Abstract
Considerable scientific progress in the pathogenesis of vascular calcification accrued in recent years will be reviewed in this monograph. Factors regulating mesenchymal cell differentiation and their role in the neointimal calcification of atherosclerosis and the vascular media calcification observed in CKD and diabetes will be discussed as will the role of bone regulatory proteins in bone mineralization and vascular calcification This will include recent studies related to fetuin-A, and the discovery of a new circulating hormone involved in regulating phosphate homeostasis and sensing skeletal hydroxyapatite precipitation. Finally, the relationship between skeletal mineralization and vascular mineralization will be discussed in terms of their links, especially through serum phosphate concentrations.
Keywords: Vascular Calcifications, Matrix Gla protein, Fetuin-A, Phosphorus, Cardiovascular Disease, Vitamin D, Vascular Smooth Muscle
Introduction
Vascular calcification is a process that has been demonstrated during the progression of atherosclerosis with neointimal calcification or with calcification of the tunica media. While intimal and medial calcification have similarities, it is not clear that they follow a common pathogenesis, and both are stimulated by chronic kidney disease (CKD). Primary medial calcification, or Mönckeberg’s sclerosis (MS), has been associated with CKD (1), aging (2), and diabetes (3). In CKD, MS was previously thought to be a passive process (1), occurring as a direct consequence of an elevated calcium x phosphorus product. However, recent evidence discussed herein suggests that this is not the case. Rather, vascular calcification appears to be an active process involving a phenotypic drift towards an osteoblast-like cell secreting and mineralizing an extracellular matrix.
Understanding the pathogenesis of vascular calcification is essential as it is a frequent cause of morbidity and mortality for patients with CKD (4). Indeed, vascular calcification occurs in all ages and stages of CKD (5,6). However, the extent of coronary calcification appears to be more pronounced with a longer time on dialysis, older age, male gender, white race, diabetes, and elevated serum calcium and phosphorous (5). Coronary calcification is especially prone to be atherosclerotic and this form of VC has been shown to be due to differentiation of neointimal cells to an osteoblastic phenotype mineralizing their extracellular matrix akin to bone formation.
Differentiation of Mesenchymal Stem Cells
Osteoblasts, smooth muscle myocytes, adipocytes, fibroblasts and chondrocytes all share a common mesenchymal progenitor stem cell. Differentiation along the osteogenic lineage requires crucial factors including the bone morphogenetic proteins (BMPs) (7) and Wnts, an amalgam of wingless (Wg) and int (Wnts) (8). The BMPs are part of the TGF-β superfamily that initiate signal transduction by binding to specific type II receptors, activating type I receptors, and affecting gene transcription through phosphorylation of regulatory Smad transcription factors. BMP-induced osteogenesis requires lineage specific transcription factors such as Runx2, Osx, and Msx1/2 that are key and include all of the proteins involved in osteoblast mediated bone formation. Mice genetically engineered to be deficient in Runx2 or Osx show a complete lack of ossification, (9) (10) while those deficient in Msx 1/2 have significant skeletal abnormalities (11) (12).
Thus, the process of osteogenic development and bone formation is critically and tightly regulated by the coordinated effects of the growth factors and transcription factors described above. Furthermore, the subsequent finding of these transcription factors expressed in calcified vessel walls has led to the theory that vascular calcification is also a tightly regulated, coordinated, and an osteoblastic process. For example, LDL receptor negative (LDLR −/−) mice fed a high fat diet developed vascular calcification associated with the expression of Msx1 and Msx2 (13). In humans, expression of Msx2 and Runx2 along with the chondrogenic transcription factor Sox9 has been described in calcified atherosclerotic samples (14). In addition, expression of the target genes of these factors such as alkaline phosphatase, osteocalcin, bone sialoprotein, and type II collagen is also increased. Other bone regulatory proteins such as matrix Gla protein (MGP) and osteoprotegerin (OPG) (both discussed below) are downregulated in calcified vessels compared with noncalcified vessels (14). Deposition of bone matrix proteins has also been described in medial calcification associated with CKD (15). These findings suggest that vascular calcification is an active process that simulates osteogenesis and bone formation.
The Vascular Smooth Muscle Cell
VSMCs normally reside in the vessel wall media in a differentiated state wherein their contractile properties regulate vascular tone. However, the VSMC phenotype is characterized by the ability to reversibly enter a synthetic state of proliferation and production of large amounts of extracellular matrix (16). The stimulus to change phenotype includes injury, various cytokines, growth factors, and certain components of the extracellular matrix. In the heightened synthetic state, VSMCs show decreased expression of contractile and adhesion proteins and a concomitant increase in cytoskeletal proteins (17). In addition to various growth factors, culture of VSMCs in medium supplemented with serum has been shown to experimentally stimulate the transition to the synthetic phenotype (18). Transition into the synthetic state is thought to be involved in the pathogenesis of atherosclerosis and MS.
