Abstract
Cigarette smoke is a complex mixture of more than 4700 chemical compounds including free radicals and oxidants. Toxicity exhibited by cigarette smoke may be due to combined action of these compounds inducing many cellular processes mediated through reactive oxygen species (ROS). Major player probably nicotine as it is present in tobacco, in higher concentrations. The compounds that induce intracellular oxidative stress recognized as the important agents involved in the damage of biological molecules. Experiments using animal and cell culture model systems suggested that moderately higher concentrations of some forms of ROS like NO and H2O2 can act as signal transducing agents. Nuclear transcription factor κ (NF-κB) an inducible transcription factor detected in neurons found to be involved in many biological processes such as inflammation, innate immunity, development, apoptosis, and antiapoptosis. Our present study demonstrates that nicotine induces ROS levels in a dose dependent manner in rat mesencephalic cells. Electro mobility shift analysis showed that nicotine activates inducible NF-κB by binding to consensus sequence of DNA. Nicotine added to cell culture stimulates the degradation of IκB-α subunit in 2 h. Further activation of c-Jun terminal kinase indicates that nicotine induces oxidative stress leading to activation of stress dependent NF-κB pathway in mesencephalic cells.
Keywords: nicotine, NF-κB, mesencephalic cells, ROS
Introduction
Nicotine was first obtained as a distillate from tobacco and used as a prescribed drug to treat rodent ulcer and constipation. Later it was realized that it is toxic to humans. Humans normally ingest nicotine by smoking or tobacco chewing in various forms. Smoking habit rapidly spread in human population especially with young people, starts as a fun becomes a habit and addictive, as it stimulates central nervous system followed by sedation [1]. The major alkaloid present in cigarettes is nicotine approximately 1–2 mg/ml and is detected in the blood of smokers [2]. Nicotine stimulates synaptic transmission in brain acting through nicotine acetylcholine receptors (nAchRe) present in specific regions controlling behavior [3, 4]. Studies have demonstrated that the nicotine promotes cell proliferation in human and rodent bronchial cells and lung epithelial cells [3, 5-8]. It has been shown that pharmacological concentrations of nicotine activates proliferation signals [9, 10] such as activation of the enzyme protein kinase C in human and mouse lung cells [3, 5, 7], activation of a kinase Raf-1 which phosphorylates Bcl-2 that antagonizes drug induced apoptosis in lung cancer cells [6, 9, 11]. Mice treated with nicotine show increased mitogenic signals, phosphorylated Akt in lung tissue, and in human lung cancer cells derived from smokers suggested that nicotine is the major compound present in cigarette largely contributed to carcinogenesis [3, 9, 10]. Nicotine induces oxidative stress and apoptosis, apart from inducing proliferative signaling pathways in number of cell types and tissues [11] Exposure of cigarette smoke induces apoptosis in rat gastric mucosa mediated by reactive oxygen species (ROS) in a p-53 independent fashion [12].
Many cell systems on exposure to short term oxidative stress lead to the accumulation of DNA damage, genomic instability, and hypomethylation of DNA. On the other hand the cells exposed to long term to oxidative stress lead to apoptosis resistance, neoplastic transformation, and tumor development [13]. Hence the ROS induced by wide variety compounds are implicated in the pathogenesis and toxicity leading to many human diseases. Experiments using both in vivo and in vitro model systems suggested that moderately higher concentrations of certain forms of ROS like NO and H2O2 can act as signal transducing agents. Numerous studies carried out on the regulation of gene expression by oxidants, antioxidants, and other determinants of the intracellular redox state [14] suggested that inducible nuclear transcription factor (NF-κB) and its activator protein IκBα have been implicated and well defined in the regulation of many processes in several cell systems. These factors control the transcription of genes involved in immune response, inflammation, apoptosis and, tumorigenesis [15-17]. NF-κB normally expressed in low levels in central nervous system [18] but plays a key role in neuronal plasticity, learning, memory consolidation, neuro protection and neurodegenaration. Recent data suggest an important role of NF-κB on proliferation, migration, and differentiation of stem cells of nervous system [18, 19]. However, number of events like seizures and cerebral ischemia induces activation of NF-κB pathway [20].
