Abstract
Background
The %carbohydrate deficient transferrin (%CDT) test offers objective evidence of unhealthy alcohol use but its cost-effectiveness in primary care conditions is unknown.
Methods
Using a decision tree and Markov model, we performed a literature-based cost-effectiveness analysis of 4 strategies for detecting unhealthy alcohol use in adult primary care patients: (i) Questionnaire Only, using a validated 3-item alcohol questionnaire; (ii) %CDT Only; (iii) Questionnaire followed by %CDT (Questionnaire-%CDT) if the questionnaire is negative; and (iv) No Screening. For those patients screening positive, clinicians performed more detailed assessment to characterize unhealthy use and determine therapy. We estimated costs using Medicare reimbursement and the Medical Expenditure Panel Survey. We determined sensitivity, specificity, prevalence of disease, and mortality from the medical literature. In the base case, we calculated the incremental cost-effectiveness ratio (ICER) in 2006 dollars per quality-adjusted life year ($/QALY) for a 50-year-old cohort.
Results
In the base case, the ICER for the Questionnaire-%CDT strategy was $15,500/QALY compared with the Questionnaire Only strategy. Other strategies were dominated. When the prevalence of unhealthy alcohol use exceeded 15% and screening age was <60 years, the Questionnaire-%CDT strategy costs less than $50,000/QALY compared to the Questionnaire Only strategy.
Conclusions
Adding %CDT to questionnaire-based screening for unhealthy alcohol use was cost-effective in our literature-based decision analytic model set in typical primary care conditions. Screening with %CDT should be considered for adults up to the age of 60 when the prevalence of unhealthy alcohol use is 15% or more and screening questionnaires are negative.
Keywords: Carbohydrate Deficient Transferring, Alcohol Use, Primary Care
THE UNITED STATES Preventive Services Task Force (USPSTF) recommends screening for unhealthy alcohol use, including at-risk drinking, problem drinking, alcohol abuse, and alcohol dependence (U.S. Preventive Services Task Force, 2004). The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines at-risk drinking as >14 drinks per week or 5 or more drinks on a single occasion for men and >7 drinks per week or 4 or more drinks on a single occasion for women or those aged over 65 (National Institute of Alcohol and Alcoholism, 2005). Among the multiple questionnaires available to screen for unhealthy alcohol use, AUDIT-Consumption (AUDIT-C) offers a 3-item inventory of the quantity and frequency of unhealthy alcohol use (Fiellin et al., 2000). It is generally sensitive (81 to 94%) and specific (82 to 86%) but can be subjected to inaccurate or untruthful responses.(Bradley et al., 2003, 2007; Gordon et al., 2001).
Serum biomarkers such as the %carbohydrate deficient transferrin (%CDT) test can provide objective evidence of unhealthy alcohol use. Heavy daily consumption of alcohol for 2 weeks or more triggers a positive test. Studies have found that %CDT has high specificity (77 to 100%) but variable sensitivity (10 to 85%) (Berner et al., 2006; Koch et al., 2004; Miller and Anton, 2004). Performance estimates vary depending on whether the goal of screening is to detect very heavy drinking (>60 to 80 g of ethanol or more than 5 to 7 drinks per day) or the at-risk amounts defined above.
%CDT has been widely used in Europe (Miller and Anton, 2004) and the United States Food and Drug Administration approved a %CDT assay in 2001 for detecting chronic heavy alcohol consumption (Food and Drug Administration, 2007). Although %CDT has the advantage of being an objective test, it has a low positive predictive value if used as the sole screening tool (Aertgeerts et al., 2001) and is expensive (Coulton et al., 2006). Nevertheless, multiple experts have suggested that alcohol biomarkers like %CDT can be useful in clinical settings including primary care (Miller et al., 2006). There are few published data about who should be tested with %CDT, how %CDT should be integrated with questionnaires such as AUDIT-C, and for which patient groups %CDT screening is most cost-effective.
Decision analysis is a systematic explicit, quantitative way of making decisions in health care that can lead to both enhanced decisions and better outcomes for patients (Hunink et al., 2001). In its most basic form, the modeler builds a decision tree and inputs the probability and value of each outcome derived from some combination of original data and the published medical literature. The modeler then associates costs and effects with each outcome. Cost-effectiveness may then be calculated as the cost divided by the benefit, the latter being expressed in disease-specific units such as the number of strokes averted, quality-adjusted life years (QALYs) gained, or in monetary units itself. When monetary units are used to calculate benefit, the analysis is termed a cost-benefit analysis. Dillie and colleagues (2005) assessed the cost-benefit of %CDT screening in primary care but focused on diabetic and hypertensive patients and did not assess the value of adding %CDT to established screening questionnaires. We conducted a comprehensive, literature-based decision analysis computer model to evaluate the cost-effectiveness of %CDT testing both alone and combined with questionnaire to screen for unhealthy alcohol use in primary care.
METHODS
Framework and Decision Model
We conducted our analysis following the recommendations of the Panel on Cost-Effectiveness in Health and Medicine (Panel on Cost-Effectiveness), (Russell et al., 1996; Siegel et al., 1996; Weinstein et al., 1996). We adopted a societal perspective, including costs and effects incurred both by patients receiving care and institutions providing care. The target population included adult men and women (ages 18 to 100 years) in primary care. The time horizon, or period over which costs and effects were aggregated, was from screening until death or age 100 years.
We modeled 4 strategies for detecting unhealthy alcohol use in primary care using TreeAge Pro 2007 Suite software (TreeAge Software Inc., Williamstown, MA). The 4 strategies were: (i) Questionnaire Only, using AUDIT-C; (ii) %CDT only; (iii) Questionnaire followed by %CDT (Questionnaire-%CDT) if the questionnaire is negative; and (iv) No Screening (case-finding only in which the clinician does not screen but discovers unhealthy use through the course of caring for a patient; Fig. 1). The Questionnaire Only strategy models current guidelines from national organizations including the USPSTF and NIAAA. The Questionnaire-%CDT strategy allows direct assessment of the cost-effectiveness of adding %CDT to the current recommended questionnaire-based screening strategy.
