Abstract
Ureaplasma respiratory tract colonization stimulates prolonged, dysregulated inflammation in the lungs of preterm infants, contributing to bronchopulmonary dysplasia (BPD) pathogenesis. Surfactant protein-A (SP-A), a lung collectin critical for bacterial clearance and regulating inflammation, is deficient in the preterm lung. To analyze the role of SP-A in modulating Ureaplasma-mediated lung inflammation, SP-A deficient (SP-A−/−) and WT mice were inoculated intratracheally with a mouse-adapted U. parvum isolate and indices of inflammation were sequentially assessed up to 28d post-inoculation. Compared to infected WT and non-infected controls, Ureaplasma-infected SP-A−/− mice exhibited an exaggerated inflammatory response evidenced by rapid influx of neutrophils and macrophages into the lung, and higher bronchoalveolar lavage TNF-α, mouse analogue of human growth-related protein alpha (KC), and monocyte chemotactic factor (MCP-1) concentrations. However, nitrite generation in response to Ureaplasma infection was blunted at 24h and Ureaplasma clearance was delayed in SP-A−/− mice compared to WT mice. Co-administration of human SP-A with the Ureaplasma inoculum to SP-A−/− mice reduced the inflammatory response, but did not improve the bacterial clearance rate. SP-A deficiency may contribute to the prolonged inflammatory response in the Ureaplasma-infected preterm lung, but other factors may contribute to the impaired Ureaplasma clearance.
The Ureaplasma parvum and U. urealyticum serovars are considered of low virulence since they are commensals in the adult female genital tract (1), but they are the most common perinatally acquired infections in preterm infants less than 30 weeks gestation and are associated with the development of bronchopulmonary dysplasia (BPD) (1). The vertical transmission rate is inversely related to gestational age (1), suggesting a developmental susceptibility to this infection.
There is compelling evidence from in vitro studies with cultured monocytes (2), as well as studies of human preterm infants (3–5), murine (6) and immature baboon (7) pneumonia models, and sheep (8) and immature baboon (9,10) intrauterine infection models that Ureaplasma is pro-inflammatory and pathogenic in the immature lung. In cultured human cord blood monocytes, Ureaplasma stimulates release of TNF-α and IL-8 (2). Moreover, in the presence of bacterial lipopolysaccharide, Ureaplasma greatly augments preterm monocyte production of pro-inflammatory cytokines while blocking expression of counter-regulatory cytokines (IL-6, IL-10). Hallmarks of the infection in human preterm infants and experimental animal models include persistent inflammatory cell influx, increased tracheal aspirate IL-1β, TNF-α, and monocyte chemoattractant protein-1 (MCP-1) concentrations during the first few weeks of life (3,11).
Lung host defense mechanisms must balance clearance of inhaled pathogens and limiting immune-mediated collateral injury to the essential gas-exchanging structures. Surfactant protein A (SP-A), a lung collectin, exhibits a dual function to enhance bacterial opsonization, phagocytosis and killing (12) and to modulate pulmonary inflammation in a microbial ligand-specific manner (13–17). Since SP-A expression is low in fetal lung and amniotic fluid prior to the third trimester (18–20), the developing lung is vulnerable to infection and dysregulated inflammation. SP-A deficiency in the immature baboon (21) and human (22) increases the risk for BPD.
The interactions of SP-A and Ureaplasma have not been previously investigated. We hypothesized that SP-A deficiency in the preterm lung increases the susceptibility to Ureaplasma infection and promotes sustained pulmonary inflammation contributing to the pathogenesis of BPD. In this initial study focused on the pulmonary inflammatory response, we analyzed indices of inflammation and pathogen clearance in SP-A-deficient and WT mice up to 4 weeks post-intratracheal inoculation with a mouse-adapted U. parvum isolate in the presence or absence of co-administered exogenous SP-A.
MATERIALS AND METHODS
Animals
Homozygous SP-A−/− and WT mice on a 129J background were obtained from Children’s Hospital (Cincinnati, OH) and were maintained as breeding colonies under pathogen-free conditions in the Central Animal Facility of the University of Maryland, Baltimore. The University of Maryland Institutional Animal Care and Use Committee approved the protocol.
SP-A
Dr. David S. Phelps (Pennsylvania State College of Medicine, Hershey, PA) generously provided human SP-A purified by the 1-butanol extraction method (23) from bronchoalveolar lavage (BAL) of patients with alveolar proteinosis that was >99% SP-A confirmed by 2 dimensional gel electrophoresis followed by Western blotting and silver staining and contained <0.01 pg endotoxin/µg SP-A.
Ureaplasma
A clinical U. parvum isolate was adapted to the 129J WT strain by serial pulmonary passage as described previously (6). For each experiment, an aliquot of this isolate was inoculated 1:10 in 10B broth and incubated overnight to obtain a titer of 106 color changing units (CCU)/ml viable organisms. The bacterial suspension was centrifuged at 14,000 × g for 30 min at 4°C and the bacterial pellet was re-suspended in PBS prior to inoculation.
Ureaplasma SP-A Binding Assay
Immunlon-2 96 well microtiter plates coated with 106 CCU mouse-adapted Ureaplasma in 0.05 M Na2CO3, pH 9.6 were incubated at 4°C overnight. After incubation, the Ureaplasma solution was removed and the plates washed. Non-specific binding was blocked by incubation for 2 h at 25°C with 200 µl/well 1% human serum albumin in 10 mM Tris (pH 7.5), 1 mM CaCl2, 0.15 M NaCl. Plates were washed five times and 100 µl 10 or 20 µg/ml purified SP-A or 10 mM EDTA in 10 mM Tris (pH 7.5), 1 mM CaCl2 and 0.1% human serum albumin was added and incubated overnight at 25°C. After washing, the plates were sequentially incubated with rabbit anti-human SP-A antibody (generous gift, David S. Phelps) (1:5000) and horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2500) for 1 h at 25°C each. After washing, color was developed using H2O2 and O-phenylenediamine dihydrochloride in 0.1 M citric acid, pH 5.0 and reaction stopped with 2.5 M H2SO4. Color intensity was measured at 490 nm.