Studies on the toxic effect of nicotine are limited and contradictory. Some labs reported that nicotine and its metabolites do not exhibit genotoxic effect [21] and DNA alteration induced by tobacco is not due to nicotine [22]. Hence the toxicity of nicotine, the major alkaloid present in cigarette smoke and tobacco is the subject of intense scientific scrutiny. It is now known that nicotine acts through nicotine acetyl choline receptors in the brain and is responsible for addictive nature of tobacco and its products [23]. In our present study we address the toxic effect of nicotine and the probable role of ROS in the activation of NF-κB in rat mesencephalic cells.
Materials and methods
Cell lines, media and chemicals
Nicotine, 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 2,7-dichlorofluoroscein diacetate (DCF-DA), Rat mesencephalic (1RB3AN27) was a gift from Dr. AG Kanthasamy, Department of Biomedical Sciences, Iowa State University, Ames, Iowa. Medium (RPMI 1640), Phosphate buffer saline (PBS), Fetal calf serum were obtained from Gibco (Invitrogen corp, C arlsbad, CA, USA), Penicillin and Streptomycin, were obtained from Sigma Chemical Co (St Louis, MO, USA).
Cell culture and treatments
Mesencephalic cells used were free from mycoplasma, confirmed by Gen-Probe Mycoplasma Rapid Detection Kit (Fisher Scientific, Pittsburgh, PA). The rat mesencephalic (1RB3AN27) cultures were maintained in Medium (RPMI 1640), supplemented with 10% fetal calf serum, l-glutamine (1%), Penicillin (100 u/ml) and Streptomycin (100 u/ml) under the atmosphere of 5% CO2, 95% air in humidified incubator at 37 °C. The cells were incubated with or without nicotine or substances in 96 well plates or six well plates for time intervals as indicated in the figure legends.
Measurement of intracellular ROS
The level of ROS present in living cells was quantified essentially as described earlier [23]. Equal number of rat mesencephalic cells (10,000/well in 96 well plate in Hanks Balanced Salt Solution) were treated with 10 μM DCF-DA (2,7-dichlorofluoroscein diacetate) for 3 h. Cells were washed with phosphate buffered saline and treated with different concentrations of nicotine and for different time intervals as mentioned in figure legends. DCF-DA penetrates into viable cells and inside the cells converted to DCF, which later reacts with ROS and fluoresce. At indicated time intervals the intensity of fluorescence is measured at excitation of wave length 485 nm and emission at wavelength of 527 nm and expressed as percent of control florescence.
Enzyme mobility shift assay (EMSA) for NF-κB
Mesencephalic cells were incubated with or without nicotine at concentrations as described in figure legends at 37 °C in a humidified atmosphere of air and 5% CO2 for 1 h. The cells were extracted with lysis buffer and electrophoretic mobility shift assay (EMSA) was carried out according to the method described earlier [23]. Briefly, nuclear extracts (8 μg of nuclear protein) prepared from cells that were exposed to different concentrations nicotine (final concentrations of 0.1, 1, 2.5, 5.0, and 10 μM) or 1 μM of nicotine concentration for different time intervals (15, 30, 60, 120, and 240 min) and were incubated with 32P labeled double-stranded NF-κB consensus sequence [23] The DNA-Protein complex supershifted was analyzed on 6% native polyacrylamide gel. The dried gel was exposed to X-ray film and protein binds to 32P radioactive NF-κB DNA activity was quantitated using Phosphor Imager (Molecular Dynamics, Sunnyvale, CA) with Quant software
Immuno blot analysis of IκB-α
Mesencephalic cells were treated with or without nicotine (1 μM) and incubated at different time intervals. Cells were washed with PBS and cell extracts were made in PBS by homogenization. Protein concentration was measured by Bradford's method. Equal quantity of protein (75 μg) was treated with sample buffer and boiled for 5 min and separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nylon membranes and probed with IκB-α antibodies washed thrice with PBS. Blots were incubated with second antibody horse radish peroxidase and bands were analyzed by chemiluminiscence kit by standard procedure as described earlier [23].
