Abstract
Although comprehensive molecular diagnostics and personalized medicine have sparked excitement among researchers and clinicians, they have yet to be fully incorporated into today’s standard of care. This is despite the discovery of disease-related oncogenes, tumor-suppressor genes and protein biomarkers, as well as other biological anomalies related to cancer. Each year, new tests are released that could potentially supplement or surpass standard methods of diagnosis, including serum, protein and gene expression analyses. All of these novel approaches have shown great promise, but initial enthusiasm has diminished as difficulties in reproducibility, expense, standardization and proof of significance beyond current protocols have emerged. This review will focus on current and novel molecular diagnostic tools applied to breast cancer with special attention to the exciting new field of microRNA analysis
Keywords: breast cancer, microarray, microRNA, molecular diagnostics, predictive value, prognostic value
Breast cancer is a major public health issue worldwide. In 2004, the most recent year available for global data, there were 1.15 million new breast cancer cases and over 500,000 deaths reported around the world [1,2]. More than half of all cases occurred in industrialized nations [1,3]. The higher incidence of breast cancer observed in more developed regions of the world is most likely attributed to the availability of screening programs used to detect breast cancer, which may otherwise have never been diagnosed [4]. The total overall cost for the treatment of patients with breast cancer increases with higher stages of the disease [5]. Therefore, screening and diagnosis of breast cancer at earlier stages both benefits the patient and minimizes the financial burden [5].
The traditional clinical approach to treat in situ and invasive breast cancer involves a combination of existing surgical-, chemical- and radiation-based therapies. Surgical options include lumpectomy, quadrantectomy, mastectomy and modified radical mastectomy. These procedures are sometimes followed by adjuvant therapy, such as hormonal and/or chemotherapy, which are administered in a case-dependent manner [6]. Hormonal therapies include selective estrogen receptor modulators (SERMs), such as tamoxifen, and aromatase inhibitors, such as anastrozole. Chemotherapy includes traditional chemotherapies as well as specific drugs such as trastuzumab, a monoclonal antibody to the HER2/neu receptor. Although these therapies have shown great promise in decreasing disease burden and preventing recurrence, like most therapies, there are a variety of toxicities associated with their administration that negatively affect the patient’s quality of life [7,8]. In addition, it is common for patients to show treatment resistance or no clinical benefit from these established therapies [6,8]. For these reasons, the future direction of breast cancer treatment is focused on developing regimens that provide the greatest clinical benefit with the least amount of toxic side effects [6].
Molecular diagnostic tests have the potential to not only provide patients with personalized diagnostic information but also allow for specifically tailored treatment plans, thus limiting resistance, nonresponse and toxicity. Certain molecular diagnostic tests can also provide prognostic information about cancer in its early stages, thereby determining whether aggressive early management is necessary. The strict definition of a prognostic molecular factor was outlined by the NIH Consensus Conference and has the following characteristics [9–12]:
The factor must provide significant and independent prognostic value, which has been validated by clinical testing;
Determination of the factor must be feasible, reproducible, widely available and include quality control;
The results should be readily interpreted by a clinician;
The measurement of the marker must not consume tissue needed for other tests, particularly histopathologic evaluation.
This review will evaluate the molecular diagnostic practices for breast cancer, including current approaches and novel RNA-based diagnostics, as well as whether these tests demonstrate the characteristics of a prognostic marker, as outlined previously. In addition, there will be a special focus on the emerging molecular field of microRNA analysis and its future role in the decision-making process for diagnostic and therapeutic approaches.
Hormone receptors: estrogen & progesterone
The presence of estrogen receptors (ER) and/or progesterone receptors (PR) is currently a component of routine evaluation of breast cancer specimens. ER, first analyzed in breast cancer in the late 1950s, was the first molecular marker evaluated for prognosis and therapy response for breast cancer. ER status has been shown to have significant predictive value on tumor response to hormone therapy in metastatic disease as well as for adjuvant therapy after local excision [13,14]. The role of PR status in predicting tumor response to therapy is still unclear, although it has shown promise. There is an indication that a ‘double-positive’ ER+/PR+ tumor responds better to hormonal therapy than ‘single-positive’ ER+/PR− or ER−/PR+ tumors [14,15]. Whether ER and PR status also have prognostic value in addition to their predictive value has been extensively studied but remains under debate.
Hormone status is typically assessed in the clinic by either the ligand binding assay (LBA) or, more commonly, by immuno-histochemical staining (IHC) of tissue sections (Table 1). The LBA, developed for estrogen receptors in 1977, involves the competitive binding of a radiolabeled steroid to the receptor of interest [16,17]. This method allows for objective quantification of the estrogen and progesterone receptors, reported as femtomoles of receptor protein per milligram of total cytosol protein (fmol/mg) [13].
Table 1.
Current molecular breast cancer diagnostic assays.
| Assay | Description | Purpose | Disease stage | Tissue requirement | Cost burden |
US FDA approval |
Turnaround | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hormone receptor and protein expression methods | ||||||||
| ER | LBA or IHC | Guide for therapy and prognostic value | All stages, at initial diagnosis | Fresh or FFPE (blocks or sections) | $ | Yes§ | 3–4 days | |
| PR | LBA or IHC | Guide for therapy and prognostic value | All stages, at initial diagnosis | Fresh or FFPE (blocks or sections) | $ | Yes§ | 3–4 days | |
| HER2 | IHC or FISH | Guide for therapy and prognostic value | All stages, at initial diagnosis | Fresh or FFPE (blocks or sections) | $ | Yes§ | 3–4 days | |
| HERmark™ | Measures total HER2 expression and HER2 homodimers | Guide for therapy and prognostic value | All stages, at initial diagnosis | FFPE (blocks or sections) | $$$ | No§ | 7 days | [201] |
| Serum methods | ||||||||
| CellSearch® | Enumerates circulating tumor cells of epithelial origin | Early detection and prognostic value | Asymptomatic patients and all stages | 7.5-ml blood sample | $$ | Yes | 1 day | [99,100] |
| Biomarker Translation Test | Detects and quantifies key biomarker levels | Screening/early detection | Asymptomatic patients | 10-ml blood sample | $ | No§ | 2 weeks | [206] |
| Genue expression profiling methods | ||||||||
| Mammaprint™ | Measures a 70-gene expression profile to predict clinical outcome of breast cancer | Prognostic value | Stage 1 or 2 | 3-mm3 fresh sample in RNA-stabilizing solution | $$$ | Yes§ | 10 days‡ | [31–33] |
| OncoVue® Test | Combines the SNP patterns of 117 genes and personal history to predict breast cancer risk | Screening | Patients without family history, before radiologically or clinically detected | Cheek cell collection by oral rinse | $ | No§ | 5 days | [101] |
| GeneSearch™ breast lymph node | Detection of gene expression markers Mammaglobin and cytokeratin 19 via qRT-PCR | Detects breast tumor cell metastasis in lymph nodes | Node-negative, T1–T3 invasive carcinoma | 2–3-mm3 fresh sentinel lymph node biopsy | $ | Yes | 30–40 min | [102] |
| Nipple fluid aspiration | Measures DNA methylation patterns of breast epithelial cells via quantitative multiplex methylation-specific PCR | Early detection | High-risk patients, before radiologically or clinically detectable | Small volume of aspirate from non-lactating women | $ | No | N/A | [103] |
| DiaGenic BCtect® | Analyzes gene expression signatures from peripheral blood | Early detection | Asymptomatic patients | Blood sample | N/A | No | N/A | [104] |
| Oncotype DX®* | Measures ER, PR and HER2 gene expression via qRT-PCR | Prognostic and predictive value | Early (stage I and II) | FFPE (block or sections) | $$$ | No§ | 10–14 days | [34] |
| Theros H/ISM and MGISM | Measures two-gene ratio and five-gene expression index | Prognostic and predictive value | Stage 1, 2 and 3 | FFPE (block or sections) | $$$ | No§ | 7–10 days | [28–30] |
Denotes recommendation by ASCO.