Fig. 1.
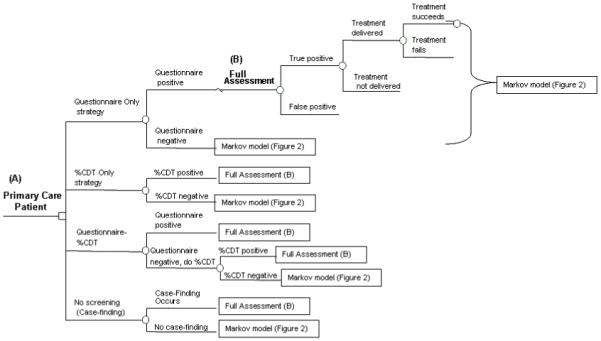
Decision tree of four strategies to screen for unhealthy alcohol use in primary care. (A) A clinician can screen a primary care patient once for unhealthy alcohol by one of four strategies. (B) Once a patient tests positive by a screening test, he or she moves into the full assessment phase. In the full assessment, clinicians ask questions to determine if the test result is a true or false positive and determine if there is an alcohol disorder. Then, there is a probability that the clinician delivers a treatment (brief intervention for at-risk drinking or abuse) or refers to specialty alcohol treatment for alcohol dependence. Finally, there is a chance that the treatment succeeds, placing the unhealthy drinker into a safer health state. Patients then enter the Markov model in one of six health states (see Fig. 2).
The initial part of the decision model simulated one-time screening, assessment, and intervention for the spectrum of unhealthy alcohol use, including at-risk drinking, alcohol abuse, and dependence. For AUDIT-C, we considered a score of ≥5 (out of a possible 12 points) positive for a man and ≥2 positive for a woman (Bradley et al., 2003, 2007; Gordon et al., 2001). The cut-off for %CDT was 2.6% as recommended by the manufacturers (Axis Shield ASA, Oslo, Norway; Berner et al., 2006). We assumed all screen-positive patients completed a full clinical assessment (i.e., the gold standard) for unhealthy alcohol use. Following this assessment, we modeled the probability that a patient would receive a brief intervention and the probability that a delivered intervention was successful. For alcohol dependence, we also modeled the probability that a patient would receive formal alcohol treatment which includes a course of cognitive behavioral (or similarly effective) therapy. If brief intervention were successful, a patient with at-risk drinking or alcohol abuse converted to safe drinking. If formal alcohol treatment worked, a patient with alcohol dependence converted to a recovery state. Such conversions are also possible in untreated groups. This “screening effect” is a beneficial reduction in drinking that occurs from the mere detection and verification of disease. Because we are uncertain if this effect would occur in real world (as opposed to research) conditions, we only applied this effect to the No Screening strategy in the base case, biasing the analysis against screening strategies.
Patients finished the initial alcohol screening and intervention part of the model in 1 of 6 mutually exclusive alcohol-related health states (Fig. 2). We then used a Markov model to track the transitions among these 6 health states from the time of screening/intervention until death.
Fig. 2.
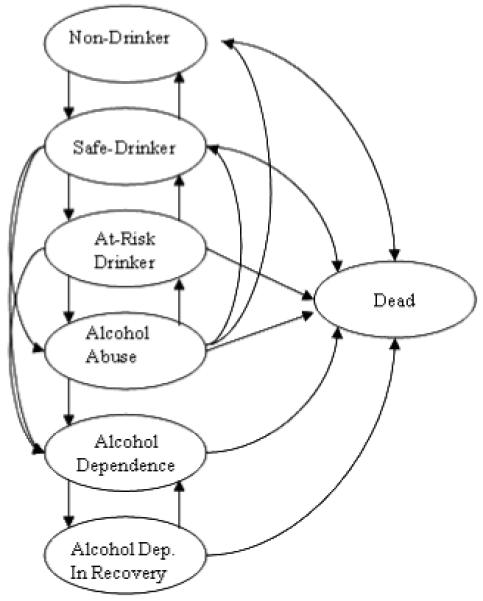
Markov model of health states defined by alcohol consumption (Non-Drinker, Safe Drinker, At-Risk Drinker) or the presence of an alcohol diagnosis (Alcohol Abuse, Alcohol Dependence, Alcohol Dependence in Recovery).
The time frame of the first part of the model (Fig. 1) is the time it takes for screening, assessment, and treatment to occur (i.e., ranging from a single clinic visit for an individual who screens negative or screens positive and receives brief intervention at the screening visit, and up to multiple visits for an individual who is alcohol-dependent and receives alcohol treatment). The “time frame” of the second part of the model, the Markov model (Fig. 2), is from the time immediately after screening/assessment/intervention until death or the age of 100 years.
Probabilities and Health State Utilities
For each probability estimate we searched Medline (1950 to spring 2007), spoke with experts, and consulted the documents of authorities such as the Centers for Disease Control and Prevention. For each parameter, we chose the highest quality evidence available but when there was uncertainty about the true value among equally good data, we made a conservative choice that biased against %CDT screening. For %CDT performance, we included data from a study of primary care patients in Germany screened for at-risk drinking (Berner et al., 2006). There was no such study from a population in the U.S. Because research on %CDT has mostly involved testing for very heavy alcohol use (e.g., >80 g ethanol or 6 drinks/day for a man and >40 g ethanol or 3 drinks/day for a woman), we also calculated the cost-effectiveness of %CDT testing using discrete diagnostic performance estimates for detecting very heavy drinkers compared with the remaining unhealthy drinking population. For these %CDT performance estimates, we used a large, multi-center international trial of patients from a range of recruitment settings (not primary care; Holder, 1998) (Table 1).
Table 1.