After stimulation with serum proliferating VSMCs grow to form a confluent monolayer (19). Subsequently, areas of the monolayer develop multicellular foci that form nodular aggregates consisting of nonproliferating, quiescent VSMCs. Cells from these nodules appear to re-express markers of smooth muscle cell differentiation. Proliferating osteoblasts also form condensing nodules in culture (20). Subpopulations of human and bovine aortic VSMCs have been shown to form these nodules and then spontaneously calcify (21).
The addition of β-donor, or high concentrations of inorganic phosphate have been shown to induce calcification in VSMCs in vitro (22), and VSMCs from atherosclerotic donors showed increased expression of Runx2, osteopontin (OPN), and alkaline phosphatase (23). Inorganic phosphate induced the expression of osterix and bone matrix proteins (23). This action is stimulated through a sodium-phosphorus cotransporter in cultured VSMCs (24,25). Thus, it appears that inorganic phosphate induces VSMCs toward an osteogenic phenotype in vitro. However, uremic serum also induces VSMCs to calcify, a process that is partly related to a factor that is independent of serum phosphate.
While the demonstration that VSMCs can be induced to calcify, and on occasion calcify spontaneously in vitro is important in the understanding of the pathogenesis of vascular calcification, the forces driving osteogenic differentiation in vivo are less clear. However, in addition to a higher incidence of vascular calcification, decreased bone formation is also a common finding in CKD, diabetes mellitus, and aging. Furthermore, recent findings in animal models suggest that decreased orthotopic bone mineralization, either by decreased bone formation or increased bone resorption, leads to increased pressure towards heterotopic mineralization. We will discuss what factors are involved in this “increased pressure”, giving particular attention to phosphate.
Bone metabolism and vascular calcification: A possible link
Matrix Gla Protein
MGP belongs to the family of mineral-binding proteins that includes coagulation factors, anticlotting factors, and osteocalcin. MGP is a vitamin K-dependent protein, requiring the vitamin for γ-carboxylation of its glutamic acid residues resulting in production of carboxyglutamic acid (Gla) residues. The Gla residues bind calcium and have been shown to inhibit hydroxyapatite precipitation (26). The affinity of MGP binding to hydroxyapatite is enhanced by calcium and decreased by phosphate and magnesium (26). MGP also appears to modulate endochondral bone formation. Overexpression of MGP blocks cartilage mineralization and inhibits endochondral ossification (27) while mice genetically engineered to be deficient in MGP have inappropriate mineralization of cartilage, disorganized chondrocyte columns, and osteopenia (28). Mice genetically engineered to be deficient in MGP also develop extensive arterial calcification and die of arterial rupture (28). Calcified arteries from these mice show decreased expression of smooth muscle cell markers and increased expression of bone-specific markers such as Runx2 and OPN (29). This finding suggests that the vascular calcification associated with this mutation is not solely related to an inability to inhibit hydroxyapatite precipitation, but rather involves a more active process resulting in a dramatic phenotypic change in VSMCs. The function of MGP to bind BMPs in the matrix may relate to BMP activity and differentiation of neointimal cells to the osteochrondrogenic pathway (30).
Osteoprotegerin
Osteoprotegerin (OPG), a soluble circulating ligand of Receptor activator of NF-κB (RANK), is an inhibitor of osteoclast differentiation and function and is part of the RANK/RANK ligand (RANKL)/OPG axis. Mice that are genetically engineered to be deficient in OPG (opg−/opg−) develop severe osteoporosis (31) probably by uninhibited osteoclast function leading to excessive bone resorption. Histomorphometric analysis of the bones of these mice reveals increased parameters of both bone resorption and bone formation, suggesting that the two processes are coupled (32). However, intravenous injection of recombinant OPG or transgenic overexpression of OPG can effectively reverse the osteoporotic phenotype (33). In addition to this phenotype, OPG-deficient mice also develop extensive vascular calcification. In contrast to the osteoporotic phenotype, only transgenic overexpression of OPG prevents vascular calcification. In other words, preventing bone resorption prevents the vascular calcification while reversing it does not. Of note, the increased bone resorption seen in these mice was not associated with an increased serum phosphate (32), which may in part be due to the increased bone formation and mineral apposition rates seen in these mice. Furthermore, OPG-deficient mice have markedly elevated RANKL as compared with wild type. Elevated RANKL may be the link between bone and vascular calcification in this model, as it has recently been shown to induce aortic myofibroblasts to form calcifying nodules with an osteogenic phenotype (34). In addition, although RANKL is not normally expressed in the vasculature, its expression was increased in both the vessels of OPG deficient mice (33) and the aortic valves in human calcific aortic stenosis (34).
The role of OPG in renal osteodystrophy and vascular calcification is less clear. Studies correlating serum OPG levels and histomorphometric parameters of bone turnover have been inconsistent.
