Abstract
Generations 5 and 6 (G5 and G6) poly(amidoamine) (PAMAM) dendrimers have been shown to be highly efficient nonviral carriers in in vitro gene delivery. However, their high toxicity and unsatisfied in vivo efficacy limit their applications. In this study, to improve their characteristics as gene delivery carriers, polyethylene glycol (PEG, molecular weight 5,000) was conjugated to G5 and G6 PAMAM dendrimers (PEG-PAMAM) at three different molar ratios of 4%, 8%, and 15% (PEG to surface amine per PAMAM dendrimer molecular). Compared with unconjugated PAMAM dendrimers, PEG conjugation significantly decreased the in vitro and in vivo cytotoxicities and hemolysis of G5 and G6 dendrimers, especially at higher PEG molar ratios. Among all of the PEG-PAMAM dendrimers, 8% PEG-conjugated G5 and G6 dendrimers (G5-8% PEG, G6-8% PEG) resulted in the most efficient muscular gene expression when polyplexes were injected intramuscularly to the quadriceps of neonatal mice. Consistent with the in vivo results, these two 8% PEG-conjugated PAMAM dendrimers could also mediate the highest in vitro transfection in 293A cells. Therefore, G5-8% PEG and G6-8% PEG possess a great potential for gene delivery both in vivo and in vitro.
Key words: intramuscular gene delivery, PAMAM dendrimer, PEG conjugation, toxicity
INTRODUCTION
The most demanding task in gene delivery systems is the development of efficient and nontoxic gene carriers. Viral vectors can mediate highly efficient gene transfer, but their immunogenicity has severely limited their application (1).
As a nonviral gene delivery carrier, poly(amidoamine) (PAMAM) dendrimers have attracted great interest due to their high efficacy in in vitro gene delivery because of their branched structure (2,3). These dendritic polymers bear primary amine groups on their branched surface, which can bind DNA, compact it into polyplexes, and promote the cellular uptake of genes (4). Therefore, PAMAM dendrimers show high levels of transfection in a wide variety of cultured cells, especially in fractured form of G5 (commercially named SuperFect) (5). However, cationic PAMAM dendrimers are associated with their cytotoxicity, hemolysis, and liver toxicity (6,7), which limit their wide applications in vitro and in vivo (8–10), as they are thought to interact with the negatively charged cell surfaces (11).
Due to its properties of biocompatibility and hydrophilicity (12,13), polyethylene glycol (PEG) was conjugated to various polymers, such as polylysine (PLL) (14–16) and poly(ethyleneimine) (PEI) (17,18), to improve the physicochemical characteristics of these polymers. PEG conjugation decreased the cytotoxicity of these polycations (19) by reducing or partially shielding the positive charge on the surface of these polycations (12). In addition, the in vitro gene delivery efficacy of PLL or PEI has indeed been greatly improved after PEG conjugation (20–22) because of the increased solubility of the polymer/DNA polyplexes and the enhancement of intracellular release of DNA molecules (23,24).
It was also verified that the chain length and the molar ratio of PEGs to PAMAM dendrimers could influence their drug-loading capacity (25–27). However, little effort has been made to study the effect of the number of PEG chains conjugated to the PAMAM dendrimers as it relates to the gene transfection efficacy and cytotoxicity of these polycations, although a fifth-generation PAMAM dendrimer (G5) conjugated with 10% PEG-3400 was shown to have a 20-fold increase of efficacy in in vitro gene transfection compared with unconjugated G5 (28).
In the current study, in order to explore the effect of PEG-conjugated molar ratios on the cytotoxicity and gene transfection of PAMAM dendrimers, PEG-5000 is conjugated to the surface of G5 and G6 PAMAM dendrimers at three different molar ratios of 4%, 8%, and 15%. The transfection efficacy of these PEG-conjugated PAMAM dendrimers (PEG-PAMAM) to a reporter gene (a plasmid encoding enhanced green fluorescent protein, pEGFP) and their toxicity are investigated.
Although some previous studies have shown that PAMAM dendrimer could facilitate local gene expression in certain organs, such as eyes, lungs, or tumors, with a certain degree of success (1,29,30),in vivo gene delivery of PAMAM dendrimers is still in its initial stages, and its limited efficacy is always associated with its unacceptable in vivo toxicity (31). Furthermore, the utility of these polymers as the vehicles for the most common intramuscular gene delivery has not been explored.
In the present study, we test the in vivo gene delivery efficacy of these PEG-PAMAM dendrimers by intramuscular injection of the polyplexes of PEG-PAMAM dendrimer and pEGFP in the quadriceps of neonatal mice. Rather than using adult mice, we carried out intramuscular gene delivery on neonatal mice since their muscle fibers are still maturing and the nuclei of their muscle cells are undergoing frequent division and mitosis. It is therefore easier to deliver genes into their muscular cells to obtain a higher gene expression than that in adult mice (32–35).
Among all of the PEG-PAMAM dendrimers, 8% PEG-conjugated G5 (G5-8% PEG) and G6 (G6-8% PEG) showed the highest intramuscular efficacy and acceptable toxicity, consistent with in vitro results. The well-known capabilities of G5-8% PEG and G6-8% PEG in intramuscular gene delivery make them potentially applicable in future clinical gene therapy on muscular disorders, such as progressive muscular dystrophy (PMD), myotonic dystrophy, and Duchenne’s muscular dystrophy (DMD).
MATERIAL AND METHODS
Materials
PAMAM dendrimers of generation 5 (theoretical molecular weight (MW) = 28,826, 128 surface amino groups) and 6 (theoretical MW = 58,048, 256 surface amino groups) and 4-nitrophenyl chloroformate were all purchased from Aldrich-Sigma Chemical Company (Milwaukee, WI, USA). Polyethylene glycol monomethyl ether of molecular weights 5,000 (MPEG-5000) was from Fluka Chemical Company (Buchs SG, Switzerland). Dulbecco’s modified Eagle’s medium (DMEM) was from Gibco Corporation (CA, USA). Tetrahydrofuran (THF), dimethyl sulfoxide, and N,N-dimethyl formamide were all from local suppliers unless otherwise mentioned.
