Abstract
Directing attention to one of two superimposing surfaces composed of dot fields rotating in opposing directions facilitates processing of brief translations of the attended surface (Valdes-Sosa et al., 1998). Here we used ERP recordings to investigate the mechanisms of endogenous attentional selection of such competing dot surfaces under conditions of dichoptic viewing (one surface to each eye) and monocular viewing (both surfaces to one eye). Under dichoptic conditions, which induced binocular rivalry, translations of the attended surface presented to one eye elicited enhanced visual P1 and N1 ERP components relative to translations of the unattended surface presented to the other eye. In comparison, during monocular viewing the attended surface translations elicited a significantly larger N1 component in the absence of any P1 modulation. These results indicate that processing of the attended surface is biased at an earlier level in extrastriate visual cortex under conditions of inter-ocular versus intra-ocular competition.
Keywords: attention, binocular rivalry, inter-ocular competition, monocular, ERPs, P1, N1
INTRODUCTION
During binocular rivalry dissimilar stimuli presented to the separate eyes are seen to alternate spontaneously between one ocular image and the other. These fluctuations in dominance and suppression are unpredictable in duration, but it is possible to bias the dominance of one rival image over the other by boosting the strength of its attributes such as contrast, luminosity, degree of motion, or contour density (Blake and Logothetis, 2002, Silver and Logothetis, 2007) or by blurring the other image (Arnold et al., 2007). Apart from explicit changes in the physical features of a stimulus, the role of top-down attention in biasing stimulus dominance has been debated for the past century. Helmholtz (1925) was the first proponent of attentional modulation of rivalry and showed that by mentally counting the contours on a rivalrous target (i.e., by focusing attention on it) he could induce sustained dominance of that stimulus. In recent years considerable evidence for the attentional control of rivalry has accrued (reviewed in Tong et al., 2006). Ooi and He (1999) showed that voluntary attention directed to a dominant stimulus in one eye made it less likely to be suppressed by a perturbing event presented to the other eye. Mitchell et al. (2004) found that exogenous attentional capture by one of two superimposed rotating non-rivalrous dot surfaces could bias dominance in favor of the selected surface during subsequent periods of rivalry. Chong and Blake (2006) obtained similar results using rivalrous gratings instead of dot patterns and showed that endogenous attention could also bias initial dominance during rivalry. Corroborating Helmholtz’s initial findings, Chong et al. (2005) found that endogenous attention indeed prolonged dominance durations during rivalry, but only when attention was deployed to the features of a rivalrous stimulus. Mere attentional engagement or spatial attention to the location of the rivalrous stimulus was insufficient to bias dominance durations. Hancock and Andrews (2007) also found evidence that involuntary as well as voluntary attention can select a rivalrous grating to be perceptually dominant. Their findings suggest competition during binocular rivalry may be an example of a more general attentional mechanism within the visual system.
Although attentional control of binocular rivalry has been established in psychophysical investigations, little is known of its neural bases. Neurophysiological investigations of attention and binocular rivalry have suggested that the two processes may involve common mechanisms (Stoner et al., 2005). Competitive stimulus selections via visual attention (reviewed in Reynolds and Chelazzi, 2004, Kastner and Pinsk, 2004) and binocular rivalry (reviewed in Tong et al., 2006, Blake and Logothetis, 2002, Moutoussis et al., 2005) are both resolved at multiple stages of processing in the visual cortex with progressively greater selectivity observed in higher cortical areas such as V4, MT and IT. Although evidence from human fMRI studies of binocular rivalry suggests that eye-based competitive selection may occur as early as area V1 (Polonsky et al., 2000, Tong and Engel, 2001, Buchert et al., 2002, Lee et al., 2005) or even LGN (Haynes et al., 2005, Wunderlich et al., 2005), stimulus feature competition occurring in higher areas is an integral part of rivalry resolution that correlates more closely with perceptual state (Leopold and Logothetis, 1996, Sheinberg and Logothetis, 1997, Haynes and Rees, 2005). Transcranial magnetic stimulation (TMS) of areas V1/V2 has been shown to induce perceptual state changes during rivalry (Pearson et al., 2007), but the authors did not consider their results to be incompatible with involvement of higher cortical areas that govern selection of the rivalrous stimuli via attention. These results together support emerging models of neural processing in binocular rivalry that involve a hierarchy of processing stages (Blake and Logothetis, 2002, Wilson, 2003, Freeman, 2005, Tong et al., 2006, Lee et al., 2007).
The time course of inter-ocular competition during rivalry has been studied by means of scalp recorded event related potentials (ERPs). ERPs allow for observations of precise temporal modulations in visual processing and reportedly have sufficient spatial resolution to distinguish neural generators in striate and extrastriate cortical areas (Martinez et al., 1999, 2001, Noesselt et al., 2002, Di Russo et al., 2003). Previous investigations of binocular rivalry using ERPs found early modulations beginning at around 100 ms (Valle-Inclán et al., 1999, Roeber and Schröger, 2004), but the neural generators of these effects were not determined. Other EEG and MEG studies of rivalry have demonstrated effects such as greater amplitudes of scalp potentials or fields and greater synchrony between sensors elicited when a stimulus is perceptually dominant vs. when it is suppressed (Lansing, 1964, Cobb et al., 1967, Brown and Norcia, 1997, Kaernbach et al., 1999, Srinivasan et al., 1999, Srinivasan and Petrovic, 2006).
In the present study we investigated the neural basis of attentional allocation to one of two rivalrous stimulus arrays using ERP recordings together with source localization of the underlying neural generators. We employed a paradigm wherein subjects viewed superimposed rotating dot surfaces, which was introduced by Valdes-Sosa et al. (1998) to investigate surface-based attentional selection in the absence of spatial cues. In this study we compared the neural bases of endogenous attentional selection of these competing dot surfaces under conditions of dichoptic viewing (with binocular rivalry) versus conditions of monocular viewing.
METHODS
Task and Stimuli
Thirteen right-handed healthy adults (7 males and 6 females, mean age 22 years) participated in the study after giving informed consent. Each participant had normal or corrected-to-normal vision.