Inoculation
Mice (6–8wks) were anesthetized with xylazine (10 mg/kg body weight) and ketamine (65 mg/kg body weight) intraperitoneally. U. parvum (106 CCU) in 50 µl PBS or PBS alone was instilled into the posterior pharynx of an anesthetized mouse while it was suspended in a vertical position and prevented from swallowing by gentle extension of the tongue. The mice were maintained in this position until aspiration was witnessed. We have previously demonstrated that Evans blue dye administered using this inoculation method stained the surface of all lobes of both lungs with only minimal amounts swallowed or retained in the trachea (24). In initial experiments, WT and SP-A−/− mice were euthanized at 6h, 1, 2, 3 or 28d post-inoculation (N=6–8 mice per condition/time point) for BAL and analysis of lung tissue for Ureaplsama by culture and PCR. At the same time points, lungs of 6–8 additional mice/condition were fixed in situ with Prefer®(Anatech, Ltd., Battle Creek, MI) fixative at 20 cm H2O constant pressure and were collected for immunohistochemical analysis. Uninoculated WT and SP-A−/− mice were euthanized at a single time point for comparisons.
Exogenous SP-A
To determine the capacity of exogenous SP-A to limit the inflammatory response and improve Ureaplasma clearance in SP-A−/− mice, purified human SP-A (100 µg) was co-administered intratracheally with the U. parvum inoculum to additional groups of SP-A−/− mice and compared to SP-A−/− mice inoculated with U. parvum alone. The animals were euthanized at 6, 24, or 48h post-inoculation (N=6 per group/time point) for BAL and analysis of lung tissue for Ureaplasma by culture and PCR.
Bronchoalveolar lavage
BAL was performed in situ as previously described (6). BAL cells were collected by centrifugation at 1,000 × g for 10 min. Cell-free supernatants were aliquoted and stored at −80°C for analysis of total protein, nitrite, and cytokine concentrations. Total cells were counted manually with a hemacytometer, and differential cell counts of Diff-Quick™-stained cytopreparations performed using morphological criteria.
Cytokine ELISA
Cytokines and chemokines that have been associated with Ureaplasma-mediated lung injury and/or BPD were selected for study (2,4,10). Murine IL-1β, TNF-α, the mouse analogue of human growth-related protein alpha (KC), MCP-1, and IL-10 concentrations were measured in duplicate using a multi-analyte immunoassay using Luminex® bead technology and reagent kits from Upstate Biotechnology (Millipore Corp., Bilerica, MA). The lower detection limits of the assays were 10.3, 6.9, 10.3, 13.7, and 16.4 pg/ml for IL-1β, TNF-α, KC, MCP-1, and IL-10, respectively. A curve was fit to the standards with a computer program (Softpro, Molecular Devices, Sunnyvale, CA), and cytokine concentrations from each sample were calculated from the standard curve.
BAL Nitrate and Nitrite
Total NO was quantified in BAL from the time course experiments by measuring its nitrate and nitrite oxidative products after enzymatic conversion to nitrite using a colorimetric assay (Cayman Chemical, Ann Arbor, MI). For the SP-A reconstitution experiments, BAL NO was analyzed with the more sensitive Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI). For both assays, the concentration for each sample was calculated from a sodium nitrite standard curve.
Lung culture
Following lavage, the lungs were removed and processed for Ureaplasma culture. The lungs were minced and then homogenized between the frosted ends of sterile glass slides and placed in 2ml of 10B media and serial 1:10 dilutions were incubated at 37°C in 95% air–5% CO2. If color change occurred, 0.2 ml of inoculum was plated on A7 agar (Northeast Laboratory, Waterville, Maine). Tube cultures and plates were examined daily for 1 week for color change and typical colonies of U. parvum, respectively.
PCR
DNA was extracted from lung homogenates with Qiagen columns according to the manufacturer’s protocol (Qiagen, Valencia, CA). PCR was performed as previously described using primers for the mba gene (6).
Immunostaining
Paraffin-embedded lung sections were immunostained for neutrophils and macrophages using commercial antibodies, the appropriate secondary HRP conjugates, diaminobenzidin staining solution, and counterstained with hematoxylin as described in the manufacturer’s protocol (BD Sciences) as previously described (6). For neutrophils, the primary antibody was 2.5 µg/ml biotinylated rat anti-mouse Gr-1 (BD-Pharmingen). For macrophages, the primary antibody was 1 µg/ml rat anti-mouse Mac-3, and the secondary antibody 2.5 µg/ml biotinylated goat anti-rat IgG1/2a (both from BD-Pharmingen; San Diego, CA).
Statistical analysis
All data are presented as means ± standard errors (SE). Differences among experimental groups at each time point and within group comparisons over time were tested by a Fisher-protected least square difference applied to a one-way analysis of variance. Chi square analysis was used to compare Ureaplasa clearance rate between infected WT and SP-A−/− groups. A p-value of <0.05 was considered significant.