Only performed at laboratory in Amsterdam.
Denotes performance in a CLIA-approved laboratory.
US$0–499
US $500–999
>US $1000
ER: Estrogen receptor; LBA: Ligand-binding assay; IHC: Immunohistochemistry; FFPE: Formalin-fixed paraffin-embedded; N/A: Not available; PR: Progesterone receptor; HER2: Human epidermal growth factor receptor 2; qRT: Quantitative reverse transcription.
The more standard approach to breast cancer diagnostics via hormone receptor analysis is IHC. IHC involves the use of antibodies and enzymes, such as horseradish peroxidase, to stain tissue sections for the tumor antigens of interest (Table 1) [18–20]. This evaluation method can be performed on both frozen or formalin-fixed paraffin-embedded (FFPE) tissue, as well as on small amounts of tissue acquired in procedures such as core biopsies. IHC also has the advantage of not only determining the percentage of positive nuclei but also the intensity of staining in individual nuclei. Unfortunately, in addition to a lack of interlaboratory standardization of the IHC technique, the process of evaluating the positivity of either ER or PR staining is performed subjectively by a pathologist, thereby introducing variability in interpretation [21]. Despite this subjectivity in staining intensity, IHC is by far the most common approach to evaluating hormone status in breast cancer today.
HER2
Another major prognostic marker that is currently recommended for the evaluation of primary invasive breast cancer is the human epidermal growth factor receptor 2, also known as HER2. HER2 is an oncogene belonging to the EGF receptor (EGFR) family [22]. Gene amplification of HER2 has been shown to occur in 10–40% of primary tumors and HER2 protein overexpression is found in almost 25% of breast cancers [23–25]. Studies have also shown that HER2 protein overexpression is associated with worse overall survival and twice the mortality rate compared with women with no HER2 expression [23].
Currently, HER2 is evaluated by either IHC for protein expression or by FISH for gene expression (Table 1) [26]. As with ER and PR, HER2 expression is reported as a percentage of stained versus unstained tumor cells. While IHC and FISH are techniques that are feasible to perform, reproducible and widely available, there has been a lack of standardization in the use and interpretation of assays, as described previously [26]. Compounding this problem, HER2 evaluation by gene amplification or protein overexpression is not recommended as the sole determinant of prognosis. Depending on their HER2 status, patients receive a variety of therapies. This makes it difficult in retrospective studies to discern if HER2 status is the single factor estimating disease progression or merely a component along with differing therapies [27].
HERmark™ assay
In an attempt to improve the current methods of HER2/neu analysis, Monogram Biosciences has recently released the HERmark™ breast cancer assay [201]. This assay measures total HER2 protein (H2T) and functional HER2 homodimer (H2D) levels on the cell surface of FFPE breast cancer tissue (Table 1). It uses a dual antibody system in which a fluorescent tag on one antibody is cleaved by a second antibody containing a photo-activated molecule. The fluorescent tags are then quantified using capillary electrophoresis (CE). HERmark reports whether a patient is HER2-negative, -positive or -equivocal based on quantified HER2 protein levels expressed as numeric values [201].
Recent studies indicate that HERmark is an accurate method for identifying breast cancer patients who are likely to benefit from trastuzumab therapy [201]. However, as a prognosticator of disease progression, studies suggest that measurements of the activated form of HER2 may be more useful than measurement of total HER2 expression. Thus, despite HERmark’s potential use as a predictive indicator of patients’ response to anti-HER2 therapy, additional studies are required to confirm these preliminary findings and to investigate whether HER2 activation measurement is a superior prognosticator of clinical outcome [201].
RNA-based tools for breast cancer diagnostics
There are several RNA-based molecular diagnostic tools that are currently commercially available for breast cancer (Table 1). Most of the tests either focus on gene expression microarrays or quantitative reverse transcription (qRT)-PCR analyses. A few of the more common gene expression tests will be highlighted here, including: Theros H/ISM and MGISM (bioTheranostics), Mammaprint™ (Agendia), and Oncotype DX® (GenomicHealth).
Theros H/ISM & MGISM
Theros H/ISM is a molecular diagnostic test that measures the ratio of HOXB13:IL17BR gene expression as a predictor of clinical outcome for breast cancer patients treated with tamoxifen [28]. A high level of expression of the two-gene ratio has been associated with tumor aggressiveness and failure to respond to tamoxifen [29]. Theros MGISM is an additional test that uses a five-gene expression index to stratify ER+ breast cancer patients into high or low risk of recurrence by reclassifying grade 2 (intermediate proliferative) tumors into grade 1-like or grade 3-like outcomes [30]. Theros H/ISM and MGISM tests require a FFPE tissue section from a breast biopsy sample. mRNA is extracted from the sample and is used to quantitatively measure gene expression via qRT-PCR. When used in combination, these two tests demonstrate potential advantages over current methodologies. First, they remove ambiguity of pathologic tumor grading by reclassifying grade 2 tumors as grade 1-like or grade 3-like. In addition, the qRT-PCR assays allow the data to be standardized in the laboratory, eliminating variability associated with subjective grading by a pathologist [30]. However, the American Society of Clinical Oncology (ASCO) is currently investigating the clinical utility and appropriate application of these assays because there have been no analyses demonstrating that this method provides superior classification of high-risk patients and their recurrence outcomes compared with conventional methods [27]. Furthermore, while Theros H/ISM and MGISM have shown predictive value for patients treated with hormonal therapy, ASCO recommends additional retrospective studies examining the ability of HOXB13:IL17BR to predict chemotherapy benefit [27].