c-JUN N-terminal Kinase assay
The c-Jun N-terminal kinase assay was performed by a modified method as described earlier [23]. Briefly, the cells (3 × 106/ml) were exposed to nicotine for 30 min, and the cells were lysed in lysis buffer containing protease inhibitors as described earlier [23]. The cell extracts were immuno-precipitated with 30 mg of anti-JNK IκB-α sepharose beads for 45 min at 4 °C and beads were then washed four times with lysis buffer (4 × 400 ml) and twice with kinase buffer (2 × 400 ml: 20 mM HEPES, pH 7.4, 1 mM DTT, 25 mM nicotine). The kinase assay was performed for 15 min at 30 °C using GST-Jun (1–79; 2 g/sample) as a substrate in 20 mM HEPES buffer, pH 7.4, containing 10 mM MgCl2, 1 mM DTT, and 10 Ci [32P] ATP. The reaction was terminated by the addition of 15 ml of 2 × SDS sample buffer, and the mixture was boiled for 5 min. The reaction mixture was analyzed on 9% SDS-PAGE. The band GST-Jun (1–79) was visualized by autoradiogram by exposing the gel to X-ray film. The radioactivity of the phosphorylated protein was quantified in Phospho Imager (Molecular Dynamics).
Statistical analysis
ROS data was expressed as mean ± SD and statistical significance was assessed by student t test. The difference between control and nicotine treated samples were considered significant if the level is p < 0.05.
Results
Nicotine induces ROS levels
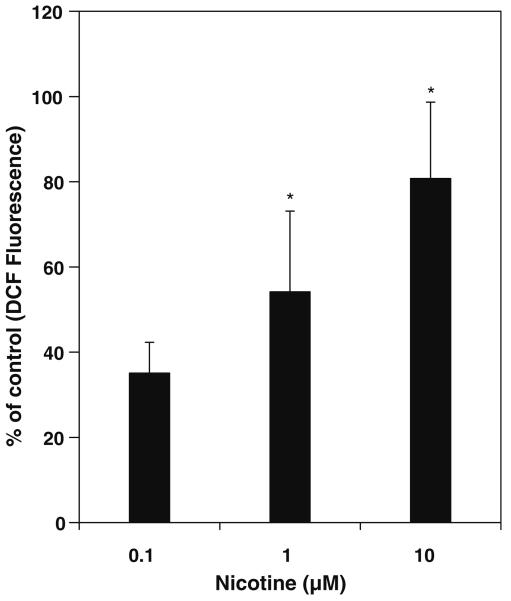
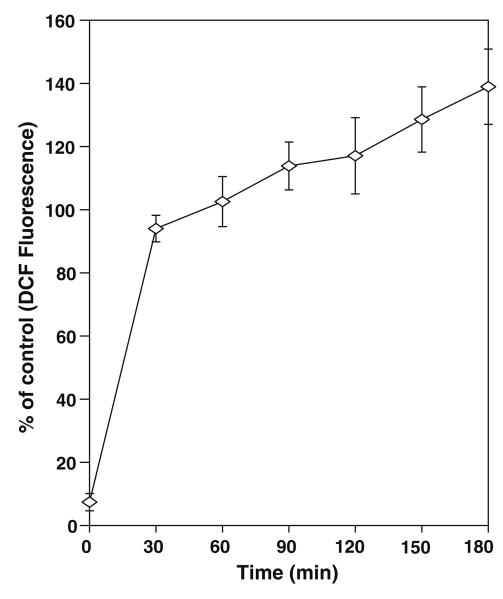
Mesencephalic cells incubated with different concentrations of nicotine increased the ROS in a dose dependent manner (Fig. 1). As low as 0.1 μM concentration of nicotine induces ROS by approximately 35%, however significant amount of increase in ROS is observed at 1 and 10 μM with 54% and 80% respectively. Nicotine also increased ROS in a time dependent manner as shown in Fig. 2. In time course experiments the increase in ROS at 30 min followed by slow increase thereafter till 180 min by nicotine.
Fig. 1.
Effect of Nicotine on ROS levels in rat Mesencephalic Cells. Cells were treated with nicotine at concentrations 0.1, 1.0 and 10.0 μM and incubated for 3 h. Fluorescence was measured at the end of 3 h. and expressed as percent of control florescence mean ± SD of 8 wells (n = 8) and the figure is a representative from three experiments performed independently. The * indicates significantly different from control samples.
Fig. 2.
Time course of induction of ROS by Nicotine in rat mesencephalic cells. Cells were incubated with 10 μM of nicotine. Fluorescence was measured at different time intervals as described in materials and methods. The values expressed as percent of control florescence mean ± SD of 8 wells (n = 8) and the figure is a representative from three experiments performed independently.
