Abstract
Multidrug resistance-associated protein 2 (MRP2/ABCC2) is mainly expressed in the apical phase of barrier membranes. It functions as a critical efflux pump in the biliary excretion of endogenous substances, such as conjugated bilirubin and bile salts, as well as many structurally diverse xenobiotics and their metabolites. Due to its important role in defining ADME/Tox properties, efforts have emerged to build the structure–activity relationship (SAR) for MRP2/ABCC2 at early stages of drug discovery process. MRP2/ABCC2 is a member of the integral membrane protein family whose high-resolution crystal structure has not been described. To overcome the obstacle of lacking detailed structural depiction, various molecular modeling approaches have been applied to derive the structural requirements for binding interactions with MRP2/ABCC2 protein, including two-dimensional (2D) and three-dimensional (3D) quantitative SAR (QSAR) analysis, pharmacophore models, and homology modeling of the transporter. Here we summarize recent progresses in understanding the SAR of MRP2/ABCC2 recognition of substrates and/or inhibitors, and describe some of the useful in vitro tools for characterizing the interactions with the transporter.
Key words: ABC transporters, ADME/Tox, MRP2/ABCC2, structure–activity relationship
INTRODUCTION
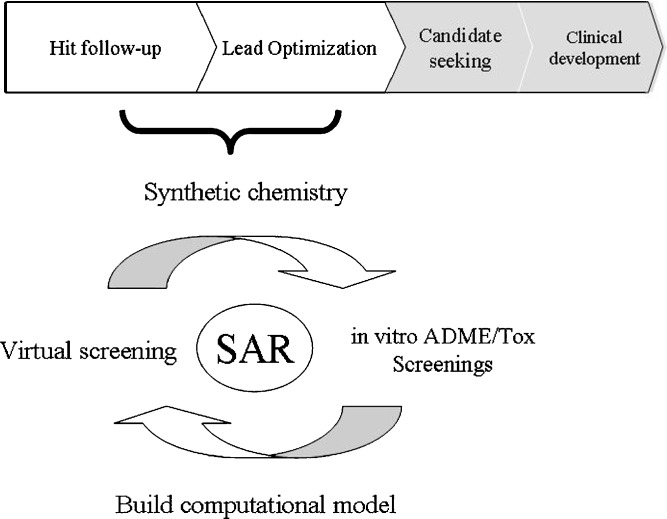
The widespread use of parallel and combinatorial chemistry as well as high-throughput screening technologies in drug discovery generates large number of candidate molecules poised for triage and selection. Since the identification of promising hits and the prediction of potential risk factors are crucial steps in the early stages of a drug discovery project (1), it is widely recognized that parallel assessment of compound ADME/Tox properties can greatly accelerate the preclinical discovery process. Due to the ever increasing pressure to improve the success rate of new chemical entity generation and to reduce the time and cost of bringing a new drug to the market, there is a large demand for new practice and technologies to be integrated into decision-making funnels, to assist that early discovery produces the best candidates in target and lead molecules while incurring the least possible amount of cost. Toward that goal, computational modeling has become a critical component in drug discovery research. Sophisticated computational methods help depict the structural requirements for ligand–protein interactions and could enable the ADME/Tox assessment at the earliest and the least expensive stages of drug discovery, such as during virtual screening prior to chemical synthesis, while screening compound libraries and in the course of hit-to-lead optimization (Fig. 1). In this review, we provide an overview of the multidrug resistance-associated protein 2 (MRP2/ABCC2)-related ADME/Tox issues, the in vitro tools for characterizing the interactions with MRP2/ABCC2, the structure–activity relationship (SAR) and quantitative SAR (QSAR) analysis of MRP2/ABCC2 substrates and/or inhibitors, and the molecular modeling of the transporter interactions.
Fig. 1.
Illustration of SAR development in drug discovery
MRP2/ABCC2 IN ADME/Tox
Representing the largest family of transmembrane proteins, adenosine triphosphate (ATP)-binding cassette (ABC) transporters ferry a wide variety of substrates across biological membranes using the energy of ATP hydrolysis, including metabolic products, lipids, sterols, and drug molecules. Phylogenically, ABC transporters are classified into seven subfamilies of 49 transporter genes (ABCA to ABCG) (2). These transporters are frequently involved in drug absorption, distribution, and excretion. Therefore, drug molecules that interact with the transporters could cause drug–drug interactions and related toxicity. Conversely, changes in the expression of transporters could affect the disposition of drugs and their metabolites, altering their pharmacokinetics and pharmacological response. Multidrug resistance-associated protein (MRP/ABCC), currently consisting of 12 members (MRP1–12/ABCC1–12), is one branch of the ABC superfamily translocating their substrates across biological membranes (3–5). MRP2/ABCC2, one member of the MRP/ABCC family, is mainly expressed in the apical membrane of liver canaliculi, renal proximal tubules, gut enterocytes, placenta, and blood–brain barriers. Physiologically, it pumps endogenous metabolites, such as conjugated bilirubin and bile salts, into bile (6,7). Caused by distinct mutations that create premature termination codons in the ABCC2/Abcc2 gene, hereditary MRP2/ABCC2 deficiency results in conjugated hyperbilirubinemia, seen in Dubin-Johnson Syndrome in humans or TR– rats (Wistar rats) and Eisai hyperbilirubinemic (EHBR) rats (Sprague–Dawley rats) (8,9). MRP2/ABCC2 also transports anionic conjugates of drugs (10,11) and many structurally diverse xenobiotics and their metabolites as part of the hepatic detoxification process (12–16). It has been recognized as a major biliary efflux transporter for anionic drugs such as methotrexate and pravastatin (17,18). Other unmodified drugs that are transported by MRP2/ABCC2 include vincristine (19), doxorubicin (20,21), HIV protease inhibitors (22), nucleoside phosphonates (23), p-aminohippuric acid (24), and fluoroquinolone antibiotics (25).
Disturbance of MRP2/ABCC2-mediated hepatobiliary transport function can result in disorder of lipid homeostasis and toxic accumulation of compounds in the liver, hence is one of the causes of drug withdrawals from the market (26,27). Most recently, it was reported that hepatobiliary secretion mediated by Mrp2/Abcc2 lead to nonlinear saturable pharmacokinetics in rats when a compensatory secretion mechanism is not sufficiently efficient (28). On the other hand, pharmacological advantage has been reported for biliary excretion of fluoroquinolone antibiotics as they are efficiently excreted into the bile followed by enterohepatic recirculation (29). Interactions between small molecule therapeutic agents and MRP2/ABCC2 protein have received increasing attention in considering their role in pharmacokinetic properties, drug–drug interactions, and/or adverse drug events (28).