Vitamin D
High-dose vitamin D causes arterial calcification (35,36,37). Vertebrae from rats treated with 1,25 – hydroxyvitamin D3 have more osteoblasts, and greater mineral appositional rate and bone formation and fewer osteoclasts than untreated controls (38). However, vitamin D induced calcification in the rat is associated with a marked increase in the cross-linked N-teleopeptides, a marker of bone resorption (37). Treatment with OPG, an inhibitor of bone resorption, prevented vascular calcification and restored serum cross-linked N-teleopeptides back to control values; agents known to inhibit bone resorption through mechanisms other than OPG also inhibited vitamin D induced vascular calcification (36) (39). Furthermore, 1,25-OH-vitamin D3 appears to promote the differentiation of osteoclasts through stimulation of RANKL in osteoblastic cells (40). Thus, at least in these animal models, vascular calcification by vitamin D appears to be related to bone resorption. Why there is such a dichotomy in the effects of vitamin D on bone metabolism is not clear, but may possibly be related to divergent effects of the various metabolites of vitamin D or to the relative dosage of vitamin D. It is important to note that vitamin D also has effects on the vasculature. Indeed, in the atherosclerotic high fat fed ldlr−/−mouse, low doses of paricalcitol and calcitriol inhibited aortic osteoblast gene expression and actually protected against vascular calcification (41).
α2-HS glycoprotein/Fetuin-A
α2-HS glycoprotein (AHSG) or fetuin-A is a bone regulatory protein that shares homologies to the fetuin superfamily, proteins present abundantly in the fetal serum (42). Fetuins have been shown to inhibit hydroxyapatite precipitation in vitro (43) and to modulate the effects of the TGF-β superfamily on osteogenesis (44). Fetuins can inhibit or stimulate osteogenesis, depending on their relative concentrations (45). Mice that are genetically engineered to be deficient in fetuin-A are phenotypically normal but develop more extensive ectopic calcification than control when fed a mineral and vitamin D-rich diet (46).
A decrease in serum fetuin-A levels in hemodialysis patients is associated with an increased risk of cardiovascular mortality and an impaired ex vivo ability to inhibit hydroxyapatite precipitation (47). This decrease in fetuin-A levels is in part due to inflammation, as fetuin-A has been shown to be a negative acute-phase reactant (48). However, one must also not discount the effects of bone metabolism in this situation. The calcium based phosphate binders and vitamin D analogues given to these patients to control secondary hyperparathyroidism may also serve to deplete fetuin-A levels via increased calcium and phosphorus incorporation into the fetuin-mineral complex.
BMP-7: Bone formation, phosphate, and vascular calcification
BMP-7, a member of the BMP family, is critical for renal, skeletal, and retinal development that is expressed in the adult primarily in the collecting tubule. Acute renal ischemia (49,50) and diabetic nephropathy (50,51) reduce BMP-7 expression. In CKD and renal osteodystrophy, treatment with BMP-7 restored normal bone turnover and osteoblast function (52,53) in either high or low turnover rates. BMP-7 has also been shown to significantly reduce intimal calcification in the low-density lipoprotein receptor-deficient high-fat fed atherosclerotic mouse (LDLR−/− high-fat) when CKD was induced (54). Furthermore, LDLR−/− high-fat fed mice with CKD have low-turnover osteodystrophy (52), and restoration of bone metabolism was associated with a significant reduction in serum phosphate (52). That renal osteodystrophy contributes to the serum phosphorus through excess bone resorption provides a link between bone turnover and vascular calcification.