Synthesis of PEG-PAMAM Dendrimers
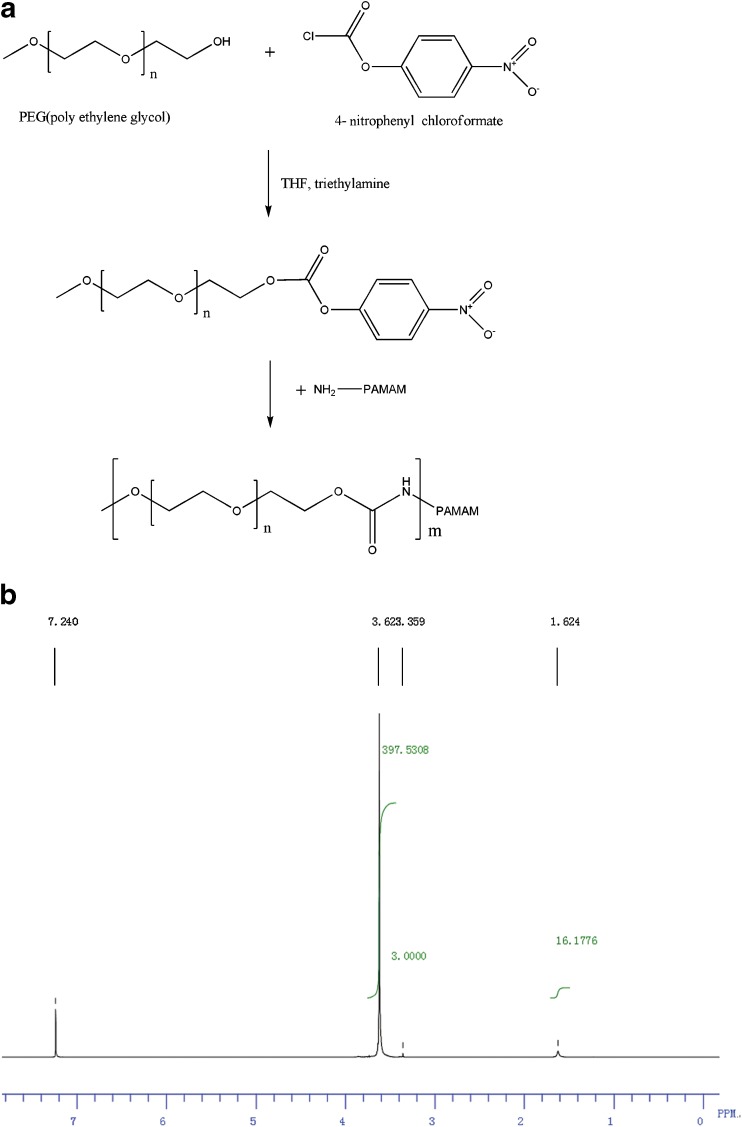
PEG-PAMAM dendrimers were synthesized via two reactions. First, MPEG-5000 was reacted with 4-nitrophenyl chloroformate to synthesize MPEG 4-nitrophenyl carbonate. Then, PEG-PAMAM dendrimers (PEG-G5 or PEG-G6) were formed by reacting G5 or G6 with MPEG 4-nitrophenyl carbonate. The synthesis scheme of PEG-PAMAM dendrimers is shown in Fig. 1a.
Fig. 1.
a The synthesis scheme of PEG-PAMAM dendrimers; b the 1H NMR spectrum of G5 with 10% theoretic modification rate of PEG
Synthesis of MPEG 4-Nitrophenyl Carbonate
THF was purified by distillation and desiccant by a 4A molecular sieve to remove water, gas, and other impure materials. Then, MPEG-5000 (0.50 mmol) was dissolved in THF (400 mL) at room temperature, and 4-nitrophenyl chloroformate (1.00 mmol) and triethylamine (1.00 mmol) were added to the solution gradually over 1 h, followed by stirring for 48 h at room temperature. MPEG 4-nitrophenyl carbonate was recovered by evaporation of the reaction mixture. The obtained MPEG 4-nitrophenyl carbonate mixture was purified by recrystallization of the products from chloroform–diethyl ether (10:1, total volume 300–400 mL).
Conjugation of MPEG to PAMAM Dendrimers
Purchased G5 (theoretical MW = 28,826) or G6 (theoretical MW = 58,048) PAMAM dendrimers were dried under a nitrogen atmosphere to remove the methanol solution and then dissolved in pure water and dialyzed against distilled water by using a dialysis bag (molecular weight 12,000–14,000 cutoff) for 72 h. Purified G5 and G6 were obtained by lyophilization.
Briefly, 0.52 μmol purified G5 or G6 was dissolved in 1 mL dimethyl sulfoxide. MPEG 4-nitrophenyl carbonate was added to the above solution at various molar ratios (5%, 10%, and 20% per PAMAM, respectively). The resulting solution was stirred to react at room temperature for 3 to 6 days (depending on the generation of the PAMAM and the desired PEG modification ratio). Then, the reaction solution was dialyzed against distilled water for 72 h, and the PEG-PAMAM dendrimer was finally obtained by lyophilization of the dialyzed solution.
Physical Characteristics of PEG-PAMAM/DNA Polyplexes
The polyplexes of PEG-PAMAM dendrimer and DNA were formed by using pEGFP as a model of DNA. Briefly, PEG-PAMAM dendrimer was dissolved in phosphate buffer solution (PBS, pH = 7.4) or DMEM (with or without serum) and mixed with the same volume of pEGFP solution at room temperature for 30 min. Depending on the experimental design, the polyplexes are performed in various conditions: PEG-G5 and PEG-G6 were at a different PEG conjugation ratio (4%, 8%, and 15% molar ratio of PEG to surface amine per dendrimer) and N/P ratios (the molar ratio of nitrogens in the dendrimer to phosphates in pEGFP) which ranged from 1:1 to 10:1.
Gel retardation was performed to assess the complexation of PEG-PAMAM dendrimers and pEGFP. Briefly, the polyplexes were prepared according to the method described above and electrophoresed on 1% (w/v) agarose gels containing ethidium bromide (0.5 μg/mL in gel) at a constant 100 V. Images of the electrophoresis results were analyzed under a UV illuminator to show the location of DNA.
To analyze the size and zeta potential of polyplexes (Zetasizer Nano-ZS Nanoseries, Malvern, USA), PEG-PAMAM/pEGFP polyplexes were formed in 1 mL PBS (0.01 M pH 7.4) at various N/P ratios and the final concentration of pEGFP was 15 μg/mL.
Cytotoxicity and Hemolysis Assay
Cytotoxicity was assessed by using CCK-8 kits (cell counting kit-8, Dojindo Laboratories, Japan). Briefly, 293A and HepG2 cells were seeded in 96-well plates at 104 cells per well in 95-μL DMEM medium containing 10% fetal bovine serum (FBS), respectively. After a 24-h incubation, cells were exposed to 5 μL of the polymer solutions (PEG-PAMAM or PAMAM) for 4 h; then, 10 μL CCK-8 reagents were added to each well. Absorbance of each well was measured at 450 nm by a microplate reader (Bio-Rad, CA, USA) and compared to untreated cells.
The hemolytic activity of the G5, G6, and their PEG-conjugated products was investigated by the modified method reported by Fischera et al. (36). Briefly, the blood of C57BL/6 mice was collected in heparinized tubes and centrifuged at about 3,000×g for 10 min to harvest erythrocytes. The cell pellet was washed three times with cold PBS (pH 7.4) by centrifugation at about 3,000×g for 10 min and resuspended in PBS to achieve a concentration of 1.5% (v/v). The suspension of erythrocytes was freshly prepared and used within 24 h after collection. PAMAM dendrimers and PEG-PAMAM dendrimers were dissolved in PBS and diluted to different concentrations of 0.2, 1, 2, 5, and 10 mg/mL. For each concentration, 100 μL polymer solutions were mixed with 100 µL 1.5% erythrocytes. The resulting suspensions were incubated at 37°C for 4 h and then centrifuged at about 13,000×g for 10 min. The release of hemoglobin was determined by photometric analysis of the supernatant at 540 nm. The hemolytic percentage of polymers was calculated by comparing the results with that of PBS (negative control) and 0.2% Triton X-100 (positive control).