Stimuli were displayed on a CRT monitor at 57 cm viewing distance in a darkened room. Subjects rested their heads on a chin-rest and viewed stimuli through a mirror stereoscope. Binocular alignment was ensured before starting the experiment by requiring the subjects to align two dichoptically presented nonius bars. During the experiment subjects fixated on a high-contrast central white annulus having inner and outer radii of 0.1º and 0.5º of visual angle (va), respectively, which was presented to both eyes and perceived as a single annulus through the stereoscope on a dark background. The luminances of the white and dark regions were 24.2 cd/m2 and 0.05 cd/m2, respectively. Surrounding the fixation annulus were two overlapping counter-rotating circular random dot patterns (diameter: 4.3º va), one green and one red. These rotating patterns created the perceptual impression of two transparent rigid surfaces sliding across each other (Valdes-Sosa et al., 1998). To induce rivalry the two dot surfaces were presented dichoptically (i.e., one in each eye) on half the experimental trials. On the remaining trials the two surfaces were presented monocularly (i.e. both surfaces to one eye). Dichoptic and monocular trials were presented in separate runs. Surface rotation direction in either eye was balanced across all trials. Surface rotation speed was 40º/s. Dot density of each dot field was 3.3 dots per square degree of visual angle, and each dot subtended 0.1º of visual angle. Red dot intensity was 5.1 cd/m2, while equiluminance of green dots was established independently for every subject prior to the experiment via heterochromatic flicker fusion (Khoe et al., 2005).
Stimulus events consisted of brief (100 ms) translations of one of the surfaces in either the upward or downward direction. Dots translated at a speed of 4º va/s. Only a subset of the dots (60%) translated coherently in order to encourage attending to the complete ensemble instead of focusing on individual dots. Interstimulus intervals (ISIs) between successive translations had a uniform distribution ranging from 400 to 700 ms (SOA 500 to 800 ms). The dot set involved in each event was randomly selected.
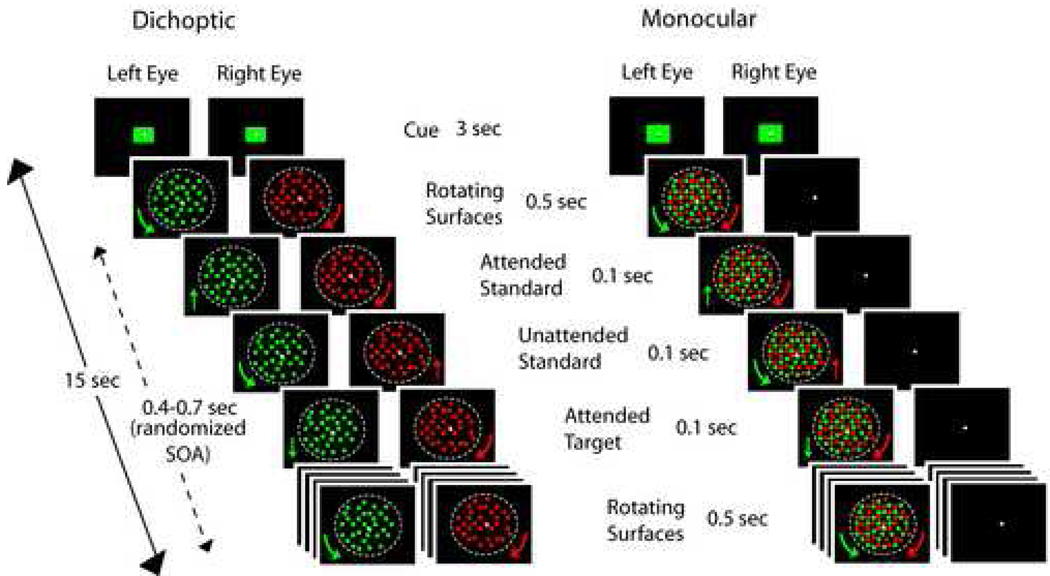
Each run was of 15 s duration with the first and last 500 ms devoid of dot translations. Four blocks of 20 runs each constituted the full experiment, with 10 s rest break after each run and several minutes of rest between blocks. Dichoptic and monocular runs were presented in counter-balanced order. Prior to each run a red or green color cue flashed on the screen for 3 s to instruct subjects to direct attention to the surface with the cued color. Attended color was randomly selected on each run and counter-balanced across all runs. During runs subjects performed a translation direction discrimination task on the attended surface and were instructed to respond with a button press every time they detected a target translation in the downward direction. Equally probable translations of the unattended surface were to be ignored. Target occurrence probability was 20% for both the attended and the unattended dot surfaces. The translations in the upward direction (80%) required no button press response and were designated as standards. A schematic of the experimental design for both dichoptic and monocular runs is shown in Fig. 1.
Figure 1.
Overview of the experimental design. Sample stimulus presentations in the dichoptic viewing condition are shown on the left, and in monocular viewing on the right. Target and standard translations of the attended and unattended dot surfaces are denoted. Time proceeds from top to bottom.
Speed and accuracy were both emphasized in the behavioral task, and correct responses were scored within a 200–800 ms period after translation onset. Correct responses to targets were categorized as ‘hits’ while responses to non-target upward translations were classified as ‘false alarms’. The hit and false alarm rates were used to derive the sensitivity estimate d’ (MacMillan & Creelman, 1991). Before the experimental recording sessions subjects practiced on the task for about 30 min. and achieved a target discrimination sensitivity of d’ ≥ 1.0.
Electrophysiological Recordings and Data Analysis
The electroencephalogram (EEG) was recorded from 64 electrode sites using a modified 10–20 system montage (Di Russo et al., 2003). Horizontal and vertical electro-oculograms (EOGs) were recorded by means of electrodes at the left and right external canthi and an electrode below the left eye, respectively. All electrodes were referenced to the right mastoid electrode during recording. Electrode impedances were kept below 5 kΩ.
The EEG was digitized at 250 Hz with an amplifier band pass of 0.1 – 80 Hz and gain of 10,000. Prior to signal averaging automated artifact rejection was performed to discard trials with eye movements, blinks or amplifier blocking. For each subject and condition ERPs were averaged time-locked to standard translations of both the attended and unattended surfaces (i.e., to non-target translations that had no associated erroneous button presses). The averages were digitally low-pass filtered with a Gaussian finite impulse function (3 dB attenuation at 46 Hz) to remove high frequency noise produced by muscle activity and external electrical sources. The filtered averages were digitally re-referenced to the average of the left and right mastoids. ERPs from all subjects were pooled to create grand-average waveforms. Attention effects were assessed by comparing the ERPs to attended standards vs. unattended standards, separately for both dichoptic and monocular viewing conditions. ERPs to the attended and unattended targets were also compared in each viewing conditions. Scalp topography maps and source localization analyses (see below) were based on attentional difference waves formed by subtracting the averaged unattended from the averaged attended waveforms for each viewing condition:
To quantify the significance of the attention effects, the prominent visual ERP components, P1 and N1 were measured as mean voltage amplitudes across 12 posterior electrode sites (6 in each hemisphere) where these components were largest. Mean amplitudes of the P1 (over 112–144 ms) and N1 (over 188–228 ms) components were tested for significance with respect to a 100 ms prestimulus baseline using t-tests. The time windows for the P1 and N1 components were centered around the peak latency of each component as measured in the grand-average waveform. Attention effects on the later P2 (240–280 ms), and the P300 (424–492 ms) component elicited by targets were also similarly characterized over 12 central electrode sites.
