Abstract
We examined the nature and timecourse of hemispheric asymmetries in verbal memory by recording event-related potentials (ERPs) in a continuous recognition task. Participants made overt recognition judgments to test words presented in central vision that were either novel (new words) or had been previously presented in the left or right visual field (old words). An ERP memory effect linked to explicit retrieval revealed no asymmetries for words repeated at short and medium retention intervals, but at longer repetition lags (20–50 intervening words) this ‘old/new effect’ was more pronounced for words whose study presentation had been biased to the right hemisphere (RH). Additionally, a repetition effect linked to more implicit recognition processes (P2 amplitude changes) was observed at all lags for words preferentially encoded by the RH but was not observed for left hemisphere (LH)-encoded words. These results are consistent with theories that the RH encodes verbal stimuli more veridically whereas the LH encodes in a more abstract manner. The current findings provide a critical link between prior work on memory asymmetries, which has emphasized general LH advantages for verbal material, and on language comprehension, which has pointed to an important role for the RH in language processes that require the retention and integration of verbal information over long time spans.
Keywords: Laterality, Verbal encoding, Retention interval, Old/new effect, P2, Dm effect
1. Introduction
Comprehending a sentence requires that the information associated with multiple words be activated and retained as new words are encountered and integrated with the prior context. For lengthy sentences or paragraphs and longer discourses, this task becomes more difficult, as the amount of information that is extracted grows and as earlier material must be remembered over longer time intervals. Thus, language comprehension relies critically upon verbal memory abilities, rendering particularly important questions about the kind of information that comes to be extracted from a verbal stimulus and the timecourse with which that information is maintained. Indeed, there may not be a single answer to these questions; recent evidence suggests that the left and right cerebral hemispheres each make unique contributions to language comprehension and that the differences in their processing may arise, at least in part, from biases in how – and for how long – each hemisphere retains word-related information.
Consonant with well-established asymmetries favoring the left hemisphere (LH) for the apprehension (e.g., Jordan, Patching, & Thomas, 2003) and processing (see Hellige, 1993, for review) of verbal material, studies using several methodologies have suggested general memory biases in which verbal information is preferentially handled by the LH and nonverbal information by the right hemisphere (RH). Memory asymmetries have often been investigated by presenting stimuli to a single hemisphere through the use of visual half-field (VF) methods. Due to the contralateral organization of the human visual system, stimuli to the left of a central fixation point (in the left visual field: LVF) are received and initially processed exclusively by the RH, and stimuli in the right visual field (RVF) are initially processed exclusively by the LH. Although interhemispheric communication is possible via the corpus callosum, any relayed information is delayed and likely to be degraded, creating processing biases that can continue well beyond the point at which the hemispheres are first able to transfer information (e.g., Zaidel, 1983). Using this technique, a number of studies have found that verbal stimuli (such as letter clusters or words) are remembered better when presented to the RVF/LH as compared with the LVF/RH, whereas nonverbal stimuli, particularly faces or line orientations, are better remembered when studied in the LVF/RH (Blanchet et al., 2001; Fontenot & Benton, 1972; Geffen, Bradshaw, & Wallace, 1971; Kimura, 1966; Leehey & Cahn, 1979). Recent hemodynamic brain imaging studies have shown a similar pattern in the activation of prefrontal cortical and medial temporal lobe regions while different types of materials are encoded. Such studies have found predominantly left-lateralized activation while verbal material is encoded, right-lateralized activation when nonverbal material (i.e., faces or patterns) is encoded, and bilateral activation during the encoding of nameable objects or scenes (Golby et al., 2001; Kelley et al., 1998).
These findings in brain-intact individuals are corroborated by data from patients with unilateral brain lesions. Verbal memory deficits are typically greater following unilateral damage to language-related areas of the LH (the temporal lobe or prefrontal cortex) than to homologous regions of the RH, damage to which has instead been linked to impaired memory for nonverbal, visuospatial stimuli (Milner, 1971, 1972; Pillon et al., 1999; Vilkki, 1987). However, the lesion studies also make clear that it would be an oversimplification to assume that verbal memory is housed in the LH and nonverbal memory in the RH. Insult to either hemisphere generally leads to some degree of memory performance deficit for both verbal and nonverbal material, as well as a preservation of some abilities for both types of information (Dobbins, Kroll, Tulving, Knight, & Gazzaniga, 1998). Furthermore, the language abilities that seem to rely most on RH processing resources are those that also seem to place some of the greatest demands on verbal memory. Patients with damage to language-related areas of the RH (particularly the temporal lobe) tend to have difficulty tracking the topic or order of a discourse (Brownell & Martino, 1998), interpreting prosody (Shapiro & Danly, 1985), and comprehending various types of nonliteral language, such as jokes, sarcasm, and indirect requests (Brownell, Simpson, Bihrle, Potter, & Gardner, 1990; Gardner, Brownell, Wopner, & Michelow, 1983; Kaplan, Brownell, Jacobs, & Gardner, 1990; see Hellige, 1993, for review). Critical to successfully comprehending all of these types of language material would seem to be the capacity to retain, and in some cases revise, prior context as new information becomes available. Thus, the RH seems important for managing complex language structures and manipulating these representations when additional information beyond the local context (such as prosodic contour, wider discourse context, and indirect or nonliteral inferences) must be incorporated. If the RH is able to maintain complex linguistic structures over time, then this suggests that the RH does contribute to verbal memory and may even have certain verbal memory advantages relative to the LH.
Indeed, recent work using a variety of tasks and stimuli suggests that both hemispheres process and remember verbal material, but that they extract different kinds of information from the same stimulus. More specifically, it has been proposed that the RH is biased to retain more information about the physical form of a verbal stimulus than the LH, and that this information sometimes proves useful in memory and language processing tasks. Some researchers have argued that the RH’s fluency for visuospatial processing (Berrini, Capitani, Della Sala, & Spinnler, 1984; Berrini, Della Sala, Spinnler, Sterzi, & Vallar, 1982; Dee & Fontenot, 1973; Kimura, 1966) biases the RH to treat even meaningful verbal stimuli primarily as physical objects (Hellige, 1980; Hellige & Webster, 1979). For example, binary (i.e., same/different) perceptual matching tasks have found more accurate detection of visual matches presented in the LVF/RH, for both single letters (presented simultaneously: Geffen, Bradshaw, & Nettleton, 1972) and whole words (presented sequentially, with the second word lateralized: Gibson, Dimond, & Gazzaniga, 1972). Similar form-processing biases have been inferred from error patterns seen in matching tasks. In a forced-choice recognition task, Pirozzolo and Rayner (1977) found that words lateralized to the RVF/LH were identified with greater accuracy overall than words presented in the LVF/RH; however, most of the errors to LVF/RH words involved choosing a physically similar word, whereas errors to RVF/LH words were split between visually confusable foils and acoustically confusable foils. Thus, whereas the RH seemed to process words primarily at the level of physical form, the LH was additionally sensitive to higher-level (in this case, phonological) aspects of the stimuli.
