Abstract
Muscle form of lactate dehydrogenase (M-LDH) physically associate with KATP channel subunits, Kir6.2 and SUR2A, and is an integral part of the ATP-sensitive K+ (KATP) channel protein complex in the heart. Here, we have shown that concomitant introduction of viral constructs containing truncated and mutated forms of M-LDH (ΔM-LDH) and 193gly-M-LDH respectively, generate a phenotype of rat heart embryonic H9C2 cells that do not contain functional M-LDH as a part of the KATP channel protein complex. The K+ current was increased in wild type cells, but not in cells expressing ΔM-LDH/193gly-M-LDH, when they were exposed to chemical hypoxia induced by 2,4 dinitrophenol (DNP; 10 mM). At the same time, the outcome of chemical hypoxia was much worse in ΔM-LDH/193gly-M-LDH phenotype than in the control one, and that was associated with increased loss of intracellular ATP in cells infected with ΔM-LDH/193gly-M-LDH. On the other hand, cells expressing Kir6.2AFA, a Kir6.2 mutant that abolishes KATP channel conductance without affecting intracellular ATP levels, survived chemical hypoxia much better than cells expressing ΔM-LDH/193gly-M-LDH. Based on the obtained results, we conclude that M-LDH physically associated with Kir6.2/SUR2A regulates the activity of sarcolemmal KATP channels as well as an intracellular ATP production during metabolic stress, both of which are important for cell survival.
Keywords: M-LDH, KATP channels, Chemical hypoxia, ATP, H9C2 cells
1. Introduction
ATP-sensitive K+ (KATP) channels are gated by intracellular ATP and are viewed as a link between cellular metabolism and membrane excitability. In the heart, the activation of these channels protects the cells against metabolic stress, such as hypoxia and ischaemia. It is generally accepted that cardiac sarcolemmal KATP channels are composed of Kir6.2, an inward rectifier, and SUR2A, an ABC protein (Inagaki et al., 1996). It has been recently suggested that in vivo these two subunits physically interact with enzymes regulating ATP production and glycolysis. One of these enzymes is a muscle form of lactate dehydrogenase (M-LDH), a minor form of LDH present in the heart (M-LDH; Carrasco et al., 2001; Crawford et al., 2002a,b; Jovanović et al., 2005; Jovanović and Jovanović, 2005; Dhar-Chowdhury et al., 2005). The physical interaction between M-LDH and Kir6.2 and SUR2A subunits has been demonstrated by co-immunoprecipitation at the recombinant and native levels, immunofluorescence and FRET analysis (Crawford et al., 2002b). However, the functional significance of M-LDH physically associated with sarcolemmal KATP channels is yet to be understood.
H9C2 cells are embryonic rat heart myocytes that have been used with success to study sarcolemmal KATP channels (Ranki et al., 2002; Crawford et al., 2003). Here, we have taken advantage of this experimental model to determine the role that species of M-LDH physically associated with Kir6.2/SUR2A play in the regulation of sarcolemmal KATP channels and cellular resistance to metabolic stress. We report that not only that M-LDH-mediated regulation of sarcolemmal KATP channels activity is crucial for cell survival during metabolic stress, but that ATP produced by sarcolemmal KATP channel protein complex mediates cytoprotection independently from the channel activity.
2. Methods
2.1. H9C2 cells and viral constructs
Rat embryonic heart H9C2 cells (ECACC, Salisbury, UK) were cultured in a tissue flask (at 5% CO2) containing Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 2 mM glutamine. For electrophysiological experiments, the cells were plated on a 35 mm × 10 mm culture dish containing 25-mm glass cover-slips. The cells were cultured in incubators (Galaxy, oxygen control model, RS Biotech, Irvine, UK). For the experiments H9C2 cells were infected with adenoviral constructs containing either green fluorescent protein (GFP, gift from C. Sunderland, University of Dundee; cells infected with GFP have served as control cells in this study), gly193-M-LDH (a catalytically inactive mutant of M-LDH, (Crawford et al., 2002b) together with truncated M-LDH (ΔM-LDH) or Kir6.2AFA (a mutant form of Kir6.2 where the pore GFG was mutated into AFA leading to largely reduced K+ conductance, Van Bever et al., 2004). When intracellular ATP levels were measured cells were infected with adenovirus containing luciferase gene. All adenoviruses were generated using the AdEasy XL Adenoviral Vector System (Stratagene) as described by the manufacturer. All of the genes were subcloned by PCR using primers containing restrict enzyme sites. Truncation of the first 19 N- and the last 48 C-terminal amino acids of mouse M-LDH was achieved using nested PCR of M-LDH gene and the following primers, sense 5′-GGCAGATCTATGCAGAACAAGATTACAGTTGT-3′, antisense, 5′-GCCTCGAGTTAATTGATTCCATAGAGACCCT-3′. gly193-M-LDH was already generated in our laboratory (Crawford et al., 2002b) and we have subcloned this gene using the following primers: sense, 5′-GGCAGATCTATGGCAACCCTCAAGGACCA-3′, antisense, 5′-GCCTCGAGTTAGAACTGCAGCTCCTTCT-3′ using gly193-M-LDH as a template. To generate Kir6.2AFA, Kir6.2 gene was