In Vitro Gene Transfection
With Lipofectamine 2000 (Invitrogen, CA, USA) being a positive control, the transfection was performed according to the recommended protocol. Briefly, pEGFP (1.5 μg) was first complexed with PAMAM or PEG-PAMAM dendrimers in 100 μL PBS (0.01 M pH 7.4) at various N/P ratios and incubated for 30 min at room temperature then added to the cells (293A cells at 70–80% confluence, transfected in serum-free DMEM). After 4 h, the cells were rinsed and incubated with 500-μL fresh culture medium containing 10% FBS and 1% penicillin/streptomycin for 48 h. The fluorescence intensity of the cells was observed by fluorescence microscope and the transfection efficacy was assessed (37).
In Vivo Gene Delivery via Intramuscular Injection
Neonatal mice with their mothers were purchased from the animal department at the Peking University Health Science Center (Beijing, China). The Laboratory Animal Care Principles (NIH publication no. 85-23, revised 1996) were followed, and the experimental protocol was approved by the Animal Care Committee, Peking University Health Science Center.
pEGFP was mixed with PEG-G5 or PEG-G6 at different PEG conjugation ratios (4%, 8%, 15% molar ratio) to form polyplexes as described above. A single dose of 100 μg/g body weight of PEG-PAMAM dendrimer and 5 μg/g body weight of pEGFP (N/P ratio of 20) was delivered by intramuscular route to the quadriceps of <1-week-old C57BL/6 mice who had a body weight around 2.5–3 g. After 48 h from the time of the injection of polyplex, the quadriceps of the mice was cryosectioned (7 μm, 300 slices) to analyze the average fluorescence intensity of muscular sections. A comparison was made with pEGFP alone at the same dose and by the same route. In order to evaluate the toxicity of polyplexes, hematoxylin–eosin (HE) stain was performed to study the morphology of the muscle at the injection position.
RESULTS AND DISCUSSION
Synthesis of PEG-PAMAM Dendrimers
Synthesis of MPEG 4-Nitrophenyl Carbonate
The average yield of MPEG 4-nitrophenyl carbonate was 78%. The 1H nuclear magnetic resonance (NMR) was recorded on a Bruker AMX-300 (300 MHz) spectrometer, and the proton chemical shifts (δ) are given relative to internal CHCl3 for CDCl3 solutions. The results were analyzed as follows: 1H NMR (CDCl3) δ 3.38 (s, OCH3), δ 3.34–3.80 (m, CH2CH2O), δ 4.42 (m, OCOOCH2), δ 7.37 (d, aromatic), δ 8.26 (d, aromatic).
Conjugation of MPEG to PAMAM Dendrimers
MPEG was conjugated to G5 and G6 dendrimers at three theoretic ratios of 5%, 10%, and 20%. After a complete dialysis process, there were no free MPEG and other small molecule compounds (MW < 5,000) left, and only PAMAM and PEG-conjugated PAMAM were anticipated to remain. The representative spectrum of 1H NMR was displayed by G5 with 10% theoretic conjugation ratio of PEG (Fig. 1b): 1H NMR (CDCl3), δ 1.62 (m, PAMAM CH2), δ 3.36 (s, OCH3), δ 3.34–3.80 (m, CH2CH2O).
A method based on the analysis of the ratio of C, H, N, and O elements of products was used to determine the exact number of the PEG chains conjugated to the PAMAM dendrimer. By comparing the C/N ratio of PEG-PAMAM determined from element analysis with the theoretical C/N values, the average conjugation ratio of PEG to PAMAM dendrimers was calculated (Table I). Among all of the synthesized products of PEG-PAMAM dendrimers, G5-20% PEG and G6-20% PEG only achieved an average PEG conjugation ratio of 15% even after a long reaction time. The result could be explained as the further reactions between the MPEG 4-nitrophenyl carbonates and the primary amine groups of PAMAM were blocked by the long and convoluted chain of MPEG-5000. It was reported that, at the molar ratio of 1:1, the maximum PEG conjugation ratios to G5 and G6 were only 69% and 63%, respectively, even if reacted at room temperature for 30 days or more (27).
Table I.
PEG Modification Ratios of PEG-PAMAM Dendrimers Determined by Element Analysis
| Theoretical PEG modification ratios (%) | Theoretical MW | Detected MW | Detected C/N ratio | The number of theoretical MPEG chains | The number of detected MPEG chains | Average PEG modification ratios (%) |
|---|---|---|---|---|---|---|
| G5-5% PEG | 60,826 | 57,326 | 4.31 | 6.4 | 5.7 | 4.5 |
| G5-10% PEG | 92,826 | 80,826 | 6.14 | 12.8 | 10.4 | 8.1 |
| G5-20% PEG | 156,826 | 125,326 | 9.53 | 25.6 | 19.3 | 15.1 |
| G6-5% PEG | 122,048 | 115,548 | 4.33 | 12.8 | 11.5 | 4.5 |
| G6-10% PEG | 186,048 | 161,548 | 6.09 | 25.6 | 20.7 | 8.1 |
| G6-20% PEG | 314,048 | 243,548 | 9.21 | 51.2 | 37.1 | 14.5 |
The detected PEG conjugation ratios of PEG-PAMAM dendrimers (4%, 8%, and 15%) were used in subsequent investigations.
