Abstract
Background
Endothelial progenitor cells (EPC) promote neovascularization and endothelial repair. Renal artery stenosis (RAS) may impair renal function by inducing intra-renal microvascular (MV) injury and remodeling. We investigated whether replenishment with EPC would protect the renal microcirculation in chronic experimental renovascular disease.
Methods and results
Single-kidney hemodynamics and function were assessed using multi-detector CT in-vivo in pigs with RAS, RAS 4 weeks after intra-renal infusion of autologous EPC, and controls. Renal MV remodeling and angiogenic pathways were investigated ex-vivo using micro-CT, histology, and Western-blotting. EPC increased renal expression of angiogenic factors, stimulated proliferation and maturation of new vessels, and attenuated renal MV remodeling and fibrosis in RAS. Furthermore, EPC normalized the blunted renal MV and filtration function.
Conclusions
The current study shows that a single intra-renal infusion of autologous EPC preserved MV architecture and function and decreased MV remodeling in experimental chronic RAS. Likely, restoration of the angiogenic cascade by autologous EPC involved not only generation of new vessels, but also acceleration of their maturation and stabilization. This contributed to preserving the blood supply, hemodynamics, and function of the RAS kidney, supporting EPC as a promising therapeutic intervention for preserving the kidney in renovascular disease.
Keywords: kidney, progenitor cells, renal blood flow, renal artery stenosis
Endothelial progenitor cells (EPC) mobilized endogenously in response to ischemia play a crucial role in augmenting neovascularization of ischemic tissues and endothelial replacement after vascular injury. Replenishment of such cells may limit vascular injury through reconstitution of the luminal barrier and cellular secretion of paracrine factors, providing a novel therapeutic option1, 2. Indeed, growing experimental and clinical evidence underscores the critical role that circulating cells play in healing the endothelium when the intrinsic system is unable to adequately support tissue repair. Targeted delivery of EPC has been shown to improve the function of the infarcted myocardium3, decrease hind-limb ischemia4, 5, rescue the kidney from acute ischemia injury6, and to participate in glomerular endothelial repair in glomerulonephritis7.
Ischemic nephropathy secondary to renal artery stenosis (RAS) represents an important cause of renovascular disease and hypertension that may induce renal injury and lead to end-stage renal disease. The presence of renovascular disease also constitutes an independent predictor for increased morbidity and mortality in cardiovascular disease and cardiac events8. We have previously shown that the kidney exposed to chronic RAS shows significant functional deterioration attended by renal inflammation, fibrosis, and microvascular (MV) rarefaction and remodeling9–12. Indeed, intra-renal MV disease likely aggravates the progression of renal injury in RAS, and may account for the failure of renal function to improve after restoration of blood flow. However, despite pressing clinical need, targeted interventions capable of protecting the kidney or reversing its injury in chronic renovascular disease are yet to be identified.
Recent evidence suggests potential for cell-based repair interventions in rodent models of renal injury13, 14. Nevertheless, the potential utility of progenitor cells for preserving the function and structure of the kidney in a model or in chronic renovascular disease has not been investigated. Therefore, the current study was designed to test the hypothesis that replenishment of progenitor cells would improve renal function by protecting the vascular integrity of the stenotic porcine kidney.
Methods
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. The Institutional Animal Care and Use Committee approved all the procedures (A17807). Twenty-one domestic pigs (55–65kg) were studied after 6 and again 10–12 weeks of observation. In 14 pigs, a local-irritant coil was placed in the main renal artery at baseline, and induced gradual development of unilateral RAS. We have previously shown that by 4–5 weeks after coil implantation this model already exhibits a chronic decrease in renal function, and that significant stenoses induce pathological renal alterations that closely resemble chronic renovascular disease in humans, including inflammation, fibrosis, and mild glomerulosclerosis10, 11, 15, 16. The pigs had renal angiography 6 weeks after induction of RAS, and were then randomized into two groups that were not further treated (RAS, n=7) or treated with an intra-renal infusion of autologous EPC (RAS+EPC, n=7). To generate EPC, peripheral mononuclear cells were isolated from each pig 3 and 5 weeks after induction of RAS, expanded in vitro, and delivered to the same RAS+EPC pig during the 6 weeks renal angiography. Four weeks later, all animals underwent repeated renal angiography, as well as in vivo studies. Throughout these 10 weeks, blood pressure was continuously monitored using a telemetry system (PhysioTel, Data Sciences) implanted at baseline in the left femoral artery. Mean arterial pressure (MAP) was recorded using telemetry at 5-minute intervals and averaged for each 24-hour period10, 11, 17, and also measured during in vivo studies using a side arm of the arterial catheter. The other seven pigs were used as controls (normal, n=7).
By six weeks after induction of RAS, all of the pigs underwent renal angiography, as mentioned above. For this, all the pigs were anesthetized with intra-muscular telazol (5 mg/kg) and xylazine (2 mg/kg), intubated, and mechanically ventilated with room air. Anesthesia was maintained with a mixture of ketamine (0.2 mg/kg/min) and xylazine (0.03 mg/kg/min) in normal saline, administered via an ear vein cannula (0.05 mL/kg/min). Under sterile conditions and fluoroscopic guidance, an 8F arterial catheter was advanced to the stenotic renal artery, proximal to the stenosis. Short bolus injections (4–6 mL) of low-osmolar non-ionic contrast media (iopamidol, Isovue-370, Squibb Diagnostics, Princeton, NJ) were used to visualize the lumen of the renal artery using a fluoroscopy system (Siemens Siremobil Compact), images were then recorded and later analyzed off-line to determine the degree of RAS, as previously described17. After angiography, in the RAS+EPC animals, EPC (106 cells/mL suspended in 10 mL of saline) were delivered into the stenotic renal artery (for details, please see the online supplement).
Four weeks later, all the animals were again similarly anesthetized for repeated renal angiography, which was followed by in vivo functional studies. After angiography, the catheter was positioned in the superior vena cava, and in vivo helical multi-detector computer tomography (MDCT) flow studies were performed for assessment of basal regional-renal perfusion, renal blood flow (RBF), and glomerular filtration rate (GFR), as previously detailed9–11, 15 18. Briefly, this involved sequential acquisition of 160 consecutive scans after a central venous injection of iopamidol (0.5 cc/kg/2 sec), and were repeated during supra-renal infusion of acetylcholine (5 µg/kg/min) to test endothelium-dependent responses. Blood samples were collected from the inferior vena cava and both renal veins for measurement of plasma renin activity (PRA, radio-immunoassay) and systemic asymmetric dimethylarginine (ADMA) levels (Euroimmun US LLC, Boonton Twp, NJ). Urine samples were collected by supra-pubic bladder puncture, and protein content measured by spectrophotometry using the Bradford method.