subcloned using sense, 5′-GCAGGATCCACCATGCTGTCCCGAAAGGGC-3′, antisense, 5′-GCATCTAG ATCAGGACAAGGAATCTGGAG-3′ and the QuickChange Site-directed mutagenesis kit (Stratagene) was used to generate Kir6.2AFA according to the manufacturer's instructions; the mutagenic primers had the following sequences, sense, 5′-TCCAGGTGACCATTGCATTCGCAGGGCGCATGGTGACA-3′, antisense, 5′-TGTCACCATGCGCCCTGCGAATGCAATGGTCACCTGGA-3′. Luciferase gene was subcloned using the following primers: sense, 5′-GCCTCGAGGCCACCATGGAAGACGCCAAA-3′, antisense, 5′-GCGTAAGCTTACACGGCGATCTTTCCGCC-3′ using pGL3-Enhancer vector (Promega) as a template. PCR was performed by using the highest fidelity PfuUltra™ DNA polymerase (Stratagene) under the following condition: the PCR was run with a hot start for 2 min at 95 °C, followed by 25 cycles of 0.5 min at 95 °C, 0.5 min at 56 °C, and 1 min at 72 °C; and a final extension 10 min at 72 °C. The PCR products were cloned between the Bgl II and Xho I sites of the pShuttle-CMV vector. All of the positive clones containing DNA inserts were verified by DNA sequencing. After construction, the shuttle vectors were linearized with Pme I and transformed into BJ5183-AD-1 competent cells to perform homologous recombination in Escherichia coli with these shuttle vectors and a large adenovirus-containing plasmid following electroporation. Recombinants were identified from single colonies, linearised, and then transfected into HEK293 cells to produce infective adenovirus virions. Adenoviral particles were obtained by cell extraction after 7–10 days of transfection, and the primary virus was further amplified by infection of AD-293 cultures. The virus titer was determined using QuickTiter Adenovirus Titer Immunoassay Kit (Cell Biolabs, Inc.) according to manufacturer's instructions. Typical virus titers were in the 109–1010 pfu/ml range. To infect H9C2 cells, a solution of recombinant adenovirus was mixed with culture medium, and cells were exposed to the virus with a multiplicity of 10 viral particles/cell for 48 h. More specifically, we have added 25 μl of 108 pfu/ml of viral solution into each well containing ∼2.5 × 105 cells.
2.2. Real time RT-PCR
To determine mRNA levels of native and mutated/truncated forms of M-LDH we have used real time RT-PCR (as described in Du et al., 2006; Jovanović et al., 2008). Total RNA was extracted from H9C2 cells using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Extracted RNA was further purified by RNeasy Plus Mini Kit (Qiagen, Crawley, UK) according to the manufacturer's instruction. The specific primers used to detect the total mRNA levels for muscle lactate dehydrogenase (M-LDH) were as follows: sense, 5′-GCAGAACAAG ATTACAGTTGT-3′, antisense, 5′-CTTGATTCCATAGAG ACCCT-3′ (the size was 795 bp of the PCR product). The primers used to detect native M-LDH were: sense, 5′-GTCCTAGCACTTCACTGTCCAG-3′ antisense, 5′-CACACTAACCAGGTCAC CACTAC-3′ (the size of the PCR product was 173 bp). The specificity of primers was tested by melting curve analysis and for their ability to produce no signal in negative controls by dimer formation (Du et al., 2006). The RT reaction was carried out with ImProm-II Reverse Transcriptase (Promega, Madison, WI). A final volume of 20 μl of RT reaction containing 4 μl of 5 × buffer, 3 mM MgCl2, 20 U of RNasin® Ribonuclease inhibitor, 1 U of ImProm-II reverse transcriptase, 0.5 mM each of dATP, dCTP, dGTP, and dTTP, 0.5 μg of oligo(dT), and 1 μg of RNA was incubated at 42 °C for 1 h and then inactivated at 70 °C for 15 min the produced cDNA were used as template for the quantitative real-time PCR. A SYBR Green I system was utilized in the reaction. The 25 μl reaction mixture contained: 12.5 μl iQ™ SYBR® Green Supermix (2×), 7.5 nM each primers, 9 μl of ddH2O, and 2 μl of cDNA. The thermal cycling conditions were as follows: an initial denaturation at 95 °C for 3 min, followed by 38 cycles of 10 s of denaturing at 95 °C, 15 s of annealing at 56 °C, and 50 s of extension at 72 °C. The real-time PCR was performed in the same wells of a 96-well plate in the iCycler iQ™ Multicolor Real-Time Detection System (Bio-Rad, Hercules, CA). The data were collected following each cycle and displayed graphically (iCycler iQ™ Real-time Detection System Software, version 3.0A, BioRad, Hercules, CA). Threshold cycle (CT) values were determined automatically by software. The melting curve data were collected to check the PCR specificity. The cDNA sample was duplicated, the corresponding no-RT mRNA sample was included as a negative control. The calculation of relative mRNA expression was performed as described (Pfaffl, 2001). The relative expression ratio (R) of gene encoding M-LDH is calculated using equation where EK is the real time PCR efficiency of M-LDH gene transcript, ER is the real time PCR efficiency of a reference gene (glyceraldehyde 3-phosphate dehydrogenase; GAPDH; primers and protocol used was as described in Jovanović et al., 2009), ΔCPK is the crossing point deviation of GFP-ΔM-LDH/gly193-M-LDH of M-LDH gene transcript while ΔCPR is the crossing point deviation of GFP-ΔM-LDH/gly193-M-LDH of a reference (GAPDH) gene transcript.