Scalp distributions of the P1, N1 and P2 components in the attentional difference waves were compared under the two viewing conditions after normalizing their amplitudes prior to ANOVA according to the method described by McCarthy and Wood (1985). For the posteriorly distributed P1 and N1 components comparisons were made over 22 occipital electrode sites (9 in each hemisphere and 4 along the midline). For the P2 component comparisons were made over 40 electrodes spanning frontal, central, parietal and occipital sites (16 in each hemisphere and 8 midline). Differences in scalp distribution were reflected in significant stimulus condition (Dichoptic/ Monocular) by electrode interactions.
Modeling of ERP Sources
Source localization was carried out to estimate the intracranial generators of each ERP component in the grand-averaged attentional difference waves. Source locations were estimated both by dipole modeling (Brain Electrical Source Analysis: BESA 2000, version 5) and by distributed linear inverse solutions based on a Local Auto-Regressive Average (LAURA, Grave de Peralta et al., 2001). The BESA algorithm estimates the location and the orientation of multiple equivalent dipolar sources by calculating the scalp distribution that would be obtained for a given dipole model (forward solution) and comparing it to the actual scalp-recorded ERP distribution (Scherg, 1990). The algorithm interactively adjusts (fits) the location and orientation of the dipole sources in order to minimize the relative variance (RV) between the model and the observed spatio-temporal ERP distribution. This analysis used the three-dimensional coordinates of each electrode site as recorded by a spatial digitizer. Symmetrical pairs of dipoles were fit sequentially to the components of interest within the same time intervals as those used for statistical testing. For example, dipole pairs to the N1 ERP component were fit after dipoles had been fit to the P1 component in the waveform. Dipole pairs were constrained to be mirror-symmetrical with respect to location but were free to vary in orientation.
LAURA (Grave de Peralta et al., 2001) estimates 3D current density distributions (rather than dipolar sources) using a realistic head model with a solution space of 4024 nodes equally distributed within the gray matter of the Montreal Neurological Institute's (MNI's) average template brain. LAURA makes no a priori assumptions regarding the number of sources or their locations and can deal with multiple simultaneously active sources (Michel et al., 2001). LAURA analyses were implemented using CARTOOL software by D. Brunet (http://brainmapping.unige.ch/cartool.php) to provide a visualization of the current source distributions underlying each component.
To estimate the anatomical brain regions giving rise to the component modulations, the current source distributions computed by LAURA and the locations of BESA source dipoles were transformed into the standardized coordinate system of Talairach and Tournoux (1988) and projected onto a structural brain image supplied by MRIcro (Rorden and Brett, 2000) using AFNI (Analysis of Functional NeuroImaging: Cox, 1996) software.
RESULTS
Behavioral results
The hit rates, sensitivity estimates (d’) and reaction times for detecting the target translations are given in Table 1. Performance under monocular viewing was significantly better than in dichoptic viewing: for hit rates (F(1,12) = 17.73, p< 0.002) and for d’ (F(1,12) = 9.16, p< 0.02). However, no difference in reaction times was found between the two conditions (F(1,12) = 0.53, p = n.s.). The eye to which the attended surface was projected did not affect either hit rate or d’ (F(1,12) = 0.002, p= n.s.), nor did the color of the attended dot surface (F(1,12) = 0.04, p= n.s.)
Table 1.
Behavioral performance (d’ scores and reaction times (RT)) for detecting the target translations on the attended dot surface in the dichoptic and monocular viewing conditions (n=13).
| Stimulus | Hit rate (%) | SEM Hit Rate (%) | d’ | SEM d’ | Mean RT (ms) | SEM RT (ms) |
|---|---|---|---|---|---|---|
| Dichoptic | 60.3 | 2.9 | 1.69 | 0.13 | 475 | 13 |
| Monocular | 68.1 | 2.9 | 1.99 | 0.14 | 471 | 11 |
ERP results
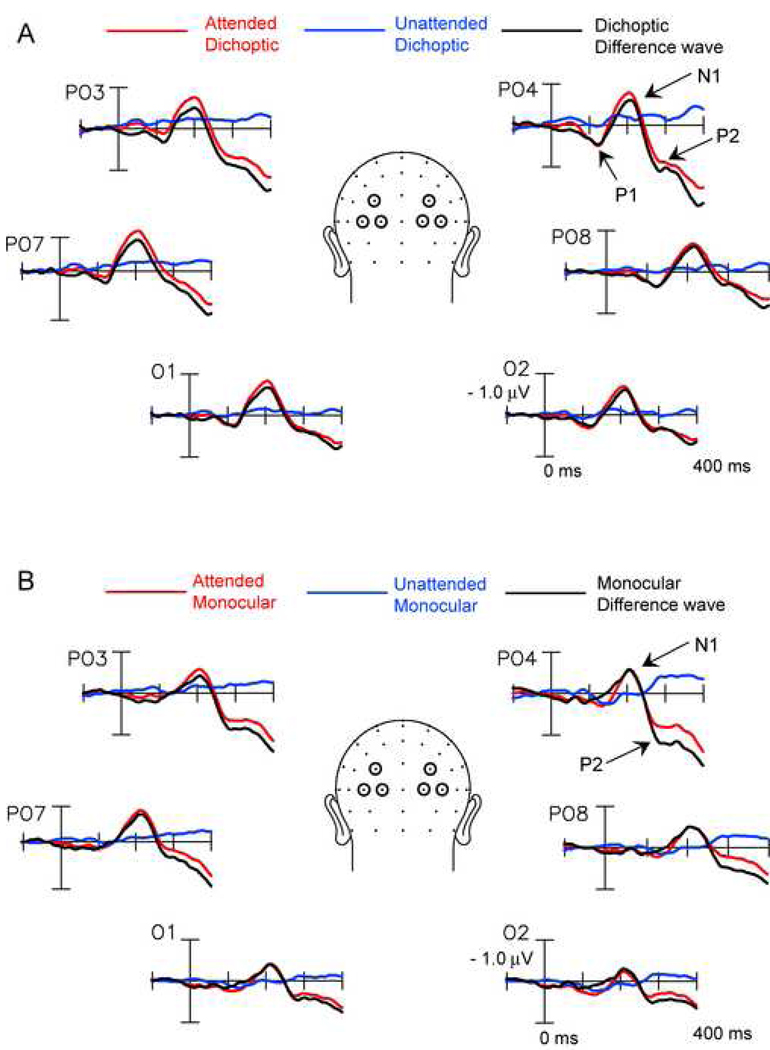
Since no behavioral differences were found based on eye of origin or color of stimulus, ERPs were collapsed across these factors. ERPs to the attended and unattended standard translations in the dichoptic and monocular viewing conditions are shown in Figure 2A and B respectively. ERPs to unattended stimuli over occipital sites showed greatly reduced amplitudes in both viewing conditions; none of the measured components (P1, N1, P2) was significantly greater than baseline in the unattended waveforms. Under dichoptic viewing ERPs elicited by attended translations showed significant P1 (peaking at 130 ms; t(12)=2.74, p< 0.02) and N1 (peaking at 210 ms; t(12) = 4.60, p< 0.0005) components. Attention effects on the P1 and N1 components seen in the dichoptic attentional difference wave (calculated as described in Methods) are listed in Table 2. A hemispheric difference was found in the P1 interval with the attention effect being larger over the right hemisphere (F(1,12) = 4.99, p< 0.05). The N1 component did not show any significant hemispheric asymmetry. As shown in Table 2, an attention effect was also obtained for the later P2 component that had a broad distribution over fronto-central electrode sites.
