Abstract
Individual variation in the experience and expression of pleasure may relate to differential patterns of lateral frontal activity. Brain electrical measures have been used to study the asymmetric involvement of lateral frontal cortex in positive emotion, but the excellent time resolution of these measures has not been used to capture second-by-second changes in ongoing emotion until now. The relationship between pleasure and second-by-second lateral frontal activity was examined with the use of hierarchical linear modeling in a sample of 128 children aged 6–10 years. Electroencephalographic activity (EEG) was recorded during “pop-out toy,” a standardized task that elicits pleasure. The task consisted of three epochs: an anticipation period sandwiched between two play periods. The amount of pleasure expressed during the task predicted the pattern of non-linear change in lateral frontal activity. Children who expressed increasing amounts of pleasure during the task exhibited increasing left lateral frontal activity during the task, whereas children who expressed contentment exhibited increasing right/decreasing left activity. These findings indicate that task-dependent changes in pleasure relate to dynamic, non-linear changes in lateral frontal activity as the task unfolds.
Keywords: pleasure, EEG, lateral frontal cortex
Pleasure, the euphoric feeling that can occur as a result of the internal representation of hedonic information, is an important ingredient of positive affect (Meehl, 1975; Klein, 1987). Activity in subcortical brain structures and multiple regions of the prefrontal cortex relate to the occurrence of pleasure (Berridge, 2003; Leon & Shadlen, 1999; Gable and Harmon-Jones, 2008). Specifically, the ability to consciously experience pleasure relates to (1) an ability to internally represent hedonic information and (2) translate represented hedonic information into conscious positive emotional experience; and these processes are likely subserved by prefrontal cortex activity. For example, recent neuroimaging data indicate that activity in several prefrontal regions contribute to positive affect, including: dorsolateral prefrontal cortex (Wallis & Miller, 2003), orbitofrontal prefrontal cortex (Kringelbach, 2005), ventromedial prefrontal cortex (Hamann, Ely, Hoffman & Kilts, 2002) and frontopolar prefrontal cortex (Pochon, Levy, Fossati, Lehericy, Poline, Pillon, Le Bihan & Dubois, 2002). In particular, it is likely that the representational (e.g. the ability to represent the hedonic value of 4 drops of a sweet liquid versus 8 drops), executive (e.g. enacting a plan to obtain a reward), and working memory functions subserved by activity in the frontal cortex—particularly the lateral prefrontal cortex—contribute to pleasure capacity (Watanabe, 1996; Hikosaka & Watanabe, 2000; Pochon et al., 2002; Kobayashi, Lauwereyns, Koizumi, Sakagami & Hikosaka, 2002; Schultz, 2006).
In addition to neuroimaging techniques, brain electrical (i.e. electroencephalographic—EEG) measures of frontal function have been extensively used to examine relations between baseline levels of frontal activity and the propensity to experience certain emotional states. Specifically, the left and right frontal regions of the cerebral hemisphere are differentially involved in the expression of approach-related emotions and withdrawal related emotions, respectively (Davidson, 1992; Wheeler, Davidson & Tomarken, 1993; Davidson, 2004a; Urry, Nitschke, Dolski, Jackson, Dalton, Mueller, Rosenkranz, Ryff, Singer & Davidson, 2004; Harmon-Jones, 2003). For example, the tendency to experience pleasure in response to pleasant stimuli has been linked to asymmetrical activity in the frontal cortices; infants respond to sweet tastes with increased relative left frontal activity (Fox & Davidson, 1986), exhibit greater relative left-sided frontal activity in response to happy video clips (Davidson & Fox, 1982), and exhibit greater relative left frontal activity during facial displays of joy (Fox & Davidson, 1988). Furthermore, adults who enjoy eating desserts to a great extent exhibit greater relative left frontal activity when they view pictures of desserts compared to when they view pictures of neutral pictures (Gable & Harmon-Jones, 2008).
A neglected, yet relevant aspect of the approach-withdrawal hypothesis of frontal EEG asymmetry is the idea that shifts in frontal asymmetry may not only mark the onset of approachrelated versus withdrawal-related emotional states, but may also carry information about whether the emotional state the individual is in is more internally-focused (introspective) or externallyfocused. Specifically, given the positive relationship between greater relative right frontal activity and the experience of withdrawal-related emotions, it seems that the types of emotions that are represented by greater relative right frontal activity would include negative and positive emotions that are more internally-focused and pensive, such as sadness and contentment (contentment can be defined as: “a calm form of happiness”). In contrast, the types of negative and positive emotions represented by greater relative left frontal activity may tend to be intense, action-oriented, and driven by external stimulation (e.g. exuberance and anger). One-half of the equation has been well articulated; adults exhibit greater relative left frontal activity when angry (Harmon-Jones, 2003). To build upon this work, it seems warranted to investigate whether individual variation in the tendency to express high intensity forms of pleasure versus lower intensity forms of pleasure relate differentially to lateral frontal asymmetries. If there is an externally-focused/internally-focused facet to the relationship between frontal EEG asymmetry and approach and withdrawal related emotions, we would expect to find that different intensities of pleasure would relate to different patterns of left and right frontal asymmetry. Specifically, if right frontal activity relates to withdrawal-related or “low approach-related” emotions that are internally-focused and pensive, then greater relative right frontal activity should relate to contentment. Why contentment? Contentment is a positive emotional state that (1) is intrapsychic and characterized by the occurrence of pleasant reveries, (2) generally involves a less dramatic outward display of positive emotion (i.e. contentment is more likely to be characterized by less intense facial signs of pleasure, such as non-Duchenne smiles, compared to higher intensity positive emotions such as exuberance) and (3) is not heavily action-oriented. There is evidence in the literature to support this hypothesis. Namely, positive emotion characterized by non-Duchenne smiles relates to greater relative right frontal activity in adults (Ekman, Davidson & Friesen, 1992). Furthermore, a greater contentment-related pleasure capacity at age 4 related to greater relative right frontal asymmetry at age 8 (Kim & Bell, 2006).