Gel Retardation Assay
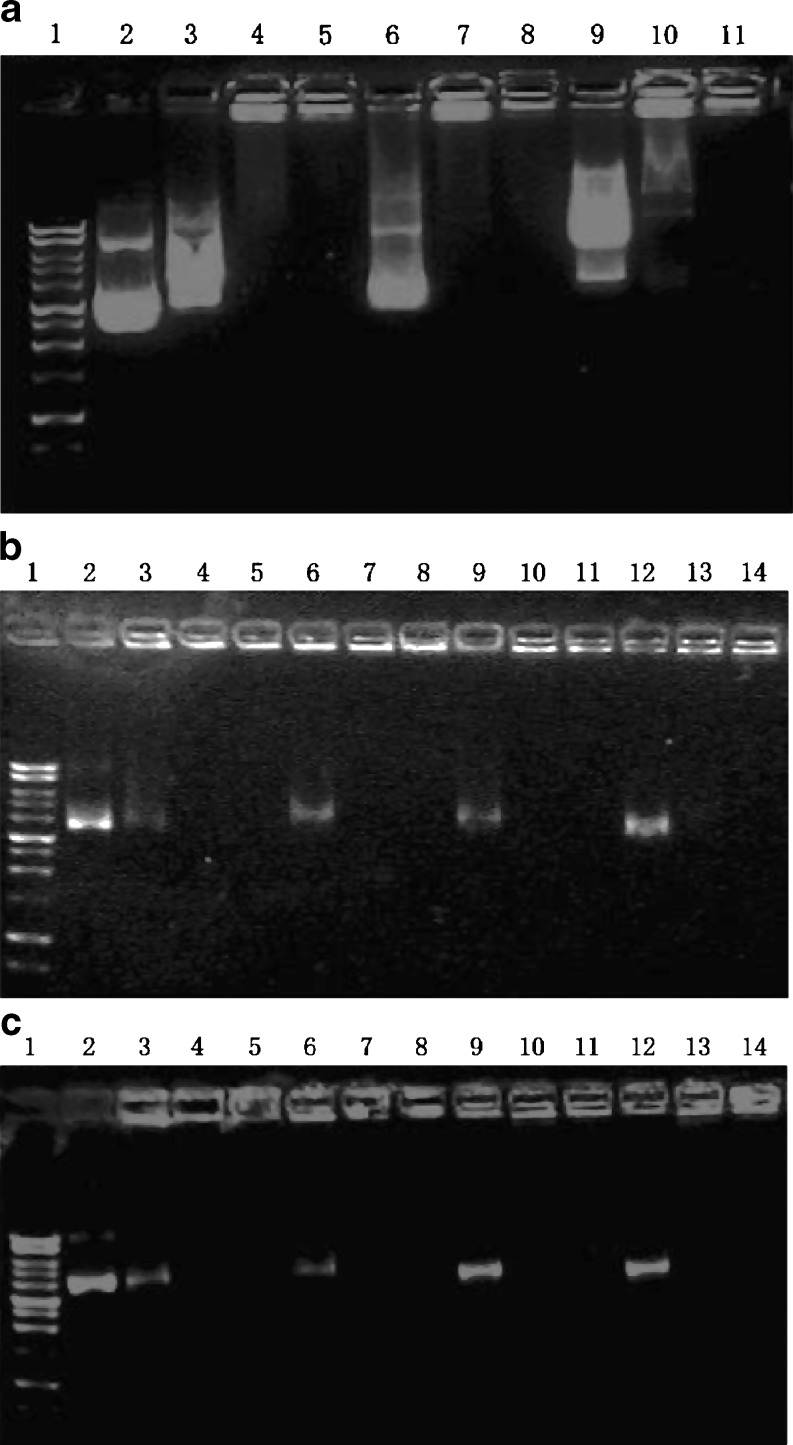
The gel retardation assay demonstrated that DNA would be effectively packed by PEG-PAMAM dendrimers at an N/P ratio greater than 10, since the mobility of the DNA was significantly retarded (Fig. 2a, lanes 3–11). Compared with the compaction of PEG-PAMAM dendrimers to DNA in PBS (Fig. 2a, lanes 3–5) and in serum-free DMEM (Fig. 2a, lanes 6–8), DMEM with 10% serum would affect the compacting process of polymers to DNA (Fig. 2a, lanes 9–11, polyplexes were formed in DMEM containing 10% FBS); therefore, serum was omitted while forming PEG-PAMAM/DNA polyplexes in the subsequent experiments.
Fig. 2.
DNA mobility retardation assays. a Lane 1: DNA marker (1-kb ladder). Lane 2: plasmid DNA (pEGFP, 1 μg). Lanes 3–11: polyplexes of G5-8% PEG and pEGFP (1 μg) formed at three different N/P ratios of 1, 5, and 10 in PBS (lanes 3, 4, 5), in serum-free DMEM (lanes 6, 7, 8), or in DMEM with 10% FBS (lanes 9, 10, 11). b Lane 1: DNA marker (1-kb ladder). Lane 2: plasmid DNA (pEGFP, 0.2 μg). Lanes 3–14: polyplexes of pEGFP (0.2 μg) with G5 (lanes 3, 4, 5), G5-4% PEG (lanes 6, 7, 8), G5-8% PEG (lanes 9, 10, 11), or G5-15% PEG (lanes 12, 13, 14). Each kind of dendrimer was used to form polyplexes with pEGFP in serum-free DMEM at three different N/P ratios of 1, 5, and 10, respectively. c Lane 1: DNA marker (1-kb ladder). Lane 2: plasmid DNA (pEGFP, 0.2 μg). Lanes 3–14: polyplexes of pEGFP (0.2 μg) with G6 (lanes 3, 4, 5), G6-4% PEG (lanes 6, 7, 8), G6-8% PEG (lanes 9, 10, 11), or G6-15% PEG (lanes 12, 13, 14). Each kind of dendrimer was used to form polyplexes with pEGFP in serum-free DMEM at three different N/P ratios of 1, 5, and 10, respectively
The influence of dendrimer generations and PEG conjugation ratios to the formation of PEG-PAMAM/DNA polyplexes is demonstrated in Fig. 2b, c. As shown in Fig. 2b, most of pEGFP was retarded in the start hole by G5 or G5-4% PEG at the lower N/P ratio of 1. However, for G5-8% PEG and G5-15% PEG, a higher N/P ratio (>5) would be needed to entirely retard the mobility of the DNA. PEG-G6 (Fig. 2c) also had the same tendency as PEG-G5. However, to all of the PEG-conjugated products, no matter which generation of PAMAM dendrimers were used, the N/P ratio above 10 would be enough to neutralize the negative charge of pEGFP and retard its mobility, suggesting that the compacting ability of PEG-PAMAM to DNA was still present after the conjugation of PEG-5000 to PAMAM dendrimers.
Size and ζ Potential of PEG-PAMAM Dendrimer/DNA Polyplexes
As shown in Table II, the smaller particle size of PEG-PAMAM/pEGFP polyplexes was generally observed at higher N/P molar ratios and formed by pEGFP with the PEG-PAMAM that had higher PEG conjugation molar ratios since PEG chains could inhibit the formation of larger aggregates and improve the dispersal of dendrimer molecules by increasing hydrophilicity (38). However, the smallest particle size was detected at the polyplexes formed by pEGFP with G5-8% PEG or G6-8% PEG in all of the PEG-PAMAM dendrimers. This suggests that PEG conjugation molar ratios greater than 15% do not contribute to forming small and compact PAMAM dendrimers/DNA polyplexes.
Table II.
Average Size and ζ Potential of the PAMAM/pEGFP or PEG-PAMAM/pEGFP Polyplexes (Formed in 1 mL PBS at Various N/P Ratios and the Final Concentration of DNA was 15 µg/mL)
| Complexes | N/P ratio | Size (nm) | ζ potential |
|---|---|---|---|
| G5/pEGFP | 10:1 | 525 ± 15 | 10.4 ± 1.9 |
| G5-4% PEG/pEGFP | 10:1 | 352 ± 17 | 7.04 ± 0.83 |
| G5-8% PEG/pEGFP | 1:1 | 278 ± 12 | −14.5 ± 1.4 |
| 10:1 | 112 ± 9 | 6.08 ± 1.80 | |
| 20:1 | 107 ± 6 | 16.5 ± 2.9 | |
| G5-15% PEG/pEGFP | 10:1 | 133 ± 10 | 5.44 ± 1.04 |
| G6/pEGFP | 10:1 | 584 ± 14 | 12.5 ± 2.8 |
| G6-4% PEG/pEGFP | 10:1 | 408 ± 20 | 8.81 ± 2.11 |
| G6-8% PEG/pEGFP | 10:1 | 196 ± 11 | 5.03 ± 1.20 |
| G6-15% PEG/pEGFP | 10:1 | 209 ± 8 | 7.72 ± 1.91 |
N/P ratios affected the particle size of G5-8% PEG/pEGFP polyplexes at constant DNA concentrations. As illustrated in Table II, the largest polyplex was observed at an N/P ratio of 1 and its net charge was negative, which indicated an insufficient compaction of G5-8% PEG to DNA at this N/P ratio. However, when the N/P ratios were higher than 10, the DNA was well compacted and the net charge of the polyplex was positive, which was consistent with the results of electrophoresis.
