Abstract
Early of onset of alcohol consumption increases the risk for the development of dependence. Whether adolescent consumption of other highly palatable solutions may also affect alcohol drinking in adulthood is not known. The purpose of this study was to determine the effects of adolescent consumption of four solutions: water, sucrose, sucrose-milk and milk on ethanol drinking in adult rats. Rats had limited access to one of the four solutions from day PND 29 to PND 51 and were subsequently trained to consume ethanol (E) using a sucrose(S) fade-out procedure. Adolescent consumption of sucrose and sucrose-milk solutions increased intake of 2.5%E when it was combined with 10%S but it had no effect on the drinking of 10%E alone. Adolescent consumption of milk and sucrose-milk significantly decreased the intake of 10%E when it was combined with 10%S, and milk significantly reduced 10%E consumption alone and when it was combined with 5%S. Adolescent exposure to the sucrose-milk and sucrose solutions was also found to increase sucrose and sucrose-milk consumption. Our findings suggest adolescent exposure to sucrose increases, whereas, exposure to milk reduces ethanol consumption in adult rats. Our results may provide a new theoretical approach to the early prevention of alcoholism.
Keywords: Adolescence, alcohol, alcoholism, sucrose, milk
1. INTRODUCTION
A rapid increase in the consumption of sweet beverages among adolescents aged 12 to 19 years in the United States has occurred with a concurrent escalation in the rates of obesity in adolescents and adults (Flegal et al., 2002; Nielsen and Popkin, 2004; Ogden et al., 2002).While evidence suggests a relationship between adolescent consumption of sweet solutions and obesity, many questions remain unanswered about the consequences of exposure to sweet solutions during adolescence. For instance, it is still unclear whether adolescent exposure to high levels of sucrose is also a risk factor for the development of substance abuse.
One of the most salient and compelling predicators of alcohol intake in animal models is the consumption of sweet-tasting solutions. Studies in rodents have shown positive correlations between consumption of alcohol and solutions sweetened with either saccharin or sucrose in rats (Dess et al., 1998; Eiler et al., 2005; Kampov-Polevoy et al., 1990, 1996; Overstreet et al., 1993; Sinclair et al., 1992; Stewart et al., 1994) and mice (Bachmanov et al., 1996; Belknap et al., 1993; Rodgers and McClearn, 1964). Sweet solutions may be reinforcing due to a number of reasons including taste and/or post-ingestive feedback. Genetic correlations between the intakes of sucrose or saccharin and alcohol seen in inbred strains could be due to factors related to taste or post-ingestive feedback or independent influences of unrelated genes for that are caused by the chance fixation of unrelated alleles during inbreeding (Blizard, 1992).
However, a positive association between the intake of sucrose and alcohol has been demonstrated in congenic mice suggesting that this correlation is not a result of fortuitous fixation of unrelated genes and provides evidence that in the B6/D2 lineage the correlation between intake of sucrose and alcohol reflects close linkage or the pleiotropic effects of the same genes (Blizard and McClearn, 2000). Additionally, studies in genetically selected rats have also demonstrated that, at least in the Indiana Preferring/Non-Preferring lines, that sucrose is more reinforcing in preferring animals both for its taste (Eiler et al., 2005) and for its appetitive and consummatory properties (Czachowski and Samson, 2002).
An association between sugar consumption and alcohol-related phenotypes has also been demonstrated in human subjects. For instance, alcohol dependent individuals prefer more concentrated sucrose solutions than control subjects (Kampov-Polevoy et al., 1997, 2004). It has also been demonstrated that subjects categorized as “sweet-likers”, based on their preference of high concentration sucrose solutions, were more likely to have a family history of alcoholism (Kampov-Polevoy et al., 2003). Additionally, abstinent alcohol-dependent individuals that stayed sober longer were found to have consumed twice the amount of sugar than other groups with shorter periods of abstinence (Yung et al., 1983). Sweet-liking may also predict response to naltrexone in alcohol dependent subjects (Garbutt et al., 2009).
Consumption of alcohol in humans is usually initiated using sweetened alcoholic beverages (Copeland et al., 2007; Fromme and Samson, 1983: Marguiles et al., 1977).Grant and Samson (1985) developed an animal model of this phenomenon that takes advantage of the rat’s preference for sweet solutions to establish alcohol self-administration in animals without food and water restriction. These investigators reported that the “sucrose fading procedure”, which consists of the introduction of sucrose solutions followed by sucrose/ethanol solutions and then the gradual elimination of sucrose, can induce rats to eventually drink a solution that is nearly 10–20% ethanol (Carillo et al., 2008; Czachowski et al., 2003; Files et al., 1995, 1996, 1997; Samson et al., 1999; Sharpe and Samson, 2003; Tolliver et al., 1988). These studies have provided evidence that the major regulation of alcohol intake in the rat using this procedure is the post-ingestional effect of alcohol (Samson et al., 2002). Taken together these findings suggest that temporary exposure to sucrose could have long-term consequences in the acquisition and maintenance of alcohol self-administration.
Studies have shown that sweet preference in most mammals is regulated by both environmental and genetic factors (Bachmanov et al., 2003; Kampov-Polevoy et al., 2004; Keskitalo et al., 2007; Sinclair et al., 1992, Stewart et al., 1994). In a series of studies, Avena and colleagues (Avena and Hoebel, 2003; Avena et al., 2004, 2008a, 2008b, 2008c, 2009) have demonstrated that rats with intermittent access to sucrose, but not with ad libitum access, became “dependent” on sugar, exhibiting somatic signs of spontaneous withdrawal when they are food deprived (Colantuoni et al., 2002). Rats given this treatment also show cross-sensitization with amphetamine after a week of abstinence (Avena and Hoebel, 2003) and further have been shown to consume greater amounts of ethanol at higher ethanol concentrations (Avena et al., 2004). These data suggest that establishing “dependence” on sucrose by using a limited access paradigm to sweet solutions can increase ethanol intake in rats and conversely rats given ethanol increase their intake of sucrose. A recent study has demonstrated that adolescent rats consume more ethanol but not more sucrose relative to adults (Maldonado et al., 2008). However, whether adolescent exposure to sucrose would increase ethanol intake in adult rats remains to be established.