The excellent time resolution of brain electrical measures—compared to the coarser time resolution of functional magnetic resonance imaging and positron emission tomography—seems particularly well suited for investigating the physiological correlates of individual differences in pleasure expression. Though electrical activity recorded from the scalp does not correspond exactly to behavioral changes, it is a measure that is well suited to capture the dynamic changes in pleasure that occur over the course of an extended pleasure-inducing task that takes place over a span of minutes. Therefore, we investigated the extent to which the growth, decay, or maintenance of contentment versus happiness over time relates to concomitant second-by-second changes in asymmetry in lateral frontal brain electrical activity. Electroencephalogram (EEG) activity was recorded while children completed the “pop-out toy” task (Pfeifer, Goldsmith, Davidson & Rickman, 2002), a task that normatively induces pleasure in children and comprises three epochs. The child plays an arousing game with the experimenter during the first epoch of the task (“Game played with experimenter”). An anticipation period, during which the child sits in a room alone and waits to play the game with their parent, makes up the second epoch (“Anticipation”). The child plays a game and surprises his or her parent during the last epoch (“Game played with parent”). Each child was video recorded during the “pop-out toy” task so that pleasure could be quantified from behavior and tracked for each child. Pleasure scores were used to predict the second-by-second lateral frontal EEG asymmetry trajectory during the “pop-out toy” task. Based upon extensive prior research and theory (Davidson, 2004b), we hypothesized that the expression of intense pleasure would be related to greater acceleration of left lateral frontal activity. The hypothesis that frontal activity in general, and lateral frontal activity in particular, may track task-dependent pleasure gains footing when it is considered in conjunction with previous research showing that activity in lateral frontal cortex relates to the online manipulation of task-dependent information (Christoff & Gabrieli, 2000; Crone, Wendelken, Donohue, van Leijenhorst, Bunge, 2006). These findings lend support to the view that activity in the lateral frontal cortex plays an important role in tracking incoming hedonic information. In order for a positive affective state to take hold, it seems likely that hedonic information must be internally represented and kept in a highly accessible state.
Method
Participants
Families were recruited from state birth records, supplemented by advertising in the local area. Children with major health problems and developmental disabilities were excluded. We did not select for risk for psychopathology. One-hundred and twenty eight individual members of twin pairs (One child from each twin pair was randomly selected for inclusion in this analysis. Each child included in the analyses presented in this report had a twin who completed the same testing, but data from each of the co-twins has not been analyzed and is not included in this report) aged 6–10 years old contributed data, but only 103 children were right-handed and had usable electrophysiological data. The 103 cases of usable data included 53 girls, 8 six-year-olds, 22 seven-year-olds, 44 eight-year-olds, 24 nine-year-olds, and 5 ten-year-olds (M age=7.96; SD=.98). There were 100 Caucasian children, 1 African-American child, 1 Asian-American child, and 1 Hispanic child. The Hollingshead Index was used to determine the socioeconomic status of each family (Hollingshead & Redlich, 1958). For this sample, the mean Hollingshead Index score was 45.86 (SD=9.58), and the mean class score was 2.18 (SD=.81).
Measurement of Pleasure
The “pop-out toy” task is from the Laboratory Temperament Assessment Battery (Pfeifer et al., 2002). During the “pop-out toy” task, pleasure was elicited in children when they played a game with an experimenter and then with their parent. The task consists of three segments. Each child engaged in each one of the three segments of the “pop-out toy” task only once. The game began when the experimenter presented the child with a can that resembled a can of edible nuts (The can actually contained a spring-loaded toy that popped out when opened). The experimenter opened the can with the child to reveal its contents (Children were generally surprised and exuded some degree of pleasure at this point). This set of events made up epoch 1 (“Game played with experimenter”). At the very end of epoch 1, the experimenter gave the child instructions on how to play the same game with their parent, and this generally evoked additional positive emotion from the child. The child was left alone in the room with the toy, while the experimenter went to go get the parent. This sequence of events made up epoch 2 (“Anticipation”). Finally, the parent, who was unaware of the surprise in the can, entered the room and the child popped the toy with his or her parent. This last series of events made up epoch 3 (“Game played with parent”).