In a series of priming and visual perception experiments, Marsolek and colleagues have directly tested the hypothesis that the two hemispheres encode different types of information from verbal material (Burgund & Marsolek, 1997; Marsolek, 1999; Marsolek, Kosslyn, & Squire, 1992; Marsolek, Nicholas, & Andresen, 2002; Marsolek, Schacter, & Nicholas, 1996). Their data show that the RH is more sensitive to changes in the physical form of a letter or word, such as letter case, suggesting that these form-specific elements are encoded as part of the stimulus. They therefore propose that the RH is biased to encode verbal information veridically, holistically, and idiosyncratically (Burgund & Marsolek, 1997; Marsolek et al., 2002), such that items that are physically different but conceptually similar (e.g., capital and lowercase letters: A vs. a) are encoded as different entities (‘A’ and ‘a’). In contrast, their work suggests that the LH is less sensitive to manipulations of letter case or other superficial characteristics; it seems to encode material at a more conceptual level. As such, LH representations for items that are perceptually distinct but categorically similar (e.g., A vs. a) would emphasize the shared elements (‘the letter a’). Thus, the LH seems biased to encode perceptual information more abstractly, perhaps even limiting encoding to generic, categorical information, such as prototypical features, and discarding unique information that does not generalize across the linguistic category.
Indeed, it may be that the RH retains some veridical information, such as physical features, that the LH does not. Support for this hypothesis has been found in verbal recognition asymmetries observed when test lures are semantically related to studied words. Using central study words and lateralized test probes, Metcalfe, Funnell, and Gazzaniga (1995) found that a commissurotomized patient (i.e., someone lacking a corpus callosum to facilitate communication between the two hemispheres) made more false alarms to semantic lures (e.g., accepting plum when it was not studied but apple and peach were) presented in the RVF/LH than in theLVF/RH. False alarms of this type – in which new, but semantically similar, items are erroneously judged to be old – are expected for a processing system that primarily stores the semantic features or ‘gist’ (e.g., ‘type of fruit’) associated with the study items. However, such errors should be less likely for a processing system that is storing more specific word form information (which clearly differentiates the repeated and lure words). Thus, the results were taken to support the hypothesis that the LH’s verbal representations are more gist-like whereas the RH’s representations are more veridical. Investigating similar processes in brain-intact individuals, Fabiani, Stadler, and Wessels (2000) further observed that, when false alarms do occur, they are more differentiated from hits when the original encoding was biased toward the RH. During the study phase of their experiment, participants viewed several themed sub-lists (e.g., a list containing nap, snore, and bed), each of which was presented within a single VF so as to bias false memory generation (e.g., incorrectly identifying the unstudied lure sleep as repeated during the test phase) to the LH or RH. Although sub-lists presented in either VF elicited frequent false alarms to the lures, comparisons of the memory-related portion of the event-related brain potential (ERP) data (which often show different brain responses to true and false memories: Gonsalves & Paller, 2000; Johnson et al., 1997) revealed differences in the brain signals associated with the false memories elicited in each hemisphere. For the RVF/LH-studied sub-lists, the ERP response (at test) to false memories overlapped with that to the true memories; that is, hits and false alarms elicited identical brain responses. However, for sub-lists that had been studied in the LVF/RH, true and false memories differed in the memory signal, as well as on early components related to sensory processing and attentional allocation. Additionally, response time (RT) data revealed that participants were slower to produce incorrect responses to the semantic lures associated with sub-lists that had been presented in the LVF/RH. Thus, both behavioral and ERP measures indicated a greater disparity between the response to true and false memories associated with RH encoding, consistent with Metcalfe et al.’s (1995) conclusions that the RH is better at discriminating semantically similar words than the LH, perhaps because of more veridical encoding.
False memories were also examined by Westerberg and Marsolek (2003), who aurally presented (to both ears) themed study lists that were subsequently tested with VF presentation. In this experiment, participants made more false alarms to semantic lures that were tested in the LVF/RH. It is worth noting, however, that this experiment differs from Metcalfe et al. (1995) and Fabiani et al. (2000) in two critical ways. First, Wester-berg and Marsolek employed a modality switch – from auditory study words to visual test words – and perhaps the results primarily indicate that the RH is less adept at matching words cross-modally. Particularly if the RH is more effective with form-specific, veridical encoding (e.g., Burgund & Marsolek, 1997; Marsolek et al., 1992), the LVF/RH processing advantages seen for within-modality matches would not be expected to extend to (and might even be reversed for) cross-modal tasks. If, instead, the LH uses abstract encoding strategies, then its representations should be more robust to modality changes. A second difference is that Westerberg and Marsolek lateralized only the test words; in contrast, Fabiani et al. lateralized the study items, and Metcalfe et al. effectively lateralized both stages (because they tested a commissurotomized patient, central study words were essentially presented to each hemisphere in isolation). This is an important consideration given evidence that lateralizing at the study phase and lateralizing at the test phase may not bias processing the same way (Berrini et al., 1982; Coney & McDonald, 1988; Leiber, 1982). Given these differences, Westerberg and Marsolek’s data are not necessarily in conflict with prior results, and could perhaps even be taken to support, rather than to challenge, the notion that the RH encodes verbal stimuli veridically whereas the LH encodes more abstractly.