The present report is part of a larger study characterizing the risks for and consequences of adolescent drinking in animal models and humans (Criado et al., 2008; Ehlers et al., 2006: Pian et al., 2008; Slawecki et al., 2001; Walker et al., 2008). The present experiments were designed to: (1) investigate whether: water, sucrose, sucrose-milk, and/or milk consumption during adolescence affected adult ethanol drinking as initiated by a sucrose fade-out procedure; and (2) subsequently, whether adolescent exposure to these four solutions also altered sucrose, sucrose-milk or milk consumption in adult rats during a single limited access test session.
2. MATERIALS AND METHODS
2.1. Animals
Postnatal 29 days (PND 29) male adolescent Wistar rats, purchased from Charles River, MA (n= 120; 77 ± 1 g (mean ± S.E.M)) were used in this study. Adolescence in these rats was defined as PND 23 to PND 55 (Spear, 2000). Rats were housed two/cage in standard plastic cages [25 (w) ×20 (h) × 45 cm (l)] during the experiment. For the duration of the experiment, a 12 h light/dark cycle (lights on at 6 am) was in effect and ad libitum food/water access was maintained. Rats were weighed in the morning 1 hour before each drinking session (9 am to 10 am) throughout experiment. Temperature of the colony room and experimental rooms were constantly maintained at 71° F. The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80–23, revised 1996) and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
2.2. Treatment of Non-Alcoholic Solutions during Adolescence
Adolescent (PND 29) rats were divided into four groups and assigned to four different solutions in a two-bottle choice limited access drinking paradigm. On each drinking day, each individual rat from each group was presented with one bottle of water and one bottle of the four assigned drinking solutions for 1-hour/day, 9 am to 10 am, Monday–Friday. The four solutions were: (1) water (W) (n=30); (2) sucrose (S) (n=29); (3) sucrose-milk (SM) (n=30); or, (4) milk (M) (n=30). Rats had limited access to these drinking solutions for 1/hr/day five days a week from 0900 to 1000 hrs until PND 51. Drinking sessions were performed in plastic cages (25cm (w) × 20cm (h) × 45cm (l)) and rats (two per cage) were separated with a transparent plastic divider. Prior to each session, two graduated cylinders (with water and a drinking solution) with stainless steel drinking tubes were mounted on each side of the cage divider. The position of the water versus the solution in the cage was alternated daily to prevent the development of a side preference. Bottles were weighed to the nearest 0.1 g before and after the 1-hour access period. The difference in weight indicated the amount of drinking solution consumed (weight in g is converted to volume in ml). The procedure used to mix each drinking solution was as followed: Sucrose (10% sucrose (w/v)), sucrose-milk (10% sucrose (w/v) + 50% (v/v) of Carnation evaporated milk from Nestle), and milk (54% (v/v) of Carnation evaporated milk from Nestle). Preference ratio was calculated by estimating the volume (ml) of sucrose and/or milk intake divided by the total volume (ml) of fluid intake. The number of calories consumed during the limited access period was also determined.
2.3. Sucrose Fade-Out Procedure
At PND 71 rats were trained to drink ethanol using a modified two-bottle sucrose substitution procedure (Samson, 1986). Sucrose fading sessions were carried out 5 days/week, 1-hour/day (9 am to 10 am, Monday–Friday). The following solutions in one of the bottles were then presented consecutively across sessions to initiate ethanol drinking: 10% sucrose (10S) for two sessions, 10% sucrose/2.5% ethanol (10S/2.5E) for one session, 10% sucrose/5% ethanol (10S/5E) for three sessions, 10% sucrose/10% ethanol (10S/10E) for four sessions, and 5% sucrose/10% ethanol (5S/10E) for five sessions. A 10% ethanol (10E) solution was then presented for 12 sessions. The second bottle contained only water. The first 6 sessions of 10E are categorized as epoch 1 and second 6 sessions are categorized as epoch 2. The position of the water versus the solution in the cage was alternated daily to prevent the development of a side preference. Bottles were weighed to the nearest 0.1 g before and after the 1-hour access period. All sucrose solutions were prepared weight/volume. All alcoholic solutions were prepared weight/volume from 95% ethanol. The amount of ethanol consumed was estimated by the grams of 95% ethanol consumed per kilogram of body weight (g/kg).
2.4. Test of Post-Adolescent Consumption of Non-Alcoholic Solutions in Adulthood
Consumption of sucrose, sucrose-milk and milk, as compared to water, was tested subsequent to the sucrose fade-out procedure at PND 105 using the two-bottle free-choice regimen with limited access, as previously described. Each group of rats was divided into three subgroups and all groups were exposed to sucrose, sucrose-milk, and milk. Rats were exposed to each solution for three consecutive days over a nine-day period in order to examine the preference ratio and volume of intake for the solutions. The order of presentation for the solutions was counterbalanced within the groups and across days such that on any given day, an equivalent number of animals from each of the original four groups (i.e., W, S, SM, and M) were exposed to the three solutions over the nine-day period. Bottles were weighed to the nearest 0.1 g before and after the 1-hour access period. The difference in weight indicated the amount of drinking solution consumed (weight in g is converted to volume in ml). Preference ratio was calculated by estimating the volume (ml) of sucrose and/or milk intake divided by the total volume (ml) of fluid intake.
2.5. Statistical Analyses
Data analyses were focused on the specific aims which were to: (1) investigate whether: water, sucrose, sucrose-milk, and/or milk consumption during adolescence affected adult ethanol drinking, as initiated by a sucrose fade-out procedure and (2) subsequently, whether adolescent exposure to these four solutions also altered sucrose, sucrose-milk or milk consumption in adult rats during a single limited access test session.