A pleasure score was generated for each child for each epoch of the “pop-out toy” task. Each pleasure score was based on ratings made by trained coders. Two separately scored indices were combined to derive 1 pleasure score for each child during each of the 3 epochs: smiles were rated on intensity/duration using a 0 (smiling absent) to 3 (intense or long lasting smiling), and vocal/facial/bodily pleasure (positive vocalizations, cheeks, eyes, etc.) were rated separately on a 0 (behavior absent) to 3 (behavior present to a strong degree) scale. Each of the two factors contributed equally to the final pleasure score. In total, the pleasure scores reflect the magnitude of the child’s smiles, and the degree of positive affect expressed vocally, facially and/or physically by the child. Pleasure scores ranged from 0 (pleasure absent) to 3 (extreme pleasure). The inter-rater reliability was high for this measure, with a kappa value of .72 (All coders were graduate level or undergraduate level research assistants trained to score vocal, facial and bodily indicators of emotion by experienced graduate level coders. During this training process, potential coders had to practice scoring videotaped cases that had already been scored by 1 or more experienced “master” graduate level coders. Once novice coders reached a level of reliability with the “master” graduate-level coders, the coder was allowed to score actual, unscored data. We chose to verify the accuracy of behavioral coding by randomly subjecting coders to reliability testing. Roughly half (55), of the cases were double coded (i.e. coded by a non-master coder and a master coder) in order to ensure that non-master coders remained at a stable level of reliable scoring over time).
Given our interest in determining whether individual differences in pleasure responsivity in-vivo during a pleasure-inducing task relate to individual differences in lateral frontal activity, we divided the sample into mild, moderate and intense pleasure groups based on the children’s emotional reactivity during the “game played with parent” (epoch 3) segment of the task because that portion of the task was best suited for capturing individual differences in pleasure since it was the portion of the task that induced the widest spread in positive emotional expression between individual children. Therefore, a mild, a moderate, and an intense pleasure group was created. All children in the mild pleasure group earned a pleasure score of 1.5 or less during “game played with parent” (epoch 3). All children in the moderate pleasure group earned a pleasure score greater than 1.5 but less than 2.5 during “game played with parent” (epoch 3), and children in the intense pleasure group earned a pleasure score greater than or equal to 2.5 during “game played with parent” (epoch 3). The mean pleasure score for each group across all three epoch is as follows: mild pleasure group, M pleasure score=1.39 (SD=.23); moderate pleasure group, M pleasure score=2.10 (SD=.20); and intense pleasure group, M pleasure score=2.64 (SD=.22).
None of the children in the sample earned a pleasure score of 0 during the “game played with parent” segment of the task. The only time any child failed to show any quantifiable positive emotion (i.e. earning a pleasure score of 0) was during the “anticipation” segment of the task, and the 1 child who earned a pleasure score of 0 during the “anticipation” segment of the task was included in the mild pleasure group.
EEG Acquisition & Analysis
29 channels of EEG activity were recorded (using a stretch-lycra electrode cap based on the 10–20 electrode system. EEG activity was recorded from FP1/2, FPF1/2, F3/4, F7/F8, FC3/4, FC7/8, F3/4, C3/4, T3/4, T5/6, CP3/4, CP5/6, P3/4, PO3/4, and a ground electrode mounted between FZ and CZ. Our intention was to identify frontal regions that showed a unique relationship to pleasure. Based upon prior data in the EEG literature showing relations between variations in lateral (F7/F8) frontal activity and emotion, we selected this frontal region for analysis. EEG from the identical time points in the parietal region (P3/P4) was selected as a comparison to determine the specificity of our findings to the frontal region. We collected eight 1-minute trials of resting EEG data referenced on-line to physically linked ears (gain=20K), four with eyes open and four with eyes closed, in one of two counterbalanced orders. EEG electrode impedances were less than 5KΩ. Eye movement and muscle artifact were removed via a regression equation fit to the raw EEG data, and a low-pass filter of 200 Hz was applied (i.e. all frequencies below 200 Hz were passed). In addition, each child’s EEG data was hand scored in order to check for, and remove any missed, EEG artifact. Alpha (8–13 Hz) power values were computed for the lateral frontal (F7/8) and parietal (P3/4) sites. On average, children tend to exhibit reliable EEG activity at a frequency of 8 Hz by the time they are 2-years-old, which reaches an average maximum of about 10 Hz by the time the child is 10-years-old (Niedermeyer, 1997; Davidson, Jackson & Larsen, 2000). Therefore, given that the bulk of the sample consisted of 7–9-year-olds, we felt that the frequency band of 8–13 Hz was adequate because it is likely that the vast majority of these children exhibit adult-like patterns of alpha activity by this stage of development. The power values calculated were based on all of the artifact-free, 1-second units of EEG data using an off-line whole-head average reference and a fast Hartley transform. Across all children, the average length of the “game played with experimenter” (epoch 1) segment of the task=38.39 seconds. On average, 75% (SD=26%) of the lateral frontal and 84% (SD=21%) of the parietal 1-second units that made up epoch 1 were usable. The average length of “anticipation”= 44.11 seconds. On average, 77% (SD=27%) of the lateral frontal and 86% (SD=20%) of the parietal 1-second units that made up epoch 2 were usable. The average length of “game played with parent”=25.45 seconds. On average, 62% (SD=29%) of the lateral frontal and 74% (SD=24%) of the parietal 1-second units that made up epoch 3 were usable. EEG asymmetry scores were computed by subtracting left alpha values from right alpha values (LogRight – LogLeft). Positive asymmetry values indicate greater relative left-sided activation and negative values indicate greater relative right-sided activation.
