Abstract
Cancer is a hyperproliferative disorder that is usually treated by chemotherapeutic agents that are toxic not only to tumor cells but also to normal cells, so these agents produce major side effects. In addition, these agents are highly expensive and thus not affordable for most. Moreover, such agents cannot be used for cancer prevention. Traditional medicines are generally free of the deleterious side effects and usually inexpensive. Curcumin, a component of turmeric (Curcuma longa), is one such agent that is safe, affordable, and efficacious. How curcumin kills tumor cells is the focus of this review. We show that curcumin modulates growth of tumor cells through regulation of multiple cell signaling pathways including cell proliferation pathway (cyclin D1, c-myc), cell survival pathway (Bcl-2, Bcl-xL, cFLIP, XIAP, c-IAP1), caspase activation pathway (caspase-8, 3, 9), tumor suppressor pathway (p53, p21) death receptor pathway (DR4, DR5), mitochondrial pathways, and protein kinase pathway (JNK, Akt, and AMPK). How curcumin selectively kills tumor cells, and not normal cells, is also described in detail.
KEY WORDS: apoptosis, cancer, curcumin, molecular targets, signaling pathways.
INTRODUCTION
There are four types of cancers; (a) carcinoma: arise from the epithelial sheet that covers the surfaces, e.g., skin, colon, etc. Approximately 90% of all human cancers are carcinomas; (b) sarcoma: these include cancer of the connective tissues such as muscle, bone, cartilage, and fibrous tissue. Approximately 2% of all cancers are sarcomas; (c) leukemia and (d) lymphoma: they originate from blood forming cells and from cells of the immune system, respectively. Approx. 8% of all cancers are leukemia and lymphomas. Based on metastatic potential, there are two classifications of cancers; (a) benign tumors or adenomas: when neoplastic growth remains clustered as a single mass, (b) malignant tumor or adenocarcinoma: when tumor invades normal tissue and spreads throughout the body.
It is estimated that human body consists of 10–13 trillion cells. Almost all of these cells get turned over within approximately 100 days, thus suggesting an apoptosis/cell death rate of 100–130 billion cells each day. What is the mechanism of this mode of cell death in normal cells is unclear. Cancer cells are distinct from normal cells in six different ways and these characteristics are shared by all cancers; self sufficiency in growth signals, insensitivity to growth inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion, and metastasis (1). The ability of tumor cell populations to expand in number is determined not only by the rate of cell proliferation but also by the rate of cell death. Apoptosis is a major source of cell death, thus agents that trigger apoptosis/cell death, could be the most promising candidates as therapeutic for cancer.
Why certain types of cancers are more prevalent in some countries than others is not clear but lifestyle, including diet, is known to play major role (2). Among the potential dietary contributors to this disparity is turmeric (Curcuma longa), a spice that is consumed frequently by people from southeast Asia, a continent with low incidence of most cancers. Powder of turmeric is extensively used in Ayurveda, Unani, and Siddha medicine as a home remedy for various diseases. This powder, curcumin (diferuloylmethane) is a yellow-colored polyphenol, first isolated in 1815, crystallized in 1870, (3,4) and identified as 1,6-heptadiene-3,5- dione-1,7-bis(4-hydroxy-3-methoxyphenyl)-(1E,6E). Curcumin analoges which made by mother nature and man have been described (2). In addition, besides diferuloylmethane or curcumin, turmeric contains minor fractions such as demethoxycurcumin (curcumin II), bisdemethoxycurcumin (curcumin III), and the recently identified cyclocurcumin (5). All these analoges suggest that while hydroxyl groups in curcumin are required for its antioxidant activity, its methoxy groups are essential for its antiinflammatory and antiproliferative activity. Various molecular targets modulated by this agent include transcription factors, growth factors and their receptors, cytokines, enzymes, and genes regulating cell proliferation and apoptosis (6)
The anticarcinogenic properties of curcumin in animals have been demonstrated by its inhibition of tumor initiation (7) and tumor promotion (8,9). Studies of curcumin have shown that it influences structurally unrelated membrane proteins across several signaling pathways (10). A recent report suggests that curcumin inserts deep into the cellular membrane in a transbilayer orientation, anchored by hydrogen bonding to the phosphate group of lipids, thus inducing negative curvature in the bilayer (11). The promotion of negative curvature by curcumin may have a direct effect on apoptosis by increasing the permeabilizing activity of the apoptotic protein tBid (12). Curcumin has been shown to suppress multiple signaling pathways and inhibit cell proliferation, invasion, metastasis, and angiogenesis. The chemopreventive action of curcumin might be due to its ability to induce apoptosis by several pathways. Curcumin directly or indirectly controls different gene or gene products involved in cell death pathways as discussed here
MODES OF CELL DEATH
There are two major pathways of cell death, apoptosis (death by suicide) and necrosis (death by injury). Apoptosis is also known as programmed cell death and involves a series of biochemical events leading to characteristic cell morphology and death. Necrosis is caused by external factors, such as infection, toxins, or trauma. Additional cell death mechanisms, such as autophagy, entosis, paraptosis, and anoikis, have been defined more recently. A brief review of these modes of cell death will serve as background for our discussion of the modes and mediators of curcumin-induced cell death.
Apoptosis
Apoptosis is a normal physiological process that is required for the maintenance of cell homeostasis. The cellular changes involved in this process are both morphological and biochemical, including disintegration of the cytoskeleton and subsequent cell shrinkage, chromatin condensation, and activation of specific proteases, called caspases (13–15). Apoptosis can be initiated by a variety of internal and external stimuli, including receptor ligation and toxic insults. Apoptosis not only plays a crucial role in tissue development and homeostasis, but is also involved in a wide range of pathological conditions (16,17). Apoptotic cell death is accompanied by a series of complex biochemical events and definite morphologic changes, which include cell shrinkage, chromatin condensation, DNA fragmentation, membrane budding, and the appearance of membrane-associated apoptotic bodies (18). Failure to accurately undergo apoptosis can cause severe anomalies, ranging from autoimmune disease to cancer.
Necrosis
Necrosis typically occurs as a result of mechanical or toxic cell injury. Necrotic cells are distinguished from apoptotic cells in that they undergo stages such as cell swelling, plasma membrane rupture, organelle breakdown, and ultimately lysis, allowing release of the cytoplasmic contents and hence induction of an inflammatory response (14).
Autophagy
Autophagy or autophagocytosis is a cellular degradation process involving the degradation of a cell's own components through the lysosomal action (19). It is a tightly regulated cell-degradation mechanism that plays a normal part in cell growth, development, and homeostasis, helping to maintain a balance between the cellular products. This process involves dynamic membrane rearrangements under a range of physiological conditions. It plays a role in cellular maintenance and development, and has been implicated in a number of genetic diseases (20).
Entosis
Entosis is a nonapoptotic cell death process that occurs in human tumors and that is provoked by loss of attachment to matrix. This cell death mechanism is initiated by an unusual process involving the invasion of one live cell into another, followed by the degradation of internalized cells by lysosomal enzymes (21). This cell death process and apoptosis can both result in the internalization of one cell inside of another; the mechanisms responsible for cell internalization are highly distinguishable. Unlike the phagocytic ingestion of apoptotic cells, cell internalization in suspension is not associated with caspase activation nor driven by phosphatidylserine exposure (22).
Paraptosis
Paraptosis, an alternative, nonapoptotic cell death program that may be induced by the insulin-like growth factor I receptor (among other inducers), is mediated by mitogen-activated protein kinases (MAPKs). Caspases are not activated in this process, nor are caspase inhibitors effective in blocking cell death (23). It is reported that TAJ/TROY, a member of the tumor necrosis factor receptor superfamily that induces nonapoptotic cell death by paraptosis, is accompanied by phosphatidylserine externalization and loss of the mitochondrial transmembrane potential and is independent of caspase activation (24).
Anoikis
One form of detachment-induced death is an apoptotic program termed anoikis that has been characterized in suspension cell cultures (25,26). Cell death induced by detachment from extracellular matrix functions as a luminal clearing mechanism during development (27) and also may function as a barrier to the development of carcinomas, which display luminal filling as a hallmark.
MECHANISMS OF CELL DEATH BY CURCUMIN
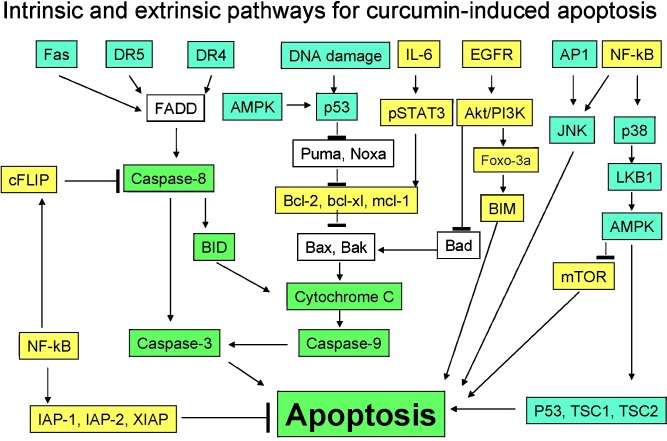
Curcumin has a diverse range of molecular targets, supporting the concept that it acts upon numerous biochemical and molecular cascades. Curcumin physically binds to as many as 33 different proteins, including thioredoxin reductase, cyclooxygenase-2, (COX2), protein kinase C, 5-lipoxygenase (5-LOX), and tubulin. Various molecular targets modulated by this agent include transcription factors, growth factors and their receptors, cytokines, enzymes, and genes regulating cell proliferation, and apoptosis (6). Curcumin has been shown to inhibit the proliferation and survival of almost all types of tumor cells. Accumulating evidence suggests that the mode of curcumin-induced cell death is mediated both by the activation of cell death pathways and by the inhibition of growth/proliferation pathways (Table I; Refs. 28–173). Many studies indicate the selective role of curcumin towards cancer cells than normal cells (Table II). We could identify more than 40 biomolecules that are involved in cell death induced by curcumin (Fig. 1). The mechanistic relationship among different signal transduction pathways, whether acting alone or together, leading to apoptosis is described. Because curcumin mediates its effect through multiple cell signaling pathways, the likelihood of developing resistance to it is less. How these interrelated pathways are activated is explained below.
Table I.
A List of Apoptotic and Growth Inhibitory Pathways Activated by Curcumin in Tumor Cells
| Pathways | References |
|---|---|
| Apoptotic pathways | |
| Caspase activation | (28–43) |
| Caspase-independent pathway | (28,42,44–46) |
| Induction of death receptors | (47–49) |
| Fas receptor aggregation | (41,50) |
| Induction of p53/p21 pathway | (51–60), |
| Release of apoptosis-releasing factor | (28,48,49,61–64) |
| Induction of DNA fragmentation | (65–76), |
| Suppression of Antiapoptotic proteins | (53,61,70,78–94) |
| Mitochondrial Activation | (28,29,31,32,35,45,47,51,53,61,65,67,69,71,83,88,95–117) |
| Autophagy | (118,119) |
| Induction of BIM | (46) |
| Activation of c-Jun kinase | (72,100,107,120–124) |
| Inhibition of IL6/STAT3 activation | (79,111,125) |
| Inhibition of NF-κB | (126–129) |
| Inhibition of Wnt/beta-catenin signaling | (130,131) |
| Growth inhibitory pathways | |
| Cell cycle regulation | (52,56,97,132,133) |
| Inhibition of PI3K-AKT activation | (134) |
| Inhibition of mTOR | (118,135,136) |
| Down-regulation of androgen receptors | (51,53,62,137–140), |
| Inhibition of growth factors and their receptors | (2,141,142) |
| Inhibition of AMP-activated protein kinase | (136,145) |
| Inhibition of COX2 and 5 LOX | (61,70,85,89,112,126–128,146–150) |
| Inhibition of Ornithine decarboxylase | (98,151) |
| Inhibition of Acidic Sphingomyelinase | (152,153) |
| Inhibition of phospholipase D | (134,152,154) |
| Activation of Thioredoxin reductase | (155–157) |
| Inhibition of p300-HAT | (158,159) |
| Intracellular [Ca(2+)](i) depletion | (117,160–162) |
| Binding and Inhibition of Glyoxylase | (163,164) |
| Binding to Microtubules | (165,166) |
| Proteasome activation | (167–169) |
| Prooxidant/antioxidant mechanism | (42,85,96,149,170–173) |
IL-6 Interleukin-6, STAT-3 signal transducer and activator of transcription 3, mTOR mammalian target of rapamycin, NF-κB nuclear factor-kappa B, COX-2 cyclooxygenase-2, 5-LOX 5-lipoxygenase, PI3K phosphoinositide 3-kinase, HAT histone acetyltransferase
Table II.