Following completion of all studies the pigs were allowed to recover for a few days (to allow for contrast media washout), and were then euthanized with a lethal intravenous dose of sodium pentobarbital (100mg/kg, Sleepaway®, Fort Dodge Laboratories, Inc, Fort Dodge, IA). Both kidneys were removed from each pig using a retroperitoneal incision and immersed in 4°C Krebs’s solution containing heparin. A lobe of tissue was immersed in 10% buffered formalin (Sigma), and a segmental artery perfusing the intact end of the stenotic kidney cannulated and prepared for micro-CT. Other lobes were shock-frozen in liquid nitrogen and stored at −80° C, or preserved in formalin10, 11, 15.
In vitro studies were then performed to assess renal histology and expression of angiogenic and fibrotic factors. Western blotting and immunohistochemistry were used to probe expression of the pro-angiogenic factors phosphorylated (p)-Akt, p-endothelial nitric oxide synthase (p-eNOS), vascular endothelial growth factor (VEGF), hypoxia-inducible factor (HIF)-α, angiopoietin-1, and integrin β3. The expression of markers and mediators of renal fibrosis such as transforming growth factor (TGF)-β, tissue inhibitor of metalloproteinases (TIMP)-1, α-smooth muscle actin (α-SMA), and matrix metalloproteinases (MMP)-2 and -9, was also investigated. Furthermore, MV and renal tissue remodeling were assessed in 5 µm mid-hilar renal paraffin-embedded slices stained with trichrome, and the presence of resident progenitor cell in the kidney by immunoreactivity of Oct-419. Double immunofluorescence for DiI and CD31 or cytokeratin was used to localize the EPC in renal vessels or tubules, respectively.
Progenitor cells
Blood collection and cell isolation
‘Late’ and ‘early’ EPC were obtained as previously described 20–24. Late EPC were cultured from peripheral mononuclear cells collected 21 days before administration, while early EPC were obtained from cells collected and cultured 7 days before in vivo CT studies. All cells were cultured in endothelial growth medium. An equal blend of early and late EPC (10×106 cells) was subsequently delivered into the renal artery (see online supplement), in line with their synergistic effect in promoting neovascularization20 23, 24 compared to each cell type alone.
Colony forming unit (CFU)
were counted to assess the availability of circulating EPC. EPC colonies consisting of multiple thin, flat cells emanating from a central cluster of rounded cells were counted after 7 days of culture in 10 random (x20) microscope fields per subject, and expressed as CFU/cm2 25, 26.
Characterization of EPC markers
Immuno-fluorescence and/or Western blotting were used to determine the monocytic (CD14), progenitor (CD34, CD133), endothelial (KDR) phenotype27, and stem cell pluripotency (Oct-4) of early and late EPC.
Growth factor and cytokine measurement
To determine the production and secretion of growth factors by EPC, the culture media of late EPC was collected for measurement of VEGF levels. The cells were then homogenized, and expression of VEGF and eNOS evaluated.
EPC function
was tested using several accepted tests28 such as acetylated LDL uptake, cell migration, proliferation, and tube formation.
Preparation and delivery of EPC
Just prior to delivery, all cells were labeled with both a fluorescent membrane dye (CM-DiI) as well as with fluorescent beads29. CM-DiI (5µl/ml) was added to the culture medium and incubated for 30 min at 37°C. Fluoresbrite plain 2 µm YG (yellow-green) polymeric beads (Polysciences, Warrington, PA) were added at a 1:25 cell-tomicrospheres ratio, and incubated 75 min at 37 °C.
EPC localization and retention
was estimated from cells observed in kidney sections from the stenotic and contralateral kidneys30. Labeled cells were manually counted under fluorescence microscopy in frozen 5µm renal cross-sections, the total area of each cross-section was calculated, and the number of cells per mm2 averaged and multiplied by the section thickness, and then by the total renal volume.
EPC engraftment
Double fluorescence of CM-DiI and CD31 or cytokeratin were examined to investigate the location and phenotypical changes of EPC into endothelial or tubular cells, respectively (see Online Supplement).
Micro-CT
A side branch of the renal artery in the dissected kidney was cannulated and infused under physiological perfusion pressure with heparinized (10 units/ml) saline, followed by the radio-opaque silicone polymer Microfil, until it filled the intra-renal vessels. For details please see the online supplement.
Renal protein expression, Western Blotting, and apoptosis
Immunohistochemistry
staining was performed in 5 µm frozen or unstained mid-hilar renal cross-sections to assess the expression of integrin β3, α-SMA, and Oct-4. For details, please see the online supplement.
Western blotting
standard blotting protocols were followed, as previously described9, using specific polyclonal antibodies against p-Akt, p-eNOS, VEGF, HIF-1α, angiopoietin-1, TGF-β, MMP-2 and -9, TIMP-1, CD-133, KDR, and Oct-4. β-actins or GADPH were used as loading controls. Protein expression (one band per animal) was quantified using densitometry and averaged in each group. For details, please see the online supplement.
Apoptosis: for quantification of apoptotic cells, DeadEnd Fluorometric TUNEL System (Promega, WI) was used in 5 µm renal mid-hilar cross-sections, as shown before11.
Data analysis
Renal angiography
The degree of RAS was measured by quantitative renal angiography, as previously described 9, 10, 15, 16, 31, and assessed as the decrease in luminal diameter of the renal artery at the most stenotic point compared to a proximal stenosis-free segment.
MDCT analysis
Manually traced regions of interest were selected in MDCT images in the aorta, renal cortex, and medulla, and time-enhancement curves were generated and analyzed to calculate RBF and GFR.
Micro-CT analysis
Images were digitized for reconstruction of 3-D volume images, and analyzed with the Analyze® software package (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN), as previously described12, 32. Renal cortical MV density, vascular volume fraction, and single-MV tortuousity were calculated.
For more details, please, see the online supplement.
Histology
Mid-hilar 5 µm trichrome-stained cross sections of each kidney (1 per animal) were examined to quantify renal fibrosis, glomerulosclerosis, and Oct-4 immunoreactivity, as previously described10, 11, and peritubular capillary density was similarly quantified in CD-31 stained slides. For details, please see Online Supplement. For quantification of angiogenic vessels, 10 fields were randomly selected from each integrin β3 stained slide (one per animal). The stained vessels were counted manually in each field, averaged, and the results expressed as number of integrin β3+ vessels per field. For apoptosis, the fraction of apoptotic cells was calculated in 10 randomly selected fields in each slide, as we previously described11.
Statistical Analysis
Results are expressed as mean ± SEM. Comparisons within groups were performed using paired student’s t-test, and among groups using one-way ANOVA, with Student Newmann-Keuls post-hoc tests for correction for multiple comparisons. Statistical significance was accepted for p≤0.05. For data measured over time (blood pressure) a two-way repeated measures ANOVA was used, and statistical significance was accepted for p≤0.05.
Results
Characterization of the EPC
Both CD 14 and CD 133 were initially expressed in early cells, but after 21 days the expression of KDR increased as CD14 and CD133 diminished in late cells (see online supplement), suggesting that late EPC acquired endothelial characteristics. The cultured EPC were also Oct-4+ (see online supplement). The number of CFU and EPC migration were similar in RAS compare to normal pigs, but interestingly, cells from RAS pigs showed increased proliferation, tube formation, and secretion of angiogenic factors compared to EPC obtained from normal pigs (see online supplement).