In sum, studies using a number of different stimulus types and tasks support the idea that the hemispheres may differ in the nature of information that each extracts from a verbal stimulus. It seems that the LH may encode verbal stimuli more abstractly and focus on the conceptual information shared by semantically similar words, whereas the RH may employ more veridical, holistic encoding strategies that include stimulus-specific information in the representation. These differences may help explain, in part, the dissociations seen in other types of language comprehension. An important part of the LH’s well-documented dominance for word and sentence processing (e.g., see Chiarello, 1988, for review; Faust, Bar-lev, & Chiarello, 2003; Faust & Chiarello, 1998; Faust, Kravetz, & Babkoff, 1993; Federmeier, Mai, & Kutas, 2005; though see Federmeier & Kutas, 1999, for a case in which stimuli in the LVF/RH show greater contextual sensitivity) may be its efficacy at rapidly extracting meaning from the physical form of a verbal stimulus. Conversely, the RH’s ability to retain a veridical trace of the original input may contribute to its role in processing lengthy or complex messages that necessitate the recall and revision of specific prior words. For example, consider the processing of jokes or puns that hinge on the existence of multiple meanings associated with a single word form; successful comprehension of these language structures seems to require RH processing resources (Bihrle, Brownell, Powelson, & Gardner, 1986; Coulson & Williams, 2005). In the example “If you want a committed man, look in a mental hospital,” revising the meaning of the word committed is critical to under-standing the joke. The literal meaning (‘dedicated, looking for love’) is related to the first-pass reading of the context, while a wholly different, humorous meaning (‘detained, locked up’) is later revealed in the punch line. In cases such as this, retaining a veridical representation of a specific word (here, committed) would seem to be critical to correctly processing the joke. If, instead, only the gist or meaning suggested by the initial context (‘dedicated, looking for love’) was extracted and the stimulus-specific information discarded, then it might prove difficult if not impossible to recover the humorous meaning. In this way, even fairly low-level differences in verbal memory might contribute to asymmetries seen at higher processing levels.
In addition to differences in the nature of the information that each hemisphere extracts from a verbal stimulus, there also seem to be differences in the timecourse with which that information is retained. Such differences could arise because of asymmetries in the timecourse of core memory processes (extending, then, to nonverbal materials as well: Bevilacqua, Capitani, Luzzatti, & Spinnler, 1979; Dee & Fontenot, 1973) or because the kind of encoding asymmetries already described interact with or influence the timecourse of memory, such that the verbal representations formed by each hemisphere are differentially sensitive to interference or decay. The question of how encoding biases interact with time has not been extensively examined. Most VF studies of verbal memory have used fairly short retention intervals or lags (number of intervening stimuli between study and test), and these have reported ubiquitous RVF/LH advantages for both the accuracy and speed of responses (Berrini et al., 1982; Blanchet et al., 2001; Hannay & Malone, 1976). The few studies that have looked at retention across a wider range of repetition intervals have found that asymmetries do change, with some finding evidence that the LH’s accuracy and speed advantages become more pronounced as the retention interval increases (Coney & McDonald, 1988; Hines, Satz, Schell, & Schmidlin, 1969). However, most of these studies lateralized not only the study words but also the test words; as such it is hard to isolate the effects of hemisphere-biased encoding from biased retrieval.
The use of lateralized tests words is further complicated by potential influences from other types of processing asymmetries, such as the established perceptual and attentional biases that lead to more rapid and accurate word reading in the RVF/LH (e.g., Jordan et al., 2003; see Chiarello, 1988, for review). Most studies find accuracy and RT differences to test words in the two VFs even with immediate repetition, when contributions from memory are likely to be relatively minimal; similar differences at longer lags are therefore difficult to interpret as memory effects. Such perceptual/attentional biases may have the strongest effects for lateralized test probes, since measurements are then made to stimuli that are likely to be apprehended more poorly in one VF (usually the LVF/RH for verbal material). Given that many previous investigations of hemispheric asymmetries in verbal memory have used lateralized test words, it is particularly problematic that the observed RVF/LH speed and accuracy advantages are also consistent with the documented reading asymmetries that favor RVF/LH words.
Federmeier and Benjamin (2005) tried to minimize the contributions of such biases by lateralizing only the study words, so that responses were instead measured to perceptually identical stimuli presented in central vision. In a continuous recognition paradigm, lateralized study words were interleaved with test words presented in central vision, spaced over a range of nine repetition lags (from immediate repetition, ‘lag 1,’ to 49 intervening words, ‘lag 50’). While there was a consistent accuracy advantage for RVF/LH-studied words (even with immediate repetition), the RT data supported the hypothesis that hemisphere-biased encoding leads to different impacts of lag on memory. At short lags (1, 2, 3, 5, 7), participants were faster to correctly accept repeated test words that had been studied in the RVF/LH than in the LVF/RH. However, at longer lags (10, 20, 30, 50), correct responses to words studied in the LVF/RH were faster (while miss RTs did not differ). That the oft-reported RVF/LH advantage was attenuated and even reversed as lag increased is striking, and suggests that something about the RH’s encoding of verbal stimuli might be more robust to interference, decay or both. It is important to note, however, that even in this case, in which study rather than test words were lateralized, behavioral asymmetries were evident at lag 1, when memory demands are obviously minimal. This suggests the presence of standing biases in initial processing (generally favoring word apprehension in the RVF/LH) that must be taken into account when trying to infer memory-related differences at longer lags. Therefore, more work is needed to flesh out the nature of and underlying causes for the lag-related VF effects obtained thus far. The current study expands upon the Federmeier and Benjamin work by examining verbal memory asymmetries with the greater functional specificity afforded by ERP measures and with particular attention to response patterns previously linked with various aspects of memory, such as the old/new, P2, and Dm effects (reviewed below). Targeting ERP effects that are known to fluctuate with memory demands (and that are generally less sensitive to apprehension difficulty) should help to elucidate the nature of the behavioral asymmetries that have been observed.
ERPs are changes in brain electrical activity that are time-locked to an event of interest, such as the visual presentation of a word. They provide a continuous and temporally fine-grained measure of processing, from sensory analysis and attentional selection to later memory and decision-making processes. Many ERP components, which are deflections in the waveform that occur in predictable time-windows and with characteristic distributions, have been identified and linked to specific cognitive and neural functions. Of particular interest in studies of memory is the “old/new effect,” characterized by increased positivity for test items that are correctly remembered (hits) relative to correctly rejected new test items (see Rugg & Doyle, 1994, for review). The old/new effect seems to involve changes that occur with repetition in at least two separable components: relative to new test words, old test words manifest amplitude reductions on the N400 and amplitude increases in the late positive complex (LPC). The N400 is a negativity that peaks around 350–400 ms post-stimulus onset and is broadly distributed, but most prominent over medial, parietal regions. The N400 occurs in response to many types of meaningful stimuli, including words, and is believed to reflect some aspect of semantic access (Kutas & Hillyard, 1980; Kutas & Federmeier, 2000). Accessing a word is easier if it has recently been retrieved, and, accordingly, N400 amplitude decreases (i.e., responses become more positive) with repetition; the magnitude of this difference is highly sensitive to repetition lag, as well as to word frequency and other semantic factors (Nagy & Rugg, 1989; Rugg, 1990; Rugg, Cox, Doyle, & Wells, 1995). Intracranial recordings have linked repetition effects on the N400 to activity in the anterior temporal lobe as well as the parietal cortex (Guillem, Rougier, & Claverie, 1999), and this is consistent with source localization analyses performed on scalp-recorded ERP data, as well as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) data (Friedman & Johnson, 2000; see Schacter & Wagner, 1999, for review). These N400 repetition effects are preserved in amnesia (Olichney et al., 2000), suggesting that they reflect implicit aspects of memory function.