Parameter List, Baseline Estimate, Range for Sensitivity Analysis, and Comment
| Parameter | Baseline | Range | Comments/citations |
|---|---|---|---|
| Probabilities | |||
| Demographics | |||
| Initial age of cohort | 50 | 18-80 | Implies all individuals at the same initial age |
| Prevalence of unhealthy drinking (%) | Includes at-risk drinkers, alcohol abuse, and alcohol dependence; prevalence; values are a weighted average assuming 50% of cohort are male (Manwell et al., 1998) | ||
| 50-year-old cohort | 22 | 10-40 | |
| 25-year-old cohort | 28 | 15-45 | |
| 75-year-old cohort | 6 | 1-20 | |
| Prevalence of abuse (%) | 11 | 7-15 | Prevalence for the base case, 50-year-old patient |
| Prevalence of at-risk drinking (%) | 4 | 2-10 | |
| Prevalence of dependence (%) | 8 | 4-12 | |
| Test performance and prevalence | |||
| Sensitivity of AUDIT-C questionnaire in women | 81 | 50-99 | Sensitivity and specificity to detect >7 drinks/wk or 4 or more drinks/day ± DSM IV disorder at a specificity of 86% (Bradley et al., 2003) |
| Specificity of AUDIT-C questionnaire in women | 86 | 50-100 | |
| Sensitivity of AUDIT-C questionnaire in men | 94 | 50-99 | Sensitivity and specificity to detect >16 drinks/wk (Gordon et al., 2001) |
| Specificity of AUDIT-C questionnaire in men | 82 | 50-100 | |
| Sensitivity of %CDT (men and women combined) | 34 | 10-99 | Sensitivity and specificity to detect individuals at-risk or more unhealthy drinking (reference standard AUDIT > 8) (Berner et al., 2006) |
| Specificity of %CDT (men and women combined) | 94 | 50-100 | |
| % delivery, treatment, and screening effects | |||
| Delivery of brief intervention (BI) (%) | |||
| At-risk | 39 | 0-59 | Probability of BI delivery by primary care provider after positive screen by questionnaire or %CDT; in the source publication (Burman et al., 2004), the 10-item AUDIT was used to categorize disease severity |
| Abuse | 59 | 39-71 | |
| Dependence | 71 | 59-100 | |
| % individuals with dependence who follow up for alcohol treatment after brief intervention or usual care | 40 | 10-90 | Preliminary data from our own work for receipt of “alcohol assistance” (ASAP Study Clinical Trials Identifier NCT00183105) after brief intervention or usual care. (Note: In the model, 0% of alcohol dependents reduce their drinking after brief intervention alone; alcohol dependents must proceed to alcohol treatment before any benefit occurs) |
| % at-risk drinkers or drinkers with alcohol abuse achieving low risk drinking after brief intervention | 39 | 0-75 | Percentage transitioning from at-risk or alcohol abuse to low risk drinking (e.g., within guidelines/suggested limits) after BI (Ockene et al., 1999) |
| % dependent drinkers achieving low risk drinking after alcohol treatment | 41 | 0-80 | Percentage transitioning from dependence to recovery after alcohol treatment (Project MATCH authors, 1997) |
| Screening effect parameters (%) | Percentage transitioning from an unhealthy to healthy state after detection but without treatment; in the base case, the screening effects only applied to the No Screening strategy but was explored in sensitivity analyses. | ||
| Abuse or at-risk to safe | 28 | 0-50 | From control arm of a randomized controlled trial for BI (Ockene et al., 1999) |
| Dependence to recovery | 14 | 0-50 | No trial data for this parameter found; 1 year spontaneous probability of transition used (Schuckit et al., 2001) |
| Percent follow up of a positive %CDT result | 50 | 10-90 | Composite probability that provider notifies patient and patient returns for full assessment |
| Percent refusal of %CDT | 0 | 0-100 | Assumed this value is zero; refusal implies no change in cost-effectiveness |
| Utilities | |||
| Nondrinker (age <65) | 0.91 | 0.74-1.00 | For all unhealthy states, we used standard gamble utilities measured in the community (Kraemer et al., 2005). For the utility of individuals with age > 65 in Nondrinker or Safe state, we used a generic, published utility for the well elderly (Gold et al., 1998) |
| Safe drinker (age <65) | 0.86 | 0.74-100 | |
| Nondrinker or safe drinker (age 65 or more) | 0.84 | 0.74-1.00 | |
| At-risk drinker (all ages) | 0.80 | 0.74-1.00 | |
| Abuse drinker (all ages) | 0.74 | 0.65-0.80 | |
| Dependent drinker (all ages) | 0.65 | 0.40-0.80 | |
| Recovery (all ages) | 0.81 | 0.74-0.86 | |
| Hazard ratios | |||
| Nondrinker | 1 | 1 | Hazard ratio (of dying) for drinking state compared with nondrinker reference state (Dawson, 2000) |
| Safe drinker | 0.8 | 0.33-2.00 | |
| At-risk | 0.92 | 0.50-4.00 | |
| Abuse drinker | 1.07 | 0.50-1.00 | |
| Dependent drinker | 1.42 | 0.50-5.00 | |
| Recovery | 1.18 | 0.50-4.00 | |
| Costs | |||
| Initial costs (in $US) | |||
| Questionnaire | 3 | 0-50 | CPT 99203 - 1 of 30 minutes assuming physician billed by time (Centers for Medicare & Medicaid, 2006b) |
| %CDT | 38 | 20-150 | Medicare reimbursement CPT 82373 + venipuncture + 0.5 hour wages = $25 (Centers for Medicare & Medicaid, 2006a)+ $3 (Centers for Medicare & Medicaid, 2006a) + $9.50 (Bureau of Labor Statistics, 2005) |
| Full assessment following positive questionnaire or case-finding | 33 | 0-250 | CPT 99203 - 10 of 30 minutes assuming physician billed based on time (Centers for Medicare & Medicaid, 2006b) |
| Full assessment following positive %CDT | 128 | 0-250 | Follow-up visit (CPT 99213) + 3 hours wage + daily travel for patient (Centers for Medicare & Medicaid, 2006b) |
| Brief intervention following positive questionnaire or case-finding | 26 | 0-200 | CPT 99203 - 7.5 of 30 minutes (Centers for Medicare & Medicaid, 2006b) |
| Brief intervention following positive %CDT | 0 | 0-200 | No additional cost after new visit cost which permits 15-25 minutes of provider time |