Figure 2.
Grand-average ERPs (n=13) associated with endogenous attention to one of two superimposed dot surfaces under dichoptic [A] and monocular [B] viewing. [A] ERPs elicited under dichoptic viewing by attended and unattended surface translations (standards) and the attend minus unattend attentional difference wave. Recordings are from three pairs of electrodes at parieto-occipital (PO3,4 and PO7,8) and occipital sites (O1,2). [B] ERPs as in [A] under monocular viewing.
Table 2.
Mean amplitudes of ERP components in the dichoptic and monocular attentional difference waves (ERPs to standard translations when attended minus when unattended). Component amplitudes were measured over scalp sites of maximal amplitude and tested for significance with respect to the 100 ms pre-stimulus baseline.
| Attention Difference Wave | Component | Amplitude (µV) | SEM (µV) | t(12) | p < |
|---|---|---|---|---|---|
| P1 (112–144 ms) | 0.25 | 0.05 | 4.68 | 0.0006 | |
| Dichoptic | N1 (188–228 ms) | −0.56 | 0.14 | 4.01 | 0.002 |
| P2 (240–280 ms) | 1.56 | 0.20 | 7.69 | 0.0001 | |
| P1 (112–144 ms) | 0.05 | 0.07 | 0.69 | n.s. | |
| Monocular | N1 (188–228 ms) | −0.46 | 0.12 | 3.80 | 0.003 |
| P2 (240–280 ms) | 1.09 | 0.20 | 5.50 | 0.0002 | |
During monocular viewing the ERP elicited by the attended stimuli showed a significant N1 component (t(12) = 4.30, p< 0.0009), but in contrast to the dichoptic viewing condition, no significant P1 was elicited (t(12) = 1.19, p = n.s.). The attentional difference wave during monocular viewing thus did not show any attention effect in the P1 latency range, while the posterior N1 and fronto-central P2 did show significant attention effects (Table2).
A highly significant P300 component was elicited by attended but not by unattended targets in both dichoptic (t(12) = 8.49, p < 0.0001) and monocular (t(12) = 8.09, p < 0.0001) viewing conditions (waveforms not shown). The amplitude of the target-elicited P300 component did not differ between the two conditions (F(1,12) = 1.42, p = n.s.).
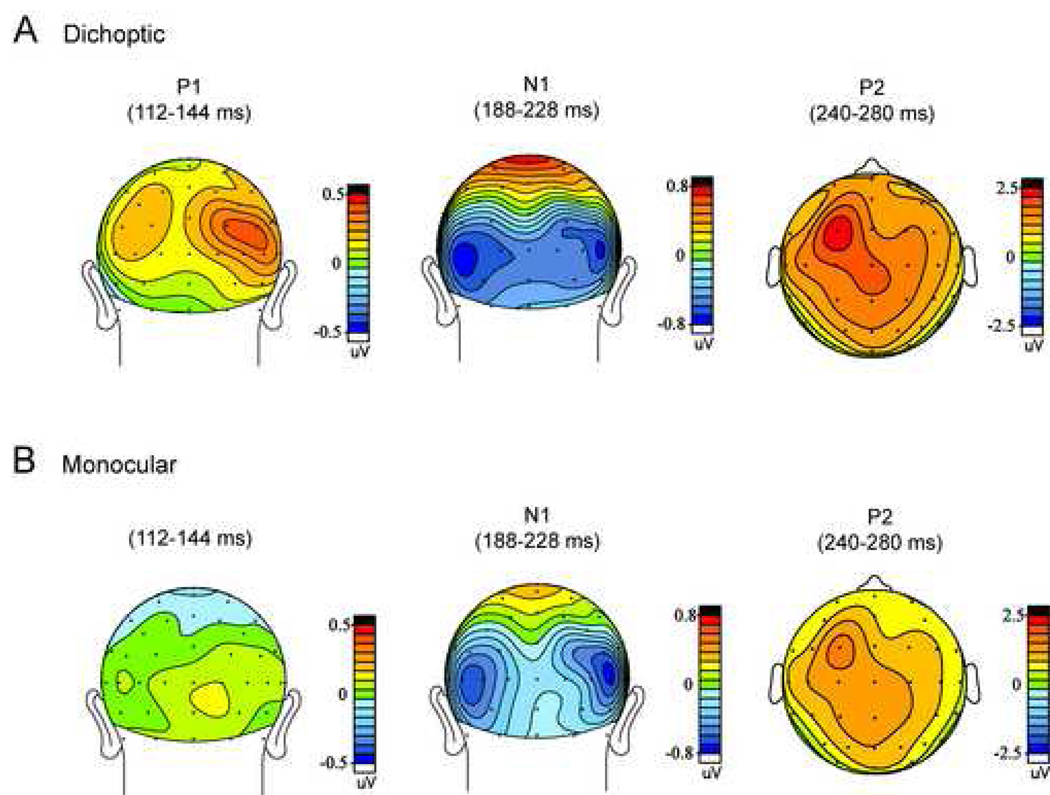
The scalp topographies of the attention effects in the P1, N1 and P2 latency windows were compared between the dichoptic and monocular viewing conditions (Fig. 3). Under dichoptic viewing (Fig. 3A), the attention-related P1 modulation was distributed over lateral occipital electrodes with greater amplitude over the right hemisphere. The enhanced N1 with attention was distributed bilaterally over lateral occipital sites, with a more ventral spread than the P1 effect. During monocular viewing (Fig. 3B) the difference wave distribution within the P1 latency window was no different from noise levels. This scalp distribution significantly differed from that of the dichoptic P1 attention effect (F(21,252) = 1.83, p<0.02). The topography of the N1 attention effect in the monocular attention difference wave did not differ from that of the dichoptic N1 effect (F(21,252) = 0.70, p = n.s.), nor did the distributions of the P2 difference wave component differ between the two conditions (F(39,468) = 1.07, p = n.s.).
