s physiological role in T cell function and development. To this end, we produced a mouse with conditional loss of furin expression in T cells by crossing mice homozygous for floxed fur alleles7 with mice expressing Cre recombinase under the control of the CD4 promoter (designated CD4cre-furf/f mice). T cell-specific deletion of fur resulted in a viable mouse in which furin mRNA and protein were virtually absent in both CD4 and CD8 compartments (supplemental Fig. 1). Furin-deficient T cells underwent normal thymic development as evidenced by normal absolute T cell numbers (data not shown), ratios of thymic subsets and TCRβ rearrangement (Fig. 1a). In young animals, the absolute numbers of T cells, proportions of CD4+ and CD8+ T cells and TCR Vβ subsets in peripheral lymphoid organs (spleen and lymph nodes) were also not significantly different from the furf/f littermate controls (data not shown and supplemental Fig. 2). In addition, partial deletion of Vβ5+ subset in peripheral CD4+ but not CD8+ T cells was evident in the absence of furin, suggesting that negative selection of thymocytes is intact (supplemental Fig. 2)8 . Thus, deletion of furin at the double positive stage of T cell development did not appear to have major developmental consequences. Intriguingly though, the numbers of thymic, natural T regulatory (CD4+Foxp3+) cells were found to be significantly elevated in CD4cre-furf/f animals (Fig. 1b).
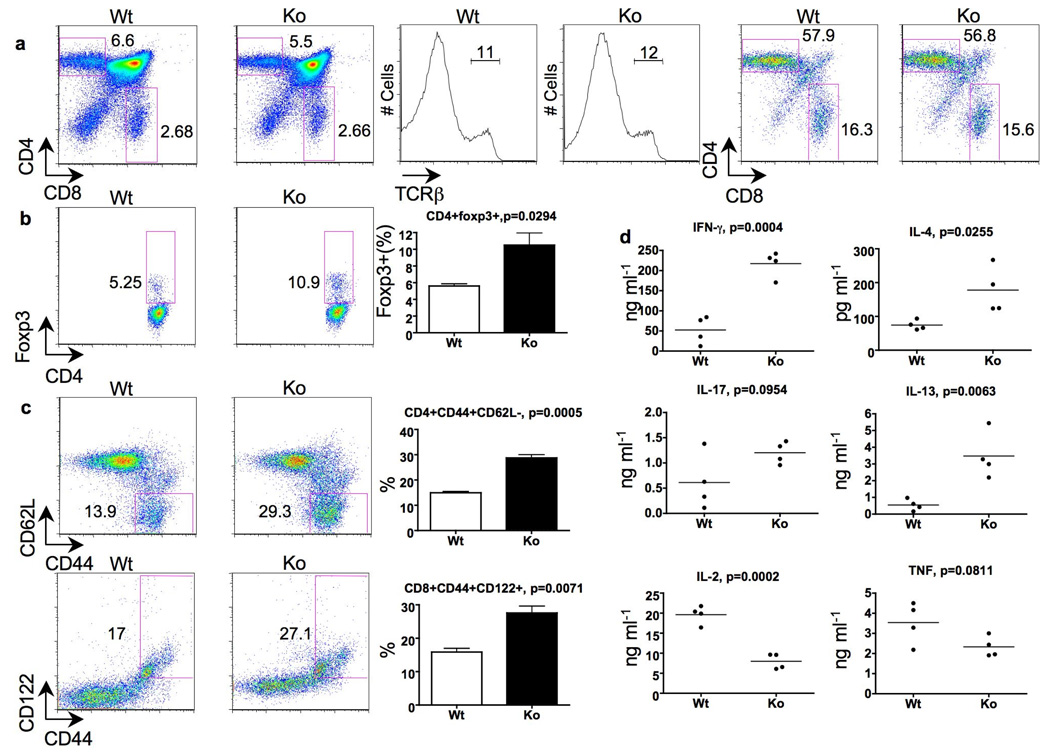
Figure 1. Normal thymic T cell development, but activated/memory phenotype of peripheral T cells in CD4cre-furf/f mice.