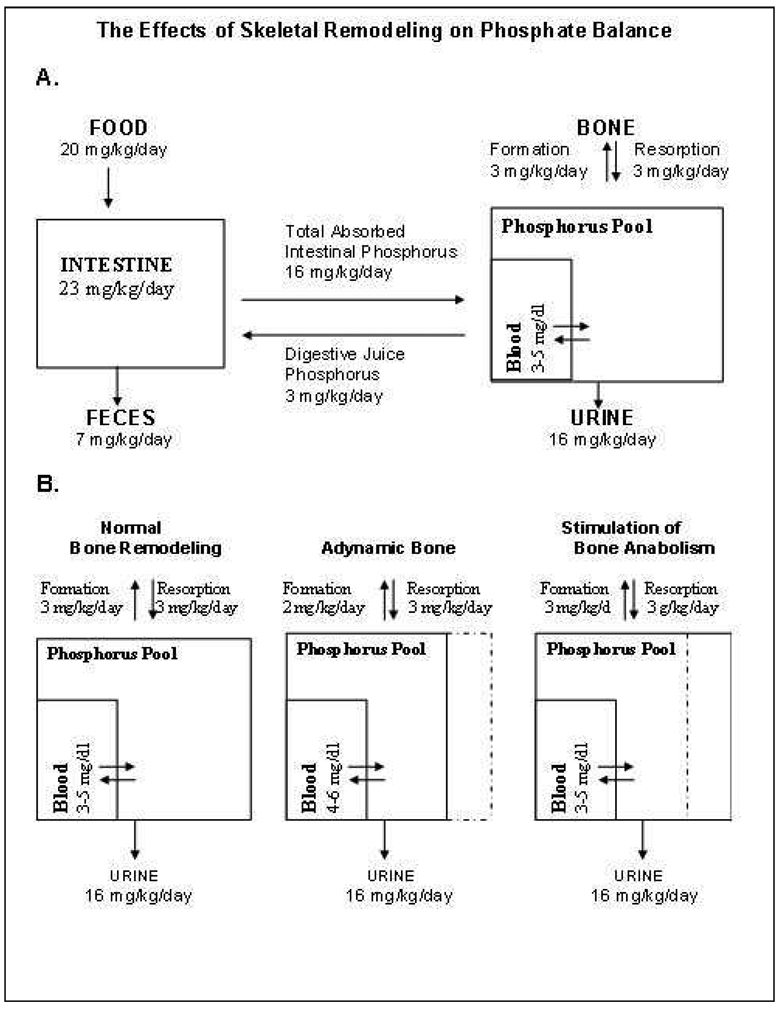
When dietary phosphorus is ingested, it enters a rapidly exchangeable pool (Figure 1A). A significant portion of this pool exists along the skeletal mineralizing surfaces where phosphorus is leaving the pool to be incorporated into bone crystals. When bone formation is reduced (Figure 1B), the skeletal mineralizing surface is reduced, effectively reducing the volume of distribution of phosphorus, resulting in an increase in the serum phosphate associated with intake. By restoring normal bone turnover, BMP-7 increased the volume of distribution of phosphorus, resulting in a decrease in serum phosphate. Since phosphorus has been shown to induce an osteogenic phenotype in VSMCs in vitro, we propose that the reduction in serum phosphate is a mechanism by which BMP-7 prevents vascular calcification. BMP-7 has also been shown to maintain the VSMC phenotype in vitro after stimulation into the synthetic phenotype (55), and BMP-7 stimulates the contractile phenotype in human VSMC derived from atherosclerotic donors (56).
Figure 1.
Effects of skeletal remodeling on phosphate balance. A, Phosphate balance diagram for a diet containing 20mg/kg body weight of elemental Pi. Absorbed Pi enters an exchangeable phosphorus pool with exits through skeletal deposition, renal excretion and enteric secretions. B, The adynamic bone disorder decreases the size of the exchangeable pool resulting in wider swings of the serum phosphorus due food ingestion. Note that the adynamic bone disorder is a state of excess bone resorption, and now the skeleton is a net contributor to exchangeable pool phosphorus (hyperphosphatemia).
FGF-23: Regulator of Phosphorus Metabolism
Since phosphate homeostasis appears to be a central component of vascular calcification, understanding its regulation is essential. In addition to parathyroid hormone (PTH) and vitamin D, another hormone, fibroblast growth factor (FGF-23), plays a critical role in homeostasis. FGF-23 is a phosphaturic hormone involved in autosomal dominant hypophosphatemic rickets (ADHR) (57) and tumor induced osteomalacia (TIO) (58). FGF-23 also inhibits expression of renal 1 alpha hydroxylase, resulting in inappropriately low levels of 1, 25-OH vitamin D3 in hypophosphatemia (59). FGF-23 levels are elevated in CKD (60,61). Elevations in the levels of FGF-23 may be secondary to osteocytic secretion and decreased clearance (60). Interestingly, targeted deletion of the FGF-23 gene results in hyperphosphatemia, hypercalcemia, suppressed PTH, and low turnover osteopenia with accumulation of osteoid (59). Mice homozygous for the null mutation died within 13 weeks of birth and autopsies revealed marked vascular calcification and calcification of the kidneys associated with an elevated BUN. Importantly, the vascular calcification associated with FGF-23 deficiency was prevented by low dietary phosphorus or by breeding in 1 alpha hydroxylase deficiency.(62) Recent human studies demonstrate that FGF-23 levels are strong predictors of mortality in hemodialysis patients, probably reflecting a role as a biomarker of the phosphate pathway in vascular calcification (63).
Bone Metabolism and Vascular Calcification: Tying it all together
Evidence suggests that changes in bone metabolism, either by increased bone resorption or decreased bone formation, leads to vascular calcification. Vascular calcification occurs in both the media and the intima, possibly by different processes. Treatment with BMP-7 in the LDLR−/− high fat mouse with CKD model resulted in reductions in intimal calcification through lowering phosphate by stimulating bone formation. If VSMC migration did not occur as part of the pathogenesis of atherosclerosis, then perhaps intimal calcification would also not occur. Indeed, intimal calcification does not occur in other animal models of vascular calcification.