Statistical Approach
Hierarchical linear modeling (Raudenbush, Bryk, Cheong & Congdon, 2004) was used to chart EEG asymmetry trajectory across the “game played with experimenter,” “anticipation,” and “game played with parent” segments of the task, with pleasure scores predicting individual differences in lateral frontal EEG asymmetry trajectory. HLM is a form of multilevel analysis that can be used to estimate average growth trajectories as well as characterize the variability of trajectories across individuals. The level-1 model estimated the association between lateral frontal EEG asymmetry scores and time elapsed from the onset of the “game played with parent” segment of the task. The second-by-second EEG asymmetry scores from each child, collected across the “game played with experimenter,” “anticipation,” and “game played with parent” segments of the task, made up the level 1 outcome variable. The shape of the overall (across all children) EEG asymmetry trajectory was modeled as nonlinear. A nonlinear model was chosen because changes in brain electrical activity do not conform to a linear pattern across time (Coan & Allen, 2004). Therefore, the simplest nonlinear model—a quadratic model—was created to accommodate the nonlinearity of EEG asymmetry patterns over time. Each within-epoch quadratic function was treated as a random factor. The linear term that is embedded in the quadratic function was treated as a random factor. Thus, the linear slope was allowed to vary between subjects. The linear term determines the basic EEG asymmetry slope that runs its course from the onset to the offset of the task. The quadratic term within each epoch represents an acceleration/deceleration parameter that accounts for non-linear change withinepoch. The EEG asymmetry trajectory from onset to offset of the task can be viewed as a compilation of the three quadratic functions that correspond to the “game played with experimenter,” “anticipation,” and “game played with parent” epochs of the task (These functions were created such that the end point of “game played with experimenter” was mathematically set equal to the start point of “anticipation.” This enabled us to graph a continuous, non-saltatory trajectory across time). Each quadratic term was treated as random, to allow for the possibility that EEG asymmetry trajectory might vary between children. The intercept was treated as a fixed factor in order to maximize our ability to discern task-dependent changes in trajectory between children. Without this feature, we would be less able to defend against the possibility that any between-subject differences in EEG asymmetry trajectory observed across the task were just a reflection of the different EEG asymmetry scores these children possessed before the task began, with the observed differences in EEG asymmetry trajectory bearing little or no relation to the occurrence of the positive stimulus.
At level-1, within each epoch, the EEG asymmetry trajectory was characterized as follows:
Lateral frontal EEG asymmetry score at time “x” of “game played with experimenter”= P0 + P1(time elapsed from the onset of “game played with experimenter”) + P2(time elapsed in “game played with experimenter”)2 + error.
Lateral frontal EEG asymmetry score at time “y” of “anticipation”= P0 + P1(time elapsed from the onset of “game played with experimenter”) + P2(total time in “game played with experimenter”)2 + P3(time elapsed in “anticipation”)2 + error.
Lateral frontal EEG asymmetry score at time “z” of “game played with parent” = P0 + P1(time elapsed from the onset of “game played with experimenter”) + P2(total time in “game played with experimenter”)2 + P3(total time in “anticipation”)2 + P4(time elapsed in “game played with parent”)2 + error.
The level-2 model was built to explain individual differences in EEG asymmetry trajectory. The level-2 model introduced pleasure scores to explain individual differences in the shape of the lateral frontal EEG asymmetry trajectory. These pleasure scores came from two sources: (1) “game played with experimenter” and “game played with parent” (Pleasure scores from the “anticipation” epoch were excluded because this epoch lacked social interaction. Therefore, the pleasure score that corresponds to this epoch may be less representative of the type of pleasure evinced during “game played with experimenter” and “game played with parent”); and (2) the period immediately preceding the onset of “game played with experimenter.” Predictors that were introduced at level-2 were restricted to variables that preceded a particular portion of the task. For example, only variables that were measurements of pleasure level before the onset of “game played with experimenter” were used to predict lateral frontal EEG asymmetry intercept values. Thus, pre-task, “game played with experimenter,” and “game played with parent” pleasure scores were entered as predictors of P0, P1, P3 and P4.
As a statistical comparison, the multilevel model built for the lateral frontal EEG asymmetry data was applied to the parietal (P3/4) EEG asymmetry data. This was done to determine whether pleasure would predict parietal EEG asymmetry trajectory. If pleasure scores predict parietal EEG asymmetry trajectory and lateral frontal EEG asymmetry trajectory in the same way, this would be evidence for a more global brain effect, not a unique lateral frontal effect.