Modulation of Proliferation and Apoptosis of Tumor Cells by Curcumin
| Cell types | References |
|---|---|
| Tumor cells | |
| Hematological cancers | |
| Leukemia | |
| ALL | (174) |
| ATL | (108,125,175,176) |
| AML | (177) |
| Promyelocytic leukemia | (32,43,98,178–180) |
| Erythromyeloblastoid leukemia | (181) |
| Lymphoma | |
| Burkitt’s Lymphoma | (47,50) |
| Hodgkin’s lymphoma | (50,79) |
| Non-Hodgkin’s lymphoma | (59,150,182) |
| Follicular lymphoma | (183) |
| Primary effusion lymphoma | (111) |
| Multiple myeloma | (84,184,185) |
| Gastrointestinal cancers | |
| Esophagus | (186) |
| Intestine | (187) |
| Liver | (97,102,114,172,188–191) |
| Pancreas | (146,148) |
| Colorectal | (31,39,54,63,78,92,95,120,131,152,167,192–198) |
| Genitourinary cancer | |
| Bladder | (199,200) |
| Kidney | (191) |
| Prostrate | (2,53,70,76,91,124,137–139,201–204) |
| Brain tumors | (34,35,52,57,68,71,88,106,110,119,205–209) |
| Breast cancer | (58,65,121,123,143,172,180,210–212) |
| Gyneoncologic cancers | |
| Cervix | (191) |
| Ovary | (191,213) |
| Thoracic/H&N cancers | |
| Lung | (29,147,188,191,211,214,215) |
| Melanoma | (36,41,133,160,216,217) |
| Normal cells | |
| Endothelial cells | (38,218) |
| Lymphocytes | (59,219,220), |
| Hepatocytes | (96,163,172) |
| Fibroblasts | (78,83,147,221–223) |
| Thymocytes | (173) |
| Mammary epithelial cells | (224) |
ALL Acute lymphoblastic leukemia, ATL Acute T cell leukemia, AML acute myelogenous leukemia
Fig. 1.
Modulation of various cell death pathways by curcumin. Targets up-regulated by curcumin are in a blue box, those down-regulated are in a yellow box, and those unaffected are in a white box. AP-1 activator protein-1, AMPK 5' adenosine monophosphate-activated protein kinase, BID BH3 interacting domain death agonist, BIM BCL2-like 11 (apoptosis facilitator), cFLIP cellular FLICE-like inhibitory protein, FADD Fas-associated protein with Death Domain, DR4 death receptor 4, DR5 death receptor 5, EGFR epithelial growth factor receptor, IAP inhibitor of apoptosis protein, IL-6 interleukin-6, JNK c-Jun N-terminal kinase, mTOR mammalian target of rapamycin, NF-kB nuclear factor-kB, PI3K phosphoinositide 3-kinase, STAT3 signal transducer and activator of transcription 3, XIAP X-linked IAP
Caspase Activation
Caspases, or cysteine-aspartic acid proteases, are a family of cysteine proteases that play essential roles in apoptosis, necrosis, and inflammation. There are numerous reports that suggests the involvement of caspases in curcumin-induced apoptosis (28,30–35). Curcumin causes DNA damage and endoplasmic reticulum (ER) stress and mitochondrial-dependent-induced apoptosis through the activation of caspase-3 (29). Combined curcumin and TRAIL treatment enhanced accumulation of hypo-diploid U87MG cells in sub G1 cell cycle phase and induced the cleavage of procaspases-3, -8, -9, and release of cytochrome c from mitochondria (35). Earlier reports suggest that curcumin activates caspases-3 and -8 but not caspase-9, supporting the rationale that apoptosis occurs via a membrane-mediated mechanism. Both a caspase-8 and broad-based caspase inhibitor, but not a caspase-9 specific inhibitor, suppressed curcumin-induced cell death (41). Curcumin induces apoptosis through mitochondrial pathway involving caspase-8, BID cleavage, cytochrome c release, and caspase-3 activation in HL-60 cells (225).
Induction of Death Receptors
Death receptors are cell surface receptors that transmit apoptotic signals initiated by specific ligands such as Fas ligand, TNF-α, and TRAIL. These receptors play a crucial role in apoptosis and can activate a caspase cascade within seconds of ligand binding. Induction of apoptosis via this mechanism is therefore very rapid.
Studies showed that curcumin inhibited the expression of Bcl-2, Bcl-xL, survivin, and XIAP, and induced the expressions Bax, Bak, PUMA, Bim, and Noxa and death receptors (TRAIL-R1/DR4 and TRAIL-R2/DR5). Curcumin is a potent enhancer of TRAIL-induced apoptosis through upregulation of DR5 expression. Both treatment with DR5/Fc chimeric protein and silencing of DR5 expression using small interfering RNA (siRNA) attenuated curcumin plus TRAIL-induced apoptosis, showing that DR5 plays a critical role in this mode of cell death. Curcumin also induced the expression of a potential proapoptotic gene, C/EBP homologous protein (CHOP), both at its mRNA and protein levels (47,48). It has also been reported that curcumin significantly induces death receptor 5 (DR5) expression both at the mRNA and protein levels, accompanying the generation of the reactive oxygen species (ROS) (49).
Fas Receptor Aggregation
Fas receptor is the ligand for Fas-activated apoptosis. Binding of the ligand promotes receptor clustering, DISC formation and the activation of the caspase cascade. In addition, the Fas receptor is generally thought only to activate apoptosis and does not play an important role in other aspects of cell signaling like the TNF receptor. Curcumin induces Fas receptor aggregation in a FasL-independent manner, and low-temperature incubation, previously shown to inhibit receptor aggregation, prevented curcumin-induced cell death (41). Apoptosis in nasopharyngeal carcinoma cell line NCE induced by curcumin was found to up-regulate the Fas receptor gene as well as the protein in a dose-dependent manner (73). Curcumin is able to inhibit the proliferation of CA46 cells and induce apoptosis by down-regulating the expression of c-myc, Bcl-2, and mutant-type p53, and up-regulating the expression of Fas (50). Curcumin induced the modulation of cell cycle and apoptosis in gastric and colon cancer cells and stimulated the activity of caspase-8, which initiates Fas signaling pathway of apoptosis (197).
Induction of p53/p21 Pathway
The transcription factor p53 has been reported to play a very important role in apoptosis (226,227). As a tumor suppressor, p53 is responsible for protecting cells from tumorigenic alterations (227,228). Mutational inactivation of p53 is frequently observed in various human cancers (229). Studies on cell death on p21(+/+) and p21(−/−) HCT-116 cells treated with curcumin showed an associated reduction in pro-caspase-3 and PARP-1 cleavage, which are indicative of apoptosis, and concluded that curcumin-induced apoptosis in HCT-116 colon cancer cells does not depend on p21 status (78). The inhibition of p21 WAF1/CIP1 by siRNA blocks curcumin-induced apoptosis, thus establishing a link between cell cycle and apoptosis (51). Results of Liu et al. (52) demonstrated that ING4 expression was almost undetectable in U251 cells, but significantly up-regulated during cell cycle arrest induced by curcumin, and p53 expression was up-regulated followed by induction of p21 WAF1/CIP1 and ING4. Experiments using p53-null as well as dominant-negative and wild-type p53-transfected cells have established that curcumin induces apoptosis in carcinoma cells via a p53-dependent pathway. The studies showed that curcumin selectively increases p53 expression at G2 phase of carcinoma cells and releases cytochrome c from mitochondria, which is an essential requirement for apoptosis (56).
Release of Apoptosis-Inducing Factor
Apoptosis-inducing factor (AIF) is a mitochondrial apoptogenic protease that up on an apoptotic stimulus translocates from mitochondria to cytosol and further to the nucleus, where it triggers chromatin condensation and large-scale DNA fragmentation (230). Curcumin-mediated apoptosis in HeLa, SiHa, and Ca Ski cells appears to be due to upregulation of proapoptotic Bax, AIF, release of cytochrome c and down-regulation of antiapoptotic Bcl-2, Bcl-xL (61). It was shown for the first time by Thayyullathil et al. that curcumin-induced rapid reactive oxygen species (ROS) generation causes the release of AIF from the mitochondria to the cytosol and nucleus, hence, leading to caspase 3-independent apoptosis (28). It is also reported that curcumin-induced release of cytochrome c, a second mitochondria-derived activator of caspase (Smac) and AIF, was also blocked in Bax−/− cells (63).
Cell Cycle Regulation
The cell cycle is set of events, resulting in cell growth and division into two daughter cells. The stages are G1-S-G2-M. The G1 stage is "GAP 1". The S stage is "Synthesis", this is the stage when DNA replication occurs. The G2 stage is "GAP 2". The M stage is "mitosis", and is when nuclear and cytoplasmic division occurs. Curcumin was found to induce G0/G1 and/or G2/M phase cell cycle arrest, up-regulate CDKIs, p21WAF1/CIP1, p27KIP1, and p53, and slightly down-regulate cyclin B1 and cdc2 in ECV304 cells (231). The cyclin D1 proto-oncogene is an important regulator of G1 to S phase transition in numerous cell types from diverse tissues. A study has demonstrated that curcumin induces apoptosis in the G2 phase of the cell cycle in deregulated cyclin D1-expressing mammary epithelial carcinoma cells, leaving its normal cells unaffected (56). Curcumin induced the expression of cyclin-dependent kinase (CDK) inhibitors p16(/INK4a), p21 WAF1/CIP1, and p27(/KIP1), and inhibited the expression of cyclin E and cyclin D1, and hyperphosphorylation of retinoblastoma (Rb) protein (51). Curcumin induces the degradation of cyclin E expression through the ubiquitin-dependent pathway and up-regulates cyclin-dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines (6). In human mantle cell lymphoma, curcumin causes cell cycle arrest at the G1/S phase of the cell cycle and induces apoptosis (150). Curcumin enhances the expression of tumor cyclin-dependent kinase (CDK) inhibitors p21 and p27 as well as tumor suppressor protein p53 but suppressed the expression of retinoblastoma protein and also induced the accumulation of cells in G1 phase of the cell cycle in multiple human tumor cell lines (232). A recent report shows the activation of ATM/Chk1 by curcumin leads to G2/M cell cycle arrest and apoptosis in pancreatic cancer cells (233)
Inhibition of PI3K-AKT Activation
Akt (protein kinase B), a serine/threonine kinase, is a critical enzyme in signal transduction pathways involved in cell proliferation, apoptosis and angiogenesis. Studies showed that curcumin concentration- and time-dependently inhibited the phosphorylation of Akt, mTOR, and their downstream substrates in human prostate cancer PC-3 cells, and this inhibitory effect acts downstream of phosphatidylinositol 3-kinase and phosphatidylinositol-dependent kinase 1(234). Curcumin causes dose-dependent apoptosis and DNA fragmentation of Caki cells, which is preceded by the sequential dephosphorylation of Akt, down-regulation of the antiapoptotic Bcl-2, Bcl-xL, and IAP proteins, release of cytochrome c and activation of caspase 3 (134). Curcumin-induced effects have been shown to be associated with the suppression of NF-κB and IKK activities but independent of the B-Raf/MEK/ERK and Akt pathways in melanoma cell lines (235).