Results
SP-A binds Ureaplasma in vitro
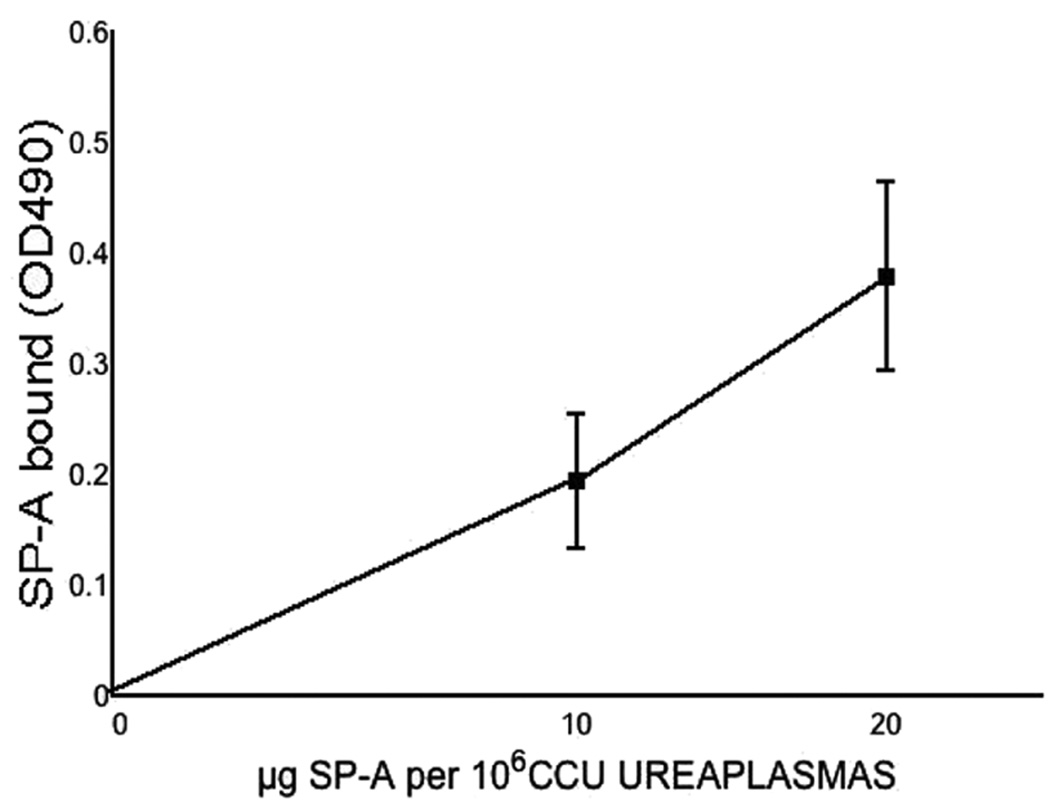
As shown in Figure 1, human SP-A bound Ureaplasma in a dose and calcium dependent manner. There was no detectable binding in the absence of calcium.
Figure 1. Binding of SP-A to Ureaplasma.
Mouse-adapted Ureaplasma (106 CCU) incubated with EDTA or SP-A (0–20 µg/ml) in presence or absence of 1 mM Ca2+. Total Ureaplasmaassociated SP-A is expressed as OD490 corrected for non-specific binding, mean ±SEM, 4 experiments. No binding was observed in the absence of Ca2+(not shown).
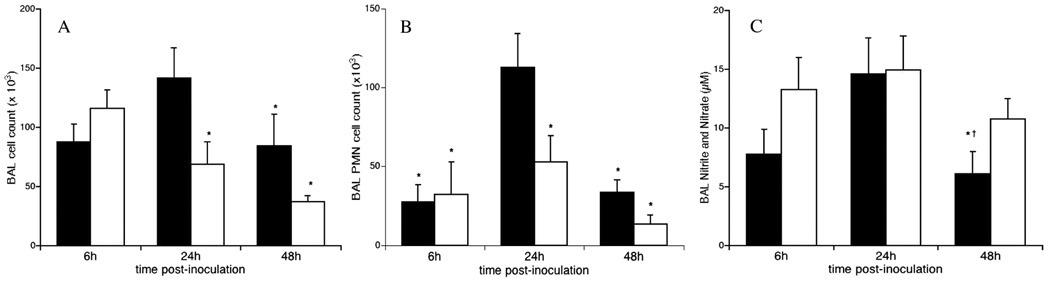
Ureaplasma infection stimulated an inflammatory cell influx into the lungs of SP-A−/− mice
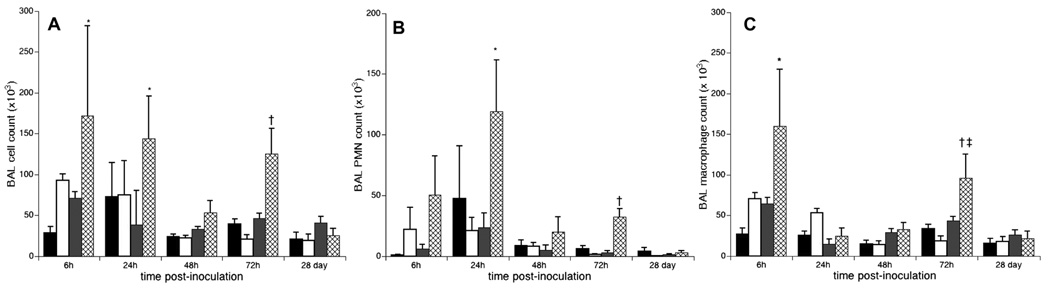
There were no differences in baseline BAL cell numbers and composition between untreated SP-A−/− and WT mice. There was a more rapid and sustained influx of inflammatory cells into the lungs of SP-A−/− than WT mice post-Ureaplasma inoculation (Fig. 2). Total BAL cell counts in the SP-A−/− infected mice were 2.4-, 3.7-, and 2.7-fold higher at 6, 24, and 72h post-inoculation (p< 0.05), respectively, than in WT mice analyzed at the same post-inoculation times (Fig 2A). There was a rapid influx of neutrophils in the Ureaplasma-infected SP-A−/− mice that peaked 24h and remained elevated at 72h post-inoculation (Fig 2B). Two significant peaks of BAL macrophage accumulation occurred at 6 and 72h post-inoculation in Ureaplasma- infected SP-A/− mice (Fig. 2C).
Figure 2. Leukocyte influx in SP-A deficient mice post-Ureaplasma inoculation.
