Abstract
We have previously reported initial clinical feasibility with our small diameter tissue engineered blood vessel (TEBV). Here we present in vitro results of the mechanical properties of the TEBVs of the first 25 patients enrolled in an arterio-venous (A-V) shunt safety trial, and compare these properties with those of risk-matched human vein and artery. TEBV average burst pressures (3,490 +/− 892 mmHg, n=230) were higher than native saphenous vein (SV) (1,599 +/− 877 mmHg, n=7), and not significantly different than native internal mammary artery (IMA) (3,196 +/− 1,264 mmHg, n=16). Suture retention strength for the TEBVs (152 +/− 50 gmf) was also not significantly different than IMA (138 +/− 50 gmf). Compliance for the TEBVs prior to implantation (3.4 +/− 1.6 %/100 mmHg) was lower than IMA (11.5 +/− 3.9 %/100 mmHg). By 6 months post-implant, the TEBV compliance (8.8 +/− 4.2 %/100 mmHg, n=5) had increased to values comparable to IMA, and showed no evidence of dilation or aneurysm formation. With clinical time points beyond 21 months as an A-V shunt without intervention, the mechanical tests and subsequent lot release criteria reported here would seem appropriate minimum standards for clinical use of tissue engineered vessels.
INTRODUCTION
For both peripheral and coronary revascularization, autologous vein and artery are clearly the gold standard for surgical reconstruction [1, 2]. When native vein and artery are not available due to previous harvest, anatomical limitations, or disease progression, synthetic materials such as Dacron or ePTFE have been used with varying degrees of success. Synthetic graft materials are used with great success in larger diameter applications such as aortic or iliac reconstruction, but they have demonstrated unacceptably poor performance in most small diameter applications (below 6 mm inside diameter). The poor efficacy of small diameter synthetics is linked to short-term thrombosis, increased rate of infection, chronic inflammatory responses to the foreign materials, and compliance mismatch between the native tissue and the prosthetic material [3–8]. These problems are well illustrated in A-V access grafts, where the intervention rates for synthetics are three-fold higher than for native vein fistulas [9]. Arguably, the problems are even more pronounced in coronary and below-knee revascularization where poor efficacy essentially precludes synthetics from widespread clinical use [2, 8].
The field of Cardiovascular Tissue Engineering has attempted to produce a clinically viable synthetic conduit by using a variety of in vitro approaches that typically combine living cells seeded into reconstituted scaffolds to create living tissue engineered blood vessels (TEBVs) [10]. One of the key limitations to cell-based approaches, however, has been a lack of mechanical strength and a subsequent reliance upon synthetic scaffolds [10–13]. The use of synthetic material, however, re-introduces the original limitations that tissue engineering was aiming to overcome in the first place. This paradox has driven the evolution towards either completely autologous approaches or scaffolds which are partially resorbable [14–17]. We have developed an approach called sheet-based tissue engineering (SBTE) that uses dermal fibroblasts cultured in conditions that promote the production of extracellular matrix (ECM) proteins [14]. The fibroblasts, embedded in their own ECM, form a robust sheet that can be rolled into tubes to make extremely strong conduits that do not rely upon any exogenous scaffolds. The multi-ply roll is matured to fuse into a cohesive tissue, which can then be seeded with endothelial cells to make a completely autologous tissue engineered blood vessel called the Lifeline™ vascular graft. Using this approach, we previously reported short to mid term in vivo results using TEBVs built with human cells derived from a single patient xenografted into various animal models [18]. Although these results were encouraging, the question remained whether or not clinically relevant vessels could be consistently created using cells taken from a broad spectrum of patients with advanced cardiovascular disease. This challenge of demonstrating functional blood vessels for an age and risk appropriate patient population has been identified as the fundamental hurdle in Cardiovascular Tissue Engineering [19]. In this study we therefore built over 250 TEBVs using cells harvested from 25 patients suffering from end-stage renal disease, lower-limb ischemia, or coronary artery disease. In order to quantitatively assess the clinical relevance of each patient’s TEBVs, we compared the burst pressure, suture retention strength, and compliance of the engineered vessels to that of native human vessels harvested from elderly patients with advanced cardiovascular disease. To confirm that an acute test such as the burst pressure test was an adequate gauge of mechanical strength, we also tested the fatigue resistance of the TEBVs by statically or dynamically loading the vessels for prolonged periods of time. Our results demonstrated that the SBTE approach can consistently produce vessels with mechanical properties similar or superior to that of native vein across a broad spectrum of patients. Based on these positive mechanical properties, we have implanted our first six patients which is the first demonstration of a completely biological TEBV to be used in humans [20]. With clinical time points beyond 21 months as an A-V shunt without intervention, the mechanical tests and subsequent lot release criteria reported here would seem appropriate minimum standards for clinical use of other engineered vessels.
MATERIALS AND METHODS
Human TEBV production
TEBVs were built using a process termed sheet-based tissue engineering as described elsewhere [18]. Skin biopsies were taken from 25 patients: 17 with end stage renal disease with failing hemodialysis grafts, 5 with lower-limb ischemia, and 3 with coronary artery disease. In brief, fibroblasts were isolated from the skin biopsy via collagenase digestion and seeded on 225cm2 tissue culture flasks. Medium was exchanged three times per week. The cultures were maintained for typically 6–8 weeks to produce collagen-rich, living, fibroblast sheets. Fibroblast sheets were detached from the cell culture substrate and rolled (4 revolutions) around a 4.75mm OD stainless steel cylinder approximately 21 cm in length. The vessels were then placed back in culture for a 12-week maturation phase to allow the layers of the roll to fuse. This tissue was dehydrated by air drying for several hours in a tissue culture hood, thus forming an acellular internal membrane (IM). A second living fibroblast sheet was then rolled (4 revolutions) around the IM, and matured in a similar fashion. At this point the vessels were removed from the stainless steel support cylinder and used for mechanical testing. For shelf life testing, the second maturation phase was extended by up to 28 weeks beyond the normal completion point. Finished vessels were 17–21 cm long, and were sectioned for mechanical testing. In all cases, the reported n refers to these test segments.
Native vessel procurement
Unused internal mammary artery (IMA) and saphenous vein (SV) segments that were harvested from patients undergoing coronary artery bypass grafting were placed in transport medium and stored on ice until testing. Twenty-two native vessels (18 IMA, 4 SV) were procured from eighteen patients. All native vessel donors (14 male, 4 female; median age 66, range 49 to 87 years) had one or more of the following cardiovascular risk factors or co-morbidities: renal disease, diabetes, hypertension, coronary artery disease, myocardial infarction, or heart failure. The study protocol was approved by the Institutional Review Board at Stanford University, and informed consent was obtained from all patients. IMA and SV were also procured from fresh cadavers through a tissue bank (LifeNet Health, Virginia Beach, VA) and transported to the lab in a similar fashion. Fat and connective tissue, if present, were dissected away to obtain access to the ends of the vessels for cannulation to the test apparatus. Collateral vessels were ligated with silk sutures and/or ligaclips, most at time of harvest.