Renal function
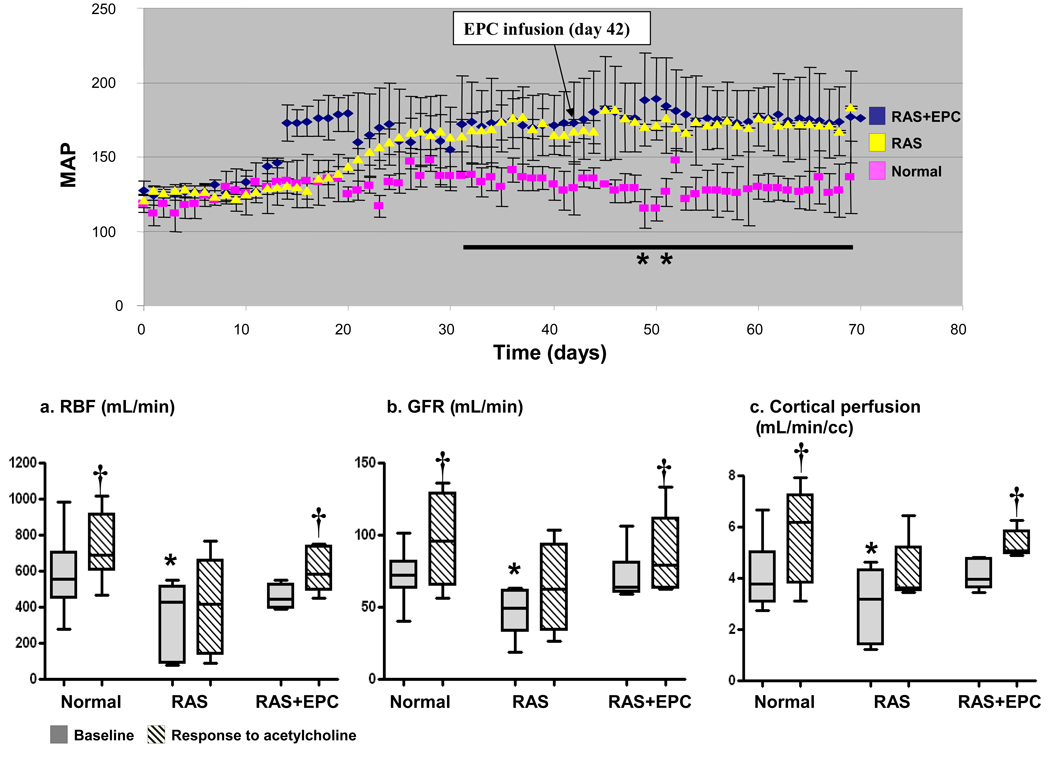
MAP and the angiographic degree of stenosis were similarly and significantly greater in RAS and RAS+EPC animals compared to normal, while systemic PRA and ADMA levels were similar among the groups, as were renal vein PRA (Table 1 and Figure 1-top). Basal RBF, cortical perfusion, and GFR were all diminished in RAS, but RBF and GFR significantly improved after EPC (ANOVA p<0.03 for RBF and GFR, ANOVA p=NS for perfusion). Similarly, RBF and GFR responses to the endothelium dependent vasodilator acetylcholine that were blunted in RAS were restored in RAS+EPC, suggesting improved renovascular endothelial function (Figure 1-bottom). These were accompanied by a significant increase in renal expression of p-eNOS in RAS+EPC, implying greater potential for NO availability.
Table 1.
Characteristics and basal stenotic kidney function (mean ± SEM) in normal, renal artery stenosis (RAS), and RAS pigs treated with autologous endothelial progenitor cells (RAS+EPC).
| Normal n=7 |
RAS n=7 |
RAS+EPC n=7 |
||
|---|---|---|---|---|
| Body weight (kg) | 47.3±2.3 | 50.7±1.9 | 51.7±2.0 | |
| Degree of stenosis (%) | 0.0±0.0 | 70.4±5.2* | 69.3±6.0* | |
| Mean arterial pressure (mmHg) | 104.7±2.2 | 140.2±7.1* | 130.3±6.9* | |
| Plasma renin activity (ng/mL/h) |
IVC | 0.22±0.04 | 0.19±0.03 | 0.21±0.04 |
| Stenotic kidney | ------- | 0.20±0.04 | 0.31±0.13 | |
| Contralateral kidney | ------- | 0.19±0.02 | 0.17±0.06 | |
| Plasma ADMA (umol/L) | 1.47±0.1 | 1.46±0.08 | 1.57±0.15 | |
| Renal volume (cc) | Cortex | 98.6±7.1 | 47.0±13.4* | 99.2±5.0 |
| Medulla | 43.9±2.4 | 21.9±6.4* | 20.5±2.5* | |
| Renal blood flow (mL/min) | 566.5±46.9 | 301.9.6±92.5* | 452.93±24.7# | |
| Glomerular filtration rate (mL/min) | 70.8±4.3 | 47.9±10.1* | 62.7±1.5 | |
| Perfusion (mL/min/cc) | Cortex | 4.1±0.3 | 2.7±0.6 | 4.1±0.2 |
| Medulla | 2.7±0.4 | 2.7±0.2 | 2.4±0.2 | |
| Proteinuria (ug/mL) | 14.4±3.7 | 21.6±4.8 | 16.8±4.1 | |
MAP: mean arterial pressure (measured during CT studies). IVC: inferior vena cava. ADMA: Asymmetric Dimethylarginine
p<0.05 vs. normal †p<0.05 vs. RAS.
p=0.06 vs. normal.
Figure 1.