Compared to all other preparations, the G5-8% PEG/pEGFP polyplex formed at an N/P ratio of 20 had the smallest particle size (107 nm) and a narrow size distribution falling within the general size requirements (100–200 nm) for efficient cellular endocytosis through clathrin-coated pits, the dominant internalization pathway for polyplexes reported in the literature (39–41).
Cytotoxicity and Hemolysis Assay
Toxicity is one of the major concerns in application of PAMAM dendrimers as a gene delivery vehicle, especially at the higher generations (>G5), which also yield higher transfection efficacy. Our results indicated that PEG conjugation could efficiently decrease the cytotoxicity and hemolysis of G5 and G6 PAMAM dendrimers.
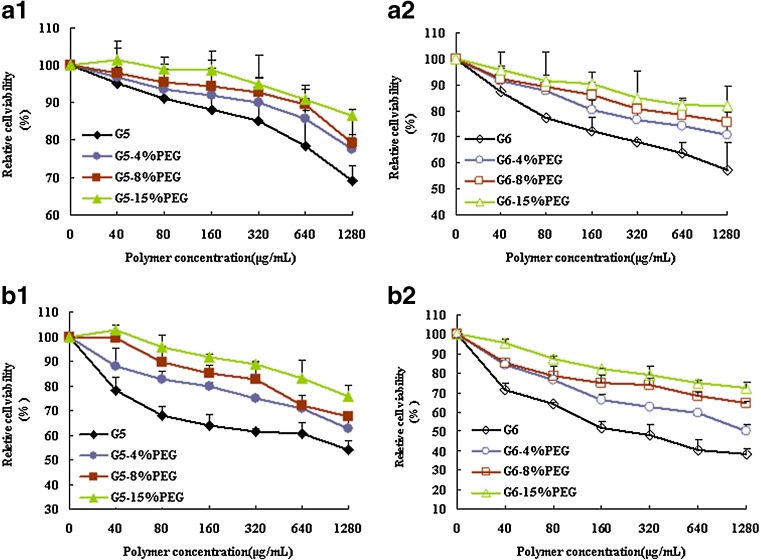
As shown in Fig. 3, all PEG-G5 and PEG-G6 dendrimers demonstrated a lower cytotoxicity than G5 or G6. At the highest concentration of 1.28 mg/mL, 15% PEG conjugation can enhance the cell viability of G6 from 57.3% to 81.9% (P < 0.005) and 38.3% to 72.4% (P < 0.001) in 293A and HepG2 cells, respectively. A similar increase in cell viability with G5-15% PEG has been documented with 21.8% (HepG2 cells, P < 0.001) and 17.4% (293A cells, P < 0.01) improvement when compared with that of unconjugated G5 dendrimers at the highest concentration tested.
Fig. 3.
Cytotoxicity of PEG-PAMAM in 293A (a1 and a2) and HepG2 (b1 and b2) cells by CCK-8 assay (n = 3). The influence of PEG-PAMAM dendrimers to the cell viability was determined after incubating the cells with different concentration of polymers for 4 h and compared with that of untreated cells
Figure 4 illustrates that PEG conjugation significantly reduced the hemolytic toxicity of the G5 and G6 dendrimers. With 0.2% TritonX-100 being a positive control, which achieves 100% hemolysis at the experimental conditions, unconjugated G5 and G6 show more than 40% hemolysis at the concentration of 2.5 mg/mL. However, after the PEG conjugation, even at the low PEG conjugation ratio of 4%, the hemolytic percentages of G5-PEG and G6-PEG are all decreased dramatically to around 5% (P < 0.005 vs unconjugated G5 and G6) at the same concentration of 2.5 mg/mL. The hemolysis of PEG-PAMAM dendrimers at the high PEG conjugation of 15% has no significant difference with that of 8% PEG conjugation.
Fig. 4.
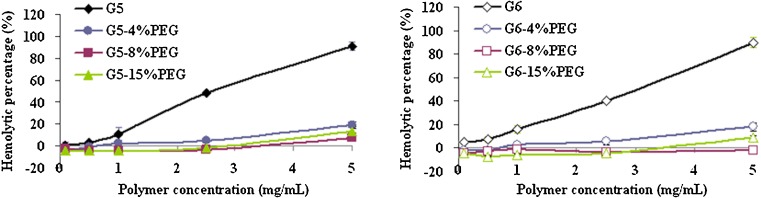
Hemolysis assay of unconjugated PAMAM and PEG-PAMAM dendrimers. Left: unconjugated G5 and PEG-G5 at PEG conjugation ratios of 4%, 8%, and 15%; right: unconjugated G6 and PEG-G6 at PEG conjugation ratios of 4%, 8%, and 15%
Effect of N/P Ratios of Polyplexes on In Vitro Gene Transfection
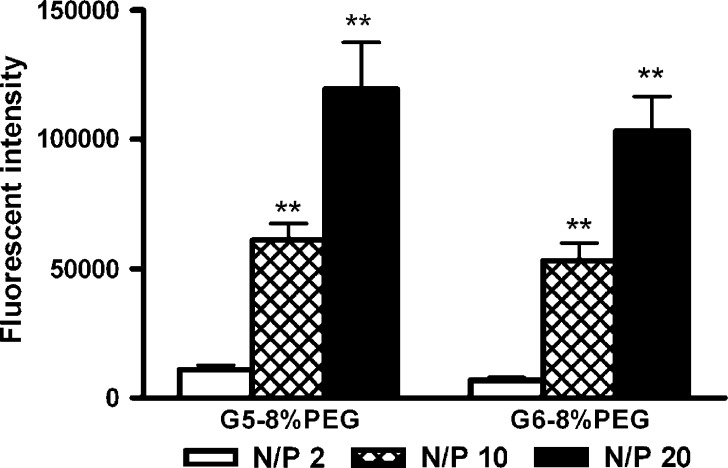
To determine the effect of N/P ratios on PEG-PAMAM-dendrimer-mediated gene transfection, the efficacy of G5-8% PEG and G6-8% PEG at three different N/P ratios of 2, 10, and 20 was assessed. Figure 5 demonstrates that G5-8% PEG and G6-8% PEG had the highest transfection efficacy to pEGFP at an N/P ratio of 20, while the lowest was at an N/P ratio of 2. However, when the N/P ratio was above 20, the viability of cells was less than 90%. The N/P ratio of 20 was, therefore, selected as the optimized ratio for the following experiments.
Fig. 5.