| Cost of alcohol dependence treatment | 1,077 | 200-10,000 | Includes provider costs as discussed by Cisler and colleagues (1998) + lost wages for 6 sessions, total 18 hours (Bureau of Labor Statistics, 2005) + 6 days of travel |
| Cost of hourly wages lost for patient | 19 | 5-30 | National mean wage adjusted for inflation (Bureau of Labor Statistics, 2005) |
| Cost of daily travel paid by patient | 15 | 0-30 | Estimated by authors |
| Future costs (in $US) | Mean annual cost including out of pocket and third party disbursements for individuals without alcohol disorder (only listed for men) (Agency for Healthcare Research and Quality, 2004) | ||
| Age 18-44 | 1,942 | 500-4,710 | |
| Ages 45-64 | 4,710 | 1,942-9657 | |
| Age 65 and over | 9,657 | 4,710-20,00 | |
| Future cost multipliers | |||
| Dependent drinker | 2 | 1-3 | Implies that future annual cost will be twice that of nondrinkers for each year lived with the disorder (Blose and Holder, 1991; Holder, 1998) |
| Abuse | 1.5 | 0.5-2 | Implies that future annual cost will be 1.5 fold that of nondrinkers (estimated by authors) |
| Recovery, at-risk, safe | 1.0 | 1.0-2.0 | Implies that future annual cost will be the same as that of nondrinkers (estimated by authors) |
For efficacy of brief intervention, we used estimates from a 5 to 10 minute brief intervention trial (Ockene et al., 1999). We operationalized efficacy with 2 variables for the transition from the at risk drinking or abuse state to safe state. The transition rate in the group receiving screening and brief intervention was 39%. The same transition in the group receiving screening alone was 28% indicating a net effect of 11%.
We derived health state transition probabilities from 2 well-established longitudinal studies conducted in the U.S. (Kerr et al., 2002; Schuckit et al., 2001). To calculate survival, we consulted the published literature (Arias, 2006; Dawson, 2000). To calculate quality-adjusted survival, we multiplied survival by health state utilities previously measured by our group (Kraemer et al., 2005). Utilities represent a degree of preference for 1 health state (scored between 0 and 1) versus a perfect health state (utility of 1).
Costs
We calculated initial costs for screening and treatment in 2006 U.S. dollars. Our estimates for screening costs represent current Medicare reimbursement for physician time and lab testing (Centers for Medicare & Medicaid, 2006a,b). To aggregate direct health care costs in the future, we used data from the Medical Expenditure Panel Survey (MEPS) (Agency for Healthcare Research and Quality, 2004). We also did not include cost incurred to people injured by the index patient nor productivity gains for treated patients experiencing improved health. For alcohol dependence and abuse, we assigned a multiplier to the baseline annual costs provided by MEPS. There are conflicting reports about the costs for at-risk drinking and so we assumed at-risk drinking had no effect on direct health care costs (multiplier = 1.0; Dillie et al., 2005; Holder, 1998; Mertens et al., 2005).
Markov Model Calibration
Transitions out of the Nondrinker state or into dependence become infrequent after the third decade of life. To calibrate transition rates for the above health states, we performed 1,000 model simulations starting with the previously mentioned transition rates. From the simulations, we calculated the proportion transitioning out of abstinence over a lifetime in 4 age and gender strata for which there was published information available (Adams and Schoenborn, 2006). Similarly, we calculated the proportions transitioning into dependence in these strata and compared these values to the published information (Dawson et al., 2005). In both cases the published data reflects the “background” rate of discovery, treatment, and transition to safer health states (either Safe, Abstinent, or Recovery depending on the starting point) and represents the natural history of unhealthy alcohol diagnosis and treatment prior to availability of %CDT screening. We then repeated simulations, each time adjusting transition rates until the proportions approximated the published data. We did not find information about the age of transition for other health states and used the published (uncalibrated) rates in those cases.
Analysis
We calculated the incremental cost-effectiveness ratio (ICER) as the difference in costs between the least expensive strategy and the next least expensive strategy divided by the difference in their effectiveness (measured both in unadjusted life years and QALYs). The ratio is expressed as how much additionally it costs (in dollars) to achieve an additional QALY. Policy makers are interested in the ICER value because it accounts for the fact that there was a less expensive option when making a selection from competing programs (Hunink et al., 2001). Interpreting the results of cost-effectiveness analysis can be problematic, making it difficult to decide whether to adopt a diagnostic test or treatment. The threshold for adoption in the Unites States is thought to be somewhere between $20,000/QALY and $100,000/QALY, with a threshold of $50,000/QALY frequently proposed (Bell et al., 2006). In the base case, we examined the cost-effectiveness in a hypothetical cohort of 50 year olds. We then repeated this analysis in 25 year olds and 75 year olds. We discounted all future health costs and QALYs by 3%.
We conducted 1-way and selected 2-way sensitivity analyses to assess the influence of uncertainty in individual parameter values on the ICER for the Questionnaire-%CDT strategy compared with the Questionnaire Only strategy. We also performed probabilistic sensitivity analysis (a process that involves specifying distributions for model input parameters and then sampling simultaneously from these distributions to assess the joint effect of input parameter uncertainty).