Top: Changes in blood pressure over time (n=3–4 animals per group)
Bottom: Renal blood flow (RBF, a), glomerular filtration rate (GFR, b), and cortical perfusion (c) at baseline and in responses to acetylcholine in normal, renal artery stenosis (RAS), and RAS treated with autologous endothelial progenitor cells (RAS+EPC) animals. EPC infusion did not decrease blood pressure in RAS. The blunted basal and challenged renal hemodynamics and function in RAS were normalized after EPC, suggesting restoration of renovascular endothelial function. *p<0.05 vs. Normal, †p<0.05 vs. baseline, ** p<0.05 vs. Normal (two-way repeated measures ANOVA)
Angiogenic factors
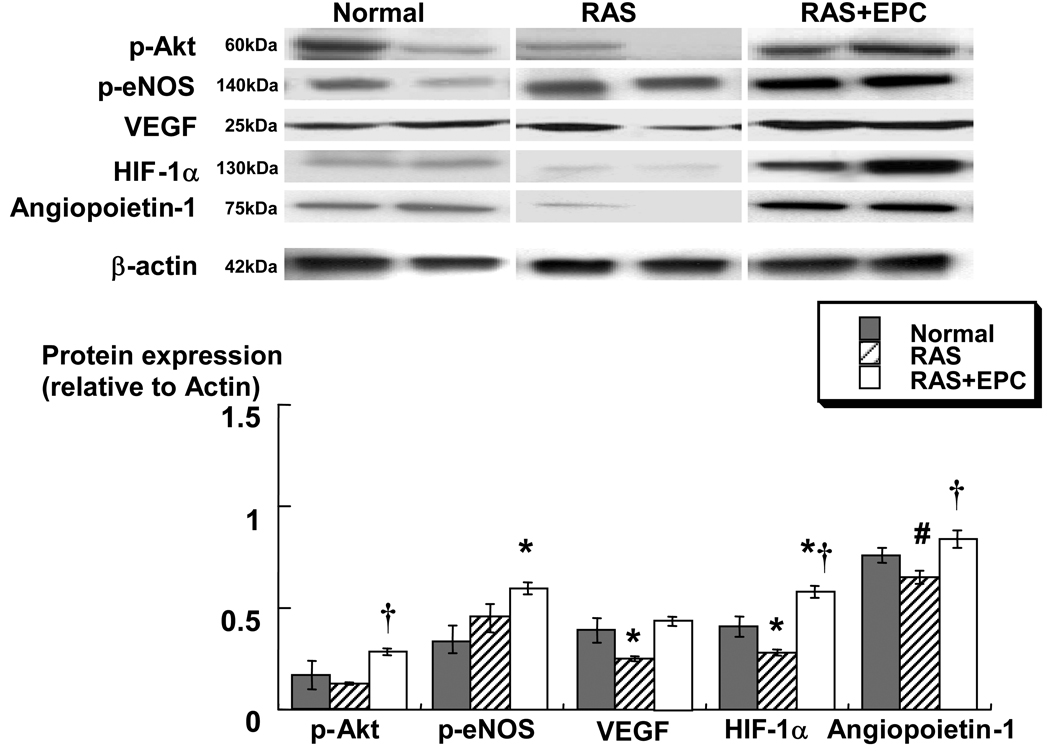
The blunted expression of pro-angiogenic HIF-1α and VEGF in RAS were increased after EPC (ANOVA p=0.004 and p=0.01, respectively), suggesting a pro-angiogenic milieu in the treated RAS kidney. Furthermore, renal expression of p-Akt and p-eNOS, key mediators of VEGF, were significantly augmented in RAS+EPC compared to untreated RAS. This was accompanied in RAS+EPC by increased renal expression of integrin β3 (19.7±3.3 vs. 10.6±2.8 and 12.9±1.5 positive vessels/field in RAS and normal, respectively, ANOVA p=0.02) and angiopoietin-1 (ANOVA p=0.03, Figure 2), suggesting that EPC not only promoted angiogenesis but also favored the maturation of the new vessels.
Figure 2.
Representative immunoblots (top) and densitometric quantification (bottom) demonstrating renal protein expression of phosphorylated Akt, phosphorylated eNOS, VEGF, HIF-1α, and angiopoietin-1 in normal, kidneys with renal artery stenosis (RAS), and RAS treated with autologous endothelial progenitor cells (RAS+EPC). Replenishment of EPC in RAS augmented the expression of vascular growth factors in the stenotic kidney, suggesting a pro-angiogenic stimulus. *p<0.05 vs. Normal. †p<0.05 vs. RAS, # p=0.079 vs. Normal.
MV 3D architecture
MV density was diminished in RAS across the renal cortex (inner, middle, and outer cortex). Notably, RAS+EPC substantially increased cortical MV density in all the cortical regions (although it remained lower than normal), resulting in improved vascular volume fraction (ANOVA p=0.0002, Figure 3). MV tortuousity was significantly increased in RAS+EPC kidneys compared to both RAS and normal controls (1.87±0.09 vs. 1.30±0.02 and 1.39±0.09, respectively, ANOVA p=0.0007), supporting the notion of abundant angiogenic vessels. Furthermore, CD-31 expression on capillaries was significantly reduced in RAS compared to normal (0.29±0.04 vs. 1.91±0.6%, p=0.01) but improved in RAS+EPC (1.13±0.6%, p=0.3 vs. Normal, and p=0.05 vs. RAS), suggesting augmented capillary proliferation in the treated kidney.
Figure 3.
Representative 3D tomographic images of renal cortex and medulla (top) and quantification (bottom) from normal, renal artery stenosis (RAS), and RAS treated with autologous endothelial progenitor cells (RAS+EPC) kidneys. EPC in RAS augmented intra-renal micro-vascular density throughout the cortex, which consequently restored renal vascular volume fraction. *p<0.05 vs. Normal. †p<0.05 vs. RAS.
Renal EPC and morphology
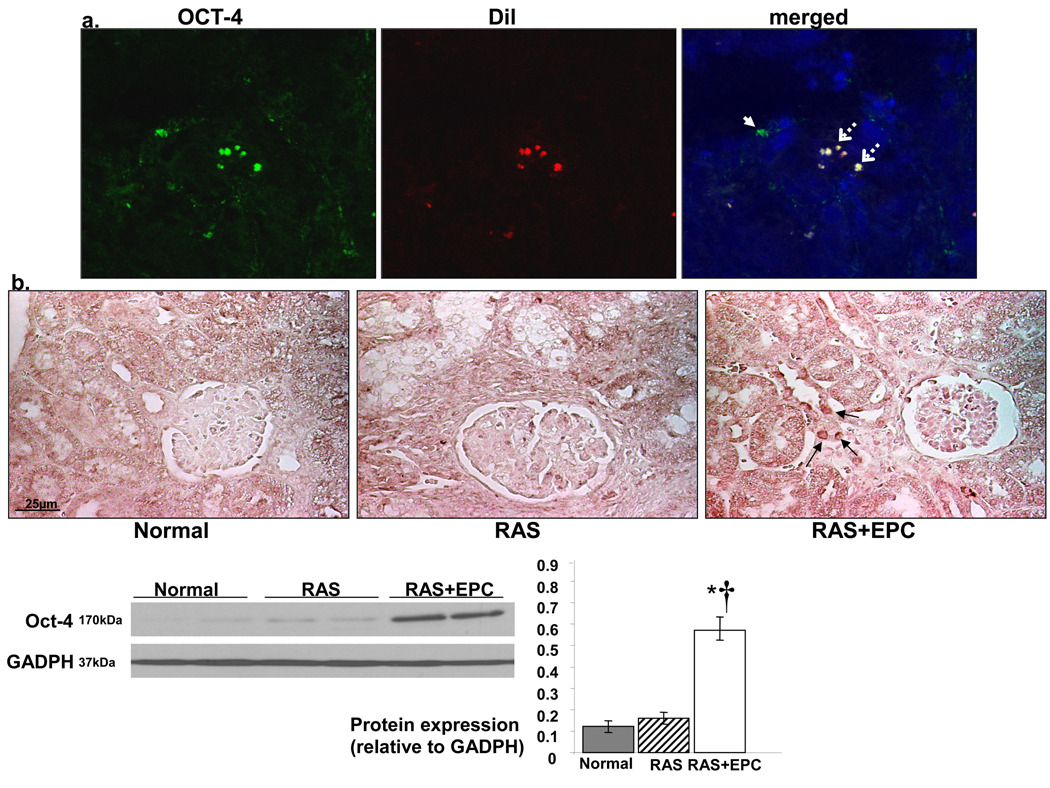
An average of 13.3±0.8% of the total injected EPC was detected 4 weeks later, the cells were evident at the tubular and vascular compartments of the treated stenotic kidneys. Some of the injected EPC were detected incorporated in very small (capillaries and vasa vasorum) MV (CD31+) and in tubules (cytokeratin+), and co-stained with these markers, suggesting that they assumed endothelial and tubular characteristics (see Figure 5s in the online supplement). Interestingly, the number of Oct-4+ cells in renal tubules was significantly increased after EPC treatment, many of which co-stained with DiI fluorescence, indicating that they originated from injected EPC. Nevertheless, only EPC-treated stenotic kidneys also showed Oct-4+ cells unlabeled with DiI (see Figure 4a), suggesting activation and mobilization of circulating or resident stem cells (Figure 4b).