Results
Task-Dependent Pleasure
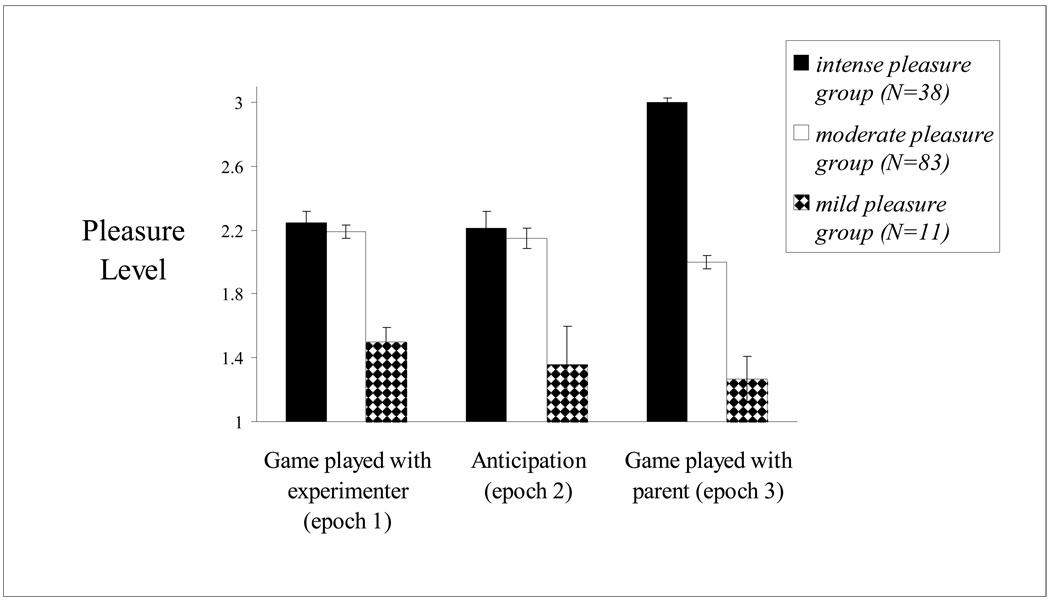
The total amount of pleasure expressed by each child was quantified by the independent ratings of trained coders who observed the facial, vocal and physical behavior of each child across all 3 epochs of the “pop-out toy” task. The pleasure scale ranged from 0 (pleasure absent) to 3 (extreme pleasure). The sample was divided into mild pleasure (M pleasure score=1.39; SD=.23),moderate pleasure (M pleasure score=2.10; SD=.20), and intense pleasure (M pleasure score=2.64; SD=.22) groups. Figure 1 provides an illustration of how the pleasure trajectories of these three pleasure groups differed across time. Notably, a significant epoch (pleasure during “game played with experimenter” / pleasure during “anticipation” / pleasure during “game played with parent”) by group (mild pleasure / moderate pleasure / intense pleasure) interaction (F(2,127)=21.37; p<.001) was present, with children in the mild and moderate pleasure groups showing no change in the amount of pleasure they expressed during the task (Figure 1; ps>.2), whereas children in the intense pleasure group showed increasing pleasure across the task. We next examined whether these different pleasure patterns related to differential patterns of second-by-second lateral frontal activity.
Figure 1.
A significant epoch (epoch 1 pleasure/epoch 2 pleasure/epoch 3 pleasure) by group (mild pleasure / moderate pleasure / intense pleasure) interaction (F(2,127)=21.37; p<.001) emerged. The pleasure scores for children in the mild pleasure group do not differ significantly from “game played with experimenter” to “game played with parent,” p>.3. Similarly, The pleasure scores for children in the moderate pleasure group did not change significantly across the task from “game played with experimenter” to “game played with parent,” (p>.2).
Baseline lateral EEG activity recorded before the task began did not predict pleasure scores during any of the three epochs of the task (all ps>.15).
Second-by-Second Lateral Frontal EEG Asymmetry and Task-Dependent Pleasure
A hierarchical growth curve model was built using lateral frontal (F7/8) second-by-second EEG asymmetry data from each child as a level-1 outcome variable. “Game played with experimenter” and “game played with parent” pleasure scores were used in the level 2 model to predict individual differences in lateral frontal EEG asymmetry trajectory during the task (table 1). (The multilevel model used in this analysis was quadratic. Thus, the formula used to calculate the lateral frontal EEG asymmetry trajectory included squared terms).
Table 1.
Hierarchical EEG Asymmetry Model for Lateral frontal cortex. The multilevel model used in this analysis was quadratic. Thus, the formula used to calculate the lateral frontal EEG asymmetry trajectory included squared terms. All factors were treated as random except for the intercept.
| Model components |
β-coefficient estimate |
predictors | predictor β-coefficient estimate |
approximate degrees of freedom |
p-value |
|---|---|---|---|---|---|
| β00 intercept |
1.97×10−2 | pleasure before the onset of “game played with experimenter” (epoch 1) |
8.26×10−3 | 7825 | .27 |
| β10 (linear component) |
2.24×10−4 | pleasure during “game played with parent” (epoch 3) |
3.19×10−4 | 101 | .18 |
| pleasure during “game played with experimenter” (epoch 1) |
4.19×10−4 | 101 | .24 | ||
| β20 (Epoch 1 quadratic component) |
−(1×10−5) | pleasure during “game played with experimenter” (epoch 1) |
−(2.7×10−5) | 102 | .21 |
| β30 (Epoch 2 quadratic component) |
2×10−6 | pleasure during “game played with experimenter” (epoch 1) |
−(1.3×10−5) | 101 | .04* |
| β40 (Epoch 3 quadratic component) |
−(1.3×10−5) | pleasure during “game played with experimenter” (epoch 1) |
5×10−5 | 101 | .01* |
| pleasure during “game played with parent” (epoch 3) |
−(1.7×10−5) | 101 | .42 |