BAL total and differential cell counts were performed. Data are expressed as the means ± SE of the total number of inflammatory cells (A), neutrophils (B), and macrophages (C). PBS-inoculated WT, black bar; PBS-inoculated SP-A−/−, white bar; U. parvum inoculated WT, gray bar; U. parvum inoculated SP-A−/−, hatched bar. *p<0.05 vs PBS controls and Ureaplasma-inoculated WT all time points; †p<0.05 vs PBS controls and Ureaplasma-inoculated WT at 72h, ‡p<0.05 vs 24h, 48h, and 28d infected SP-A−/−
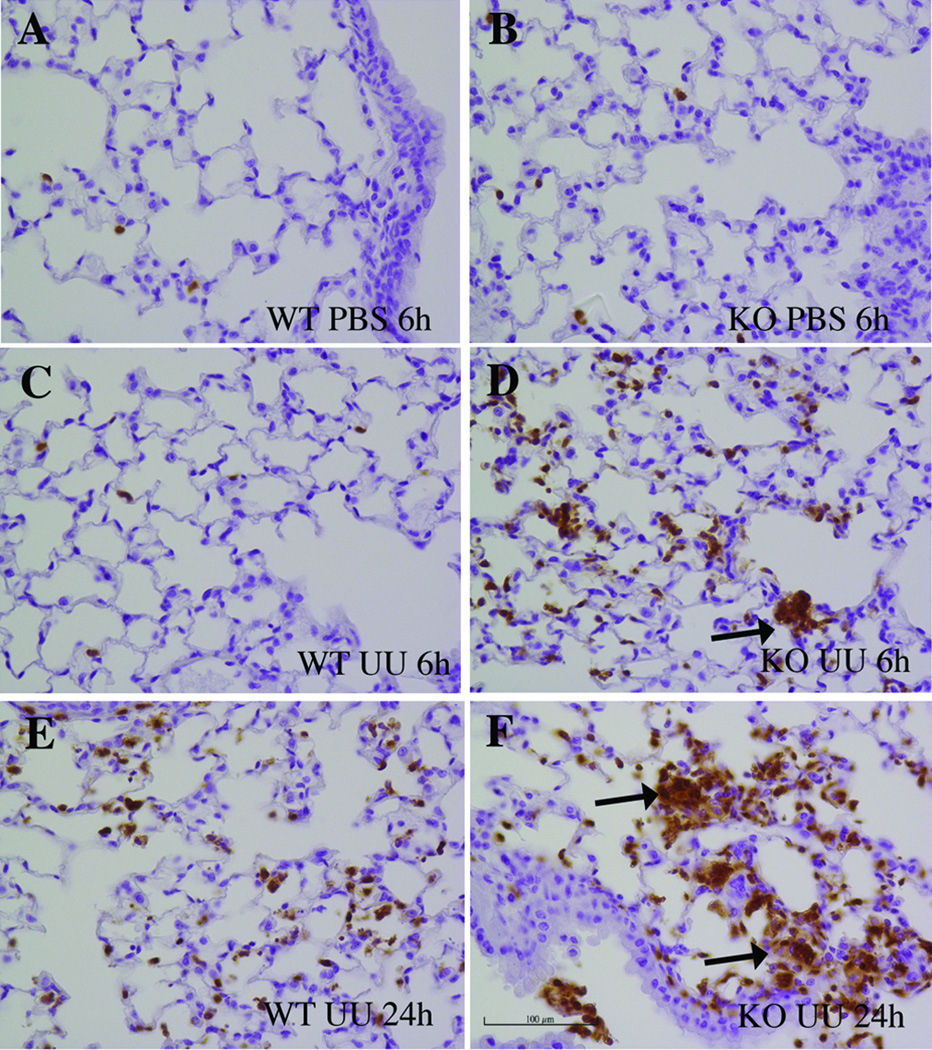
There were a greater number of immunoreactive PMNs throughout the lung interstitium and within focal areas by 6 to 24h post-inoculation in SP-A−/− mice compared to infected WT mice (Fig. 3). At 72h post-inoculation, macrophages were noted within the airway in the Ureaplasma- inoculated SP-A−/− mice with focal peribronchiolar infiltrates (data not shown). In contrast, there were few alveolar and interstitial inflammatory cells in untreated and PBS-inoculated controls.
Figure 3. PMN immunostaining of Ureaplasma-infected lungs.
Lung sections from PBS and Ureaplasma-inoculated WT and SP-A−/− mice were immunostained with biotinylated rat anti-mouse Gr-1 antibody and counterstained with hematoxylin. (A to F) Representative immunostained sections of lungs 6h (A–D) and 24h (E–F) post-inoculation. SP-A−/− mice demonstrated extensive focal PMN infiltrates in interstitium and peribronchi (arrows indicate immunoreactive PMN). Original magnification 400×, scale 100 µm. Abbreviations: PMN: polymorphonuclear cells KO, SP-A−/− knockout; UU, Ureaplasma-inoculated.