Figure 4.
a) Representative double fluorescence of CM-DiI (red) and immunoreactivity of Oct-4 (green, x40) in frozen renal sections. b) Representative staining (x40), Western blotting bands, and densitometry (bottom) of renal immunoreactivity to Oct-4 in normal, renal artery stenosis (RAS), and RAS treated with autologous endothelial progenitor cells (RAS+EPC) kidneys. Some Oct-4+ cells are of EPC origin (dashed arrow) but others are not (white arrow), suggesting recruitment of endogenous progenitor cells. *p<0.05 vs. Normal, †p<0.05 vs. RAS.
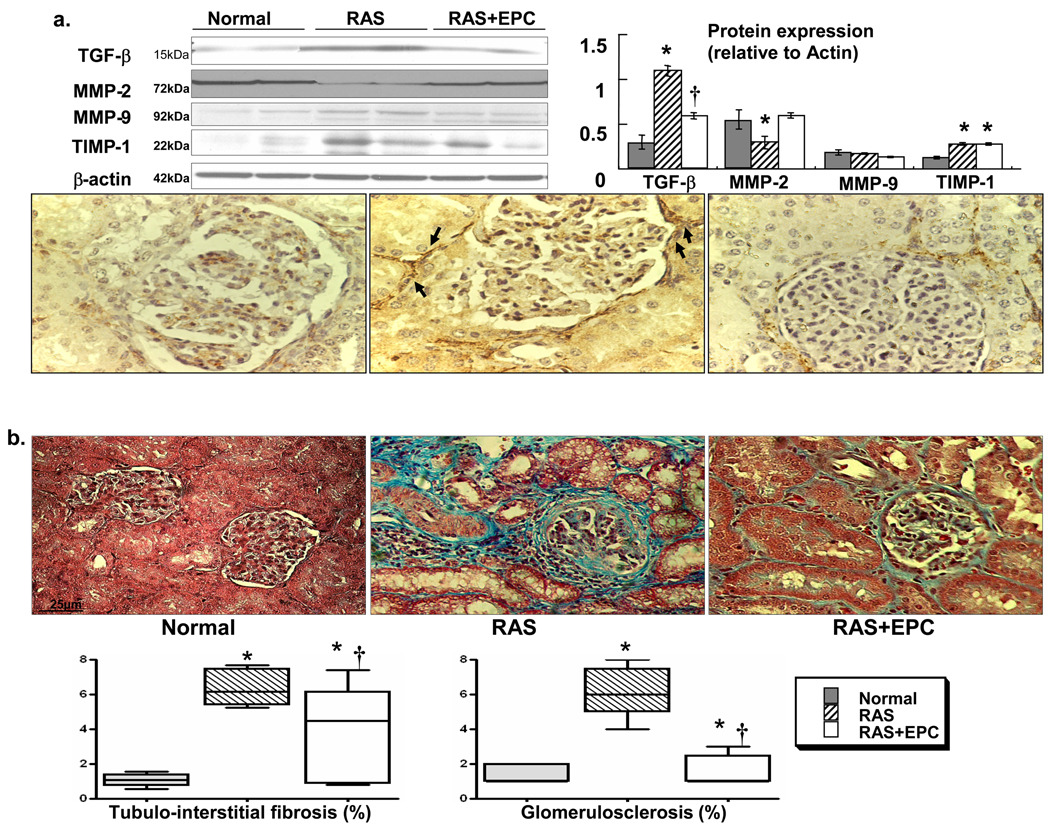
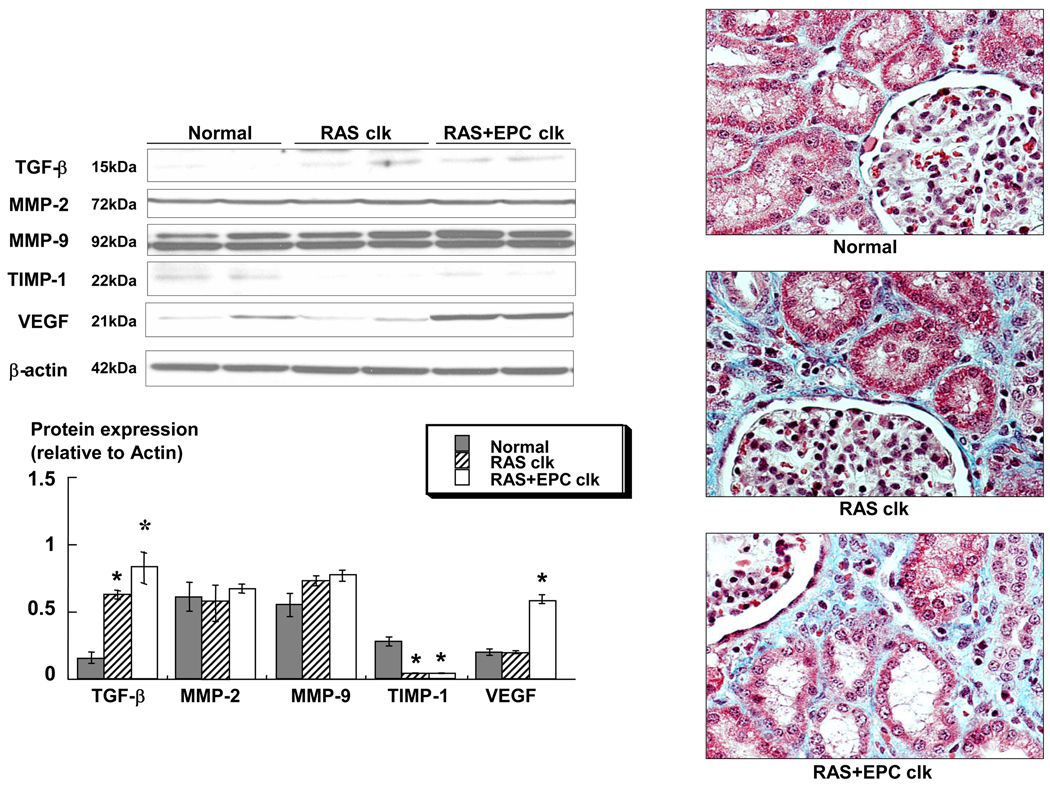
RAS kidneys showed increased expression of TGF-β and α-SMA and decreased MMP-2 (Figure 5a), which were accompanied by increased glomerulosclerosis, perivascular and tubulointerstitial fibrosis compared to normal controls (Figure 5b), overall suggesting renal remodeling. Importantly, EPC improved the expression of those factors and decreased fibrosis in the stenotic kidney, without fully normalizing it (ANOVA p=0.001). Conversely, EPC did not affect apoptosis, as the fraction of apoptotic cells was significantly and similarly elevated in RAS and RAS+EPC compared to normal (0.59±0.1, 0.52±0.2, and 0.09±0.01%, respectively, p<0.04).