Figure 3.
Topographical maps of ERP amplitudes in the P1, N1 (back view) and P2 (top view) latency windows in the attentional difference waves under [A] dichoptic and [B] monocular viewing.
Source Analysis
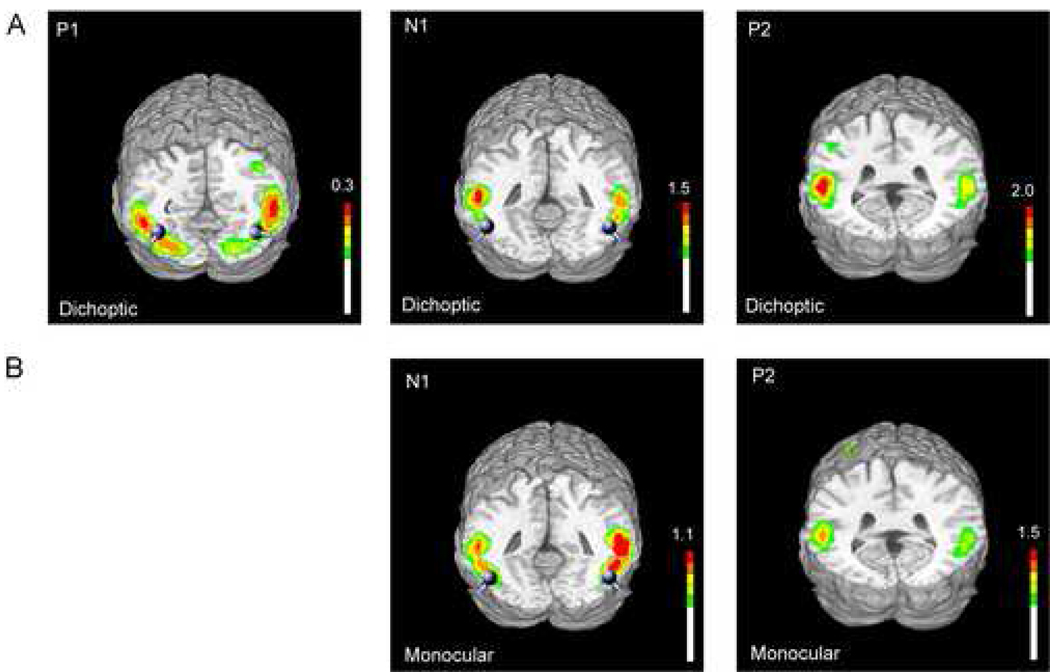
Using the BESA algorithm pairs of dipoles were fit to the scalp topographies of the P1 and N1 components in the grand-average dichoptic attentional difference wave and to the N1 component in the monocular attentional difference wave. The P1, N1 and P2 difference wave components were also modeled using a distributed minimum-norm approach (LAURA, Grave de Peralta et al., 2001). The location of the BESA dipoles and the generator sites estimated by LAURA were transformed into the standardized coordinate system of Talairach and Tournoux (1988) and superimposed on the rendered cortical surface of a single individual’s brain (Fig. 4). Talairach coordinates of the dipole pairs and an estimate of their goodness of fit as reflected by residual variance are listed in Table 3. In the case of the P2 component a satisfactory dipole model with low residual variance could not be achieved, and hence only the current source maxima as modeled by LAURA are reported.
Figure 4.
Estimated sources for the major visual ERP components in the grand-average attention difference waveforms in the [A] dichoptic (P1, N1 and P2 components) and [B] monocular (N1 and P2 components) viewing conditions modeled using LAURA and BESA. Sources and dipoles are projected onto a standard MRI rendered brain in Talairach space. BESA dipole fits are shown in gray. LAURA inverse solutions are represented in units of current source intensity (nA/mm3).
Table 3.
Talairach coordinates of BESA dipole pairs that modeled ERP components in the dichoptic and monocular attention difference waves. For the P2 component coordinates of the current source maxima as modeled by LAURA are shown. Percent residual variance not accounted for by the model over the time interval 112–228 ms is shown for each component.
| Attention Difference Wave | Component | x (mm) | y (mm) | z (mm) | Residual Variance (%) |
|---|---|---|---|---|---|
| P1 | ± 36 | − 58 | − 9 | 9 | |
| Dichoptic | N1 | ± 41 | − 67 | − 6 | 8 |
| P2 | ± 51 | − 35 | 6 | (LAURA only) | |
| Monocular | N1 | ± 49 | − 70 | 1 | 12 |
| P2 | ± 51 | − 36 | 6 | (LAURA only) | |
The dipole pair accounting for the P1 component in the dichoptic attentional difference wave was localized to ventro-lateral extrastriate visual cortex (Fig. 4A). The N1 components in both dichoptic and monocular difference waves were accounted for by dipole pairs in the same general region, about 10 mm more posterior than the P1 dipoles. As seen in Fig. 4, the LAURA estimates of current source intensity for each of these components corresponded well with the BESA source dipoles. For each modeled component, the locations of the maximum current source foci of the LAURA solution were within 10–15 mm of the dipoles fit using BESA. The later P2 components in the dichoptic and monocular difference waves as modeled by LAURA had bilateral sources in the vicinity of the superior temporal gyri.
DISCUSSION
This study used ERPs to investigate the neural correlates of endogenous attentional allocation to superimposed rivalrous counter-rotating dot surfaces. When the two surfaces were presented dichoptically (one to each eye), brief translations of the attended surface elicited much larger P1 (112–144 ms), N1 (188–228 ms) and P2 (240–280 ms) components of the ERP than did translations of the unattended surface. On the other hand, during monocular viewing (both surfaces presented to the same eye) the attended surface translations elicited enlarged N1 and P2 components relative to the unattended surface translations, but the P1 was not modulated by attention. These results indicate that attentional selection can bias neural activity at an earlier level of processing when attended and unattended surfaces are presented to the separate eyes.