MammaPrint™
The MammaPrint test is a molecular diagnostic tool that assesses a breast cancer patient’s chance for tumor recurrence. The MammaPrint uses a 70-gene signature that has been shown to have independent prognostic value over clinicopathologic risk assessment in patients with node-negative breast cancer [31,32]. The test requires a fresh sample (at least 3 mm in diameter) obtained during a surgical biopsy to be sent to the Agendia laboratory in Amsterdam in an RNA-stabilizing solution for analysis. RNA is isolated from the sample, amplified and co-hybridized with a standard reference to the MammaPrint microarray to obtain the 70-gene expression profile [33]. This method has been shown to have an extremely high correlation of prognostic prediction to tumor recurrence (p < 0.0001) [33]. In 2007, the US FDA approved the MammaPrint test for use on freshly frozen tissue.
Although the MammaPrint gene expression profile has the potential to be a useful diagnostic tool, there are many limitations that need to be taken into consideration. MammaPrint screening is only recommended for patients with stage 1 or 2 invasive breast cancer who are younger than 61 years old with a lymph node-negative tumor measuring less than 5 mm3 [33]. In addition, because the MammaPrint test requires a large amount of sample, there may not be enough tissue remaining for routine histological evaluation. The MammaPrint assay is currently only performed in the Agendia laboratory in Amsterdam, The Netherlands; therefore, a variety of laboratories do not have the ability to perform the assay. The tissue collection and handling requirements make the assay difficult for use in clinical practice. Samples taken for analysis must be taken from regions clear of both necrotic and stromal tissue, which may be impossible to obtain from a biopsy. Furthermore, specimens must contain at least 30% malignant cells on hematoxylin and eosin staining to be eligible to proceed for analysis on the MammaPrint assay [27]. For these reasons, ASCO determined that additional data from retrospective studies are required for more definitive recommendations for use of this assay in clinical practice [27].
Oncotype DX®
Oncotype DX is a 21-gene expression assay that uses qRT-PCR and microarray technologies to identify patients who may be successfully treated with chemotherapy and estimate the likelihood that invasive breast cancer will recur after treatment (Table 1) [34,35]. The Oncotype DX assay uses FFPE tissue blocks that can be shipped from anywhere in the USA and internationally. Currently, Oncotype DX is the standard breast cancer screening test for women with early-stage (Stage I or II), node-negative, ER+ invasive breast cancer [34,36]. The assay reports a recurrence score that ranges from 0 to 100, indicating the probability of cancer recurring within 10 years of the original diagnosis [34]. The recurrence score is then categorized into one of three groups: low, intermediate or high risk. There is a particular urgency for such information in women with early-stage breast cancer, where the great variety of treatment options can be narrowed down and tailored to each patient.
Both ASCO and the National Comprehensive Cancer Network (NCCN) have incorporated the Oncotype DX assay into their guidelines, highlighting the assay’s ability to predict a patient’s risk of recurrence and benefit from chemotherapy [26,27,202]. According to the 2007 ASCO Clinical Guidelines, once tamoxifen-treated patients have been stratified by the Oncotype DX assay to the low risk of recurrence group, they can be spared adjuvant chemotherapy [27]. The 2008 NCCN Breast Cancer Treatment Guidelines advise inclusion of this gene expression assay in the systemic adjuvant treatment decision pathway for patients with node-negative, hormone receptor-positive, HER2-negative tumors that are 0.6–1.0 cm with unfavorable features or tumors that measure over 1 cm [202]. Although these newer diagnostic tests have made incremental progress in breast cancer diagnosis and management, researchers and healthcare providers are still searching for a universal, reproducible and effective test to predict therapeutic response and outcome.
MicroRNA
The first microRNA was discovered in C. elegans in 1993 while screening for genes involved in developmental timing [37]. One of the genes discovered in the screening did not encode a protein, but rather a small 22-nucleotide RNA. By 2001, more than 100 of these small regulatory RNAs, later named microRNAs or miRNAs, were identified in various species including humans [38–40]. Today, there are approximately 700 known microRNAs in humans (ranging from 18–24 nucleotides in length) as cataloged by miRBase at the Wellcome Trust Sanger Institute [41–43].
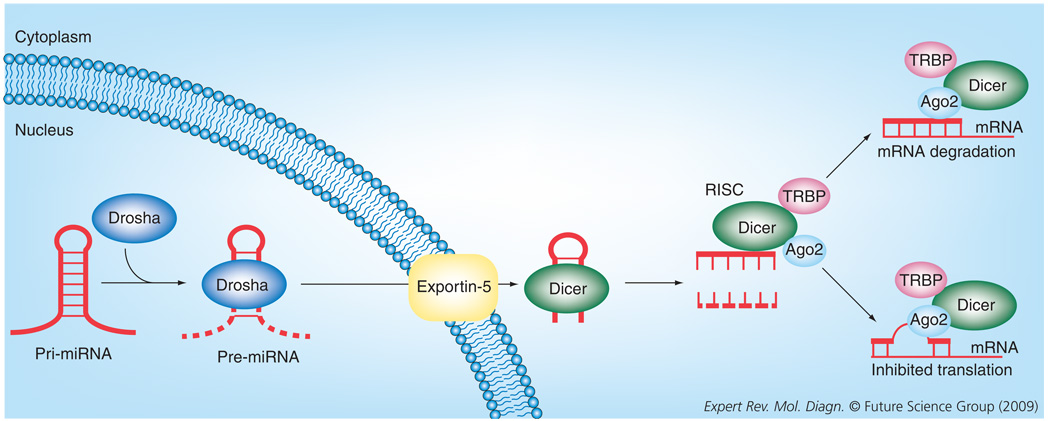
MicroRNAs are initially transcribed in the nucleus as pri-miRNAs, which are approximately 500–3000 bases long containing one or more stem-loop structures (Figure 1) [44]. A ribonuclease, Drosha, converts the pri-miRNA to pre-miRNA, which is then exported via Exportin-5 to the cytoplasm [45]. The pre-miRNA is then converted by another ribonuclease, Dicer, to a short RNA duplex [46,47]. After untwisting, one strand becomes the mature single-stranded microRNA and the other is typically degraded [48]. This mature microRNA then combines with the RNA-induced silencing complex (RISC), which is composed of Dicer, the HIV transactivating response RNA-binding protein (TRBP) and Argonaute2 (Ago2) [48]. The microRNA/RISC complex attaches to the messenger RNA (mRNA) in one of two ways: when the sequences are perfectly complementary, the microRNA/ RISC complex binds tightly to the mRNA and, via the enzyme Ago2, the mRNA is degraded [48,49]. More commonly, when the sequences are imperfectly complementary, the microRNA/RISC complex binds and inhibits translation of the mRNA without degradation. The final outcome of either of these pathways is a decrease in the protein level of the target gene.