To investigate whether water, sucrose, sucrose-milk, and/or milk consumption during adolescence affected adult ethanol drinking, as initiated by a sucrose fade-out procedure, first the averaged intake of solutions and preference ratio of the solutions consumed in the two-bottle choice paradigm during adolescence was calculated by estimating the volume (ml) of the solution in one bottle for the four groups (e.g. water, sucrose, sucrose-milk and/or milk intake) divided by the total volume (ml) of both the solution bottle and the water bottle for all the drinking sessions during adolescence. The data were analyzed by one way ANOVA with repeated measures, with post hoc testing (Fisher’s Least Significance Difference test). Volumes of solutions of sucrose, sucrose-ethanol, and ethanol consumed by the S, SM, and M groups as compared to the W group were compared during the sucrose fade-out procedure using one-way ANOVA with repeated measures, with post hoc testing (Fisher’s Least Significance Difference test). Evaluation of differences in 10% ethanol consumption for the two different drinking epochs in the four adolescent drinking groups (W, S, SM and M) were compared using a two-way ANOVA with post hoc testing (Fisher’s Least Significance Difference test). Pearson’s linear correlation was used to determine the relationship between adolescent caloric (calories) and total fluid (ml) intakes with adult ethanol intakes (g/kg).
To test whether adolescent exposure to these four solutions during adolescence also altered sucrose, sucrose-milk or milk consumption in adult rats during a single limited access test session, the averaged intake of 3-day drinking sessions and preference ratio of the solutions consumed in the two-bottle choice paradigm was calculated by estimating the volume (ml) of the solution in one bottle for the four groups (e.g. water, sucrose, sucrose-milk and/or milk intake) divided by the total volume of both the solution bottle and the water bottle for the single drinking session and the data were analyzed by two way ANOVA (group × solution) with post hoc testing (Fisher’s Least Significance Difference test).
3. RESULTS
3.1. Consumption of Sucrose, Sucrose-Milk and Milk during Adolescence
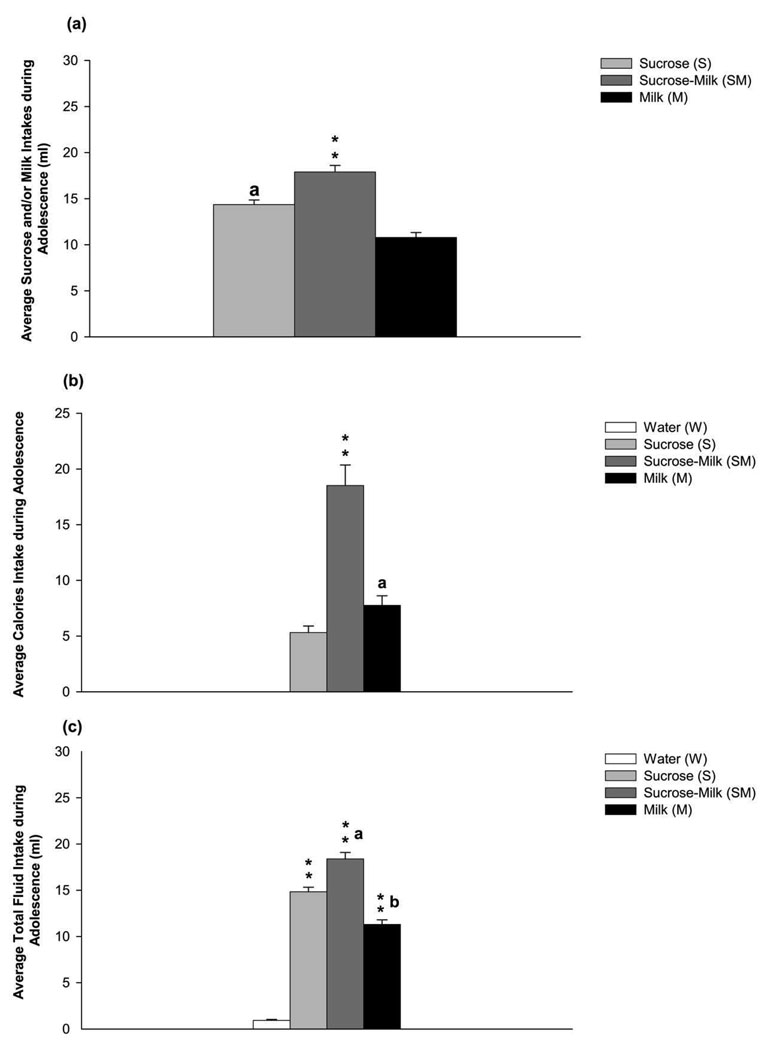
Analysis of the average solution intake (ml) for the three solutions (sucrose, sucrose-milk and milk) consumed during the 1-hr limited access period during adolescence showed significant differences among groups [F(2,89) = 36.06, p < 0.001; Figure 1a]. Post hoc assessment revealed that SM group of rats consumed significantly more solution than S and M groups (p < 0.01). In addition, post hoc assessment also indicated that S group of rats consumed more solution than M group (p < 0.01). Analyses of the preference ratio during the 1-hr limited access period indicated significant differences among the groups of rats exposed to sucrose, sucrose-milk or milk [F(2,89) = 9.71, p < 0.001]. Post hoc analyses revealed that the preference ratios of S [0.97 ± 0.03] and SM [0.97 ± 0.03] groups were significantly higher than the ratio of M group [0.95 ± 0.03]. Average calories and total fluid intake during the 1-hr limited access sessions were also calculated and are shown in Figures 1 b–c. These results showed that the caloric intake of SM group was significantly higher than S or M group [F(2,89) = 202.6, p < 0.001] (see Figure 1b). Similarly, the total fluid intake of SM group was higher than S or M groups [F(3,119) = 219.8, p < 0.001] (see Figure 1c). In contrast, W group during the 1-hr limited access sessions consumed only small amount of fluid (0.9 ± 0.1 ml average per session) with no caloric intake (Figures 1b–c). No significant differences in body weight measured on the last day of the drinking session (PND 51) were observed among treatment groups [F(3,119) = 0.40, NS].
Figure 1.
(a) The group of adolescent rats that had limited exposed to sucrose-milk (SM) consumed higher averaged volume (ml) of solution than adolescent rats in the S or M group. Data are the mean ± SEM; “a “indicates significance different from M group. ** p< 0.01 indicates significance from S and M groups. (b) The group of adolescent rats that had limited exposed to sucrose-milk (SM) consumed higher averaged calories than adolescent rats in the S or M group. Data are the mean ± SEM; “a “indicates significance different from S group. ** p< 0.01 indicates significance from S and M groups. (c) The groups of adolescent rats that had limited exposed to sucrose (S), sucrose-milk (SM) and milk (M) consumed higher averaged volumes (ml) of solutions than adolescent rats in the W group. Data are the mean ± SEM; “a “indicates significance different from S and M groups. “b “indicates significance different from S group. ** p< 0.01 indicates significance from W group.