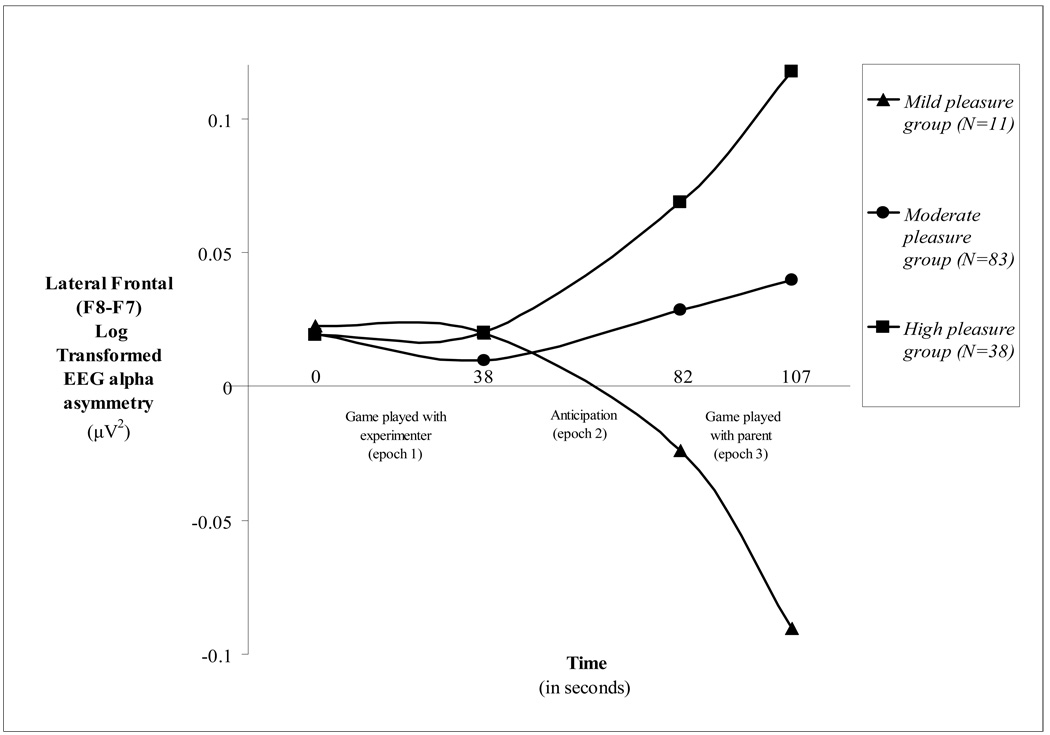
Across the sample as a whole, lateral frontal EEG asymmetry differed significantly from onset to offset of the task (p<.01), indicating that significant change occurred in lateral EEG asymmetry from “game played with experimenter” to “game played with parent.” Overall, the lateral frontal EEG asymmetry trajectory for the intense pleasure group is characterized by a positive growth curve (Figure 2); indicating increasing relative left lateral frontal activation over time. This pattern emerged because the amount of pleasure expressed during “game played with experimenter” positively predicted lateral frontal EEG asymmetry during “game played with parent” (β=5×10−5 ; p<.05; table 1; figure 2). This neurophysiological pattern corresponds to the behavioral pleasure pattern exhibited by children in the intense pleasure group across the task (Figure 1). Thus, left lateral frontal activity appears to increase on a moment-to-moment basis with increases in behavioral signs of pleasure as the task evolves.
Figure 2.
Lateral frontal EEG asymmetry trajectories during the “pop-out toy” task for the mild, moderate and intense pleasure groups. The first data point on the graph represents where each group’s lateral frontal EEG asymmetry score was at the beginning of “game played with experimenter”. The last 3 data points represent where each group’s EEG asymmetry score was at the end of “game played with experimenter,” “anticipation,” and “game played with parent,” respectively.
The negative curvature in the lateral frontal EEG asymmetry trajectory of the mild pleasure group during “game played with parent” is due to these children expressing less pleasure during “game played with experimenter” than children in the moderate and intense pleasure groups. This neurophysiological pattern seems to be a marker for the propagation of a calm pleasure state into subsequent moments of a task.
The relatively stable pattern of lateral frontal activation exhibited by children in the moderate pleasure group contrasts with the mild and intense pleasure groups’ pattern. This neurophysiological pattern may mark the propagation of a moderately intense—yet unyielding—pleasure state into subsequent moments of a task.
We conducted additional analyses to determine whether changes in left or right lateral frontal activation accounts for the observed shifts in asymmetry score at a between-groups level. We regressed mean whole-head power from the alpha power for the right (F8) and left (F7) lateral frontal electrode site for each child in order to index the alpha power unique to each site (Davidson, Jackson & Larson, 2000), providing a means to determine whether activity in the left versus right hemisphere contributes to the observed changes in asymmetry score during the task. Then we tested the difference between dependent rs using Cohen & Cohen’s (1983) method in order to determine whether the observed positive frontal asymmetry in the intense pleasure group is driven by (1) increasing left frontal activity, (2) decreasing right activity or (3) both increasing left and decreasing right frontal activity. Similarly we were interested in deciphering whether the negative lateral frontal asymmetry values observed in the mild pleasure group was driven by (1) increasing right activity, (2) decreasing left activity, or (3) decreasing left frontal activity and increasing right frontal activity. We found that increasing left lateral frontal activity accounts for the changes in the intense pleasure group (r=−.29 versus r=−.03, p<.05). We found that the combination of decreasing left frontal and increasing right frontal activity accounts for the asymmetry in the mild pleasure group (r=.35 versus r=.03; ns), and the combination of increasing left and decreasing right frontal activity accounts for the observed asymmetry in the moderate pleasure group (r=−.12 versus r=−.08, ns).