2.3. Patch clamp electrophysiology
To monitor whole cell K+ current the giagaohm seal patch-clamp technique was applied in the whole cell configuration. For whole-cell electrophysiology applied on H9C2 cells were superfused with Tyrode solution (in mM: 136.5 NaCl; 5.4 KCl; 1.8 CaCl2; 0.53 MgCl2; 5.5 glucose; 5.5 HEPES-NaOH; pH 7.4). All pipettes (resistance 3–5 MΩ), were filled with (in mM): KCl 140, MgCl2 1, EGTA-KOH 5, HEPES-KOH 5 (pH 7.3). Depending whether open or closed KATP channels were required, 3 μM (for ATP-free pipette solution; this small amount of ATP was added to prevent the channel run-down) or 3 mM (to keep KATP channels closed) of ATP was added. When the effect of LDH substrates on whole cell K+ current was assessed NADH plus pyruvate (20 mM each) was added into the pipette solution. The effect of 2,4-dinitrophenol (DNP; 10 mM) on K+ current in H9C2 cells was measured using perforated patch clamp electrophysiology with essentially the same pipette solution as above just ATP was omitted and amphotericin B (Sigma, 240 μg/ml; Lippiat, 2008) added. For all cells monitored, the membrane potential was normally held at −40 mV and the currents evoked by a series of 400 ms depolarising and hyperpolarising current steps (−100 mV to +80 mV in 20 mV steps) recorded directly to hard disk using an Axopatch-200B amplifier, Digidata-1321 interface and pClamp8 software (Axon Instruments, Inc., Forster City, CA). The capacitance compensation was adjusted to null the additional whole-cell capacitative current. The slow capacitance component measured by this procedure was used as an approximation of the cell surface area and allowed normalisation of current amplitude (i.e. current density). Currents were low pass filtered at 2 kHz and sampled at 100 μs intervals.
2.4. Cell survival assay
The survival of H9C2 cells was assayed using Multitox-Fluor Multiplex Cytotoxicity Assay (Promega). Briefly, H9C2 cells were plated in complete media (DMEM containing 10% FCS) in 96-well plate, the recombinant adenovirus (GFP or gly193-M-LDH/ΔM-LDH or Kir6.2AFA) was added to the wells at the multiplicity of infection of 10. After 48 h infection, the DNP was added to each well at final concentration of 10 mM. To measure cell survival 6 h later, the peptide substrate (GF-AFC) that can be cleaved only by live cells was added to the each well. Following 30 min-long incubation at 37 °C, plates were measured using 1420 Multibabel Counter (Victor) plate reader, with excitation at 370 nm and emissions of 480 nm. The percentage of live cells was calculated based on the intensity of fluorescence according to the manufacturer instructions.
2.5. Luciferase assay
H9C2 cells were infected with GFP/luciferase or gly193-M-LDH/ΔM-LDH/luciferase with Kir6.2AFA/luciferase 48 h before luciferase assay. To measure luciferase luminescence cells were mounted in 96-well plate in buffer with the following composition (in mM): 30 HEPES, 3 ATP, 15 MgSO4, 10 DTT; pH: 7.4. Some of the cells were untreated while the others were treated with 10 mM DNP. The reaction for luciferase luminescence measurement was initiated by adding 100 μM of luciferin and the luminescence was measured on a plate reader 1420 Multibabel Counter (Victor). Luminescence was measured in the absence of DNP and after 1 h of cell incubation with 10 mM DNP.
2.6. Immunoprecipitation/Western blotting and lactate dehydrogenase assays
Total lactate dehydrogenase (LDH) activity was measured in H9C2 cell extracts. In brief, the cells were homogenised in buffer (TRIS 10 mM, NaH2PO4 20 mM, EDTA 1 mM, PMSF 0.1 mM, pepstatin 10 μg/ml, leupeptin 10 μg/ml, at pH 7.8) and centrifugated at 500 × g (to remove large particles from the homogenate), the supernatant was kept at 4 °C for 18 h and the LDH activity was measured using Roche/Hitachi MODULAR analyzer P1800 and Cobas Roche/Hitachi kit according to the manufacturer instructions. To obtain cellular membrane fraction cells were homogenised in buffer I (TRIS 10 mM, NaH2PO4 20 mM, EDTA 1 mM, PMSF 0.1 mM, pepstatin 10 μg/ml, leupeptin 10 μg/ml, at pH 7.8) and incubated for 20 min (at 4 °C). The osmolarity was restored with KCl, NaCl and sucrose and the obtained mixture was centrifugated at 500 × g. The supernatant was diluted in buffer II (imidazole 30 mM, KCl 120 mM, NaCl 30 mM, NaH2PO4 20 mM, sucrose 250 mM, pepstatin 10 μg/ml, leupeptin 10 μg/ml, at pH 6.8) and centrifugated at 7000 × g, pellet removed and supernatant centrifugated at 30,000 × g. The obtained pellet contains membrane fraction. Protein concentration was determined using the method of Bradford; 10 μg of the anti-Kir6.2 antibody was prebound to Protein-G Sepharose beads and used to immunoprecipitate from 50 μg of membrane fraction protein extract. Lactate dehydrogenase activity was determined in a reagent solution containing 0.2 M Tris–HCl (100 ml), 6.6 M NADH (1 ml), 30 mM sodium pyruvate (10 ml); pH was adjusted to 7.3 at 25 °C. LDH activity was measured using a spectrophotometer (WPA lightwave, Jencons) set at wavelength 340 nm on pellets (20 μl) dissolved in PBS (total volume was 100 μl) and put in reagent solution (Tris–HCl 1.4 ml, NADH 50 μl, sodium pyruvate 50 μl). Following 5 min incubation of the reagent solution in the spectrophotometer pellets were added and the absorbance at 340 nm was measured every min until steady-state is reached. The reaction rate was determined by a decrease in absorbance at 340 nm, resulting from the oxidation of NADH indicative of LDH activity. For some experiments, the pellets of the precipitation were run on SDS-polyacrylamide gels for Western analysis. Western blot probing was performed using 1/1000 dilutions of anti-LDH antibody and detection was achieved using Protein-G HRP and ECL reagents.