a, b, Proportions of CD4+, CD8+, TCR-β+ and Foxp3+ thymocytes in 8 week old mice. Panels on the right show CD4+/CD8+ proportions in TCR-β rearranged cells. Three mice per group were analyzed. Representative flow cytometry blots and plotted mean values are shown. c, Activated/memory splenic T cells in 7–9 week old mice. Representative flow cytometry blots and plotted mean values are shown (n=3 per group). d, Cytokine production. Mesenteric lymph node cells were stimulated with plate-bound CD3 and soluble CD28 antibodies for 48 hours (n=4, nine weeks old CD4cre-furf/f and furf/f mice).
While depleting furin at the double positive stage of the thymic development did not grossly affect T cell development, furin deficiency in T cells was associated with increased numbers of activated, memory-like CD4+CD44hiCD62L− and CD8+CD44hiCD122+ T cells in the periphery even in 7–9 weeks old mice (Fig. 1c). To gain more insight into the biological consequence of the absence of furin in T cells, we performed microarray analysis on sorted naïve, CD4+CD44lowCD62L+ CD4cre-furf/f and littermate furf/f T cells (>98% purity). Although the cells were isolated based on their naïve phenotype, the absence of furin was associated with the upregulation of a number of genes typically associated with T cell activation, including Fos, Jun and Ifng (supplemental Fig. 3). Moreover, upon activation furin-deficient T cells were observed to produce greater amounts of Th1 (IFN-γ) and Th2 (IL-4 and IL-13) type cytokines, less IL-2 and unaltered levels of TNF or IL-17 (Fig. 1d and supplemental Fig. 4).
At approximately 6 months of age, CD4cre-furf/f, but not littermate furf/f or CD4cre-fur+/+ mice became overtly ill, at which point they developed a progressive wasting disease characterized by weight loss, ruffled hair and hunched appearance. Gross pathologic examination of the large intestine and stomach of CD4cre-furf/f mice revealed macroscopic evidence of inflammation and fibrosis (Fig. 2a). Mesenteric lymph nodes were enlarged, but obvious splenomegaly was rarely observed. Histologically, mice had severe inflammatory bowel disease characterized by dense chronic inflammation with reactive epithelial atypia and architectural distortion; scattered neutrophils were also observed. Nodules of lymphoid infiltrates were also noted in the liver, lung and kidney, and CD4cre-furf/f mice were also found to have high levels of anti-nuclear and anti-DNA antibodies, indicative of systemic autoimmune disease (Fig. 2b and c, supplemental Fig. 4 and 5). Analysis of serum cytokines in CD4cre-furf/f mice revealed elevated levels of circulating pro-inflammatory IL-6, and the hallmark Th1 and Th2 cytokines IFN-γ and IL-13, respectively, and lower levels of the anti-inflammatory cytokine IL-10 (Fig. 2d). In addition, there was secondary activation of B cells, as evidenced by elevated levels of the serum immunoglobulins, IgG1, IgG2 and IgE (Fig. 2e). In the gut and mesenteric lymph nodes of older, autoimmune CD4cre-furf/f animals, we also observed expansion of CD4+ and CD8+ effector cells, as well as upregulation of the activation marker CD69 on CD4+ effector cells (Fig. 3a and 3b). Together with the evidence of T cell activation, autoantibody production and hyperproduction of Th1 and Th2 cytokines, these data suggest that CD4cre-furf/f mice have a breakdown in immune tolerance.