Effect of different N/P ratios on transfection efficacy of G5-8% PEG and G6-8% PEG on 293A cell with pEGFP. N/P ratios with statistically significant differences (P < 0.01) from N/P ratio of 2 are denoted by ** (n = 3)
Effect of the PEG Conjugation Ratios on In Vitro Gene Transfection
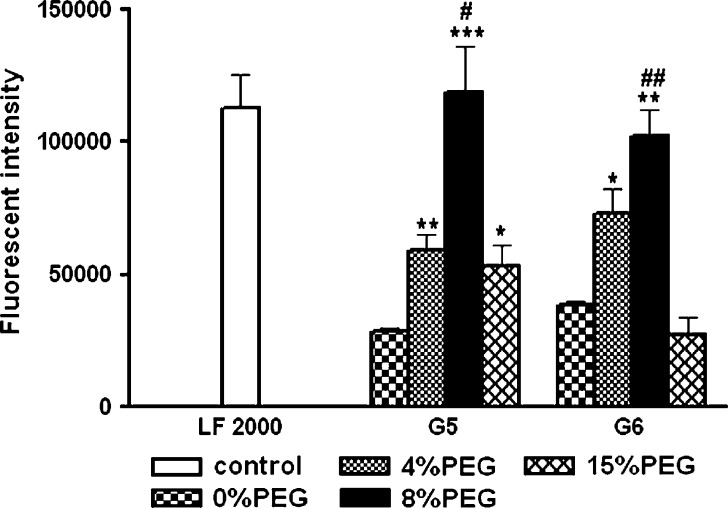
Figure 6 indicates that PEG conjugation ratios could significantly influence the transfection efficacy mediated by both PEG-G5 and PEG-G6. Among all of the PEG-PAMAM dendrimers, G5-8% PEG and G6-8% PEG showed the highest capability of mediating gene transfection at an N/P ratio of 20 in 293A cells and at a comparable extent as that mediated by Lipofectamine 2000, which is widely used as a commercial transfection reagent.
Fig. 6.
Effect of different PEG conjugation ratios on the transfection efficacy of PEG-G5 and PEG-G6 on 293A cell, with Lipofectamine 2000 (LF 2000) as a positive control and pEGFP as a model of DNA. Statistically significant differences are denoted by * (P < 0.05), ** (P < 0.01), and *** (P < 0.005), compared with unconjugated G5 or G6 PAMAM dendrimer (0% PEG), and # (P < 0.05) and ## (P < 0.01), compared with G5-15% PEG or G6-15% PEG (n = 3)
A possible reason for the lower transfection extent mediated by unconjugated G5 and G6 and their 4% PEG conjugations vs their 8%PEG conjugations might be due to the higher cytotoxicity of former preparations. Furthermore, the aggregations of polyplexes formed by these unconjugated and 4% PEG-conjugated dendrimers (Table II) may also make polyplexes difficult to penetrate into cells.
At the high PEG conjugation ratio of 15%, the transfection efficacy was paradoxically reduced by 50% and 75% for PEG-G5 and PEG-G6, respectively. The lower transfection efficacy compared to their 8%PEG conjugations may result from a greater shielding of PEG chains to the surface amine groups of the PAMAM dendrimers (28) at the higher PEG conjugation ratio of 15%. This increases the difficulty of polyplexes to bind with the cell membrane (15). In addition, it could also be due to a wrapping of the PEG chains to DNA, thereby blocking the intracellular release of DNA.
Our studies demonstrated that 8% PEG conjugation, among the three PEG conjugation ratios (4%, 8%, and 15%), achieved the highest transfection with acceptable toxicity in both G5-PEG and G6-PEG.
Intramuscular Gene Delivery
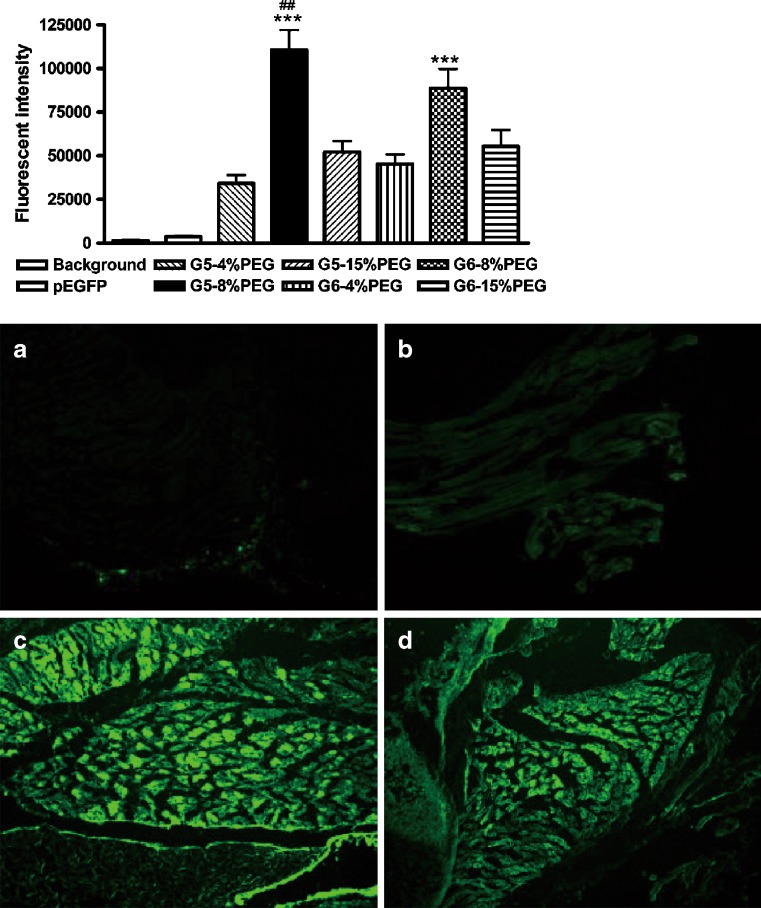
In vivo gene delivery via intramuscular injection of PEG-G5/pEGFP or PEG-G6/pEGFP polyplexes to neonatal mice resulted in robust expression of EGFP in the quadriceptic muscles near the injection sites (Fig. 7). All of the PEG-G5 and PEG-G6 products could greatly enhance the expression of pEGFP, compared with naked pEGFP (over several orders of magnitude in general).
Fig. 7.
Intramuscular gene delivery efficacy of PEG-PAMAM dendrimers to pEGFP on neonatal mice (n = 5 in each group). The fluorescence of EGFP was detected by cryosection of the muscles near the injection point after 48 h via intramuscular injection of a single dose of PEG-PAMAM/pEGFP polyplexes (the dose of dendrimers and pEGFP was100 and 5 μg/g of body weight, respectively, and the N/P ratio was around 20), with same dose of pEGFP as control. Statistically significant differences are denoted by *** (P < 0.005 G5-8% PEG or G6-8% PEG versus other PEG conjugation ratios at the same generation of PAMAM), and ## (P < 0.01 G5-8% PEG versus G6-8% PEG). The representative fluorescence pictures of GFP expression in the muscles (×100) near the injection point after 48 h are shown as follows: a background; b naked plasmid eGFP; c G5-8% PEG/pEGFP; d G6-8% PEG/pEGFP
Consistent with the findings in cell cultures, G5-8% PEG resulted in the highest expression of GFP among all of the PEG-PAMAM dendrimers. Besides G5-8% PEG, the second most efficient PEG-PAMAM was G6-8% PEG, which yielded a higher efficacy than other PEG-PAMAM polyplexes (P < 0.01). Unconjugated G5 and G6 were not included in the in vivo studies because of their hemolysis toxicity or lethality to neonatal mice at the experimental dose of 0.1 mg/g of body weight.