Primary medial calcification occurs primarily in patients with CKD or diabetes mellitus, and as a part of aging. These three conditions also share in some instances a common characteristic of decreased bone formation. Indeed, animal models and human studies of aging (64,65) and diabetes (66,67) have demonstrated decreased bone volume and bone formation rates. Treatment of LDLR −/− high fat fed mice (who have the metabolic syndrome or diabetes) with another bone anabolic, PTH (1–34) fragment also results in reduced vascular calcification (68). While it is not known what effect PTH (1–34) had on serum phosphate (presumably it would decrease it), this fragment did result in an elevation of skeletal and serum OPN, a known inhibitor of vascular calcification. While other mechanisms may be involved in this protective effect of PTH (1–34), these findings further support the link between bone metabolism and vascular calcification.
With excess bone resorption, vascular calcification also occurs. We hypothesize that the pathogenesis involves the effects of phosphorus and reduced levels of serum fetuin-A, a known inhibitor of ectopic mineralization. However, since mice genetically engineered to be deficient in fetuin-A do not have significant ectopic calcification, reduced levels are probably not the sole factor involved in its pathogenesis. For example, high levels of serum RANKL may also be involved in vascular calcification in the OPG deficient mouse. Furthermore, serum phosphate does not appear to be elevated in these animal models of vascular calcification, perhaps due to a concomitant increase in bone formation rates in these models.
Conclusion: Implications for Chronic Kidney Disease
We have shown that prevention of secondary hyperparathyroidism results in low turnover renal osteodystrophy (adynamic bone disorder) in CKD (69). We propose that the adynamic bone disorder develops in part due to a deficiency in Wnt and BMP activity in the skeleton and that secondary hyperparathyroidism develops as an adaptive process (70). Furthermore, we propose that with the adynamic bone disorder (low-turnover state), there is a decreased volume of distribution for phosphorus, resulting in an increase in the serum phosphate and greater stimulation of FGF23. Inorganic phosphate has been shown to induce an osteogenic phenotype in VSMC, resulting in a more active process of mineralization. The extent of vascular calcification indeed has been shown to be associated with higher serum phosphorus in humans with CKD (5). Thus, treatment of positive phosphate balance in CKD may be required to decrease the high rates of associated cardiovascular mortality. Clearly vascular calcification is a significant problem for patients with CKD. In this manuscript we have provided evidence that bone metabolism affects the vasculature. While vascular calcification occurs without CKD, we propose that the central mechanism in most cases is abnormal bone metabolism. Complications likely specific to CKD include chronic inflammation leading to atherosclerosis and low serum fetuin-A levels and hyperphosphatemia from decreased excretion. Finally, one must also consider the effects of risk factors for atherosclerosis such as hypertension, hyperlipidemia, diabetes, and smoking on vascular calcifications.
Given that there is such significant morbidity and mortality associated with vascular calcification, it is essential to develop treatments to prevent or slow the progression of this process. While attempts to adjust oral calcium, calcitriol analogues, and calcium in the dialysate are key to management of renal osteodystrophy, there is no easy way to measure the success of these approaches except for bone biopsy. New and improved assessments are required and are reviewed in detail by Dr. Mary Leonard in a subsequent manuscript in this Seminars in Nephrology. We propose that bone anabolics such as BMP-7 may be novel therapeutic options for the treatment of low turnover osteopenia and the prevention of vascular calcification. Human studies must be performed to further assess this concept.
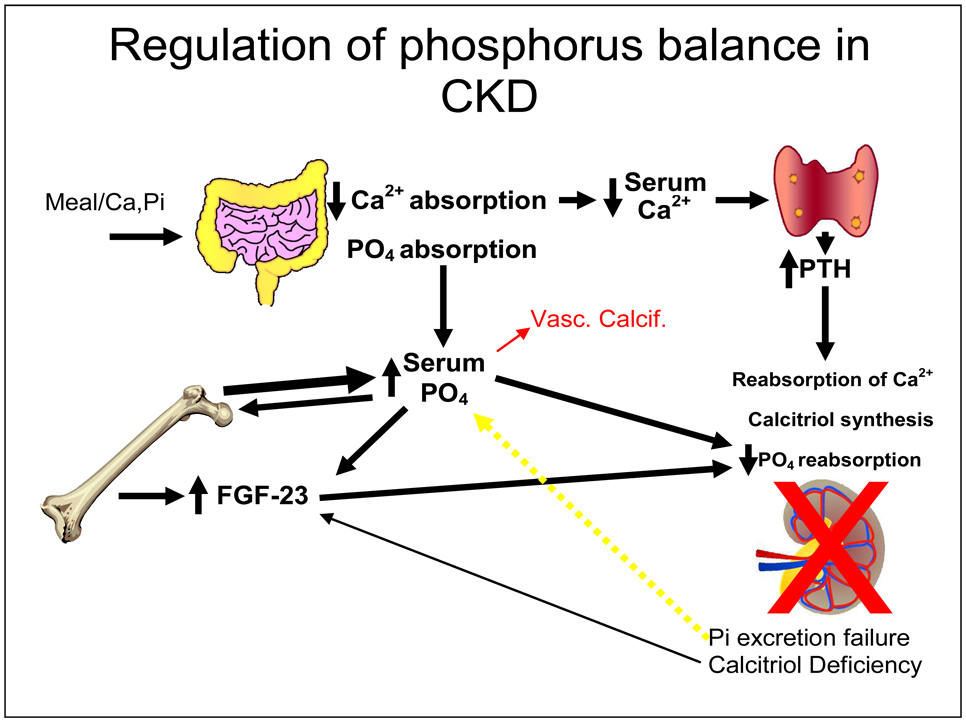
Figure 2.
