Abstract
To better understand the implications for considering delayed graft function (DGF) a performance measure, we compared outcomes associated with a 2- to 3-fold difference in the incidence of DGF at 2 transplant centers. We analyzed 5072 kidney transplantations between 1984–2006 at the University of Minnesota Medical Center (UMMC) and Hennepin County Medical Center (HCMC). In logistic regression the adjusted odds ratio for DGF at HCMC versus UMMC was 3.11 (95% Confidence Interval [CI]=2.49–3.89) for deceased donors and 2.24 (CI=1.45–3.47) for living donors. In Cox analysis of 4957 transplantations, slow graft function [SGF; creatinine >3.0 mg/dL (230 μmol/L) on day 5 without dialysis] was associated with graft failure at UMMC (Relative Risk [RR] =1.43, CI=1.25–1.64), but not HCMC (RR=0.99, CI=0.77–1.28). RR’s of DGF were similar at both centers. Thus, the lower incidence of DGF at UMMC likely resulted in a higher incidence and higher risk of SGF compared to HCMC. Indeed, graft survival for recipients with DGF at HCMC was similar (p=0.3741) to that of recipients with SGF at UMMC. We conclude that dialysis per se is likely not a cause of worse graft outcomes. A better definition is needed to measure early graft dysfunction and its effects across transplant programs.
INTRODUCTION
Delayed graft function (DGF) is usually defined as the need for dialysis during the first week after kidney transplantation.(1) Analyses of registry (2–4) and single-center data (5–7) have shown that DGF is associated with graft failure. However, it is not clear how much the incidence of DGF and its association with graft failure is due to inherent differences in the functional capacity of the transplanted kidney, or to center-specific differences in the clinical threshold for the use of dialysis after transplantation. Indeed, the need for dialysis is often subjective. Physicians and surgeons may have different thresholds for administering dialysis, in part influenced by an untested hypothesis that dialysis per se may be harmful to the transplanted kidney. Few studies have examined clinical correlates of early graft function determined by more objective measures than the perceived need for dialysis in the first week after transplantation.(8–11)
Since 1963 there have been two independent kidney transplantation programs in Minneapolis, Minnesota, one at the University of Minnesota Medical Center (UMMC) and one at Hennepin County Medical Center (HCMC). Interestingly, the incidence of DGF has been substantially higher at HCMC than at the UMMC. We questioned whether this difference in the rates of DGF was due to differences in patient populations, or to different practice patterns between the two institutions. We also questioned whether the higher incidence of DGF after kidney transplantation at HCMC was associated with a proportionally higher rate of graft failure at HCMC compared to UMMC.
In this analysis, we first compared the incidence and clinical correlates of DGF at HCMC and UMMC. In particular, we examined whether there were differences in the rates of DGF between HCMC and UMMC that could not be explained by differences in patient populations and transplantation characteristics, thereby inferring differences in DGF attributable to other causes. We then determined whether there was an independent association between DGF and graft failure, and if the differences in the rates of DGF at HCMC and UMMC were associated with proportional differences in rates of graft failure. We also examined whether other markers of early graft function, such as slow graft function [SGF; defined as serum creatinine >3.0 mg/dL (230 μmol/L) at 5 days after transplantation without dialysis] were better clinical correlates of graft failure and helped explain differences in DGF between HCMC and UMMC.
METHODS
Protections and Approvals
This study was approved by the institutional review boards of UMMC and HCMC. Separate data sets were created at UMMC and HCMC. These data sets were then de-identified and pooled for analysis. Only the investigators had access to these data.
Study Population
We included all kidney transplantations in adults (ages 18 and older) between January 1, 1984, and December 31, 2006. We included both first and subsequent kidney transplantations. We excluded patients who had transplantation with an organ other than a kidney, either before or at the time of kidney transplantation. Patients who underwent transplantation with an organ other than a kidney, after the kidney transplantation, were included, but analyzed only up until the time of receiving the non-kidney organ, i.e. they were censored at the time they received a non-kidney organ transplantation. Follow-up was until graft failure or through September 1, 2007.
Measurement of Early Kidney Function
In addition to DGF (the need for dialysis during the first week after transplantation), we defined SGF as serum creatinine greater than 3.0 mg/dL (230 μmol/L) on day 5 after transplantation in patients without DGF.(12) If serum creatinine at day 5 was missing, we used the mean of serum creatinine on days 4 and 7 to substitute for creatinine on day 5 in determining SGF. If serum creatinine on day 4 was also missing, we used the value for day 7 to determine SGF (values for day 6 were not prospectively collected in the database).