3.2. Effects of Adolescent Consumption of Sucrose, Sucrose-Milk, Milk on Ethanol Intake during the Sucrose Fade-Out
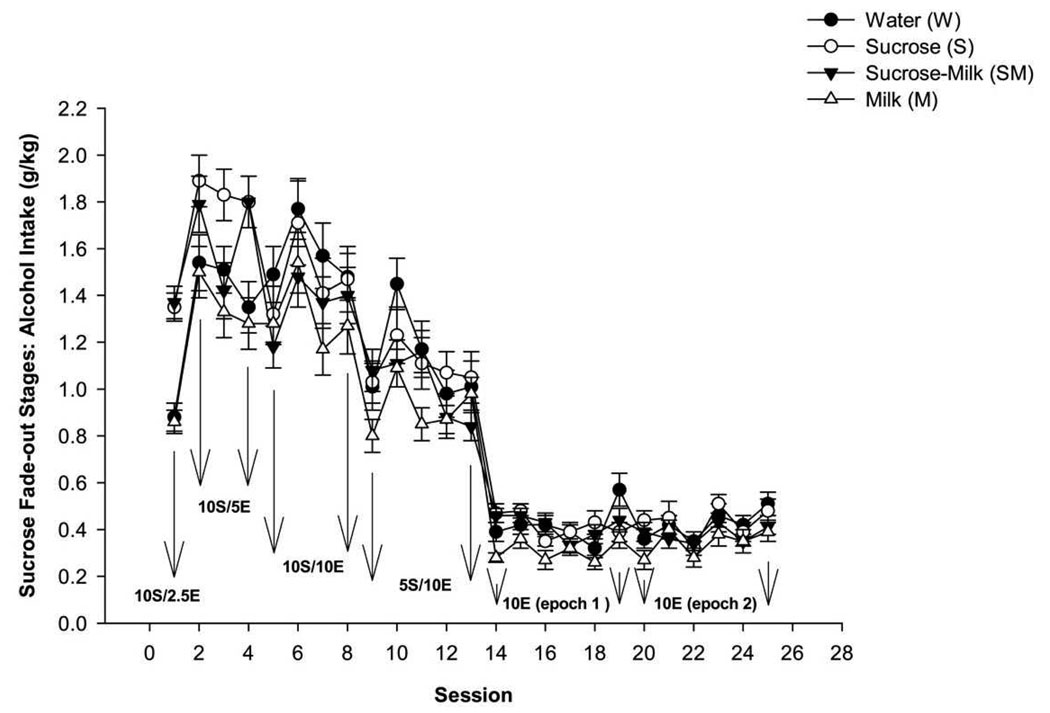
Figure 2 graphically presents the ethanol intake for each day the animals were evaluated during the sucrose fade-out and during the 10% E drinking sessions for the four treatment groups.
Figure 2.
Mean ethanol intake (g/kg) for each individual session across the 25 days of the sucrose fade-out. Data points represent means; bar represent standard errors.
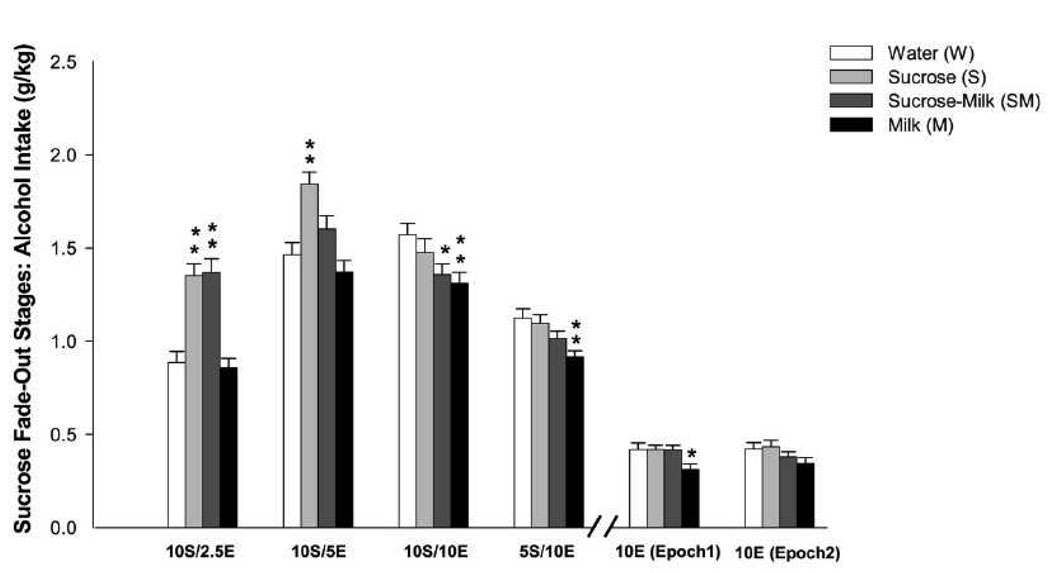
One way ANOVA with post-hoc analysis of the averaged alcohol intake during the sucrose fade out sessions revealed that the solution drank during adolescence was associated with altered ethanol intake. Ethanol intake was increased in the 10S/2.5E condition in the S and SM groups as compared to W group [F(3,118) = 20.38, p < 0.01; Figure 3]. Analysis of averaged ethanol intake during the 10S/5E 1-hr sessions also showed significant differences among groups [F(3,356) = 9.79, p < 0.01; Figure 3]. Post hoc analysis showed that only S group exhibited higher ethanol intake than W group (p < 0.01).
Figure 3.
Mean ethanol intake (g/kg) during different stages of the sucrose fade-out: 10% sucrose + 2.5% ethanol (10S2.5E); 10% sucrose + 5% ethanol (10S5E); 10% sucrose + 10% ethanol (10S10E); 5% sucrose + 10% ethanol (5S10E). Data are the mean ± SEM; * p< 0.05 indicates significant different from W group. ** p< 0.01 indicates significant different from W group. 10% ethanol (10E) (Epochs 1 and 2). Data are the mean ± SEM; * p< 0.05 indicates significant different from rest of experimental groups.