Regulation of phosphorus balance in CKD. Meal associated Ca and PO4 are absorbed. Decreased Ca absorption and hypocalcemia stimulate parathyroid hormone secretion. Absorbed PO4 is deposited in the skeleton through bone formation or excreted by the kidney. Skeletal osteocytes read bone formation, and when the available PO4 exceeds skeletal deposition (bone formation), they secrete FGF23 to have the kidney excrete the excess PO4. In CKD, renal excretion of PO4 fails to maintain balance despite PTH and FGF23 influence and positive PO4 balance results (yellow arrow) and the serum PO4 begins to rise. This is a direct stimulus to heterotopic mineralization (red arrow and vascular calcification as a form of heterotopic mineralization).
Acknowledgements
This work was supported by NIH grants (DK070790, AR41677, T32-DK062705) to KAH and research support from Shire, Genzyme, Abbott and Fresenius. We wish to thank Mat Davies, Richard Lund and Song Wang, past fellows in the Hruska laboratory for their hard work and research contributions to this effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ejerblad S, Ericsson JLE, Eriksson I. Arterial lesions of the radial artery in uraemic patients. Acta Chir Scand. 1979;145:415–428. [PubMed] [Google Scholar]
- 2.Elliott RJ, McGrath LT. Calcification of the human aorta during aging. Calcif Tissue Int. 1994;54:268–273. doi: 10.1007/BF00295949. [DOI] [PubMed] [Google Scholar]
- 3.Edmonds ME.Medial arterial calcification and diabetes mellitus Z Kardiol 200089:pII/101-II/104, [DOI] [PubMed] [Google Scholar]
- 4.Blacher Jacques, Safar MichelE, Guerin AlainP, Pannier Bruno, Marchais SylvainJ, London GerardM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003 May 1;63:1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 5.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 6.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Eng J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Hongwei, Jiang Wei, Phillips FrankM, Haydon RexC, Peng Ying, Zhou Lan, Luu HueH, An Naili, Breyer Benjamin, Vanichakarn Pantila, Szatkowski JanPaul, Park JaeYoon, He TongChuan. Osteogenic Activity of the Fourteen Types of Human Bone Morphogenetic Proteins (BMPs) Journal of Bone and Joint Surgery. 2003 Aug 1;85:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Kato Masaki, Patel MillanS, Levasseur Regis, Lobov Ivan, Chang BennyHJ, Glass DonaldA, II, Hartmann Christine, Li Lan, Hwang TaeHo, Brayton CoryF, Lang RichardA, Karsenty Gerard, Chan Lawrence. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biology. 2002 Apr 15;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao Y-H, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, De Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 11.Satokata I, Mass R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nature Genetics. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- 12.Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nature Genetics. 2004;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 13.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. Journal of Biological Chemistry. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 14.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 15.Moe SharonM, O'Neill KalishaD, Duan Danxia, Ahmed Sadiq, Chen NealX, Leapman StephenB, Fineberg Naomi, Kopecky Kenyon. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney International. 2002 Feb 1;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 16.Hedin U, Roy J, Tran PK, Lundmark K, Rahman A. Control of smooth muscle cell proliferation - the role of the basement membrane. Thromb Haemost. 1999;82 Suppl:23–26. [PubMed] [Google Scholar]
- 17.Worth NF, Rolfe BE, Song J, Campbell R. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganization of contractile and cytoskeletal proteins. Cell Motility and the Cytoskeleton. 2001;49:130–145. doi: 10.1002/cm.1027. [DOI] [PubMed] [Google Scholar]
- 18.Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- 19.Brennan MJ, Millis AJ, Fritz KE. Fibronectin inhibits morphological changes in vascular smooth muscle cells. J Cell Physiol. 1982;112:284–290. doi: 10.1002/jcp.1041120219. [DOI] [PubMed] [Google Scholar]
- 20.Barone LM, Owen TA, Tassinari MS. Developmental expression and hormonal regulation of the rat matrix gla protein (MGP) gene in chondrogenesis and osteogenesis. J Cell Biochem. 1991;46:351–365. doi: 10.1002/jcb.240460410. [DOI] [PubMed] [Google Scholar]
- 21.Boström K, Watson KE, Horn S, Worthman C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. β-Glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995;15:2003–2009. doi: 10.1161/01.atv.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 23.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in chronic kidney disease. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circulation Research. 2000;87:e10–e17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 25.Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 26.Roy ME, Nishimoto SK. Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment:calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone. 2002;31:296–302. doi: 10.1016/s8756-3282(02)00821-9. [DOI] [PubMed] [Google Scholar]