Structure–Activity Approaches to Efflux Transporter Interactions
Recently, efforts have been made to solve ABC transporter-related issues in the areas of (1) circumventing multidrug resistance (MDR) mediated by P-gp/ABCB1, BCRP/ABCG2, and MRPs/ABCCs; (2) achieving favorable pharmacokinetic profiles; and (3) targeting in specific organs, e.g., CNS penetration. The common strategy is to build the SAR within the exploratory chemical space and to identify the structural features that are critical for interacting with the ABC transporters. Since the response caused by a substrate and/or an inhibitor is a result of complementary recognition between transporter protein and small molecule via its multiple functional groups, addition or removal of a key group may alter the efflux outcome in in vitro and/or in vivo settings (30). QSAR analysis furthers the understanding by establishing quantitative correlations between the transport property and the molecular descriptors (e.g., lipophilicity, hydrogen-bond forming capacities, molecular weight, polar and non-polar surface areas, and steric and comformational factors, etc.). Pharmacophore models have proven useful in the prediction of the drug–transporter interactions in rational drug design (31). In cancer therapy, attempts have been made to overcome MDR by either developing inhibitory modulators of ABC transporters to co-administer with the anticancer drugs, or modifying the interactions between drugs and the ABC transporters. For example, substantial amount of work has been done to understand the P-gp recognition and to identify key molecular elements within chemical scaffold (32–36). Such recognition consists of hydrophobic interaction (aromatic ring stacking, van der Waals interaction of aliphatic groups) and polar interaction (hydrogen bonds, electrostatic interaction). Furthermore, the recognition of a substrate and/or inhibitor molecule by transporter depends on not only the presence of strong interacting polar or non-polar groups but also their 3D-spatial arrangement (37).
In addition to P-gp/ABCB1 interactions, developing SAR for MRP/ABCC interaction has been an ongoing effort during the lead optimization stage in drug discovery. For example, Wang and co-workers identified potent and selective MRP1/ABCC1 inhibitors of pyrrolopyrimidine analogs that are potentially useful as MDR reversal agent in chemotherapy (38). Boumendjel et al. reported the discovery of a MRP1/ABCC1 inhibitor in an effort to mitigate MDR in anticancer treatment (39). However, SAR for the interaction of ABC transporters, in particular MRP/ABCC interactions, is generally complicated. Based on the transport mechanism, two types of molecules have been identified as MRP/ABCC inhibitors or substrates: (1) compounds co-transported with glutathione (GSH) and cytosolic glutathione S-transferases (GST) and (2) compounds interacting with MRP independent of GSH and GST. Though the interactions are poorly understood for the compounds that co-transport with GSH and GST, certain structural requirements have been drawn for the GSH/GST independent compounds (39). By analyzing a group of structurally diverse compounds, some common structural features were identified for possible MRP1/ABCC1 inhibition, in which aromatic/heteroaromatic moieties, nitrogen atom, and carbonyl groups are frequently observed in MRP1/ABCC1 inhibitors and/or substrates (39). The SAR of transporter interactions has been also applied in drug design to improve pharmacokinetic profile by reducing the effects of efflux pump in gastrointestinal tract. For example, saquinavir (SQV) is a protease inhibitor for the treatment of HIV-1 infection and its low and variable bioavailability is attributed to membrane-bound efflux pumps, including MRP2/ABCC2 (40). Jain and co-workers converted SQV to dipeptide prodrugs valine–valine–saquinavir (Val-Val-SQV) and glycine–valine–saquinavir (Gly-Val-SQV) via an ester linkage between the hydroxyl group of SQV and the carboxylic group of amino acid (41). They found that the Val-Val-SQV modification successfully eradicates the MRP2/ABCC2 interaction and exhibits about five-fold enhanced intestinal absorption in rat (41). Other SAR efforts on ABC transporters can also be found in reviews of this AAPS Journal. These works lay ground as examples for better understanding transporter recognition and provide practical approaches to establish SAR on ABC transporters.
QSAR ANALYSIS AND MOLECULAR MODELING OF MRP2/ABCC2 INTERACTIONS
The “old fashioned” drug discovery was an expensive and laborious process, as it consisted of massive testing of natural and synthetic compounds. In modern drug discovery, computational chemistry has become an essential component in designing and selecting compounds of desirable pharmacological properties via statistical analysis and/or molecular modeling. Computational models can be built using known knowledge and experimental data to evaluate the suitability of compounds before an expensive and time-consuming synthesis attempt is made.
QSAR analysis has been largely applied to discern physico-chemical parameters responsible for the ABCC2/MRP2 substrate specificity and/or inhibition activity. By 2D-QSAR modeling, Han et al. found that non-polar surface area and the calculated 1-octanol–water partition coefficient (ClogP) followed by molecular weight to a lesser extent are important characteristics that correlate with rat Mrp2/Abcc2 affinity of methotrexate and two dozen of its derivatives (42). In a follow-up study, simulated annealing partial least squares analysis reinforce the importance of ClogP and PSA, and additionally identify negative charge as important factors for binding affinity to Mrp2/Abcc2 (43). Further analysis using 3D-QSAR method yields a pharmacophore model consisting of two hydrophobic regions (two aromatic rings) and an anionic ionizable group as critical molecular features. Attempting to map the 3D receptor interaction of rat Mrp2/Abcc2, comparative molecular field analysis (CoMFA) has been applied to a set of substrates of diverse chemical structures (44). Using a number of molecular properties (i.e., steric field, electrostatic field, and ClogP) the 3D-QSAR model shows the capabilities in assessing the feasibility of Mrp2 binding, as well as estimating binding affinity (Km) of chemical compounds. A recent classification model is able to differentiate inhibitors from non-inhibitors with 77% accuracy by support vector machine, and 74% by pharmacophore modeling (45). Similar to the previous findings, lipophilicity, aromatic functions, hydrogen bond features, and polarizability are among the most important molecular properties that sensitize the inhibitory recognition of MRP2/ABCC2 (45).