Inhibition of mTOR
mTOR is a large (>250 kDa) class IV PI-3 kinase family member with protein kinase activity. mTOR forms a complex with the 12-kDa cytosolic protein, FKBP-12, and rapamycin and functions to arrest the cell cycle in the G1 phase. mTOR regulates Akt activity, a crucial downstream effector in the PI-3K–PTEN pathway, which controls cell proliferation and survival. Targeting this function of mTOR may also have therapeutic potential. For example, curcumin was shown to inhibit the Akt/mammalian target of rapamycin/p70 ribosomal protein S6 kinase pathway and activate the extracellular-signal-regulated kinases (ERK) 1/2, thereby inducing autophagy (118).
Down-Regulation of Androgen Receptors
Various studies evaluated the role of curcumin in cell growth and activation of signal transduction pathways in both androgen-dependent and -independent prostate cancer cell lines (35,53,62,138,139). The results showed that curcumin down-regulated transactivation and expression of AR, AP-1, NF-κB, and CBP. These studies showed that curcumin has enormous potential as an anticancer agent against prostate cancer (140). Curcumin enhanced the apoptosis-inducing potential of TRAIL in androgen-unresponsive PC-3 cells and sensitized androgen-responsive TRAIL-resistant LNCaP cells (62).
Inhibition of Growth Factors and their Receptors
Growth factors play a critical role in the proliferation of tumor cells. EGF, HER2, FGF, VEGF, PDGF, insulin growth factor (IGF)-1, and others have been associated with cell proliferation. The suppression of these growth factors or their receptors results in suppression of tumor growth. For example, the inhibition of EGFR expression and decreased ERK1/2 activity decreased survival and enhanced induction of apoptosis in lung and pancreatic adenocarcinoma cells (148). Curcumin inhibited EGF-stimulated phosphorylation of EGFR in MDA-MB-468 cells and phosphorylation of ERK 1 and -2, as well as ERK activity and levels of nuclear c-fos in MDA-MB-468 and HBL100 cells (143). Recently it was reported that curcumin inhibits 253JB-V and KU7 bladder cancer cell growth, which could be attributed to induction of apoptosis and decreased expression of the proapoptotic protein survivin and VEGF and VEGFR1 (144)
Inhibition of AMP-Activated Protein Kinase (AMPK)
AMP-activated protein kinase is a heterotrimeric serine/threonine protein kinase comprising a catalytic subunit (α) and two regulatory subunits (β and γ). Curcumin strongly activates AMPK in a p38-dependent manner in CaOV3 ovarian cancer cells, thus inducing cell death (145). Stimulation of AMPK by curcumin down-regulated peroxisome proliferator-activated receptor-gamma (PPAR-γ) in 3T3-L1 adipocytes and decreased COX2 expression in MCF-7 cells, which in turn affects the proliferation rate.
Inhibition of COX2 and 5 LOX
Cyclooxygenase-2 (COX), also known as prostaglandin (PG) H2 synthase, is the rate-limiting enzyme in the conversion of arachidonic acid into PGs. The two known forms of COX are referred to as COX1 and COX2. Overexpression of COX2 has been frequently observed in colon tumors, and COX2 plays a major role in colon carcinogenesis Curcumin was found to modulate both the expression and the activity of these enzymes. A number of studies were carried out to establish the role of curcumin in mediating COX2 and 5 LOX (61,70,85,89,112,126–128,146–150). In human colon epithelial cells, curcumin inhibits COX2 induction by the colon tumor promoters tumor necrosis factor alpha and fecapentaene-12, thus inhibiting proliferation and inducing apoptosis (236). Curcumin has been shown to regulate the eicosanoid pathway involving COX and LOX. The details of how 5LOX and COX2 are regulated by curcumin are not fully established; however, we do know that curcumin regulates LOX and COX2 predominately at the transcriptional level and, to a certain extent, the posttranslational level (237). There is a report that the inhibition of COX2 and Wnt/EGFR/NF-κB-signaling pathways induces apoptosis in colon cancer cells (238).
Inhibition of Ornithine Decarboxylase
A recent study has shown that a chemopreventive effect of curcumin could be due to the hyperproduction of ROS, which would in turn induce apoptosis in tumor cells. The study found that enzyme activity and protein expression of ornithine decarboxylase (ODC) were reduced during curcumin treatment. Overexpression of ODC in human promyelocytic leukemia HL-60 parental cells could reduce curcumin-induced apoptosis, which would lead to loss of mitochondrial membrane potential, through reducing intracellular ROS. Moreover, ODC over expression prevented cytochrome c release and the activation of caspase-9 and caspase-3 following curcumin treatment (98). An earlier study showed the effects of curcumin on renal carcinoma. In the study, Fe-NTA, a known complete renal carcinogen, which generates ROS in vivo, was given intraperitoneally to mice, and curcumin was tested for its ability to inhibit oxidative stress and the ODC activity and histopathological changes in the kidney. ODC activity in the kidney was significantly increased but remained at normal levels in curcumin-pretreated mice (239).
Inhibition of Acidic Sphingomyelinase
Curcumin's apoptosis-inducing effects in colon cancer cell lines are accompanied by ceramide generation. This increase occurs through de novo synthesis, as much as the increase in ceramide could be attenuated by pre-incubation of the cells with myriocin, and no changes were observed in sphingomyelin levels or in either acidic or neutral sphingomyelinase activities. However, the inhibition of ROS using N-acetylcysteine led to an inhibition of JNK activation. Hence, the study concluded that curcumin induces apoptosis via a ROS-associated mechanism that converges on JNK activation, and to a lesser extent via a parallel ceramide-associated pathway (153). Curcumin reduced the hydrolytic capacity of the cells against choline-labeled sphingomyelin, associated with a mild increase in cellular sphingomyelin in the cells. Curcumin also inhibited acid sphingomyelinase an effect that may account for its antiproliferative effects against colon cancer cells (152).
Inhibition of Phospholipase D
The enzymatic activity of phospholipase D (PLD) is known to be essential for cell survival and protection from apoptosis. During apoptosis, a small fraction of PLD1 is cleaved by caspases in a p53-independent manner and NF-PLD1 amplifies apoptotic signaling through inhibition of the remaining PLD1 activity (217). In a cell-free system, curcumin inhibited several types of phospholipases, most effectively PLD. It also inhibited 12-O-tetradecanoylphorbol-13-acetate-induced PLD activation in intact J774.1 cells in a dose-dependent manner (154).
Activation of Thioredoxin Reductase
The thioredoxin reductase (TrxR) isoenzymes, TrxR1 in cytosol or nucleus and TrxR2 in mitochondria, are essential mammalian selenocysteine (Sec)-containing flavoenzymes with a -Gly-Cys-Sec-Gly active site. TrxRs are the only enzymes catalyzing the NADPH-dependent reduction of the active site disulfide in thioredoxins (Trxs), which play essential roles in substrate reductions, defense against oxidative stress, and redox regulation by thiol redox control. Trx can scavenge reactive oxygen species (ROS) and directly inhibits proapoptotic proteins such as apoptosis signal-regulating kinase 1 (ASK1). There are a number of inhibitors with chemotherapy applications that target either Trx or TrxR to induce apoptosis in cancer cells. A study has shown that rat TrxR1 activity in Trx-dependent disulfide reduction was inhibited by curcumin (156). Different curcumin analogs were also investigated for their inhibitory effects on thioredoxin reductase (TrxR). Most of them were more potent TrxR inhibitors than natural curcumin (157).
Inhibition of STAT3 Activation
Signal transducers and activators of transcription (STATs) play important roles in numerous cellular events, including differentiation, inflammation, and the immune response. Furthermore, constitutive STAT activation can be observed in a high number of tumors. Curcumin is incorporated into H-RS cells and inhibits both NF-κB and STAT3 activation, leading to decreased expression of proteins involved in cell proliferation and apoptosis, e.g., Bcl-2, Bcl-xL, cFLIP, XIAP, c-IAP1, survivin, c-myc, and cyclin D1 (79). Curcumin treatment induced a decrease of nuclear STAT3, -5a, and -5b, without affecting either STAT1 or the phosphorylation state of STAT1, -3 or -5 in the K562 cell line. Most interestingly, the decrease of nuclear STAT5a and -5b after curcumin treatment was accompanied by an increase of truncated STAT5 isoforms, indicating that curcumin is able to induce the cleavage of STAT5 into its dominant-negative variants lacking the STAT5 C-terminal region (240). The constitutive phosphorylation of STAT3 found in certain multiple myeloma cells was abrogated by treatment with curcumin (241), and inhibition of STAT3 by curcumin leads to the induction of apoptosis (185)
Activation of c-Jun Kinase
In vitro experiments indicated that inhibition of c-Jun/AP-1 binding to its cognate motif by curcumin may be responsible for the inhibition of c-Jun/AP-1-mediated gene expression (242). Curcumin, a natural inhibitor of JNK signaling, protected the HT22 cells from glutamate-induced death at nanomolar concentrations (243). Curcumin time- and dose-dependent induction of apoptosis was accompanied by sustained phosphorylation and activation of c-jun N-terminal kinase (JNK) and p38 MAPK as well as inhibition of constitutive NF-κB transcriptional activity (195).
Induction of DNA Fragmentation
Curcumin inhibits activation of Vgamma9Vdelta2 T cells by phosphoantigens and induces apoptosis involving apoptosis-inducing factor and large-scale DNA fragmentation. This cytotoxicity was associated with increased annexin V reactivity, nuclear expression of active caspase-3, cleavage of poly(ADP-ribose) polymerase, translocation of apoptosis-inducing factor to the nucleus, and morphological evidence of nuclear disintegration (244). Curcumin activates the apoptotic pathway in human renal Caki cells. Treatment of Caki cells with 50 μM curcumin resulted in the activation of caspase 3, cleavage of phospholipase C-γ1, and DNA fragmentation (134). Curcumin treatment protected cells from UVC-induced oligonucleosomal DNA degradation. Experiments using recombinant DFF activated with caspase-3 showed that curcumin inhibits plasmid DNA and chromatin degradation although it does not prevent activation of DFF40/CAD endonuclease after its release from the inhibitor (33). Curcumin-treated Jurkat cells exhibited DNA splitting into high- but not low-molecular-weight fragments. These cells retained their high mitochondrial Delta psi, and the content of Ca2+ in endoplasmic reticulum stores remained at the level typical for untreated cells (45).
Direct DNA Damage
Drug- or radiation-induced injury to DNA may produce deviations in DNA’s normal double helical conformation. These changes include structural distortions that interfere with replication and transcription, as well as point mutations that disrupt base pairing and exert damaging effects on future generations through changes in DNA sequence. If the damage is minor, it can often be repaired (DNA repair). If the damage is extensive, it can induce apoptosis. Curcumin induces such DNA damage to both the mitochondrial and nuclear genomes in human hepatoma G2 cells. The study demonstrated dose-dependent damage in both the mitochondrial and nuclear genomes and the greater extent of the mitochondrial damage. The mechanism underlies the elevation in ROS and lipid peroxidation generated by curcumin (245). Studies with mouse–rat hybrid retina ganglion cell line N18 cells showed a dose- and time-dependent increase in DNA damage with curcumin treatment, which was confirmed by comet assay as well as agarose gel electrophoresis (246).
Intracellular [Ca (2±)](i) Depletion
Curcumin induced a marked depletion of [Ca(2+)](i) in Caki cells bathed with both Ca(2+)-containing and -free solutions. This indicates that curcumin acts as a stimulator of intracellular Ca(2+) uptake into mitochondria via uniporter pathway and may involve the execution of apoptosis (162). Reports suggest that curcumin may induce the expression of the HSP70 gene through the initial depletion of intracellular Ca(+2), followed by the suppression of p53 gene function in the target cells (247).