Figure 1.
MicroRNA processing in the cell.
Despite information currently known, the functional role of microRNA in mammals is still emerging. To date, they have been associated with embryogenesis and stem cell maintenance [50], hematopoietic stem cell differentiation [51], brain development [52,53] and cancers [54–56]. Whether the changes in microRNA expression are a cause or effect of the disease is not yet known for many microRNA species. The role of microRNAs in cancer was first suspected when it was observed in C. elegans and Drosophila that microRNA controlled aspects of cell proliferation and apoptosis [37,57]. When microRNA genes were being studied in humans, it was noted that many genes were located at fragile sites in the genome, which are frequently amplified or deleted in human cancers [58]. Finally, microRNA expression appeared to be deregulated in cancer versus normal tissue [59–61]. Since those initial studies, many examples of microRNA deregulation have been shown in chronic lymphocytic leukemia [62], B-cell lymphoma [63,64] and breast cancer.
MicroRNA & breast cancer
MicroRNA deregulation in breast cancer was first demonstrated by Iorio and colleagues in 2005 [65]. Since this first study, there has been a surge of data accumulated on the expression of various microRNAs and their roles in breast cancer (Table 2). More recent studies have gone further to determine downstream targets or associate microRNA expression with prognostic information or response to treatment. The microRNAs described in the folllowing sections have either been shown to be consistently upregulated or downregulated and/or have been shown to potentially possess prognostic or predictive value.
Table 2.
Summary of microRNA associated with breast cancer.
| microRNA | RNA source (number of cell lines and/or cases) | Method of analysis | Expression stauts in tumor | Ref. |
|---|---|---|---|---|
| miR-7 | Fresh frozen tissue (184) | miRNA microarray and qRT-PCR | Expression associated with tumor aggressiveness (ER+) | [89] |
| miR-10b | Cell lines (11) | qRT-PCR | Highly expressed in metastatic cells | [76] |
| Fresh frozen tissue (219) | qRT-PCR | Downregulated in patients with distant and regional relapse and local recurrence | [77] | |
| Cell lines (14)Fresh frozen tissue (76) | miRNA microarray and northern blot | Downregulated in tumors | [65] | |
| miR-Let-7/a | Cell lines (4) | qRT-PCR | Downregulated in tumors | [105] |
| miR-21 | Cell lines (14) and fresh frozen tissue (76) | miRNA microarray and northern blot | Upregulated in tumors | [65] |
| Fresh frozen tissue (8) FFPE tissue (137) | miRNA microarray and qRT-PCR | Overexpression correlated with advanced clinical stage, lymph node metastasis and poor prognosis | [66] | |
| Cell lines (13) and FFPE Tissue (>100) |
In situ hybridization | Increased expression in tumors | [69] | |
| Tissue* (540) | miRNA microarray | Overexpressed in tumors | [72] | |
| Tissue* (40) | qRT-PCR | Overexpression may indicate aggressive phenotype | [74] | |
| Fresh frozen tissue (344) | qRT-PCR | Increased expression may aid tumor progression | [75] | |
| miR-125a | Cell lines (14) and fresh frozen tissue (76) | miRNA microarray and northern blot | Downregulated in tumors | [65] |
| miR-125b | Cell lines (14) and fresh frozen tissue (76) | miRNA microarray and northern blot | Downregulated in tumors | [65] |
| miR-128 | Fresh frozen tissue (184) | miRNA microarray and qRT-PCR | Expression associated with tumor aggressiveness (ER+) | [89] |
| miR-145 | Cell lines (14) and fresh frozen tissue (76) | miRNA microarray and northern blot | Downregulated in tumors | [65] |
| miR-155 | Cell lines (14) and fresh frozen tissue (76) | miRNA microarray and northern blot | Upregulated in tumors | [65] |
| miR-205 | Cell lines (1) and fresh frozen tissue | qRT-PCR and northern blot | Downregulated in tumors | [106] |
| Cell lines (5) and fresh frozen tissue (1) | qRT-PCR | Downregulated in tumors | [107] | |
| miR-206 | Cell lines (1) and fresh frozen tissue () | qRT-PCR | Downregulated in ER+tumors | [78] |
| miR-210 | Fresh frozen tissue (219) | miRNA microarray and qRT-PCR | Expression inversely correlated with overall and disease-free survival | [88] |
| Fresh frozen tissue (298) | miRNA microarray and qRT-PCR | Expression associated with tumor aggressiveness (ER+), early relapse (ER−) and poor outcome (triple negative) | [89] | |
| miR-221/222 | Cell lines (12) | miRNA microarray, qRT-PCR and northern blot | Upregulated in ER-tumors | [94] |
| Cell lines (2) | miRNA microarray and qRT-PCR | Upregulated in HER2+and tamoxifen-resistant tumors | [93] | |
| miR-93 | Cell lines (21) and fresh frozen tissue (93) | miRNA microarray and qRT-PCR | Highly expressed in high-grade tumors | [54] |
| miR-106b | Cell lines (21) and fresh frozen tissue (93) | miRNA microarray and qRT-PCR | Highly expressed in high-grade tumors | [54] |
| miR-25 | Cell lines (21) and fresh frozen tissue (93) | miRNA microarray and qRT-PCR | Highly expressed in high-grade tumors | [54] |
| miR-335 | Cell lines (7) and fresh frozen tissue (20) | miRNA microarray and qRT-PCR | Increased expression led to metastasis suppression | [80] |
| miR-126 | Cell lines (7) and fresh frozen tissue (20) | miRNA microarray and qRT-PCR | Increased expression led to metastasis suppression | [80] |
| miR-328 | Cell lines (2) | qRT-PCR | Increased expression decreased expression of breast cancer resistance protein (BCRP/ABCG2) | [108] |
| miR-373 | Cell lines (4) and fresh frozen tissue (94) | qRT-PCR | Increased expression stimulated cell migration and invasion | [109] |
| miR-516-3p | Fresh Frozen Tissue (184) | miRNA microarray and qRT-PCR | Expression associated with tumor aggressiveness (ER+) | [89] |
| miR-520c | Cell lines (4) and fresh frozen tissue (94) | qRT-PCR | Increased expression stimulated cell migration and invasion | [109] |
| miR-27a | Cell lines (6) | qRT-PCR | Upregulated in tumors | [110] |
| miR-27b | Cell lines (4) and fresh frozen tissue (24) | miRNA microarray and qRT-PCR | Downregulated in tumors | [86] |
| miR-17-5p | Cell lines (12) and fresh frozen tissue (16) | miRNA microarray, northern blot and qRT-PCR | Downregulated in tumors | [111] |
| miR-9-1 | Cell lines (5) and fresh frozen tissue (71) | qRT-PCR | Downregulated in tumors | [112] |
Tissue fixation not specified.