Variables Analyzed
Baseline demographic data for donors and recipients included age (18.0–30.0, reference=30.1–45.0, 45.1–60, >60.0 years), sex, ethnicity (reference=Caucasian, African-American, Native American, Asian, Hispanic, other), and body mass index (lean<18.5, reference=normal 18.5–24.9, overweight 25.0–29.9, obese 30.0–39.9, massively obese ≥40 kg/m2). Additional donor factors include type of kidney (living or deceased) and cause of death (trauma/no trauma), Other recipient factors included pre-emptive transplantation before chronic dialysis, duration of end-stage kidney disease (0, reference=1–365, 366–730, >730 days), type of dialysis used before transplant (peritoneal/hemodialysis), prior kidney transplantation, and primary cause of kidney failure (reference=glomerulonephritis, type 1 diabetes, type 2 diabetes, polycystic kidney disease, nephrosclerosis including hypertension and vascular disease, other, unknown). Recipient co-morbidities at time of transplant include hepatitis B and C, diabetes (including those as cause of primary disease), and smoking. Transplantation characteristics analyzed were transplant center (UMMC vs. HCMC), transplantation era (year of transplantation, reference = 1984–1991, 1992–1996, 1997–2001, or 2002–2006), induction and initial maintenance immunosuppressive medication (cyclosporine with azathioprine or mycophenolate mofetil, tacrolimus with mycophenolate mofetil, tacrolimus with sirolimus, interleukin-2 receptor antagonist, and anti-lymphocyte antibody), pretransplant splenectomy or bilateral native nephrectomy, cold ischemia time (<12.0, reference=12.0–23.9, 24.0–47.9, ≥48.0 hours), use of pump perfusion, number of major histocompatibility (MHC) mismatches, donor laparoscopic nephrectomy, cytomegalovirus (CMV) risk [donor CMV antibody(+)/recipient(−), donor(+)/recipient(+), donor(−)/recipient(+), reference = donor(−)/recipient(−)], peak or current panel reactive antibody (PRA) >30%, historical B-cell crossmatch positive, treatment with acyclovir (or valcyclovir) prophylaxis, treatment with ganciclovir (or valganciclovir) prophylaxis, more than one donor renal artery, if the donor kidney was from the left side or if two kidneys were transplanted en bloc.
Statistical Analysis
Logistic regression was used to determine which of the clinical characteristics described above were independently associated with DGF. A forward selection process was used with P<0.05 entry criteria. Cox proportional hazards models were used to determine which measures of early kidney allograft function were associated with graft failure (return to dialysis, re-transplantation without returning to dialysis or death with a functioning kidney allograft), death-censored graft failure (return to dialysis or re-transplantation without returning to dialysis) and death. Relative risk (RR) and 95% confidence intervals (CI) were calculated for each measure. Since we were examining early graft function, kidneys labeled as primary non-function were removed from the survival analyses.
To test the hypothesis that the effects of DGF may be different at UMMC and HCMC, we examined interactions between HCMC vs. UMMC and the associations between 1) SGF, 2) short DGF (requiring only 1 or 2 dialysis treatments), and 3) prolonged DGF (requiring 3 or more dialysis treatments) and graft failure. For graft survival, event curves were based on Kaplan-Meier analysis and groups were compared using the log-rank test. All analyses were done with SAS version 9.1 (Cary, NC, SAS Institute).
Missing values were handled in multivariate analyses by including a category for missing values. If only the missing value category was significantly associated with the outcome, the missing value indicator was then dropped from the analysis.
RESULTS
Study Population
From 1984–2006, there were 5072 kidney-only transplantations at HCMC and UMMC. There were missing values for: SGF before (N=918; 18.1%) and after (N=34, 0.7%) substituting creatinine values at 4 days and 1 week for day 5 posttransplant, historical B-cell crossmatch (N=1654, 32.6%), smoking at the time of transplantation (N=396, 7.8%), recipient body mass index (N=594, 11.7%), donor body mass index (N=2240, 44.2%), use of en bloc kidneys (N=1734, 34.2%), maintenance dialysis modality at transplantation (N=1666, 32.4%), and left donor kidney used (N=76, 1.5%).
Incidence of DGF and SGF
Of the 5072 transplants, 3349 were performed at UMMC and 1723 at HCMC. The unadjusted incidence of DGF was 13.9% at UMMC and 33.7% at HCMC (Chi-square P<0.0001). Among 2538 recipients of living donor kidneys the unadjusted incidence of DGF was 3.7% at UMMC and 7.3% at HCMC (P<0.0001). Among 2534 recipients of deceased donor kidneys the unadjusted incidence of DGF was 27.2% at UMMC and 49.7% at HCMC (P<0.0001). The adjusted odds ratio for DGF at HCMC compared to UMMC was 3.11 (CI 2.49–3.89) for deceased donors and 2.24 (CI 1.45–3.47) for living donors. Overall, the odds ratio for DGF at HCMC was 2.64 (CI 2.17–3.21) compared to UMMC for living and deceased donors.