Nicotine activates NFκB transcription factor
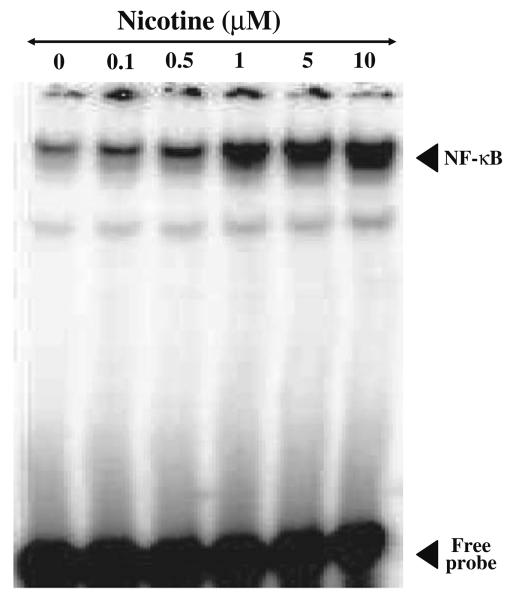
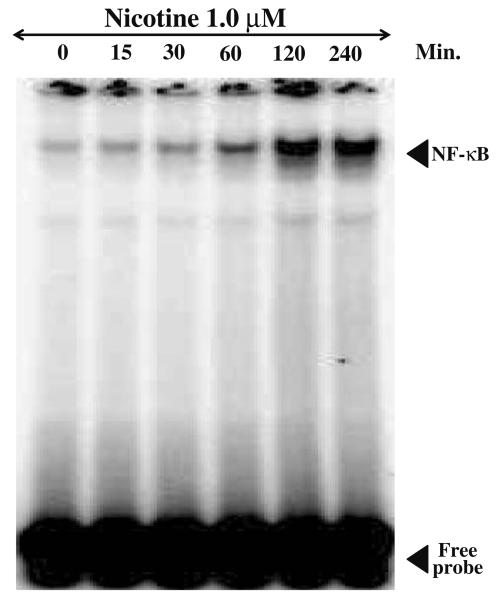
The results of EMSA for NF-κB DNA binding activity in mesencephalic cells exposed to different doses of nicotine (0.01–10 μM) for 2 h is shown in Fig. 3. Nicotine enhanced NF-κB DNA binding activity in a dose-dependent manner. NF-κB DNA binding activity was found to be more than two fold at low concentration of 0.1 μM concentration and stimulated more than 8 fold at 1.0 μM concentration and saturated by 5.0 μM concentration of nicotine compared to control values. To investigate the time required for NF-κB activation mesenceplalic cells were exposed to 1 μM concentration of nicotine for different time intervals (0, 30, 60, 120, and 240 min) and EMSA was carried out. The result of such as study is shown in Fig. 4. It suggests that nicotine induces NF-κB transcription factor within 15 min and maximum DNA binding activity was observed by more than sevenfold by 120 min. compared to control.
Fig. 3.
Effect of nicotine on NF-κB DNA binding activity in mesencephalic cells (dose). Nuclear proteins (20 μg) were analyzed on 7.5% native PAGE to detect NF-κB by electrophoretic mobility shift assay. The data shown is a representative of three independent experiments performed on extracts obtained from control and different concentrations of nicotine (0.1, 1.0, 5.0, 10 μM) treated mesencephalic cells.
Fig. 4.
Effect of nicotine on NF-κB DNA binding activity in mesencephalic cells (time). Nuclear proteins (20 μg) were analyzed on 7.5% native PAGE to detect NF-κB by electrophoretic mobility shift assay. The data shown is a representative of three independent experiments performed on extracts obtained from control and 1 μM nicotine treated mesencephalic cells for different time periods.