Compliance measurements
Compliance measurements were made following the general recommendations defined in ANSI/AAMI/ISO 7198:1998/2001 “Cardiovascular implants – tubular vascular prostheses” (ANSI 7198) [21], which describes standardized testing methods for vascular grafts. Segments of vessels approximately 6 cm in length were tensioned to 0.460 N, and pressurized with phosphate-buffered saline (PBS). High resolution digital images were recorded at 50 and 200mmHg, and used to measure the external diameter. Small ringlets from the same vessels were fixed in formalin prior to testing to obtain geometry at rest from histology cross sections. The inside diameter and wall thickness values at rest were obtained by analyzing calibrated magnified pictures of the histology slides. Assuming an incompressible wall, compliance was calculated as follows, and reported as % per 100mmHg as specified in the ANSI 7198 standard:
where:
p1 = lower pressure
p2 = higher pressure
Ripx = internal radius at pressure x, and is calculated from:
where:
Ropx = measured external radius at pressure x
Ri = measured internal radius at rest
t0 = measured wall thickness at rest
Compliance measurements in human patients implanted with the Lifeline™ TEBV were made by analyzing the change in diameter of the vessel throughout the cardiac cycle using ultrasound to visualize the graft near the midpoint. Blood pressure during the ultrasound exam was recorded.
Burst pressure measurements
Burst pressure tests were conducted as specified in ANSI 7198. Segments of vessels approximately 6 cm in length were cannulated and pressurized with PBS at a rate of 80 to 100 mmHg/sec until failure. A custom LabView (National Instruments, Inc.) data acquisition system in conjunction with a digital pressure gauge (PG10000, by PSI-Tronix,) and a computer was used to record the pressure at a sampling rate of 3Hz. In all cases, rupture occurred at a location away from the cannulation site.
Suture retention testing
Suture retention tests were conducted as specified in ANSI 7198. Segments of vessels approximately 15 mm in length were cannulated onto a vertical metal mandrel which itself was attached to a base weighing 2 kg, and placed on a digital scale (Navigator, by Ohaus). A single 2mm bite of 5-0 prolene suture with BV-1 needle (Ethicon Inc.) was placed at the end of the vessel segment, and pulled out at a constant rate of 120 mm/min until failure. The force curve was measured digitally using a LabView data acquisition system sampling the scale output at 5Hz. The test was repeated two more times on the same sample at locations 120 degrees apart to obtain three values for each vessel test segment.
Dynamic fatigue test
Controlled cyclic loading of the vessels was accomplished via a closed, sterile flow loop that was fed by a pressurized reservoir. The pressure in the reservoir was controlled by a mechanically-actuated valve and a regulated pressure source. Needle valves up- and downstream of the reservoir tuned the fill and leak rates of the reservoir, which allowed fine control of the pressure wave across the vessel. Transmural pressure across the test segment was recorded using a pressure transducer (PX-26, by Omega, Inc.) and a LabView data acquisition system. Pressure was cycled at 1 Hz at either 120/80 mmHg for 14 days or 600/300 mmHg for 3 days. Dynamically fatigued test segments were then burst as described above and compared to unloaded controls.
Static fatigue test
Static loading of the vessels was accomplished by using an elevated reservoir. Pressure across the vessel test segment was intermittently monitored using a digital pressure gauge (Model 68370-02, Cole-Parmer, Inc.). Pressure was maintained at 250 mmHg for 5 days and then test segments were burst as described above and compared to unloaded controls.
Step-wise fatigue test
All step-wise fatigue tests were carried out on internal membranes alone; i.e. the living adventitia layers were not added to the vessel, thus burst pressures were approximately half of what would be expected with a full TEBV. Vessel segments were loaded into the burst pressure apparatus and pressurized to 1,200 mmHg, which was expected to produce approximately 80% of the ultimate yield stress for a saphenous vein. If after 15 minutes the vessel had not ruptured, the pressure was increased to 1,400 mmHg. If after an additional 15 minutes the vessels still had not ruptured, the pressure was increased to 1,500 mmHg until failure. Time and pressure at failure were recorded.
Vessel ringlet pull test
A uniaxial tensioning apparatus that pulls to failure a small ringlet of tissue was used as described by Seliktar et al [22], and an estimated burst pressure was calculated using Laplace’s relationship as described by Nieponice et al [16]. To conduct the test, a 5 mm long segment of vessel was placed around two parallel hooks made of stainless steel wire 1.5 mm in diameter. The hooks were then pulled apart at a rate of 40 mm/min until tissue failure. The force curve was measured digitally using a LabView data acquisition system sampling at 5Hz. The tests were filmed to verify that failure occurred in the middle of the tissue away from the hooks.
Student’s t test was used to evaluate statistical difference between groups.
RESULTS
TEBV and native vessel morphology
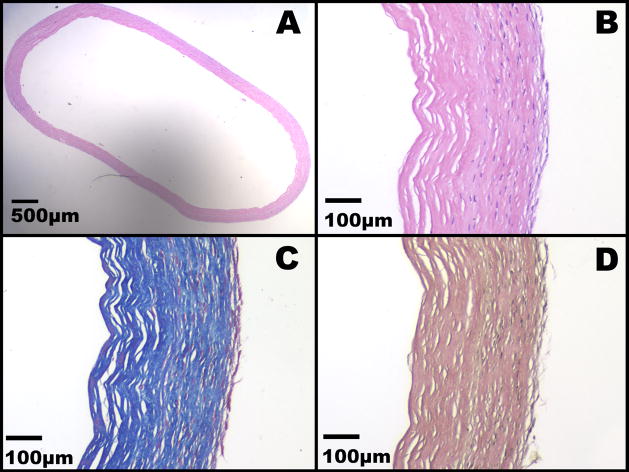
A total of 282 TEBVs were produced from cells harvested from a total of twenty five patients. There were 14 males and 11 females; median age 62, range 26 to 89 years. Each TEBV was approximately 21 cm in length. The inside diameter of the TEBVs were either 2.4 mm, 4.8 mm, or 6.6 mm for radial artery, A-V shunt, and lower limb indications, respectively. The internal diameter of the native vessels ranged from 1.5mm to 4.5mm. The wall thickness of the TEBV’s ranged from 0.2mm (IMs only) to 0.6mm. The wall thickness of the native vessels ranged from 0.3mm to 0.8mm. Native vessels and TEBV’s were from similar, risk-appropriate populations; they were older patients suffering from advanced vascular disease. There were 18 native vessel donors (14 male, 4 female; median age 66, range 49 to 87 years. TEBV patient and native vessel patient demographics are listed in Tables 1 and 2, respectively. Macroscopically, the TEBV was as a flexible tubular tissue with a homogenous appearance and an even diameter. Histological appearance of the TEBV’s showed an acellar IM wrapped by a living adventitia (Figure 1). The histology revealed the laminated aspect of the construct and confirmed the fusion of individual layers of both the IM and the adventitia, as well as the fusion of the IM with the adventitia. As expected, living fibroblasts (as judged by the absence of signs of necrosis or apodosis such as nuclear fragmentation and pyknosis) were observed only in the adventitial layers. While cell distribution was generally homogenous, a cell-rich layer of fibroblast of various thickness was see on the outer surface of the construct (better seen on Figure 1c). No living cells were observed in the devitalized IM. Endothelial cells are not observed as TEBVs used for mechanical testing were not endothelialized. Both the IM and the adventitia were comprised of a dense collagen network as judged by the Masson’s Tri-chrome (Figure 1c). The IM appears to have more lacunae possibly caused by the removal of the fibroblast. No elastic fibers were evident prior to implantation based on Verhoeff-Van Gieson stain (Figure 1d).