The unadjusted incidence of SGF was 14.8% at UMMC and 6.3% at HCMC (Chi-square P<0.0001). Among 2538 living donor kidneys, the unadjusted incidence of SGF was 7.3% at UMMC and 5.3% at HCMC (Chi-square P=0.079). For the 2534 deceased donor kidneys, the unadjusted incidence of SGF was 24.5% at UMMC and 7.0% at HCMC (Chi-square P<0.0001). The adjusted odds ratio for SGF at HCMC compared to UMMC was 0.328 (CI 0.24–0.44) for deceased donors and 0.78 (CI 0.52–1.19) for living donors. Overall, the odds ratio of SGF at HCMC was 0.39 (CI 0.29–0.53) compared to UMMC for living and deceased donors.
Primary Non-Function
Patients with kidneys that were subsequently considered to have never functioned (“primary non-function”) were included in the analysis of DGF. Of 115 (2.2%) considered to have had graft failure before the end of the first week after transplantation, 87 (75.6%) had DGF, but 28 (24.4%) had death with function or graft nephrectomies prior to dialysis, and thus did not meet the definition of DGF.
Graft Failure
After eliminating patients with primary non-function, there were 4957 kidney transplants considered to have survived beyond the first week. We used the Cox proportional hazards models to determine the associations of graft failure with SGF, short DGF, and prolonged DGF compared to normal functioning grafts for each hospital. Three analyses were done with HCMC and UMMC combined (Table 1) and 2 with the hospitals analyzed separately. With both approaches, the results were similar (data not shown). SGF was not associated with graft failure at HCMC compared to normal functioning grafts (RR 0.99, CI, 0.77–1.28) whereas SGF at UMMC was associated with increased graft failure (RR 1.43, CI 1.25–1.64). Short DGF (1–2 dialysis runs) had similar risk at both hospitals with a RR of 1.27 (CI 1.04–1.56) at HCMC and a RR 1.27 (CI 1.02–1.57) at UMMC. Prolonged DGF (3 or more dialysis runs) was associated with worse graft survival at both HCMC (RR 1.51, CI 1.27–1.79) and UMMC (RR 2.12, CI 1.78–2.51).
Table 1.
Clinical correlates for graft failure occurring more than 1 week after transplantation.
| Graft Function and Institution | UMMC+HCMC (N=4957) |
|
|---|---|---|
| N1 | RR (95% CI) P-Value2 | |
| Transplanted at HCMC | 1687 | 1.03 (0.92–1.17) P=0.5921 |
| SGF3 v. IF4 at HCMC | 172 | 0.99 (0.77–1.28) P=0.9686 |
| SGF v. IF at UMMC | 502 | 1.43 (1.25–1.64) P<0.0001 |
| Short DGF5 v. IF at HCMC | 214 | 1.27 (1.04–1.56) P=0.0170 |
| Short DGF v. IF at UMMC | 180 | 1.27 (1.02–1.57) P=0.0296 |
| Prolonged DGF v. IF at HCMC | 342 | 1.51 (1.27–1.79) P<0.0001 |
| Prolonged DGF v. IF at UMMC | 224 | 2.12 (1.78–2.51) P<0.0001 |
The number of transplantations having the characteristic described in column 1.
P values > 0.05 are underlined.
SGF was defined as not having DGF but serum creatinine on posttransplant day 5 > 3.0 mg/dL without dialysis. When values for serum creatinine were missing on day 5, an average of days 4 and 7 was substituted, or when day 4 was missing day 7 was substituted. Of the 918 (18.1%) SGF missing values, 884 were substituted. A missing value indicator for the remaining 34 missing included in the model was not statistically significant.
IF was defined as not having either DGF or SGF.
DGF was defined as the need for dialysis within the first week after transplantation. It was called short if only 1 or 2 dialysis treatments were required, and prolonged if 3 or more treatments were required.
Abbreviations: UMMC, University of Minnesota Medical Center; HCMC, Hennepin County Medical Center; CI, confidence interval; SGF, slow graft function; IF, immediate function; DGF, delayed graft function.