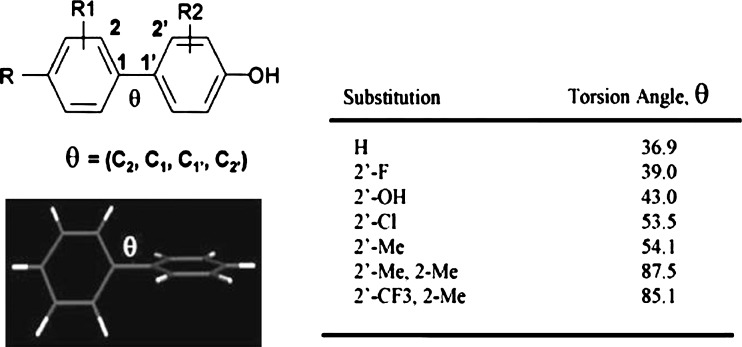
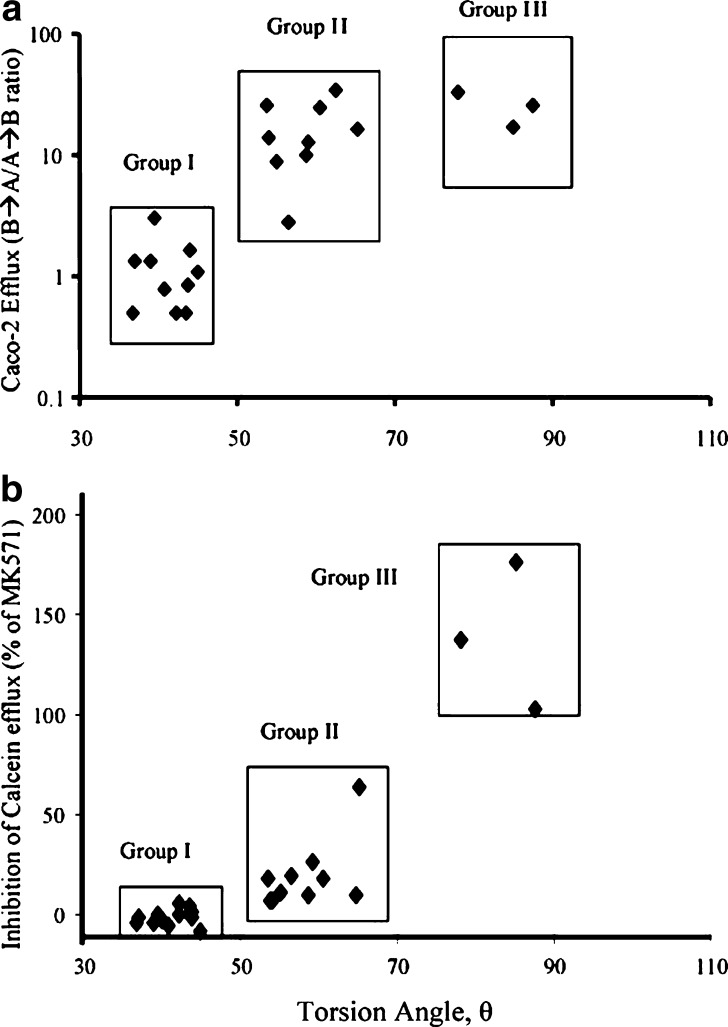
ABCC2/MRP2 interactions are highly specific. In a comparative study of MRP1- and MRP2-mediated calcein transport in MDCKII gene transfected cells, van Zanden et al. analyzed large series of flavonoids to define the structural requirements needed for potent inhibition (46). Coincidently, the dihedral angle between the B- and C-ring of flavonoids is suggested to be one of the three structural characteristics for MRP1/ABCC1 inhibition, along with the total number of methoxylate and hydroxyl groups. In their report, the MRP2/ABCC2 recognition demonstrates higher selectivity than MRP1/ABCC1, exhibiting a strong reliance on the presence of a flavonol B-ring pyrogallol group. Such high specificity of ABCC2/MRP2 interactions is also observed in a group of bi-phenyl-substituted heterocyclic compounds. In the study, both the nature and the magnitude of the ABCC2/MRP2 transport and/or inhibition profiles are tightly mediated by the torsion angles of the bi-phenyl system (47). Given the number and the size of the ortho-substituents that the bi-phenyl moiety bears, the MRP2/ABCC2 transport and inhibition profile of this series of compounds correlate well with the torsion angle between the two phenyl rings calculated at the density functional theory (DFT B3LYP/6-31G*) level. The DFT accurately calculate the dihedral angles, which successfully classifies the compounds into three groups. As the torsion angle increases, the compounds transit from non-substrate and/or non-inhibitor to substrate and/or weak inhibitor, and eventually to substrate and/or potent inhibitor (Figs. 2 and 3). In a separate study of discriminating inhibitors from non-inhibitors of MRP2/ABCC2, the orthogonal partial least squares discriminant analysis is applied to over a hundred structurally diverse drug-like compounds (48). An overlapping but narrower inhibitor space is found for MRP2/ABCC2 than P-gp/ABCB1 and BCRP/ABCG2. The inhibitors are generally larger and more lipophilic and present more aromatic features than the non-inhibitors. The multivariate model is successful in distinguishing more than 70% of the MRP2/ABCC2 inhibitors from non-inhibitors (48).
Fig. 2.
Illustration of torsion angles for a group of substituted bi-phenyl ring system. The torsion angles increase with increasing bulkiness of the substituents in the following order: H < F < OH < Cl < Me. The bi-phenyl ring systems are of the smallest torsion angles (37–45o) when they are unsubstituted or substituted with small atoms such as fluorine or oxygen. Chloride or 2-methyl substituted bi-phenyl ring systems exhibit moderate torsion angles (54–65o). The torsion angles of the 2, 2’-dimethyl substituted biphenyls are further increased (78–87o). Reproduced with permission from Lai et al. (47)
Fig. 3.
The correlation plots between torsion angles and the interactions of MRP2/ABCC2: a the inhibition of MRP2/ABCC2-mediated calcein efflux, b MRP2/ABCC2-mediated efflux. Based on the inhibition degree and the torsion angle range, the compounds were further classified into group I (37–45˚), group II (54–65o), and group III (78–87o). Reproduced with permission from Lai et al. (47)
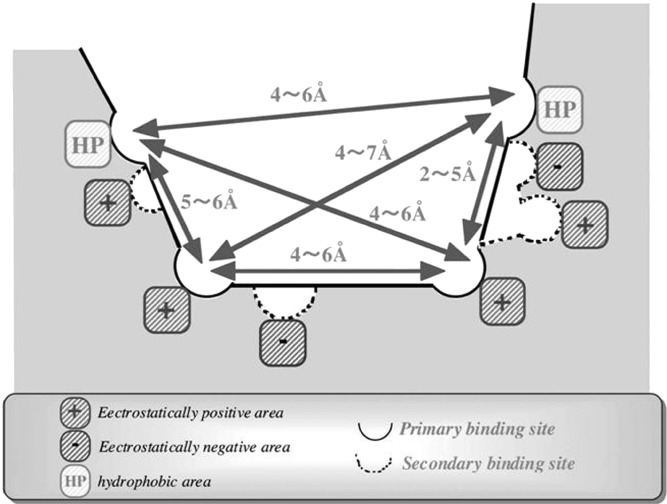
Based on the 3D-pharmacophore and the contour map obtained from the CoMFA calculation, Hirono et al. proposed the structure of the ligand-binding site of rat Mrp2/Abcc2 (44). In their models, the primary binding site is proposed to be assembled by two hydrophobic and two electropositive sub-pockets, augmented by the secondary ligand sites via two electropositive and two electronegative sites (Fig. 4). Ligand recognition is speculated to be achieved through interactions in the two primary binding sites, while the secondary binding sites are responsible for the broad substrate specificity of Mrp2/Abcc2 (44). In light of the ligand-derived charge interactions, two amino acids in the transmembrane domain of rat Mrp2, conserved among MRP families, have been shown critical for their positive electrostatics by site-directed mutagenesis. K325M and R586L mutations of rat Mrp2/Abcc2 resulted in marked reduction in the transport for glutathione conjugates and leukotriene C4 (49).