Figure 5.
a) Representative immunoblots and densitometric quantification demonstrating renal protein expression of TGF-β, MMP-2, MMP-9, and TIMP-1, and pictures showing renal immunoreactivity of α-SMA+ cells (arrows), likely myofibroblasts. b) Renal trichrome staining (x40) and quantification of renal fibrosis and glomerulosclerosis in normal, renal artery stenosis (RAS), and RAS treated with autologous endothelial progenitor cells (RAS+EPC) kidneys. EPC significantly decreased pro-fibrotic activity and attenuated renal remodeling in RAS. *p<0.05 vs. Normal, †p<0.05 vs. RAS.
Contralateral kidney
The presence of labeled cells and changes in morphology in the contralateral kidney were determined. Interestingly, although few EPC were observed in the contralateral kidney of RAS+EPC animals (1–2 cells per slide), VEGF expression was increased compared to both normal and untreated RAS pigs. However, unlike the RAS+EPC kidney, the contralateral kidney of RAS and RAS+EPC animals did not show any changes in tubulo-interstitial fibrosis (2.09±0.1 and 1.83±0.05 %, respectively, p<0.01 vs. Normal), blunted number of capillaries (0.64±0.04 and 0.70±0.04 %, respectively, p<0.05 vs. Normal) or renal expression of TGF-β (elevated compared to normal), MMP-2, and -9 (unchanged) and TIMP-1 (attenuated compared to normal), implying lack of effect of these cells remodeling of the contralateral kidney (Figure 6). Apoptosis also remained evident (1.22±0.47 and 1.07±0.30 respectively p<0.05 vs. Normal).
Figure 6.
Left: Representative immunoblots and densitometry demonstrating protein expression of TGF-β, MMP-2, MMP-9, TIMP-1, and VEGF in the contralateral kidney (CLK). Right: Renal trichrome staining (x40) of normal kidneys and CLK of renal artery stenosis (RAS) and RAS treated with endothelial progenitor cells (RAS+EPC) animals. Intra-renal infusion of EPC in the stenotic kidney only augmented VEGF in the CLK, but did not have any effect in attenuating renal fibrosis or regulating the expression of its mediators. *p<0.05 vs. Normal
Discussion
The current study shows, for the first time, the feasibility of a cell-based approach to treat the ischemic kidney distal to the stenosis. A single intra-renal infusion of autologous EPC during the evolution of RAS restored the hemodynamics and function of the ischemic kidney, preserved MV architecture, and attenuated renal remodeling. The capability of EPC to restore vascular integrity and preserve the stenotic kidney may therefore enable development of novel therapeutic renoprotective strategies in chronic renovascular disease.
Renovascular disease is often associated with atherosclerosis, but constitutes a strong predictor for increasing morbidity and mortality independent of other cardiovascular risk factors8, as is a decrease in GFR33. Renal disease is often characterized by decreased MV density as well as tubulo-interstitial fibrosis, which are determinants of renal outcomes34. Regression of intra-renal MV accompanies many forms of renal disease like diabetes and aging, and MV remodeling correlates with development of renal scarring35. Indeed, we have previously shown that renal injury is evident by 1 month after development of the RAS 16, 31, and that deterioration of renal hemodynamics and function is paralleled by significant fibrosis10, 11, MV rarefaction, and remodeling9, 12. MV and parenchymal damage distal to the obstruction likely contributes to renal dysfunction observed in humans with chronic renovascular disease despite revascularization, but few therapeutic options are available to restore renal viability and vascular integrity.
In many ischemic and injured organs, mobilization, homing, and trans-differentiation of EPC play an important role in augmenting neovascularization36 and endothelial replacement after vascular injury22, 24. EPC augment angiogenesis by stimulating both the secretion of angiogenic growth factors and by providing a source of progenitor cells that can differentiate into mature vascular endothelial cells. They appear to confer their beneficial effect not only by their long-term engraftment and local retention in the injured tissue, but also by transient secretion of vascular growth factors within this region37. Yet, in spite of the promise of EPC delivery in treating diseases associated with blood vessel disorders, the potential of this strategy to salvage the ischemic kidney in RAS has not been explored. The current study indicates that this strategy is feasible and effective. In the current study we delivered into the stenotic kidney a combination of early EPC, which retain monocytic characteristics and avid angiogenic activity, and late EPC, which exhibit more mature endothelial-like features. Previous studies have demonstrated that these cell types synergistically enhance angiogenesis more effectively than each cell type alone20. This intervention elicited new vessel formation and reversed most of the functional and structural deterioration of the stenotic kidney. We observed that some of the injected cells assumed endothelial and tubular phenotype and incorporated into renal structures. However, considering the relatively small number of EPC retained in the tissue, their autocrine and paracrine activity were likely key for the increase in protein expression and improvement in renal function.
