Abstract
Background
Bacterial vaginosis is a very common vaginal infection. The lack of endogenous lactobacilli and overgrowth of pathogens facilitate numerous gynecological complications.
Methods
A phase I dose-ranging safety trial tested the safety, tolerability and acceptability of Lactobacillus crispatus CTV-05 (LACTIN-V) administered by vaginal applicator. Twelve healthy volunteers were enrolled in three blocks of four (5 × 108, 1 × 109 and 2 × 109 cfu/dose). Each block was randomized in a 3:1 ratio of active product to placebo. Participants used study product for 5 consecutive days, returned for follow up on Days 7 and 14, and had phone interviews on Days 2 and 35.
Results
All 12 participants took 5 doses and completed study follow-up.
Overall, 45 adverse events (AEs) occurred, of which 31 (69%) were genitourinary (GU) AEs. GU AEs appeared evenly distributed between the three treatment blocks and between LACTIN-V and placebo arms. The most common GU AEs were vaginal discharge in 5 subjects (42%), abdominal pain in 4 subjects (33%), metrorrhagia in 4 subjects (33%), vulvovaginitis in 4 subjects (33%), vaginal candidiasis in 3 subjects (25%), and vaginal odor in 3 subjects (25%). Forty one (91%) AEs were mild (grade 1) in severity. All four moderate AEs (grade 2) were unrelated to product use. No grade 3 or 4 AEs or serious adverse events (SAE) occurred. Laboratory parameters and colposcopy findings were within normal limits or clinically insignificant. The product was well tolerated and accepted.
Conclusion
All three dose levels of LACTIN-V appeared to be safe and acceptable in healthy volunteers.
Keywords: Probiotic, Lactobacillus crispatus, bacterial vaginosis, safety, clinical trial phase 1
INTRODUCTION
Bacterial vaginosis (BV), characterized by an imbalanced vaginal flora deficient in naturally occurring hydrogen peroxide-producing lactobacilli is one of the most frequent vaginal infections and affects about 15 – 50% of reproductive aged women globally. 1 2 3 4 5 While symptoms of BV consist of a thin, homogeneous discharge and a strong vaginal odor, most women with BV are unaware of their condition. However, BV has been associated with significant gynecologic and obstetric complications including pelvic inflammatory disease 6 7, endometritis 8, and post-operative infections including post-partum endometritis.9 10 Strong associations have also been reported between BV and pre-term delivery, miscarriage 11 12, and amniotic fluid infections.13 14 Recent studies have also linked BV with increased susceptibility to HIV infection.4 15 16 17 Furthermore, 20 - 30% of women with BV relapse within 1-3 months following standard antibiotic treatment.18 19 These sequelae clearly justify investigational studies of agents like probiotics 20 that may be effective in the improved treatment and/or prevention of BV.
Reconstituting a normal Lactobacillus-predominant vaginal flora has been promoted for many years to provide a microbial defense against genital colonization by pathogens. LACTIN-V developed by Osel, Inc. (Santa Clara, CA) contains a naturally occurring human vaginal strain of L. crispatus CTV-05. This product is designed to replenish the vaginal lactobacilli population in women with BV following conventional antibiotic treatment with metronidazole gel (0.75%). Previous formulations of LACTIN-V at lower concentrations administered in a gelatin capsule appeared to be safe in clinical trials when tested for prevention of BV and recurrent urinary tract infection.21 However, the gelatin capsules dissolved slowly in the vagina, probably impeding vaginal colonization by L. crispatus CTV-05 which reached 40% and was below expectation (Osel Inc. Unpublished data. 2006). Consequently, Osel Inc. increased the product dose and designed a novel applicator to facilitate delivery of the powder formulation directly into the vagina in an attempt to improve vaginal colonization of the product. The objective of this phase 1 trial was to assess the safety, tolerability and acceptability of three different doses of this new LACTIN-V formulation using the new vaginal delivery device in healthy volunteers.
MATERIALS AND METHODS
Design
This was a Phase 1, placebo-controlled, dose-ranging, randomized, double blind study in sexually-abstinent women conducted at the Clinical Translational Science Institute (CTSI) Clinical Research Center at the University of California, San Francisco (UCSF), USA. The study protocol and informed consent form were approved by the Committee on Human Research at UCSF (# 43476-30970-02A). Safety oversight was provided by a Safety Monitor. This trial is registered at www.ClinicalTrials.gov (NCT00537576). The trial was conducted from December 2007 through April 2008.