The LPC occurs later than the N400; it is a broad positivity that varies in its time course but often occurs from about 500 to 800 ms after stimulus onset, and is primarily distributed over medial, parietal sites (see Rugg & Doyle, 1994, for review). The amplitude of this positivity is sensitive to factors that change memory strength, such that, for example, it decreases with lag and increases with frequent study exposures (Finnigan, Humphreys, Dennis, & Geffen, 2002; Kim, Kim, & Kwon, 2001; Nagy & Rugg, 1989). ERP studies using intracranial or source localization techniques suggest that important generators for the LPC portion of the old/new effect reside in anterior temporal and frontal lobe areas (Guillem et al., 1999; Friedman & Johnson, 2000), and LPC effects are compromised in amnesia (Olichney et al., 2000), suggesting a link with explicit memory processes. Although some experiments have sought to disentangle the processes underlying the N400 and LPC, they are often examined together in memory tasks, particularly given their overlapping distributions and temporal proximity. This combined old/new effect provides an index of memory strength, which can then be compared as a function of experimental manipulations such as, in the present study, VF of presentation or repetition lag.
In addition to the type of memory effects seen on the N400 and the LPC, several experiments have also found repetition-based amplitude changes on the P2, a frontally maximal, positive-going component peaking around 200 ms. The P2 constitutes part of the normal sensory-evoked response to a visual stimulus and has been associated with higher-order perceptual and attentional processing, specifically visual feature analysis (Luck & Hillyard, 1994). In studies of implicit priming and explicit memory at short retention intervals, the P2 is enhanced for repeated, relative to novel, visual stimuli (Curran & Dien, 2003; Misra & Holcombe, 2003). Additionally, evidence from highly predictive sentence contexts suggests that P2 amplitude may be linked to top-down matching processes, as perceptual input is compared to contextually derived expectations (Federmeier et al., 2005). Though a precise specification of the functional and neural basis of the P2 is lacking, the extant evidence suggests that the P2 may index implicit, higher-order perceptual processes that occur when a stimulus is being compared with mental representations that were either stored in memory or built from a linguistic context. If so, in a recognition experiment, P2 amplitude should be enhanced for words repeated at short lags, and perhaps even at longer lags if sufficient form-based information is retained and utilized during memory retrieval.
The ERP effects discussed so far concern comparisons among test stimuli. However, it is also possible to examine differences at the encoding stage by comparing ERPs to the study trials, sorted by later performance. Words that are correctly recognized at test have been associated with greater positivity at study, particularly in the 400–800 ms range. Although its distribution is variable, this difference due to memory, or Dm effect, is often widespread, having maximal effects over central and sometimes frontal channels (Paller, Kutas, & Mayes, 1987; see Münte, Urbach Düzel, & Kutas, 2000, for review). Hemodynamic measures have noted the importance of medial temporal lobe structures (particularly the hippocampus, but also perirhinal, entorhinal, and parahippocampal cortices) as well as the left prefrontal cortex in successful encoding (see Paller & Wagner, 2002, or Schacter & Wagner, 1999, for review). Data from intracranial and scalp recordings further suggest that left inferior prefrontal and medial temporal lobe structures may generate Dm activity (Fernández et al., 1999; Friedman & Johnson, 2000). Dm amplitude has often been taken to represent the depth or strength of encoding (Paller et al., 1987;Van Petten & Senkfor, 1996). Comparisons of Dm effects based on VF can thus provide an index of encoding asymmetries, relatively independent of retention and retrieval factors. Dm effects for lateralized study words have not been examined before, so it is not yet known whether differences in encoding strength, as indexed by this late positivity, are predictive of later recognition performance for stimuli presented to a single VF.
ERPs thus provide a set of functionally specified measures that can be used to examine verbal memory. As already discussed, there has been very little work of any kind looking at the timecourse of memory in the two hemispheres and no research on verbal memory asymmetries using ERPs. There-fore, the present experiment uses ERP measures in conjunction with a VF design to examine memory asymmetries that arise due to encoding biases, and howthese asymmetries interact with repetition lag. Following Federmeier and Benjamin (2005), the experiment employs a continuous recognition task, with interleaved lateralized study words and central test words, spaced by a range of repetition lags. Three particular aspects of the ERP response are examined. The old/new effect (a point-by-point subtraction of the ERP response to hits and correct rejections) is expected to show a general decrease with lag, as previous studies have commonly found for centrally presented study and test words (e.g., Nagy & Rugg, 1989). The timecourse of the P2 repetition effect has not been extensively examined in explicit recognition, so it is possible that it will only obtain at short lags (as has been found in implicit priming, Misra & Holcolmbe, 2003). Of critical interest is whether the timecourse of these retrieval-related memory effects differs for words that were originally studied in the RVF/LH as compared with the LVF/RH. If the RT effects observed by Federmeier and Benjamin (2005) were driven by asymmetries in the strength of the memory signal, a similar pattern might be seen on the old/new effect, with hits to RVF/LH-studied words eliciting more positive ERP responses at short and medium lags, but hits to LVF/RH-studies items showing more positive responses at long lags. If, however, the RT difference reflects contributions from an implicit and perceptually based memory signal, then VF-based differences might be manifest instead on components such as the P2. Dm effects are also examined to see whether behavioral performance is more closely yoked to encoding-stage brain activity for items processed in one of the two VFs.
2. Methods
2.1. Participants
Data were obtained from a final set of 24 University of Illinois undergraduates (12 female and 12 male), 1 who participated in the study for course credit or monetary compensation. Mean age of the participants was 19 years (range: 18–24). All were native speakers of English with no early exposure (<5 years) to a second language and were right handed, as indexed by the Edinburgh handedness inventory (Oldfield, 1971). Mean laterality quotient was 0.73 (range: 0.19–1.00), where 1.0 is strongly right-handed and–1.0 is strongly left-handed; six participants reported having left-handed members of their immediate family. All participants gave informed consent prior to participation, and the experiment was approved by the University of Illinois Institutional Review Board.