ER: Estrogen receptor; FFPE: Formalin-fixed, paraffin-embedded; qRT: Quantitative reverse transcription.
miR-21
miR-21 has surfaced in multiple studies as having consistent and significant increased expression in breast cancer cell lines and human tissue when compared with normal cells and tissues [65–72]. Many groups have begun researching the downstream targets of miR-21. Si et al. created MCF-7 cells with various amounts of miR-21 knockdown, which resulted in reduced cell growth and increased apoptosis in a dose-dependent manner [71]. In mouse xenograft tumors, knockdown of miR-21 inhibited tumor proliferation as demonstrated by reduction in Ki-67 immunoreactivity and tumor growth [71]. This same group went on to compare protein expression profiles in the xenograft tumors with and without miR-21 knockdown and found that the tumor suppressor tropomyosin 1 (TPM1) was a potential target [70]. Zhu et al. also found a binding site for miR-21 in the 3′ untranslated region (3′-UTR) of the TPM1 transcript, which was shown to be necessary for miR-21-mediated translational repression [70]. Another target for miR-21 appears to be tumor-suppressor gene programmed cell death (PDCD)4 [73]. miR-21 appears to downregulate PDCD4 at the mRNA and protein level [73]. More recently, Huang et al. found a negative correlation between miR-21 and the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which suggests PTEN is another potential target of miR-21 [74]. In this same study, the authors compared miR-21 expression to markers of aggressive phenotype. They found correlation between increased expression and lymph node positivity, higher proliferation index and advanced TNM stage [74]. This supports the results of two earlier studies, which also identified a correlation between increased miR-21 expression and poor disease-free survival in early-stage patients and advanced clinical stage, lymph node metastases and shortened survival [66,75].
miR-10b
miR-10b was one of the three microRNAs in the Iorio et al. study that demonstrated significant downregulation in breast cancer cells compared with primary human mammary epithelial cells (HMECs) [65]. However, in a subsequent study, miR-10b appeared to be highly expressed in metastatic cancer cells [76]. Functional studies have demonstrated that miR-10b overexpression promotes cell migration and invasion in vitro, and initiates tumor invasion and metastasis in vivo [76]. The same authors performed both upstream and downstream studies for miR-10b and found potential players in both directions [76]. Upstream, studies on the transcription factor Twist, which has been previously shown to be associated with invasive lobular carcinoma, suggest that it directly induces miR-10b expression [76]. Downstream, it appears miR-10b inhibits translation of homeobox D10 (HOXD10) resulting in the induction of the pro-metastatic gene product, ras homologue gene family member C (RHOC) [76]. However, in a letter in response to this study, Gee et al. found, when studying miR-10b expression in patients with primary tumors and nodal metastases versus primary tumors without nodal metastases, there was no significant association between miR-10b levels and metastasis or prognosis [77]. Ma et al. counterargued in response that their finding was an increase of miR-10b expression in breast cancers that had already metastasized, not that increased miR-10b in early breast cancers could predict whether the tumor would metastasize or not [77]. Differences in microRNA expression between studies may be attributed to the current technique employed in their isolation [77]. Most analyses to this date have been on whole tumors, which include malignant cells, normal cells, stroma and lymphocytes [77]. This, therefore, can underestimate or entirely miss microRNAs that are expressed in a very small population of cells within the whole sample [77].
miR-206
Multiple studies have also shown a significant association between expression of miR-206 and the expression of estrogen receptors in breast cancer [65,78,79]. Iorio et al. were the first to show that miR-206 expression was elevated in those tumors that were ER− [65]. Functional studies have since shown that miR-206 can silence translation of the ER mRNA by interacting with two sites in its 3′-UTR [79]. A recent article by Kondo et al. likewise demonstrated that miR-206 expression was decreased in ER+ breast tumors [78]. Further supporting the importance of miR-206, it has also been shown to decrease metastatic activity in mice for two breast cancer cell lines: BOM1 (highly metastatic to bone) and LM2 (highly metastatic to lung) [80]. Restoration of expression of miR-206 in BOM1 and LM2 cells significantly decreased their metastatic activity in mouse models [80].
miR-125a/b
miR-125a and miR-125b were first demonstrated in a microRNA profile study to be significantly downregulated in HER2-positive breast cancers [81]. Computation analysis then confirmed target sites at the 3′UTR regions of HER2 and HER3 for these microRNAs [81]. A tissue culture analysis demonstrated that overexpression of miR-125a or miR-125b in an ErbB2-dependent cancer cell line (SKBR3) suppressed HER2 and HER3 transcript and protein levels, which decreased cell motility and invasiveness [82].
miR-27a/b
miR-27a and miR-27b appear to have opposite roles in breast cancer. miR-27a is upregulated in breast cancer, and appears to target the transcriptional cofactor, ZBTB10 [83]. ZBTB10 represses the specificity protein 1 (SP1) transcription factor, which is overexpressed in many cancers and plays a role in the G0–G1 to S phase progression in breast cancer cells [84]. Therefore, by reducing expression of ZBTB10, miR-27a indirectly upregulates SP1 thus increasing S phase progression and functions as an oncogene. miR-27b, on the other hand, was first shown by sequence comparison to be potentially associated with cytochrome P4501B1 (CYP1B1), which is known to catalyze the metabolism of certain procarcinogens and is overexpressed in a wide range of cancers [85]. The expression level of both miR-27b and CYP1B1 was analyzed in breast and normal tissue, which demonstrated that as miR-27b expression decreases in breast cancer, the expression of CYP1B1 increases [86]. In a subsequent functional study in cells from the MCF-7 cell line, miR-27b was also shown to transcriptionally downregulate CYP1B1 expression, thus assuming the role of a tumor-suppressor gene [86].
Expert commentary
Today’s commercially available molecular diagnostic tests show great promise in enhancing the standard methods of assessing disease status and treatment options for breast cancer patients. It is clear from the conservative recommendations of the American Society of Clinical Oncology that these tests still have some hurdles to overcome. As outlined earlier, many of the newer tests are limited to a very specific subset of breast cancer patients, which constrains their wide-scale clinical effectiveness. Strict requirements of tissue amount, collection and handling and even analysis location, in the case of Mammaprint, restrict their value further. While breast cancer is a heterogeneous disease, which may ultimately require many tests at various stages, this increases the cost and hinders the accessibility to breast cancer patients globally [87]. The most important question is whether these new methods are superior to the standard methods of stratifying breast cancer. Once further validation of these assays is completed, they certainly have the potential to equal, if not surpass, the traditional ER, PR and HER2 detection methods in terms of prognostic and predictive value. When this is achieved, it will not be long until these assays are fully integrated into the clinical assessment of each breast cancer patient.