Mitochondrial Activation
Mitochondria play a pivotal role in the process of apoptosis. The intrinsic pathway of apoptosis involves the activation of proapoptotic members of the Bcl-2 family that exert their function through mitochondria. Role of mitochondria is well established in a curcumin-induced apoptosis (28,31,32,47,53,61,65,67,69,88,95,97–118). Curcumin induces the release of cytochrome c from mitochondria, causing activation of caspase 3 and concomitant PARP cleavage, which is the hallmark of caspase-dependent apoptosis (28). Curcumin-induced rapid ROS generation causes the release of AIF from the mitochondria to the cytosol and nucleus, hence leading to caspase 3-independent apoptosis (69). HepG2 cells exposed to curcumin for 1 h showed a transient elevation of mitochondrial membrane potential (MMP), followed by cytochrome c release into the cytosol and disruption of MMP after 6 h exposure to curcumin. These results suggest that mitochondrial hyperpolarization is a prerequisite for curcumin-induced apoptosis and that mtDNA damage is the initial event triggering a chain of events leading to apoptosis in HepG2 cells (102). A study on curcumin protection of PC12 cells against MPP (+)-induced apoptosis suggested that the cytoprotective effects of curcumin might be mediated by the Bcl-2-mitochondria-ROS-iNOS pathway (108). Curcumin induced an increase in rat liver mitochondrial membrane permeability, resulting in swelling, loss of membrane potential and inhibition of ATP synthesis. These effects were mediated by the opening of the permeability transition pore (117). Curcumin targets proliferative cells more efficiently than differentiated cells and induces apoptosis via mitochondrial pathways. Addition of curcumin to neuro 2a cells induces a rapid decrease in mitochondrial membrane potential and the release of cytochrome c into cytosol, followed by activation of caspase-9 and caspase-3 (116).
Binding and Inhibition of Glyoxalase
Glyoxalases (Glo1 and Glo2) are involved in the glycolytic pathway by detoxifying the reactive methylglyoxal (MGO) into d-lactate in a two-step reaction using glutathione (GSH) as cofactor. Inhibitors of glyoxalases are considered antiinflammatory and anti-tumor agents. Curcumin inhibits Glo1, resulting in non-tolerable levels of MGO and GSH. As a result, various metabolic pathways are disturbed, so that for example, cellular ATP and GSH content are depleted (163). The depletion of cellular ATP and GSH may in turn decrease cell survival.
Suppression of Antiapoptotic Proteins
The inhibitors of apoptosis (IAP) proteins are a recently discovered class of caspase inhibitors that selectively bind and inhibit caspase-3, -7, and -9; these inhibitors have great potential in the treatment of malignancy (248). Curcumin is reported to inhibit the expression of these caspases inhibitors both in vitro and in vivo (89). Other antiapoptotic proteins that are inhibited by curcumin include Bcl-2, Bcl-xL, X-linked inhibitors of apoptosis (XIAP), and survivin (61,77–94,127). Curcumin sensitizes prostate cancer cells to TRAIL by inhibiting Akt-regulated NF-κB and NF-kappaB-dependent antiapoptotic Bcl-2, Bcl-xL, and XIAP (91). Curcumin-induced apoptosis in human androgen-independent (DU145) and -dependent (LNCaP) prostate cancer cell lines (124) and HL-60 cells (225), which correlated with down-regulation of the expression of Bcl-2 and Bcl-xL
Binding to Microtubules
Curcumin may inhibit cancer cell proliferation by perturbing microtubule assembly dynamics. At higher inhibitory concentrations (>10 μM), curcumin induced significant depolymerization of interphase microtubules and mitotic spindle microtubules of HeLa and MCF-7 cells. However, at low inhibitory concentrations there were minimal effects on cellular microtubules. It disrupted microtubule assembly in vitro, reduced GTPase activity, and induced tubulin aggregation (165). In one study, curcumin-down-regulated Taxol induced phosphorylation of the serine/threonine kinase Akt, a survival signal which in many instances is regulated by NF-kappaB, but tubulin polymerization and cyclin-dependent kinase Cdc2 activation induced by Taxol was not affected by curcumin (249).
Proteasome Activation
It has been reported that curcumin-induced apoptosis is mediated through the impairment of ubiquitin proteasome system (UPS). Curcumin disrupts UPS function by directly inhibiting the enzyme activity of the proteasome's 20S core catalytic component. The direct inhibition of proteasome activity also causes an increase in half-life of IκBα that ultimately leads to the down-regulation of NF-κB activation (169), thus activating the apoptotic pathway. Exposure of mouse neuro 2a cells to curcumin causes a dose-dependent decrease in proteasome activity and an increase in ubiquitinated proteins. Curcumin targets proliferative cells more efficiently than differentiated cells and induces apoptosis via mitochondrial pathways (116).
Pro- and Antioxidant Mechanisms
Studies have shown that curcumin mediates its apoptotic and antiinflammatory activities through modulation of the redox status of the cell (42,149,169). TNF-mediated NF-κB activation was inhibited by curcumin; and glutathione reversed the inhibition. Glutathione also counteracted the inhibitory effects of curcumin on TNF-induced NF-κB-regulated antiapoptotic (Bcl-2, Bcl-xL, IAP1), proliferative (cyclin D1), and proinflammatory (COX2, iNOS, and MMP-9) gene products (85). Reports suggest that curcumin modifies TrxR by shifting the enzyme from an antioxidant to a prooxidant (156). Low concentrations of curcumin may protect hepatocytes by reducing lipid peroxidation and cytochrome c release. Conversely, higher concentrations provoke glutathione depletion, caspase-3 activation, and hepatocytotoxicity (96). Curcumin pretreatment significantly decreased ROS and resulted in survival of copper-injured BRL cells, possibly by anti-oxidation and inhibition of p-JNK expression (170). Curcumin induces apoptosis via ROS generation in malignant cells, but not in normal cells (172). In Jurkat cells, curcumin prevents glutathione decrease, thus protecting cells against caspase-3 activation and oligonucleosomal DNA fragmentation (42). In normal cells, curcumin induces apoptosis in a glutathione-independent pathway (173).
Autophagy
Autophagy is a response of cancer cells to various anticancer therapies. It is designated programmed cell death type II and characterized by the formation of autophagic vacuoles in the cytoplasm. In one study, curcumin induced G2/M arrest and nonapoptotic autophagic cell death in U87-MG and U373-MG malignant glioma cells. It inhibited the Akt/mTOR/p70S6K pathway and activated the ERK1/2 pathway, resulting in induction of autophagy. In the subcutaneous xenograft model of U87-MG cells, curcumin inhibited tumor growth significantly (P < 0.05) and induced autophagy (119). It inhibited the Akt/mammalian target of rapamycin/p70 ribosomal protein S6 kinase pathway and activated the extracellular-signal-regulated kinases and induced autophagy (118).
Inhibition of NF-κB
The transcription factor NF-κB is constitutively expressed in almost all cancer types and suppresses apoptosis in a wide variety of tumors. The constitutive expression of NF-κB has been reported in human cancer cell lines in culture, carcinogen-induced mouse mammary tumors, and biopsies from cancer patients. There are various reports that curcumin inhibits the activation of NF-κB by inducers such as TNF, H2O2. phorbol ester, cigarette smoke condensate, interleukin (IL)-1, 12-O-tetradecanoylphorbol-13-acetate (TPA), and anticancer drugs (6) . Curcumin inhibits the activation of NF-κB and the expression of various oncogenes regulated by NF-κB, including c-jun, c-fos, c-myc, NIK, MAPKs, ERK, ELK, PI3K, Akt, CDKs, and iNOS (250). Curcumin prevents the entry of NF-κB into the nucleus, thereby decreasing the expression of cell cycle regulatory proteins and survival factors such as Bcl-2 and survivin. Curcumin arrested the cell cycle by preventing the expression of cyclin D1, cdk-1, and cdc-25 (126). In hepatic cancer HA22T/VGH cell line, curcumin inhibited constitutively activated NF-κB and NF-κB regulated gene products such as apoptosis proteins (IAPs) and other target genes (128). It inhibits TNF-induced NF-κB-regulated gene products involved in cellular proliferation (COX2, cyclin D1, and c-myc), antiapoptosis (IAP1, IAP2, XIAP, Bcl-2, and Bcl-xL; 127). Curcumin could prevent tumor-induced thymic atrophy by restoring the activity of NF-κB. Further investigations suggest that neutralization of tumor-induced oxidative stress and restoration of NF-κB activity along with the reduction of the TNF-α signaling pathway can be the mechanism behind curcumin-mediated thymic protection (251). Curcumin down-regulated NF-κB in human multiple myeloma cells, leading to the suppression of proliferation and induction of apoptosis (184)
Inhibition of Wnt/beta-catenin Signaling
The Wnt/beta-catenin signaling pathway plays a major role in development, tissue homeostasis, and regeneration. The deregulation of this pathway is found in various human cancers. Numerous reports suggest that curcumin is a good inhibitor of b-catenin/Tcf signaling in gastric, colon, and intestinal cancer cells (131,231). It has been reported that inhibition of Wnt-2 had synergistic effects on suppressing Wnt signaling and inducing apoptosis, suggesting that aberrant canonical Wnt/beta-catenin signaling in colorectal cancer can be regulated at multiple levels (252). Thus, the proapoptotic effects of curcumin could be mediated through this pathway
Activation of Nrf2
Nuclear erythroid 2 p45-related factor 2 (Nrf2) is a redox-sensitive basic leucine zipper transcription factor that is involved in the transcriptional regulation of many antioxidant genes. The Nrf2/antioxidant response element (ARE) signaling pathway plays a key role in activating cellular antioxidants, including heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase-1 (NQO1), and glutathione. Curcumin treatment results in ROS generation, activation of Nrf2 and MAP kinases and the inhibition of phosphatase activity in hepatocytes, and when curcumin is not administered in toxic doses, these multiple pathways converge to induce HO-1 (253). Another study further suggests that curcumin has the ability to induce HO-1 expression, presumably through Nrf2-dependent ARE activation in rat vascular smooth muscle cells and human aortic smooth muscle cells, and provide evidence that the antiproliferative effect of curcumin is considerably linked to its ability to induce HO-1 expression (254).
Inhibition of hTERT
Another possible activity of curcumin able to induce cell death processes is the inhibition of hTERT, the active subunit of telomerase. Increasing concentrations of curcumin caused a decrease in the level of hTERT mRNA in MCF-7 cells. hTERT is activated in cancer cells and prevents telomere shortening and thus the activation of apoptotic processes. The inhibition of hTERT is an additional mechanism by which curcumin can induce cell death in cancer cells (255)
EFFECT OF CURCUMIN ON NORMAL CELLS
Curcumin has been shown to have differential effects on normal cells such as endothelial cells, lymphocytes, hepatocytes, fibroblasts, thymocytes, and mammary epithelial cells (78,96,110–115,163,172,173,224). Why curcumin kills tumor cells and not normal cells is not fully understood, but several reasons have been suggested. First, absorption and fluorescence spectroscopic methods showed that cellular uptake of curcumin is higher in tumor cells than in normal cells (256). The studies also showed that curcumin was maximally distributed in the cell membrane and the nucleus. Second, the glutathione levels in tumor cells tend to be lower than normal cells, thus enhancing the sensitivity of tumor cells to curcumin (172). Third, most tumor cells, but not normal cells, express constitutively active NF-κB and mediate their survival (150). Curcumin can suppress the survival and proliferation of tumor cells by suppressing NF-κB-regulated gene products. Apart from this, human epidermal keratinocytes have been shown to undergo apoptosis when exposed to curcumin through the inhibition of AP-1 (104). Low concentrations of curcumin may protect hepatocytes by reducing lipid peroxidation and cytochrome c release. Conversely, higher concentrations provoke glutathione depletion, caspase-3 activation, and hepatocytotoxicity (96). Interestingly, curcumin had no effect on normal rat hepatocytes, which showed no superoxide generation and therefore no cell death (172). Primary cultures of human dermal fibroblasts were less sensitive to lower doses of curcumin (78). Studies have shown that curcumin causes fibroblast apoptosis and that this could be inhibited by co-administration of antioxidants N-acetyl-l-cysteine , biliverdin or bilirubin, suggesting that ROS are involved (221). In endothelial cells, curcumin inhibited MAP kinase activity and enhanced TNF-induced apoptosis (218). Studies suggests that curcumin induces the apoptosis in human retinal endothelial cells by the regulation of intracellular ROS generation, VEGF expression and release, and VEGF-mediated PKC-beta II translocation (257). Curcumin can induce cell death in both quiescent and proliferating normal human lymphocytes, without oligonucleosomal DNA degradation, which is considered a main hallmark of apoptotic cell death (258). Curcumin induces cell death in lymphocytes that can be classified as apoptosis-like cell death (220).