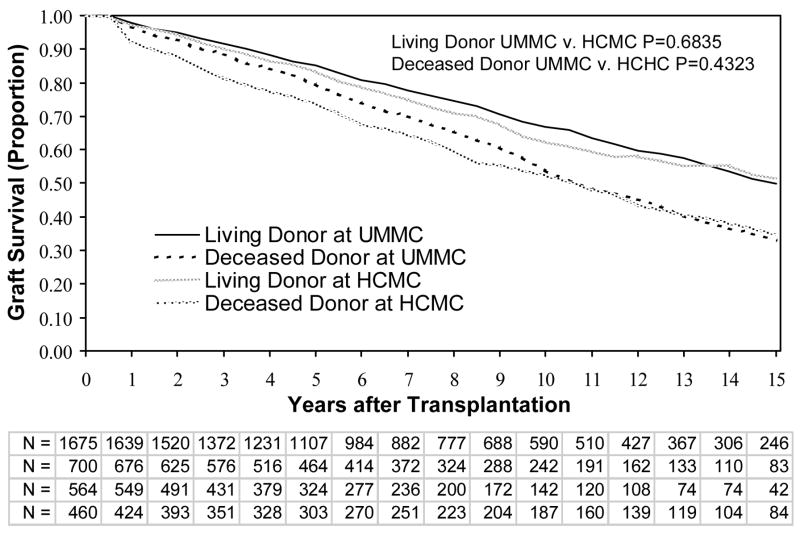
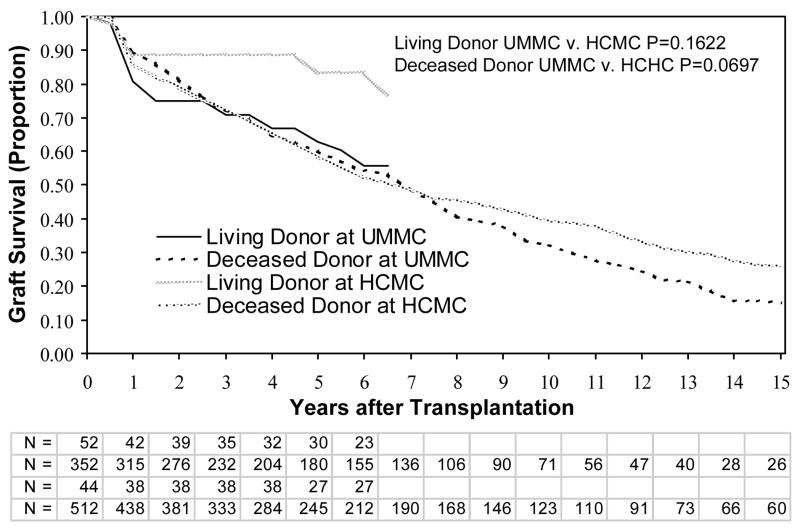
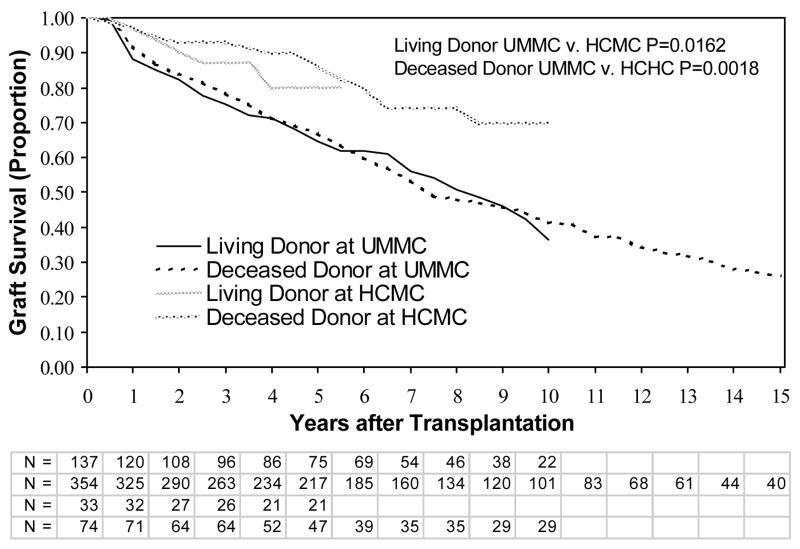
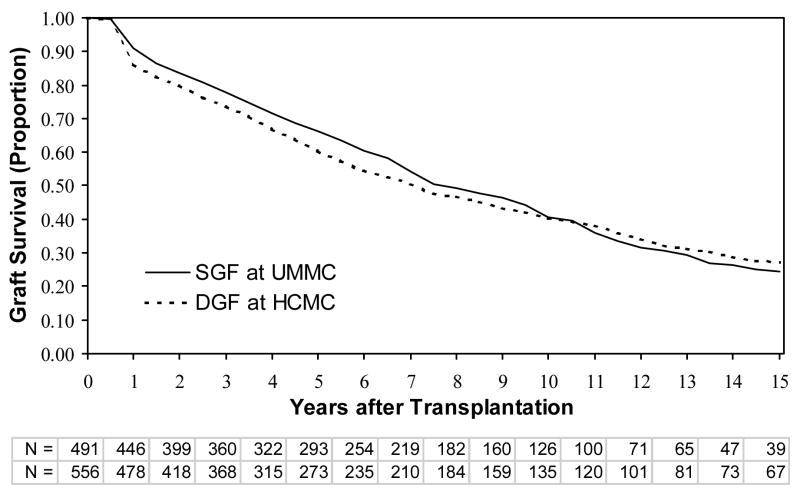
We next analyzed graft survival by site and type of kidney (living versus deceased donor). There were no differences in graft survival for HCMC versus UMMC, for either living donors or deceased donors, when all individuals were included (data not shown). Similarly, there were no differences in graft survival between HCMC and UMMC among only patients without SGF or DGF (Figure 1). For individuals with DGF (short and prolonged), graft survival was not significantly different at the 2 sites between living donors or deceased donors (Figure 2). However, patients with SGF had significantly better graft survival at HCMC than UMMC for both living and deceased donors (Figure 3). We then compared the graft survival of individuals with SGF at UMMC and DGF at HCMC and found they had similar outcomes (Figure 4).