Behaviorally, subjects were able to attend effectively to one surface under both dichoptic and monocular viewing conditions, although target detection rates were significantly higher during monocular trials. It is likely that target detection was hampered during dichoptic viewing because attention could not completely eliminate the spontaneous rivalry process, which caused the attended surface to be occasionally suppressed from view. Such an assumption is supported by previous research showing that endogenous attentional control of rivalry is relatively weaker than is attentional modulation of other forms of visual competition such as ambiguous figures (Meng and Tong, 2004, van Ee et al., 2006) or even exogenous attentional capture of a rivalrous surface (Chong and Blake, 2006). The small difference in target detection rates (68 vs. 60%) between the two viewing conditions might also be attributed to an overall difference in task difficulty resulting from interference with the attentional selection process by spontaneous rivalry. While a difference in difficulty might possibly affect the ERP attention effects, it seems unlikely that the modest difference in difficulty observed here would engender the substantial difference in attentional modulation of the P1 that was obtained between the dichoptic and monocular conditions.
The earliest effect of attentional selection during dichoptic viewing was found on the P1 component in the 112–144 ms range that was localized to lateral extrastriate visual cortex. In contrast, attentional selection during monocular viewing was not associated with modulation of the P1. This difference between the two viewing conditions was due to the presence of a significant P1 component in the ERPs to the attended stimuli under dichoptic viewing but not under monocular viewing. ERPs to the unattended stimuli did not show any significant components under either viewing condition. These results suggest that selective processing of the attended surface begins at an earlier stage (manifested in the P1 component) when the competing surfaces are presented to the separate eyes as opposed to the same eye. It should be cautioned, however, that the presence of P1 modulation only under dichoptic conditions might reflect an interaction between attentional selection and inter-ocular competition rather than an earlier level of selection.
In contrast with the present findings, an earlier ERP investigation of endogenous attention to superimposed rotating dot surfaces (presented under binocular viewing conditions) did in fact observe attentional modulation of the P1 component (Valdes-Sosa et al., 1998). Their differing results might have been due to much longer periods of sustained attention and greater attentional demands in a more difficult task of translation discrimination in all cardinal directions. Another possibility is that binocular viewing, while being perceptually similar to monocular presentation, may activate additional neural populations that are susceptible to attentional modulation in the P1 latency range.
The present results provide further evidence that the visual P1 attention effect is dissociable from attention effects on the N1, which has been demonstrated in previous ERP studies of visual spatial attention (Luck et al., 1990, 1994, reviewed in Hopfinger et al., 2004). The source localization of the P1 component obtained here is consistent with previous studies that localized the generators of the P1 to the lateral extrastriate visual cortex, which includes areas V3, V3a and V4 (Clark et al., 1995, Clark and Hillyard, 1996, Martinez et al., 1999, 2001, Di Russo et al., 2002). Within these extrastriate cortical regions inhibitory interactions between the eyes implemented in binocular neurons have been found to be very efficient, especially in areas V3 and V4. Dichoptic masking studies suggest that such inhibition builds up incrementally in strength at successively higher areas and is relatively weak in striate cortex itself (Hubel and Wiesel, 1961, Macknik and Martinez-Conde, 2004, Tse e al., 2005, Macknik, 2006). The present results suggest that attentional allocation may employ these inhibitory circuits to enhance inter-ocular selectivity during dichoptic viewing.
It should be noted, however, that the present results do not entirely rule out the possibility of an earlier attentional selection at the level of striate cortex, as suggested by many fMRI studies of binocular rivalry (Polonsky et al., 2000, Tong and Engel, 2001, Buchert et al., 2002, Lee et al., 2005). If such early selection did occur in primary visual cortex but did not produce an organized ERP field over the scalp (either due to low current strength, poor time-locking, or non-optimal cellular geometry), ERP recordings may fail to register the selection process. The consequences of such an early selection might then only become detectable at higher levels where neural activity was organized in such a way as to produce an enhanced ERP detectable at the surface.
Translations of the attended surface elicited enlarged N1 components (188–228 ms) relative to the unattended translations in both the dichoptic and monocular viewing conditions. The N1 source generators in both viewing conditions were localized to the ventro-lateral extrastriate visual cortex. These results fit well with previous source estimations of the N1 component elicited in association with object-selective attention (Martinez et al., 2006, 2007). In several studies of attentional selection, the N1 has been found to be associated with discriminative processing of visual information in the extrastriate cortex (Ritter et al., 1988, Vogel and Luck, 2000, Hopf et al., 2002, Martinez et al., 2006). The posterior N1 has also been consistently enhanced by attention in previous investigations of attentional selection of superimposed rotating dot surfaces (Valdes-Sosa et al., 1998, 2003, Pinilla et al., 2001, Lopez et al., 2004, Khoe et al., 2005, Rodríguez and Valdes-Sosa, 2006). Our results are in accord with these findings and provide further evidence that object (surface) selection is manifested in the N1 component generated within specialized regions of visual cortex where objects are represented (Martinez et al., 2007; Murray et al., 2002), not only under binocular viewing but also with dichoptic and monocular viewing.
The later P2 component was found to localize primarily to superior temporal cortex with some activity also present in the inferior parietal lobule. The origins of this component are not well understood, and its exact role in visual selection has remained elusive. Here we found that a robust P2 was elicited by the attended surface under both dichoptic and monocular viewing. Interestingly, the P2 attention effect was found to be somewhat greater under dichoptic viewing. Although a convincing interpretation of this result is not within reach, it could be that dichoptic attentional selection requires greater involvement of higher cortical areas to resolve competition than does monocular selection. Further investigations are required to decipher this effect.
In summary, we found that the earliest influence of attention on inter-ocular competition during rivalry was evident in an amplitude modulation of the P1 component of the visual ERP generated within extrastriate visual areas, followed by attention effects on the N1 and P2 components. In contrast, during monocular viewing only the later N1 and P2 components were modulated by attentional selection of competing surfaces. This is in line with recent evidence (Khoe et al., in press) that both the P1 and N1 components are modulated by exogenous attentional cueing of competing dot surfaces during rivalry, but only the N1 is modulated by exogenous cueing of monocularly presented surfaces. It appears from these parallel findings that attentional biasing of rivalrous surfaces affects visual processing in a similar way whether induced via top-down instructions to attend or bottom-up capture of attention. The neural processes that underlie this differential modulation of the P1 component under the two viewing conditions need further investigation using higher resolution recording techniques.