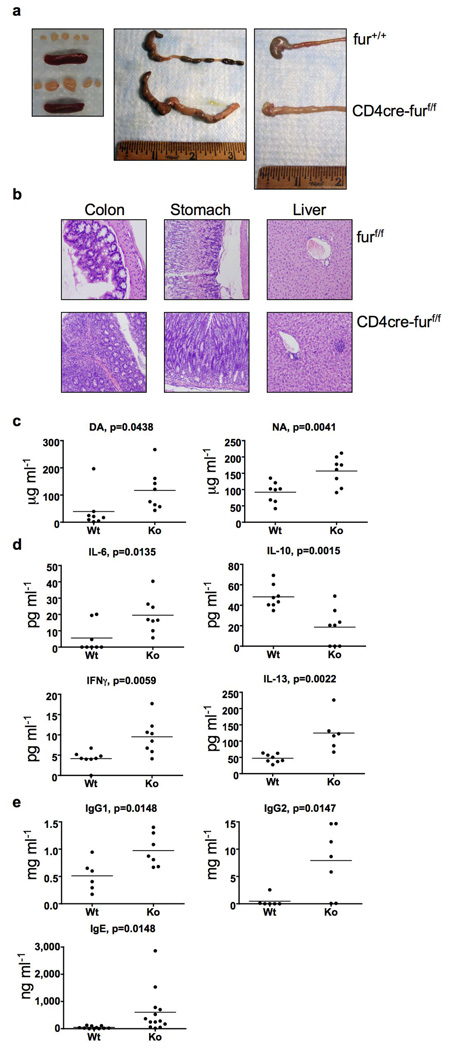
Figure 2. Development of age-related autoimmunity in CD4cre-furf/f mice.
a, Lymphoid organs, colon and stomach/duodenum of CD4cre-furf/f and age-matched wild-type animals (6 months). b, Haematoxylin- and eosin-stained sections of colon, stomach and liver (6 months old, CD4cre-furf/f and furf/f mice). c, Anti-dsDNA (DA) and nuclear antibody (NA) titers in CD4cre-furf/f animals compared with age-matched wild-type and furf/f animals (n=8, 5–7 months). d, Serum cytokines in CD4cre-furf/f animals compared with age-matched wild-type and furf/f animals (n=6–8, 5–7 months). e, ELISA for serum immunoglobulins of CD4cre-furf/f animals compared with age matched wild-type and furf/f animals (n=6–13, 5–7 months).
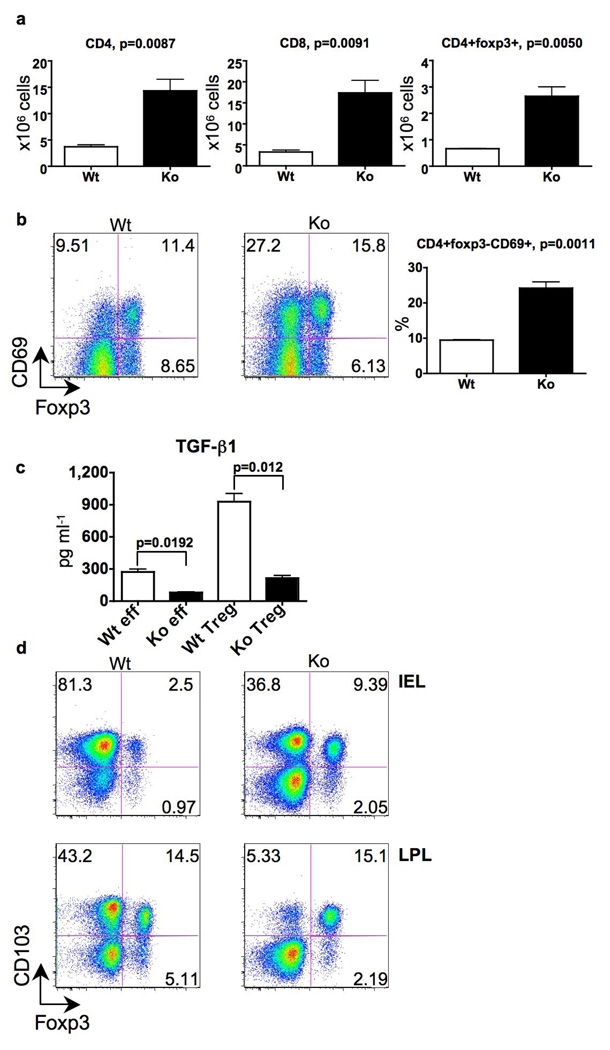
Figure 3. Deletion of furin in T cells results in T cell expansion/activation, and impairs TGF-β1 production, and CD103 expression.