2.2. Stimuli
A set of 567 highly concrete and imageable (both with ratings of 500–700 on a scale from 100 to 700) singular nouns, 4–6 letters in length and with a written frequency of 2–60 (Francis & Kucera, 1982), were selected from the MRC Psycholinguistic Database (Coltheart, 1981). Words were randomly selected from this set to create two, nonoverlapping experimental lists for each participant (one list to be used in each of two experimental blocks). Each list contained 112 new test words (tested but not studied) and 144 repeated words (seen twice: at study and at test; all study items were later tested). Lists were matched between participants so that across the experiment identical words appeared in each VF for each condition.
Words were presented serially in a continuous recognition design. Old test words were separated from their study presentation by lags of 1 (immediate repetition), 2, 3, 5, 7, 10, 20, 30, and 50. For each participant, 32 old test words appeared at each lag, with 16 studied in each VF. A different lag structure was used in each of the experimental blocks. In order to have sufficient trials in each condition to obtain stable ERPs, lags were grouped at analysis into three classes: short (1, 2, 3), medium (5, 7, 10), and long (20, 30, 50).
2.3. Procedure
Participants were tested individually in a quiet, dimly lit room, and seated 100 cm in front of a computer screen. They were instructed to focus on a central fixation point that remained throughout the experiment and to try to minimize saccadic eye movements and blinks. As words appeared, participants were to read and remember the lateralized study items, without making a response. They were told to respond as accurately as possible2 to the centrally presented words, by pressing the “yes” button in response to words that they had previously seen at any prior point in the experiment (old test words), and the “no” button to unfamiliar test words (new test words). One response button was held in each hand, and the hand used to respond “yes” was counterbalanced across participants. After demonstrating proficiency with the task and with eye control in a brief practice block, participants completed two test blocks lasting approximately 20 min each, with a 5–10 min interim break.
A small, black fixation point remained in the horizontal center (0.5 visual degrees below the vertical center) of a white background during both study and test trials. Words were presented in black sans serif font in capital letters. Laterally presented study words were presented with their central-facing edge two visual degrees from the horizontal center and remained on the screen for 200 ms, followed by an interstimulus interval of 2300 ms. Test words appeared just above the fixation cross and remained on the screen until a response was registered. They were followed by a 2500 ms interstimulus interval.
2.4. EEG recording
The electrooculogram (EOG) signal was recorded with a bipolar montage of silver/silver-chloride (Ag/AgCl) electrodes placed on the outer canthus of each eye to detect saccades. Additionally, one electrode placed below the left eye detected blinks. Scalp-recorded electroencephalography (EEG) data were collected from 26 geodesically arranged electrodes embedded in an electrocap. All impedances were kept under 5 kΩ. Excepting the bipolar montage detecting saccades, all electrodes were referenced online to the left mastoid process and later rereferenced to the algebraic mean of the left and right mastoids. Data were bandpass filtered from 0.02 to 100 Hz online and sampled at 250 Hz.
Prior to averaging, trials containing eye movements (abrupt voltage shifts in the EOG) or other artifacts (signal drift, amplifier blocking, etc.) were rejected with thresholds individualized to each participant by visual inspection. Trials containing eye blinks were corrected for 21 subjects, using a procedure developed by Dale (1994); blink trials were rejected for the remaining three subjects, who had an insufficient number of blinks to obtain a stable filter. In addition to the removal of test trials that contained saccades, study trials that elicited eye movements during the 200 ms of stimulus presentation were marked, and the corresponding test trials were also excluded from further analysis. Average total trial loss was 5.9 percent. ERPs were computed from 100 ms before to 920 ms after the onset of each word. After subtracting the100 ms pre-stimulus baseline, averages of artifact-free ERPs were calculated for each experimental condition. For test stimuli, these conditions were: correct rejections; RVF-studied short lag hits; LVF-studied short lag hits; RVF-studied medium lag hits; LVF-studied medium lag hits; RVF-studied long lag hits; LVF-studied long lag hits. ERP averages for the Dm effect analyses were created by sorting study trials based on recognition performance at test. Because it is likely that different factors contribute to recognizing or not recognizing test words that are repeated at different lags, comparisons were only done within lag groups. However, sufficient numbers of both hits and misses were needed to obtain stable ERP data, and therefore averages could be created only for words later repeated at long repetition lags, resulting in four conditions: RVF study/later remembered at long lags; RVF study/later forgotten at long lags; LVF study/later remembered at long lags; LVF study/later forgotten at long lags. Measurements were taken after a digital bandpass filter of 0.2–20 Hz was applied. For all ERP analyses, the reported p-values reflect the Greenhouse-Geisser epsilon correction for repeated measures with more than 1 degree of freedom. Interactions with electrode sites are only reported when of theoretical significance.
3. Results
3.1. Behavioral responses
Mean accuracy and RT data are presented in Table 1 and Table 2, respectively. Mean accuracy for centrally presented new test words was 86.00 percent. For old test words, a 2 (Study VF: RVF, LVF) × 3 (Lag Group: short, medium, long) repeated measures ANOVA on the accuracy data revealed main effects of Study VF (F(1,23) = 29.97, p < .001) and Lag Group (F(2,46) = 52.17, p < .001), as well as a significant interaction (Study VF × Lag Group: F(2,46) = 4.21, p < .05). As expected, accuracy decreased with lag, and was better for words originally studied in the RVF/LH at all lags. The accuracy difference between the VFs increased between the short and medium lags (possibly reflecting the influence of a ceiling effect at the shortest lags), but decreased again at the longest lags. This same numerical pattern was present in the Federmeier and Benjamin (2005) data.
Table 1.
Mean accuracy (percent correct) across conditions
| Short lag repetitions |
Medium lag repetitions |
Long lag repetitions |
|
|---|---|---|---|
| Studied in RVF/LH | 84.04 (2.12) | 78.13 (1.91) | 64.83 (2.36) |
| Studied in LVF/RH | 77.33 (1.98) | 63.88 (2.84) | 55.50 (2.67) |
| Difference: RVF – LVF | 6.71 | 14.25 | 9.33 |
| t-Values | 2.31a | 4.16b | 2.62a |
Note. Mean square errors are given in parenthesis.
p < .05.
p < .01.
Table 2.