Is the Relationship Between Change in Lateral Frontal EEG Asymmetry and Pleasure Unique to the Frontal Region?
The level-1 and level-2 models that were combined and applied to the lateral frontal EEG asymmetry data were also applied to the parietal EEG asymmetry data. The significant predictive effects that were present in the lateral frontal model were absent when this model was applied to the parietal EEG data (all ps>.70). This result suggests that the observed relationship between pleasure and task-dependent change in lateral frontal EEG asymmetry during a positive stimulus is unique to the frontal region and does not reflect whole-brain changes.
Furthermore, we conducted an additional analysis to determine whether the shift in lateral frontal asymmetry observed during the task was significantly stronger than any shift in parietal asymmetry. To this end, we computed a “change” score by subtracting each child’s lateral frontal EEG asymmetry score and parietal asymmetry score at the onset of “game played with experimenter” from their lateral frontal EEG asymmetry score or parietal EEG asymmetry score at the end of “game played with parent.” We then used a repeated measures ANOVA that included all children in the sample to determine whether the change in asymmetry score differed by region; we found that it did. The average amount of change in lateral frontal EEG asymmetry from onset to offset of the task was .03 (SEM=.007), whereas the average change in parietal EEG asymmetry from onset to offset of the task was 0 (SEM=.005). The degree of change in the two regions is significantly different (p<.001), indicating that dynamic change in asymmetry score was stronger in the lateral frontal region relative to the parietal region.
Discussion
The results from this study suggest that the second-by-second changes in the expression of pleasure during a positive stimulus is related to dynamic growth in left lateral frontal activation over the course of the task. Baseline lateral frontal EEG asymmetry (a trait-like marker) did not relate to the amount of pleasure expressed during the “pop-out toy” task. We predicted that it would be related to pleasure during this task. It may be that responses to this task are more situational or state governed rather than reflecting more trait-like variation associated with individual differences in baseline frontal asymmetry.
Increasing left lateral frontal activity during a positive stimulus was predicted by the amount of pleasure displayed. Previous research involving children supports the idea that left lateral frontal activity is related to positive emotion (Davidson & Fox, 1982; Fox & Davidson, 1986). The present data suggest that the dynamic non-linear growth in the second-by-second sequence of lateral frontal EEG asymmetry tracks the chronometry of task-dependent pleasure. These findings indicate that an important component of individual differences in affective style (Davidson, 2000) is the dynamic unfolding of pleasure over time. Those children whose expression of pleasure accelerates more rapidly show greater growth in relative left lateral frontal activation as the task evolves. The data underscore the utility of harnessing the temporal information in the EEG to characterize individual differences in affective chronometry.
The task we studied in this report provided an excellent basis for examining how pleasure does and does not build-up as a result of contact with pleasure inducing stimuli. This feature of the task may be a key reason for the changes in activation observed in this frontal region as the task evolved. The increasing left lateral frontal activation observed in a subset of the children tested may be an indication that this region plays a role in the propagation of an increasingly intense positive emotional state over time, whereas the combination of decreasing left/increasing right lateral frontal activation over time may mark the propagation of a less intense, calm positive emotional state—likely characterized by contentment or some variant of it—over time. The propagation of an intense or calm positive emotional state over the course of a task may be conceptualized as a form of affective working memory (Davidson, 2004b; Pizzagalli, Sherwood, Henriques & Davidson, 2005) because it seems likely that humans possess a neurophysiological mechanism for making positive emotional states last as long as possible. Our findings are consistent with the notion that the frontal cortex supports this form of affective working memory and more specifically the data indicate that accelerating growth in left prefrontal activation is associated with a pattern of growing pleasure as the task develops. In contrast, those children whose expression of pleasure remains moderate and unchanging tend to exhibit both increasing left-sided and decreasing right-sided lateral frontal activation as the task develops. In contrast, those children whose expression of pleasure remains relatively mild and unchanging across the task are characterized by a pattern of combined decreasing left and increasing right lateral frontal activation as the task unfolds. We believe that the decreasing left frontal activity/increasing right frontal activity observed in the mild pleasure group may signal that these children experienced a non-approach oriented form of positive emotion such as contentment during the task. This formulation fits with the approach-withdrawal model of lateral frontal EEG asymmetry—and highlights the need to consider the internally-focused versus externally-focused facet of the approach-withdrawal spectrum—because the combination of increasing right frontal activity and decreasing left frontal activity may relate to internally-focused, self-reflective positively valenced emotions such as contentment just as left frontal activity has been found to relate to externally-focused, approach-related negative emotions such as anger (Harmon-Jones, 2003).
The differences in frontal brain activation observed during this novel, positive affect eliciting task suggest that individual children differ in the degree to which they become engaged by highly active forms of positive emotion (e.g. joy) versus calmer forms of positive emotion (e.g. contentment) in the moment. If the concept of positive affect is partitioned in a similar manner as negative affective states have been, then one can ask the question: how might different positive emotional states be differentiated neurophysiologically? Our data suggest that individual differences in the way one child versus another manifests positive affect in response to the same stimulus may be linked to differences in their frontal brain activity during the task. Further study will be necessary to determine whether this state effect may relate to a more long-term, trait-like tendency to engage in high intensity forms of positive emotion or calm forms of positive emotion on a regular basis (i.e. individual children may tend to fall on one end of a positive affect continuum or the other, tending to routinely express exuberance rather than contentment—or vice versa—in response to positive stimuli). Whether the state effects observed in the present study map onto trait differences should be investigated empirically.