Averaged ethanol intake during the 10S/10E sessions was also significantly different among groups [F(3,475) = 3.54, p < 0.05]. However, post hoc analysis revealed that SM (p < 0.05) and M (p < 0.01) groups exhibited significantly lower ethanol intake than W group. Analysis of averaged ethanol intake during the 5S/10E drinking sessions also revealed significant differences among groups [F(3,591) = 4.85, p < 0.01; Figure 3]. Post hoc assessment showed that M group showed significantly lower ethanol intake than W group (p < 0.01).
Pearson’s linear analysis was used to determine the relationship between adolescent caloric intakes (calories) and adult ethanol intakes (g/kg). These findings suggested a significant correlation between adolescent caloric intake and adult ethanol intake during the 10S/2.5E session (p < 0.01). However, no correlation was observed between adolescent caloric intake and adult ethanol intake for any of the other sessions. The relationship between adolescent total fluid intake (ml) and adult ethanol intake (g/kg) was also tested. Results suggested a significant correlation between adolescent total fluid intake and adult ethanol intake during the 10S/2.5E and 10S/5E sessions (p < 0.01). However, no correlation was observed between adolescent total fluid intake and adult ethanol intake during any other sessions.
3.2.1. Adolescent Exposure to Milk Reduces Ethanol Intake in Adult Rats
Analysis of the averaged ethanol intake during the first 6 sessions of access to 10% ethanol alone (epoch 1) revealed significant differences among treatment groups [F(3,118) = 3.33, p < 0.05; Fig. 3]. Post hoc analysis showed that M group consumed significant less ethanol than W, S and SM groups (p < 0.05). Analysis of the averaged ethanol intake during the second 6 sessions of access to 10% E (epoch 2) revealed no significant differences among treatment groups [F(3,118) = 1.67, NS; Fig. 3]. However, analysis of ethanol intake during the 12 sessions of access to 10%E (combined averaged of epochs 1 and 2) showed no significant differences among treatment groups [F(3,118) = 2.47, NS; data not shown].
3.3. Effects of Adolescent Exposure of Sucrose, Sucrose-Milk or Milk on Consumption of Three Solutions in Adult Rats
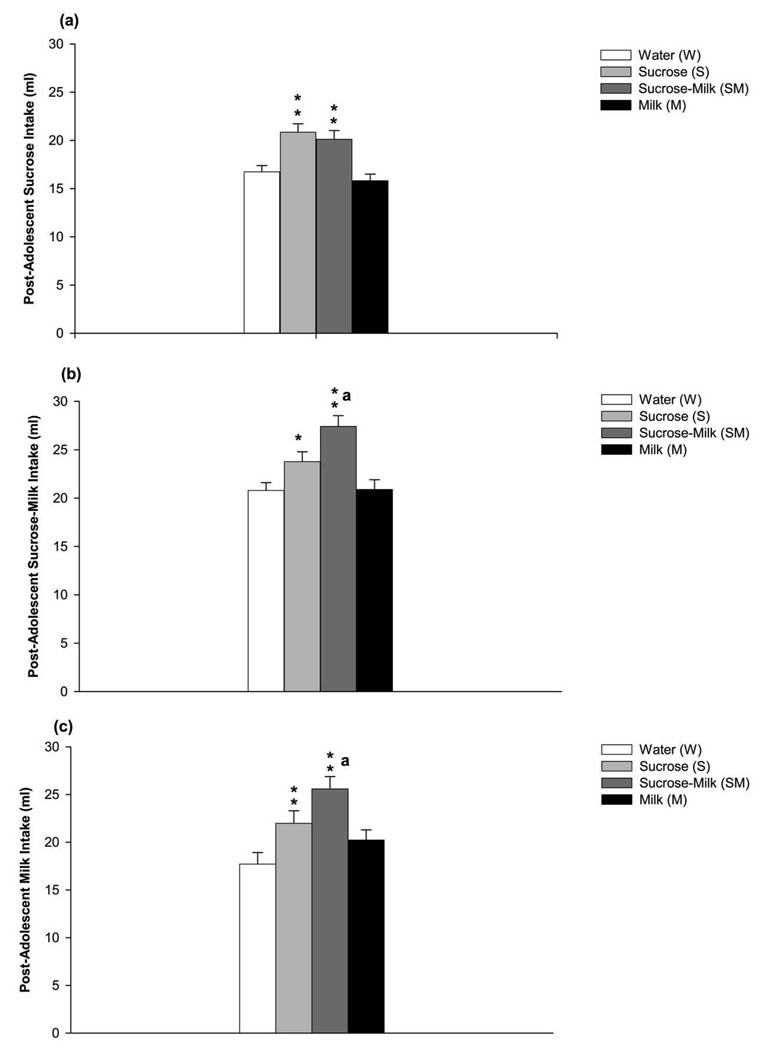
Two way (group × solution) ANOVA of the sucrose intake in adult rats after adolescent exposure to water, sucrose, sucrose-milk or milk indicated a main effect of group on post-adolescent sucrose consumption over a 3-day period [F(3,114) = 13.40, p < 0.01]. Post hoc one way ANOVA revealed that S or SM groups consumed significantly more sucrose than M or W groups [F(3,118) = 13.75, p < 0.01; Figure 4a]. Analysis of sucrose-milk intake in adult rats also showed a main effect of group on post-adolescent sucrose-milk over a 3-day period [F(3,112) = 14.26, p < 0.01]. Post hoc one way ANOVA demonstrated that S and SM groups consumed significantly more sucrose-milk than the M and W groups [F(3,118) = 11.15, p < 0.01; Figure 4b]. Analysis of adult milk intake showed a main effect of group on post-adolescent milk consumption over a 3-day period [F(3,112) = 14.26, p < 0.01]. Post hoc one way ANOVA showed that S and SM groups consumed more milk than the W group [F(3,118) = 8.45, p < 0.01; Figure 4c]. However, adult consumption of milk was not different between S and M groups. Results also indicated that SM group consumed more milk than M group [F(3,118) = 8.45, p < 0.01; Figure 4c].