RESULTS
Model predicted proportions of transition out of the non-drinker state and of dependence onset calibrated well, falling within 2% of published probabilities (Table A1). The base-case model results for cost, effectiveness, and ICER are shown in Table 2. The No Screening and %CDT Only strategies were both more costly and less effective than (i.e., they were dominated by) the other strategies in the base case and the other scenarios described in Table 2. In the base case 50-year-old cohort, the Questionnaire-%CDT strategy cost $15,500/QALY compared with the Questionnaire Only strategy. The ICER for the same comparison in a 25-year-old cohort was substantially lower at $3,380/QALY and in a 75-year-old cohort was substantially higher at $243,000/QALY (Table 3).
Table 2.
Cost and Effectiveness of 4 Strategies for Alcohol Screening in a Cohort of 50-Year-Old Primary Care Patients
| Strategy | cost (in $) | Incremental Cost (in $) |
Effectiveness (in QALYs) |
Incremental effectiveness (in QALYs) |
ICER (in $/QALY) |
|---|---|---|---|---|---|
| Questionnaire Only | 143,568 | 16.013 | |||
| %CDT Only | 144,104 | 523 | 15.984 | -0.031 | (Dominateda) |
| Questionnaire-%CDT | 143,581 | 13 | 16.014 | 0.001 | 15,500 |
| No Screening | 143,780 | 199 | 15.999 | -0.015 | (Dominated) |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Dominated implies this strategy cost more and is less effective.
Table 3.
Incremental Cost-Effectiveness Ratio for Questionnaire-%CDT Strategy Versus Questionnaire Only as a Function of Age, Use of Life Years, Patient Costs Inclusion, and Screening Effects
| Age | ICER ($/QALY) |
ICER ($/LY) |
ICER without patient costs included ($/QALY) |
ICER with screening effects included in intervention strategiesa |
|---|---|---|---|---|
| 25 | 3,380 | 18,200 | Dominatesb | Dominates |
| 50 | 15,500 | 58,600 | 5,030 | 2,290 |
| 75 | 243,000 | 441,000 | 164,000 | 128,000 |
ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; LY, life year.
Screening effect pertains to the transition from unhealthy to healthy state after positive screening but without formal intervention. In the base case, we applied this effect only to the No Screening strategy. In sensitivity analysis, we applied this to all strategies.
Dominates implies that the Questionnaire-%CDT strategy cost less and gained more QALYs compared with the Questionnaire Only strategy.
Sensitivity Analyses
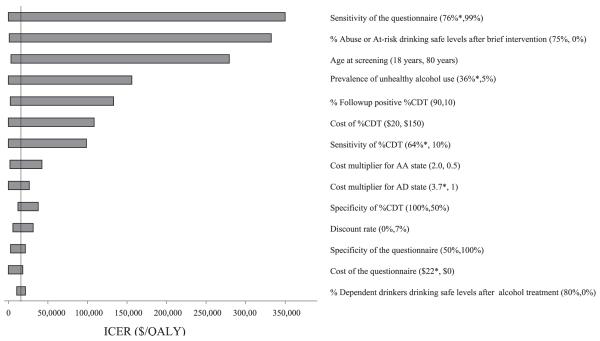
The base line ICE Restimate was sensitive to the percentage of at-risk drinkers or drinkers with alcohol abuse achieving safe drinking levels after brief intervention, questionnaire and %CDT sensitivity, age at screening, prevalence of unhealthy alcohol use, and the follow-up rate of positive %CDT results (Fig. 3). The Questionnaire-%CDT strategy dominated the Questionnaire Only strategy when questionnaire sensitivity was less than 76% or %CDT sensitivity was greater than 64%. In order for the ICER to cross the $50,000/QALY threshold, the % at-risk drinkers or drinkers with alcohol abuse achieving low risk drinking after brief intervention would have to drop from 39% to 17%, the sensitivity of %CDT would have to drop from 34% to 17%, or follow-up after ordering the %CDT test would have to drop from 50% to 23%. In the analysis that looked at the effect of using discrete %CDT diagnostic performance data for very heavy alcohol use, the ICER for the Questionnaire-%CDT strategy increased to $27,800/QALY and the %CDT only strategy was still dominated.
Fig. 3.
Tornado diagram of one-way sensitivity analyses on important model parameters. The horizontal bars indicate the incremental cost-effectiveness ratio (ICER) of the Questionnaire-%CDT strategy compared with the Questionnaire Only strategy. Values in parentheses for each variable represent the range over which sensitivity analysis was performed as shown in Table 1. If the Questionnaire-%CDT strategy the Questionnaire Only strategy, then one end of the range is replaced by the value at which dominance occurs and is shown by an asterisk; the vertical line represents the ICER using the baseline value. An asterisk denotes the value for which the Questionnaire-%CDT strategy dominates the Questionnaire Only strategy. ICER, incremental cost-effectiveness ratio; QALY, quality adjusted life year.
In a 2-way sensitivity analysis varying age and prevalence of unhealthy alcohol use (Fig. 4), the ICER for Questionnaire-%CDT remains below a $50,000/QALY threshold if unhealthy alcohol use is ≥15% and screening age is ≤60. The Questionnaire Only strategy dominated the No Screening strategy in virtually all age cohorts. Probabilistic sensitivity analysis indicates that at the $50,000/QALY threshold, the Questionnaire-%CDT strategy was favored in 64% of the simulations compared to the Questionnaire Only which was favored 35% of the time (Fig. 5).
Fig. 4.
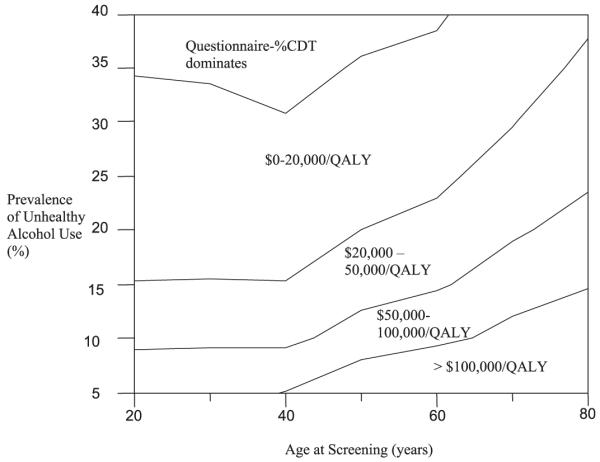
Two-way sensitivity analysis on the incremental cost-effectiveness ratio (ICER) as a function of the prevalence of unhealthy alcohol use and age at screening. The $/QALY values indicate the ICER range for the Questionnaire-%CDT strategy compared to Questionnaire Only strategy at specific combinations. QALY, quality adjusted life year.