Acknowledgements
This work was supported by an NSF-IGERT Grant and by NIH Grant 9EY016984-33. The Cartool software (http://brainmapping.unige.ch/Cartool.php) was programmed by Denis Brunet, from the Functional Brain Mapping Laboratory, Geneva, Switzerland, and is supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnold DH, Grove PM, Wallis TS. Staying focused: a functional account of perceptual suppression during binocular rivalry. J Vis. 2007;7(7):1–8. doi: 10.1167/7.7.7. 7. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nat Rev Neurosci. 2002;3(1):13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Norcia AM. A method for investigating binocular rivalry in realtime with the steady-state VEP. Vision Res. 1997;37(17):2401–2408. doi: 10.1016/s0042-6989(97)00045-x. [DOI] [PubMed] [Google Scholar]
- Buchert M, Greenlee MW, Rutschmann RM, Kraemer FM, Luo F, Hennig J. Functional magnetic resonance imaging evidence for binocular interactions in human visual cortex. Exp Brain Res. 2002;145(3):334–339. doi: 10.1007/s00221-002-1121-x. [DOI] [PubMed] [Google Scholar]
- Chong SC, Tadin D, Blake R. Endogenous attention prolongs dominance durations in binocular rivalry. J Vis. 2005;5(11):1004–1012. doi: 10.1167/5.11.6. [DOI] [PubMed] [Google Scholar]
- Chong SC, Blake R. Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vision Res. 2006;46(11):1794–1803. doi: 10.1016/j.visres.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp. 1995;2:170–187. [Google Scholar]
- Clark VP, Hillyard SA. Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J Cogn Neurosci. 1996;8:387–402. doi: 10.1162/jocn.1996.8.5.387. [DOI] [PubMed] [Google Scholar]
- Cobb WA, Morton HB, Ettlinger G. Cerebral potentials evoked by pattern reversal and their suppression in visual rivalry. Nature. 1967;216(5120):1123–1125. doi: 10.1038/2161123b0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2002;15(2):95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cereb Cortex. 2003;13(5):486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- Freeman AW. Multistage model for binocular rivalry. J Neurophysiol. 2005;94(6):4412–4420. doi: 10.1152/jn.00557.2005. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta R, Gonzalez Andino S, Lantz G, Michel CM, Landis T. Noninvasive localization of electromagnetic epileptic activity. I. Method descriptions and simulations. Brain Topogr. 2001;14:131–137. doi: 10.1023/a:1012944913650. [DOI] [PubMed] [Google Scholar]
- Hancock S, Andrews TJ. The role of voluntary and involuntary attention in selecting perceptual dominance during binocular rivalry. Perception. 2007;36(2):288–298. doi: 10.1068/p5494. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Deichmann R, Rees G. Eye-specific effects of binocular rivalry in the human lateral geniculate nucleus. Nature. 2005;438(7067):496–499. doi: 10.1038/nature04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Predicting the stream of consciousness from activity in human visual cortex. Curr Biol. 2005;15(14):1301–1307. doi: 10.1016/j.cub.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Helmholtz Hv. In: Treatise on physiological optics. 3. Southhall JP, editor. New York: Dover; 1925. (original work published 1866) [Google Scholar]
- Hopf JM, Vogel E, Woodman G, Heinze HJ, Luck SJ. Localizing visual discrimination processes in time and space. J Neurophysiol. 2002;88(4):2088–2095. doi: 10.1152/jn.2002.88.4.2088. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Luck SJ, Hillyard SA. Selective attention: Electrophysiological and neuromagnetic studies. In: Gazzaniga MS, editor. The cognitive neurosciences. 3rd ed. Cambridge: MIT Press; 2004. [Google Scholar]
- Hubel DH, Wiesel TN. Integrative action in the cat's lateral geniculate body. J Physiol. 1961;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaernbach C, Schroger E, Jacobsen T, Roeber U. Effects of consciousness on human brain waves following binocular rivalry. Neuroreport. 1999;10(4):713–716. doi: 10.1097/00001756-199903170-00010. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA. Visual attention as a multilevel selection process. Cogn Affect Behav Neurosci. 2004;4(4):483–500. doi: 10.3758/cabn.4.4.483. [DOI] [PubMed] [Google Scholar]
- Khoe W, Mitchell JF, Reynolds JH, Hillyard SA. Exogenous attentional selection of transparent superimposed surfaces modulates early event-related potentials. Vision Res. 2005;45(24):3004–3014. doi: 10.1016/j.visres.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Khoe W, Mitchell JF, Reynolds JH, Hillyard SA. J Vis. ERP evidence that surface-based attention biases interocular competition during rivalry. in press. [DOI] [PubMed] [Google Scholar]
- Lansing RW. Electroencephalographic Correlates of Binocular Rivalry in Man. Science. 1964;146:1325–1327. doi: 10.1126/science.146.3649.1325. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blake R, Heeger DJ. Traveling waves of activity in primary visual cortex during binocular rivalry. Nat Neurosci. 2005;8(1):22–23. doi: 10.1038/nn1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Blake R, Heeger DJ. Hierarchy of cortical responses underlying binocular rivalry. Nat Neurosci. 2007;10(8):1048–1054. doi: 10.1038/nn1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys' percepts during binocular rivalry. Nature. 1996;379(6565):549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- Lopez M, Rodriguez V, Valdes-Sosa M. Two-object attentional interference depends on attentional set. Int J Psychophysiol. 2004;53(2):127–134. doi: 10.1016/j.ijpsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalogr Clin Neurophysiol. 1990;75(6):528–542. doi: 10.1016/0013-4694(90)90139-b. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20(4):887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Macknik SL, Martinez-Conde S. Dichoptic visual masking reveals that early binocular neurons exhibit weak interocular suppression: implications for binocular vision and visual awareness. J Cogn Neurosci. 2004;16(6):1049–1059. doi: 10.1162/0898929041502788. [DOI] [PubMed] [Google Scholar]
- Macknik SL. Visual masking approaches to visual awareness. Prog Brain Res. 2006;155:177–215. doi: 10.1016/S0079-6123(06)55011-3. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. New York: Cambridge University Press; 1991. Detection theory: A user's guide. [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2(4):364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Martinez A, DiRusso F, Anllo-Vento L, Sereno MI, Buxton RB, Hillyard SA. Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Res. 2001;41(10–11):1437–1457. doi: 10.1016/s0042-6989(00)00267-4. [DOI] [PubMed] [Google Scholar]