Figure 4.
(a) Post-adolescent consumption of sucrose (ml) during a 3-day drinking session. Rats in the S and SM groups consumed more sucrose than M and W groups. Data are the mean ± SEM; ** p< 0.01 indicates significance from W and M groups. (b) Post-adolescent consumption of sucrose-milk (ml) during a 3-day drinking session. Rats in the S and SM groups consumed more sucrose-milk than M and W groups. Data are the mean ± SEM; “a” indicates significance different from S group. ** p< 0.01 and * p< 0.05 indicate significance from W and M groups. (c) Post-adolescent consumption of milk (ml) during a 3-day drinking session. Rats in the S and SM groups consumed more milk than W group. Data are the mean ± SEM; “a” indicates significance different from S and M groups. ** p< 0.01 indicates significance from W group.
Preference ratios were determined for adult rats exposed to the three different solutions. Analysis of the preference ratio during adult sucrose exposure indicated significant differences among groups [F(3,118) = 2.90, p < 0.05]. Post hoc analysis demonstrated that the preference ratio for sucrose consumption in M group was lower than the ratio in S and SM groups (p < 0.05; Data not shown).
Analyses of the preference ratio during adult sucrose-milk exposure indicated no significant differences among groups [F(3,118) = 1.69, NS]. Statistical analysis of the preference ratio during adult milk exposure also indicated no significant differences among groups [F(3,118) = 2.01, NS]. No significant differences in body weight measured on the last day of post-adolescence exposure to the three solutions (PND 114) were observed among treatment groups [F(3,118) = 0.53, NS].
4. DISCUSSION
Studies of food likes and dislikes in humans have clearly demonstrated that prior exposure to a particular flavor enhances the “liking” for that flavor and that this “liking” may generalize to other flavors particularly those associated with calories and sweet tastes (Crandall, 1985; Pliner,1982; Yeomans et al., 2006). In animal studies, sucrose feeding at weaning has been found to increase the preference for sucrose in adolescence (Sato et al., 1991). Data from the present study extend those findings by exploring the effect of limited access drinking of sucrose and milk during adolescence on the consumption of those solutions in adulthood. As predicted, adolescent exposure to sucrose and sucrose-milk solutions was found to increase sucrose and sucrose-milk consumption in adult rats.
In contrast, in the present study, limited access to milk during adolescence had no effect on adult consumption of sweet solutions or milk, as compared to rats that only had access to water during adolescence. How milk may influence ingestion of other solutions in the environment is not entirely known. One study reported that inhibition of rostral opioid receptors by naloxone increased the time of oral grasp response, decreased total time on the nipple, and eliminated ingestion of milk from an artificial surrogate nipple (Petrov et al., 1998). In contrast, naloxone had no effect on the ingestion of water (Petrov et al., 1998). Moreover, another study found that milk consumption is positively correlated with DA function in the nucleus accumbens in rats (Richardson and Gratton, 1996). These studies demonstrated that milk consumption can activate reward systems, and this is consistent with our present findings demonstrating that adolescent rats prefer milk as compared to water during limited access. However, the present study’s results did not provide evidence to suggest that exposure to limited access milk in adolescence modifies milk drinking in adults.
The effect of early exposure to a solution enhancing consumption of that solution later in life has also been shown in the case of exposure to ethanol. In one study fetal or infantile exposure to ethanol in rats was found to promote ethanol ingestion in adolescence and adulthood (see Spear and Molina, 2005). In another study, ethanol exposure during the periadolescent period in rats was found to lessen conditioned aversion to ethanol in adulthood (Diaz-Granados and Graham, 2007). What is not clear is whether exposure to specific fats, sugars, or special flavors during adolescence can enhance other solutions, such as ethanol intake, in adulthood.
The present study explored the effect of limited access to milk, sucrose-milk and sucrose exposure during adolescence on ethanol drinking in adults. The results of the present study demonstrate that adolescent rats that had limited access sucrose solutions consumed significantly more of an ethanol/sugar solution (10% sugar and 2.5 or 5% ethanol concentrations) than control rats as adults. These data are consistent with human studies that have found that drinks with chocolate milk and sugary/ fruity tastes that had between 5% and 9% ethanol had the highest acceptability, especially among children and adolescents (Copeland et al., 2007). Thus these data suggest that exposure to sucrose during adolescence may increase the tolerability and/or enhance the reinforcing properties of drinking low concentrations of ethanol combined with sucrose as an adult.
Unexpectedly, our results indicated that adolescent rats exposed to milk consumed significantly less ethanol as adults than rats exposed to water during the last three stages of the sucrose fading procedure (10S/10E, 5S/10E, and 10E). These results suggest that reduced ethanol intake in adulthood may not be due to a “hedonic effect” of palatable solutions during adolescence, as only the rats exposed to milk showed changes in ethanol-alone intake in adulthood. If the observed reduced ethanol intake in rats exposed to milk is due to a hedonic effect, then the other two groups of rats exposed to sweet solutions should have also shown a reduction in ethanol intake. It is possible that milk may possess a specific property that alters the taste or “mouth feel” of ethanol following adolescent exposure to milk.
The perception of flavors in milk is one of the mammal’s earliest sensory experiences and milk flavor has an effect on later food acceptance (Sullivan and Birch, 1994). However, it is still unknown why rats exposed to milk, but not sucrose-milk, during adolescence showed a reduction in ethanol intake. Milk contains substantial macronutrients such as fatty acids and proteins, which provide a considerable quantity of calories (Berkey et al., 2005). It is possible that adolescent caloric and fluid intake played an important role in reducing adult ethanol intake in the present study. However if that was true then adolescent rats exposed to sucrose-milk should have also shown higher ethanol intake during the 10S5E drinking sessions, which was not the case. Therefore the present results suggest that adult ethanol intake does not solely reflect a history of higher caloric intake. Another possible explanation is that the macronutrients in milk (e.g. fats and/or amino acids) could produce long-term direct effects on systems regulating ethanol preference. Some studies have shown that maternal nutrition can modify the neural systems that regulate appetite in the brain of the offspring (Breier et al., 2001; McMillen et al., 2005; Muhlhauser et al., 2006), perhaps such systems may also impact ethanol intake.