2.7. Statistical analysis
Data are presented as mean ± S.E.M, with n representing the number of independent experiments. Mean values were compared by the ANOVA followed by Student's t-test or by Student's t-test alone where appropriate using SigmaStat program (Jandel Scientific, Chicago, IL). P < 0.05 was considered statistically significant.
3. Results
3.1. Infection with ΔM-LDH/gly193-M-LDH act as a dominant negative strategy for M-LDH physically associated with sarcolemmal KATP channels in H9C2 cells
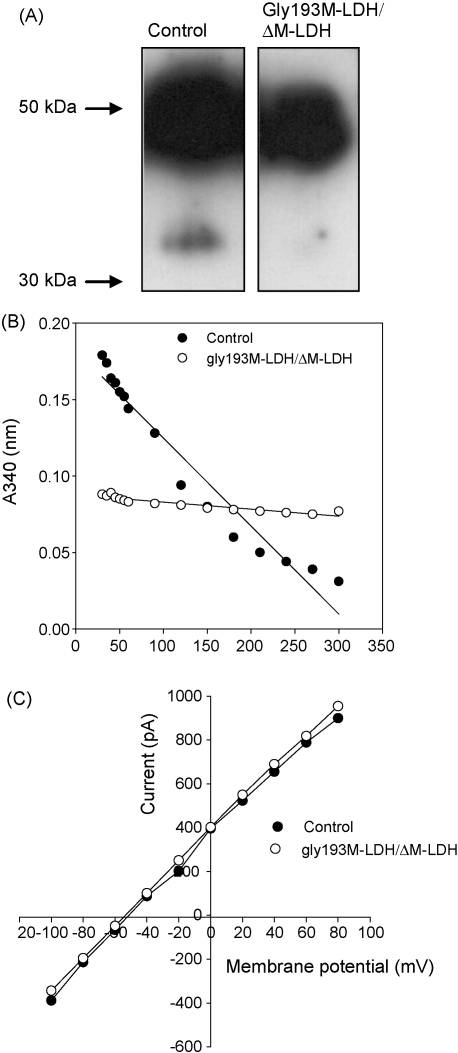
It has been shown ΔM-LDH retain M-LDH catalytic activity but can not physically associate with Kir6.2/SUR2A while gly193M-LDH can physically associate with Kir6.2/SUR2A but has no catalytic activity (Crawford et al., 2002b). In order to neutralize both the physical interaction with KATP channel subunits and M-LDH catalytic activity, we have co-infected H9C2 cells with ΔM-LDH and gly193M-LDH. Infection with GFP or ΔM-LDH/gly193-M-LDH did not significantly affect the mRNA levels of native M-LDH (cycling threshold was 15.90 ± 0.08 in control cells and 15.90 ± 0.16 in ΔM-LDH/gly193-M-LDH cells, n = 4 for each, P = 1). However, M-LDH total mRNA levels were significantly increased in cells infected with ΔM-LDH/gly193-M-LDH than in cells infected with GFP (cycling threshold was 21.12 ± 0.11 in control cells and 17.67 ± 0.06 in ΔM-LDH/gly193-M-LDH cells, n = 4 for each, P < 0.001; cycling threshold for GAPDH was 14.10 ± 0.14 in control cells and 14.05 ± 0.06 in ΔM-LDH/gly193-M-LDH cells, P = 0.75, n = 4 for each). Cells infected with ΔM-LDH/gly193-M-LDH had 11.3 times more M-LDH mRNA than the cells infected with GFP. It is calculated that in cells infected with ΔM-LDH/gly193-M-LDH only 7.1% of M-LDH mRNA was native and 92.9% of mRNA was ΔM-LDH/gly193-M-LDH. The immunoprecipitation with anti-Kir6.2 antibody followed by Western blotting with anti-LDH antibody or LDH assay has revealed that infection with ΔM-LDH/gly193M-LDH has disrupted physical interaction between the channel subunit with M-LDH and that no LDH catalytic activity was found in anti-Kir6.2 precipitate (Fig. 1A and B). As M-LDH regulates the activity of sarcolemmal KATP channels by producing lactate, a KATP channel opener, we have tested whether infection of ΔM-LDH/gly193-M-LDH would interfere with M-LDH-mediated regulation of KATP channels. In cells expressing wild type of M-LDH, the presence of pyruvate (20 mM) and NADH (20 mM) has increased whole cell K+ current despite intracellular presence of 3 mM ATP (the current at 80 mV was 821 ± 91 pA in the absence and 1740 ± 221 pA in the presence of pyruvate and NADH, n = 5–6, P < 0.01). In contrast, in cells infected with ΔM-LDH/gly193M-LDH, pyruvate (20 mM) and NADH (20 mM) did not increase whole cell K+ current (Fig. 1C; the current at 80 mV was 899 ± 121 pA in cells expressing wild type of M-LDH and 952 ± 104 pA in cells expressing 193gly-M-LDH, n = 5–6, P < 0.01). Taken all together, the performed experiments suggested that expression of ΔM-LDH/gly193M-LDH has effectively generated a KATP channel protein complex that is devoid of M-LDH regulation. At the same time, infection of H9C2 cells with ΔM-LDH/gly193M-LDH did not affect total LDH activity (data not shown).
Fig. 1.