Mean response time (ms) across conditions
| Short lag hits | Medium lag hits | Long lag hits | |
|---|---|---|---|
| Studied in RVF/LH | 869.87 (35.94) | 976.78 (41.66) | 1091.28 (61.32) |
| Studied in LVF/RH | 932.25 (47.91) | 1003.60 (50.26) | 1087.07 (81.32) |
| Difference: RVF – LVF | −62.38 | −26.82 | 4.21 |
| t-Values | −1.04 | −0.41 | 0.04 |
Note. Mean square errors are given in parenthesis.
No t-tests were significant.
The mean RT for correct rejections was 1146.41 ms. For correctly identified repeated words (hits), a 2 (Study VF: RVF, LVF) × 3 (Lag Group: short, medium, long) repeated measures ANOVA revealed a main effect of Lag Group (F(2,46) = 14.22, p < .001), but not of Study VF (F(1,23) = 1.42, p = ns); there was no interaction (Study VF × Lag Group: F(2,46) = 1.59, p = ns). As expected, RTs increased with increasing lag. Because the instructions emphasized accuracy over speed, it is perhaps not surprising that VF effects were not significant in this data set. However, the numerical pattern replicated that in Federmeier and Benjamin (2005): at the shortest lags, response times favored words studied in the RVF/LH, but this advantage diminished and ultimately disappeared with increasing lag.
3.2. ERPs
Fig. 1 shows the grand average ERPs to all correctly classified old test words for each Study VF condition, along with correctly classified new words. Both new words and old words elicited the expected early perceptual and attentional components. At posterior electrode sites, these include a positive peak maximal around 140 ms (P1), a negative peak maximal around 195 ms (N1), and a positivity that was strongest around 320 ms (P2). Both the N1 and the P2 can also be seen at frontal electrodes, but earlier in time, with the frontal N1 peaking around 60 ms, and the frontal P2 peaking around 200 ms. These early components are followed by a negativity peaking around 350 ms (N400), and a positivity occurring between 500 and 800 ms (LPC); both have fairly broad distributions, but are strongest over medial, centro-parietal sites. These effects, and their differences across conditions, will be discussed in more detail below.
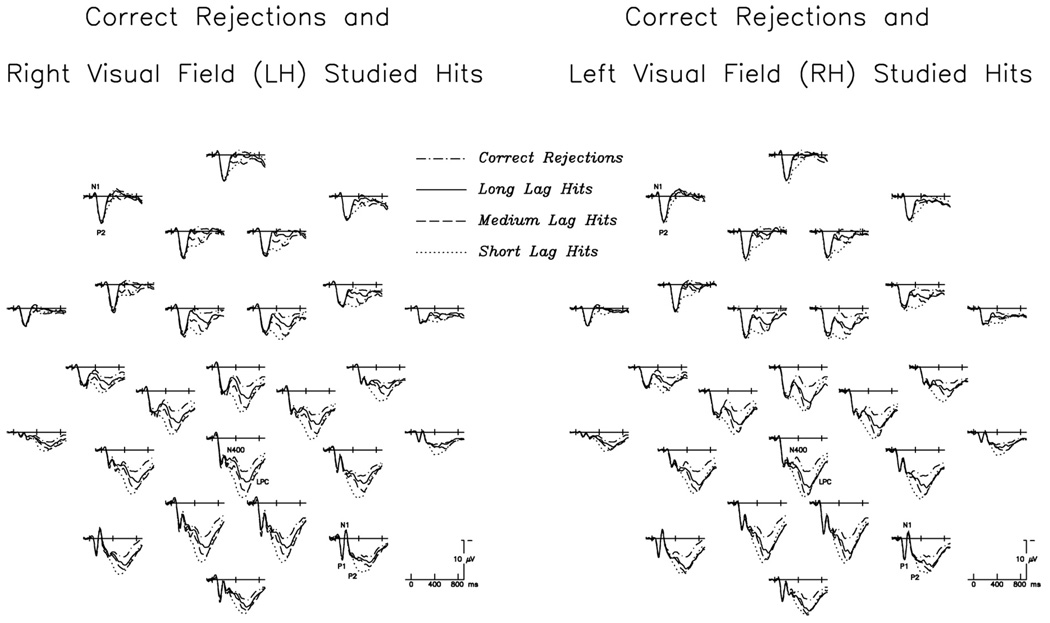
Fig. 1.
Grand average ERP waveforms to correctly identified test words, at all 26 scalp electrodes. At left are RVF/LH-studied hits (repeated at short, medium, and long lags) plotted with correct rejections; at right are LVF/RH-studied hits (repeated at short, medium, and long lags) plotted with correct rejections (note that test words appeared in central vision, and correct rejection waveforms are therefore identical in the two figures). Here, and in all subsequent figures, negative is plotted up. Latencies and amplitudes of early perceptual components (P1, N1, P2) are similar for all conditions, except for frontal P2s, which are larger for RH-studied hits. Old/new effects are visible at most electrode sites and for items studied in either VF, with greater positivity to hits than to correct rejections; this difference is strongest over centro-parietal sites and apparent in timewindows encompassing both the N400 and the LPC.