Milk and sucrose-milk may differentially activate overlapping neural pathways that regulate feeding and reward (e.g., opioid and DA) (Olszewski and Levine, 2007) as well as taste preferences. There is ample evidence to suggest that the reinforcing properties of both alcohol and sweet substances may be mediated by overlapping neurotransmitter systems such as DA and endogenous opioids (Avena et al., 2004, 2006; Eiler et al., 2003; Froehlich and Li, 1994; Hodge et al., 1994; June et al., 2004; Kelley et al., 2002; Wise, 1998; Wise and Rompre, 1989). Human studies have also shown that opioid antagonist can change the taste of sweet substances (Bertino et al., 1991; Drewnowski et al., 1992; Fantino et al., 1986).
Orexigenic neuropeptides such as neuropeptide Y and Galanin could also be impacted by early dietary experiences. Galanin has been shown to stimulate the consumption of high-fat foods as well as increase the intake of ethanol (Barton et al., 1995; Leibowitz and Kim, 1992; Lewis et al., 2004; Rada et al., 2004; Tempel et al., 1988). The consumption of fat or ethanol can, alternatively, increase the expression of galanin in the hypothalamus (Akabayashi et al., 1994; Chang et al., 2004). Studies have shown that the molecular function of galanin receptors is age dependent (Planas et al., 1994a, 1994b; Rossmanith et al., 1994;). Therefore, it is possible that galanin’s role in reducing or stimulating ethanol intake could also be age-dependent. Further studies aimed at more directly evaluating the mechanisms underlying the effects of early dietary experiences on ethanol intake will be necessary to isolate the neural systems responsible fort this phenomena.
The present study is the first report to show a link between sugar and milk consumption in adolescence and subsequent consumption of ethanol. Limited access exposure to sugar appears to enhance ethanol consumption at low doses when it is combined with sucrose whereas milk consumption reduces intake of ethanol alone. Studies characterizing the mechanisms underlying these phenomena may also provide another approach to the early prevention of alcoholism.
Acknowledgements
This study was supported in part by National Institute on Alcoholism and Alcohol Abuse grants awarded to CLE, AA006059 andAA014339. Jerry P. Pian is supported by NIAAA training grant 5T32 AA007456. The authors thank Greta Berg and Derek Wills for their assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A. 1994;91:10375–10379. doi: 10.1073/pnas.91.22.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Avena NM, Carrillo CA, Needham L, Leibowitz SF, Hoebel BG. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol. 2004;34:203–209. doi: 10.1016/j.alcohol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008a;94:309–315. doi: 10.1016/j.physbeh.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008b;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008c;156:865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, et al. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C, Lin L, York DA, Bray GA. Differential effects of enterostatin, galanin and opioids on high-fat diet consumption. Brain Res. 1995;702:55–60. doi: 10.1016/0006-8993(95)00966-8. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159:543–550. doi: 10.1001/archpedi.159.6.543. [DOI] [PubMed] [Google Scholar]
- Bertino M, Beauchamp GK, Engelman K. Naltrexone, an opioid blocker, alters taste perception and nutrient intake in humans. Am J Physiol. 1991;261:R59–R63. doi: 10.1152/ajpregu.1991.261.1.R59. [DOI] [PubMed] [Google Scholar]
- Blizard DA. Analyzing phenotypic correlations in studies with selected lines. Behav Genet. 1992;22:29–33. doi: 10.1007/BF01066790. [DOI] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res. 2000;24:253–258. [PubMed] [Google Scholar]
- Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WP. Fetal programming of appetite and obesity. Mol Cell Endocrinol. 2001;185:73–79. doi: 10.1016/s0303-7207(01)00634-7. [DOI] [PubMed] [Google Scholar]
- Carrillo J, Howard EC, Moten M, Houck BD, Czachowski CL, Gonzales RA. A 3-day exposure to 10% ethanol with 10% sucrose successfully initiates ethanol self-administration. Alcohol. 2008;42:171–178. doi: 10.1016/j.alcohol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Copeland J, Stevenson RJ, Gates P, Dillon P. Young Australians and alcohol: the acceptabllity of ready-to-drink (RTD) alcoholic beverages among 12–30-year-olds. Addiction. 2007;102:1740–1746. doi: 10.1111/j.1360-0443.2007.01970.x. [DOI] [PubMed] [Google Scholar]
- Crandall CS. The liking of foods as a result of exposure: eating donuts in Alaska. J Soc Psychol. 1985;125:187–194. [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of Dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcohol Clin Exp Res. 2002;26:1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiol Behav. 2003;78:51–59. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav. 1992;51:371–379. doi: 10.1016/0031-9384(92)90155-u. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, Seyoum R, Foster KL, Mailey C, June HL. D1 dopamine receptor regulates alcohol-motivated behaviors in the bed nucleus of the stria terminalis in alcohol-preferring (P) rats. Synapse. 2003;48:45–56. doi: 10.1002/syn.10181. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, Woods JE, Masters J, McKay PF, Hardy L, III, Goergen JJ, et al. Brain stimulation reward performance and sucrose maintained behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res. 2005;29:571–583. doi: 10.1097/01.alc.0000158934.50534.b7. [DOI] [PubMed] [Google Scholar]
- Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–R96. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Brice GT. Sucrose, ethanol, and sucrose/ethanol reinforced responding under variable-interval schedules of reinforcement. Alcohol Clin Exp Res. 1995;19:1271–1278. doi: 10.1111/j.1530-0277.1995.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Brice GT, Deitrich RA, Draski LJ. Initiation of ethanol self-administration by the sucrose-substitution method with HAS and LAS rats. Alcohol Clin Exp Res. 1996;20:677–681. doi: 10.1111/j.1530-0277.1996.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Files FJ, Denning CE, Hyytia P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcohol Clin Exp Res. 1997;21:749–753. [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among U.S. adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Li TK. Opioid involvement in alcohol drinking. Ann N Y Acad Sci. 1994;739:156–167. doi: 10.1111/j.1749-6632.1994.tb19817.x. [DOI] [PubMed] [Google Scholar]