Figure 1.
Graft survival in patients without delayed graft function or slow graft function. Patients thought to have graft failure in the first week (primary nonfunction) were excluded. Shown are comparisons between living and deceased donor transplants, at the UMMC and HCMC. P-values are by the log-rank test. Abbreviations: UMMC, University of Minnesota Medical Center; HCMC, Hennepin County Medical Center; N, the number remaining with functioning grafts at each year of follow up for living and deceased donor transplantations at UMMC and HCMC, respectively.
Figure 2.
Graft survival in patients with delayed graft function. Patients thought to have graft failure in the first week (primary nonfunction) were excluded. Shown are comparisons between living and deceased donor transplants, at the UMMC and HCMC. P-values are by the log-rank test. Abbreviations: UMMC, University of Minnesota Medical Center; HCMC, Hennepin County Medical Center; N, the number remaining with functioning grafts at each year of follow up for living and deceased donor transplantations at UMMC and HCMC, respectively.
Figure 3.
Graft survival in patients with slow graft function. Patients thought to have graft failure in the first week (primary nonfunction) were excluded. Shown are comparisons between living and deceased donor transplants, at the UMMC and HCMC. P-values are by the log-rank test. Abbreviations: UMMC, University of Minnesota Medical Center; HCMC, Hennepin County Medical Center; N, the number remaining with functioning grafts at each year of follow up for living and deceased donor transplantations at UMMC and HCMC, respectively.
Figure 4.
Graft survival comparing patients with SGF at UMMC to patients with DGF at HCMC. Patients thought to have graft failure in the first week (primary nonfunction) were excluded. Shown are comparisons between living and deceased donor transplants, at the UMMC and HCMC. P-values are by the log-rank test. Abbreviations: UMMC, University of Minnesota Medical Center; HCMC, Hennepin County Medical Center; N, the number remaining with functioning grafts at each year of follow up for living and deceased donor transplantations at UMMC and HCMC, respectively.
Patient Outcomes
In the adjusted models, DGF was associated with increased graft failure, death-censored graft failure, and mortality. Graft failure and death-censored graft failure were also associated with SGF (Table 2).
Table 2.
Independent associations between DGF and SGF and outcomes among patients who had a functioning kidney at 1 week, or graft failure more than 1 week after transplantation.1
| Early Kidney Function Variables | Graft Failure (N=2007) | Death-Censored Graft Failure (N=965) | Death (N=1042) |
|---|---|---|---|
| Relative Risk (95% Confidence Interval) P-Value | |||
| Association of DGF2 with Outcomes (Unadjusted and Adjusted) | |||
| Delayed graft function (N=572) | 1.97 (1.75–2.22) P<.0001 | 1.85 (1.55–2.21) P<.0001 | 2.07 (1.77–2.43) P<.0001 |
| Delayed graft function (N=572) | 1.42 (1.24–1.63) P<.0001 | 1.40 (1.14–1.72) P=0.0013 | 1.46 (1.21–1.76) P<.0001 |
| Association of SGF3 with Outcomes (Unadjusted and Adjusted) | |||
| Slow graft function (N=592) | 1.68 (1.49–1.90) P<.0001 | 2.01 (1.71–2.37) P<.0001 | 1.39 (1.16–1.66) P=0.0003 |
| Slow graft function (N=592) | 1.34 (1.17–1.52) P<.0001 | 1.59 (1.33–1.90) P<.0001 | 1.12 (0.92–1.35) P=0.2535 |
Results of Cox proportional hazards analyses, both unadjusted (with only 2 covariates) and adjusted for multiple covariates (see text for details). There were 4434 patients with complete data on DGF and SGF analyzed, and “N “refers to the number of events. P values >0.05 are underlined.
DGF was defined as the need for dialysis within the first week after transplantation.