a,b, Absolute CD4+Foxp3, CD4+Foxp3+ and CD8+ cell numbers and the proportion of CD4+Foxp3CD69+ cells in the mesenteric lymph nodes of CD4cre-furf/f animals were compared with age-matched wild-type and furf/f animals (n=3, 5–6 months). c, TGFβ-1 production. Purified CD4cre-furf/f and furf/f CD4+CD25 and CD4+CD25+ cells were activated with plate-bound CD3 and soluble CD28 antibodies; TGFβ-1 was measured by ELISA in duplicate and a representative of three experiments is shown. d, Expression of Foxp3 and CD103 in lamina propria and intraepithelial CD4+ cells; shown is a representative flow cytometry plot of three mice per group analyzed (5–6 months).
The importance of mechanisms that maintain peripheral tolerance to prevent autoimmunity have become increasingly evident. In particular, adequate numbers and functionality of regulatory T (Treg) cells are critical components of T cell-dependent peripheral tolerance. We found that mice in which furin was deleted in T cells had elevated numbers and proportions of peripheral CD4+Foxp3+ cells in the small intestine and mesenteric lymph nodes (Fig. 3a and d). Production of the anti-inflammatory cytokine TGFβ-1 by Treg and effector T cells is also critical in the maintenance of peripheral tolerance and prevention of autoimmune disease9–11. TGFβ-1 is initially synthesized as a proprotein that requires multiple steps to generate the mature, biologically active cytokine12. The pro-protein is enzymatically cleaved to generate an amino-terminal Latency Associated Peptide (LAP), but this product remains non-covalently associated with TGFβ-1 keeping it in a biologically inactive state. Previous studies with recombinant furin have argued for a role as a pro-TGFβ-1 converting enzyme, although other PC family members have also been reported to have this activity13. We first explored furin’s criticality in the production of biologically active TGFβ-1 by assessing whether furin-deficient T cells generated normal levels of TGFβ-1 in vitro. To this end, we measured the secretion of TGFβ-1 by CD4+CD25− and CD4+CD25+ T cells and found that furin-deficient T cells secreted considerably reduced levels of activated TGFβ-1 (Fig. 3c). We confirmed this deficit by measuring TGFβ-1 by ELISA and Western blot, using antibodies that selectively detect only active TGFβ-1 and not the unprocessed cytokine (supplemental Fig. 6c and d). In contrast, the levels of surface-expressed of LAP and total Tgfb1 mRNA were not reduced in furin-deficient T cells consistent with normal production of pro-TGFβ-1 (Fig. 3c, supplemental Fig.6a, b and data not shown).
The conversion of naïve CD4+ T cells in to Foxp3+ Treg cells is also dependent upon TGFβ-114. This conversion can be accomplished by addition of exogenous TGFβ-1 or by incubation of naïve CD4+ T cells with TGFβ-1-producing Treg cells. As shown in supplementary Figure 7, addition of TGFβ-1 or wild-type Treg cells induced the expression of Foxp3 in both wild-type and furin-deficient CD4+ naïve T cells. However, furin-deficient Tregs were defective in their ability to upregulate Foxp3+ expression in normal CD4+ T cells in vitro (supplemental Fig. 7). This latter finding is consistent with the reduced capacity of furin-deficient T cells to produce normal levels of biologically active TGFβ-1; however, it is also clear that furin-deficient T cells can respond to exogenously added TGFβ-1.
To assess whether bioactive TGFβ-1 was reduced in the absence of furin in vivo, we first examined the expression of the integrin CD103 on T lymphocytes. This integrin has been linked to T cell gut homing and retention within epithelial compartments and is known to be induced by TGFβ-19, 15. We found that numbers of CD4+CD103+Foxp3− cells in the lamina propria and intraepithelial compartments were reduced in small intestine of CD4cre-furf/f mice compared to wild-type mice (Fig. 3d).