Objectives
The primary objective of this study was to assess the safety, tolerability and acceptability of vaginal applicators prefilled with three doses (5 × 108, 1 × 109 and 2 × 109 colony forming units (cfu)/dose) of L. crispatus CTV-05 versus placebo when applied vaginally once daily for 5 consecutive days.
Outcomes
Safety was assessed by comparing the incidence of adverse events (AE), including genitourinary (GU) clinical signs and symptoms, colposcopic findings of the genital tract, renal and liver function among participants randomized to LACTIN-V and those randomized to placebo. The DAIDS Toxicity Table Addendum for Vaginal Microbicide Studies (November 2007) was used to grade GU abnormalities 22, the definition of colposcopic findings was based on the 2004 WHO/CONRAD colposcopy manual 23, and the DAIDS Adult Toxicity Table (December 2004) 24 was used to grade serum chemistry, hematology, and other abnormalities. Clinical signs and symptoms were evaluated at enrollment, and 2, 7, 14 and 35 days following initiation of product use; vaginal microflora was assessed at enrollment and 7 and 14 days following initiation of product use, and blood samples for serum chemistry and hematology were collected at enrollment and 14 days following initiation of product use. Tolerability was evaluated by comparing the number of participants in the LACTIN-V and placebo arms who prematurely discontinued themselves from product use due to an AE. Acceptability was assessed using a self-administered questionnaire.
Participants
Volunteers were recruited through advertising in health centers, educational institutions, community bulletin boards and online forums. They were eligible if they were 18-40 years of age and in good health, had regular menstrual cycles of 21-35 days in length, were sexually experienced and willing to abstain from the use of other vaginal products, as well as willing to be sexually abstinent 72 hours prior to enrollment and until the last clinical visit on Day 14. Volunteers who met any of the following criteria were not enrolled: pregnant or within 2 months after pregnancy, currently breastfeeding, clinically detectable genital epithelial disruption; urinary tract infection (UTI); HIV antibodies; history of recurrent genital herpes; syphilis; vaginal candidiasis; symptomatic bacterial vaginosis (BV) as diagnosed per Nugent score ≥ 7 at screening visit; Trichomonas vaginalis; Neisseria gonorrhoeae, or Chlamydia trachomatis at screening visit; abnormal cervical cytology (Pap smear); significantly abnormal serum chemistry or hematology test results; allergy to any known component of study product or latex; initiation of a new long-acting treatment (e.g. depomedroxyprogesterone) within the past 3 months; history of recurrent vaginal infections (≥2 in past 6 months); active uncontrolled medical condition; use of a new investigational drug within 30 days or an immunosuppressive drug within 60 days prior to using the study product. Women who met all eligibility criteria were consented prior to enrollment.
Study procedures
Each volunteer was seen at four scheduled visits, and underwent two telephone interviews. At the first visit (screening), informed consent was obtained as well as a medical history. A urine sample was taken to test for β-hCG and for urine dipstick analysis. A urinalysis including urine culture was performed if dipstick analysis revealed abnormalities greater than trace. HIV and STI counseling was performed, and a blood sample was collected to test for HIV, syphilis, as well as testing for hematology, liver and renal function. A physical examination including pelvic examination followed. The pelvic examination included direct visual inspection, as well as vaginal pH testing, vaginal wet mount for candidiasis, BV by Amsel’s criteria and Nugent’s Gram stain testing, as well as cervicovaginal swabs specimens for T. vaginalis, N. gonorrhoeae, C. trachomatis testing, and a Pap smear. The volunteer was instructed not to use vaginal products and to abstain from sexual intercourse during the 72 hours prior to the enrollment visit and for the first 14 days of the study.
Qualified subjects were further evaluated for enrollment into the study on their second visit (study Day 1) which was scheduled 5-14 days after the first day of the next menses and within 30 days after the screening visit. Participants were provided with post-test counseling and screening test results. Eligibility criteria were reviewed and a urine specimen was taken for pregnancy testing and dipstick urinalysis. A review of symptoms was conducted, and a pelvic exam including direct visual inspection, colposcopy, vaginal pH and vaginal wet mount were performed. If the volunteer still met the eligibility criteria, she was enrolled and a blood sample was collected for hematology, liver and renal function testing.