Increased pulmonary cytokine response in Ureaplasma-infected SP-A−/− mice
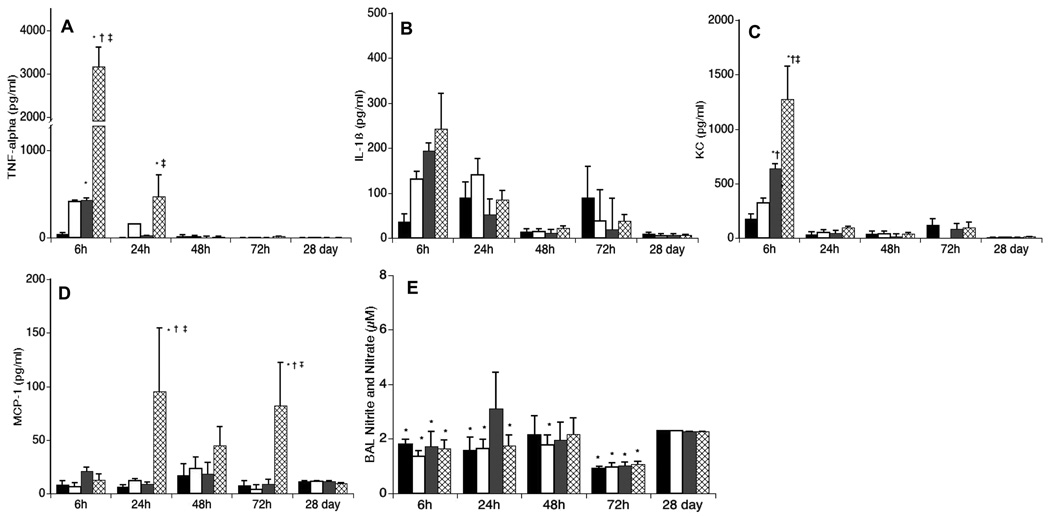
There were no differences in BAL cytokine concentrations between uninoculated WT and SP-A−/−control mice. However, infected WT and SP-A−/− mice differed in kinetics and concentrations of the pro-inflammatory cytokines. TNF-α, IL-1β, and KC increased rapidly in both infected WT and SP-A−/− mice (Fig 4A–C). However, TNF-α and KC concentrations in BAL were 7- and 2-fold higher in infected SP-A−/− mice 6h post-inoculation compared with infected WT mice and TNFα levels remained elevated longer in the SPA−/− mice (24 vs. 6h). MCP-1 levels were also higher in infected SPA−/− than WT mice, but expression occurred later than for TNFα and KC, first increasing at 24h post-inoculation, but remaining elevated for 72h (Fig. 4D). There were no significant differences in IL-10 concentrations among the groups (data not shown).
Figure 4. Effects of Ureaplasma inoculation on BAL cytokine and NO concentrations.
BAL cytokines were measured by a multi-analyte immunoassay using Luminex® bead technology. (A) TNF-α (B) IL-1β; (C) KC; and (D) MCP-1. Nitrite in BAL was measured with the Griess reaction after nitrate was reduced to nitrite with nitrate reductase (E). Data are expressed as means ± SE. PBS-inoculated WT, black bar; PBS-inoculated SP-A−/−, white bar; U. parvum inoculated WT, gray bar; U. parvum inoculated SP-A−/−, hatched bar. *p <0.05 vs PBS-inoculated WT; †p<0.05 vs PBS-inoculated SP-A−/−; ‡p< 0.05 vs Ureaplasma-inoculated WT. (E) *p< 0.05 vs Ureaplasma-inoculated WT 24h post-inoculation.
Blunted NO response in Ureaplasma-infected SP-A−/− mice
Despite the increase in BAL inflammatory cells and cytokines in Ureaplasma-infected SP-A−/−mice during the first 24h post-inoculation, the BAL nitrite concentration (after conversion of nitrate to nitrite as described in Methods and Materials) at 24h in these mice was lower than the concentration in BAL of infected WT mice (Fig. 4E).
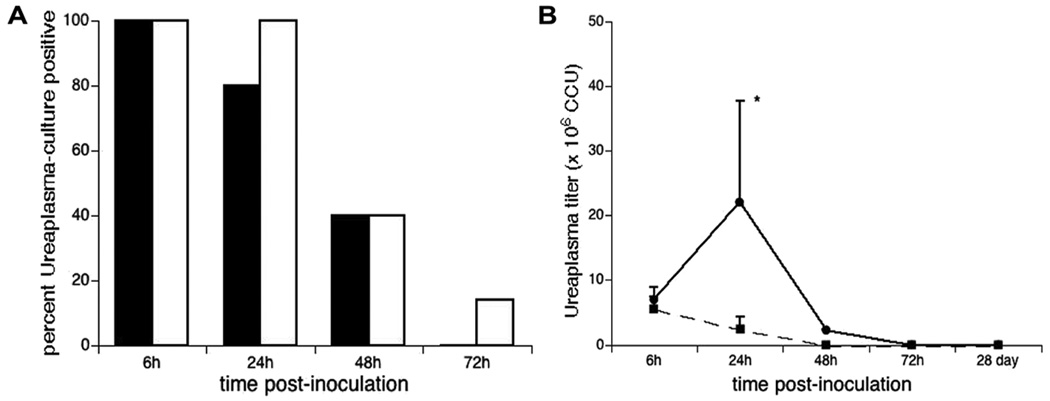
Ureaplasma clearance is delayed in infected SP-A−/− mice
None of the Ureaplasma-inoculated mice appeared ill and there was no mortality associated with the infection in either group. Lung cultures confirmed the presence of Ureaplasma in 100% of SPA−/− and WT mice 6h post-inoculation, but clearance was modestly delayed in the SPA−/− mice (Fig 5A). 100%, 40%, and 15% of SP-A−/− mice were culture positive at 24, 48, and 72h post-inoculation compared with 80%, 40%, and 0% of WT mice (p=0.036). Unlike the Ureaplasma culture titers in the inoculated WT animals, which rapidly decreased following inoculation (Fig 5B), the Ureaplasma titers in the SPA−/− mice increased during the first 24h post-inoculation before decreasing to titers comparable to WT by 48h.
Figure 5. Percent of WT and SP-A−/− mice culture-positive post-Ureaplasma inoculation (A) and lung homogenate titers (CCU) (B).
Lungs of Ureaplasma-inoculated mice were homogenized and cultured in 10B broth. Data are expressed as the percentages of animals that were Ureaplasma positive by culture and confirmed by PCR in WT (black bar) and SP-A−/− mice (white bars) at each day post-inoculation. *p<0.05 vs WT at 24h and all other time points both strains.