Table I.
TEBV mechanical properties and source patient characteristics
Patient demographics and individual mechanical properties of TEBVs
| Patient Demographics |
Comorbidities |
Graft Indication | Mechanical Properties |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Age | Sm | Diab | CH | PGI | PGF | Internal Diameter (mm) | Burst Pressure (mmHg) | Suture Retention (grams-force) | Compliance (%/100mmHg) | |
| 1 | F | 56 | x | x | x | AV | 4.8 | 3,123 +/− 340 (n=12) (range: 2,596–3,759) | 188 +/− 36 (n=36) (range: 103–250) | 2.8 +/− 1.5 (n=5) (range: 0.9–4.0) | ||
| 2 | M | 61 | CS | x | x | x | x | AV | 4.8 | 2,664 +/− 347 (n=10) (range: 2,102–3,386) | 142 +/− 16 (n=30) (range: 113–173) | 4.7 +/− 1.0 (n=5) (range: 3.6–5.5) |
| 3 | F | 68 | x | x | x | AV | 4.8 | 4,617 +/− 426 (n=12) (range: 3,969–5,193) | 210 +/− 27 (n=36) (range: 163–268) | 2.8 +/− 0.7 (n=6) (range: 2.0–3.5) | ||
| 4 | M | 77 | x | x | x | AV | 4.8 | 3,019 +/− 594 (n=12) (range: 1,675–3,708) | 149 +/− 21 (n=36) (range: 87–186) | 2.9 +/− 1.9 (n=6) (range: 1.4–6.0) | ||
| 5 | M | 29 | x | x | x | AV | 4.8 | 3,657 +/− 268 (n=12) (range: 3,143–4,040) | 162 +/− 23 (n=36) (range: 103–203) | 2.5 +/− 1.9 (n=6) (range: 0.3–5.3) | ||
| 6 | M | 78 | PS | x | x | x | AV | 4.8 | 2,348 +/− 221 (n=12) (range: 2,000–2,840) | 75 +/− 10 (n=36) (range: 56–103) | 4.1 +/− 3.7 (n=6) (range: 0.9–9.3) | |
| 7 | M | 89 | x | x | x | AV | 4.8 | 4,140 +/− 690 (n=10) (range: 3,171–4,945) | 221 +/− 14 (n=30) (range: 191–256) | 3.8 +/− 1.0 (n=5) (range: 2.7–5.2) | ||
| 8 | F | 62 | CS | x | x | x | AV | 4.8 | 2,582 +/− 330 (n=6) (range: 2,111–2,915) | 98 +/− 13 (n=15) (range: 77–128) | 2.1 +/− 1.8 (n=3) (range: 0.8–4.1) | |
| 9 | F | 58 | x | x | AV | 4.8 | 4,424 +/− 898 (n=10) (range: 2,255–5,159) | 199 +/− 23 (n=30) (range: 149–266) | 2.6 +/− 1.2 (n=6) (range: 1.1–4.5) | |||
| 10 | F | 54 | CS | x | x | x | x | AV | 4.8 | 4,542 +/− 453 (n=10) (range: 3,719–5,315) | 112 +/− 12 (n=30) (range: 87–146) | 3.5 +/− 0.7 (n=5) (range: 2.4–4.1) |
| 11 | M | 71 | x | x | x | x | AV | 4.8 | 2,412 +/− 319 (n=10) (range: 1,855–2,959) | 180 +/− 21 (n=30) (range: 132–218) | 3.4 +/− 0.7 (n=5) (range: 2.8–4.4) | |
| 12 | F | 67 | x | x | AV | 4.8 | 4,994 +/− 594 (n=6) (range: 4,170–5,763) | 212 +/− 19 (n=18) (range: 188–250) | 3.1 +/− 0.3 (n=3) (range: 2.9–3.4) | |||
| 13 | F | 81 | x | x | x | x | AV | 4.8 | 3,426 +/− 271 (n=8) (range: 2,922–3,726) | 165 +/− 22 (n=24) (range: 110–221) | 4.6 +/− 0.2 (n=4) (range: 2.9–3.4) | |
| 14 | M | 26 | CS | x | x | x | AV | 4.8 | 4,341 +/− 506 (n=8) (range: 3,698–4,957) | 187 +/− 20 (n=24) (range: 158–227) | 3.6 +/− 1.4 (n=4) (range: 2.7–5.7) | |
| 15 | F | 59 | x | x | x | AV | 4.8 | 2,803 +/− 208 (n=4) (range: 2,600–3,045) | 141 +/− 12 (n=12) (range: 122–163) | 6.1 +/− 0.1 (n=2) (range: 6.0–6.1) | ||
| 16 | F | 76 | PS | LL | 6.6 | 3,358 +/− 499 (n=5) (range: 2,870–4,122) | 291 +/− 55 (n=15) (range: 159–359) | 2.5 +/− 2.6 (n=3) (range: 0.4–5.3) | ||||
| 17 | M | 62 | PS | x | LL | 6.6 | 3,035 +/− 315 (n=5) (range: 2,691–3,454) | 157 +/− 18 (n=12) (range: 130–184) | 2.1 +/− 1.1 (n=3) (range: 0.9–3.0) | |||
| 18 | M | 75 | PS | x | x | x | LL | 6.6 | 3,101 +/− 364 (n=8) (range: 2,683–3,770) | 131 +/− 12 (n=24) (range: 108–152) | 3.8 +/− 2.0 (n=5) (range: 1.6–6.2) | |
| 19 | M | 47 | PS | x | x | x | AV | 4.8 | 4,549 +/− 380 (n=4) (range: 4,120–5,032) | 184 +/− 20 (n=12) (range: 154–220) | 4.5 +/− 0.2 (n=2) (range: 4.4–4.7) | |
| 20 | M | 77 | PS | x | x | x | LL | 6.6 | 3,693 +/− 457 (n=9) (range: 2,726–4,238) | 119 +/− 15 (n=24) (range: 82–142) | 4.1 +/− 0.4 (n=3) (range: 3.7–4.5) | |
| 21 | M | 73 | x | x | x | LL | 6.6 | 3,650 +/− 338 (n=7) (range: 3,124–3,988) | 171 +/− 12 (n=18) (range: 132–187) | 2.3 +/− 0.3 (n=3) (range: 2.0–2.7) | ||
| 22 | M | 67 | x | x | x | x | RAD | 2.4 | 2,285 +/− 220 (n=8) (range: 2,001–2,617) | 95 +/− 24 (n=24) (range: 61–155) | not performed | |
| 23 | F | 59 | x | RAD | 2.4 | 3,467 +/− 422 (n=10) (range: 2,835–4,163) | 138 +/− 27 (n=12) (range: 96–182) | 4.1 +/− 0.4 (n=3) (range: 3.0–5.3) | ||||
| 24 | F | 45 | x | AV | 4.8 | 3,970 +/− 515 (n=20) (range: 2,842–4,817) | 104 +/− 14 (n=60) (range: 70–148) | 3.0 +/− 1.5 (n=10) (range: 0.7–5.8) | ||||
| 25 | M | 57 | PS | RAD | 2.4 | 4,832 +/− 897 (n=8) (range: 3,880 + 6,269) | 123 +/− 23 (n=24) (range: 57–170) | 3.2 +/− 1.2 (n=4) (range: 2.1–4.3) | ||||
Sm: Smoker PS: Previous smoker CS: Current smoker Diab: Diabetes CH: Controlled hypertension PGI: Previous graft intervention PGF: Prev. graft failure LL: Lower limb AV: A-V shunt RAD: Radial artery replacement
Table II.