3.2.1. Old/new effect
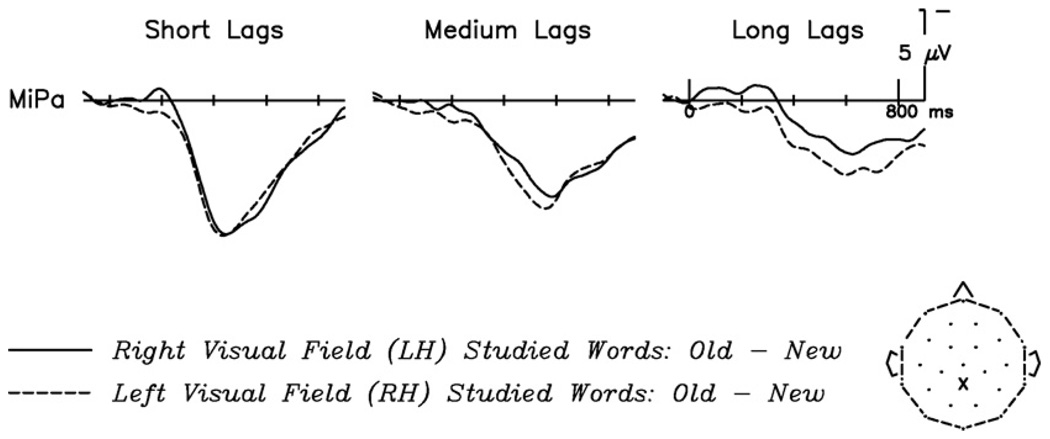
Old/new effects, which have been shown to be modulated by explicit recognition (Rugg & Doyle, 1994), were examined using nine medial, centro-posterior electrode sites, where such responses tend to be most pronounced (see Fig. 1). Old/new difference waves were derived by subtracting, on a point-by-point basis, the electrical response to correctly rejected new test words from the response elicited to correctly recognized repeated words. This was done separately for each VF and lag group, yielding six difference waves: RVF-studied short lag hits-correct rejections; LVF-studied short lag hits-correct rejections; RVF-studied medium lag hits-correct rejections; LVF-studied medium lag hits-correct rejections; RVF-studied long lag hits-correct rejections; LVF-studied long lag hits-correct rejections. Fig. 2 shows old/new difference waves at a representative channel for RVF/LH-studied words and for LVF/RH-studied words, across lag conditions. To compare the Study VF and Lag Group effects, mean amplitude measures from 250 to 800 ms for each of the six difference waves were submitted to a 2 (Study VF: RVF, LVF)×3 (Lag Group: short, medium, long) × 9 (Electrode) repeated measures ANOVA. A main effect of Lag Group (F(2,46) = 15.05, p < .001) showed, as predicted, that the size of the old/new effect decreased with increasing lag. There was no main effect of Study VF (F(1,23) = 2.14, p = ns), nor did the two terms interact (F(2,46) = 1.21, p = ns). However, planned comparisons at each lag condition, using a 2 (Study VF: RVF, LVF) × 9 (Electrode) ANOVA, revealed a significant effect of Study VF on the size of the old/new difference wave for words repeated at long lags (F(1,23) = 4.33, p < .05), indicating a stronger memory signal for words that had originally been studied in the LVF. No effects of Study VF were seen at either short (F(1,23) = 0.00, p = ns) or medium (F(1,23) = 0.36, p = ns) lags.
Fig. 2.
Old/new difference waves across lag conditions as a function of study VF. Effect is shown at the midline parietal channel (MiPa), where the old/new effect and modulations thereof are strongest. At both short and medium lags, the old/new effect does not differ by study VF. (Note, however, the amplitude differences around 200 ms, which reflect P2 repetition effects exhibited only by RH-studied words; see Fig. 3) At long lags, the old/new effect is greater for LVF/RH-studied words throughout the recording epoch.
3.2.2. P2 repetition effect
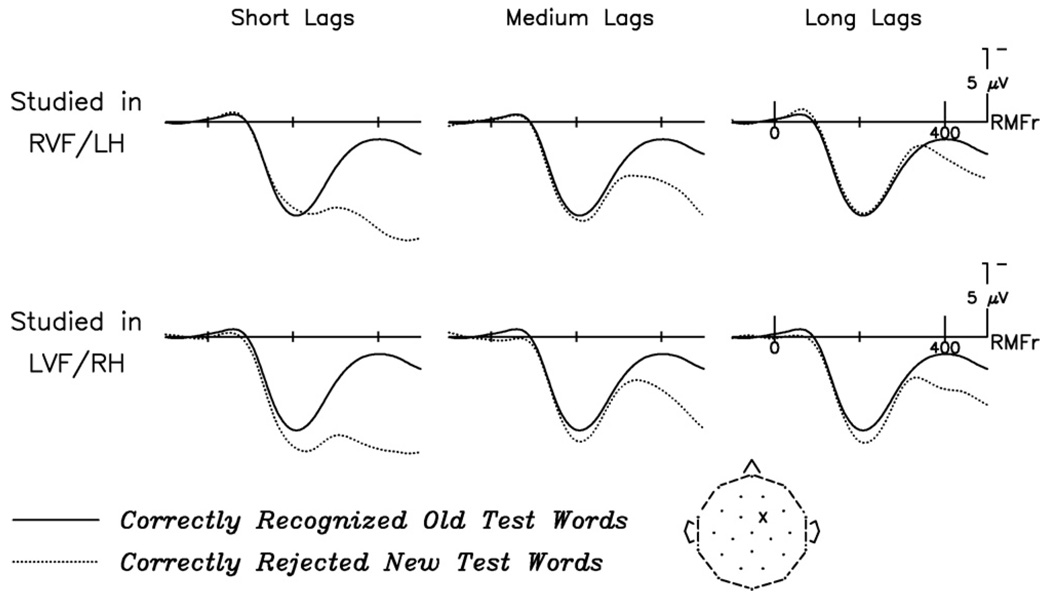
The P2 component was also analyzed, as its amplitude has been shown to increase for repeated words and may be linked to perceptual matching processes. Fig. 3 shows early responses to new, RVF-studied, and LVF-studied test words, at a representative channel (see also Fig. 1, for effect distribution). Analyses were conducted using six frontal electrode sites where P2 effects are typically most prominent; additionally, this selection reduces the potential for contamination from overlapping components (such as the N400), which are more prominent over posterior sites. Because the P2 repetition effect is a difference between new and old test words, this effect was directly compared using the old/new difference waves for words studied by each VF and repeated in each of the three lag conditions minus correctly rejected words (i.e., the same six difference waves from the old/new effect analyses were used, but now focused on an earlier time-window and more anterior electrodes sites). Mean amplitudes were measured from 150 to 250 ms, and compared in a 2 (Study VF: RVF, LVF) × 3 (Lag Group: short, medium, long) × 6 (Electrode) ANOVA. This analysis revealed a main effect of Study VF (F(1,23) = 5.13, p < .05), indicating a stronger repetition effect for LVF-studied words. However, there was no main effect of Lag Group (F(2,46) = 0.49, p = ns) and no Study VF by Lag Group interaction (F(2,46) = 2.15, p = ns), indicating that the magnitude of the P2 repetition effect was consistent across lag for both Study VFs. To follow up on the Study VF effect, comparisons of P2 mean amplitudes to correctly recognized test words and to correctly rejected new words (now single condition waveforms, rather than difference waves) were conducted separately for each VF: 2 (Test Condition: new, old) × 6 (Electrode). These analyses revealed no effect of Test Condition for RVF-studied words (F(1,23) = 0.03, p = ns) but a significant difference for LVF-studied words (F(1,23) = 9.29, p < .01). Thus, words that had been initially studied in the LVF elicited a reliable P2 repetition effect, which was retained across lag, while words that had been initially studied in the RVF did not elicit P2 repetition effects at any lag.
Fig. 3.