Fig. 4.
Proposed primary and secondary ligand-binding sites of rat Mrp2/Abcc2. The primary binding site is assembled by two hydrophobic and two electropositive sub-pockets. The secondary binding site consists of two electropositive and two electronegative sites. Reproduced with permission from Springer Science (Hirono et al. (44))
It has been proposed that MRP2/ABCC2 contains two distinct binding sites: one site for substrate transport and a second site that allosterically regulates the affinity of the transport site for the substrate (50). Limited mutagenesis study revealed four basic residues in the transmembrane domain important for transport activity, specifically K324, K483, R1210, and R1257 (51). Additionally, on the last transmembrane segment a highly conserved Trp residue (Trp1254) was shown to be essential for MRP2-mediated transport of methotrexate, but the 17-beta-estradiol glucuronide transport is affected by only non-conserved mutations (51). Due to exceeding challenges on the purification and crystallization of the ABC superfamily of proteins, the only structural information available to date is a bacterial ABC transporter (52). An earlier structure of a bacterial MDR-ABC transporter MsbA has been retracted due to incorrect topological assignments resulted from low resolution of the X-ray diffraction data (53). Nevertheless, using the obsolete PDB entry a homology model of ABCC2/MRP2 has been built to predict the binding of known MRP2/ABCC2 inhibitors and a series of quercetin glucuronide conjugates (54). The postulated binding site is composed of amino acids Phe550, Val557, Asn587, Ser558, Trp1144, Phe1140, Arg1257, and Glu1260, which are important for close contact interactions with the inhibitors and/or substrates. The calculated binding affinities by molecular docking seem to correlate with the experimental EC50 values. In agreement with an independent study, the major phase II metabolites of quercetin are equally potent or even better inhibitors of MRP2/ABCC2 than quercetin itself (55). Suggested by preliminary success, homology modeling could be a more amenable approach to derive 3D interactions for MRP2/ABCC2 than the other ABC family members, possibly due to its stricter substrate specificity of MRP2/ABCC2.
IN VITRO TOOLS FOR IDENTIFYING SUBSTRATES/INHIBITORS OF MRP2/ABCC2 IN DRUG DISCOVERY
In SAR and QSAR analysis, the reliability of models depends in part on the algorithms used to generate the relationships. More importantly, the predictive ability of a model relies on the quality and quantity of the data used for training; the larger the training set is, the broader chemical space the model can apply. A variety of in vitro tools have been developed to identify a new chemical entity as a substrate/inhibitor of ABCC2/MRP2, which can be incorporated into the consideration of favorable or adverse impact on clinical drug ADME/Tox properties and efficacy. While the in vivo models offer the definitive information, the in vitro methods are amenable to high-throughput and automation formats. The data generated from these assays are valuable as they provide the necessary elucidation of transporter involvement and the potential clinical impact, and are useful for developing computational models.
Bi-directional Transport Through a Polarized Cell Monolayer
Bi-directional drug transport through a polarized cell monolayer grown on a permeable filter support is the most direct method for testing transporter function. Various cell lines can form monolayers with tight junction, such as HT29 and Caco-2 cells, and may refer to bi-directional cell monolayer transport (56). Derived from a human colon carcinoma, Caco-2 monolayers form tight monolayers and express drug transporters that mimic the human intestinal epithelia cells (57). The bi-directional assays in Caco-2 cells have been shown to be a valuable tool for identifying substrates and/or inhibitors and understanding the mechanistic function. This model has been successfully used for analyzing the structure–activity relationships of MRP2/ABCC2 substrates (47) or for rapidly identifying the MRP2/ABCC2 inhibitors using fluorescent substrates (58). However, as mentioned above, Caco-2 cells express the most transporters in intestinal epithelia cells, and are not a “pure” model for identification of single transporter interaction. The gene transfected cell lines overexpressing single transporter gene are useful tools in transporter functional assays due to the unambiguous identification of the transporter involved (20,59–62). The MRP2/ABCC2-mediated fluorescent substrates efflux in gene transfected cell line has been used for compound profiling (63). However, the single MRP2/ABCC2 gene transfected cell model is difficult to demonstrate the vectorial transport of ABCC2/MRP2 substrates due to the lack of the corresponding basolateral membrane transport mediated by uptake transporters (64,65). To overcome the limitations, the MDCK cells have also been doubly transfected with transporters (i.e., OATP1B1 or OATP1B3, and MRP2/ABCC2) or even quadruply transfected (66), to allow active uptake and efflux to be characterized, especially for anionic substrates with poor permeability. These models describe the cooperative functions of uptake transporters on basal membrane and efflux transporters on apical membrane for governing the vectorial transport (64,67). Nonetheless, the multiple gene transfected cell lines have not been generally accepted, due to the unknown expressing efficiency and substrate specificity of corresponding transporters. The bi-directional transport assays can be used as second tier studies to determine the kinetic parameters of substrate or inhibitor for generating Km or IC50 values (68).
Membrane Vesicular Uptake Assay
Through monitoring the accumulation of substrate within the vesicles, inside-out vesicles preparations from transporter gene overexpressed sf9 insect cells or tissues are popularly used to identify substrates and inhibitors of ABC transporters (69–71). Vesicle preparations are exposed to test compound in the presence or absence (as negative controls) of ATP. The accumulation of compound in the vesicle is determined by rapid filtration in a cell harvester to remove remaining external compound (28,72). For MRP2/ABCC2-mediated uptake, the rate of ATP-dependent estradiol-17β-D-glucuronide (E17G) transport is linear for up to 30 min (48). The addition of glutathione is required for MRP2/ABCC2 transport, due to the potential requirement for its co-transport with substrates of these proteins. Since the experiments could be performed in a 96-well plate in a rapid filtration technique (28,48), the inverted membrane vesicles assay is considered as an effective method for determining MRP2/ABCC2 interactions and is amenable to high-throughput screening (73,74).