Demographics of donor patients for native vessels
| Patient Demographics |
Comorbidities |
||||
|---|---|---|---|---|---|
| ID | Gender | Age | CAD | Diab | CH |
| A | M | 61 | x | ||
| B | M | 75 | x | ||
| C | M | 50 | x | ||
| D | M | 72 | x | x | |
| E | M | 87 | x | ||
| F | M | 75 | x | ||
| G | M | 73 | x | x | x |
| H | M | 58 | x | ||
| I | M | 62 | x | ||
| J | M | 73 | x | ||
| K | F | 65 | x | ||
| L | F | 49 | x | x | |
| M | F | 59 | x | ||
| N | M | 53 | x | ||
| O | M | 72 | x | ||
| P | M | 57 | x | x | |
| Q | F | 70 | x | ||
| R | M | 68 | x | ||
CAD: Coronary artery disease Diab: Diabetes CH: Controlled hypertension
Figure 1. Histological analysis of the TEBVs.
A) H&E stain of the TEBV at 2x original magnification. B) H&E stain of the TEBV at 10x original magnification. C) Masson’s trichrome stain of the TEBV at 10x original magnification. D) Verhoeff-Van Gieson stain of the TEBV at 10x original magnification.
Mechanical Properties
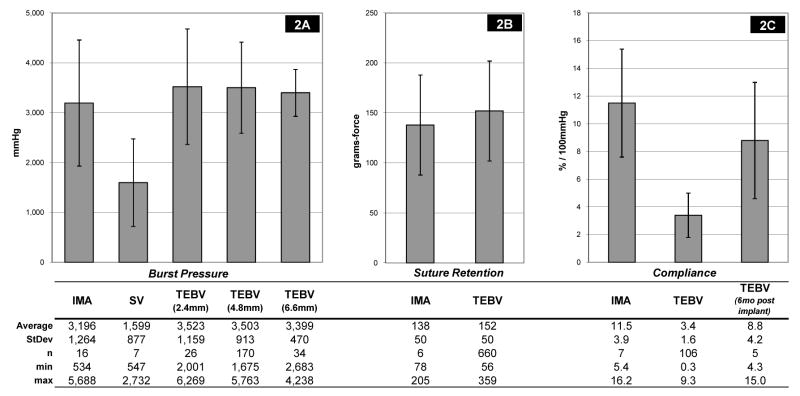
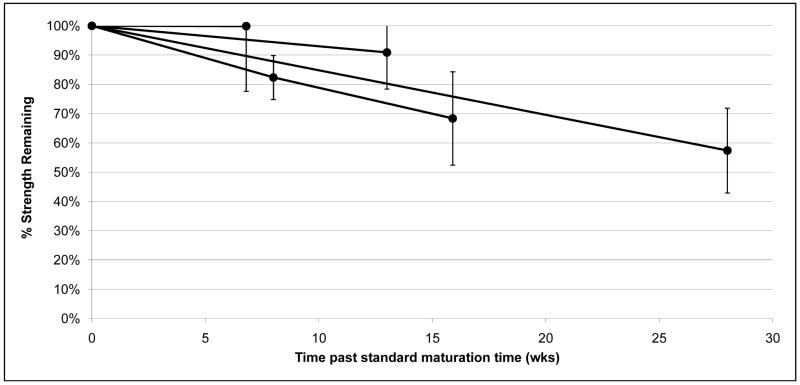
Individual TEBV burst pressure, compliance, and suture retention strength for all patients, are included in Table 1. TEBV average burst pressures [mmHg] were higher than native SV (3,523+/−1,159, 3,503+/−913, 3,399+/−470, for: 2.4mm, 4.8mm and 6.6 mm internal diameter (i.d.), respectively vs. 1,599+/−877, p < 0.05), but not significantly different than native IMA (Figure 2a; 3,523+/−1,159, 3,503+/−913, 3,399+/−470, for: 2.4mm, 4.8mm and 6.6 mm i.d., respectively vs. 3,196+/−1264, p > 0.2). Suture retention strength [grams-force, gmf] for the TEBVs was not significantly different than IMA (Figure 2b; 152+/−50 vs. 138+/−50, p > 0.5). Compliance [%/100mmHg] for the TEBVs prior to implantation was lower than IMA (Figure 2c; 3.4+/−1.6 vs. 11.5+/−3.9). By 6 months post-implant, the TEBVs compliance, measured by Doppler ultrasound, had increased to values comparable to IMA without concomitant evidence of dilation of aneurysm formation (Figure 2c; 8.8+/−4.2 vs. 11.5+/−3.9). In shelf life tests, burst pressure decreased by 1–2% per week in a linear fashion through time points out to 28 weeks after the standard vessel maturation phase (Figure 3).
Figure 2. Mechanical Test Results.
Comparison of burst pressure, suture retention force, and compliance values between native vessels and TEBVs.
Figure 3. Shelf life tests.
Burst pressure tests were performed at different time points after the standard maturation time on TEBVs from four different patients. Vessel strength declined linearly by about 1–2% in burst pressure per week.