SGF was defined as not having DGF, but serum creatinine on day 5 ≥ 3.0 mg/dL.
Abbreviations: DGF, delayed graft function; SGF slow graft function.
Dialysis Indications
Although it was not possible to objectively discern indications for dialysis in this retrospective study, we examined differences between UMMC versus HCMC in laboratory parameters on the day of first post-transplant dialysis. We chose 20 consecutive patients with DGF from each site, a total of 40 patients who were transplanted 2000–06, when complete laboratory records were available. We examined pre-dialysis serum creatinine, blood urea nitrogen, bicarbonate, and potassium on the day of first dialysis, as well as the time from transplant to first dialysis. No statistically significant differences were found between UMMC and HCMC in serum potassium (5.59 ± 0.82 mmol/L versus 5.60 ± 0.88 mmol/L, respectively, p=0.96), bicarbonate (21.5 ± 3.37 mmol/L versus 21.3 ± 3.38 mmol/L, p=0.85), blood urea nitrogen (65.7 ± 23.7 mg/dL versus 54.2 ± 21.5 mg/dL, p=0.12), or creatinine (9.06 ± 3.14 mg/dL versus 9.12 ± 3.15 mg/dL, p=0.96) at time of first dialysis. The time to dialysis was 1.57 ± 1.08 days at UMMC versus 1.55 ± 1.05 days at HCMC (p=0.95). For SGF, we did find that serum creatinine on day 5 was higher at UMMC compared to HCMC (5.58 ± 2.45 mg/dL versus 5.03 ± 1.84, p=0.0117). We were unable to identify subjective reasons for dialysis including volume overload and uremia from the medical records.
DISCUSSION
A large difference in the incidence of DGF between HCMC and UMMC prompted us to better understand the causes and consequences of DGF, by comparing and contrasting DGF at the two programs. The major findings were that 1) the adjusted odds for DGF were 2- to 3-fold higher at HCMC than at UMMC, 2) despite the higher incidence of DGF, graft survival (adjusted and unadjusted) was not different for patients transplanted at HCMC and UMMC, 3) the adjusted rate of SGF was lower at HCMC, 4) SGF was a risk factor for graft failure at UMMC, but not at HCMC, and 5) the risk for graft failure of SGF at UMMC was similar to the risk of DGF at HCMC. The most parsimonious explanation for these differences is that there was a lower threshold for prescribing dialysis after transplantation at HCMC compared to UMMC. This could result in more patients who would otherwise have SGF receiving dialysis at HCMC, thereby reducing the risk for graft failure associated with both DGF and SGF at HCMC compared to UMMC. In any case, these results have potentially important implications for using DGF as a performance measure, and for interpreting the results of single-center and registry analyses of outcomes associated with DGF and SGF.(1)
The unadjusted incidence of DGF was almost 2-fold higher at HCMC than UMMC, for both living and deceased donor transplantations. This difference could not be explained by differences in CIT or other donor and recipient characteristics. In a multivariate analysis, the odds of DGF were 3-fold higher at HCMC compared to UMMC for deceased donor recipients, and more than 2-fold higher for living donor transplantations. One difference that could not be accounted for in this analysis was the delayed use of CsA at UMMC and not HCMC. At HCMC, the practice has been to initiate CsA at the time of transplantation regardless of the level of initial kidney function. At UMMC, calcineurin-inhibitors (CNI) are delayed until function has improved. However, it is unlikely that the earlier initiation of CsA at HCMC explains a 2-fold higher incidence of DGF at HCMC than UMMC. Indeed, a small (N=100) randomized controlled trial at HCMC failed to find a difference in the incidence or severity of DGF, whether CsA was initiated early or delayed with the use of antithymocyte globulin.(13) Likewise, Belitsky, et al., randomly allocated 110 first deceased donor recipients to immediate CsA versus delayed CsA with antilymphocyte globulin and found no difference in the incidence of DGF.(14) Similarly, Lebranchu, et al., randomly allocated 100 first deceased donor recipients to basiliximab plus early CsA versus antilymphocyte globulin plus delayed CsA and found no difference in DGF between the two groups.(15) Charpentier, et al., randomly allocated 555 patients to immediate CsA versus delayed CsA with antithymocyte globulin versus delayed tacrolimus with antithymocyte globulin and found no differences between the 3 groups in the need for dialysis.(16) Finally, a recent randomized trial (N=197), also failed to find that delaying CNI initiation reduced the incidence of DGF.(17) Thus, there is little evidence that delaying CNI initiation reduces the incidence of DGF and could thereby explain the large difference in incidence in DGF between HCMC and UMMC.