Angiogenesis involves a sequence of events regulated by numerous factors that results in development of new vessels. Among those factors, VEGF is crucial for preservation of the microvasculature, and in concert with other factors, stimulates processes responsible for cell division, migration and survival, extra-cellular matrix degradation, and tube formation that generate, repair, and maintain MV networks. We have previously shown both HIF-1α and VEGF to be paradoxically down-regulated in chronic RAS9, 12, attended by MV rarefaction and renal fibrosis. HIF-1α is the most important transcription factor driving VEGF mRNA expression and production, and is considered a crucial primary defense mechanism for the adaptive response to ischemia in the kidney38. Remarkably, intra-renal administration of EPC in the stenotic kidney restored HIF-1α, VEGF, eNOS, Akt, and angiopoietin-1, all of which stimulate or mediate angiogenesis. Angiopoietin-1 is an endothelial cell survival factor that in concert with VEGF can promote angiogenesis, maturation of the new vessels, and vascular repair39, and the concurrent improvement of both may have had an additive effect on vascular proliferation and maturation in RAS+EPC40. Augmented neovascularization was also reflected by the increases in renal expression of integrin β3, peritubular CD31+ capillaries, MV density, and tortuousity, which are all indices of angiogenic vessels. Therefore, EPC not only attenuated MV dysfunction and rarefaction in RAS, but also improved maturation and stabilization of the new vessels, thereby improving the overall hemodynamics and function of the stenotic kidney. Although apoptosis remained elevated in RAS+EPC, this therapeutic approach significantly decreased the scarring in the stenotic kidney, possibly as a result of increased blood supply and NO availability after EPC treatment. Furthermore, it may have also been related to a decreased fibrogenic activity and improved matrix turnover, as suggested by the improved expression of TGF-β and MMP-2 and decreased fibrosis and glomerulosclerosis in RAS+EPC, which in turn may have facilitated restoration of the MV network as well.
The number of CFU that we found is comparable to previous studies using similar techniques26. We observed that CFU were similar in normal and RAS animals, suggesting that circulating EPC were not depleted in RAS, yet RAS EPC showed increased proliferation potential and better angiogenic function (tube formation) compared to controls. These characteristics differ from EPC function in humans with chronic refractory hypertension41 but are more consistent with other clinical studies on EPC in essential or pregnancy-induced hypertension42, 43 or with recent studies in comparable models 44. It is possible that duration and nature of the disease, as well as species differences, may have contributed to these differences. For example, a preferential increase in PRA at the early stage (4–5 weeks) of the disease 31 might favor angiotensin II-mediated angiogenic activity45 that would gradually dissipate as the disease progresses and oxidative stress increases31. Interestingly, the current study shows that delivery of autologous EPC into the stenotic kidney also increased number of cells positive for Oct-4, a stem cell transcription factor and marker suggestive of pluripotency19. While many of these were likely the Oct-4+ injected EPC, at least some of them were not labeled with the EPC markers, suggesting that this intervention also promoted mobilization of resident or homing of endogenous circulating progenitor cells. Either by incorporating and differentiating in the tissue host and/or by autocrine and paracrine activity, EPC are capable of stimulating the function and proliferation of surrounding progenitor and mature cells20. Importantly, some of the injected EPC were detected engrafted into blood vessels and tubules and assumed at least some endothelial and tubular features (CD31 and cytokeratin expression), although it remains to be determined what level of functionality the engrafted cells achieved. Interestingly, EPC were observed incorporated only in very small MV, likely peritubular capillaries and vasa vasorum, but not in larger vessels. Presumably, longer transit times and greater surface area facilitate their contact, adherence, retention, and migration into tissue structures.
Importantly, our study showed that EPC restored the blunted RBF and GFR of the RAS kidney, which might be a result of the preserved microvasculature, but also likely due to augmented availability of eNOS-derived NO, as implied by the increased renal expression of activated eNOS5. In turn, NO is indispensable for MV sprouting by maintaining vasodilation during the early steps of angiogenesis46 and by promoting VEGF-induced capillary proliferation47. Furthermore, eNOS-derived NO might have contributed to enhance Oct-4 expression in resident progenitor cells and promoted endothelial differentiation48 in the RAS+EPC kidney. Hence, upregulated eNOS not only favored renal MV endothelial function, but may have also contributed to angiogenesis in the RAS+EPC kidney. Moreover, VEGF is also a potent vasodilator that may have contributed to recovering endothelial function in RAS+EPC49. Interestingly, the degree of stenosis and hypertension in RAS remained unchanged by EPC, although renal function improved with intra-renal EPC therapy. While the remaining obstruction of the renal artery and decrease in renal perfusion pressure might have been sufficient to activate the intra-renal renin-angiotensin system, renal vein PRA did not lateralize to the stenotic side. Alternatively, an increase in oxidative stress may also sustain hypertension at the chronic phase of untreated RAS when PRA declines31. In addition, we cannot rule out the possibility that disease in the contralateral kidney results in volume retention and thereby mediates hypertension. Indeed, the sustained fibrosis and decreased capillary density in both the stenotic and contralateral kidneys indicate residual injury that might have also contributed to the persistent hypertension.
In summary, the current study shows the renoprotective effects of EPC in a model of chronic RAS. A targeted intervention using autologous EPC during the evolution of the disease reversed most of the functional and structural deterioration of the stenotic kidney in this otherwise progressive disease. Likely, restoration of the angiogenic cascade by autologous EPC involved not only generation of new vessels, but also acceleration of their maturation and stabilization. This contributed to preserving the blood supply, hemodynamics, and function of the RAS kidney, and thereby decreased renal remodeling. Future studies are needed to examine the feasibility of this approach after longer duration of chronic renal ischemia in humans, and in the presence of additional cardiovascular risk factors.
Supplementary Material
Acknowledgments
The authors are grateful to Mark E. Rosenberg, MD, University of Minnesota, for his advice on 19 the Oct-4 staining technique.
Funding Sources: Supported by grant numbers DK-73608, HL-77131, PO1HL085307, HL-75566, and HL-76611 from the NIH, and by an unrestricted grant from The GlaxoSmithKline Research & Education Foundation for Cardiovascular Disease.
Footnotes
Significant attention has been directed to the biologic and therapeutic capabilities of progenitor cells. Endothelial progenitor cells (EPC) mobilized endogenously in response to ischemia play a crucial role in augmenting neovascularization of ischemic tissues and repair of the vessel wall following endothelial cell denudation. A large body of experimental and clinical evidence accumulated over the last 10 years that administration of EPC could improve the function of the ischemic tissues. The current study tested the feasibility of using progenitor cells to treat the obstructed kidney in a model of chronic renovascular disease. This disease is difficult to treat and may induce hypertension and renal injury, leading to end-stage renal disease. In this model, a single intra-renal administration of EPC restored renal hemodynamics and function, preserved renal microvascular architecture, and attenuated fibrosis of the ischemic kidney. The demonstrated capability of EPC to restore vascular integrity and preserve the stenotic kidney may constitute an important step for designing novel therapeutic measures for management of patients with renovascular disease.
Disclosures: None.