- 27.Yagami Kimitoshi, Suh JoYoung, Enomoto-Iwamoto Motomi, Koyama Eiki, Abrams WilliamR, Shapiro IrvingM, Pacifici Maurizio, Iwamoto Masahiro. Matrix GLA Protein Is a Developmental Regulator of Chondrocyte Mineralization and, When Constitutively Expressed, Blocks Endochondral and Intramembranous Ossification in the Limb. The Journal of Cell Biology. 1999 Nov 29;147:1097–1108. doi: 10.1083/jcb.147.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 29.Steitz SA, Speer MY, Curinga G, Yang Hsueh-Ying, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification. Circulation Research. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 30.Bostrom Kristina, Tsao David, Shen Sam, Wang Ying, Demer Linda L. Matrix GLA Protein Modulates Differentiation Induced by Bone Morphogenetic Protein-2 in C3H10T1/2 Cells. Journal of Biological Chemistry. 2001 Apr 20;276:14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 31.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpleyl J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes & Development. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Midori, Udagawa Nobuyuki, Matsuura Sachiko, Mogi Makio, Nakamura Hiroshi, Horiuchi Hiroshi, Saito Naoto, Hiraoka BYukihiro, Kobayashi Yasuhiro, Takaoka Kunio, Ozawa Hidehiro, Miyazawa Hiroo, Takahashi Naoyuki. Osteoprotegerin Regulates Bone Formation through a Coupling Mechanism with Bone Resorption. Endocrinology. 2003 Dec 1;144:5441–5449. doi: 10.1210/en.2003-0717. [DOI] [PubMed] [Google Scholar]
- 33.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sankoc A, Kιlιc R, Brueckmann M, Lang S, Zahn I, Vahl C. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulate aortic valve calcification*1. Journal of Molecular and Cellular Cardiology. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol. 2000;20:317–327. doi: 10.1161/01.atv.20.2.317. [DOI] [PubMed] [Google Scholar]
- 36.Price PaulA, Faus SamuelA, Williamson MatthewK. Bisphosphonates Alendronate and Ibandronate Inhibit Artery Calcification at Doses Comparable to Those That Inhibit Bone Resorption. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001 May 1;21:817–824. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- 37.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by Warfarin and by Vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 38.Erben ReinholdG, Scutt AndrewM, Miao Dengshun, Kollenkirchen Uwe, Haberey Martin. Short-Term Treatment of Rats with High Dose 1,25-Dihydroxyvitamin D3 Stimulates Bone Formation and Increases the Number of Osteoblast Precursor Cells in Bone Marrow. Endocrinology. 1997 Nov 1;138:4629–4635. doi: 10.1210/endo.138.11.5511. [DOI] [PubMed] [Google Scholar]
- 39.Price PaulA, June HelenH, Buckley JessicaR, Williamson MatthewK. SB 242784, a Selective Inhibitor of the Osteoclastic V-H+ATPase, Inhibits Arterial Calcification in the Rat. Circulation Research. 2002 Sep 20;91:547–552. doi: 10.1161/01.res.0000033987.22436.50. [DOI] [PubMed] [Google Scholar]
- 40.Kitazawa S, Kajimoto K, Kondo T, Kitazawa R. Vitamin D3 supports osteoclastogenesis via functional vitamin d response element of human RANKL gene promoter. J Cell Biochem. 2003;89:771–777. doi: 10.1002/jcb.10567. [DOI] [PubMed] [Google Scholar]
- 41.Mathew S, Strebeck F, Hruska KA. The protective actions of vitamin D analogs in the vascular calcification of chronic kidney disease (CKD) J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 42.Elzanowski A, Barker WC, Hunt LT, Seibel-Ross E. Cystatin domains in alpha-2-HS glycoprotein and fetuin. FEBS Lett. 1988;227:167–170. doi: 10.1016/0014-5793(88)80890-1. [DOI] [PubMed] [Google Scholar]
- 43.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein α2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 44.Demetriou M, Binkert C, Sukhu B, Tenenbaum HD, Dennis JW. Fetuin/alpha2-HS glycoprotein is transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 45.Binkert C, Demetriou M, Sukhu B, Szweras M, Tennenbaum HC, Dennis JW. Regulation of osteogenesis by Fetuin. J Biol Chem. 1999;274:28514–28520. doi: 10.1074/jbc.274.40.28514. [DOI] [PubMed] [Google Scholar]