Participants received five identical, pre-filled, single-use applicators containing LACTIN-V or placebo. The first four participants received applicators filled with 5 × 108 colony forming units (cfu)/dose (150mg) of LACTIN-V or 150 mg placebo; the next four participants received 1 × 109 cfu/dose (300mg) of LACTIN-V or 300 mg placebo; and the final four participants received applicators filled with 2 × 109 cfu/dose (600mg) of LACTIN-V or 600 mg placebo. The first application of study product was supervised in the clinic. The participant was instructed to insert the study product once daily for the first five days of the study and to place the used applicator back into the overwrap and plastic envelope, and return them to the study clinic at the next visit. The participant was again instructed not to use any other vaginal products and to abstain from sexual intercourse. She was given a diary card on which to record the date and time of product application, as well as any symptoms experienced (including, for example, genital tract itching, vaginal odor, abnormal vaginal discharge, nausea, cramping, headache, constipation, diarrhea, common cold symptoms, etc).
A follow up telephone call on Day 2 inquired about experiences with the product and possible symptoms. Participants returned to the clinic for follow-up visits on Day 7 and Day 14 following the enrollment visit. During follow-up visits, study staff collected used applicators, which were tested for exposure to vaginal mucus using trypan blue staining technique.25 A review of symptoms, a pelvic exam including direct visual inspection and colposcopy, and collection of vaginal samples for pH and Gram stain were performed at each follow-up visit. Additionally, a urine specimen was taken for pregnancy testing and dipstick urinalysis. During the Day 14 follow-up visit, a blood sample was collected for hematology, liver and renal function testing. A questionnaire evaluating acceptability was completed. A final follow up telephone call on Day 35 assessed possible symptoms, recent medical history and menstruation since the last clinical visit.
Lab methods
Pregnancy was tested by rapid urine β-hCG assays (QuickVue+ Pregnancy Test, Quidel Corporation, San Diego, CA). Urine was evaluated by urine dipstick for evidence of urinary tract infection (Chemstrips, Roche Diagnostics Limited, West Sussex, UK). HIV antibody testing was conducted using an ELISA (Genetic Systems HIV-1/HIV-2 Plus O EIA, Bio-Rad Laboratories, Hercules, CA) with confirmatory Western Blot (Genetic Systems Reflex Western Blot by Bio-Rad) for ELISA positives. Syphilis was assessed by rapid plasma reagin (Becton-Dickinson, Franklin Lakes, NJ) for screening with a confirmatory agglutination test (Fujirebio Serodia T. pallidum Passive Particle Agglutination, Fujirebio Diagnostics, Malvern, PA). BV was diagnosed through the evaluation of Gram stained vaginal smears using Nugent’s criteria. 26 Cervicovaginal swabs were evaluated for T. vaginalis, N. gonorrhoeae, and C. trachomatis using nucleotide amplification tests (Gen-Probe Aptima Combo 2 assay by Gen-Probe Inc., San Diego, CA). Pap smears were conducted on cervical specimens (ThinPrep pap test, Cytyc Corporation, Marlborough, MA). Returned used applicators were assessed for exposure to vaginal mucus using 1% trypan blue staining technique. 25 Serum was tested for liver and kidney function and whole blood was used for complete blood counts and differentials.
Randomization
The randomization scheme was developed by a UCSF pharmacist not otherwise directly involved with study participants. Subjects were randomized in a 3:1 LACTIN-V to placebo ratio in blocks of four corresponding to each dosing group. Individual assignments were concealed in sequentially numbered, sealed study drug kits. The placebo contained the same amount of preservation matrix as the active product, and the participants, investigators, sponsor, and study staff did not know which product a participant received.