Recent evidence argues for an essential protective role for T cell-derived TGFβ-1 in suppressing autoimmunity10. Experimentally, it was found TGFβ-1-deficient Treg cells fail to suppress the inflammatory bowel disease caused by homeostatic expansion of naïve CD4+CD45Rbhi cells. To assess their function, furin-deficient naïve CD4+CD25− CD45Rbhi cells (effectors) were injected into T cell-deficient host animals alone, or in combination with CD4+CD25+ Treg cells. Mice that received either wild-type or furin-deficient effectors cells with no Treg cells lost weight and developed severe gut inflammation macroscopically and histologically (Fig. 4a, b and c). Furin-deficient effector T cells exhibited more aggressive phenotype, as evidenced by a significant increase in the absolute number of knock-out CD4+ cells in mesenteric lymph nodes (Fig. 4d).
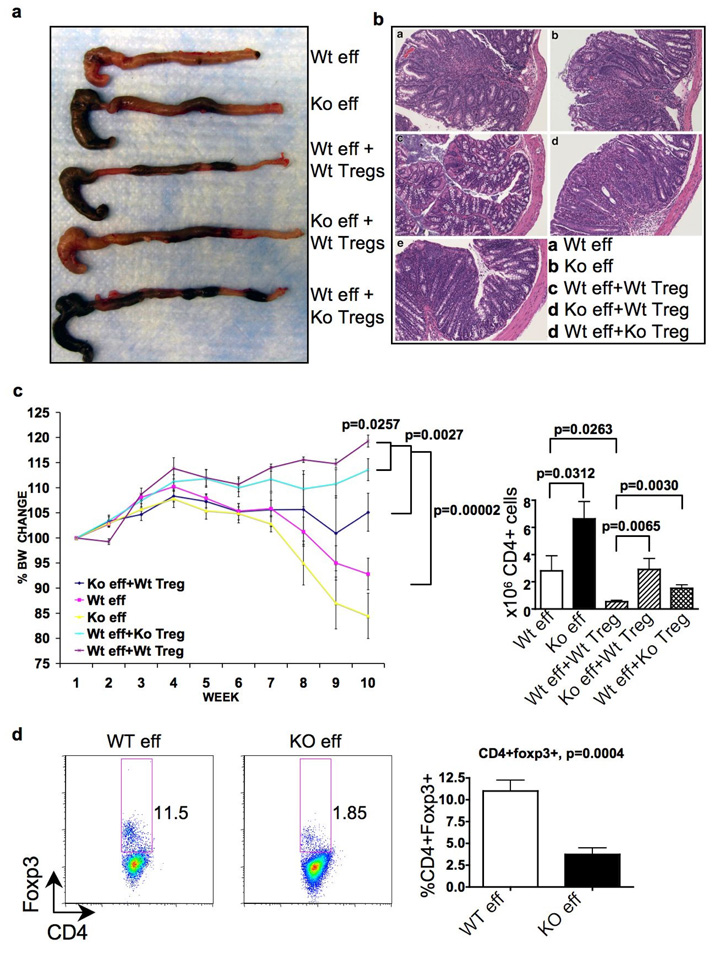
Figure 4. Furin deficiency results in increased T effector cells aggressiveness and impaired T regulatory cell protection in suppressing colitis.
Purified wild-type (Wt) CD4cre-furf/f (Ko) CD4+CD25CD45Rbhi naïve T cells were transferred alone or in combination with wild-type or furin-deficient CD4+CD25+ T regulatory cells into TCRα−/− recipients. Mice were analyzed on week 10 (n=5 per group). a, Representative images of colons. b, Representative colon histology. c, Body weight change during the experiment .d, Absolute CD4+ cell numbers in mesenteric lymph nodes. e, Spontaneous conversion of adoptively transferred naïve CD4+CD25CD45Rbhi cells into CD4+Foxp3+ cells. Representative flow cytometry blots and plotted mean values are shown. In e data are pooled from two identical experiments.