Cellular Uptake or Efflux Assay
Monitoring drug accumulation in ABC transporter overexpressing cells/xenopus Laevis oocytes can be used to characterize transporter substrates or inhibitors either through compound uptake or by inhibition of compound efflux (75–81). Experimentally, the probe substrate needs to be pre-loaded into the cells during a preincubation period prior to efflux assay (82). For a single transporter characterization, the choice of a probe compound is the key and should be based on substrate efficiency for the specific transporter involved, ease of measurement, and range of sensitivity and responsiveness to inhibition (83,84). The efflux of florescent substrate, e.g., calcein and GS-MF (5-chloromethylfluorescein diacetate GSH conjugate), has been used for measured MRP/ABCC function or the inhibitory effects of testing compounds (46,47). Inhibition of the relevant transporter results in the retention of fluorescent substrate within the cell (81). The use of specific fluorescent substrate as a marker for functional efflux inhibition is desirable due to its intracellular accumulation that the trapped fluorescence level is directly correlated with the inhibition of transporter function. This fluorescent substrate can also be detected by a plate reader to reduce the labor involved and allowing real-time measurements.
ATPase Activation Assay
As the ABC superfamily of transporters activate through ATP hydrolysis to adenosine diphosphate releasing of inorganic phosphate (Pi), the membrane preparations can also be used to determine the interactions with ABC transporters by measurement of ATP hydrolysis (85). This ATP conversion is quantified either directly through colorimetric detection of Pi liberation (85–87) or indirectly by spectrophotometric monitoring of NADH turnover in a coupled enzyme cycling assay (87,88). The assay has successfully been used for determining the P-gp affinity (89) and is expanded to other ABC transporters. However, as the release of Pi is correlated to the activation of the transporter ATPase, and presumably to a transport event, the assay is an indirect measure of transport. Thus it is not for the direct measurement of transporter function. In addition, some drugs can stimulate Pi release at low concentrations, but inhibit the effect at higher concentrations (90–92) or result in a false negative due to significant nonspecific binding (92).
CONCLUSIONS
The advancements in screening technologies and the vast volume of the ADME/Tox data provide opportunities to probe the interaction between MRP2/ABCC2 transporter and its substrates and inhibitors. Various computational models have been developed and offered the promise of depicting the requirements for MRP2/ABCC2 interaction at the molecular level. These include: (1) physico-chemical properties, (2) key functional groups, (3) 3D spatial arrangement, and (4) the primary and secondary binding sites of Mrp2/Abcc2. However, most of the studies are based on series of structurally related compounds; thus the conclusions drawn are limited to the specific chemical spaces they represent. The need to develop more general QSAR models to predict broad transport properties for MRP2/ABCC2 remains largely unmet. Advancement of MRP2/ABCC2 structural biology could significantly accelerate revelation of the biological function of this transporter and provide further insights into developing more versatile and predictive computational models.
Acknowledgements
We would like to thank Drs. Gennadiy I. Poda and Joseph C. Fleishaker for their helpful comments and suggestions on our review.
Abbreviations
- ADME/Tox
absorption, distribution, metabolism, excretion, and toxicity
- QSAR
quantitative structure–activity relationship
- SAR
structure–activity relationship
Footnotes
The uppercase characters identify the human protein, i.e., MRP2/ABCC2, while lowercase characters indicate that the transporter derives from preclinical species, i.e. Mrp2/Abcc2.
Reference
- 1.Guido RV, Oliva G, Andricopulo AD. Virtual screening and its integration with modern drug design technologies. Curr Med Chem. 2008;15:37–46. doi: 10.2174/092986708783330683. [DOI] [PubMed] [Google Scholar]
- 2.Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001;33:475–479. doi: 10.1023/A:1012823120935. [DOI] [PubMed] [Google Scholar]
- 3.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 4.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/S0169-409X(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 5.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 6.Gerk PM, Vore M. Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition. J Pharmacol Exp Ther. 2002;302:407–415. doi: 10.1124/jpet.102.035014. [DOI] [PubMed] [Google Scholar]
- 7.Meier PJ, Stieger B. Bile salt transporters. Annu Rev Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- 8.Buchler M, Konig J, Brom M, Kartenbeck J, Spring H, Horie T, Keppler D. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271:15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am J Physiol. 1997;272:G16–22. doi: 10.1152/ajpgi.1997.272.1.G16. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cMOAT/MRP2. Semin Liver Dis. 1998;18:359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- 11.Keppler D, Konig J. Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis. 2000;20:265–272. doi: 10.1055/s-2000-9391. [DOI] [PubMed] [Google Scholar]
- 12.Paulusma CC, van Geer MA, Evers R, Heijn M, Ottenhoff R, Borst P, Oude Elferink RP. Canalicular multispecific organic anion transporter/multidrug resistance protein 2 mediates low-affinity transport of reduced glutathione. Biochem J. 1999;338(Pt 2):393–401. doi: 10.1042/0264-6021:3380393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keppler D, Arias IM. Hepatic canalicular membrane. Introduction: transport across the hepatocyte canalicular membrane. FASEB J. 1997;11:15–18. doi: 10.1096/fasebj.11.1.9034161. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Suzuki H, Hirohashi T, Kume K, Shimizu T, Sugiyama Y. Functional analysis of a canalicular multispecific organic anion transporter cloned from rat liver. J Biol Chem. 1998;273:1684–1688. doi: 10.1074/jbc.273.3.1684. [DOI] [PubMed] [Google Scholar]