Statistical analyses
The sample size of 12 subjects was chosen based on safety and clinical considerations. Because of the small sample the results are descriptive in nature. Data was entered and managed using the DataFax Clinical Database Management System (version 3.7) and was exported to R (version 2.61) for analysis. The analysis sample was defined as participants who were randomized, and received at least one dose of study product, irrespective of whether they completed the study. AEs were classified following a standard toxicity table. 22 Analyses for primary safety study endpoints were based on treatment group-specific summaries of frequency, severity and relationship to product use of AEs within each of the three dosing blocks. AEs were tabulated both as frequencies and proportions of participants experiencing at least one event, and overall frequency of observed events pooled over participants.
RESULTS
Adherence to study protocol
All 12 subjects attended all visits and reported to have inserted all 5 vaginal applicators within the allowable time windows. The mean age was 29.1 (SD = 7.0) years in the LACTIN-V arm vs. 27.3 (SD = 1.2) years in the placebo arm. Ten of the 12 participants were Caucasian. Lifetime number of sexual partners was 9.9 (SD = 6.8) and 13 (SD = 8.5), and the number of sexual partners in the last 6 months was 1.2 (SD = 1.2) and 1.0 (SD = 1.0) in the LACTIN-V and placebo arms, respectively.
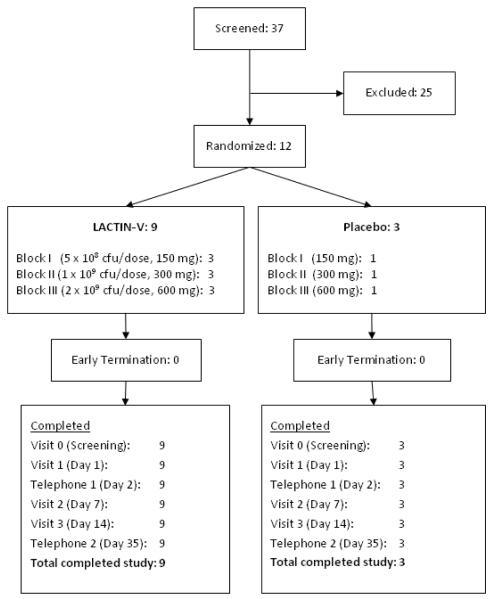
No participant discontinued study product herself or was discontinued from study product by study staff due to an AE (Figure 1). All applicators were used and returned by study participants. Ninety three percent of the LACTIN-V applicators as well as 100% of the placebo applicators stained positive for exposure to vaginal mucus.
Figure 1.
Adverse events
By definition AEs occurred any time after the first applicator was administered and within 35 days of enrollment. All 12 randomized subjects (100%) reported at least one AE. AEs reported in two or more subjects included: vaginal discharge (5 subjects, 42%), abdominal pain (4 subjects, 33%), metrorrhagia (4 subjects, 33%), vulvovaginitis (4 subjects, 33%), vaginal candidiasis (3 subjects, 25%), vaginal odor (3 subjects, 25%) and urinary tract infection (2 subjects, 17%). AEs were mostly mild in severity (41 out of 45 total AEs, 91%) and occurred among 11 subjects. Four of 45 total AEs (9%) were moderate in severity, occurred among 4 subjects and were determined to be unrelated or probably unrelated to study product: in the LACTIN-V arm one woman suffered from gastroenteritis and another woman had a urinary tract infection while in the placebo arm one woman reported ear pain and another woman experienced an upper respiratory tract infection (Table 1). One unexpected AE occurred at the highest dose when an applicator tip separated from the barrel during product administration; this event was not associated with any clinical signs or symptoms. No grade 3 or 4 severity AEs or SAEs occurred during the study.
Table 1. Summary and relatedness of adverse events.
(number of AEs and number of participants experiencing AE denoted by [ ])
| Adverse Event | LACTIN-V | Placebo | ||||
|---|---|---|---|---|---|---|
| Possibly, Probably & Definitely Related |
Unrelated & Probably Unrelated |
Total | Possibly, Probably & Definitely Related |
Unrelated & Probably Unrelated |
Total | |
| All AEs | 22 [8] | 9 [5] | 31 [9] | 6 [3] | 8 [3] | 14 [3] |
| All genitourinary AEs | 21 [8] | 2 [2] | 23 [8] | 5 [3] | 3 [2] | 8 [3] |
| AEs Grade 1 | 22 [8] | 7 [3] | 29 [8] | 6 [3] | 6 [3] | 12 [3] |
| AEs Grade 2 | 0 [0] | 2 [2] | 2 [2] | 0 [0] | 2 [2] | 2 [2] |
Twenty three AEs (51%) occurred between enrollment and the Day 7 visit (i.e. dosing phase), 9 AEs (20%) were reported between Day 7 and Day 14 (post-dosing phase) and 13 AEs (29%) occurred between Day 14 and the telephone interview on Day 35. About half of the AEs occurred during the first seven days of follow-up which included the five-day dosing phase; the other half took place between Days 8 – 35.