Exogenous SP-A reduced inflammation, but did not improve Ureaplasma clearance
Since the greatest differences in the inflammatory response and bacterial clearance occurred in the first 48h post-inoculation, studies of the effect of exogenous SP-A focused on this time period. Co-administration of purified human SP-A with the Ureaplasma inoculum to SP-A−/− mice significantly reduced the total number of BAL inflammatory cell and neutrophils at 24h (Fig. 6A–B) and BAL TNF-α and KC concentrations (Table 1), but did not affect other measured cytokines or chemokines. There was also a trend towards higher BAL nitrite concentration (Fig. 6C) concentration at 6h post-inoculation in exogenous SP-A treated mice. Exogenous SP-A did not significantly affect Ureaplasma clearance in the SP-A−/− mice 24 and 48h post-inoculation (data not shown).
Figure 6. Exogenous SP-A reduced inflammatory cell influx and partially restored the NO response in Ureaplasma-inoculated SP-A−/− mice.
SP-A−/− mice were inoculated intratracheally with 106 CCU Ureaplasma ± 100 µg purified human SP-A and lungs harvested after 6, 24 or 48h. Total and differential cell counts were performed. Nitrite in BAL was measured after nitrate was reduced to nitrite with nitrate reductase, U. parvum inoculation alone, black bars; U. parvum plus SP-A inoculation, white bars. Data are expressed as the means ± SE of the total number of inflammatory cells (A), neutrophils (B), or, total NO (C). *p<0.05 vs Ureaplasma-inoculated SPA−/− 24h post-inoculation.
Table 1.
Bronchoalveolar lavage cytokine concentrations in Ureaplasma-infected SP-A−/− mice with and without co-administered exogenous SP-A*
| No SP-A | Plus SP-A | ||
|---|---|---|---|
| TNF-α (pg/ml) | 6 h | 2267 ±119‡§ | 716 ± 211†‡§ |
| 24 h | 217.1 ± 44 | 376.5 ± 45.2 | |
| 48 h | 104.0 ± 38.3 | 198.1 ± 87.9 | |
| IL-1β (pg/ml) | 6 h | 78.4 ± 9.1‡ | 51.2 ± 12§ |
| 24 h | 73.0 ± 3.0‡ | 87.6 ±11.7 | |
| 48 h | 33.0 ±6.7 | 60.9 ± 18.2 | |
| KC (pg/ml) | 6 h | 1677 ± 152‡§ | 1013 ±228†‡§ |
| 24 h | 417 ± 26.8 | 543 ± 49.7 | |
| 48 h | 415 ± 32.3 | 466 ± 94.7 | |
| MCP-1 (pg/ml) | 6 h | 91.0 ± 17.5 | 53.2 ± 5.0 |
| 24 h | 96.4 ± 14.2 | 79.3 ± 13.5 | |
| 48 h | 467 ± 276 | 590 ± 194 |
Data are expressed as mean ± SEM, n=8 each time point per condition
p<0.05 vs Ureaplasma-infected SP-A−/− mice, same time point
p <0.05 vs 48 h same condition
p <0.05 vs 24 h same condition
DISCUSSION
SP-A gene and protein expression are low early in gestation and the postnatal increase in expression is blunted after preterm birth (18,25). SP-A concentrations in human fetal lung during the second trimester are less than 0.5% of adult concentrations (18,19). During fetal lung development in the baboon, SP-A is undetectable at 125 d (68% term) and 140 d (76% term) gestation, but increases to adult levels near term (26). In the ventilated 125d immature baboon model, low SP-A levels (<20% adult level) are associated with increased risk of pulmonary infections and enhanced expression of inflammatory cytokines (27). In humans, Ureaplasma has been detected in amniotic fluid as early as the time of genetic amniocentesis (16–20 weeks) (28) when SP-A levels are low. We speculate that SP-A deficiency may contribute to the susceptibility of the developing lung to intrauterine Ureaplasma infection.
Several lines of evidence implicate SP-A deficiency in the pathogenesis of BPD. Preterm infants less than 1000 g birthweight with low SP-A/dipalmitoly phosphatidylcholine ratio (<25 ng/nmol) during the first week of life were more likely to die or develop BPD (22,29). SP-A polymorphisms are associated with RDS, suggesting that alterations in SP-A protein affect surfactant function, predisposing to lung disease in prematurely born infants (30). In the 140 d immature baboon model, ventilation-induced lung injury was associated with increased lung tissue SP-A, but decreased lung lavage SP-A concentration, suggesting perturbations in SP-A secretion in these animals contributes to abnormal surfactant function and dysregulated inflammation (21).
The critical role of SP-A in limiting inflammation has been clearly shown in short-term experimental bacterial (13), mycoplasmal (14) and viral (15,16) infection and intratracheal endotoxin models (17) in SP-A deficient mice. In the present study, we demonstrated for the first time that SP-A bound the mucosal commensal Ureaplasma in vitro and intratracheal inoculation with t Ureaplasma stimulated an early (6–24h), exaggerated increase in pro-inflammatory cytokines and influx of leukocytes into the lungs of SP-A deficient mice compared to a relatively mild inflammatory response in infected WT mice. Reconstitution with exogenous SP-A did not enhance bacterial clearance, but reduced the early influx of inflammatory cells and blunted expression of the pro-inflammatory cytokines. Thus, SP-A appears to be an important modulator of Ureaplasma-mediated lung inflammation in the murine pneumonia model.
There was a rapid (within 6h) and sustained (up to 72h) influx of inflammatory cells into the airways and interstitium of infected SP-A−/− mice not observed in non-infected controls or infected WT mice. Neutrophils increased during the first 24h while macrophages were the dominant cells thereafter. The focal intra-alveolar and peribronchiolar infiltrates observed in the Ureaplasma-infected SP-A−/− mice are similar to the histologic appearance of other experimental Ureaplasma infection models, including the mycoplasma-susceptible C3H/HeN mouse strain (6), newborn mice (31), newborn immature baboons infected postnally (7) and antenatally (9,10), and post-mortem findings in Ureaplasma-infected human preterm (4,5) and full-term (32) infants dying with acute pneumonia.