- 15.Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/S0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 16.Kusuhara H, Sugiyama Y. Role of transporters in the tissue-selective distribution and elimination of drugs: transporters in the liver, small intestine, brain and kidney. J Control Release. 2002;78:43–54. doi: 10.1016/S0168-3659(01)00480-1. [DOI] [PubMed] [Google Scholar]
- 17.Hulot JS, Villard E, Maguy A, Morel V, Mir L, Tostivint I, William-Faltaos D, Fernandez C, Hatem S, Deray G, Komajda M, Leblond V, Lechat P. A mutation in the drug transporter gene ABCC2 associated with impaired methotrexate elimination. Pharmacogenet Genomics. 2005;15:277–285. doi: 10.1097/01213011-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Keppler D, Kamisako T, Leier I, Cui Y, Nies AT, Tsujii H, Konig J. Localization, substrate specificity, and drug resistance conferred by conjugate export pumps of the MRP family. Adv Enzyme Regul. 2000;40:339–349. doi: 10.1016/S0065-2571(99)00022-9. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- 21.Koike K, Abe T, Hisano T, Kubo T, Wada M, Kohno K, Kuwano M. Overexpression of multidrug resistance protein gene in human cancer cell lines selected for drug resistance to epipodophyllotoxins. Jpn J Cancer Res. 1996;87:765–772. doi: 10.1111/j.1349-7006.1996.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutmann H, Torok M, Fricker G, Huwyler J, Beglinger C, Drewe J. Modulation of multidrug resistance protein expression in porcine brain capillary endothelial cells in vitro. Drug Metab Dispos. 1999;27:937–941. [PubMed] [Google Scholar]
- 23.Miller MD, Margot NA, Hertogs K, Larder B, Miller V. Antiviral activity of tenofovir (PMPA) against nucleoside-resistant clinical HIV samples. Nucleosides Nucleotides Nucleic Acids. 2001;20:1025–1028. doi: 10.1081/NCN-100002483. [DOI] [PubMed] [Google Scholar]
- 24.Leier I, Hummel-Eisenbeiss J, Cui Y, Keppler D. ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int. 2000;57:1636–1642. doi: 10.1046/j.1523-1755.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 25.Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, Tsuji A. Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob Agents Chemother. 2002;46:344–349. doi: 10.1128/AAC.46.2.344-349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isley WL. Hepatotoxicity of thiazolidinediones. Expert Opin Drug Saf. 2003;2:581–586. doi: 10.1517/14740338.2.6.581. [DOI] [PubMed] [Google Scholar]
- 27.Tang W. Drug metabolite profiling and elucidation of drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2007;3:407–420. doi: 10.1517/17425255.3.3.407. [DOI] [PubMed] [Google Scholar]
- 28.Y. Hu, K.E. Sampson, B.R. Heyde, K.M. Mandrell, N. Li, A. Zutshi, and Y. Lai. Saturation of Mrp2/Abcc2 Mediated Hepatobiliary Secretion: Nonlinear Pharmacokinetics of A Heterocyclic Compound in Rats after I.V Bolus Administration. Drug Metab Dispos doi:10.1124/dmd.108.024059 (2009). [DOI] [PubMed]
- 29.Suzuki H, Sugiyama Y. Transporters for bile acids and organic anions. Pharm Biotechnol. 1999;12:387–439. doi: 10.1007/0-306-46812-3_14. [DOI] [PubMed] [Google Scholar]
- 30.Raub TJ. P-glycoprotein recognition of substrates and circumvention through rational drug design. Mol Pharm. 2006;3:3–25. doi: 10.1021/mp0500871. [DOI] [PubMed] [Google Scholar]
- 31.Stouch TR, Gudmundsson O. Progress in understanding the structure-activity relationships of P-glycoprotein. Adv Drug Deliv Rev. 2002;54:315–328. doi: 10.1016/S0169-409X(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 32.Fong WF, Shen XL, Globisch C, Wiese M, Chen GY, Zhu GY, Yu ZL, Tse AK, Hu YJ. Methoxylation of 3′, 4′-aromatic side chains improves P-glycoprotein inhibitory and multidrug resistance reversal activities of 7, 8-pyranocoumarin against cancer cells. Bioorg Med Chem. 2008;16:3694–3703. doi: 10.1016/j.bmc.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 33.Choi CH, Kim JH, Kim SH. Reversal of P-glycoprotein-mediated MDR by 5, 7, 3′, 4′, 5′-pentamethoxyflavone and SAR. Biochem Biophys Res Commun. 2004;320:672–679. doi: 10.1016/j.bbrc.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Ayral-Kaloustian S, Nguyen T, Hernandez R, Lucas J, Discafani C, Beyer C. Synthesis and SAR of 6-chloro-4-fluoroalkylamino-2-heteroaryl-5-(substituted) phenylpyrimidines as anti-cancer agents. Bioorg Med Chem. 2009;17:111–118. doi: 10.1016/j.bmc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Coburger C, Wollmann J, Baumert C, Krug M, Molnar J, Lage H, Hilgeroth A. Novel insight in structure-activity relationship and bioanalysis of P-glycoprotein targeting highly potent tetrakishydroxymethyl substituted 3, 9-diazatetraasteranes. J Med Chem. 2008;51:5871–5874. doi: 10.1021/jm800480y. [DOI] [PubMed] [Google Scholar]
- 36.Colabufo NA, Berardi F, Cantore M, Perrone MG, Contino M, Inglese C, Niso M, Perrone R, Azzariti A, Simone GM, Porcelli L, Paradiso A. Small P-gp modulating molecules: SAR studies on tetrahydroisoquinoline derivatives. Bioorg Med Chem. 2008;16:362–373. doi: 10.1016/j.bmc.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 37.Pajeva IK, Wiese M. Pharmacophore model of drugs involved in P-glycoprotein multidrug resistance: explanation of structural variety (hypothesis) J Med Chem. 2002;45:5671–5686. doi: 10.1021/jm020941h. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Folkes A, Chuckowree I, Cockcroft X, Sohal S, Miller W, Milton J, Wren SP, Vicker N, Depledge P, Scott J, Smith L, Jones H, Mistry P, Faint R, Thompson D, Cocks S. Studies on pyrrolopyrimidines as selective inhibitors of multidrug-resistance-associated protein in multidrug resistance. J Med Chem. 2004;47:1329–1338. doi: 10.1021/jm031011g. [DOI] [PubMed] [Google Scholar]
- 39.Boumendjel A, Baubichon-Cortay H, Trompier D, Perrotton T, Di Pietro A. Anticancer multidrug resistance mediated by MRP1: recent advances in the discovery of reversal agents. Med Res Rev. 2005;25:453–472. doi: 10.1002/med.20032. [DOI] [PubMed] [Google Scholar]