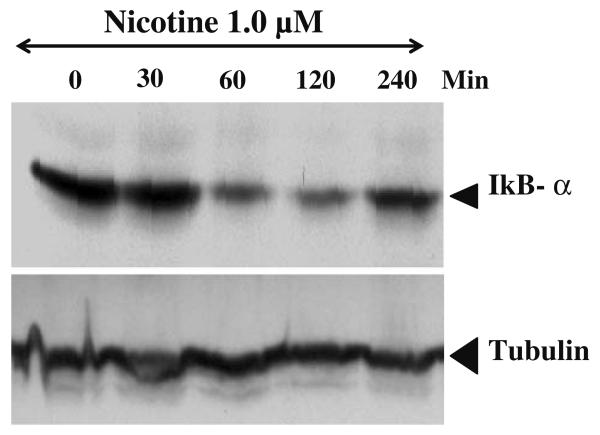
IκB-α degradation in response to nicotine
Mesencephalic cells show maximum expression of IκB-α protein levels in control and 30 min exposure to 1 μM concentration of nicotine. However, the levels of IκB-α significantly decreased by 1 h and maximum decrease is seen by 2 h (Fig. 5). This Result also suggests that the inhibitory effect of IκB-α on NF-κB is temporally relieved following the addition of nicotine and hence maximum binding activity of NF-κB is observed at 1 and 2 h (Fig. 5). The levels IκB -α almost comes back to control levels by the end of 4 h (Fig. 5).
Fig. 5.
Effect of nicotine on IκB-α degeradation. Mesencephalic cells were treated with or without nicotine (1 μM) for different time intervals as described in materials and methods. Cells were washed with PBS and cell extracts were made in PBS by homogenization. Equal quantity of protein (75 μg) was separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nylon membranes and probed with IκB-α antibodies washed thrice with PBS and incubated with second antibody horse radish peroxidase and bands were analyzed by chemiluminiscence kit.
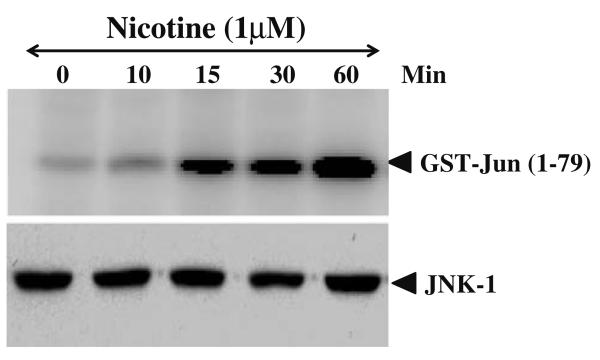
Nicotine activates c-Jun kinase
NF-κB DNA binding activity induced by nicotine was regulated by the phosphorylation of oncogene c-Jun at the N-terminus. Phsophorylated c-Jun subsequently activates NF-κB and its transcriptional activity. Phosphorylation of c-Jun was mediated by a MAP kinase called Jun N-terminal kinase (JNK). Whole cell extracts from control and nicotine treated cells were immuno-precipitated with anti-JNK antibody and kinase activity was measured using GST-Jun as a substrate. The result of such a study is presented in Fig. 6. Mesencephalic cells incubated with 1 μM nicotine show increased JNK activity by 15 min. and more than six fold increase in the kinase activity was observed by 60 min.
Fig. 6.
Effect of nicotine on JNK activation. Cell extracts from control and nicotine treated mesencephalic cells were prepared as described in materials and methods and 300 μg of total protein extract were immuno-precipitated with anti-JNK (0.3 μg/sample) antibody, and the kinase reaction was performed using 32P-labeled ATP and GST-Jun as substrate protein. The radiolabeled GST-Jun was detected in 9% SDS-PAGE. The level of JNK was detected using 50 μg of same extract proteins and analyzed in 10% SDS-PAGE by Western blot.
Discussion
Cigarette smoke is a complex mixture of more than 4700 chemical compounds while the major one is nicotine. Besides these compounds cigarette smoke also contains free radicals and the other oxidants that add up to induced levels of ROS. Toxicity exhibited by cigarette smoke may be due to combined action of many of these compounds inducing many cellular processes mediated by ROS. Major candidate probably nicotine, as it is present in higher concentrations in tobacco. In the present study as low as 1 μM concentration of nicotine induces ROS levels in significant amounts in rat mesencephalic cells. Studies from our laboratory on mouse brain also report that long term environmental tobacco smoke (ETS) induces ROS in different regions of brain [23]. Chronic administration of nicotine significantly depleted GSH content in liver and testes. Nicotine also decreased catalase activity in liver while increased catalase activity in kidney and testes. It has also been shown that ethanol or nicotine produce oxidative tissue injuries in rat [24]. Cigarette smoke induce ROS production, and this ROS play a critical role in signaling of the cellular inflammatory response by activating the transcription factor, NF-κB in cancer cells in vitro [25].