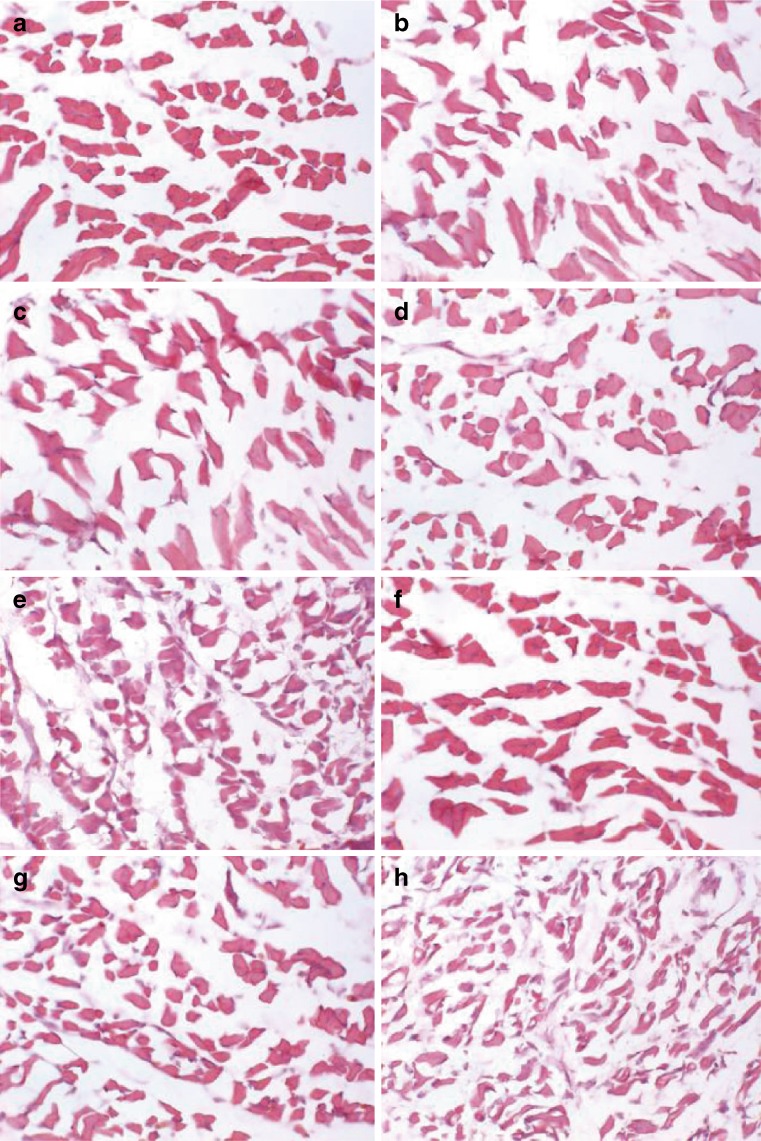
PEG conjugation dramatically decreased the in vivo toxicities of G5 and G6 dendrimers. Following a period of 48 h after intramuscular injection, the HE staining of the muscles near the injection sites demonstrated that several preparations had a minimal visible adverse effect on muscular cells; these were: the untreated mice (control, panel a), naked pEGFP (panel b), the polyplexes of pEGFP and PEG-PAMAM dendrimers (G5 and G6) with PEG conjugation ratios above 8% (panels c, d, f and g). The G5-4% PEG (panel e) and G6-4% PEG (panel h) resulted in a slight amount of damage to the muscle cells in terms of the disorganization of muscle fibers and the increase of intercellular matrix, compared with that of controls (Fig. 8). The results indicated that the significant intramuscular GFP expression mediated by G5-8% PEG and G6-8% PEG was not at the expense of muscle damage.
Fig. 8.
Morphology of the muscles around the injection point after 48 h via intramuscular injection of a single dose of pEGFP (5 μg/g of body weight) with or without PEG-PAMAM dendrimers (100 μg/g of body weight) by hematoxylin and eosin staining (×400). a Control (untreated normal muscles); b naked pEGFP; c G5-15% PEG/pEGFP; d G5-8% PEG/pEGFP; e G5-4% PEG/pEGFP; f G6-15% PEG/pEGFP; g G6-8% PEG/pEGFP; h G6-4% PEG/pEGFP
The highest in vivo EGFP expression mediated by PEG-PAMAM dendrimers was achieved 2 days after a single injection and was short term since EGFP could not be detected after 5 days (data not shown). Multiple intramuscular injections at various time points in adult mice are currently being examined.
CONCLUSION
The study shows PEG-G5 and PEG-G6 dendrimers, with PEG conjugation molar ratio at 8% (PEG to surface amine per PAMAM), can facilitate dramatic intramuscular gene delivery in neonatal mice. The significant intramuscular GFP expression mediated by G5-8% PEG and G6-8% PEG was not at the expense of muscle damage, consistent with similar findings observed in vitro in cultured 293A and HepG2 cells.
Although it has not been verified on other animals, the excellent capability of G5-8% PEG and G6-8% PEG in intramuscular gene delivery in neonatal mice appears to warrant further investigation on adult animals and on other therapeutic genes. Future clinical gene therapy application on muscular disorders, such as PMD, myotonic dystrophy, and DMD, may be increasingly within our sights.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30500197).
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- G5
PAMAM dendrimer at generation 5
- G6
PAMAM dendrimer at generation 6
- MW
molecular weight
- PAMAM
polyamidoamine
- PEI
poly(ethyleneimine)
- PEG
poly(ethylene glycol)
- PLL
polylysine
- pEGFP
plasmid enhanced green fluorescent protein
- THF
tetrahydrofuran
Contributor Information
Rong Qi, Phone: +86-10-82802769, FAX: +86-10-82802769, Email: ronaqi@gmail.com.
George Liu, Phone: +86-10-82802769, FAX: +86-10-82802769, Email: vangeorgeliu@gmail.com.