We found a rapid increase in BAL concentrations of the pro-inflammatory cytokines TNF-α and KC in infected SP-A−/− mice. As observed in human infants in vivo (3), and cord blood monocytes in vitro (2), Ureaplasma infection did not stimulate an increase in the anti-inflammatory cytokine IL-10 at any time point in SP-A−/− or WT mice. These observations suggest that SP-A deficiency in the preterm Ureaplasma–infected lung may result in an imbalance between pro- and anti-inflammatory processes, thereby contributing to dysregulated inflammation.
Whether exogenous SP-A treatment will reduce inflammation in the preterm lung is unknown. In a newborn rabbit model of acute lung injury achieved by intratracheal instillation of fibrinogen, bovine surfactant enriched with 5% SP-A treatment reduced surfactant inactivation and limited neutrophil infiltration compared to surfactant alone (33). In contrast, ovine SP-A plus human SP-C surfactant treatment of preterm lambs with ventilation-induced lung injury stimulated acute neutrophil influx into the lung (34). In the current study, SP-A reconstitution limited inflammatory cytokine release and inflammatory cell influx. Differences in response to exogenous SP-A in these models may be due to differences in the context of lung injury (fibrinogen, ventilation, or infection) or differences in timing of SP-A treatment relative to initial injury (post-injury vs. concomitant exposure).
There are a number of limitations of the current study. In SP-A reconstitution experiments, SP-A was co-administered as a single dose with the Ureaplasma inoculum. A single dose may have been insufficient to optimally suppress inflammation or improve microbial clearance due to rapid clearance of exogenous SP-A (13). Although pre-inoculation with exogenous SP-A may have altered susceptibility to a subsequent infection and multiple dosing may have improved microbial clearance, we did not perform these experiments to avoid adverse responses to serial anesthesia and multiple intratracheal instillations. Although this study did not include the component of lung immaturity, the data suggests that SP-A deficiency may predispose the preterm lung to a more exaggerated inflammatory response to Ureaplasma infection and subsequent development of BPD.
Our findings indicate that SP-A may be more important in limiting inflammation than in accelerating bacterial clearance in response to Ureaplasma pulmonary infection. This may have particular relevance for the SP-A deficient preterm human lung, which is exposed to multiple pro-inflammatory stimuli (e.g. infection, hyperoxia, volutrauma). In transgenic mice, over-expression of TNF-α̣ IL-6, or IL-11 (35), or conditional IL-1β overexpression in fetal and neonatal lung (36) inhibits alveolarization, indicating that prolonged exposure of the preterm lung to a pro-inflammatory environment contributes to abnormal alveolar septation, the hallmark pathologic feature of BPD. The impact of the interaction of SP-A deficiency and Ureaplasma infection on developmental signaling in the lung will need to be assessed in intrauterine or newborn infection models in future studies.
Acknowledgments
Financial support: This work was funded by a Grant-in-Aid by the Mid-Atlantic American Heart Association and NIH grants HL071113 and HL087166.
ABBREVIATIONS
- BAL
bronchoalveolar lavage
- BPD
bronchopulmonary dysplasia
- CCU
color-changing units
- KC
mouse analogue of human growth-related protein alpha
- MCP-1
monocyte chemoattractant protein-1
- SP-A
surfactant protein A
REFERENCES
- 1.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001;69:3906–3915. doi: 10.1128/IAI.69.6.3906-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson AM, Taciak V, Lovchik J, Fox RE, Campbell AB, Viscardi RM. Ureaplasma urealyticum respiratory tract colonization is associated with an increase in IL-1β and TNF-α relative to IL-6 in tracheal aspirates of preterm infants. Pediatr Infect Dis J. 1998;17:321–328. doi: 10.1097/00006454-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Viscardi RM, Manimtim WM, Sun CC, Duffy L, Cassell GH. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Dev Pathol. 2002;5:141–150. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 5.Viscardi R, Manimtim W, He JR, Hasday JD, Sun CC, Joyce B, Pierce RA. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol. 2006;9:143–151. doi: 10.2350/10-05-0112.1. [DOI] [PubMed] [Google Scholar]
- 6.Viscardi RM, Kaplan J, Lovchik JC, He JR, Hester L, Rao S, Hasday JD. Characterization of a murine model of Ureaplasma urealyticum pneumonia. Infect Immun. 2002;70:5721–5729. doi: 10.1128/IAI.70.10.5721-5729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh WF, Butler J, Coalson J, Hensley D, Cassell GH, deLemos RA. A primate model of Ureaplasma urealyticum infection in the premature infant with hyaline membrane disease. Clin Infect Dis. 1993;17:S158–S162. doi: 10.1093/clinids/17.supplement_1.s158. [DOI] [PubMed] [Google Scholar]
- 8.Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, Ikegami M, Jobe AH. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198:122.e1–122.e8. doi: 10.1016/j.ajog.2007.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with Ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]
- 10.Viscardi RM, Atamas SP, Luzina IG, Hasday JD, He JR, Sime PJ, Coalson JJ, Yoder BA. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res. 2006;60:141–146. doi: 10.1203/01.pdr.0000228322.73777.05. [DOI] [PubMed] [Google Scholar]
- 11.Baier RJ, Loggins J, Kruger TE. Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relation to isolation of Ureaplasma urealyticum. J Investig Med. 2001;49:362–369. doi: 10.2310/6650.2001.33902. [DOI] [PubMed] [Google Scholar]