CONCLUSION
Overall, our review shows that curcumin can kill a wide variety of tumor cell types through diverse mechanisms. Because of numerous mechanisms of cell death employed by curcumin, it is possible that cells may not develop resistance to curcumin-induced cell death. Furthermore, its ability to kill tumor cells and not normal cells makes curcumin an attractive candidate for drug development. Although numerous animal studies and clinical trials have been done, additional studies are needed to gain the full benefit from curcumin.
ACKNOWLEDGMENTS
We thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments. Dr. Aggarwal is Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267:133–64. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Vogel P (1815) J Pharm 50
- 4.Daybe FV (1870) Uber Curcumin, den Farbstoff der Curcumawurzzel. 609
- 5.Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull (Tokyo) 1993;41:1640–3. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102:580–92. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 7.Huang MT, Wang ZY, Georgiadis CA, Laskin JD, Conney AH. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7, 12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13:2183–6. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 8.Conney AH, Lysz T, Ferraro T, et al. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv Enzyme Regul. 1991;31:385–96. doi: 10.1016/0065-2571(91)90025-H. [DOI] [PubMed] [Google Scholar]
- 9.Huang MT, Smart RC, Wong CQ, Conney AH. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–6. [PubMed] [Google Scholar]
- 10.Bilmen JG, Khan SZ, Javed MH, Michelangeli F. Inhibition of the SERCA Ca2+ pumps by curcumin. Curcumin putatively stabilizes the interaction between the nucleotide-binding and phosphorylation domains in the absence of ATP. Eur J Biochem. 2001;268:6318–27. doi: 10.1046/j.0014-2956.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- 11.Barry J, Fritz M, Brender JR, Smith PE, Lee DK, Ramamoorthy A. Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin. J Am Chem Soc. 2009;131:4490–8. doi: 10.1021/ja809217u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epand RF, Martinou JC, Fornallaz-Mulhauser M, Hughes DW, Epand RM. The apoptotic protein tBid promotes leakage by altering membrane curvature. J Biol Chem. 2002;277:32632–9. doi: 10.1074/jbc.M202396200. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/S0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 17.Fadeel B, Orrenius S, Zhivotovsky B. Apoptosis in human disease: a new skin for the old ceremony? Biochem Biophys Res Commun. 1999;266:699–717. doi: 10.1006/bbrc.1999.1888. [DOI] [PubMed] [Google Scholar]
- 18.Wyllie AH, Beattie GJ, Hargreaves AD. Chromatin changes in apoptosis. Histochem J. 1981;13:681–92. doi: 10.1007/BF01002719. [DOI] [PubMed] [Google Scholar]
- 19.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 20.Stromhaug PE, Klionsky DJ. Approaching the molecular mechanism of autophagy. Traffic. 2001;2:524–31. doi: 10.1034/j.1600-0854.2001.20802.x. [DOI] [PubMed] [Google Scholar]
- 21.Overholtzer M, Mailleux AA, Mouneimne G, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 23.Sperandio S, Poksay K, de Belle I, et al. Paraptosis: mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004;11:1066–75. doi: 10.1038/sj.cdd.4401465. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li X, Wang L, et al. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci. 2004;117:1525–32. doi: 10.1242/jcs.00994. [DOI] [PubMed] [Google Scholar]
- 25.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM regulates apoptosis during mammary ductal morphogenesis, and its absence reveals alternative cell death mechanisms. Dev Cell. 2007;12:221–34. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 2008;45:1403–12. doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Lin SS, Huang HP, Yang JS et al. (2008) DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett [DOI] [PubMed]
- 30.Park K, Lee JH. Photosensitizer effect of curcumin on UVB-irradiated HaCaT cells through activation of caspase pathways. Oncol Rep. 2007;17:537–40. [PubMed] [Google Scholar]
- 31.Su CC, Lin JG, Li TM, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–89. [PubMed] [Google Scholar]
- 32.Tan TW, Tsai HR, Lu HF, et al. Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res. 2006;26:4361–71. [PubMed] [Google Scholar]
- 33.Sikora E, Bielak-Zmijewska A, Magalska A, et al. Curcumin induces caspase-3-dependent apoptotic pathway but inhibits DNA fragmentation factor 40/caspase-activated DNase endonuclease in human Jurkat cells. Mol Cancer Ther. 2006;5:927–34. doi: 10.1158/1535-7163.MCT-05-0360. [DOI] [PubMed] [Google Scholar]
- 34.Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–74. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- 35.Gao X, Deeb D, Jiang H, Liu YB, Dulchavsky SA, Gautam SC. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol. 2005;5:39–48. [PubMed] [Google Scholar]
- 36.Qiu S, Tan SS, Zhang JA, et al. Apoptosis induced by curcumin and its effect on c-myc and caspase-3 expressions in human melanoma A375 cell line. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:1517–21. [PubMed] [Google Scholar]
- 37.Wu Q, Chen Y, Li XG. Effect of curcumin on caspase 8- and caspase 9-induced apoptosis of lymphoma Raji cell. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13:624–7. [PubMed] [Google Scholar]
- 38.Belakavadi M, Salimath BP. Mechanism of inhibition of ascites tumor growth in mice by curcumin is mediated by NF-kB and caspase activated DNase. Mol Cell Biochem. 2005;273:57–67. doi: 10.1007/s11010-005-7717-2. [DOI] [PubMed] [Google Scholar]
- 39.Rashmi R, Santhosh Kumar TR, Karunagaran D. Human colon cancer cells differ in their sensitivity to curcumin-induced apoptosis and heat shock protects them by inhibiting the release of apoptosis-inducing factor and caspases. FEBS Lett. 2003;538:19–24. doi: 10.1016/S0014-5793(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 40.Anto RJ, Maliekal TT, Karunagaran D. L-929 cells harboring ectopically expressed RelA resist curcumin-induced apoptosis. J Biol Chem. 2000;275:15601–4. doi: 10.1074/jbc.C000105200. [DOI] [PubMed] [Google Scholar]
- 41.Bush JA, Cheung KJ, Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–14. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 42.Piwocka K, Jaruga E, Skierski J, Gradzka I, Sikora E. Effect of glutathione depletion on caspase-3 independent apoptosis pathway induced by curcumin in Jurkat cells. Free Radic Biol Med. 2001;31:670–8. doi: 10.1016/S0891-5849(01)00629-3. [DOI] [PubMed] [Google Scholar]
- 43.Pan MH, Chang WL, Lin-Shiau SY, Ho CT, Lin JK. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J Agric Food Chem. 2001;49:1464–74. doi: 10.1021/jf001129v. [DOI] [PubMed] [Google Scholar]
- 44.Piwocka K, Bielak-Mijewska A, Sikora E. Curcumin induces caspase-3-independent apoptosis in human multidrug-resistant cells. Ann N Y Acad Sci. 2002;973:250–4. doi: 10.1111/j.1749-6632.2002.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 45.Piwocka K, Zablocki K, Wieckowski MR, et al. A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp Cell Res. 1999;249:299–307. doi: 10.1006/excr.1999.4480. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh AK, Kay NE, Secreto CR, Shanafelt TD. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 2009;15:1250–8. doi: 10.1158/1078-0432.CCR-08-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain AR, Ahmed M, Al-Jomah NA, et al. Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt's lymphoma cell lines. Mol Cancer Ther. 2008;7:3318–29. doi: 10.1158/1535-7163.MCT-08-0541. [DOI] [PubMed] [Google Scholar]
- 48.Jung EM, Park JW, Choi KS, Lee HI, Lee KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–17. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- 49.Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–13. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Chen Y, Xu J, Lu L. Anticancer activities of curcumin on human Burkitt's lymphoma. Zhonghua Zhong Liu Za Zhi. 2002;24:348–52. [PubMed] [Google Scholar]
- 51.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell Cycle. 2007;6:2953–61. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 52.Liu E, Wu J, Cao W, et al. Curcumin induces G2/M cell cycle arrest in a p53-dependent manner and upregulates ING4 expression in human glioma. J Neurooncol. 2007;85:263–70. doi: 10.1007/s11060-007-9421-4. [DOI] [PubMed] [Google Scholar]
- 53.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3'-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–18. [PubMed] [Google Scholar]
- 54.Song G, Mao YB, Cai QF, Yao LM, Ouyang GL, Bao SD. Curcumin induces human HT-29 colon adenocarcinoma cell apoptosis by activating p53 and regulating apoptosis-related protein expression. Braz J Med Biol Res. 2005;38:1791–8. doi: 10.1590/S0100-879X2005001200007. [DOI] [PubMed] [Google Scholar]
- 55.Tsvetkov P, Asher G, Reiss V, Shaul Y, Sachs L, Lotem J. Inhibition of NAD(P)H:quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc Natl Acad Sci U S A. 2005;102:5535–40. doi: 10.1073/pnas.0501828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280:20059–68. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 57.Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–98. [PubMed] [Google Scholar]
- 58.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–40. doi: 10.1016/S0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 59.Han SS, Chung ST, Robertson DA, Ranjan D, Bondada S. Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53. Clin Immunol. 1999;93:152–61. doi: 10.1006/clim.1999.4769. [DOI] [PubMed] [Google Scholar]
- 60.Jee SH, Shen SC, Tseng CR, Chiu HC, Kuo ML. Curcumin induces a p53-dependent apoptosis in human basal cell carcinoma cells. J Invest Dermatol. 1998;111:656–61. doi: 10.1046/j.1523-1747.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 61.Singh M, Singh N (2009) Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Mol Cell Biochem [DOI] [PubMed]
- 62.Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rashmi R, Kumar S, Karunagaran D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis. 2005;26:713–23. doi: 10.1093/carcin/bgi025. [DOI] [PubMed] [Google Scholar]
- 64.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Bcl-XL or Ku70 protects human colon cancer cells (SW480) against curcumin-induced apoptosis while their down-regulation potentiates it. Carcinogenesis. 2004;25:1867–77. doi: 10.1093/carcin/bgh213. [DOI] [PubMed] [Google Scholar]
- 65.Cheah YH, Nordin FJ, Sarip R, et al. Combined xanthorrhizol-curcumin exhibits synergistic growth inhibitory activity via apoptosis induction in human breast cancer cells MDA-MB-231. Cancer Cell Int. 2009;9:1. doi: 10.1186/1475-2867-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Priya S, Sudhakaran PR. Cell survival, activation and apoptosis of hepatic stellate cells: modulation by extracellular matrix proteins. Hepatol Res. 2008;38:1221–32. doi: 10.1111/j.1872-034X.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 67.Das R, Roy A, Dutta N, Majumder HK. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis. 2008;13:867–82. doi: 10.1007/s10495-008-0224-7. [DOI] [PubMed] [Google Scholar]
- 68.Freudlsperger C, Greten J, Schumacher U. Curcumin induces apoptosis in human neuroblastoma cells via inhibition of NFkappaB. Anticancer Res. 2008;28:209–14. [PubMed] [Google Scholar]
- 69.Hail N., Jr Mitochondrial reactive oxygen species affect sensitivity to curcumin-induced apoptosis. Free Radic Biol Med. 2008;44:1382–93. doi: 10.1016/j.freeradbiomed.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 70.Hoque A, Chen H, Xu XC. Statin induces apoptosis and cell growth arrest in prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 2008;17:88–94. doi: 10.1158/1055-9965.EPI-07-0531. [DOI] [PubMed] [Google Scholar]
- 71.Zhu YG, Chen XC, Chen ZZ, et al. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol Sin. 2004;25:1606–12. [PubMed] [Google Scholar]