Our results also demonstrate that nicotine activates NF-κB DNA binding activity to consensus sequence in a dose dependent manner. NF-κB is a family of DNA binding proteins regulates transcription of many genes and control many cellular processes [19]. Preliminary requirement for activation of NF-κB is homo or heterologous dimerization of p50 and p65 Proteins [25]. Dimerization of NF-κB may occur between nascent polypeptide emerging from successive ribosomes of mRNA [19]. NF-κB activation in different cells occurs following specific stimuli such as cytokines, chemokines, bacterial LPS, or viral infection. Cigarette smoke, which has variety of compounds also stimulates NF-κB over a period of time [23]. It is known that nicotine acts through nicotine acetyl choline receptors in the brain and is responsible for addictive nature of tobacco and its products [26]. Nicotine activation of NF-κB may be through binding to nicotine acetyl choline receptors. Earlier study from our laboratory also shows activation of NF-κB in mouse brain stem, cerebellum, frontal cortex, hippocampus, and straitum following the long-term exposure of ETS [23].
NF-κB is normally present in inactive form in cytosol by binding to IκB. IκB kinase (IKK) complex catalyze the signal dependent phosphorylation of IκB. Phosphorylated IκB undergoes proteosomal degradation activating NF-κB [19]. Transfected mesencephalic cells show maximum expression of IκB-α protein levels and on exposure to nicotine, the levels of IκB-α significantly decreased and maximum decrease in levels were seen by 2 h (Fig. 5). Activated NF-κB transported to nucleus and increase in binding activity of NF-κB to consensus sequence of DNA could be seen in Fig. 4.
NFκB activation and DNA binding activity regulated by the phosphorylation of oncogene c-Jun at the N-terminal portion of the protein [23]. Phsophorylated c-Jun subsequently activates NF-κB and its transcriptional activity. phosphorylation c-Jun was mediated by a MAP kinase called JNK [23]. Most agents that activates NF-κB also activates AP-1 transcription factor and it was reported that cigarette smoke activates AP1 [27, 28]. Our recent studies show that mouse exposed to ETS activated AP-1 DNA binding activity in brain tissues [23]. Studies carried out by other laboratory also show AP-1 activation in rat lung tissue after 8 weeks of exposure to cigarette smoke [29]. This observation was in agreement with, the recent reports from our laboratory as well as others that activation of AP-1 requires the action of JUN kinase, which is an early event in this signaling pathway [29, 30]. Nicotine treated mesencephalic cells induces JNK enzyme activity by phosphorylating the substrate, suggested clearly that nicotine activation of NF-κB is mediated through MAP kinase pathway. Cigarette smoke has been shown to induce MAPK and this activation was co-related with concomitant increased in histone 3 phospho-acetylation, histone 4 acetylation, and increased DNA binding of the redox-sensitive transcription factor NF-κB in rat lung [29].
Our studies using in vitro mesencephalic cell model suggest that nicotine induce ROS production in a dose dependent manner and ROS in turn activate redox-sensitive transcription factor NF-κB. We found that nicotine exerts a specific effect on cell signal transduction pathways mediated by NF-κB, possibly triggered by ROS. Cigarette smoke exposure induces inflammatory response by induction of cytokines mediated by NF-κB in rat lungs [29] and in lung cells was observed [28]. ROS and NF-κB activation may be one of the initial events transcription and expression of cytokines involved in the inflammatory response as it regulates the expression of many genes involved in immune and inflammatory response. This also leads to the coordinated expression of many genes such as cytokines, chemokines, adhesion molecules, and enzymes involved in mediator synthesis and the further amplification and perpetuation of the inflammatory response [27].
Nicotine induced significant increase in ROS that resulted in the activation of NF-κB by activating signal responsive kinases. Further studies are underway to understand the genes that are activated and regulated by nicotine.
Acknowledgment
This work was supported by NSF HRD0401587: NASA NCC 9–165: NIH/RCMI RR03045–19 (GR).