Infection of H9C2 cells with gly193M-LDH/ΔM-LDH has a dominant-negative effect on M-LDH physically associated with KATP channel subunits. (A) Original Western blot of anti-Kir6.2 precipitate of H9C2 control cells and cells infected with gly193M-LDH/ΔM-LDH (the size of M-LDH is 36 kDa; similar results were obtained in three blots). (B) LDH assay with anti-Kir6.2 immunoprecipitate of membrane fraction of control cells (control) and cells infected with gly193M-LDH/ΔM-LDH (similar results were obtained in three experiments). (C) Current–voltage relationships in control cells and cells infected with gly193M-LDH/ΔM-LDH filled with pipette solution containing ATP (3 mM) plus pyruvate (20 mM) plus NADH (20 mM). Each point represent mean (n = 5–6).
3.2. Infection with ΔM-LDH/gly193-M-LDH inhibits the activation of sarcolemmal KATP channels during chemical hypoxia
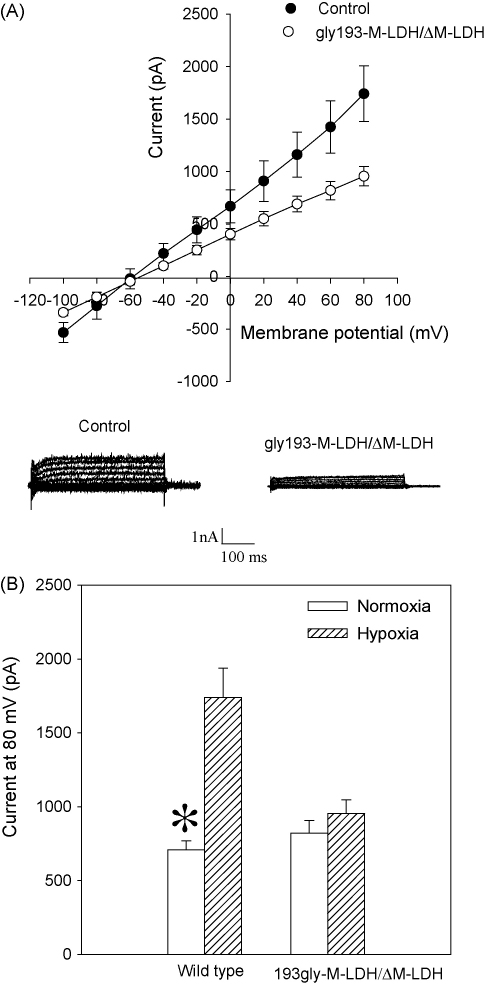
2,4-Dinitrophenol (DNP) is a known metabolic inhibitor that was used with success to induce metabolic stress in different cell types (Brady et al., 1996; Jovanović et al., 1998). When applied, this compound decreases intracellular ATP production and induces chemical hypoxia, which activates KATP channels (Han et al., 1996). We have applied perforated patch clamp electrophysiology to test whether M-LDH physically associated with Kir6.2/SUR2A regulates the opening of KATP channels when cells are challenged with DNP. This method preserves the intracellular milieu during the whole cell recordings (Lippiat, 2008), which allowed us to monitor the behaviour of KATP channels-conducted K+ current during stress under conditions of intact intracellular environment. In control H9C2 cells, DNP (10 mM) induced the activation of KATP channels, as reflected by the increase in whole cell K+ current (Fig. 2; the current at 80 mV was 710 ± 58 pA under control conditions and 1720 ± 187 pA in the presence of DNP, n = 5, P < 0.01), while this was not observed in cells infected with ΔM-LDH/gly193-M-LDH (Fig. 2; the current at 80 mV was 826 ± 91 pA under control conditions and 956 ± 102 pA in the presence of DNP, n = 5, P = 0.36). The DNP-induced current was significantly lower in cells expressing ΔM-LDH/gly193-M-LDH then in control cells (Fig. 2).
Fig. 2.
M-LDH physically associated with Kir6.2/SUR2A is required for the KATP channels activation in chemical hypoxia. (A) Current–voltage relationships with corresponding original membrane currents in control cells and cells infected with gly193M-LDH/ΔM-LDH when exposed to DNP (10 mM). Each point represents mean ± SEM (n = 5). (B) Membrane current at 80 mV under control conditions (normoxia) and when exposed to DNP (10 mM) in wild type cells and cells infected with gly193M-LDH/ΔM-LDH. Each bar represents mean ± SEM (n = 5).
3.3. Infection with ΔM-LDH/gly193-M-LDH exacerbates the outcome of chemical hypoxia
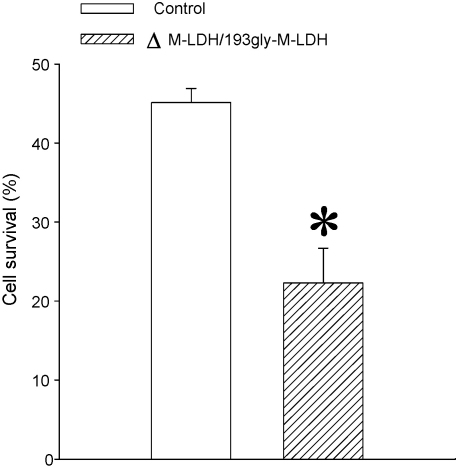
In some studies, failure to activate KATP channels has been associated with exacerbation of the outcome of the metabolic stress (Brady et al., 1996), but in some others that was not the case (Saavadra et al., 2004). Here, we have assessed whether a removal of functional connection between M-LDH and KATP channels would have any effect on the outcome of chemical hypoxia. Under control conditions, 45.1 ± 1.8% (n = 11) of H9C2 cells survived exposure to DNP (10 mM) (Fig. 3A). Cells infected with ΔM-LDH/gly193-M-LDH had suffered much worse outcome, as only 22.3 ± 4.4% cells have survived 10 mM DNP (Fig. 3, n = 6).