- 72.Kamath R, Jiang Z, Sun G, Yalowich JC, Baskaran R. c-Abl kinase regulates curcumin-induced cell death through activation of c-Jun N-terminal kinase. Mol Pharmacol. 2007;71:61–72. doi: 10.1124/mol.106.026575. [DOI] [PubMed] [Google Scholar]
- 73.Yang FW, Huang JZ, Lin XL, Zhen ZN, Chen XM. Apoptosis in nasopharyngeal carcinoma cell line NCE induced by curcumin and its molecular mechanism. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006;41:612–6. [PubMed] [Google Scholar]
- 74.Mosieniak G, Sliwinska M, Piwocka K, Sikora E. Curcumin abolishes apoptosis resistance of calcitriol-differentiated HL-60 cells. FEBS Lett. 2006;580:4653–60. doi: 10.1016/j.febslet.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 75.Wolanin K, Magalska A, Mosieniak G, et al. Curcumin affects components of the chromosomal passenger complex and induces mitotic catastrophe in apoptosis-resistant Bcr-Abl-expressing cells. Mol Cancer Res. 2006;4:457–69. doi: 10.1158/1541-7786.MCR-05-0172. [DOI] [PubMed] [Google Scholar]
- 76.Deeb DD, Jiang H, Gao X, Divine G, Dulchavsky SA, Gautam SC. Chemosensitization of hormone-refractory prostate cancer cells by curcumin to TRAIL-induced apoptosis. J Exp Ther Oncol. 2005;5:81–91. [PubMed] [Google Scholar]
- 77.Chen ZQ, Jie X, Mo ZN. Curcumin inhibits growth, induces G1 arrest and apoptosis on human prostatic stromal cells by regulating Bcl-2/Bax. Zhongguo Zhong Yao Za Zhi. 2008;33:2022–5. [PubMed] [Google Scholar]
- 78.Watson JL, Hill R, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin induces apoptosis in HCT-116 human colon cancer cells in a p21-independent manner. Exp Mol Pathol. 2008;84:230–3. doi: 10.1016/j.yexmp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Mackenzie GG, Queisser N, Wolfson ML, Fraga CG, Adamo AM, Oteiza PI. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin's lymphoma cells. Int J Cancer. 2008;123:56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 80.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 81.Shankar S, Ganapathy S, Chen Q, Srivastava RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest New Drugs. 2008;26:289–97. doi: 10.1007/s10637-007-9099-7. [DOI] [PubMed] [Google Scholar]
- 83.Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28:1277–86. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- 84.Liu B, Bai QX, Chen XQ, Gao GX, Gu HT. Effect of curcumin on expression of survivin, Bcl-2 and Bax in human multiple myeloma cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:762–6. [PubMed] [Google Scholar]
- 85.Sandur SK, Ichikawa H, Pandey MK, et al. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free Radic Biol Med. 2007;43:568–80. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu Z, Shah DM. Curcumin down-regulates Ets-1 and Bcl-2 expression in human endometrial carcinoma HEC-1-A cells. Gynecol Oncol. 2007;106:541–8. doi: 10.1016/j.ygyno.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 87.Karunagaran D, Joseph J, Kumar TR. Cell growth regulation. Adv Exp Med Biol. 2007;595:245–68. doi: 10.1007/978-0-387-46401-5_11. [DOI] [PubMed] [Google Scholar]
- 88.Karmakar S, Banik NL, Ray SK. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32:2103–13. doi: 10.1007/s11064-007-9376-z. [DOI] [PubMed] [Google Scholar]
- 89.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–61. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 90.Bhattacharyya S, Mandal D, Saha B, Sen GS, Das T, Sa G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007;282:15954–64. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- 91.Deeb D, Jiang H, Gao X, et al. Curcumin [1, 7-bis(4-hydroxy-3-methoxyphenyl)-1–6-heptadine-3, 5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;321:616–25. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 92.Su CC, Chen GW, Lin JG, Wu LT, Chung JG. Curcumin inhibits cell migration of human colon cancer colo 205 cells through the inhibition of nuclear factor kappa B /p65 and down-regulates cyclooxygenase-2 and matrix metalloproteinase-2 expressions. Anticancer Res. 2006;26:1281–8. [PubMed] [Google Scholar]
- 93.Balasubramanian S, Eckert RL. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J Biol Chem. 2007;282:6707–15. doi: 10.1074/jbc.M606003200. [DOI] [PubMed] [Google Scholar]
- 94.Chan WH, Wu HY, Chang WH. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol. 2006;44:1362–71. doi: 10.1016/j.fct.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Kwon Y, Magnuson BA. Age-related differential responses to curcumin-induced apoptosis during the initiation of colon cancer in rats. Food Chem Toxicol. 2009;47:377–85. doi: 10.1016/j.fct.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 96.Ghoneim AI. Effects of curcumin on ethanol-induced hepatocyte necrosis and apoptosis: implication of lipid peroxidation and cytochrome c. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:47–60. doi: 10.1007/s00210-008-0335-2. [DOI] [PubMed] [Google Scholar]
- 97.Wang WZ, Cheng J, Luo J, Zhuang SM. Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma cells to apoptosis. FEBS Lett. 2008;582:2689–95. doi: 10.1016/j.febslet.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 98.Liao YF, Hung HC, Hour TC, et al. Curcumin induces apoptosis through an ornithine decarboxylase-dependent pathway in human promyelocytic leukemia HL-60 cells. Life Sci. 2008;82:367–75. doi: 10.1016/j.lfs.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 99.Gopinath P, Ghosh SS. Apoptotic induction with bifunctional E. coli cytosine deaminase-uracil phosphoribosyltransferase mediated suicide gene therapy is synergized by curcumin treatment in vitro. Mol Biotechnol. 2008;39:39–48. doi: 10.1007/s12033-007-9026-3. [DOI] [PubMed] [Google Scholar]
- 100.Wan XH, Luo XP. Relationship between copper injury and apoptosis and the effect of curcumin on copper-injured BRL cells. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:567–70. [PubMed] [Google Scholar]
- 101.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol. 2008;226:161–8. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 102.Cao J, Liu Y, Jia L, et al. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic Biol Med. 2007;43:968–75. doi: 10.1016/j.freeradbiomed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Wahl H, Tan L, Griffith K, Choi M, Liu JR. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol. 2007;105:104–12. doi: 10.1016/j.ygyno.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 104.Balasubramanian S, Eckert RL. Keratinocyte proliferation, differentiation, and apoptosis-differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicol Appl Pharmacol. 2007;224:214–9. doi: 10.1016/j.taap.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang XR, Qi MX, Kang KR. Apoptosis of lens epithelial cell induced by curcumin and its mechanism. Zhonghua Yan Ke Za Zhi. 2006;42:649–53. [PubMed] [Google Scholar]
- 106.Karmakar S, Banik NL, Patel SJ, Ray SK. Curcumin activated both receptor-mediated and mitochondria-mediated proteolytic pathways for apoptosis in human glioblastoma T98G cells. Neurosci Lett. 2006;407:53–8. doi: 10.1016/j.neulet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 107.Chan WH, Wu HJ. Protective effects of curcumin on methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Acta Pharmacol Sin. 2006;27:1192–8. doi: 10.1111/j.1745-7254.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 108.Chen J, Tang XQ, Zhi JL, et al. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11:943–53. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- 109.Banjerdpongchai R, Wilairat P. Effects of water-soluble antioxidants and MAPKK/MEK inhibitor on curcumin-induced apoptosis in HL-60 human leukemic cells. Asian Pac J Cancer Prev. 2005;6:282–5. [PubMed] [Google Scholar]
- 110.Wang Q, Sun AY, Simonyi A, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82:138–48. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 111.Uddin S, Hussain AR, Manogaran PS, et al. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene. 2005;24:7022–30. doi: 10.1038/sj.onc.1208864. [DOI] [PubMed] [Google Scholar]
- 112.Sen S, Sharma H, Singh N. Curcumin enhances Vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem Biophys Res Commun. 2005;331:1245–52. doi: 10.1016/j.bbrc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 113.Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–29. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- 114.Nair J, Strand S, Frank N, et al. Apoptosis and age-dependant induction of nuclear and mitochondrial etheno-DNA adducts in Long–Evans Cinnamon (LEC) rats: enhanced DNA damage by dietary curcumin upon copper accumulation. Carcinogenesis. 2005;26:1307–15. doi: 10.1093/carcin/bgi073. [DOI] [PubMed] [Google Scholar]
- 115.Ligeret H, Barthelemy S, Zini R, Tillement JP, Labidalle S, Morin D. Effects of curcumin and curcumin derivatives on mitochondrial permeability transition pore. Free Radic Biol Med. 2004;36:919–29. doi: 10.1016/j.freeradbiomed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 116.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–5. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 117.Morin D, Barthelemy S, Zini R, Labidalle S, Tillement JP. Curcumin induces the mitochondrial permeability transition pore mediated by membrane protein thiol oxidation. FEBS Lett. 2001;495:131–6. doi: 10.1016/S0014-5793(01)02376-6. [DOI] [PubMed] [Google Scholar]
- 118.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–7. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 119.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 120.Collett GP, Campbell FC. Overexpression of p65/RelA potentiates curcumin-induced apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2006;27:1285–91. doi: 10.1093/carcin/bgi368. [DOI] [PubMed] [Google Scholar]
- 121.Ramachandran C, Rodriguez S, Ramachandran R, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25:3293–302. [PubMed] [Google Scholar]
- 122.Sawada H, Ibi M, Kihara T, et al. Estradiol protects dopaminergic neurons in a MPP + Parkinson's disease model. Neuropharmacology. 2002;42:1056–64. doi: 10.1016/S0028-3908(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 123.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–75. [PubMed] [Google Scholar]
- 124.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 125.Rajasingh J, Raikwar HP, Muthian G, Johnson C, Bright JJ. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem Biophys Res Commun. 2006;340:359–68. doi: 10.1016/j.bbrc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 126.Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv Exp Med Biol. 2007;595:173–84. doi: 10.1007/978-0-387-46401-5_6. [DOI] [PubMed] [Google Scholar]
- 127.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 128.Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- 129.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 130.Park CH, Hahm ER, Park S, Kim HK, Yang CH. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005;579:2965–71. doi: 10.1016/j.febslet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 131.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–27. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 132.Weir NM, Selvendiran K, Kutala VK, et al. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6:178–84. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zheng M, Ekmekcioglu S, Walch ET, Tang CH, Grimm EA. Inhibition of nuclear factor-kappaB and nitric oxide by curcumin induces G2/M cell cycle arrest and apoptosis in human melanoma cells. Melanoma Res. 2004;14:165–71. doi: 10.1097/01.cmr.0000129374.76399.19. [DOI] [PubMed] [Google Scholar]
- 134.Woo JH, Kim YH, Choi YJ, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 135.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–96. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 136.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S. Curcumin disrupts the mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69:1000–8. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo H, Yu JH, Chen K, Ye ZQ, Liu GC. Curcumin-induced apoptosis in androgen-dependent prostate cancer cell line LNCaP in vitro. Zhonghua Nan Ke Xue. 2006;12:141–4. [PubMed] [Google Scholar]