Consistent with previous reports, transfer of wild-type Treg cells with wild-type effectors prevented the wasting disease, gut inflammation, CD4+ cell expansion and IFN-γ production (Fig. 4 and supplemental Fig. 8).16. However, furin-deficient Treg cells were found to be significantly impaired in their ability to prevent gut pathology, weight loss, as well as inhibiting homeostatic proliferation and cytokine production by CD4+ cells. Intriguingly, furin-deficient effectors could not be completely suppressed by wild-type Tregs (Fig 4c and supplemental Fig. 8) During homeostatic expansion of naїve CD4+ cells, spontaneous conversion of CD4+ T cells to Foxp3+ cells occurs in a TGFβ-1 dependent manner (typically 10–15%)17, 18. We found that adoptive transfer of naïve furin-deficient CD4+ cells in T cell deficient hosts resulted in the generation of fewer Foxp3+ cells than wild-type cells, perhaps due to lack of autocrine TGFβ-1 production (Fig 4d). Lack of spontaneous conversion may contribute to the enhanced aggressiveness of furin-deficient effectors in vivo.
Based on previous in vitro studies using recombinant proteins, furin has been suggested to process a variety of substrates. Fundamental, non-redundant roles in cell biology are suggested by the early lethality of fur germ-line knockout. In this regard, it was striking that T cell-specific furin-deficient animals have grossly normal T cell development, arguing that furin is not a required PC for a plethora of substrates, consistent with the observed partial redundancy of furin in liver7.
To our surprise, the overt phenotype associated with selectively deleting furin in T cells was autoimmunity. Like mice in which TGFβ-1 was deleted in a T cell-specific manner10, CD4cre-furf/f mice had elevated numbers of thymic/natural T regulatory cells, hyperproduction of both Th1 and Th2 cytokines, and expansion of CD4+ and CD8+ cells. Similar to TGFβ-1-deficient T cells, in vitro suppression activity was not impaired (supplemental Fig. 9), but in vivo suppressive activity was reduced and effector T cells were more aggressive. These findings were accompanied by the development of age-related systemic autoimmunity and inflammatory bowel disease. Thus CD4cre-furf/f mice largely phenocopy the abnormalities seen in CD4cre-tgfbf/n mice. The phenotype of CD4cre-furf/f mice also mimics the pathology seen in mice in which another molecule involved in the activation of TGFβ-1, integrin αvβ8, is deleted in dendritic cells19. It is noteworthy though that unlike TGFβ-1-deficient Tregs, CD4cre-furf/f CD4+CD25+ cells are partially functional in vivo and furin-deficient effector cells are more resistant to the Treg suppression (Fig. 4). Our results are consistent with the idea that furin is a critical PC in vivo for the proper endoproteolytic processing of TGF-β1. While other PCs have also been reported to cleave TGFβ-1 in vitro, they evidently cannot replace furin in T cells. However, it should also be emphasized that furin probably has many important substrates in T cells other than TGFβ-1. While our data evidently argue for a role of T cell-expressed furin in maintenance of peripheral tolerance and there was no gross deficit in thymic selection, more work on furin’s role in T cell development and central tolerance is clearly warranted.
Our results have additional implications. Furin activity has been linked to the pathogenesis of several diseases including metastatic cancers, cystic fibrosis and infectious diseases3, 6, 20, 21. Consequently, furin inhibitors have been proposed as possible therapies for such diseases. However, our findings suggest that interfering with furin activity might have the unexpected consequence of promoting autoimmunity. In principle, this might be beneficial in that it might boost T cell mediated immune responses, and be advantageous in treating cancer and infections.
METHODS SUMMARY
Mice
Mice that express floxed fur7 alleles were backcrossed six times with C57/BL6 mice. Wild-type, CD4-Cre, and TCRα−/− mice on C57/BL6 background were from Taconic. Mice were maintained and housed under pathogen-free conditions in accordance with the NIH Animal Care and Use Committee.
Cell purification and flow cytometry
Cells were purified by magnetic separation (Miltenyi) and sorted with MoFlo cell sorter (Dako). Flow cytometry was performed with FACSCANTO or FACSCALIBUR instruments (Becton Dickinson) and data were analyzed using FlowJo software (Treestar).