Fig. 3.
M-LDH physically associated with Kir6.2/SUR2A is crucial for cell survival in chemical hypoxia. Bar graph showing a percentage of control cells and cells infected with gly193M-LDH/ΔM-LDH that survived treatment with DNP (10 mM). Each bar represents mean ± SEM (n = 6–11). *P < 0.05.
3.4. Infection with ΔM-LDH/gly193-M-LDH potentiate decrease of intracellular ATP during chemical hypoxia
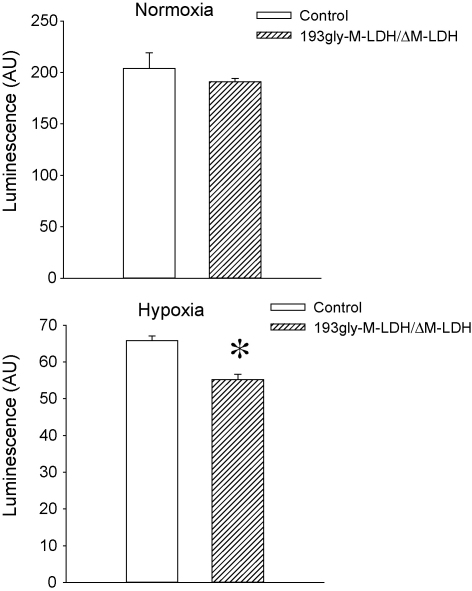
Maintaining intracellular ATP is crucial for cell survival during any type of stress. Here, we have elucidated how expression of ΔM-LDH/gly193-M-LDH affects the ATP levels in H9C2 cells. To be able to measure intracellular ATP in living cells, we have infected cells with the construct encoding luciferase gene; this strategy allows measurement of ATP levels in intracellular environment. The infection of cells with ΔM-LDH/gly193-M-LDH did not affect total ATP levels under control conditions (total ATP: the intensity of luminescence was 204.0 ± 15.1 AU in control and 191.1 ± 3.3 AU in ΔM-LDH/gly193-M-LDH cells, n = 5, P = 0.14; Fig. 4 A). However, the magnitude of DNP-induced decrease of intracellular ATP was significantly higher in cells infected with ΔM-LDH/gly193-M-LDH (65.8 ± 1.3 AU, n = 5, Fig. 4B) then in control cells (55.2 ± 1.5 AU, n = 5, P = 0.01; Fig. 4B).
Fig. 4.
M-LDH physically associated with Kir6.2/SUR2A is required for counteracting chemical hypoxia-induced decrease in intracellular ATP. Bar graphs showing luciferase luminescence in control cells and cells infected with gly193M-LDH/ΔM-LDH under control conditions (normoxia) and after treatment with 10 mM DNP (hypoxia). Each bar represents mean ± SEM (n = 5). *P < 0.05.
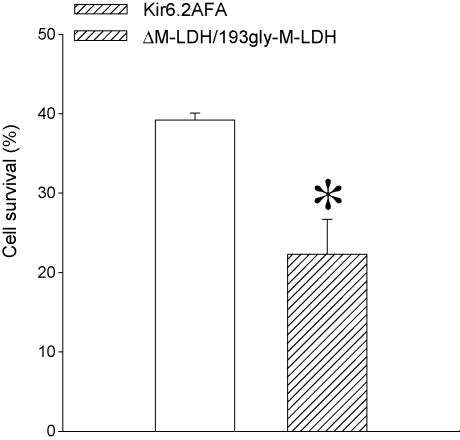
3.5. Infection with Kir6.2AFA has much weaker effect on cell survival in chemical hypoxia when compared to ΔM-LDH/gly193-M-LDH
The results with mutant and truncated M-LDH forms have demonstrated that M-LDH physically associated with Kir6.2/SUR2A regulate KATP channels activity and intracellular ATP levels. It has remained unclear whether these two effects are interconnected and how they contribute to the cellular survival. Therefore, to clarify these points, we have used Kir6.2AFA, a mutant form of Kir6.2 with largely decreased K+ conductance (Van Bever et al., 2004). It has been already shown in H9C2 cells that infection with Kir6.2AFA abolishes KATP channels-mediated K+ current induced by DNP (10 mM) without affecting DNP-induced decrease in intracellular ATP (Jovanović et al., 2009). In cells infected with Kir6.2AFA, cell survival was significantly higher then in cells infected with ΔM-LDH/gly193-M-LDH (39.2 ± 0.9% versus 22.3 ± 4.4% respectively, n = 6–8, P < 0.01; Fig. 5).
Fig. 5.
A comparison of susceptibility to chemical hypoxia in cells infected with Kir6.2AFA and gly193M-LDH/ΔM-LDH. Bar graph showing survival of cells infected with Kir6.2AFA and gly193M-LDH/ΔM-LDH when exposed to DNP (10 mM). Each bar represents mean ± SEM (n = 6–8). *P < 0.05.
4. Discussion
In the present study, we have shown that the species of M-LDH that physically associate with KATP channels is cytoprotective due to regulation of the activity of sarcolemmal KATP channels and intracellular level of ATP. These two M-LDH effects do not seem to be interconnected and are likely to be independent of each other.