Despite the higher rates of DGF at HCMC, graft survival was similar at HCMC and UMMC (Table 1). This was true for recipients of living or deceased donor transplantations, in unadjusted analyses (data not shown). Thus, either graft survival was unaffected by the higher rate of DGF at HCMC, or other factors mitigated the effects of DGF on graft failure at HCMC. In analyses adjusted for SGF and DGF, graft survival remained similar at HCMC and UMMC (Table 2).
The severity of DGF, as defined by the number of dialysis treatments, was associated with graft failure. Short DGF (1–2 dialysis treatments) was associated with a 27% higher risk of graft failure at both UMMC and HCMC (Table 1). However, prolonged DGF (≥3 dialysis treatments) at UMMC and HCMC were associated with an even greater likelihood of graft failure (Table 1). Schneulle, et al., showed that DGF requiring >2 dialysis treatments (N=78) was associated with worsened graft survival compared to both immediate function (N=189) and DGF requiring only 1–2 dialysis treatments (N=43).(11) Similarly, Giral-Classe, et al., reported that patients with DGF lasting ≤6 days (N=442) had a similar risk for graft failure as those with immediate function (N=221), but DGF lasting > 6 days (N=401) was associated with a higher risk of graft failure.(8)
Slow graft function was associated with graft failure at UMMC, but not at HCMC (Table 1). The definition of SGF used in this analysis was previously shown by Humar, et al., to correlate with graft failure at UMMC.(9) Johnson, et al., reported that a day 1–7 creatinine reduction ratio of <70% (N=202), but without dialysis requirement, was associated with lower graft survival than immediate function (N=668), but not as low as that associated with DGF (N=102).(10)
While there may be other explanations for the differences in the association between DGF and SGF with graft failure at UMMC versus HCMC, we believe that differences in the clinical threshold for dialysis between the two institutions most likely explain this observation. Unfortunately, in this retrospective study, we were unable to document differences in indications for dialysis, or differences between the two institutions in laboratory parameters on the day of dialysis in a limited sample of patients with DGF. Nevertheless, medical practice at UMMC has been such that dialysis is used only if there is perceived to be a strong need, while at HCMC dialysis is used if the need (strong or otherwise) is present. The lower threshold for dialysis at HCMC could “dilute” the association of prolonged DGF with graft failure at HCMC.
Similarly, a lower threshold for dialysis at HCMC could mean that many HCMC patients, who were comparable to those having SGF at the UMMC, instead received dialysis at HCMC. Consistent with this hypothesis is the fact that the incidence of graft failure in HCMC patients with DGF (short or prolonged) was similar to the incidence of graft failure in UMMC patients with SGF (Figure 4). A lower threshold for starting dialysis after transplantation at HCMC compared to UMMC could also explain why SGF was not a clinical correlate of graft failure at HCMC, while SGF was associated with graft failure at the UMMC.
Although our analysis focused on associations between DGF and graft failure, associations between DGF and death-censored graft failure, and associations between DGF and mortality, were similar to the associations between DGF and graft failure (Table 2). On the other hand, SGF was associated with graft failure and death-censored graft failure, but not with mortality in the adjusted model (Table 2).
There are several limitations to this study. Although we believe the higher rate of DGF is related to a lower threshold for dialysis at HCMC, we were unable to examine subjective indications for dialysis. Similarly, allograft function immediately prior to dialysis initiation was also not available to allow us to determine whether dialysis was started at a higher level of function at HCMC than UMMC. As mentioned above, it is unlikely that differences in induction immunosuppressive medication regimens, e.g. delayed CNI use, led to differences in the incidence and severity of DGF at HCMC compared to UMMC. Finally, we did not examine the costs associated with extended hospitalizations from DGF versus SGF. Patients with DGF are likely to stay in the hospital longer, which would lead to higher hospitalization costs. However, patients with SGF can be discharged with close follow-up in clinic which would likely lead to lower costs.
In summary, marked differences in the incidence of DGF between two transplant centers were likely due to differences in the threshold for initiating dialysis treatment early after transplantation. This would, in turn, account for the fact that the higher rate of DGF did not result in worse graft survival. If true, this may suggest that dialysis per se does not cause graft dysfunction as has sometimes been suggested.(18–20) These data also suggest that if DGF is to be used as a performance indicator for transplant programs, a more objective measurement of kidney allograft function than “dialysis in the first week after transplantation” should be considered. Additional studies are required to determine the best measure of early graft function.
Acknowledgments
Funding sources: This study was supported by the National Institutes of Health Program Project Grant DK13083, National Institutes of Health Prefaculty Training in Pediatric Nephrology T32 DK007087-35, and a research grant from the American Society of Transplantation. Ajay Israni, MD, is a Robert Wood Johnson Foundation Physician Faculty Scholar.