Likewise, microRNA demonstrates potential as a novel biomarker of disease state and progression. If confirmed, this entity may be the newest component in the next generation of breast cancer diagnostic assays. As described earlier, many individual microRNAs have been linked to molecules currently used for prognosis in breast cancer, such as ER and HER2 status [65,78,79,81,82]. In addition, a few microRNAs studied thus far have also shown differences in expression according to breast tumor staging [76]. For example, several members of the miR-let-7 family were found to be downregulated in breast cancer samples with lymph node metastases or higher proliferation indices [65]. miR-210 expression has been shown to be inversely correlated with overall and disease free survival, and positively correlated with tumor aggressiveness, early relapse and poor outcome [88,89]. Two other microRNAs demonstrated opposite expression changes with increasing proliferation indices and tumor stage: miR-145 was progressively downregulated with increasing proliferation index, and miR-21 was progressively upregulated with increasing tumor stage [65]. Therefore, microRNAs show great promise as new potential prognostic markers in breast cancer. The immediate task presented is to determine whether they have independent prognostic value beyond their associations with current prognostic molecules.
MicroRNAs have also been evaluated for their importance in predicting response to hormone therapies and chemotherapies [90,91]. For example, miR-451 has been found to be associated with multi-drug-resistance genes in MCF-7 cells [92]. Other microRNAs, such as miR-221/222, have been found to be upregulated in tamoxifen-resistant cells compared with tamoxifen-sensitive cells [93,94]. These studies indicate that microRNAs also hold promise as entities to distinguish response to treatment.
Perhaps the most important issue is that of microRNA normalization in the laboratory. There is still uncertainty whether a microRNA ‘housekeeping’ molecule exists, similar to GAPDH when analyzing mRNA. Recently, Davoren et al. addressed this question, specifically in breast cancer, to help establish endogenous control genes for microRNA research in this particular tissue type [95]. In their study, miR-let-7a and miR-16 were found to provide a reliable system for normalization in breast [95]. However, as reported in Table 2, miR-let-7a has also been shown to be downregulated in breast cancer in other studies. Therefore, these studies need to be repeated and validated prior to general acceptance in the laboratory.
Another major concern with microRNA profiling to date has been the inconsistency that has been observed from study to study. For example, Mattie et al. were able to demonstrate that a certain microRNA signature was able to discriminate between HER2 positive and negative tumors [81]; however, Iorio et al. and Blenkiron et al. were unable to show such an association [54,65]. The type of sample used as the RNA source, such as cell lines versus tissue samples, has a large influence on reported outcomes. Since microRNAs are regulating elements, their expression may be altered in immortalized cells grown on plastic compared with actual human tissue samples. In addition, the microRNA distribution may be heterogeneous within the tumor. This heterogeneity may contribute to some of the inconsistencies observed thus far.
In its favor, unlike mRNA, the difference in microRNA quality between frozen and FFPE tissue does not appear to be as much of a concern. mRNA studies typically require snap frozen tissue to ensure intact, unmodified biomolecules. The formalin fixation process can introduce nonspecific crosslinks between nucleic acids and proteins, which can distort results. Yet, the small size of the microRNA (18–24 nucleotides) allows them to be more easily retrieved and studied from both frozen and FFPE tissue. There have been numerous studies assessing the microRNA quality from FFPE tissue compared with frozen tissue using both microarray and qRT-PCR platforms [96–98]. The ability to utilize FFPE stored tissue blocks is a distinct advantage for microRNA, and opens a large number of cataloged tissue specimens for study. Furthermore, the ability to study microRNA in FFPE tissue introduces the possibility of in situ hybridization analysis to complement the microRNA amplification-based approaches [69].
Five-year view
The explosion of microRNA data in the past few years has begun to shed light on the involvement of these molecules in the progression of breast cancer. One question that has arisen from recent studies is how much of microRNA expression is a local phenomenon? For example, due to the small size of a breast biopsy, is it possible that an area of significant microRNA expression would be missed? Most of the studies completed to date are of either cell line or whole-tumor tissue samples, which contain stroma and inflammatory cells in addition to malignant tumor cells. Analyzing these samples directly could potentially dilute microRNAs that would otherwise demonstrate significant expression in malignant tumor cells only. This is an area of interest that may be better explored in future studies.
A significant drawback to microRNA analysis is the current lack of a consistent, universal control. However, if a normalizer is established, microRNAs have the potential to resemble current commercially available assays, and could easily be interpreted by physicians to assist with decisions on patient care. There are several companies that have recently released diagnostic tests that are based on microRNA platform data. These companies include Exiqon, Rosetta Genomics and Asuragen [203–205]. Although these tests are for colon, lung and pancreatic cancer, respectively, this is an indication that a microRNA test for breast cancer may be on the horizon.
Since most of the microRNA studies on breast cancer samples have been completed in the past few years, it may take some time for reliable tests to be developed. However, the stability of the microRNA, its ability to be extracted and analyzed from FFPE tissue, and the straightforward assays that have already been developed make it a very attractive molecule for newer, more accurate testing.
Key issues.
The main goals of molecular diagnostics will continue to be the enhancement and fine-tuning of oncologic decision pathways and treatment options for patients with breast cancer in order to maximize clinical benefit and minimize unnecessary toxicity and nonresponse.
MicroRNAs show great promise as new potential prognostic markers in breast cancer. The immediate task presented is to determine whether they have independent prognostic value beyond their associations with current prognostic molecules.
MicroRNAs also hold promise in predicting response to hormone therapies and chemotherapies, thereby becoming unique predictive factors in breast cancer.
However, microRNA assays do not currently have an accepted control against which they can be compared, nor have they demonstrated ease in reproducibility.
MicroRNAs have the potential to resemble current commercially available assays and, therefore, could be easily interpreted by physicians to assist with patient care.
MicroRNA assays will most likely be commercially available for breast cancer diagnostics within the next 5 years.
Acknowledgments
Financial & competing interests disclosure
This research was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Christine K Zoon, Tumor Angiogenesis Section, Surgery, Branch National Cancer Institute, National, Institutes of Health, Bethesda, MD, USA.
Elizabeth Q Starker, Tumor Angiogenesis Section, Surgery, Branch National Cancer Institute, National, Institutes of Health, Bethesda, MD, USA.
Arianne M Wilson, Tumor Angiogenesis Section, Surgery, Branch National Cancer Institute, National, Institutes of Health, Bethesda, MD, USA.
Michael R Emmert-Buck, Pathogenetics Unit, Laboratory of, Pathology and Urological Oncology, Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Steven K Libutti, Montefiore Medical Center/Albert Einstein, College of Medicine, Bronx, NY, USA.