It has been recently shown that M-LDH, a minor LDH isoform in the heart, is an integral part of the sarcolemmal KATP channel protein complex (Crawford et al., 2002b). The present study confirmed this notion, as Western blotting/LDH assay/patch clamp electrophysiology has shown both physical and functional connection between channel subunits and M-LDH in H9C2 cells. To address the functional significance of M-LDH species that physically interact with KATP channels we have generated adenoviral constructs containing truncated and mutant forms of M-LDH that do not physically associate with the channel subunit (ΔM-LDH) and is catalytically inactive (193gly-M-LDH). The real time RT-PCR as well as Western blotting/LDH assay/patch clamp electrophysiology has demonstrated that co-infection with ΔM-LDH and 193gly-M-LDH effectively disrupts physical interaction between M-LDH and KATP channels and inhibits M-LDH activity in microenvironment surrounding the channels in H9C2 cells.
It is well established that KATP channels are activated during metabolic stress, but the role of M-LDH in this activation remained elusive. Originally, it has been thought that the activity of KATP channels is regulated solely by the intracellular levels of ATP. However, more recent research has demonstrated that the activity of KATP channels is controlled by a complex interaction of many intracellular factors and signalling pathways. In addition to ATP, the activity of these channels may be regulated by other nucleotides, intracellular pH, lactate, cytoskeleton, protein kinase C, phosphatidylinositol-4,5-bisphosphate, and by the operative condition of the channel itself (reviewed by Zingman et al., 2007). It has been shown that lactate activates sarcolemmal KATP channels despite physiological, millimolar, levels of intracellular ATP (Crawford et al., 2002b). A physical interaction between M-LDH and KATP channel subunits secure such level of proximity between LDH and the channel proteins that a high level of lactate in the microenvironment surrounding the channel can be achieved, which can then activate the channel. The present study has revealed that M-LDH as a component of sarcolemmal KATP channel protein complex is required for the activation of KATP channels during chemical hypoxia, and this has never been shown before.
It has been reported in some studies that the inhibition of KATP channels exacerbates the outcome of the stress, but in many other studies this has been disputed (reviewed by Kane et al., 2005). In the present study, we have shown that a cellular phenotype lacking M-LDH has become dramatically more susceptible to chemical hypoxia. In cells where KATP channels activity was inhibited, exacerbation of the cellular survival has never been reported to reach a magnitude that we have obtained with ΔM-LDH/gly193-M-LDH. This has brought to our attention a possibility that a regulation of sarcolemmal KATP channels activity might not be the only cytoprotective mechanism afforded by M-LDH. In principle, the main function of LDH is production of lactate, which is a KATP channel opener, and ATP, which is the main source of energy in the cell (Van Hall, 2000; Crawford et al., 2002b). It was, therefore, possible that ATP produced by M-LDH contributes to the cell survival during stress. In our recent study, we have shown that the inhibition of M-LDH catalytic activity in H9C2 cells not only inhibits hypoxia-induced KATP channels activation, but also facilitates a decrease in subsarcolemmal ATP level (Jovanović et al., 2009). Here, a decrease of intracellular ATP in cellular phenotype expressing ΔM-LDH/gly193-M-LDH was more pronounced then those in the wild type suggesting that a species of M-LDH physically associated with KATP channels produces ATP during chemical hypoxia and that this could be an important factor in M-LDH-mediated cytoprotection.
To elucidate whether M-LDH-mediated regulation of KATP channels activity and ATP production is interconnected, we have compared the effects of Kir6.2AFA on cell survival with those of ΔM-LDH/gly193-M-LDH. Kir6.2AFA is a mutant form of Kir6.2 that has largely diminished K+ conductance (Van Bever et al., 2004). The cellular H9C2 phenotype expressing Kir6.2AFA does not respond to chemical hypoxia by increasing K+ current, which is in accord with the idea that this current is flowing through KATP channels. At the same time, Kir6.2AFA does not affect dynamics of intracellular ATP during chemical hypoxia suggesting that the activity of KATP channels per se does not regulate intracellular levels of ATP (Jovanović et al., 2009). This also suggests that the observed effect of ΔM-LDH/gly193-M-LDH on ATP levels is independent from the ΔM-LDH/gly193-M-LDH action on the KATP channel activity. The effect of ΔM-LDH/gly193-M-LDH on cellular survival was clearly more deleterious then those of Kir6.2AFA showing that the ATP producing properties of M-LDH are probably important for cell survival during stress.
The mechanism of cardioprotection afforded by KATP channels is a long standing issue. The structural studies of these channels have shown that channel subunits in vivo are physically associated with enzymes that catalyse ATP production (Carrasco et al., 2001; Crawford et al., 2002a,b; Jovanović et al., 2005; Jovanović and Jovanović, 2005; Dhar-Chowdhury et al., 2005), including M-LDH, which catalytic activity is particularly important under anaerobic conditions (reviewed in Van Hall, 2000). Such composition of sarcolemmal KATP channels would indicate that KATP channel might serve as an ATP-producing machinery. The fact that Kir6.2AFA did not influence ATP levels would stand against a possibility that the change in the channel activity per se regulates ATP production. It seems that M-LDH physically associated with Kir6.2/SUR2A is involved in (1) the activation of sarcolemmal KATP channels and (2) maintaining levels of ATP during stress. As M-LDH is an integral part of the sarcolemmal KATP channel protein complex, this implies that the KATP channels-mediated cytoprotection involves not only regulation of membrane potential (Jovanović and Jovanović, 2001a,b), but also a regulation of intracellular ATP levels. The obtained findings would support the idea that KATP channels could have a role in regulating cardiac bioenergetics that is yet beyond the channel activity.