Fig. 5.

Percentage of simulations for which four strategies to screen for unhealthy alcohol use are cost-effective in a 50-year-old cohort of primary care patients. QALY, quality adjusted life year.
DISCUSSION
Our analysis indicates that adding %CDT to questionnaire based one-time screening is cost-effective in typical primary care conditions. In 50 year olds, the Questionnaire-%CDT strategy costs $15,500 per QALY gained when compared to the Questionnaire Only strategy. Compared with the Questionnaire Only strategy, the Questionnaire-%CDT strategy was favored at a threshold of $50,000/QALY when the prevalence of unhealthy alcohol use exceeded 15% and the age at screening was <60 years. The Questionnaire Only strategy dominated the No Screening strategy in virtually all age cohorts. Screening with the %CDT test alone was not cost-effective.
We provide evidence for intensifying screening to detect unhealthy alcohol use in primary care by adding a %CDT test when questionnaire screening is negative. Our analysis differs from a cost-benefit analysis conducted by Dillie and colleagues (2005) which suggested that adding %CDT to physician interview is cost saving, meaning that it achieved better outcome at a lower cost. We found that adding %CDT to questionnaire screening was cost-effective (achieved better outcome but at a higher, though generally acceptable, cost) but not cost-saving. Some people informally summarize such interventions as “good value.” Unlike the previous analysis, we accounted for incomplete follow-up of %CDT, potential need for a second visit to address the positive result, and poor provider performance in delivering treatment (Burman et al., 2004; Saitz et al., 2003). Our analysis also included the long term cost and effects of screening with %CDT and included patient time costs and out of pocket expenses (i.e., the societal perspective). The base case ICER value of $15,500/QALY compares favorably with the cost-effectiveness of other currently accepted screening programs—e.g. one-time HIV screening ($33,000/QALY) (Paltiel et al., 2006) or colonoscopy every 10 years compared with annual fecal occult blood testing or no screening ($12,000 to 18,000/life year; Pignone et al., 2002).
Our conclusion also differed from Coulton and colleagues (2006) who found the cost per patient screened was 20-fold greater for %CDT compared with questionnaire based screening in Welsh males. This group did not, however, analyze the incremental cost-effectiveness of adding %CDT to questionnaire-based screening as in the current study and did not account for potential downstream costs saved, mortality avoided, and quality of life improved.
There are several limitations to this work. There is no single estimate for the prevalence of unhealthy alcohol use in primary care. Prevalence varies by gender, race, ethnicity, geography, and duration but has been reported in multiple studies (Manwell et al., 1998; Taj et al., 1998) to be more than 20% using the current NIAAA definition we adopted for our analysis. We chose prevalence estimates from a study by Manwell and colleagues (1998) in which 21,282 patients in Wisconsin were screened for unhealthy alcohol use. That study reported a 90-day prevalence of unhealthy alcohol use of 23%, combined for all ages and both genders. The study included one of the largest U.S. primary care samples available and it provided data about the spectrum of unhealthy alcohol use. Our sensitivity analysis suggests the Questionnaire-%CDT strategy would still be cost-effective (at the $50,000/QALY threshold) in a lower prevalence scenario when the age at screening is less than 60 years.
There is also no single way to administer brief intervention and therefore no single estimate for the transition rate from at-risk drinking to safe drinking levels. We believe our choice for the value of the transition rate (i.e., 39%) after brief intervention was conservative. Other studies such as Project Treat (Fleming et al., 1997) using a longer initial BI and incorporating follow-up contacts have described the effect to be larger but we believe a one-time, 5 to 10 minute intervention was the one most likely to resemble how physicians actually conduct brief intervention. Comparisons with other brief intervention trials such as those included in a recent systematic review (Beich et al., 2003) are limited by exclusion of subjects with lower levels of risky alcohol.
Another limitation of the Markov modeling technique we used is that the transition probabilities depend only on the current state and not on the history of past states. For example, individuals in the at-risk drinking state in a given 1-year cycle had the same probability of transitioning into other states regardless of their drinking state in prior cycles. We did not have information about the rate of transition from safe to at-risk drinking for an individual with a prior history of atrisk drinking compared with someone without this history. We obtained information about transitions in drinking behavior from a study by Kerr and colleagues (2002) based on the National Health Nutrition Examination Survey. Transition rates provided by Kerr and colleagues represent the rate of transitions at the aggregate level. This includes individuals with and without a prior history of at-risk drinking. We therefore believe that the transition rates we used are an accurate representation of the transitions from safe to at-risk drinking, at the aggregate level. For individuals with a history of alcohol dependence, this “amnestic” property of Markov models was mitigated by the high rate of relapse built into the Recovery state.
Other limitations include absence of conditional diagnostic test performance data for %CDT (i.e., the sensitivity and specificity in a population already having tested negative by questionnaire). We believe biomarker screening has a diagnostic performance that is independent from questionnaire performance. Our estimate for %CDT performance to detect unhealthy alcohol use was a conservative choice from the limited trials set in general primary care. Had we chosen to use discrete diagnostic performance data for detecting very heavy drinkers, as in the previously mentioned sensitivity analysis, the economic implications would not have changed substantially.
We also did not have information about the effectiveness of brief intervention or alcohol treatment in a group testing negative by questionnaire. Brief intervention is likely to be less successful in a group testing negative by questionnaire. Such individuals may be feigning low risk use or they may be infrequent risky drinkers, and in either case less likely to change, although the exact magnitude of the differential effectiveness is not known.