- Martinez A, Teder-Salejarvi W, Vazquez M, Molholm S, Foxe JJ, Javitt DC, Di Russo F, Worden MS, Hillyard SA. Objects are highlighted by spatial attention. J Cogn Neurosci. 2006;18(2):298–310. doi: 10.1162/089892906775783642. [DOI] [PubMed] [Google Scholar]
- Martinez A, Ramanathan DS, Foxe JJ, Javitt DC, Hillyard SA. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963–7973. doi: 10.1523/JNEUROSCI.0031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol. 1985;62(3):203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Meng M, Tong F. Can attention selectively bias bistable perception? Differences between binocular rivalry and ambiguous figures. J Vis. 2004;4(7):539–551. doi: 10.1167/4.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Thut G, Morand S, Khateb A, Pegna AJ, Grave de Peralta R, Gonzalez S, Seeck M, Landis T. Electric source imaging of human brain functions. Brain Res Brain Res Rev. 2001;36(2–3):108–118. doi: 10.1016/s0165-0173(01)00086-8. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Stoner GR, Reynolds JH. Object-based attention determines dominance in binocular rivalry. Nature. 2004;429(6990):410–413. doi: 10.1038/nature02584. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Keliris G, Kourtzi Z, Logothetis N. A binocular rivalry study of motion perception in the human brain. Vision Res. 2005;45(17):2231–2243. doi: 10.1016/j.visres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Murray MM, Wylie GR, Higgins BA, Javitt DC, Schroeder CE, Foxe JJ. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. J Neurosci. 2002;22(12):5055–5073. doi: 10.1523/JNEUROSCI.22-12-05055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ. Delayed striate cortical activation during spatial attention. Neuron. 2002;35(3):575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- Ooi TL, He ZJ. Binocular rivalry and visual awareness: the role of attention. Perception. 1999;28(5):551–574. doi: 10.1068/p2923. [DOI] [PubMed] [Google Scholar]
- Pearson J, Tadin D, Blake R. The effects of transcranial magnetic stimulation on visual rivalry. J Vis. 2007;7(7):1–11. doi: 10.1167/7.7.2. 2. [DOI] [PubMed] [Google Scholar]
- Pinilla T, Cobo A, Torres K, Valdes-Sosa M. Attentional shifts between surfaces: effects on detection and early brain potentials. Vision Res. 2001;41(13):1619–1630. doi: 10.1016/s0042-6989(01)00039-6. [DOI] [PubMed] [Google Scholar]
- Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3(11):1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Ritter W, Simson R, Vaughan HG., Jr Effects of the amount of stimulus information processed on negative event-related potentials. Electroencephalogr Clin Neurophysiol. 1988;69(3):244–258. doi: 10.1016/0013-4694(88)90133-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez V, Valdes-Sosa M. Sensory suppression during shifts of attention between surfaces in transparent motion. Brain Research. 2006;1072:110–118. doi: 10.1016/j.brainres.2005.10.071. [DOI] [PubMed] [Google Scholar]
- Roeber U, Schröger E. Binocular rivalry is partly resolved at early processing stages with steady and with flickering presentation: a human event-related brain potential study. Neurosci Lett. 2004;371(1):51–55. doi: 10.1016/j.neulet.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Scherg M. Fundamentals of dipole source analysis. In: Grandori F, Hoke M, Roman GL, editors. Auditory evoked magnetic fields and electric potentials. 1990. pp. 40–69. [Google Scholar]
- Sheinberg DL, Logothetis NK. The role of temporal cortical areas in perceptual organization. Proc Natl Acad Sci U S A. 1997;94(7):3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Logothetis NK. Temporal frequency and contrast tagging bias the type of competition in interocular switch rivalry. Vision Res. 2007;47(4):532–543. doi: 10.1016/j.visres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Russell DP, Edelman GM, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J Neurosci. 1999;19(13):5435–5448. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Petrovic S. MEG phase follows conscious perception during binocular rivalry induced by visual stream segregation. Cereb Cortex. 2006;16(5):597–608. doi: 10.1093/cercor/bhj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner GR, Mitchell JF, Fallah M, Reynolds JH. Interacting competitive selection in attention and binocular rivalry. Prog Brain Res. 2005;149:227–234. doi: 10.1016/S0079-6123(05)49016-0. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York: Thieme; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Tong F, Engel SA. Interocular rivalry revealed in the human cortical blind-spot representation. Nature. 2001;411(6834):195–199. doi: 10.1038/35075583. [DOI] [PubMed] [Google Scholar]
- Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci. 2006;10(11):502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Tse PU, Martinez-Conde S, Schlegel AA, Macknik SL. Visibility, visual awareness, and visual masking of simple unattended targets are confined to areas in the occipital cortex beyond human V1/V2. Proc Natl Acad Sci U S A. 2005;102(47):17178–17183. doi: 10.1073/pnas.0508010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Sosa M, Bobes MA, Rodriguez V, Pinilla T. Switching attention without shifting the spotlight object-based attentional modulation of brain potentials. J Cogn Neurosci. 1998;10(1):137–151. doi: 10.1162/089892998563743. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa M, Bobes MA, Rodriguez V, Acosta Y, Perez JI, Borrego M. The influence of scene organization on attention: Psychophysics and electrophysiology. In: Duncan NKaJ., editor. Functional neuroimaging of visual cognition. Oxford, UK: Oxford University Press; 2003. pp. 321–344. [Google Scholar]
- Valle-Inclan F, Hackley SA, de Labra C, Alvarez A. Early visual processing during binocular rivalry studied with visual evoked potentials. Neuroreport. 1999;10(1):21–25. doi: 10.1097/00001756-199901180-00004. [DOI] [PubMed] [Google Scholar]
- van Ee R, Noest AJ, Brascamp JW, van den Berg AV. Attentional control over either of the two competing percepts of ambiguous stimuli revealed by a two-parameter analysis: means do not make the difference. Vision Res. 2006;46(19):3129–3141. doi: 10.1016/j.visres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37(2):190–203. [PubMed] [Google Scholar]
- Wilson HR. Computational evidence for a rivalry hierarchy in vision. Proc Natl Acad Sci U S A. 2003;100(24):14499–14503. doi: 10.1073/pnas.2333622100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Schneider KA, Kastner S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat Neurosci. 2005;8(11):1595–1602. doi: 10.1038/nn1554. [DOI] [PMC free article] [PubMed] [Google Scholar]