- Fromme K, Samson HH. A survey analysis of first intoxication experiences. J Stud Alcohol. 1983;44:905–910. doi: 10.15288/jsa.1983.44.905. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M, et al. Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol Alcohol. 2009;44:293–300. doi: 10.1093/alcalc/agn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Samson HH. Induction and maintenance of ethanol self-administration without food deprivation in the rat. Psychopharmacology (Berl) 1985;86:475–479. doi: 10.1007/BF00427912. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Tolliver GA, Haraguchi M. Effects of intraaccumbens injections of dopamine agonists and antagonists on sucrose and sucrose-ethanol reinforced responding. Pharmacol Biochem Behav. 1994;48:141–150. doi: 10.1016/0091-3057(94)90510-x. [DOI] [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ, Foster KL, McKay PF, Seyoum R, et al. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharm. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry. 1997;154:269–270. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Overstreet DH, Rezvani AH, Viglinskaya IV, Badistov BA, et al. Pain sensitivity and saccharin intake in alcohol-preferring and - nonpreferring rat strains. Physiol Behav. 1996;59:683–688. doi: 10.1016/0031-9384(95)02110-8. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Khalitov E. Family history of alcoholism and response to sweets. Alcohol Clin Exp Res. 2003;27:1743–1749. doi: 10.1097/01.ALC.0000093739.05809.DD. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Eick C, Boland G, Khalitov E, Crews FT. Sweet liking, novelty seeking, and gender predict alcoholic status. Alcohol Clin Exp Res. 2004;28:1291–1298. doi: 10.1097/01.alc.0000137808.69482.75. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Keskitalo K, Knaapila A, Kallela M, Palotie A, Wessman M, Sammalisto S, et al. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr. 2007;86:55–63. doi: 10.1093/ajcn/86.1.55. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Kim T. Impact of a galanin antagonist on exogenous galanin and natural patterns of fat ingestion. Brain Res. 1992;599:148–152. doi: 10.1016/0006-8993(92)90863-5. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Johnson DF, Waldman D, Leibowitz SF, Hoebel BG. Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res. 2004;28:1822–1828. doi: 10.1097/01.alc.0000148099.12344.c8. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Alipour KK, Kirstein CL. Voluntary ethanol consumption differs in adolescent and adult male rats using a modified sucrose-fading paradigm. Alcohol Clin Exp Res. 2008;32:1574–1582. doi: 10.1111/j.1530-0277.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- Margulies RZ, Kessler RC, Kandel DB. A longitudinal study of onset of drinking among high-school students. J Stud Alcohol. 1977;38:897–912. doi: 10.15288/jsa.1977.38.897. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Adam CL, Muhlhausler BS. Early origins of obesity: programming the appetite regulatory system. J Physiol. 2005;565:9–17. doi: 10.1113/jphysiol.2004.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006;20:1257–1259. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among U.S. children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Levine AS. Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav. 2007;91:506–512. doi: 10.1016/j.physbeh.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res. 1993;17:366–369. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. Endogenous opioids and the first suckling episode in the rat. Dev Psychobiol. 1998;33:175–183. [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA. Activation of galanin pathways across puberty in the male rat: assessment of regional densities of galanin binding sites. Neuroscience. 1994a;63:859–867. doi: 10.1016/0306-4522(94)90530-4. [DOI] [PubMed] [Google Scholar]
- Planas B, Kolb PE, Raskind MA, Miller MA. Activation of galanin pathways across puberty in the male rat: galanin gene expression in the bed nucleus of the stria terminalis and medial amygdala. Neuroscience. 1994b;63:851–858. doi: 10.1016/0306-4522(94)90529-0. [DOI] [PubMed] [Google Scholar]
- Pliner P. The effects of mere exposure on liking for edible substances. Appetite. 1982;3:283–290. doi: 10.1016/s0195-6663(82)80026-3. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Gratton A. Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study in rat. J Neurosci. 1996;16:8160–8169. doi: 10.1523/JNEUROSCI.16-24-08160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers DA, McClearn GE. Sucrose versus ethanol appetite in inbred strains of mice. Q J Stud Alcohol. 1964;25:26–35. [PubMed] [Google Scholar]
- Rossmanith WG, Marks DL, Clifton DK, Steiner RA. Induction of galanin gene expression in gonadotropin-releasing hormone neurons with puberty in the rat. Endocrinology. 1994;135:1401–1408. doi: 10.1210/endo.135.4.7523097. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C. Chronic ethanol self-administration in a continuous-access operant situation: the use of a sucrose/ethanol solution to increase daily ethanol intake. Alcohol. 1999;19:151–155. doi: 10.1016/s0741-8329(99)00032-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Legg B. Effects of self-administered alcohol or sucrose preloads on subsequent consumption in the rat. J Stud Alcohol. 2002;63:107–113. [PubMed] [Google Scholar]
- Sato N, Shimizu H, Shimomura Y, Uehara Y, Takahashi M, Negishi M. Sucrose feeding at weaning alters the preference for sucrose in adolescence. Exp Clin Endocrinol. 1991;98:201–206. doi: 10.1055/s-0029-1211118. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Samson HH. Ethanol and sucrose self-administration components: effects of drinking history. Alcohol. 2003;29:31–38. doi: 10.1016/s0741-8329(02)00318-x. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol. 1992;9:155–160. doi: 10.1016/0741-8329(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Sullivan SA, Birch LL. Infant dietary experience and acceptance of solid foods. Pediatrics. 1994;93:271–277. [PubMed] [Google Scholar]
- Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–314. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH. Ethanol preference following the sucrose-fading initiation procedure. Alcohol. 1988;5:9–13. doi: 10.1016/0741-8329(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Mobini S, Elliman TD, Walker HC, Stevenson RJ. Hedonic and sensory characteristics of odors conditioned by pairing with tastants in humans. J Exp Psychol Anim Behav Process. 2006;32:215–228. doi: 10.1037/0097-7403.32.3.215. [DOI] [PubMed] [Google Scholar]
- Yung L, Gordis E, Holt J. Dietary choices and likelihood of abstinence among alcoholic patients in an outpatient clinic. Drug Alcohol Depend. 1983;12:355–362. doi: 10.1016/0376-8716(83)90007-8. [DOI] [PubMed] [Google Scholar]