- 46.Schafer Cora, Heiss Alexander, Schwarz Anke, Westenfeld Ralf, Ketteler Markus, Floege Jurgen, Muller-Esterl Werner, Schinke Thorsten, Jahnen-Dechent Willi. The serum protein {alpha}2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. Journal of Clinical Investigation. 2003 Aug 1;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ketteler M, bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis a cross-ssectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 48.Lebreton JP, Joisel F, Raoult JP, Lannuzel B, Rogez JP, Humbert G. Serum concentration of human alpha 2-HS glycoprotein during the inflammatory process: evidence that alpha 2-HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64:1118–1129. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon M, Maresh JG, Harris SE, Hernandez JD, Arar M, Olson MS, Abboud HE. Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. American Journal of Physiology. 1999;276:F382–F389. doi: 10.1152/ajprenal.1999.276.3.F382. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Chen Q, Simon TC, Strebeck F, Chaudhary L, Morrissey J, Liapis H, Klahr S, Hruska KA. Bone morphogenetic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 2003;63:2037–2049. doi: 10.1046/j.1523-1755.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 51.Wang S, Hirschberg R. Loss of renal tubular BMP7 during the evolution of experimental diabetic nephropathy. J Am Soc Nephrol. 2000;11:655A. [Google Scholar]
- 52.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16:917–928. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez EA, Lund RJ, Martin KJ, McCartney JE, Tondravi MM, Sampath TK, Hruska KA. Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int. 2002;61:1322–1331. doi: 10.1046/j.1523-1755.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 54.Davies MR, Lund RJ, Hruska KA. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol. 2003;14:1559–1567. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 55.Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cellular Physiol. 2000;184:37–45. doi: 10.1002/(SICI)1097-4652(200007)184:1<37::AID-JCP4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 56.Kokubo T, Uchida H, Chasnoff SE, Matthew S, Hruska KA, Choi ET. Chronic kidney disease (CKD) accelerates development of neointimal hyperplasia in a mouse arteriovenous fistula model. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White KE, Evans WE, O'Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, Grabowski M, Meitinger T, Strom TM. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genetics. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 58.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. PNAS. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimada T, Kakitani Makoto, Yamazaki Yuji, Hasegawa Hisashi, Takeuchi Yasuhiro, Fujita Toshiro, Fukumoto Seiji, Tomizuka Kazuma, Yamashita Takeyoshi. Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. Journal of Clinical Investigation. 2004 Feb 15;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Larsson Tobias, Nisbeth Ulf, Ljunggren Osten, Juppner Harald, Jonsson KennethB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 61.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 62.Razzaque Mohammed S, Sitara Despina, Taguchi Takashi, St-Arnaud Rene, Lanske Beate. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. The FASEB Journal05-5432fje. 2006 Jan 25; doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gutierrez OrlandoM, Mannstadt Michael, Isakova Tamara, Rauh-Hain JoseAlejandro, Tamez Hector, Shah Anand, Smith Kelsey, Lee Hang, Thadhani Ravi, Juppner Harald, Wolf Myles. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. The New England Journal of Medicine. 2008 Aug 7;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Banu J, Mcmahan CA, Kalu DN. Male rodent model of age-related bone loss in men. Bone. 2001;29:141–148. doi: 10.1016/s8756-3282(01)00483-5. [DOI] [PubMed] [Google Scholar]
- 65.Clarke BL, Ebeling PR, Jones JD, Wahner HW, O'Fallon WM, Riggs BL, Fitzpatrick LA. Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endo Metab. 1996;81:2264–2270. doi: 10.1210/jcem.81.6.8964862. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki K, Miyakoshi N, Tsuchida T, Kasukawa Y, Sato K, Itoi E. Effects of combined treatment of insulin and human parathyroid hormone(1–34) on cancellous bone mass and structure in streptozotocin-induced diabetic rats. Bone. 2003;33:108–114. doi: 10.1016/s8756-3282(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 67.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 68.Shao Jian Su, Cheng SuLi, Charlton-Kachigian Nichole, Loewy Arleen P, Towler Dwight A. Teriparatide (Human Parathyroid Hormone (1–34)) Inhibits Osteogenic Vascular Calcification in Diabetic Low Density Lipoprotein Receptor-deficient Mice. Journal of Biological Chemistry. 2003 Dec 12;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 69.Lund RJ, Davies MR, Brown AJ, Hruska KA. Successful treatment of an adynamic bone disorder with bone morphogenetic protein-7 in a renal ablation model. J Am Soc Nephrol. 2004;15:359–369. doi: 10.1097/01.asn.0000109671.99498.08. [DOI] [PubMed] [Google Scholar]
- 70.Hruska KA, Saab G, Chaudhary LR, Quinn CO, Lund RJ, Surendran K. Kidney-bone, bone-kidney, and cell-cell communications in renal osteodystrophy. Seminars in Nephrology. 2004;24:25–38. doi: 10.1053/j.semnephrol.2003.08.010. [DOI] [PubMed] [Google Scholar]