Of the 45 AEs in the study, 31 (69%) occurred among the nine participants (75% of all participants) receiving LACTIN-V at three different dose levels. Of these, 22 AEs (71%) were possibly, probably or definitely related with study product use. The three subjects receiving placebo at three different dose levels (25% of all participants) experienced 14 AEs (31%), of which 6 (43%) were possibly, probably or definitely related with study product use.
Thirty one of the 45 AEs (69%) were genitourinary (GU) AEs. Of these 31 GU AEs, 26 (84%) were classified to be either possibly, probably or definitely related to the investigational product. GU AEs were evenly distributed between the treatment blocks as well as between the LACTIN-V and placebo arms (Table 2). Four women experienced 5 GU tract infections during the study. One woman receiving placebo (Block II) was diagnosed with asymptomatic candidiasis at visit 2 (Day 7); another participant receiving LACTIN-V (Block III) reported symptomatic candidiasis at visit 2 (Day 7) which was confirmed by wet mount. A third participant receiving LACTIN-V (Block II) had asymptomatic candidiasis at visit 3 (Day 14) and also experienced an asymptomatic urinary tract infection with positive results per urine dipstick confirmed by urine culture (>100,000 cfu/ml) at visit 2 (Day 7). During the Day 35 telephone interview one woman receiving LACTIN-V (Block II) reported a urinary tract infection that had already resolved.
Table 2. Most Frequent Adverse Events.
(number of AEs and number of participants experiencing AE denoted by [ ])
| Adverse Event* |
Block I (5 × 108 cfu/dose) |
Block II (1 ×109 cfu/dose) |
Block III (2 × 109 cfu/dose) |
Total | % of affected subjects | ||||
|---|---|---|---|---|---|---|---|---|---|
| LACTIN-V | Placebo | LACTIN-V | Placebo | LACTIN-V | Placebo | LACTIN-V | Placebo | ||
| Vaginal Discharge |
1 [1]† | - | 2 [2] | - | 2 [2] | - | 5 [5] | - | 42% |
| Abdominal Pain |
1 [1] | - | 2 [2] | - | - | 1 [1] | 3 [3] | 1 [1] | 33% |
| Metrorrhagia | - | 1 [1] | - | - | 2 [2] | 1 [1] | 2 [2] | 2 [2] | 33% |
| Vulvovaginitis | - | - | 1 [1] | - | 2 [2] | 1 [1] | 3 [3] | 1 [1] | 33% |
| Headache | 1 [1] | - | 1 [1] | 1 [1] | - | - | 2 [2] | 1 [1] | 25% |
| Vaginal Candidiasis |
- | - | 1 [1] | 1 [1] | 1 [1] | - | 2 [2] | 1 [1] | 25% |
| Vaginal Odor | 1 [1] | - | 2 [2] | - | - | - | 3 [3] | - | 25% |
perdefinition, AEs were new conditions occurring after enrollment. Conditions already existing at baseline were only classified as an AE if condition worsened during the study.
no participant experienced a similar AE twice
Colposcopy Findings
Colposcopy was performed at the enrollment visit (Day 1), visit 2 (Day 7) and visit 3 (Day 14). Colposcopic findings were evenly distributed between the treatment blocks as well as between the LACTIN-V and placebo arms. No deep epithelial disruption was detected by colposcopy (Table 3).
Table 3. Colposcopic findings.