- 40.Huisman MT, Smit JW, Crommentuyn KM, Zelcer N, Wiltshire HR, Beijnen JH, Schinkel AH. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. Aids. 2002;16:2295–2301. doi: 10.1097/00002030-200211220-00009. [DOI] [PubMed] [Google Scholar]
- 41.Jain R, Agarwal S, Mandava NK, Sheng Y, Mitra AK. Interaction of dipeptide prodrugs of saquinavir with multidrug resistance protein-2 (MRP-2): evasion of MRP-2 mediated efflux. Int J Pharm. 2008;362:44–51. doi: 10.1016/j.ijpharm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Han Y-H, Kato Y, Haramura M, Ohta M, Matsuoka H, Sugiyama Y. Physicochemical parameters responsible for the affinity of methotrexate analogs for rat canalicular multispecific organic anion transporter (cMOAT/MRP2) Pharm Res. 2001;18:579–586. doi: 10.1023/A:1011064806507. [DOI] [PubMed] [Google Scholar]
- 43.Ng C, Xiao Y-D, Lum BL, Han Y-H. Quantitative structure-activity relationships of methotrexate and methotrexate analogues transported by the rat multispecific resistance-associated protein 2 (rMrp2) Eur J Pharm Sci. 2005;26:405–413. doi: 10.1016/j.ejps.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Hirono S, Nakagome I, Imai R, Maeda K, Kusuhara H, Sugiyama Y. Estimation of the three-dimensional pharmacophore of ligands for rat multidrug-resistance-associated protein 2 using ligand-based drug design techniques. Pharm Res. 2005;22:260–269. doi: 10.1007/s01869-005-1869-8. [DOI] [PubMed] [Google Scholar]
- 45.Zhang H, Xiang ML, Zhao YL, Wei YQ, Yang SY. Support vector machine and pharmacophore-based prediction models of multidrug-resistance protein 2 (MRP2) inhibitors. Eur J Pharm Sci. 2009;36:451–457. doi: 10.1016/j.ejps.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 46.van Zanden JJ, Wortelboer HM, Bijlsma S, Punt A, Usta M, Bladeren PJ, Rietjens IM, Cnubben NH. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem Pharmacol. 2005;69:699–708. doi: 10.1016/j.bcp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Lai Y, Xing L, Poda GI, Hu Y. Structure-activity relationships for interaction with multidrug resistance protein 2 (ABCC2/MRP2): the role of torsion angle for a series of biphenyl-substituted heterocycles. Drug Metab Dispos. 2007;35:937–945. doi: 10.1124/dmd.106.013250. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen JM, Matsson P, Bergstrom CA, Norinder U, Hoogstraate J, Artursson P. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2) J Med Chem. 2008;51:3275–3287. doi: 10.1021/jm7015683. [DOI] [PubMed] [Google Scholar]
- 49.Ito K, Suzuki H, Sugiyama Y. Charged amino acids in the transmembrane domains are involved in the determination of the substrate specificity of rat Mrp2. Mol. Pharmacol. 2001;59:1077–1085. doi: 10.1124/mol.59.5.1077. [DOI] [PubMed] [Google Scholar]
- 50.Zelcer N, Huisman MT, Reid G, Wielinga P, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel AH, Borst P. Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2) J Biol Chem. 2003;278:23538–23544. doi: 10.1074/jbc.M303504200. [DOI] [PubMed] [Google Scholar]
- 51.Ryu S, Kawabe T, Nada S, Yamaguchi A. Identification of basic residues involved in drug export function of human multidrug resistance-associated protein 2. J Biol Chem. 2000;275:39617–39624. doi: 10.1074/jbc.M005149200. [DOI] [PubMed] [Google Scholar]
- 52.Dawson R, Locher K. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 53.Chang G, Roth C, Reyes C, Pornillos O, Chen Y-J, Chen A. Retraction. Science. 2006;314:1875. doi: 10.1126/science.314.5807.1875b. [DOI] [PubMed] [Google Scholar]
- 54.Williamson G, Aeberli I, Miguet L, Zhang Z, Sanchez MB, Crespy V, Barron D, Needs P, Kroon PA, Glavinas H, Krajcsi P, Grigorov M. Interaction of positional isomers of quercetin glucuronides with the transporter ABCC2 (cMOAT, MRP2) Drug Metab Dispos. 2007;35:1262–1268. doi: 10.1124/dmd.106.014241. [DOI] [PubMed] [Google Scholar]
- 55.van Zanden JJ, van der Woude H, Vaessen J, Usta M, Wortelboer HM, Cnubben NHP, Rietjens IMCM. The effect of quercetin phase II metabolism on its MRP1 and MRP2 inhibiting potential. Biochem Pharmacol. 2007;74:345–351. doi: 10.1016/j.bcp.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001;46:27–43. doi: 10.1016/S0169-409X(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 57.Putnam WS, Pan L, Tsutsui K, Takahashi L, Benet LZ. Comparison of bidirectional cephalexin transport across MDCK and caco-2 cell monolayers: interactions with peptide transporters. Pharm Res. 2002;19:27–33. doi: 10.1023/A:1013647114152. [DOI] [PubMed] [Google Scholar]
- 58.Siissalo S, Hannukainen J, Kolehmainen J, Hirvonen J, Kaukonen AM. A Caco-2 cell based screening method for compounds interacting with MRP2 efflux protein. Eur J Pharm Biopharm. 2009;71:332–338. doi: 10.1016/j.ejpb.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 59.Bakos E, Evers R, Szakacs G, Tusnady GE, Welker E, Szabo K, de Haas M, van Deemter L, Borst P, Varadi A, Sarkadi B. Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem. 1998;273:32167–32175. doi: 10.1074/jbc.273.48.32167. [DOI] [PubMed] [Google Scholar]
- 60.Evers R, Zaman GJ, van Deemter L, Jansen H, Calafat J, Oomen LC, Oude Elferink RP, Borst P, Schinkel AH. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J Clin Invest. 1996;97:1211–1218. doi: 10.1172/JCI118535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi S, Zhao YL, Nadai M, Yoshizumi H, Cen X, Torita S, Takagi K, Takagi K, Hasegawa T. Involvement of the drug transporters p glycoprotein and multidrug resistance-associated protein Mrp2 in telithromycin transport. Antimicrob Agents Chemother. 2006;50:80–87. doi: 10.1128/AAC.50.1.80-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao Y, Davidson R, Smith A, Pereira D, Zhao S, Soglia J, Gebhard D, de Morais S, Duignan DB. A 96-well efflux assay to identify ABCG2 substrates using a stably transfected MDCK II cell line. Mol Pharm. 2006;3:45–54. doi: 10.1021/mp050088t. [DOI] [PubMed] [Google Scholar]
- 63.Forster F, Volz A, Fricker G. Compound profiling for ABCC2 (MRP2) using a fluorescent microplate assay system. Eur J Pharm Biopharm. 2008;69:396–403. doi: 10.1016/j.ejpb.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki M, Suzuki H, Ito K, Abe T, Sugiyama Y. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and Multidrug resistance-associated protein 2 (MRP2/ABCC2) J Biol Chem. 2002;277:6497–6503. doi: 10.1074/jbc.M109081200. [DOI] [PubMed] [Google Scholar]