We did not have information about the clinical effect of ordering a blood test in patients denying unhealthy alcohol use. Patients who take offense from being asked to confirm their reported drinking behavior with %CDT may decide not to discuss their alcohol use or other medical problems as freely with their provider. They may even decide to sever relations with this provider. We believed the frequency of these untoward consequences would be low and therefore did not model any costs for the deterioration or discontinuation in the patient-provider relationship. We feel the decision to not to model these costs, however, was still a conservative choice given that mention of objective corroboration of a person’s report with %CDT will likely prime an admission of unhealthy use for a large percentage of primary care patients, thereby obviating the need and cost for the test. In addition, at least 1 study suggests that the use of %CDT can provide motivation for some patients to reduce their alcohol use (Fleming et al., 2004). The exact direction of the bias imposed by our balanced modeling assumptions (i.e., that all patients who screened negative by the questionnaire would undergo the blood test and that no patient would voluntarily disclose their drinking status upon broaching the issue of biomarker screening) is unknown and represents area for future inquiry.
Lastly, we did not model all possible consequences of a false-positive %CDT result. There is no consensus for the workup of elevated %CDT results and false-positive results may occur in patients underreporting alcohol use (i.e., the gold standard interviews used to assess performance are imperfect). Future research should assess %CDT performance and treatment effectiveness in a cohort testing negative by questionnaire, patient and provider acceptability of the Questionnaire-%CDT strategy, and the implications of false-positive %CDT results.
In conclusion, adding %CDT to questionnaire based one-time screening for unhealthy alcohol use was cost-effective in typical primary care conditions and, at minimum, clinicians should screen all patients with a questionnaire. Some clinicians may consider ordering %CDT after a negative screening questionnaire for adults up to age 60 when the prevalence of unhealthy alcohol use is 15% or more. However, despite its cost-effectiveness, issues around effectiveness of brief intervention in a questionnaire negative group, patient acceptability of blood testing in this same group, and management of false-positive results should be better studied before we can recommend widespread use of %CDT.
ACKNOWLEDGMENTS
This study was supported by National Research Service Award T32 HP 10028-08 and the National Institute on Alcohol Abuse and Alcoholism R01 AA12617.
APPENDIX
Table A1.
Predicted versus published proportions for transition out of nondrinker state and transition into dependence in 4 age and gender strata
| Proportion transitioning out of nondrinker statea |
Proportion transitioning into dependence in the future compared with the total ever being dependentb |
|||
|---|---|---|---|---|
| Stratum | Model predicted proportion |
Published proportion |
Model predicted proportion |
Published proportion |
| 25-year-old men | 0.56 | 0.55 | 0.33 | 0.33 |
| 25-year-old women | 0.35 | 0.37 | ||
| 50-year-old men | 0.05 | 0.06 | 0.06 | 0.05 |
| 50-year-old women | 0 | 0 | ||
Analysis adjusting for gender as published in the National Household Interview Survey (Adams and Schoenborn, 2006).
Analysis unadjusted for gender as published in the National Epidemiologic Survey of Alcohol Related Conditions (Dawson et al., 2005).
REFERENCES
- Adams PF, Schoenborn CA. Health behaviors of adults: United States, 2002-2004. Vital Health Stat Ser. 2006;10:1–140. [PubMed] [Google Scholar]
- Aertgeerts B, Buntinx F, Ansoms S, Fevery J. Screening properties of questionnaires and laboratory tests for the detection of alcohol abuse or dependence in a general practice population. Br J Gen Pract. 2001;51:206–217. [PMC free article] [PubMed] [Google Scholar]
- Agency for Healthcare Research and Quality [Accessed June 13, 2007];Medical Expenditure Panel Survey. 2004 Available at: http://www.meps.ahrq.gov/mepsweb/ [PubMed]
- Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- Beich A, Thorsen T, Rollnick S. Screening in brief intervention trials targeting excessive drinkers in general practice: systematic review and meta-analysis. BMJ. 2003;327:536–542. doi: 10.1136/bmj.327.7414.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, Neumann PJ. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332:699–703. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner MM, Bentele M, Kriston L, Manz C, Clement H-W, Harter M, Mundle G. DOVER and QUVER-new marker combinations to detect and monitor at-risk drinking. Alcohol Clin Exp Res. 2006;30:1372–1380. doi: 10.1111/j.1530-0277.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- Blose JO, Holder HD. The utilization of medical care by treated alcoholics: longitudinal patterns by age, gender, and type of care. J Sub Abuse. 1991;3:13–27. doi: 10.1016/s0899-3289(05)80003-3. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, Maynard C, Burman ML, Kivlahan DR. Two brief alcohol-screening tests from the Alcohol Use Disorders Identification Test (AUDIT): validation in a female veterans affairs patient population. Arch Intern Med. 2003;163:821–829. doi: 10.1001/archinte.163.7.821. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Debenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics [Accessed June 13, 2007];National Compensation Survey. 2005 Available at: http://data.bls.gov/PDQ/outside.jsp?survey=nc.
- Burman ML, Kivlahan D, Buchbinder M, Broglio K, Zhou XH, Merrill JO, Mcdonell MB, Fihn SD, Bradley KA, Ambulatory Care Quality Improvement Project I Alcohol-related advice for Veterans Affairs primary care patients: who gets it? Who gives it? J Stud Alcohol. 2004;65:621–630. doi: 10.15288/jsa.2004.65.621. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid [Accessed June 6, 2007];Clinical Diagnostic Laboratory Fee Schedule. 2006a Available at: http://www.cms.hhs.gov/ClinicalLabFeeSched.
- Centers for Medicare & Medicaid [Accessed June 6, 2007];Physician Fee Schedule. 2006b Available at: http://www.cms.hhs.gov/pfslookup/02_PFSearch.asp.