It will be interesting to determine whether individual differences in positive emotional tendencies relate to different social, cognitive, emotional, or educational/vocational outcomes. For example, Masters, Barden & Ford (1979) found that four year old children who were asked to either spend 30 seconds remembering “something that happened that made you feel so happy you just wanted to jump up and down” (p. 382)—a condition designed to elicit exuberance—or 30 seconds remembering “something that happened that made you feel so happy that you just wanted to sit and smile” (p. 382)—a condition designed to induce contentment—engaged in more efficient problem solving than children who were not asked to think about a happy moment. Importantly, both the contentment and happiness conditions led to statistically even elevations in problem solving ability relative to the control condition. This result suggests that children’s experience of different types of positive affect in certain contexts may lead to similar outcomes. However, it is also likely that children will differ in their propensity to experience different types of positive affect and more refined analyses may reveal different effects of contentment and exuberance. Our findings underscore the critical need to dissect the neural underpinnings of these different forms of positive affect. As it happens, we may come to find that eliciting/increasing contentment has distinct positive effects relative to eliciting/increasing happiness, and designing methods to elicit contentment may be a useful tool for promoting learning and general well-being in certain children, whereas eliciting/increasing happiness may be more useful for promoting learning and well-being in other children.
It is important to note that the scalp-recorded EEG is a coarse reflection of integrated regional brain activity and thus the localization of the underlying sources of the scalp-recorded signals is approximate at best. However, the virtue of these measures lies in their temporal resolution which is on the same time scale as the dynamic changes in pleasure that can be reliably coded from the behavior of children. Our findings show that the dynamic time course of these prefrontal electrical changes may reflect important features of individual variation in affective chronometry. The fact that our findings are derived from children in the six to ten year age range is noteworthy. Children of this age show rich expression of affect that tends to be more constrained and socially managed in adults. Thus, in addition to their intrinsic worth in illustrating early manifestations of affective style and temperament, the use of children to study the brain electrical changes that dynamically vary with affect may be particularly significant since overt, reliably coded behavioral expressions of pleasure can easily be quantified in subjects this age.
Acknowledgments
This research was supported in part by a National Institute of Mental Health institutional training grant (T32-MH18931). This research was also supported by a grant from the National Institute of Mental Health (P50-MH069315). We thank Andrew T. Herdina for his contribution to this research project, and we thank those children who participated in this study.
Contributor Information
Sharee N. Light, Department of Psychology, University of Wisconsin-Madison
James A. Coan, Department of Psychology, University of Virginia
Corrina Frye, Department of Psychology, University of Wisconsin-Madison.
H. Hill Goldsmith, Department of Psychology, University of Wisconsin-Madison.
Richard J. Davidson, Department of Psychology, University of Wisconsin-Madison
References
- Berridge K. Pleasures of the brain. Brain & Cognition. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proceedings of the National Academy of Science. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style—hemispheric substrates. Psychological Science. 1992;3:39–43. [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Well-being and affective style: Neural substrates and biobehavioral correlates. Philosophical Transactions of the Royal Society (London) 2004a;359:1395–1411. doi: 10.1098/rstb.2004.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: Perspectives in frontal EEG asymmetry research. Biological Psychology. 2004b;67:219–234. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218:1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of physiology. 2nd Ed. New York: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;38:242–353. [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia. 1986;24:417–422. doi: 10.1016/0028-3932(86)90028-x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10-month-old infants. Developmental Psychology. 1988;24:230–236. [Google Scholar]
- Gable PA, Harmon-Jones E. Relative left frontal cortical activation to appetitive stimuli: considering the role of individual differences. Psychophysiology. 2008;45:275–278. doi: 10.1111/j.1469-8986.2007.00627.x. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13:135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness. New York: John Wiley & Sons; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Bell MA. Frontal EEG asymmetry and regulation during childhood. Annals New York Academy of Sciences. 2006;1094:308–312. doi: 10.1196/annals.1376.040. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Lauwereyns J, Koizumi M, Sakagami M, Hikosaka O. Influence of reward expectation on visuospatial processing in macaque lateral frontal cortex. Journal of Neurophysiology. 2002;87:1488–1498. doi: 10.1152/jn.00472.2001. [DOI] [PubMed] [Google Scholar]
- Klein DF. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and affect deficit states. New York: PMA Publishing; 1987. pp. 1–14. [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the lateral frontal cortex of the Macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Masters JC, Barden C, Frod ME. Affective states, expressive behavior, and learning in children. Journal of Personality and Social Psychology. 1979;37:380–390. [Google Scholar]
- Meehl P. Hedonic capacity: Some conjectures. Bulletin of the Menninger Clinic. 1975;39:295–306. [PubMed] [Google Scholar]
- Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. International Journal of Psychophysiology. 1997;26:31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Development. 2002;73:1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: A source localization study. Psychological Science. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan B, Dubois B. The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM 6: Hierarchical Linear and Non-Linear Modeling. Lincolnwood, IL: Scientific Software International, Inc; 2004. [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, Rosenkranz MA, Ryff CD, Singer BH, Davidson RJ. Psychological Science. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbitofrontal prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: a biological substrate of affective style. Psychophysiology. 1993;30:82–89. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]