- 12.Wright JR, Borron P, Brinker KG, Folz RJ. Surfactant Protein A: regulation of innate and adaptive immune responses in lung inflammation. Am J Respir Cell Mol Biol. 2001;24:513–517. doi: 10.1165/ajrcmb.24.5.f208. [DOI] [PubMed] [Google Scholar]
- 13.LeVine AM, Kurak KE, Wright JR, Watford WT, Bruno MD, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein-A binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol. 1999;20:279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 14.Hickman-Davis JM, Gibbs-Erwin J, Lindsey JR, Matalon S. Role of surfactant protein-A in nitric oxide production and mycoplasma killing in congenic C57BL/6 mice. Am J Respir Cell Mol Biol. 2004;30:319–325. doi: 10.1165/rcmb.2003-0246OC. [DOI] [PubMed] [Google Scholar]
- 15.Harrod KS, Trapnell BC, Otake K, Korfhagen TR, Whitsett JA. SP-A enhances viral clearance and inhibits inflammation after pulmonary adenoviral infection. Am J Physiol. 1999;277:L580–L588. doi: 10.1152/ajplung.1999.277.3.L580. [DOI] [PubMed] [Google Scholar]
- 16.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 17.Borron P, McIntosh JC, Korfhagen TR, Whitsett JA, Taylor J, Wright JR. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L840–L847. doi: 10.1152/ajplung.2000.278.4.L840. [DOI] [PubMed] [Google Scholar]
- 18.Ballard PL, Hawgood S, Liley H, Wellenstein G, Gonzales LW, Benson B, Cordell B, White RT. Regulation of pulmonary surfactant apoprotein SP 28–36 gene in fetal human lung. Proc Natl Acad Sci USA. 1986;83:9527–9531. doi: 10.1073/pnas.83.24.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitsett JA, Pilot T, Clark JC, Weaver TE. Induction of surfactant protein in fetal lung. Effects of cAMP and dexamethasone on SAP-35 RNA and synthesis. J Biol Chem. 1987;262:5256–5261. [PubMed] [Google Scholar]
- 20.Miyamura K, Malhotra R, Hoppe HJ, Reid KB, Phizackerley PJ, Macpherson P, Lopez Bernal A. Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta. 1994;1210:303–307. doi: 10.1016/0005-2760(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 21.Awasthi S, Coalson JJ, Crouch E, Yang F, King RJ. Surfactant proteins A and D in premature baboons with chronic lung injury (Bronchopulmonary dysplasia). Evidence for an inhibition of secretion. Am J Respir Crit Care Med. 1999;160:942–949. doi: 10.1164/ajrccm.160.3.9806061. [DOI] [PubMed] [Google Scholar]
- 22.Hallman M, Merritt TA, Akino T, Bry K. Surfactant protein A, phosphatidylcholine, and surfactant inhibitors in epithelial lining fluid. Correlation with surface activity, severity of respiratory distress syndrome, and outcome in small premature infants. Am Rev Respir Dis. 1991;144:1376–1384. doi: 10.1164/ajrccm/144.6.1376. [DOI] [PubMed] [Google Scholar]
- 23.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–L553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- 24.Rice P, Martin E, He JR, Frank M, DeTolla L, Hester L, O'Neill T, Manka C, Benjamin I, Nagarsekar A, Singh I, Hasday JD. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol. 2005;174:3676–3685. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 25.Minoo P, Segura L, Coalson JJ, King RJ, DeLemos RA. Alterations in surfactant protein gene expression associated with premature birth and exposure to hyperoxia. Am J Physiol. 1991;261:L386–L392. doi: 10.1152/ajplung.1991.261.6.L386. [DOI] [PubMed] [Google Scholar]
- 26.Coalson JJ, King RJ, Yang F, Winter V, Whitsett JA, Delemos RA, Seidner SR. SP-A deficiency in primate model of bronchopulmonary dysplasia with infection. In situ mRNA and immunostains. Am J Respir Crit Care Med. 1995;151:856–866. doi: 10.1164/ajrccm/151.3_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- 27.Awasthi S, Coalson JJ, Yoder BA, Crouch E, King RJ. Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons. Am J Respir Crit Care Med. 2001;163:389–397. doi: 10.1164/ajrccm.163.2.2004168. [DOI] [PubMed] [Google Scholar]
- 28.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 29.Stevens PA, Schadow B, Bartholain S, Segerer H, Obladen M. Surfactant protein A in the course of respiratory distress syndrome. Eur J Pediatr. 1992;151:596–600. doi: 10.1007/BF01957730. [DOI] [PubMed] [Google Scholar]
- 30.Kala P, Ten Have T, Nielsen H, Dunn M, Floros J. Association of pulmonary surfactant protein A (SP-A) gene and respiratory distress syndrome: interaction with SP-B. Pediatr Res. 1998;43:169–177. doi: 10.1203/00006450-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Crouse DT, Cassell GH, Waites KB, Foster JM, Cassady G. Hyeroxia potentiates Ureaplasma urealyticum pneumonia in newborn mice. Infect Immun. 1990;58:3487–3493. doi: 10.1128/iai.58.11.3487-3493.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brus F, van Waarde WM, Schoots C, Oetomo SB. Fatal ureaplasmal pneumonia and sepsis in a newborn infant. Eur J Pediatr. 1991;150:782–783. doi: 10.1007/BF02026711. [DOI] [PubMed] [Google Scholar]
- 33.Sun B, Curstedt T, Lindgren G, Franzen B, Alaiya AA, Calkovska A, Robertson B. Biophysical and physiological properties of a modified porcine surfactant enriched with surfactant protein A. Eur Respir J. 1997;10:1967–1974. doi: 10.1183/09031936.97.10091967. [DOI] [PubMed] [Google Scholar]
- 34.Kramer BW, Jobe AH, Bachurski CJ, Ikegami M. Surfactant protein A recruits neutrophils into the lungs of ventilated preterm lambs. Am J Respir Crit Care Med. 2001;163:158–165. doi: 10.1164/ajrccm.163.1.2005084. [DOI] [PubMed] [Google Scholar]
- 35.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 36.Bry K, Whitsett JA, Lappalainen U. IL-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol. 2007;36:32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]