Michael A Tangrea, Pathogenetics Unit, Laboratory of Pathology and Urological Oncology, Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA, tangream@mail.nih.gov.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, de la Garza Salazar J, Pritchard K, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Fernandez LM. Use of statistics to assess the global burden of breast cancer. Breast J. 2006;12 Suppl. 1:S70–S80. doi: 10.1111/j.1075-122X.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 4.Breast Cancer Screening. Lyon, France: IARC Press; 2002. [Google Scholar]
- 5. Groot MT, Baltussen R, Uyl-de Groot CA, Anderson BO, Hortobagyi GN. Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North America, and Asia. Breast J. 2006;12 Suppl. 1:S81–S90. doi: 10.1111/j.1075-122X.2006.00206.x.• Overview of the global ramifications of breast cancer.
- 6.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 7.Moore S. Managing treatment side effects in advanced breast cancer. Semin. Oncol. Nurs. 2007;23(4 Suppl 2):S23–S30. doi: 10.1016/j.soncn.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N. Engl. J. Med. 2009;360(1):63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- 9.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265(3):391–395. [PubMed] [Google Scholar]
- 10.National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1–3, 2000. J. Natl Cancer Inst. Monogr. 2001;30:5–15. [PubMed] [Google Scholar]
- 11.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J. Natl Cancer Inst. 2001;93(13):979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 12.Consensus conference 2000: adjuvant therapy for breast cancer. National Institutes of Health Consensus Development Conference Statement November 1–3, 2000. Cancer Control. 2001;8(1):55. [PubMed] [Google Scholar]
- 13.Buzdar A. The place of chemotherapy in the treatment of early breast cancer. Br. J. Cancer. 1998;78 Suppl. 4:16–20. doi: 10.1038/bjc.1998.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowsett M, Allred C, Knox J, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin. Oncol. 2008;26(7):1059–1065. doi: 10.1200/JCO.2007.12.9437. [DOI] [PubMed] [Google Scholar]
- 15.Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005;103(11):2241–2251. doi: 10.1002/cncr.21030. [DOI] [PubMed] [Google Scholar]
- 16.McGuire WL, De La Garza M, Chamness GC. Evaluation of estrogen receptor assays in human breast cancer tissue. Cancer Res. 1977;37(3):637–639. [PubMed] [Google Scholar]
- 17.Hull DF, 3rd, Clark GM, Osborne CK, Chamness GC, Knight WA, 3rd, McGuire WL. Multiple estrogen receptor assays in human breast cancer. Cancer Res. 1983;43(1):413–416. [PubMed] [Google Scholar]
- 18.Greene GL, Sobel NB, King WJ, Jensen EV. Immunochemical studies of estrogen receptors. J. Steroid Biochem. 1984;20(1):51–56. doi: 10.1016/0022-4731(84)90188-2. [DOI] [PubMed] [Google Scholar]
- 19.Jensen EV, Greene GL, DeSombre ER. Immunochemical studies of estrogen receptors. Prog. Clin. Biol. Res. 1987;249:283–305. [PubMed] [Google Scholar]
- 20.Taylor CR, Shi SR, Chaiwun B, Young L, Imam SA, Cote RJ. Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum. Pathol. 1994;25(3):263–270. doi: 10.1016/0046-8177(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 21.Layfield LJ, Gupta D, Mooney EE. Assessment of tissue estrogen and progesterone receptor levels: a survey of current practice, techniques, and quantitation methods. Breast J. 2000;6(3):189–196. doi: 10.1046/j.1524-4741.2000.99097.x. [DOI] [PubMed] [Google Scholar]
- 22.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229(4717):974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J. Clin. Oncol. 1990;8(1):103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 24.van de Vijver MJ, Mooi WJ, Wisman P, Peterse JL, Nusse R. Immunohistochemical detection of the neu protein in tissue sections of human breast tumors with amplified neu DNA. Oncogene. 1980;2(2):175–178. [PubMed] [Google Scholar]
- 25.Slamon DJ. Proto-oncogenes and human cancers. N. Engl. J. Med. 1987;317(15):955–957. doi: 10.1056/NEJM198710083171509. [DOI] [PubMed] [Google Scholar]
- 26. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007;131(1):18. doi: 10.5858/2007-131-18-ASOCCO.•• Outlines the guidelines and requirements for laboratory testing of HER2.
- 27. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin. Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364.•• Provides the most recent recommendations for breast cancer diagnostics
- 28.Ma XJ, Wang Z, Ryan PD, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5(6):607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Jansen MP, Sieuwerts AM, Look MP, et al. HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: a retrospective study. J Clin. Oncol. 2007;25(6):662–668. doi: 10.1200/JCO.2006.07.3676. [DOI] [PubMed] [Google Scholar]
- 30.Ma XJ, Salunga R, Dahiya S, et al. A five-gene molecular grade index and HOXB13:IL17BR are complementary prognostic factors in early stage breast cancer. Clin. Cancer Res. 2008;14(9):2601–2608. doi: 10.1158/1078-0432.CCR-07-5026. [DOI] [PubMed] [Google Scholar]
- 31.van ‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 32.Buyse M, Loi S, van ‘t Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98(17):1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 33.Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278. doi: 10.1186/1471-2164-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin. Chem. 2007;53(6):1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 35.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 36.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 37.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 38.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 39.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294(5543):858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 40.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294(5543):862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 41.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 45.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17(24):3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 47.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293(5531):834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 48.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 49.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3(3):e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bernstein E, Kim SY, Carmell MA, et al. Dicer is essential for mouse development. Nat. Genet. 2003;35(3):215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 51.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 52.Miska EA. How microRNAs control cell division, differentiation and death. Curr. Opin. Genet. Dev. 2005;15(5):563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Miska EA, Alvarez-Saavedra E, Townsend M, et al. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5(9):R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blenkiron C, Goldstein LD, Thorne NP, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8(10):R214. doi: 10.1186/gb-2007-8-10-r214.• MicroRNA profile study that classifies breast cancer into established mRNA molecular subtypes.
- 55.Verghese ET, Hanby AM, Speirs V, Hughes TA. Small is beautiful: microRNAs and breast cancer-where are we now? J. Pathol. 2008;215(3):214–221. doi: 10.1002/path.2359. [DOI] [PubMed] [Google Scholar]
- 56. Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch. 2008;452(1):1–10. doi: 10.1007/s00428-007-0532-2.• Recent review of association between microRNA and cancer.
- 57.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113(1):25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 58.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl Acad. Sci. USA. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 60.Gaur A, Jewell DA, Liang Y, et al. Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 2007;67(6):2456–2468. doi: 10.1158/0008-5472.CAN-06-2698. [DOI] [PubMed] [Google Scholar]
- 61.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 62.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He L, Thomson JM, Hemann MT, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ota A, Tagawa H, Karnan S, et al. Identification and characterization of a novel gene, C13orf25, as a target for 13q31–q32 amplification in malignant lymphoma. Cancer Res. 2004;64(9):3087–3095. doi: 10.1158/0008-5472.can-03-3773. [DOI] [PubMed] [Google Scholar]
- 65. Iorio MV, Ferracin M, Liu CG, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783.•• First comprehensive microRNA microarray analysis completed on breast cancer tissue.