- 65.Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101:1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kopplow K, Letschert K, Konig J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol. 2005;68:1031–1038. doi: 10.1124/mol.105.014605. [DOI] [PubMed] [Google Scholar]
- 67.Cui Y, Konig J, Keppler D. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol Pharmacol. 2001;60:934–943. doi: 10.1124/mol.60.5.934. [DOI] [PubMed] [Google Scholar]
- 68.Gao J, Murase O, Schowen RL, Aube J, Borchardt RT. A functional assay for quantitation of the apparent affinities of ligands of P-glycoprotein in Caco-2 cells. Pharm Res. 2001;18:171–176. doi: 10.1023/A:1011076217118. [DOI] [PubMed] [Google Scholar]
- 69.Tamai I, Tsuji A, Kin Y. Carrier-mediated transport of cefixime, a new cephalosporin antibiotic, via an organic anion transport system in the rat renal brush-border membrane. J Pharmacol Exp Ther. 1988;246:338–344. [PubMed] [Google Scholar]
- 70.Yasumiba S, Tazuma S, Ochi H, Chayama K, Kajiyama G. Cyclosporin A reduces canalicular membrane fluidity and regulates transporter function in rats. Biochem J. 2001;354:591–596. doi: 10.1042/0264-6021:3540591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aslamkhan A, Han YH, Walden R, Sweet DH, Pritchard JB. Stoichiometry of organic anion/dicarboxylate exchange in membrane vesicles from rat renal cortex and hOAT1-expressing cells. Am J Physiol Renal Physiol. 2003;285:F775–783. doi: 10.1152/ajprenal.00140.2003. [DOI] [PubMed] [Google Scholar]
- 72.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71:619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- 73.Tabas LB, Dantzig AH. A high-throughput assay for measurement of multidrug resistance protein-mediated transport of leukotriene C4 into membrane vesicles. Anal Biochem. 2002;310:61–66. doi: 10.1016/S0003-2697(02)00282-8. [DOI] [PubMed] [Google Scholar]
- 74.Sakurai A, Kurata A, Onishi Y, Hirano H, Ishikawa T. Prediction of drug-induced intrahepatic cholestasis: in vitro screening and QSAR analysis of drugs inhibiting the human bile salt export pump. Expert Opin Drug Saf. 2007;6:71–86. doi: 10.1517/14740338.6.1.71. [DOI] [PubMed] [Google Scholar]
- 75.Feng B, Mills J, Davidson R, Mireles R, Janiszewski J, Troutman M, de Morais S. In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos. 2008;36:268–275. doi: 10.1124/dmd.107.017434. [DOI] [PubMed] [Google Scholar]
- 76.Eneroth A, Astrom E, Hoogstraate J, Schrenk D, Conrad S, Kauffmann HM, Gjellan K. Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction. Eur J Pharm Sci. 2001;12:205–214. doi: 10.1016/S0928-0987(00)00117-2. [DOI] [PubMed] [Google Scholar]
- 77.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 78.Sekine T, Cha SH, Hosoyamada M, Kanai Y, Watanabe N, Furuta Y, Fukuda K, Igarashi T, Endou H. Cloning, functional characterization, and localization of a rat renal Na+-dicarboxylate transporter. Am J Physiol. 1998;275:F298–305. doi: 10.1152/ajprenal.1998.275.2.F298. [DOI] [PubMed] [Google Scholar]
- 79.Cha SH, Sekine T, Fukushima JI, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001;59:1277–1286. doi: 10.1124/mol.59.5.1277. [DOI] [PubMed] [Google Scholar]
- 80.Wang EJ, Casciano CN, Clement RP, Johnson WW. In vitro flow cytometry method to quantitatively assess inhibitors of P-glycoprotein. Drug Metab Dispos. 2000;28:522–528. [PubMed] [Google Scholar]
- 81.Tiberghien F, Loor F. Ranking of P-glycoprotein substrates and inhibitors by a calcein-AM fluorometry screening assay. Anticancer Drugs. 1996;7:568–578. doi: 10.1097/00001813-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 82.Li M, Yuan H, Li N, Song G, Zheng Y, Baratta M, Hua F, Thurston A, Wang J, Lai Y. Identification of interspecies difference in efflux transporters of hepatocytes from dog, rat, monkey and human. Eur J Pharm Sci. 2008;35:114–126. doi: 10.1016/j.ejps.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Wang EJ, Casciano CN, Clement RP, Johnson WW. Active transport of fluorescent P-glycoprotein substrates: evaluation as markers and interaction with inhibitors. Biochem Biophys Res Commun. 2001;289:580–585. doi: 10.1006/bbrc.2001.6000. [DOI] [PubMed] [Google Scholar]
- 84.Schwab D, Fischer H, Tabatabaei A, Poli S, Huwyler J. Comparison of in vitro P-glycoprotein screening assays: recommendations for their use in drug discovery. J Med Chem. 2003;46:1716–1725. doi: 10.1021/jm021012t. [DOI] [PubMed] [Google Scholar]
- 85.Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992;267:4854–4858. [PubMed] [Google Scholar]
- 86.Drueckes P, Schinzel R, Palm D. Photometric microtiter assay of inorganic phosphate in the presence of acid-labile organic phosphates. Anal Biochem. 1995;230:173–177. doi: 10.1006/abio.1995.1453. [DOI] [PubMed] [Google Scholar]
- 87.Sauna ZE, Smith MM, Muller M, Kerr KM, Ambudkar SV. The mechanism of action of multidrug-resistance-linked P-glycoprotein. J Bioenerg Biomembr. 2001;33:481–491. doi: 10.1023/A:1012875105006. [DOI] [PubMed] [Google Scholar]
- 88.Garrigues A, Nugier J, Orlowski S, Ezan E. A high-throughput screening microplate test for the interaction of drugs with P-glycoprotein. Anal Biochem. 2002;305:106–114. doi: 10.1006/abio.2002.5650. [DOI] [PubMed] [Google Scholar]
- 89.Xia CQ, Xiao G, Liu N, Pimprale S, Fox L, Patten CJ, Crespi CL, Miwa G, Gan LS. Comparison of species differences of P-glycoproteins in beagle dog, rhesus monkey, and human using Atpase activity assays. Mol Pharm. 2006;3:78–86. doi: 10.1021/mp050034j. [DOI] [PubMed] [Google Scholar]
- 90.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 91.Markowitz JS, Devane CL, Liston HL, Boulton DW, Risch SC. The effects of probenecid on the disposition of risperidone and olanzapine in healthy volunteers. Clin Pharmacol Ther. 2002;71:30–38. doi: 10.1067/mcp.2002.119815. [DOI] [PubMed] [Google Scholar]
- 92.Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–628. [PubMed] [Google Scholar]