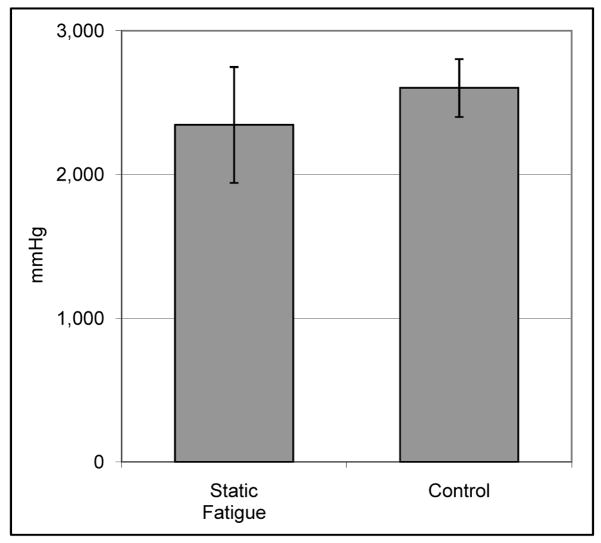
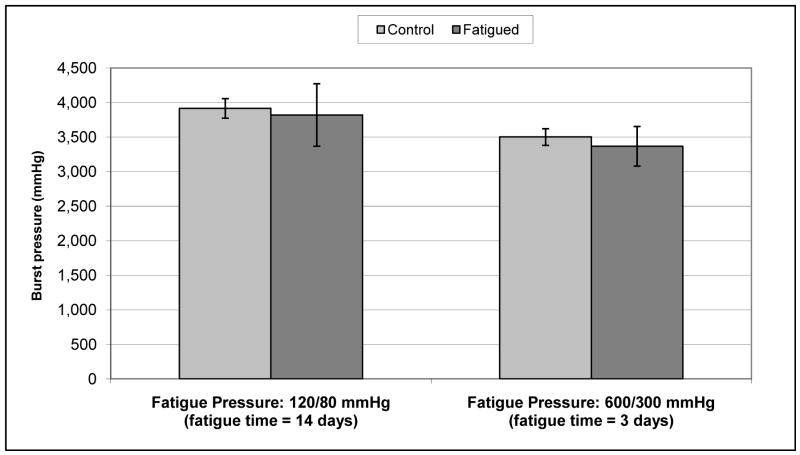
In static fatigue tests (Figure 4), the burst pressure for vessels pressurized to 250mmHg and held for 5 days (2,346 +/− 404 mmHg, n=4) was not significantly different (p = 0.37) than unloaded controls (2,602 +/− 202 mmHg, n=3). Similarly, cyclic loading (Figure 5) did not decrease the burst pressure relative to static controls for vessels loaded at 120/80 mmHg for 14 days (3,820 +/− 453 mmHg versus 3,916 +/− 142 mmHg, p = 0.74) or 600/300 mmHg for 3 days (3,369 +/− 287 mmHg versus 3,502 +/− 121 mmHg, p = 0.43). In step-wise fatigue tests, IMs alone burst, on average, after 25 minutes; 15 minutes held at 1,200 mmHg, then 10 minutes 45 seconds at 1,400 mmHg. Unfatigued IM controls burst at 1,762 +/− 70 mmHg (n=4), which was approximately half that of standard TEBVs which include an adventitial layer.
Figure 4. Static fatigue burst pressure versus unloaded controls.
Burst pressure of TEBVs statically fatigued at 250mmHg for 5 days was not significantly different than unloaded controls (n = 3 for control, n = 4 for fatigue).
Figure 5. Dynamic fatigue burst pressure versus unloaded controls.
Burst pressure of TEBVs dynamically fatigued (black) at 120/80 mmHg for 14 days or 600/300 mmHg for 3 days, respectively, was not significantly different than unloaded controls (grey) (n = 3 for fatigue and control for 120/80 mmHg, n = 4 for fatigue and control for 600/300 mmHg)
Burst pressure strength of vessels fatigued under cyclic pressure at 1Hz as compared to unfatigued controls. (n=3 for 120/80, n=4 for 600/300)
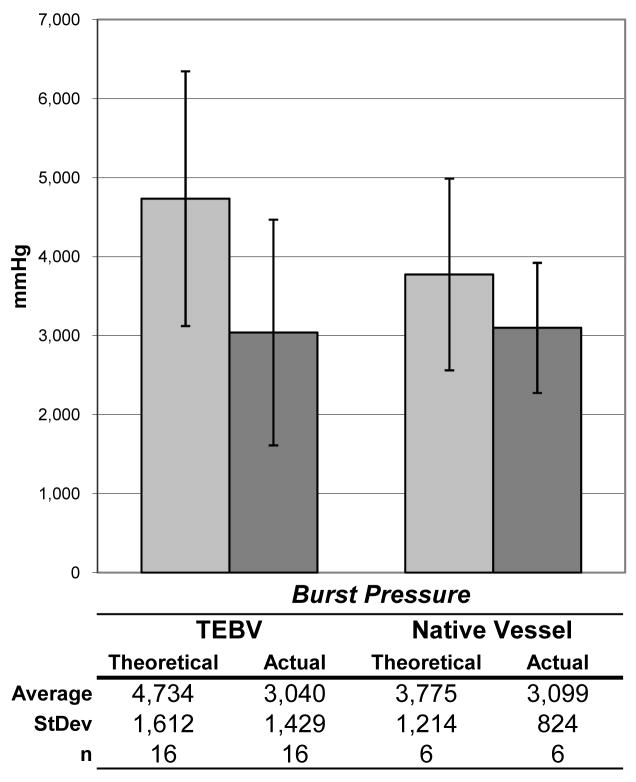
Ringlets of TEBVs were pulled in tension to rupture. Tensile rupture force was 1,545 +/− 526 gmf (n=16). The ringlet test gives a theoretical burst strength (using Laplace’s relationship) of 4,734 mmHg. Actual burst pressure from other segments taken from the same TEBV was 3,040 mmHg. By comparison, for native vessel ringlet tests, theoretical versus actual burst strength was 3,775 (n=6) versus 3,099 mmHg, respectively (Figure 6).
Figure 6. Calculated versus measured burst pressure.
Burst pressure of TEBVs and native vessels (4 IMA segments and 2 saphenous vein segments) as calculated (grey) via ringlet rupture force overestimated actual burst pressure (black).