References
- 1.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 3.Cecka JM. The UNOS Scientific Regristry. In: Cecka JM, Terasaki PI, editors. Clinical Transplants 1998. Los Angeles: UCLA Tissue Typing Laboratory; 1999. pp. 1–16. [Google Scholar]
- 4.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66(12):1697–1701. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 5.Feldman HI, Gayner R, Berlin JA, Roth DA, Silibovsky R, Kushner S, et al. Delayed function reduces renal allograft survival independent of acute rejection. Nephrol Dial Transplant. 1996;11(7):1306–1313. [PubMed] [Google Scholar]
- 6.Moreso F, Seron D, Gil-Vernet S, Riera L, Fulladosa X, Ramos R, et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrol Dial Transplant. 1999;14(4):930–935. doi: 10.1093/ndt/14.4.930. [DOI] [PubMed] [Google Scholar]
- 7.Quiroga I, McShane P, Koo DD, Gray D, Friend PJ, Fuggle S, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21(6):1689–1696. doi: 10.1093/ndt/gfl042. [DOI] [PubMed] [Google Scholar]
- 8.Giral-Classe M, Hourmant M, Cantarovich D, Dantal J, Blancho G, Daguin P, et al. Delayed graft function of more than six days strongly decreases long-term survival of transplanted kidneys. Kidney Int. 1998;54(3):972–978. doi: 10.1046/j.1523-1755.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 9.Humar A, Ramcharan T, Kandaswamy R, Gillingham K, Payne WD, Matas AJ. Risk factors for slow graft function after kidney transplants: a multivariate analysis. Clin Transplant. 2002;16(6):425–429. doi: 10.1034/j.1399-0012.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnston O, O’kelly P, Spencer S, Donohoe J, Walshe JJ, Little DM, et al. Reduced graft function (with or without dialysis) vs immediate graft function--a comparison of long-term renal allograft survival. Nephrol Dial Transplant. 2006;21(8):2270–2274. doi: 10.1093/ndt/gfl103. [DOI] [PubMed] [Google Scholar]
- 11.Schnuelle P, Gottmann U, Koppel H, Brinkkoetter PT, Krzossok S, Weiss J, et al. Comparison of early renal function parameters for the prediction of 5-year graft survival after kidney transplantation. Nephrol Dial Transplant. 2007;22(1):235–245. doi: 10.1093/ndt/gfl530. [DOI] [PubMed] [Google Scholar]
- 12.Humar A, Johnson EM, Payne WD, Wrenshall L, Sutherland DE, Najarian JS, et al. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant. 1997;11(6):623–627. [PubMed] [Google Scholar]
- 13.Kasiske BL, Johnson HJ, Goerdt PJ, Heim-Duthoy KL, Rao KV, Dahl DC, et al. A randomized trial comparing cyclosporine induction with sequential therapy in renal transplant recipients. Am J Kidney Dis. 1997;30(5):639–645. doi: 10.1016/s0272-6386(97)90487-x. [DOI] [PubMed] [Google Scholar]
- 14.Belitsky P, MacDonald AS, Cohen AD, Crocker J, Hirsch D, Jindal K, et al. Comparison of antilymphocyte globulin and continuous i.v. cyclosporine A as induction immunosuppression for cadaver kidney transplants: a prospective randomized study. Transplant Proc. 1991;23(1 Pt 2):999–1000. [PubMed] [Google Scholar]
- 15.Lebranchu Y, Bridoux F, Buchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant. 2002;2(1):48–56. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 16.Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation. 2003;75(6):844–851. doi: 10.1097/01.TP.0000056635.59888.EF. [DOI] [PubMed] [Google Scholar]
- 17.Kamar N, Garrigue V, Karras A, Mourad G, LeFrancois N, Charpentier B, et al. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. Am J Transplant. 2006;6(5 Pt 1):1042–1048. doi: 10.1111/j.1600-6143.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 18.Woo YM, Craig AM, King BB, Junor BJ, McMillan MA, Briggs JD, et al. Biocompatible membranes do not promote graft recovery following cadaveric renal transplantation. Clin Nephrol. 2002;57(1):38–44. doi: 10.5414/cnp57038. [DOI] [PubMed] [Google Scholar]
- 19.Romao JE, Jr, Abensur H, de Castro MC, Ianhez LE, Massola VC, Sabbaga E. Effect of dialyser biocompatibility on recovery from acute renal failure after cadaver renal transplantation. Nephrol Dial Transplant. 1999;14(3):709–712. doi: 10.1093/ndt/14.3.709. [DOI] [PubMed] [Google Scholar]
- 20.Valeri A, Radhakrishnan J, Ryan R, Powell D. Biocompatible dialysis membranes and acute renal failure: a study in post-operative acute tubular necrosis in cadaveric renal transplant recipients. Clin Nephrol. 1996;46(6):402–409. [PubMed] [Google Scholar]