- 138.Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer-I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 139.Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293–303. doi: 10.1002/pros.1074. [DOI] [PubMed] [Google Scholar]
- 140.Nakamura K, Yasunaga Y, Segawa T, et al. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21:825–30. [PubMed] [Google Scholar]
- 141.Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by curcumin. Arch Pharm Res. 2004;27:683–92. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- 142.Lev-Ari S, Strier L, Kazanov D, et al. Curcumin synergistically potentiates the growth-inhibitory and pro-apoptotic effects of celecoxib in osteoarthritis synovial adherent cells. Rheumatology (Oxford) 2006;45:171–7. doi: 10.1093/rheumatology/kei132. [DOI] [PubMed] [Google Scholar]
- 143.Squires MS, Hudson EA, Howells L, et al. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol. 2003;65:361–76. doi: 10.1016/S0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 144.Chadalapaka G, Jutooru I, Chintharlapalli S, et al. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345–54. doi: 10.1158/0008-5472.CAN-07-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pan W, Yang H, Cao C, et al. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol Rep. 2008;20:1553–9. [PubMed] [Google Scholar]
- 146.Swamy MV, Citineni B, Patlolla JM, Mohammed A, Zhang Y, Rao CV. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer. 2008;60(Suppl 1):81–9. doi: 10.1080/01635580802416703. [DOI] [PubMed] [Google Scholar]
- 147.Park C, Moon DO, Choi IW, et al. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med. 2007;20:365–72. [PubMed] [Google Scholar]
- 148.Lev-Ari S, Starr A, Vexler A, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–30. [PubMed] [Google Scholar]
- 149.Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer Res. 2005;25:4029–36. [PubMed] [Google Scholar]
- 150.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–13. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 151.Leu TH, Maa MC. The molecular mechanisms for the antitumorigenic effect of curcumin. Curr Med Chem Anticancer Agents. 2002;2:357–70. doi: 10.2174/1568011024606370. [DOI] [PubMed] [Google Scholar]
- 152.Cheng Y, Kozubek A, Ohlsson L, Sternby B, Duan RD. Curcumin decreases acid sphingomyelinase activity in colon cancer Caco-2 cells. Planta Med. 2007;73:725–30. doi: 10.1055/s-2007-981540. [DOI] [PubMed] [Google Scholar]
- 153.Moussavi M, Assi K, Gomez-Munoz A, Salh B. Curcumin mediates ceramide generation via the de novo pathway in colon cancer cells. Carcinogenesis. 2006;27:1636–44. doi: 10.1093/carcin/bgi371. [DOI] [PubMed] [Google Scholar]
- 154.Yamamoto H, Hanada K, Kawasaki K, Nishijima M. Inhibitory effect on curcumin on mammalian phospholipase D activity. FEBS Lett. 1997;417:196–8. doi: 10.1016/S0014-5793(97)01280-5. [DOI] [PubMed] [Google Scholar]
- 155.Adams BK, Cai J, Armstrong J, et al. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–75. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 156.Fang J, Lu J, Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: a novel molecular mechanism for its anticancer activity. J Biol Chem. 2005;280:25284–90. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 157.Liu Z, Du ZY, Huang ZS, Lee KS, Gu LQ. Inhibition of thioredoxin reductase by curcumin analogs. Biosci Biotechnol Biochem. 2008;72:2214–8. doi: 10.1271/bbb.80229. [DOI] [PubMed] [Google Scholar]
- 158.Marcu MG, Jung YJ, Lee S, et al. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2:169–74. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 159.Hu J, Wang Y, Chen Y. Curcumin-induced histone acetylation in malignant hematologic cells. J Huazhong Univ Sci Technolog Med Sci. 2009;29:25–8. doi: 10.1007/s11596-009-0105-5. [DOI] [PubMed] [Google Scholar]
- 160.Bakhshi J, Weinstein L, Poksay KS, Nishinaga B, Bredesen DE, Rao RV. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis. 2008;13:904–14. doi: 10.1007/s10495-008-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bentzen PJ, Lang E, Lang F. Curcumin induced suicidal erythrocyte death. Cell Physiol Biochem. 2007;19:153–64. doi: 10.1159/000099203. [DOI] [PubMed] [Google Scholar]
- 162.Bae JH, Park JW, Kwon TK. Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem Biophys Res Commun. 2003;303:1073–9. doi: 10.1016/S0006-291X(03)00479-0. [DOI] [PubMed] [Google Scholar]
- 163.Santel T, Pflug G, Hemdan NY, et al. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoS ONE. 2008;3:e3508. doi: 10.1371/journal.pone.0003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choudhary D, Chandra D, Kale RK. Modulation of radioresponse of glyoxalase system by curcumin. J Ethnopharmacol. 1999;64:1–7. doi: 10.1016/S0378-8741(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 165.Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. Febs J. 2006;273:5320–32. doi: 10.1111/j.1742-4658.2006.05525.x. [DOI] [PubMed] [Google Scholar]
- 166.Dempe JS, Pfeiffer E, Grimm AS, Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol Nutr Food Res. 2008;52:1074–81. doi: 10.1002/mnfr.200800029. [DOI] [PubMed] [Google Scholar]
- 167.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–92. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang H, Landis-Piwowar KR, Chen D, Milacic V, Dou QP. Natural compounds with proteasome inhibitory activity for cancer prevention and treatment. Curr Protein Pept Sci. 2008;9:227–39. doi: 10.2174/138920308784533998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dikshit P, Goswami A, Mishra A, Chatterjee M, Jana NR. Curcumin induces stress response, neurite outgrowth and prevent NF-kappaB activation by inhibiting the proteasome function. Neurotox Res. 2006;9:29–37. doi: 10.1007/BF03033305. [DOI] [PubMed] [Google Scholar]
- 170.Wan XH, Li YW, Luo XP. Curcumin attenuated the lipid peroxidation and apoptotic liver injury in copper-overloaded rats. Zhonghua Er Ke Za Zhi. 2007;45:604–8. [PubMed] [Google Scholar]
- 171.Yoshino M, Haneda M, Naruse M, et al. Prooxidant activity of curcumin: copper-dependent formation of 8-hydroxy-2'-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol In Vitro. 2004;18:783–9. doi: 10.1016/j.tiv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 172.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–8. [PubMed] [Google Scholar]
- 173.Jaruga E, Bielak-Zmijewska A, Sikora E, et al. Glutathione-independent mechanism of apoptosis inhibition by curcumin in rat thymocytes. Biochem Pharmacol. 1998;56:961–5. doi: 10.1016/S0006-2952(98)00144-0. [DOI] [PubMed] [Google Scholar]
- 174.William BM, Goodrich A, Peng C, Li S. Curcumin inhibits proliferation and induces apoptosis of leukemic cells expressing wild-type or T315I-BCR-ABL and prolongs survival of mice with acute lymphoblastic leukemia. Hematology. 2008;13:333–43. doi: 10.1179/102453308X343437. [DOI] [PubMed] [Google Scholar]
- 175.Hussain AR, Al-Rasheed M, Manogaran PS, et al. Curcumin induces apoptosis via inhibition of PI3'-kinase/AKT pathway in acute T cell leukemias. Apoptosis. 2006;11:245–54. doi: 10.1007/s10495-006-3392-3. [DOI] [PubMed] [Google Scholar]
- 176.Du J, Suzuki H, Nagase F, et al. Methylglyoxal induces apoptosis in Jurkat leukemia T cells by activating c-Jun N-terminal kinase. J Cell Biochem. 2000;77:333–44. doi: 10.1002/(SICI)1097-4644(20000501)77:2<333::AID-JCB15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 177.Chen Y, Wu Y, He J, Chen W. The experimental and clinical study on the effect of curcumin on cell cycle proteins and regulating proteins of apoptosis in acute myelogenous leukemia. J Huazhong Univ Sci Technolog Med Sci. 2002;22:295–8. doi: 10.1007/BF02896768. [DOI] [PubMed] [Google Scholar]
- 178.Pae HO, Jeong SO, Jeong GS, et al. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem Biophys Res Commun. 2007;353:1040–5. doi: 10.1016/j.bbrc.2006.12.133. [DOI] [PubMed] [Google Scholar]
- 179.Mukherjee Nee Chakraborty S, Ghosh U, Bhattacharyya NP, Bhattacharya RK, Dey S, Roy M. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol Cell Biochem. 2007;297:31–9. doi: 10.1007/s11010-006-9319-z. [DOI] [PubMed] [Google Scholar]
- 180.Roy M, Chakraborty S, Siddiqi M, Bhattacharya RK. Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac J Cancer Prev. 2002;3:61–7. [PubMed] [Google Scholar]
- 181.Duvoix A, Morceau F, Schnekenburger M, et al. Curcumin-induced cell death in two leukemia cell lines: K562 and Jurkat. Ann N Y Acad Sci. 2003;1010:389–92. doi: 10.1196/annals.1299.071. [DOI] [PubMed] [Google Scholar]
- 182.Sun C, Liu X, Chen Y, Liu F. Anticancer effect of curcumin on human B cell non-Hodgkin's lymphoma. J Huazhong Univ Sci Technolog Med Sci. 2005;25:404–7. doi: 10.1007/BF02828208. [DOI] [PubMed] [Google Scholar]
- 183.Skommer J, Wlodkowic D, Pelkonen J. Cellular foundation of curcumin-induced apoptosis in follicular lymphoma cell lines. Exp Hematol. 2006;34:463–74. doi: 10.1016/j.exphem.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 184.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 185.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 186.Tian F, Song M, Xu PR, Liu HT, Xue LX. Curcumin promotes apoptosis of esophageal squamous carcinoma cell lines through inhibition of NF-kappaB signaling pathway. Ai Zheng. 2008;27:566–70. [PubMed] [Google Scholar]
- 187.Collett GP, Robson CN, Mathers JC, Campbell FC. Curcumin modifies Apc(min) apoptosis resistance and inhibits 2-amino 1-methyl-6-phenylimidazo[4, 5-b]pyridine (PhIP) induced tumour formation in Apc(min) mice. Carcinogenesis. 2001;22:821–5. doi: 10.1093/carcin/22.5.821. [DOI] [PubMed] [Google Scholar]
- 188.Huang AC, Lin SY, Su CC, et al. Effects of curcumin on N-bis(2-hydroxypropyl) nitrosamine (DHPN)-induced lung and liver tumorigenesis in BALB/c mice in vivo. In Vivo. 2008;22:781–5. [PubMed] [Google Scholar]
- 189.Priya S, Sudhakaran PR. Curcumin-induced recovery from hepatic injury involves induction of apoptosis of activated hepatic stellate cells. Indian J Biochem Biophys. 2008;45:317–25. [PubMed] [Google Scholar]
- 190.Chan WH, Wu HJ, Hsuuw YD. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann N Y Acad Sci. 2005;1042:372–8. doi: 10.1196/annals.1338.057. [DOI] [PubMed] [Google Scholar]
- 191.Khar A, Ali AM, Pardhasaradhi BV, Varalakshmi CH, Anjum R, Kumari AL. Induction of stress response renders human tumor cell lines resistant to curcumin-mediated apoptosis: role of reactive oxygen intermediates. Cell Stress Chaperones. 2001;6:368–76. doi: 10.1379/1466-1268(2001)006<0368:IOSRRH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hsu YC, Weng HC, Lin S, Chien YW. Curcuminoids-cellular uptake by human primary colon cancer cells as quantitated by a sensitive HPLC assay and its relation with the inhibition of proliferation and apoptosis. J Agric Food Chem. 2007;55:8213–22. doi: 10.1021/jf070684v. [DOI] [PubMed] [Google Scholar]
- 193.Lev-Ari S, Maimon Y, Strier L, Kazanov D, Arber N. Down-regulation of prostaglandin E2 by curcumin is correlated with inhibition of cell growth and induction of apoptosis in human colon carcinoma cell lines. J Soc Integr Oncol. 2006;4:21–6. [PubMed] [Google Scholar]
- 194.Scott DW, Loo G. Curcumin-induced GADD153 gene up-regulation in human colon cancer cells. Carcinogenesis. 2004;25:2155–64. doi: 10.1093/carcin/bgh239. [DOI] [PubMed] [Google Scholar]
- 195.Collett GP, Campbell FC. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis. 2004;25:2183–9. doi: 10.1093/carcin/bgh233. [DOI] [PubMed] [Google Scholar]
- 196.Rashmi R, Kumar S, Karunagaran D. Ectopic expression of Hsp70 confers resistance and silencing its expression sensitizes human colon cancer cells to curcumin-induced apoptosis. Carcinogenesis. 2004;25:179–87. doi: 10.1093/carcin/bgh001. [DOI] [PubMed] [Google Scholar]