DISCUSSION
The poor performance of synthetics such as ePTFE and Dacron in small diameter revascularization applications has driven 50 years of research into strategies to bridge the gap between synthetic materials and native vein and artery. One of the key milestones in this effort was Bell’s landmark paper in 1986 which described the concept of a completely biological and cell-based graft produced in vitro [10]. While this research spawned the field of Cardiovascular Tissue Engineering, both Bell’s approach and other early tissue engineered attempts have been hampered by poor mechanical strength and a subsequent reliance upon permanent synthetic scaffolds to provide requisite strength. Indeed, the vast majority of the approaches described over the last 25 years are dependent upon synthetic materials or chemically derived biological scaffolds [23, 24]. The inclusion of these exogenous materials, however, defeats the elegance of the cell-based approach by re-introducing materials that are thrombogenic, pro-inflammatory, or harbor infections. Recognizing the deleterious effects of permanent synthetic scaffolds, several groups utilize either completely autologous approaches [14, 15, 18, 20], or partially resorbable scaffolds [16, 17, 25, 26]. While only two groups have recently transitioned to human use [17, 20, 26], the literature is replete with TEBV models that claim clinically relevant mechanical properties. There is, however, no clear precedent or guideline that defines appropriate properties to justify transition to human use. Moreover, the only test standard designed for vascular grafts was originally written for synthetic (i.e. ePTFE or Dacron) grafts [21]. There are also unique challenges for lot release testing given the generally small lot sizes associated with cell-based therapeutics. As the various research efforts advance toward clinical use, it is therefore important to establish consistent testing guidelines and targets that might justify transition to human use. While few would argue that TEBVs should ideally mimic the key mechanical properties of native vessels, surprisingly few studies have reported the key functional parameters of burst pressure, compliance and suture retention for native human vessels harvested from an age and risk appropriate population (see Holzapfel et al. 2005 [27] for review). Earlier such data on human vessels has been focused on arteries/veins harvested from younger, healthier patients [14, 28, 29]. The ‘target’ for the mechanical properties of TEBVs is therefore poorly defined, and thus one of the objectives of this study was to document the mechanical properties of vessels harvested from risk appropriate patients (older patients with cardiovascular disease).
Investigators have previously proposed that the fundamental challenge remaining in Cardiovascular Tissue Engineering is to demonstrate appropriate mechanical strength using age and risk appropriate human cells [19], since the response of cells in culture can be highly dependent on age, disease status and species [19, 30, 31, 32]. Previously, we published initial feasibility work using human cells and demonstrated that mechanically robust vessels could be built from human cells absent any other exogenous support scaffold. While this was an important breakthrough, the work was comprised from cells harvested from only a few patients. The secondary objective of this study was therefore to expand and detail our mechanical data from a broad base of patients with advanced cardiovascular disease. Here we present detailed mechanical data and our lot release criteria for the first 25 adult patients enrolled in a trial implanting a completely autologous tissue engineered blood vessel as either an A-V shunt or a lower limb bypass. It should be noted that for both trials, one of the key inclusion criteria was a previously failed graft. This not only suggests that the patients had an advanced state of disease, but that they have an increased risk for graft failure [33].
Burst Pressure
Burst pressure is clearly one of the key parameters that determines a vessel’s suitability for implantation. While several groups have recently reported burst pressures for TEBVs in excess of 2,000 mmHg [15, 16, 18, 25], the results must be weighed in light of the geometry of the test specimens utilized and the longevity of the scaffolds used. With respect to geometry, Laplace’s law states that burst pressure increases linearly with decreasing diameter if the wall thickness is kept constant (burst pressure = material yield stress × thickness/radius). Assuming that TEBVs are built at or near the diffusion limitation of thickness, a 2 mm inside diameter vessel would therefore have twice the burst pressure of a similarly built vessel of 4 mm diameter. When reporting burst pressures, it is therefore important to list the diameter, and to exercise caution when extrapolating burst strength to larger diameters. In most cases, minimum threshold burst strength would not be maintained at larger diameters unless thickness can be increased without reaching diffusion limitations. While our mechanical test results do not match exactly with the engineering expectations defined by Laplace, we have exceeded 6,000 mmHg for vessels at 2.4 mm, while averaging only about 3,400 mmHg for vessels at 6.6 mm. With our own data, the lack of a more dramatic loss of burst strength with increasing diameter is likely caused by a combination of patient to patient variability and the fact that thickness increased slightly with diameter (average thickness was 248 +/− 69, 264 +/− 41 and 278 +/− 28 mm for 2.4, 4.8, and 6.6 mm i.d. TEBVs respectively). Patient-to-patient variations make these trends difficult to assess, but clearly diameter is an important variable that must be addressed in evaluating each TEBV model.
Test specimen length may also play an important role in assessing manufacturing consistency and resistance to rupture. A 3 cm long segment built in a miniaturized bioreactor may not accurately capture the variations in strength throughout the length of a longer TEBV of clinically-relevant length. In our case, we tested three segments that were each at least 6 cm long taken from TEBVs that were approximately 21 cm long. Intermediate segments were then used for histology, suture retention testing, ringlet testing, or archival.
In this study, native veins with a mean diameter of 2.3 mm demonstrated an average burst pressure of just under 1,600 mmHg. This is slightly lower than the ranges defined in previous studies [14, 28, 29], which is likely due to the advanced age and disease state of the donors in this study. Using the clinical efficacy of vein grafts as a justification, we have defined our minimum lot acceptance criteria for burst pressure testing as 2,000 mmHg. Intra-patient variability of the sheet-based TEBVs was quite low (average standard deviation is 435 mmHg), suggesting that the manufacturing process had a high degree of consistency and reproducibility. However, patient to patient variation is quite high. This variation, however, is similar to that which was observed for the native vessels. Of note is that just over 10% of the native vessel test samples failed below 1,000 mmHg. These failures were associated with branches that were insufficiently ligated or segments that may have had harvest related damage. This observation underscores the possibility that harvest related damage to the structure of the vein or to the endothelium may contribute to both early and mid-term in vivo failures.
Other groups have reported ringlet or strip tensile testing in place of burst pressure to define structural properties [16, 22, 34–39]. Given the simplicity of these tests and the small volume of material destroyed for the test, we were motivated to validate this approach to see if it could realistically be used in place of burst pressure as a means of judging suitability for implantation. Our data with both native vessel segments and TEBVs suggests that the ringlet test overestimates actual burst pressure by just over 50% for the TEBVS and 20% for the native tissue (Figure 6). If we analyze the testing done by others, we see that this moderate tendency to overestimate burst pressure is consistent across several groups with varying tissue engineering approaches (Table 3). This overestimation is likely due to the fact that the short ringlets are unlikely to reflect tissue weaknesses due to small manufacturing flaws or material heterogeneity as effectively as a burst pressure test on a longer segment. Moreover, the assumption of tissue incompressibility may slightly overestimate the actual burst pressure, particularly for materials with a Poisson’s ratio significantly less than 0.5. We conclude that while these tests may be useful for early developmental testing, before being used to justify clinical use of vascular grafts there must be strong statistical evidence linking the two tests for any given TEBV approach. There must also be ample demonstration of manufacturing consistency using actual burst pressure measurements on longer segments of tissue. We are currently collecting such data in anticipation of a reduced dependence upon burst pressure measurement for lot release testing. The lot release threshold based on theoretical burst strength (derived from ringlet test data), however, will likely be somewhat higher than that for the actual burst pressure to account for the tendency for ringlet data to overestimate actual strength.