Taken all together this study has demonstrated that M-LDH physically associated with KATP channels is crucial in regulating KATP channels activity, ATP production and cellular resistance to metabolic stress.
Acknowledgements
This research was supported by grants from the British Heart Foundation, Biotechnology and Biological Sciences Research Council, Medical Research Council, TENOVUS-Scotland, Wellcome Trust and Anonymous Trust.
References
- Brady P.A., Zhang S., Lopez J.R., Jovanović A., Alekseev A.E., Terzic A. Dual effect of glyburide, an antagonist of KATP channels, on metabolic inhibition-induced Ca2+ loading in cardiomyocytes. Eur J Pharmacol. 1996;308:343–349. doi: 10.1016/0014-2999(96)00327-5. [DOI] [PubMed] [Google Scholar]
- Carrasco A.J., Dzeja P.P., Alekseev A.E., Pucar D., Zingman L.V., Abraham M.R. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.M., Ranki H.J., Botting C.H., Budas G.R., Jovanović A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+channel in vivo. FASEB J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.M., Budas G.R., Jovanović S., Ranki H.J., Wilson T.J., Davies A.M. M-LDH serves as a sarcolemmal K(ATP) channel subunit essential for cell protection against ischemia. EMBO J. 2002;21:3936–3948. doi: 10.1093/emboj/cdf388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R.M., Jovanović S., Budas G.R., Davies A.M., Lad H., Wenger R.H. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channels. J Biol Chem. 2003;278:31444–31455. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar-Chowdhury P., Harrell M.D., Han S.Y., Jankowska D., Parachuru L., Morrissey A. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J Biol Chem. 2005;18:38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q., Jovanović S., Clelland A., Sukhodub A., Budas G.R., Phelan K. Overexpression of SUR2A generates a cardiac phenotype resistant to ischaemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Kim E., Ho W.K., Earm Y.E. Blockade of the ATP-sensitive potassium channel by taurine in rabbit ventricular myocytes. J Mol Cell Cardiol. 1996;28:2043–2050. doi: 10.1006/jmcc.1996.0197. [DOI] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J.P., Wang C.Z., Aguilar-Bryan L., Bryan J. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Jovanović S., Jovanović A. Pinacidil prevents membrane depolarisation and intracellular Ca2+ loading in single cardiomyocytes exposed to severe metabolic stress. Int J Mol Med. 2001;7:639–643. doi: 10.3892/ijmm.7.6.639. [DOI] [PubMed] [Google Scholar]
- Jovanović S., Jovanović A. Delivery of genes encoding KATP channel subunits in conjuction with pinacidil prevents membrane depolarisation in cells exposed to chemical hypoxia-reoxygenation. Biochem Biophys Res Commun. 2001;282:1098–1102. doi: 10.1006/bbrc.2001.4691. [DOI] [PubMed] [Google Scholar]
- Jovanović S., Jovanović A. High glucose regulates the activity of cardiac sarcolemmal KATP channels via 1 3-bisphosphoglycerate: a novel link between cardiac membrane excitability and glucose metabolism. Diabetes. 2005;54:383–393. doi: 10.2337/diabetes.54.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović S., Du Q., Crawford R.M., Budas G.R., Stagljar I., Jovanović A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. EMBO Rep. 2005;6:848–852. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović S., Du Q., Mukhopadhyay S., Swingler R., Buckley R., McEachan J. A patient suffering from hypokalemic periodic paralysisis is deficient in skeletal muscle ATP-sensitive K+ channels. Clin Transl Sci. 2008;1:71–74. doi: 10.1111/j.1752-8062.2008.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović S, Du Q, Sukhodub A, Jovanović A. A dual mechanism of cytoprotection afforded by M-LDH in embryonic rat heart H9C2 cells. Biochim Biophys Acta 2009, doi:10.1016/j.bbamcr.2009.04.007 [DOI] [PMC free article] [PubMed]
- Jovanović A., Jovanović S., Lorenz E., Terzic A. Recombinant cardiac ATP-sensitive K+ channel subunits confer resistance towards chemical hypoxia-reoxygenation injury. Circulation. 1998;98:1548–1555. doi: 10.1161/01.cir.98.15.1548. [DOI] [PubMed] [Google Scholar]
- Kane G.C., Liu X.K., Yamada S., Olson T.M., Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiat J.D. Whole-cell recording using the perforated patch clamp technique. Methods Mol Biol. 2008;491:141–149. doi: 10.1007/978-1-59745-526-8_11. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acid Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki H.J., Budas G.R., Crawford R.M., Davies A.M., Jovanović A. 17β-estradiol regulates expression of KATP channels in heart-derived H9c2 cells. J Am Coll Cardiol. 2002;40:367–374. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Saavadra W.F., Paolocci N., Kass D.A. Effects of cardioselective KATP channel antagonism on basal, stimulated, and ischaemic myocardial function in in vivo failing canine heart. Br J Pharmacol. 2004;135:657–662. doi: 10.1038/sj.bjp.0704510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bever L., Poitry S., Faure C., Norman R.I., Roatti A., Baertschi A.J. Pore loop-mutated rat KIR6 1 and KIR6. 2 suppress KATP current in rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2004;287:H850–H859. doi: 10.1152/ajpheart.00054.2004. [DOI] [PubMed] [Google Scholar]
- Van Hall G. Lactate as a fuel for mitochondrial respiration. Acta Physiol Scand. 2000;168:643–656. doi: 10.1046/j.1365-201x.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- Zingman L.V., Alekseev A.E., Hodgson-Zingman D., Terzic A. ATP-sensitive K+ channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]