References
- 1.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 2.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto A, Murayama T, Kusano K, Ii M, Tkebuchava T, Shintani S, Iwakura A, Johnson I, von Samson P, Hanley A, Gavin M, Curry C, Silver M, Ma H, Kearney M, Losordo DW. Synergistic effect of bone marrow mobilization and vascular endothelial growth factor-2 gene therapy in myocardial ischemia. Circulation. 2004;110:1398–1405. doi: 10.1161/01.CIR.0000141563.71410.64. [DOI] [PubMed] [Google Scholar]
- 4.Napoli C, Williams-Ignarro S, de Nigris F, de Rosa G, Lerman LO, Farzati B, Matarazzo A, Sica G, Botti C, Fiore A, Byrns RE, Sumi D, Sica V, Ignarro LJ. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci U S A. 2005;102:17202–17206. doi: 10.1073/pnas.0508534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, Vajkoczy P, Hofmann WK, Peters C, Pennacchio LA, Abolmaali ND, Chavakis E, Reinheckel T, Zeiher AM, Dimmeler S. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 6.Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 7.Abe-Yoshio Y, Abe K, Miyazaki M, Furusu A, Nishino T, Harada T, Koji T, Kohno S. Involvement of bone marrow-derived endothelial progenitor cells in glomerular capillary repair in habu snake venom-induced glomerulonephritis. Virchows Arch. 2008;453:97–106. doi: 10.1007/s00428-008-0618-5. [DOI] [PubMed] [Google Scholar]
- 8.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med. 2005;165:207–213. doi: 10.1001/archinte.165.2.207. [DOI] [PubMed] [Google Scholar]
- 9.Chade AR, Zhu X, Mushin OP, Napoli C, Lerman A, Lerman LO. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 10.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 11.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 12.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical Microvascular Remodeling in the Stenotic Kidney. Role of Increased Oxidative Stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 13.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 14.Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, Saruta T, Hishikawa K, Fujita T. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol. 2005;16:997–1004. doi: 10.1681/ASN.2004050367. [DOI] [PubMed] [Google Scholar]
- 15.Chade AR, Bentley MD, Zhu X, Rodriguez-Porcel M, Niemeyer S, Amores-Arriaga B, Napoli C, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol. 2004;15:1816–1825. doi: 10.1097/01.asn.0000130428.85603.6b. [DOI] [PubMed] [Google Scholar]
- 16.Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999;10:1455–1465. doi: 10.1681/ASN.V1071455. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Rodriguez-Porcel M, Herrmann J, Zhu X, Grande JP, Napoli C, Lerman A, Lerman LO. Antioxidant Intervention Blunts Renal Injury in Experimental Renovascular Disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 18.Daghini E, Primak AN, Chade AR, Krier JD, Zhu X, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function using 64-slice multidetector CT: comparison with EBCT. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J, Heremans Y, Lund T, Blackstad M, Jiang Y, Luttun A, Rosenberg ME. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 20.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 21.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 22.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 23.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 24.Gulati R, Jevremovic D, Witt TA, Kleppe LS, Vile RG, Lerman A, Simari RD. Modulation of the vascular response to injury by autologous blood-derived outgrowth endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H512–H517. doi: 10.1152/ajpheart.00063.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 26.Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 29.Pislaru SV, Van Ranst M, Pislaru C, Szelid Z, Theilmeier G, Ossewaarde JM, Holvoet P, Janssens S, Verbeken E, Van de Werf FJ. Chlamydia pneumoniae induces neointima formation in coronary arteries of normal pigs. Cardiovasc Res. 2003;57:834–842. doi: 10.1016/s0008-6363(02)00787-3. [DOI] [PubMed] [Google Scholar]
- 30.Moore XL, Lu J, Sun L, Zhu CJ, Tan P, Wong MC. Endothelial progenitor cells' "homing" specificity to brain tumors. Gene Ther. 2004;11:811–818. doi: 10.1038/sj.gt.3302151. [DOI] [PubMed] [Google Scholar]
- 31.Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37:541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109:2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]
- 33.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050–1065. doi: 10.1161/01.HYP.0000102971.85504.7c. [DOI] [PubMed] [Google Scholar]
- 34.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001;16:765–770. doi: 10.1093/ndt/16.4.765. [DOI] [PubMed] [Google Scholar]
- 35.Kang DH, Kanellis J, Hugo C, Truong L, Anderson S, Kerjaschki D, Schreiner GF, Johnson RJ. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 37.Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 38.Manotham K, Tanaka T, Ohse T, Kojima I, Miyata T, Inagi R, Tanaka H, Sassa R, Fujita T, Nangaku M. A biologic role of HIF-1 in the renal medulla. Kidney Int. 2005;67:1428–1439. doi: 10.1111/j.1523-1755.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 39.Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- 40.Chae JK, Kim I, Lim ST, Chung MJ, Kim WH, Kim HG, Ko JK, Koh GY. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–2578. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 41.Oliveras A, Soler MJ, Martinez-Estrada OM, Vazquez S, Marco-Feliu D, Vila JS, Vilaro S, Lloveras J. Endothelial progenitor cells are reduced in refractory hypertension. J Hum Hypertens. 2008;22:183–190. doi: 10.1038/sj.jhh.1002304. [DOI] [PubMed] [Google Scholar]
- 42.Buemi M, Allegra A, D'Anna R, Coppolino G, Crasci E, Giordano D, Loddo S, Cucinotta M, Musolino C, Teti D. Concentration of circulating endothelial progenitor cells (EPC) in normal pregnancy and in pregnant women with diabetes and hypertension. Am J Obstet Gynecol. 2007;196(68):e61–e66. doi: 10.1016/j.ajog.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Delva P, Degan M, Vallerio P, Arosio E, Minuz P, Amen G, Di Chio M, Lechi A. Endothelial progenitor cells in patients with essential hypertension. J Hypertens. 2007;25:127–132. doi: 10.1097/HJH.0b013e3280109271. [DOI] [PubMed] [Google Scholar]
- 44.Salguero G, Akin E, Templin C, Kotlarz D, Doerries C, Landmesser U, Grote K, Schieffer B. Renovascular hypertension by two-kidney one-clip enhances endothelial progenitor cell mobilization in a p47phox-dependent manner. J Hypertens. 2008;26:257–268. doi: 10.1097/HJH.0b013e3282f09f79. [DOI] [PubMed] [Google Scholar]
- 45.Hu C, Dandapat A, Mehta JL. Angiotensin II induces capillary formation from endothelial cells via the LOX-1 dependent redox-sensitive pathway. Hypertension. 2007;50:952–957. doi: 10.1161/HYPERTENSIONAHA.107.096446. [DOI] [PubMed] [Google Scholar]
- 46.Kon K, Fujii S, Kosaka H, Fujiwara T. Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester retards vascular sprouting in angiogenesis. Microvasc Res. 2003;65:2–8. doi: 10.1016/s0026-2862(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 47.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Nitric oxide, VEGF, and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2005;289:H336–H343. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 48.Chu L, Jiang Y, Hao H, Xia Y, Xu J, Liu Z, Verfaillie CM, Zweier JL, Liu Z. Nitric oxide enhances Oct-4 expression in bone marrow stem cells and promotes endothelial differentiation. Eur J Pharmacol. 2008;591:59–65. doi: 10.1016/j.ejphar.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 49.Schrijvers BF, Flyvbjerg A, Tilton RG, Lameire NH, De Vriese AS. A neutralizing VEGF antibody prevents glomerular hypertrophy in a model of obese type 2 diabetes, the Zucker diabetic fatty rat. Nephrol Dial Transplant. 2006;21:324–329. doi: 10.1093/ndt/gfi217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.