References
- 1.Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Deliv Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Gao Y, Gao G, He Y, Liu TL, Qi R. Recent advances of dendrimers in delivery of genes & drugs. Mini-Rev Med Chem. 2008;8:889–900. doi: 10.2174/138955708785132729. [DOI] [PubMed] [Google Scholar]
- 3.Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR., Jr Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci U S A. 1996;93:4897–4902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CC, Mackay JA, Szoka JF. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 6.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/S0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 7.Neerman MF, Zhang W, Parrish AR, Simanek EE. In vitro and in vivo evaluation of a melamine dendrimer as a vehicle for drug delivery. Int J Pharm. 2004;281:129–132. doi: 10.1016/j.ijpharm.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Chen HT, Neerman MF, Parrish AR, Simanek EE. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J Am Chem Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 9.Qiu LY, Bae YH. Polymer architecture and drug delivery. Pharm Res. 2006;23:1–30. doi: 10.1007/s11095-005-9046-2. [DOI] [PubMed] [Google Scholar]
- 10.Zinselmeyer BH, Mackay SP, Schatzlein AG, Uchegbu IF. The lower-generation polypropylenimine dendrimers are effective gene-transfer agents. Pharm Res. 2002;19:960–967. doi: 10.1023/A:1016458104359. [DOI] [PubMed] [Google Scholar]
- 11.Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) starburst dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu MJ, Kono K, Fréchet JM. Water soluble dendrimer–poly(ethylene glycol) starlike conjugates as potential drug carriers. J Polym Sci A Polym Chem. 1999;37:3492–3503. doi: 10.1002/(SICI)1099-0518(19990901)37:17<3492::AID-POLA7>3.0.CO;2-0. [DOI] [Google Scholar]
- 14.Bhadra D, Bhadra S, Jain NK. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J Pharm Pharm Sci. 2005;8:467–482. [PubMed] [Google Scholar]
- 15.Choi YH, Liu F, Kim JS, Choi YK, Park JS, Kim SW. Polyethylene glycol-grafted poly-l-lysine as polymeric gene carrier. J Control Rel. 1998;54:39–48. doi: 10.1016/S0168-3659(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 16.Bikram M, Ahn CH, Chae SY, Lee M, Yockman JW, Kim SW. Biodegradable poly(ethylene glycol)-co-poly(l-lysine)-g-histidine multiblock copolymers for nonviral gene delivery. Macromolecules. 2004;37:1903–1916. doi: 10.1021/ma035650c. [DOI] [Google Scholar]
- 17.Kursa M, Walker GF, Roessler V, Ogris M, Roedl W, Kircheis R. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjug Chem. 2003;14:222–231. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- 18.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–160. doi: 10.1016/S0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 19.Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D'Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–266. doi: 10.1016/S0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 20.Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ, et al. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem. 2002;13:845–854. doi: 10.1021/bc025529v. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Han SO, Ko KS, Koh JJ, Park JS, Yoon JW, et al. Repression of GAD autoantigen expression in pancreas beta-cells by delivery of antisense plasmid/PEG-g-PLL complex. Mol Ther. 2001;4:339–346. doi: 10.1006/mthe.2001.0458. [DOI] [PubMed] [Google Scholar]
- 22.Toncheva V, Wolfert MA, Dash PR, Oupicky D, Ulbrich K, Seymour LW, et al. Novel vectors for gene delivery formed by self-assembly of DNA with poly(l-lysine) grafted with hydrophilic polymers. Biochim Biophys Acta. 1998;1380:354–368. doi: 10.1016/s0304-4165(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 23.Huang RQ, Qu YH, Ke WL, Zhu JH, Pei YY, Jiang C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethylene glycol-modified polyamidoamine dendrimer. FASEB J. 2007;21:1117–1125. doi: 10.1096/fj.06-7380com. [DOI] [PubMed] [Google Scholar]
- 24.Kim T, Seo HJ, Choi JS, Jang HS, Baek J, Kim K, et al. PAMAM-PEG-PAMAM: novel triblock copolymer as a biocompatible and efficient gene delivery carrier. Biomacromolecules. 2004;5:2487–2492. doi: 10.1021/bm049563j. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Morris JJ, Lopina ST. Polyethylene glycol-polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J Colloid Interface Sci. 2004;273:148–154. doi: 10.1016/j.jcis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers having poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjug Chem. 2000;11:910–917. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 27.Hedden RC, Bauer BJ. Structure and dimensions of PAMAM/PEG dendrimer-star polymers. Macromolecules. 2003;36:1829–1835. doi: 10.1021/ma025752n. [DOI] [Google Scholar]
- 28.Luo D, Haverstick K, Belcheva N, Han E, Saltzman WM. Poly(ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules. 2002;35:3456–3462. doi: 10.1021/ma0106346. [DOI] [Google Scholar]
- 29.Eichman JD, Bielinska AU, Kukowska-Latallo JF, Baker JR., Jr The use of PAMAM dendrimers in the efficient transfer of genetic material into cells. Pharm Sci Technol Today. 2000;3:232–245. doi: 10.1016/S1461-5347(00)00273-X. [DOI] [PubMed] [Google Scholar]
- 30.van de Wetering P, Schuurmans-Nieuwenbroek NM, Hennink WE, Storm G. Comparative transfection studies of human ovarian carcinoma cells in vitro, ex vivo and in vivo with poly(2-(dimethylamino) ethyl methacrylate)-based polyplexes. J Gene Med. 1999;1:156–165. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<156::AID-JGM29>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Marano RJ, Wimmer N, Kearns PS, Thomas BG, Toth I, Brankov M, et al. Inhibition of in vitro VEGF expression and choroidal neovascularization by synthetic dendrimer peptide mediated delivery of a sense oligonucleotide. Exp Eye Res. 2004;79:525–535. doi: 10.1016/j.exer.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Sapru MK, McCormick KM, Thimmapaya B. High-efficiency adenovirus-mediated in vivo gene transfer into neonatal and adult rodent skeletal muscle. J Neurosci Methods. 2002;114:99–106. doi: 10.1016/S0165-0270(01)00518-0. [DOI] [PubMed] [Google Scholar]
- 33.Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K, et al. A differential efficiency of adenovirus-mediated in vivo gene transfer into skeletal muscle cells of different maturity. Hum Mol Genet. 1994;3:579–584. doi: 10.1093/hmg/3.4.579. [DOI] [PubMed] [Google Scholar]
- 34.Feero WG, Rosenblatt JD, Huard J, Watkins SC, Epperly M, Clemens PR, et al. Viral gene delivery to skeletal muscle: insights on maturation dependent loss of fiber infectivity for adenovirus and herpes simplex type 1 viral vectors. Hum Gene Ther. 1997;8:317–380. doi: 10.1089/hum.1997.8.4-371. [DOI] [PubMed] [Google Scholar]
- 35.Nalbantoglu J, Pari G, Karpati G, Holland PC. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus- mediated gene delivery to muscle cells. Hum Gene Ther. 1999;10:1009–1019. doi: 10.1089/10430349950018409. [DOI] [PubMed] [Google Scholar]
- 36.Fischera D, Lib Y, Ahlemeyerc B, Krieglsteinc J, Kissela T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24:1121–1131. doi: 10.1016/S0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 37.Desrumaux C, Risold PY, Schroeder H, Deckert V, Masson D, Athias A, et al. Phospholipid transfer protein (PLTP) deficiency reduces brain vitamin E content and increases anxiety in mice. FASEB J. 2005;19:296–297. doi: 10.1096/fj.04-2400fje. [DOI] [PubMed] [Google Scholar]
- 38.Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–832. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 39.Azzam T, Domb AJ. Current developments in gene transfection agents. Curr Drug Deliv. 2004;1:165–193. doi: 10.2174/1567201043479902. [DOI] [PubMed] [Google Scholar]
- 40.Grosse S, Aron Y, Thevenot G, Francois D, Monsigny M, Fajac I. Potocytosis and cellular exit of complexes as cellular pathways for gene delivery by polycations. J Gene Med. 2005;7:1275–1286. doi: 10.1002/jgm.772. [DOI] [PubMed] [Google Scholar]
- 41.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]