(number of AEs and number of participants experiencing AE denoted by [ ])
| Block I 5 × 108 cfu/dose) |
Block II (1 × 109 cfu/dose) |
Block III (2 × 109 cfu/dose) |
Total | |||||
|---|---|---|---|---|---|---|---|---|
| Colposcopic finding |
LACTIN-V | Placebo | LACTIN-V | Placebo | LACTIN-V | Placebo | LACTIN-V | Placebo |
| Erythema | - | - | - | 1 | 1 | - | 1 | 1 |
| Petechiae | - | - | 1 | - | - | - | 1 | - |
| Edema | - | 1 | - | - | - | - | - | 1 |
| Abrasion * | - | - | 1 | - | - | - | 1 | - |
| Laceration * | - | 1 | - | - | - | - | - | 1 |
| Other | - | - | - | 1 | - | - | - | 1 |
| Total number of findings [women with at least one finding] † |
- | 2 [1] | 2 [2] | 2 [1] | 1 [1] | - | 3 [3] | 4 [2] |
Findings with disrupted epithelium, all of which were considered superficial.
The total number of women may not equal the column sum because a given woman may have more than one type of finding.
Only those Colposcopic findings which were discovered after (but not at) visit 1 (enrollment visit) were included in Table 1 as earlier findings could not possibly have been related to the study product.
Laboratory Findings
Blood for additional assessment of safety was drawn at the screening visit, enrollment visit and final visit at Day 14. The changes of hematology and serum chemistry results were minor and clinically insignificant.
Only women with a Nugent Score of < 7 were eligible to participate in the study. At the screening visit, 11 of 12 randomized participants (92%) had Nugent Scores of 0-3 (normal vaginal flora) and one had an intermediate Nugent Score of 4-6 (8%). At the Day 14 visit all 12 subjects (100%) had a Nugent Scores consistent with normal vaginal flora.
Tolerability and Acceptability
No participant prematurely discontinued the trial due to AEs. Ten of 12 women (83%) agreed or strongly agreed with statements regarding the overall satisfaction with the study product and the comfort of using the study product.
DISCUSSION
This small phase I study was designed to assess the safety and acceptability of LACTIN-V in healthy women using a new delivery system and at a higher dose than previously tested in other studies (Osel Inc. unpublished data. 2006) 21. No grade 3 or 4 severity AEs or SAEs occurred. The reported AEs were evenly distributed between treatment blocks and study arms. Forty-one of the 45 AEs were graded as mild and 28 AEs were classified as related to product use. A third of all AEs occurred among placebo users who represented a quarter of all study participants. Vaginal discharge was the most common adverse event. The highest dose of 600 mg required more forceful ejection of study product which led to the disengagement of the applicator tip in a single occasion. Following this incident, the applicator was redesigned for future trials.
In summary, the data collected in this study suggest that vaginal administration of L. crispatus CTV-05 administered once daily by a vaginal applicator for 5 days, at a dose level up to 2 × 109 cfu/dose, is safe and well-tolerated in pre-menopausal healthy women.
The authors recognize the limitations of the small sample size in this trial that enrolled healthy women. The assessment of product safety in women with BV is an important objective in ongoing and planned future trials of LACTIN-V.
A high recurrence rate of BV after conventional antibiotic treatments 18 19 illustrates the need for enhanced therapy to prevent recurrent BV. LACTIN-V was designed to facilitate the repopulation of normal vaginal Lactobacillus strains. Previous formulations of lower-dose L. crispatus CTV-05 had good safety outcomes, but the rates of L. crispatus CTV-05 colonization were lower than expected. We hypothesize that the administration of the higher-dose LACTIN-V by a vaginal applicator will lead to increased colonization of L. crispatus CTV-05 following treatment of BV and thereby could reduce the risk of BV recurrence. A phase 2 trial to continue the assessment of product safety and determine the colonization efficiency of LACTIN-V at 2 × 109 cfu/dose in women with BV following metronidazole gel (0.75%) treatment is underway.
ACKNOWLEGMENTS
We wish to express our gratitude to all participants, the study clinicians performing the clinical visits, the staff of the CTSI Clinical Research Center at the San Francisco General Hospital, the data management team at DF/Net Research in Seattle, as well as the study sponsor Osel Inc. of Santa Clara, CA, USA.
This study was supported by a grant from Osel Inc. Jeanna Park was supported by the Doris Duke Charitable Foundation Clinical Research Fellowship at UCSF.
In addition, this study was supported by grant number UL1 RR024131-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/.
Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp
REFERENCES
- 1.Pastore LM, Thorp JM, Jr., Royce RA, et al. Risk score for antenatal bacterial vaginosis: BV PIN points. J Perinatol. 2002;22(2):125–32. doi: 10.1038/sj.jp.7210654. [DOI] [PubMed] [Google Scholar]
- 2.Gibney L, Macaluso M, Kirk K, et al. Prevalence of infectious diseases in Bangladeshi women living adjacent to a truck stand: HIV/STD/hepatitis/genital tract infections. Sex Transm Infect. 2001;77(5):344–50. doi: 10.1136/sti.77.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Klebanoff MA, Nugent R, et al. Bacterial colonization of the vagina during pregnancy in four ethnic groups. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1996;174(5):1618–21. doi: 10.1016/s0002-9378(96)70617-8. [DOI] [PubMed] [Google Scholar]
- 4.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350(9077):546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 5.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109(1):114–20. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 6.Eschenbach DA, Hillier S, Critchlow C, et al. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158(4):819–28. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 7.Larsson PG, Platz-Christensen JJ, Thejls H, et al. Incidence of pelvic inflammatory disease after first-trimester legal abortion in women with bacterial vaginosis after treatment with metronidazole: a double-blind, randomized study. Am J Obstet Gynecol. 1992;166(1 Pt 1):100–3. doi: 10.1016/0002-9378(92)91838-2. [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Kiviat NB, Hawes SE, et al. Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol. 1996;175(2):435–41. doi: 10.1016/s0002-9378(96)70158-8. [DOI] [PubMed] [Google Scholar]
- 9.Newton ER, Prihoda TJ, Gibbs RS. A clinical and microbiologic analysis of risk factors for puerperal endometritis. Obstet Gynecol. 1990;75(3 Pt 1):402–6. [PubMed] [Google Scholar]
- 10.Watts DH, Krohn MA, Hillier SL, et al. Bacterial vaginosis as a risk factor for post-cesarean endometritis. Obstet Gynecol. 1990;75(1):52–8. [PubMed] [Google Scholar]
- 11.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333(26):1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 12.Kurki T, Sivonen A, Renkonen OV, et al. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstet Gynecol. 1992;80(2):173–7. [PubMed] [Google Scholar]
- 13.Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319(15):972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 14.Silver HM, Sperling RS, Clair PJ, et al. Evidence relating bacterial vaginosis to intraamniotic infection. Am J Obstet Gynecol. 1989;161(3):808–12. doi: 10.1016/0002-9378(89)90406-7. [DOI] [PubMed] [Google Scholar]
- 15.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12(13):1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180(6):1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 17.Myer L, Denny L, Telerant R, et al. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192(8):1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11):1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 19.Myer L, Kuhn L, Denny L, et al. Recurrence of symptomatic bacterial vaginosis 12 months after oral metronidazole therapy in HIV-positive and -negative women. J Infect Dis. 2006;194(12):1797–9. doi: 10.1086/509625. [DOI] [PubMed] [Google Scholar]
- 20.Bolton M, van der Straten A, Cohen CR. Probiotics: potential to prevent HIV and sexually transmitted infections in women. Sex Transm Dis. 2008;35(3):214–25. doi: 10.1097/OLQ.0b013e31815b017a. [DOI] [PubMed] [Google Scholar]
- 21.Czaja CA, Stapleton AE, Yarova-Yarovaya Y, et al. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol. 2007;2007:35387. doi: 10.1155/2007/35387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Division of AIDS DAIDS Toxicity Table Addendum for Vaginal Microbicide Studies (November 2007) Nov, 2007.
- 23.CONRAD/WHO [Update 2004]; [Accessed 09/19/2008];Manual for the standardization of colposcopy for the evaluation of vaginal products. 2004 http://www.conrad.org/colpo/Revised_Manual.PDF.
- 24.Division of AIDS [Accessed 09/19/2008];Table for Grading Severity of Adult and Pediatric Adverse Events. 2004 December; http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/DAIDSAEGradingTable.pdf.
- 25.Wallace A, Thorn M, Maguire RA, et al. Assay for establishing whether microbicide applicators have been exposed to the vagina. Sex Transm Dis. 2004;31(8):465–8. doi: 10.1097/01.olq.0000135986.35216.ba. [DOI] [PubMed] [Google Scholar]
- 26.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]