Table 3.
Calculated and measured burst pressures for different groups using either ringlet or strip tensile rupture testing.
| Tensile Strength |
||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Specimen Geometry | Reported | Converted to grams force | Diameter (mm) | Wall Thickness (mm) | Length (mm) | Calc. Burst Pressure (mmHg) | Actual Burst Pressure (mmHg) |
| Donovan et al. 1990 [37] | Ring | 10.45 N | 1,066 | 5.4 | 0.37 | 5.0 | NR: 2,903* | NM |
| Seliktar et al. 2000 [22] | Ring | 58 KPa | NR: 30* | 3.5 | 0.5 | 5.0 | NR: 62* | < 500** |
| Vorp et al. 2003 [34] | Strip | 180 N/cm2 | 2,643* | 3.3 | 1.8 | 8.0 | NR: 1,473* | NM |
| McKee et al. 2003 [36] | Ring | NR | NR: 96.8* | NR: 4.0** | 0.36 | NR: 5.0** | NR: 356* | NM |
| Stankus et al. 2007 [35] | Ring | 18 MPa | NR: 897* | 4.7 | 0.163 | 1.5 | NR: 4,815* | 1,750 |
| Nieponice et al. 2008 [16] | Strip | 2.6 MPa | NR: 239* | 4.0 | 0.3 | 3.0 | NR: 2,127* | NM |
| Liu et al. 2007 [38] | Ring | 75 KPa | NR: 36* | 4.0 | 0.93 | NR: 5.0** | NR: 130* | 160 |
| Native Vessels | Ring | 922 gf | 922 | 3.5 | 0.39 | 5.0 | 3,876 | 3,099 |
| TEBVs | Ring | 1,545 gf | 1545 | 4.8 | 0.32 | 5.0 | 4,735 | 3,040 |
our calculation
our assumption
NM: not measured NR: not recorded
We also show that the TEBVs are relatively stable at time points well beyond the optimum implantation date (Figure 3). The very slow decrease of mechanical strength observed in prolong storage may be due to the secretion of matrix metalloproteinases (MMP). We have previously shown that MMPs are produced by the adventitial component of the TEBV [14]. Addition of an MMP inhibitor to the storage media may be an interesting improvement to enhance the mechanical stability of the TEBV. Living cell-based TEBVs require somewhat cumbersome clinical logistics in terms of scheduling implantation, thus the stability of burst pressure as a function of time gives moderate flexibility for coordinating surgeries.
Suture Retention and Compliance
Like burst pressure testing, there is a wide range of testing techniques used to establish suture retention strength and compliance. We have used the international ANSI 7198 [21] standard as a guideline for both tests. Changes in bite depth, suture thickness or the number of sutures will change the observed strength. It is therefore important to clearly identify these variables. Both suture retention and compliance for our TEBVs were somewhat lower than that tested for native vessels, but we have implanted vessels into humans with a suture retention test strength as low as 75 gmf without surgical or post-implantation anastomotic complication. Given the flexibility that the surgeon has to use different suture, increased bite depth or, most importantly, the number of sutures placed, we have therefore established 50 gmf as our lot release criteria for further clinical trials. Compliance, while implicated in long-term graft failure [40], is probably the least important pre-implantation mechanical property, as vessel remodeling processes will likely have profound effects on the measured compliance [41]. In clinical studies we have demonstrated that the compliance of our vessels increase from approximately 2% to approximately 9% compliance per 100 mmHg within the first 6 months without concomitant dilation [20]. While we test pre-implant compliance to establish a base value and then track it in vivo at 3 month intervals, we do not use it as a release test parameter. We previously hypothesized that this increase in compliance is associated with either migration or transdifferentiation of cells to form a functional smooth muscle cell media [18]. This remodeling process may be induced by the mechanical signaling provided by the pulsatile blood flow. Additional in vivo studies are underway to explore this hypothesis in greater detail.
It is important to note that the compliance in native vessels is likely linked to both elastin content and the characteristics of the vascular media. In excluding the vascular media from our TEBV in favor of production simplicity, we sacrifice both compliance and vasoactivity at the time of implant. While there are clear limitations to the interpretation of our changes in compliance post-implant (low n, different method of measuring compliance), in vivo remodeling may eventually restore vasoactivity and increase compliance.
Fatigue Testing
While the burst pressure test is an important indicator of the initial strength of the vessel, it does not predict longer-term strength which can be negatively impacted by fatigue or graft degradation. While immune-mediated degradation is essentially impossible to accurately model in preclinical studies (due to species-dependent differences in cell biology and immune responses), graft degradation due to fatigue or hydrolysis of resorbable scaffolds can be modeled in vitro [42]. We therefore developed static, step-wise, and dynamic fatigue testing protocols to ensure that there were no inherent fatigue-dependent loss of strength. With tests ranging from a few hours to 14 days, we saw that in each case, burst strength was maintained relative to static controls. In all tests performed pressure was cycled at 1Hz, which is a good approximation of physiological pulse. One possible limitation to this test, however, is that in vivo pulse rate can range from 0.7 to 3Hz. While these in vitro tests do not account for immune mediated degradation, they suggest that the TEBV’s burst strength is not compromised by fatigue loading or a rate dependent application of pressure. These results, coupled with positive animal studies, justified our ultimate transition to human use.
Lot Release Testing
As cell-based therapies increasingly become a clinical reality, it is important to establish appropriate tests to justify clinical use and to define appropriate acceptance criteria for tests for validating the mechanical and biological properties of each patient’s device prior to implantation. Obviously, destructive lot release tests cannot be run on vessels intended for surgery, so it is necessary to grow parallel vessels for the release tests. In this study we establish the burst, compliance, and suture retention properties of native vessels harvested from humans with advanced cardiovascular disease. We also demonstrate that the SBTE approach can consistently produce vessels with mechanical properties that exceed that of native veins. Finally, given the initial safety demonstrated in our clinical implants, we propose that these tests are appropriate standards for further human use of TEBVs.
CONCLUSIONS
In this study, we have demonstrated that burst pressures exceeding that of native vein can be achieved for a tissue engineered blood vessel for a wide spectrum of clinically relevant patients. Contrary to popular belief, no external scaffolding or synthetic supports are required to provide the requisite mechanical properties. We also propose specific mechanical testing criteria that may provide an appropriate benchmark for future Cardiovascular Tissue Engineering efforts that attempt to transition to human clinical trials. Given the positive results in this study coupled with positive initial clinical efficacy, we are now expanding our clinical trials to include other indications, and, importantly, are now shifting effort toward shortening production time, and using allogeneic tissues for select patient populations that cannot tolerate the relatively long production times for the fully autologous TEBV.
Acknowledgments
This work was supported in part by grant 5R44HL64462-6 from the NHLBI.