- 197.Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001;21:873–8. [PubMed] [Google Scholar]
- 198.Chen H, Zhang ZS, Zhang YL, Zhou DY. Curcumin inhibits cell proliferation by interfering with the cell cycle and inducing apoptosis in colon carcinoma cells. Anticancer Res. 1999;19:3675–80. [PubMed] [Google Scholar]
- 199.Kamat AM, Sethi G, Aggarwal BB. Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human blad. Mol Cancer Ther. 2007;6:1022–30. doi: 10.1158/1535-7163.MCT-06-0545. [DOI] [PubMed] [Google Scholar]
- 200.Tong QS, Zheng LD, Lu P, et al. Apoptosis-inducing effects of curcumin derivatives in human bladder cancer cells. Anticancer Drugs. 2006;17:279–87. doi: 10.1097/00001813-200603000-00006. [DOI] [PubMed] [Google Scholar]
- 201.Deeb D, Jiang H, Gao X, et al. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004;3:803–12. [PubMed] [Google Scholar]
- 202.Andrzejewski T, Deeb D, Gao X, et al. Therapeutic efficacy of curcumin/TRAIL combination regimen for hormone-refractory prostate cancer. Oncol Res. 2008;17:257–67. doi: 10.3727/096504008786991611. [DOI] [PubMed] [Google Scholar]
- 203.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 204.Deeb D, Xu YX, Jiang H, et al. Curcumin (diferuloyl-methane) enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cells. Mol Cancer Ther. 2003;2:95–103. [PubMed] [Google Scholar]
- 205.Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aravindan N, Madhusoodhanan R, Ahmad S, Johnson D, Herman TS. Curcumin inhibits NFkappaB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther. 2008;7:569–76. doi: 10.1158/1535-7163.MCT-07-2132. [DOI] [PubMed] [Google Scholar]
- 207.Belkaid A, Copland IB, Massillon D, Annabi B. Silencing of the human microsomal glucose-6-phosphate translocase induces glioma cell death: potential new anticancer target for curcumin. FEBS Lett. 2006;580:3746–52. doi: 10.1016/j.febslet.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 208.Khajavi M, Inoue K, Wiszniewski W, Ohyama T, Snipes GJ, Lupski JR. Curcumin treatment abrogates endoplasmic reticulum retention and aggregation-induced apoptosis associated with neuropathy-causing myelin protein zero-truncating mutants. Am J Hum Genet. 2005;77:841–50. doi: 10.1086/497541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nagai S, Kurimoto M, Washiyama K, Hirashima Y, Kumanishi T, Endo S. Inhibition of cellular proliferation and induction of apoptosis by curcumin in human malignant astrocytoma cell lines. J Neurooncol. 2005;74:105–11. doi: 10.1007/s11060-004-5757-1. [DOI] [PubMed] [Google Scholar]
- 210.Kim HI, Huang H, Cheepala S, Huang S, Chung J. Curcumin inhibition of integrin (alpha6beta4)-dependent breast cancer cell motility and invasion. Cancer Prev Res (Phila Pa) 2008;1:385–91. doi: 10.1158/1940-6207.CAPR-08-0087. [DOI] [PubMed] [Google Scholar]
- 211.Aggarwal BB, Shishodia S, Takada Y, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–8. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 212.Kim MS, Kang HJ, Moon A. Inhibition of invasion and induction of apoptosis by curcumin in H-ras-transformed MCF10A human breast epithelial cells. Arch Pharm Res. 2001;24:349–54. doi: 10.1007/BF02975105. [DOI] [PubMed] [Google Scholar]
- 213.Zheng LD, Tong QS, Wu CH. Inhibitory effects of curcumin on apoptosis of human ovary cancer cell line A2780 and its molecular mechanism. Ai Zheng. 2002;21:1296–300. [PubMed] [Google Scholar]
- 214.Radhakrishna Pillai G, Srivastava AS, Hassanein TI, Chauhan DP, Carrier E. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett. 2004;208:163–70. doi: 10.1016/j.canlet.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 215.Tourkina E, Gooz P, Oates JC, Ludwicka-Bradley A, Silver RM, Hoffman S. Curcumin-induced apoptosis in scleroderma lung fibroblasts: role of protein kinase cepsilon. Am J Respir Cell Mol Biol. 2004;31:28–35. doi: 10.1165/rcmb.2003-0354OC. [DOI] [PubMed] [Google Scholar]
- 216.Marin YE, Wall BA, Wang S, et al. Curcumin downregulates the constitutive activity of NF-kappaB and induces apoptosis in novel mouse melanoma cells. Melanoma Res. 2007;17:274–83. doi: 10.1097/CMR.0b013e3282ed3d0e. [DOI] [PubMed] [Google Scholar]
- 217.Jang YH, Namkoong S, Kim YM, Lee SJ, Park BJ, Min DS. Cleavage of phospholipase D1 by caspase promotes apoptosis via modulation of the p53-dependent cell death pathway. Cell Death Differ. 2008;15:1782–93. doi: 10.1038/cdd.2008.111. [DOI] [PubMed] [Google Scholar]
- 218.Furusu A, Nakayama K, Xu Q, Konta T, Kitamura M. MAP kinase-dependent, NF-kappaB-independent regulation of inhibitor of apoptosis protein genes by TNF-alpha. J Cell Physiol. 2007;210:703–10. doi: 10.1002/jcp.20881. [DOI] [PubMed] [Google Scholar]
- 219.Magalska A, Sliwinska M, Szczepanowska J, Salvioli S, Franceschi C, Sikora E. Resistance to apoptosis of HCW-2 cells can be overcome by curcumin- or vincristine-induced mitotic catastrophe. Int J Cancer. 2006;119:1811–8. doi: 10.1002/ijc.22055. [DOI] [PubMed] [Google Scholar]
- 220.Bielak-Zmijewska A, Koronkiewicz M, Skierski J, Piwocka K, Radziszewska E, Sikora E. Effect of curcumin on the apoptosis of rodent and human nonproliferating and proliferating lymphoid cells. Nutr Cancer. 2000;38:131–8. doi: 10.1207/S15327914NC381_18. [DOI] [PubMed] [Google Scholar]
- 221.Scharstuhl A, Mutsaers HA, Pennings SW, Szarek WA, Russel FG, Wagener FA (2008) Curcumin-Induced Fibroblast Apoptosis and in Vitro Wound Contraction Are Regulated by Antioxidants and Heme Oxygenase: Implications for Scar Formation. J Cell Mol Med [DOI] [PMC free article] [PubMed]
- 222.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang M, Bian F, Wen C, Hao N. Inhibitory effect of curcumin on proliferation of human pterygium fibroblasts. J Huazhong Univ Sci Technolog Med Sci. 2007;27:339–42. doi: 10.1007/s11596-007-0332-6. [DOI] [PubMed] [Google Scholar]
- 224.Cucuzza LS, Motta M, Miretti S, Accornero P, Baratta M. Curcuminoid-phospholipid complex induces apoptosis in mammary epithelial cells by STAT-3 signaling. Exp Mol Med. 2008;40:647–57. doi: 10.3858/emm.2008.40.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–50. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 226.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 227.Ko LJ, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–72. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 228.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 229.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21:335–48. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 230.Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett. 2004;557:14–20. doi: 10.1016/S0014-5793(03)01464-9. [DOI] [PubMed] [Google Scholar]
- 231.Park MJ, Kim EH, Park IC, et al. Curcumin inhibits cell cycle progression of immortalized human umbilical vein endothelial (ECV304) cells by up-regulating cyclin-dependent kinase inhibitor, p21WAF1/CIP1, p27KIP1 and p53. Int J Oncol. 2002;21:379–83. [PubMed] [Google Scholar]
- 232.Aggarwal BB, Banerjee S, Bharadwaj U, Sung B, Shishodia S, Sethi G. Curcumin induces the degradation of cyclin E expression through ubiquitin-dependent pathway and up-regulates cyclin-dependent kinase inhibitors p21 and p27 in multiple human tumor cell lines. Biochem Pharmacol. 2007;73:1024–32. doi: 10.1016/j.bcp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 233.Sahu RP, Batra S, Srivastava SK. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br J Cancer. 2009;100:1425–33. doi: 10.1038/sj.bjc.6605039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7:2609–20. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer. 2005;104:879–90. doi: 10.1002/cncr.21216. [DOI] [PubMed] [Google Scholar]
- 236.Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 237.Rao CV. Regulation of COX and LOX by curcumin. Adv Exp Med Biol. 2007;595:213–26. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 238.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–7. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Okazaki Y, Iqbal M, Okada S. Suppressive effects of dietary curcumin on the increased activity of renal ornithine decarboxylase in mice treated with a renal carcinogen, ferric nitrilotriacetate. Biochim Biophys Acta. 2005;1740:357–66. doi: 10.1016/j.bbadis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 240.Blasius R, Reuter S, Henry E, Dicato M, Diederich M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem Pharmacol. 2006;72:1547–54. doi: 10.1016/j.bcp.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 241.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 242.Huang TS, Lee SC, Lin JK. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1991;88:5292–6. doi: 10.1073/pnas.88.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Suh HW, Kang S, Kwon KS. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol Cell Biochem. 2007;298:187–94. doi: 10.1007/s11010-006-9365-6. [DOI] [PubMed] [Google Scholar]
- 244.Cipriani B, Borsellino G, Knowles H, et al. Curcumin inhibits activation of Vgamma9Vdelta2 T cells by phosphoantigens and induces apoptosis involving apoptosis-inducing factor and large scale DNA fragmentation. J Immunol. 2001;167:3454–62. doi: 10.4049/jimmunol.167.6.3454. [DOI] [PubMed] [Google Scholar]
- 245.Cao J, Jia L, Zhou HM, Liu Y, Zhong LF. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol Sci. 2006;91:476–83. doi: 10.1093/toxsci/kfj153. [DOI] [PubMed] [Google Scholar]
- 246.Lu HF, Yang JS, Lai KC et al (2009) Curcumin-Induced DNA Damage and Inhibited DNA Repair Genes Expressions in Mouse-Rat Hybrid Retina Ganglion Cells (N18). Neurochem Res [DOI] [PubMed]
- 247.Chen YC, Kuo TC, Lin-Shiau SY, Lin JK. Induction of HSP70 gene expression by modulation of Ca(+2) ion and cellular p53 protein by curcumin in colorectal carcinoma cells. Mol Carcinog. 1996;17:224–34. doi: 10.1002/(SICI)1098-2744(199612)17:4<224::AID-MC6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 248.Dean EJ, Ranson M, Blackhall F, Holt SV, Dive C. Novel therapeutic targets in lung cancer: Inhibitor of apoptosis proteins from laboratory to clinic. Cancer Treat Rev. 2007;33:203–12. doi: 10.1016/j.ctrv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 249.Bava SV, Puliappadamba VT, Deepti A, Nair A, Karunagaran D, Anto RJ. Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-kappaB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J Biol Chem. 2005;280:6301–8. doi: 10.1074/jbc.M410647200. [DOI] [PubMed] [Google Scholar]
- 250.Lin YG, Kunnumakkara AB, Nair A, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 251.Bhattacharyya S, Mandal D, Sen GS, et al. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: protection by curcumin. Cancer Res. 2007;67:362–70. doi: 10.1158/0008-5472.CAN-06-2583. [DOI] [PubMed] [Google Scholar]
- 252.Shi Y, He B, Kuchenbecker KM, et al. Inhibition of Wnt-2 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. Int J Cancer. 2007;121:1175–81. doi: 10.1002/ijc.22848. [DOI] [PubMed] [Google Scholar]
- 253.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–72. [PubMed] [Google Scholar]
- 254.Pae HO, Jeong GS, Jeong SO, et al. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp Mol Med. 2007;39:267–77. doi: 10.1038/emm.2007.30. [DOI] [PubMed] [Google Scholar]
- 255.Ramachandran C, Fonseca HB, Jhabvala P, Escalon EA, Melnick SJ. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Lett. 2002;184:1–6. doi: 10.1016/S0304-3835(02)00192-1. [DOI] [PubMed] [Google Scholar]
- 256.Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta. 2008;1780:673–9. doi: 10.1016/j.bbagen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 257.Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Ophthalmol Vis Sci. 2006;47:2179–84. doi: 10.1167/iovs.05-0580. [DOI] [PubMed] [Google Scholar]
- 258.Magalska A, Brzezinska A, Bielak-Zmijewska A, Piwocka K, Mosieniak G, Sikora E. Curcumin induces cell death without oligonucleosomal DNA fragmentation in quiescent and proliferating human CD8+ cells. Acta Biochim Pol. 2006;53:531–8. [PubMed] [Google Scholar]