- Cisler R, Holder HD, Longabaugh R, Stout RL, Zweben A. Actual and estimated replication costs for alcohol treatment modalities: case study from Project MATCH. J Stud Alcohol. 1998;59:503–512. doi: 10.15288/jsa.1998.59.503. [DOI] [PubMed] [Google Scholar]
- Coulton S, Drummond C, James D, Godfrey C, Bland JM, Parrott S, Peters T, Stepwice Research T. Opportunistic screening for alcohol use disorders in primary care: comparative study. BMJ. 2006;332:511–517. doi: 10.1136/bmj.38743.421574.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Alcohol consumption, alcohol dependence, and all-cause mortality. Alcohol Clin Exp Res. 2000;24:72–81. [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001-2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Dillie KS, Mundt M, French MT, Fleming MF. Cost-benefit analysis of a new alcohol biomarker, carbohydrate deficient transferrin, in a chronic illness primary care sample. Alcohol Clin Exp Res. 2005;29:2008–2014. doi: 10.1097/01.alc.0000187606.54854.db. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160:1977–1989. doi: 10.1001/archinte.160.13.1977. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Barry KL, Manwell LB, Johnson K, London R. Brief physician advice for problem alcohol drinkers. A randomized controlled trial in community-based primary care practices. JAMA. 1997;277:1039–1045. [PubMed] [Google Scholar]
- Fleming M, Brown R, Brown D. The efficacy of a brief alcohol intervention combined with %CDT feedback in patients being treated for type 2 diabetes and/or hypertension. J Stud Alcohol. 2004;65:631–637. doi: 10.15288/jsa.2004.65.631. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration [Accessed August 1, 2007];Device Listing. 2007 Available at: http://www.fda.gov/search/databases.html.
- Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical Care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Maisto SA, Mcneil M, Kraemer KL, Conigliaro RL, Kelley ME, Conigliaro J. Three questions can detect hazardous drinkers. J Fam Pract. 2001;50:313–320. [PubMed] [Google Scholar]
- Holder HD. Cost benefits of substance abuse treatment: an overview of results from alcohol and drug abuse. J Ment Health Policy Econ. 1998;1:23–29. doi: 10.1002/(sici)1099-176x(199803)1:1<23::aid-mhp3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hunink M, Glasziou P, Siegel J. Decision Making in Health and Medicine. Cambridge University Press; Cambridge UK: 2001. [Google Scholar]
- Kerr WC, Fillmore KM, Bostrom A. Stability of alcohol consumption over time: evidence from three longitudinal surveys from the United States. J Stud Alcohol. 2002;63:325–333. doi: 10.15288/jsa.2002.63.325. [DOI] [PubMed] [Google Scholar]
- Koch H, Meerkerk GJ, Zaat JO, Ham MF, Scholten RJ, Assendelft WJ. Accuracy of carbohydrate-deficient transferrin in the detection of excessive alcohol consumption: a systematic review. Alcohol Alcohol. 2004;39:75–85. doi: 10.1093/alcalc/agh031. [DOI] [PubMed] [Google Scholar]
- Kraemer KL, Roberts MS, Horton NJ, Palfai T, Samet JH, Freedner N, Tibbetts N, Saitz R. Health utility ratings for a spectrum of alcohol-related health states. Med Care. 2005;43:541–550. doi: 10.1097/01.mlr.0000163644.97251.14. [DOI] [PubMed] [Google Scholar]
- Manwell LB, Fleming MF, Johnson K, Barry KL. Tobacco, alcohol, and drug use in a primary care sample: 90-day prevalence and associated factors. J Addict Dis. 1998;17:67–81. doi: 10.1300/J069v17n01_07. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Miller PM, Anton RF. Biochemical alcohol screening in primary health care. Addict Behav. 2004;29:1427–1437. doi: 10.1016/j.addbeh.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Miller PM, Spies C, Neumann T, Javors MA, Hoyumpa AM, Roache J, Webb A, Kashi M, Sharkey FE, Anton RF, Egan BM, Basile J, Nguyen S, Fleming MF, Dillie KS. Alcohol biomarker screening in medical and surgical settings. Alcohol Clin Exp Res. 2006;30:185–193. doi: 10.1111/j.1530-0277.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- National Institute of Alcohol and Alcoholism [Accessed April 6, 2007];Assessing Alcohol Problems: A Guide for Clinicians and Researchers. 2005 Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/PocketGuide/pocket_guide5.htm.
- Ockene JK, Adams A, Hurley TG, Wheeler EV, Hebert JR. Brief physician- and nurse practitioner-delivered counseling for high-risk drinkers: does it work? Arch Intern Med. 1999;159:2198–2205. doi: 10.1001/archinte.159.18.2198. [DOI] [PubMed] [Google Scholar]
- Paltiel AD, Walensky RP, Schackman BR, Seage GR, III, Mercincavage LM, Weinstein MC, Freedberg KA. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- Pignone M, Saha S, Hoerger T, Mandelblatt J, Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1172–1177. [PubMed] [Google Scholar]
- Saitz R, Horton NJ, Sullivan LM, Moskowitz MA, Samet JH. Addressing alcohol problems in primary care: a cluster randomized, controlled trial of a systems intervention. The screening and intervention in primary care (SIP) study. Ann Intern Med. 2003;138:372–382. doi: 10.7326/0003-4819-138-5-200303040-00006. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Bucholz KK, Reich T, Bierut L. Five-year clinical course associated with DSM-IV alcohol abuse or dependence in a large group of men and women. Am J Psychiatry. 2001;158:1084–1090. doi: 10.1176/appi.ajp.158.7.1084. [DOI] [PubMed] [Google Scholar]
- Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- Taj N, Devera-Sales A, Vinson DC. Screening for problem drinking: does a single question work? J Fam Pract. 1998;46:328–335. [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force [Accessed April 6, 2007];Screening for Alcohol Misuse. 2004 Available at: http://www.ahcpr.gov/clinic/uspstf/uspsdrin.htm.
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]