We also gratefully acknowledge LifeNet Health Tissue Bank for providing tissue samples for mechanical testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Ann Vasc Surg. 2004;18:66–73. doi: 10.1007/s10016-003-0094-y. [DOI] [PubMed] [Google Scholar]
- 2.Canver CC. Conduit options in coronary artery bypass surgery. Chest. 1995;108:1150–1155. doi: 10.1378/chest.108.4.1150. [DOI] [PubMed] [Google Scholar]
- 3.Muller KM, Dasbach G. The pathology of vascular grafts. Curr Top Pathol. 1994;86:273–306. doi: 10.1007/978-3-642-76846-0_7. [DOI] [PubMed] [Google Scholar]
- 4.Formichi MJ, Guidoin RG, Jausseran JM, Awad JA, Johnston KW, King MW, et al. Expanded PTFE prostheses as arterial substitutes in humans: late pathological findings in 73 excised grafts. Ann Vasc Surg. 1988;2:14–27. doi: 10.1016/s0890-5096(06)60773-5. [DOI] [PubMed] [Google Scholar]
- 5.Rahlf G, Urban P, Bohle RM. Morphology of healing in vascular prostheses. Thorac Cardiovasc Surg. 1986;34:43–48. doi: 10.1055/s-2007-1020371. [DOI] [PubMed] [Google Scholar]
- 6.Hagerty RD, Salzmann DL, Kleinert LB, Williams SK. Cellular proliferation and macrophage populations associated with implanted expanded polytetrafluoroethylene and polyethyleneterephthalate. J Biomed Mater Res. 2000;49:489–497. doi: 10.1002/(sici)1097-4636(20000315)49:4<489::aid-jbm7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Murray-Wijelath J, Lyman DJ, Wijelath ES. Vascular graft healing. III. FTIR analysis of ePTFE graft samples from implanted bigrafts. J Biomed Mater Res B Appl Biomater. 2004;70:223–232. doi: 10.1002/jbm.b.30044. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. Eur J Vasc Endovasc Surg. 2006;31:627–636. doi: 10.1016/j.ejvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Renal Data System. 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2007. [Google Scholar]
- 10.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 11.Tschoeke B, Flanagan TC, Cornelissen A, Koch S, Roehl A, Sriharwoko M, et al. Development of a Composite Degradable/Nondegradable Tissue-engineered Vascular Graft. Artif Organs. 2008;32:800–9. doi: 10.1111/j.1525-1594.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 12.Torikai K, Ichikawa H, Hirakawa K, Matsumiya G, Kuratani T, Iwai S, et al. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg. 2008;136:37–45. 45 e31. doi: 10.1016/j.jtcvs.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 13.Rashid ST, Fuller B, Hamilton G, Seifalian AM. Tissue engineering of a hybrid bypass graft for coronary and lower limb bypass surgery. FASEB J. 2008;22:2084–2089. doi: 10.1096/fj.07-096586. [DOI] [PubMed] [Google Scholar]
- 14.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 15.Chue WL, Campbell GR, Caplice N, Muhammed A, Berry CL, Thomas AC, et al. Dog peritoneal and pleural cavities as bioreactors to grow autologous vascular grafts. J Vasc Surg. 2004;39:859–867. doi: 10.1016/j.jvs.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Nieponice A, Soletti L, Guan J, Deasy BM, Huard J, Wagner WR, et al. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials. 2008;29:825–833. doi: 10.1016/j.biomaterials.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 18.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poh M, Boyer M, Solan A, Dahl SL, Pedrotty D, Banik SS, et al. Blood vessels engineered from human cells. Lancet. 2005;365:2122–2124. doi: 10.1016/S0140-6736(05)66735-9. [DOI] [PubMed] [Google Scholar]
- 20.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 21.ANSI/AAMI/ISO 7198:1998/2001. Cardiovascular implants—Tubular vascular prostheses. 2001.
- 22.Seliktar D, Black RA, Vito RP, Nerem RM. Dynamic mechanical conditioning of collagen-gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28:351–362. doi: 10.1114/1.275. [DOI] [PubMed] [Google Scholar]
- 23.Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- 24.Bordenave L, Menu P, Baquey C. Developments towards tissue-engineered, small-diameter arterial substitutes. Expert Rev Med Devices. 2008;5:337–347. doi: 10.1586/17434440.5.3.337. [DOI] [PubMed] [Google Scholar]
- 25.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 26.Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 27.Holzapfel GA, Sommer G, Gasser CT, Regitnig P. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am J Physiol Heart Circ Physiol. 2005;289:H2048–2058. doi: 10.1152/ajpheart.00934.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lamm P, Juchem G, Milz S, Schuffenhauer M, Reichart B. Autologous endothelialized vein allograft: a solution in the search for small-caliber grafts in coronary artery bypass graft operations. Circulation. 2001;104(12 Suppl 1):I108–114. [PubMed] [Google Scholar]
- 29.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, et al. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- 31.DiMuzio P, Tulenko T. Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg. 2007;45 (Suppl A):A99–103. doi: 10.1016/j.jvs.2007.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heydarkhan-Hagvall S, Esguerra M, Helenius G, Soderberg R, Johansson BR, Risberg B. Production of extracellular matrix components in tissue-engineered blood vessels. Tissue Eng. 2006;12:831–842. doi: 10.1089/ten.2006.12.831. [DOI] [PubMed] [Google Scholar]
- 33.Rosas SE, Joffe M, Burns JE, Knauss J, Brayman K, Feldman HI. Determinants of successful synthetic hemodialysis vascular access graft placement. J Vasc Surg. 2003;37:1036–1042. doi: 10.1067/mva.2003.257. [DOI] [PubMed] [Google Scholar]
- 34.Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003;75:1210–1214. doi: 10.1016/s0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- 35.Stankus JJ, Soletti L, Fujimoto K, Hong Y, Vorp DA, Wagner WR. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28:2738–2746. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKee JA, Banik SS, Boyer MJ, Hamad NM, Lawson JH, Niklason LE, et al. Human arteries engineered in vitro. EMBO Rep. 2003;4:633–638. doi: 10.1038/sj.embor.embor847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donovan DL, Schmidt SP, Townshend SP, Njus GO, Sharp WV. Material and structural characterization of human saphenous vein. J Vasc Surg. 1990;12:531–537. [PubMed] [Google Scholar]
- 38.Liu JY, Swartz DD, Peng HF, Gugino SF, Russell JA, Andreadis ST. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res. 2007;75:618–628. doi: 10.1016/j.cardiores.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 40.Nerem RM. Role of mechanics in vascular tissue engineering. Biorheology. 2003;40:281–287. [PubMed] [Google Scholar]
- 41.Opitz F, Schenke-Layland K, Cohnert TU, Starcher B, Halbhuber KJ, Martin DP, et al. Tissue engineering of aortic tissue: dire consequence of suboptimal elastic fiber synthesis in vivo. Cardiovasc Res. 2004;63:719–730. doi: 10.1016/j.cardiores.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348–355. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]