References
- 1.Pailer M. Chemistry of nicotine and related alkaloids (including biosynthetic aspects) In: EulerVon US, editor. Tobacco Alkaloids and Related Compounds. The McMillan Co; New york: 1964. pp. 15–36. [Google Scholar]
- 2.Hammond D, Collishaw NE, Callard C. Secret science: tobacco industry research on smoking behaviour and cigarette toxicity. Lancet. 2006;367:781–787. doi: 10.1016/S0140-6736(06)68077-X. [DOI] [PubMed] [Google Scholar]
- 3.Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell growth Diff. 1994;5:1033–1040. [PubMed] [Google Scholar]
- 4.Mandelzys A, Cooper E. Effects of ganglionic satellite cells and NGF on the expression of nicotine acetylcholine currents by rat sensory neurons. J Neurophysiol. 1992;67:1213–1221. doi: 10.1152/jn.1992.67.5.1213. [DOI] [PubMed] [Google Scholar]
- 5.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 6.Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogen. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 7.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- 8.Wright SC, Zhong J, Zhong H, Larrick JW. Nicotine inhibition of apoptosis suggests a role in tumor promotion. FASEB J. 1993;7:1045–1051. [PubMed] [Google Scholar]
- 9.Minna JD. Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer. J Clin Investig. 2003;111:31–33. doi: 10.1172/JCI17492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo MG, D'Atri F, Vicentini IM. Mechanisms of mitogen-activated protein kinase activation by nicotine in small-cell lung carcinoma cells. Biochem J. 1997;328:499–503. doi: 10.1042/bj3280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by {beta}-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;3:7–20. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta (1–42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholinereceptors. J Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 13.Baeuerle PA, Kenkel T. Function and activation of NF-kappa B in the immune system. Ann Rev immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 14.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radical Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 15.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 16.Lin A, Karin M. NF-κB in cancer a marked target. Semin Cancer Biol. 2004;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 17.Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nature Rev Mol Cell Biol. 2004;5:392–491. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 18.Kaltschmidt C, Kaltschmidt B, Neumann H, Wekerle H, Baeuerle PA. Constitutive NF-κB activity in neurons. Mol Cell Biol. 1994;14:3981–3992. doi: 10.1128/mcb.14.6.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widera D, Mikenberg I, Kaltschmidt B, Kaltschmidt C. Potential role of NF-κB in adult neural stem cells; the understand steersman. Int J Devl Neurosci. 2006;24:91–102. doi: 10.1016/j.ijdevneu.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 21.Doolittle DJ, Winegar R, Lee CK, Caldwell WS, Hayes AW, de Bethizy JD. The genotoxic potential of nicotine and its major metabolites. Mutat Res. 1995;344:95–102. doi: 10.1016/0165-1218(95)00037-2. [DOI] [PubMed] [Google Scholar]
- 22.Mizusaki S, Okamoto H, Akiyama A, Fukuhara Y. Relation between chemical constituents of tobacco and mutagenic activity of cigarette smoke condensate. Mut Res. 1977;48:319–325. doi: 10.1016/0027-5107(77)90175-0. [DOI] [PubMed] [Google Scholar]
- 23.Manna SK, Rangaswamy T, Wise K, Sarkar S, Shishodia S, Biswal S, Ramesh GT. Long term environmental tobacco smoke activates nuclear transcription factor-kappa B, activator protein-1 and stress responsive kinases in mouse brain. Biochem Pharmacol. 2006;71:1602–1609. doi: 10.1016/j.bcp.2006.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain K, Scotttt BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001;2:89–97. doi: 10.1016/s0741-8329(01)00176-8. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal BB. Nuclear factor kappa the enemy with in. Cancer cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Campain JA. Nicotine potentially a multifunctional carcinogen. Toxicol Sci. 2004;79:1–3. doi: 10.1093/toxsci/kfh106. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 28.Gensch E, Gallup M, Sucher A, Li D, Gebremichael A, Lemjabbar H, Mengistab A, Dasari V, Hotchkiss J, Harkema J, Basbaum C. Tobacco smoke control of mucin production in lung cells requires oxygen radicals AP-1 and JNK. J Biol Chem. 2004;279:39085–39093. doi: 10.1074/jbc.M406866200. [DOI] [PubMed] [Google Scholar]
- 29.Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I. Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol. 2004;31:633–642. doi: 10.1165/rcmb.2004-0006OC. [DOI] [PubMed] [Google Scholar]
- 30.Manna SK, Ramesh GT. Interleukin-8 induces nuclear transcription factor-κB through TRAF6- dependent pathway. J Biol Chem. 2005;280:7010–7021. doi: 10.1074/jbc.M410994200. [DOI] [PMC free article] [PubMed] [Google Scholar]