- 66.Yan LX, Huang XF, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008;27(31):4373–4379. doi: 10.1038/onc.2008.72. [DOI] [PubMed] [Google Scholar]
- 68.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cancer Res. 2008;18(3):350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 69.Sempere LF, Christensen M, Silahtaroglu A, et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67(24):11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 70.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J. Biol. Chem. 2007;282(19):14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 71.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26(19):2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 72.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl Acad. Sci. USA. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem. 2008;283(2):1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 74.Huang GL, Zhang XH, Guo GL, et al. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncol. Rep. 2009;21(3):673–679. [PubMed] [Google Scholar]
- 75.Qian B, Katsaros D, Lu L, et al. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-β1. Breast Cancer Res. Treat. 2008 doi: 10.1007/s10549-008-0219-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 77.Gee HE, Camps C, Buffa FM, et al. MicroRNA-10b and breast cancer metastasis. Nature. 2008;455(7216):E8–E9. doi: 10.1038/nature07362. [DOI] [PubMed] [Google Scholar]
- 78.Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. miR-206 Expression is down-regulated in estrogen receptor α-positive human breast cancer. Cancer Res. 2008;68(13):5004–5008. doi: 10.1158/0008-5472.CAN-08-0180. [DOI] [PubMed] [Google Scholar]
- 79.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-α (ERa) and represses ERα messenger RNA and protein expression in breast cancer cell lines. Mol. Endocrinol. 2007;21(5):1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 80.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mattie MD, Benz CC, Bowers J, et al. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol. Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125aormiR-125b. J. Biol. Chem. 2007;282(2):1479–1486. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- 83.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66(3):1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 84.Abdelrahim M, Samudio I, Smith R, 3rd, Burghardt R, Safe S. Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J. Biol. Chem. 2002;277(32):28815–28822. doi: 10.1074/jbc.M203828200. [DOI] [PubMed] [Google Scholar]
- 85.Murray GI, Taylor MC, McFadyen MC, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57(14):3026–3031. [PubMed] [Google Scholar]
- 86.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66(18):9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 87.Bertucci F, Birnbaum D. Reasons for breast cancer heterogeneity. J. Biol. 2008;7(2):6. doi: 10.1186/jbiol67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Camps C, Buffa FM, Colella S, et al. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin. Cancer Res. 2008;14(5):1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 89.Foekens JA, Sieuwerts AM, Smid M, et al. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc. Natl Acad. Sci. USA. 2008;105(35):13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi M, Guo N. MicroRNA expression and its implications for the diagnosis and therapeutic strategies of breast cancer. Cancer Treat. Rev. 2009 doi: 10.1016/j.ctrv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Lowery AJ, Miller N, McNeill RE, Kerin MJ. MicroRNAs as prognostic indicators and therapeutic targets: potential effect on breast cancer management. Clin. Cancer Res. 2008;14(2):360–365. doi: 10.1158/1078-0432.CCR-07-0992. [DOI] [PubMed] [Google Scholar]
- 92.Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008;7(7):2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 93.Miller TE, Ghoshal K, Ramaswamy B, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008;283(44):29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao JJ, Lin J, Yang H, et al. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008;283(45):31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Davoren PA, McNeill RE, Lowery AJ, Kerin MJ, Miller N. Identification of suitable endogenous control genes for microRNA gene expression analysis in human breast cancer. BMC Mol. Biol. 2008;9:76. doi: 10.1186/1471-2199-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Chen J, Radcliffe T, Lebrun DP, Tron VA, Feilotter H. An array-based analysis of microRNA expression comparing matched frozen and formalin-fixed paraffin-embedded human tissue samples. J. Mol. Diagn. 2008;10(6):513–519. doi: 10.2353/jmoldx.2008.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li J, Smyth P, Flavin R, et al. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007;7:36. doi: 10.1186/1472-6750-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siebolts U, Varnholt H, Drebber U, Dienes HP, Wickenhauser C, Odenthal M. Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J Clin. Pathol. 2009;62(1):84–88. doi: 10.1136/jcp.2008.058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 100.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 2005;23(7):1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 101.Ralph DA, Zhao LP, Aston CE, et al. Age-specific association of steroid hormone pathway gene polymorphisms with breast cancer risk. Cancer. 2007;109(10):1940–1948. doi: 10.1002/cncr.22634. [DOI] [PubMed] [Google Scholar]
- 102.Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann. Surg. 2005;241(2):319–325. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suijkerbuijk KP, van der Wall E, Vooijs M, van Diest PJ. Molecular analysis of nipple fluid for breast cancer screening. Pathobiology. 2008;75(2):149–152. doi: 10.1159/000123853. [DOI] [PubMed] [Google Scholar]
- 104.Sharma P, Sahni NS, Tibshirani R, et al. Early detection of breast cancer based on gene-expression patterns in peripheral blood cells. Breast Cancer Res. 2005;7(5):R634–R644. doi: 10.1186/bcr1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 106.Iorio MV, Casalini P, Piovan C, et al. microRNA-205 regulates HER3 in human breast cancer. Cancer Res. 2009;69(6):2195–2200. doi: 10.1158/0008-5472.CAN-08-2920. [DOI] [PubMed] [Google Scholar]
- 107.Wu H, Zhu S, Mo YY. Suppression of cell growth and invasion by miR-205 in breast cancer. Cell Res. 2009;19(4):439–448. doi: 10.1038/cr.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol. Pharmacol. 2009 doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang Q, Gumireddy K, Schrier M, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008;10(2):202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 110.Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67(22):11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 111.Yu Z, Wang C, Wang M, et al. A cyclin D1/microRNA 17/20 regulatory feedback loop in control of breast cancer cell proliferation. J. Cell Biol. 2008;182(3):509–517. doi: 10.1083/jcb.200801079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lehmann U, Hasemeier B, Christgen M, et al. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J. Pathol. 2008;214(1):17–24. doi: 10.1002/path.2251. [DOI] [PubMed] [Google Scholar]
Websites
- 201.HERmark, Monogram Biosciences, Inc. www.hermarkassay.com.
- 202.NCCN Clinical Practice Guidelines in Oncology™ Breast Cancer, (Version 2.2008) www.nccn.org.
- 203.Exiqon. www.exiqon.com.
- 204.Rosetta Genomics. www.rosettagenomics.com/index.asp.
- 205.Asuragen, Inc. www.asuragen.com.
- 206.Provista Biomarker Translation Test. www.provistals.com/BTTestOverview.aspx.