Cytokine and antibody measurements
Mesenteric lymph node T cells (1×106 ml−1) were activated with plate-bound anti-CD3 (10 µg ml−1) and soluble anti-CD28 (2 µg ml−1) (BD Pharmingen). Cytokine levels in supernatants and sera were determined with IL-13 and IL-17 ELISA (R&D Systems) or with Cytometric Bead Array (BD Pharmingen). Serum auto-antibodies and immunoglobulins were determined by ELISA (Alpha Diagnostic International). TGFβ-1 production by purified CD4+CD25− and CD4+CD25+ cells was measured after two rounds of activation using a multispecies TGFβ-1 ELISA kit (Invitrogen).
Gut cell preparation
Intraepithelial lymphocytes were purified from the small intestine using mechanical separation and 30% Percol centrifugation; lamina propria cells were purified as described22.
In vivo suppression assay
The T cell suppression assay was performed as previously described with small modifications16. Briefly, TCRα−/− mice were injected intravenously with purified wild-type or furin-deficient naïve CD4+CD25−CD45Rbhi cells with or without wild-type or furin-deficient CD4+CD25+ Treg cells. Mice were monitored for signs of disease and analyzed on week 10.
Statistical analysis
P values were calculated using Student’s t-test, error bars in graphs represent s.e.m.
Supplementary Material
Acknowledgements
We thank Drs. Juan Bonifacino (NICHD) and Sharon Wahl (NIDCR) for providing helpful comments and critically reading the manuscript, Drs. Alfred Singer (NCI) and Leonid Pobenzinsky (NCI) for providing helpful suggestions and Dr. Mohammed Oukka (Harvard Medical School) for providing Foxp3-GFP mice. This work has been supported by the Intramural Research Program of NIAMS, Academy of Finland, the Finnish Medical Foundation and Maud Kuistila Memorial Foundation, the “Fonds voor Wetenschappelijk Onderzoek Vlaanderen” and “Geconcerteerde Onderzoeksactie van de Vlaamse Gemeenschap”
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
REFERENCES
- 1.Roebroek AJ, et al. Failure of ventral closure and axial rotation in embryos lacking the proprotein convertase Furin. Development. 1998;125:4863–4876. doi: 10.1242/dev.125.24.4863. [DOI] [PubMed] [Google Scholar]
- 2.Pesu M, Muul L, Kanno Y, O'Shea JJ. Proprotein convertase furin is preferentially expressed in T helper 1 cells and regulates interferon gamma. Blood. 2006;108:983–985. doi: 10.1182/blood-2005-09-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiryaev SA, et al. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J Biol Chem. 2007;282:20847–20853. doi: 10.1074/jbc.M703847200. [DOI] [PubMed] [Google Scholar]
- 4.Scamuffa N, et al. Selective inhibition of proprotein convertases represses the metastatic potential of human colorectal tumor cells. J Clin Invest. 2008;118:352–363. doi: 10.1172/JCI32040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor NA, Van De Ven WJ, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. Faseb J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- 6.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roebroek AJ, et al. Limited redundancy of the proprotein convertase furin in mouse liver. J Biol Chem. 2004;279:53442–53450. doi: 10.1074/jbc.M407152200. [DOI] [PubMed] [Google Scholar]
- 8.Fink PJ, Fang CA, Turk GL. The induction of peripheral tolerance by the chronic activation and deletion of CD4+V beta 5+ cells. J Immunol. 1994;152:4270–4281. [PubMed] [Google Scholar]
- 9.Nakamura K, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 10.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 12.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 13.Dubois CM, et al. Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme. Am J Pathol. 2001;158:305–316. doi: 10.1016/s0002-9440(10)63970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schon MP, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 16.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang S, et al. Conversion of CD4+ CD25- cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 19.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: "master switches" in the regulation of tumor growth and progression. Mol Carcinog. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- 21.Ornatowski W, Poschet JF, Perkett E, Taylor-Cousar JL, Deretic V. Elevated furin levels in human cystic fibrosis cells result in hypersusceptibility to exotoxin A-induced cytotoxicity. J Clin Invest. 2007;117:3489–3497. doi: 10.1172/JCI31499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.