P2 repetition effects at each lag, for RVF/LH-studied hits (first row) and LVF/RH-studied hits (second row). Effects are shown at the right medial frontal electrode site (RMFr), where P2 effects are prominent, with an abbreviated timescale (100 ms pre-stimulus to 500 ms post-stimulus onset). For RVF/LH-studied test words, P2 responses to hits and correct rejections did not differ at any lag. For LVF/RH-studied test words, in contrast, P2 responses to hits were more positive than P2 responses to correct rejections at all lags. (Note that correct rejections do not have a lag condition, but are overplotted at each to illustrate the repetition effect.)
3.2.3. Dm effect
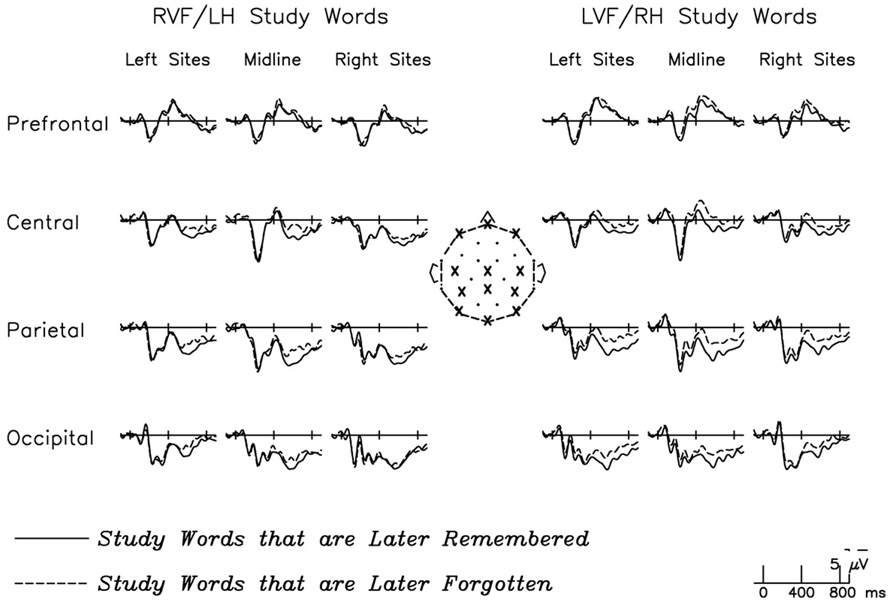
Encoding strength was examined in the Dm effect by comparing RVF and LVF study words that were later remembered to those that were later forgotten (Fig. 4). Because relatively few words are missed with short and medium lag repetitions, these effects could be examined only for the long lag group. Dm effects have never been examined for lateralized study stimuli, and therefore it was important to empirically determine the distribution of the effect (if any). Thus, initial analyses were conducted from 400 to 800 ms using a repeated measures ANOVA that included distributional factors: 2 (Study VF: RVF, LVF) × 2 (Memory: later forgotten, later remembered) × 2 (Hemisphere: LH, RH electrode sites) × 2 (Laterality: lateral, medial electrode sites) × 4 (Anteriority: prefrontal, frontal, central/ parietal, occipital electrode sites).3 There was a significant effect of Memory (F(1,23) = 6.15, p < .05), such that lateralized study words that were later remembered were associated with increased positivity in this time-window. This effect was fairly widespread, but tended to have a medial, posterior, slightly LH-biased distribution (Memory × Laterality: F(1,23) = 4.80, p < .05; Memory × Hemisphere × Anteriority: F(3,69) = 3.64, p < .05; Memory × Laterality × Anteriority: F(3,69) = 6.05, p < .01; Memory × Hemisphere × Laterality × Anteriority: F(3,69) = 3.30, p < .05). Critically, there was no Study VF by Memory interaction (F(1,23) = .37, p = ns), nor did these two factors interact with any distributional factors.4
Fig. 4.
Dm effects for RVF/LH study words (left side of figure) and LVF/RH study words (right side of figure). Selected channels present the distribution of the Dm effect over left hemisphere, midline, and right hemisphere electrodes, at prefrontal regions, central regions, parietal regions, and occipital regions. For both VFs, encoding phase activity is predictive of later memory performance, in the form of greater central-posterior positivity for words that are later remembered at long lags as compared with that for words that are later forgotten.
As recommended by the distributional interactions, subsequent analyses of the Dm effect in each visual field used the eight medial posterior sites where the effect seemed to be most prominent. The magnitude of the Dm effect for words studied in each VF was examined in a 2 (Memory: later forgotten, later remembered) × 8 (Electrode) ANOVA, which revealed a marginal difference for RVF study words (F(1,23) = 2.82, p = .11) but a significant difference for LVF study words (F(1,23) = 13.54, p < .01). As these results hinted that there may be VF differences, a direct comparison of the effect across the two VFs (using difference waves: RVF study words that were later remembered minus RVF study words later forgotten; LVF study words later remembered minus LVF study words later forgotten) was conducted: 2 (Study VF: LVF, RVF) × 8 (Electrode). However, this analysis revealed no significant difference in the size of the effect (F(1,23) = 0.19, p = ns), consistent with the lack of a significant interaction in the distributional analysis. Thus, study phase activity to words in both VFs was predictive of later memory, with a tendency for this difference to be more robust for LVF/RH presented words.
4. Discussion
The goal of this experiment was to assess the timecourse of hemispheric asymmetries that arise during the encoding of verbal material, and, from this, to develop an understanding of the type(s) of representation each hemisphere builds and maintains from a verbal stimulus. Memory asymmetries have been examined previously, but this was the first study to collect electrophysiological measures in addition to behavioral indices, thus providing data from multiple stages of processing. In particular, this experiment examined VF asymmetries for three ERP effects linked to memory: the old/new effect, which has been linked to the strength of the explicit memory signal at test; the P2 repetition effect, which is sensitive to early implicit memory and perceptual matching processes during retrieval; and the Dm effect, which captures brain activity during the encoding stage that is predictive of later recognition performance.
As expected, the accuracy data favored words initially studied in the RVF/LH, and this was true at all repetition lags (largest for medium lags). This is consistent with previous experiments (Berrini et al., 1982; Hannay & Malone, 1976; Hines et al., 1973), including a recent study using the same stimuli and lag structure (Federmeier & Benjamin, 2005). Despite the pervasiveness of this effect, accuracy advantages for RVF/LH stimuli may not reflect superior memory per se. In particular, it has been shown that word apprehension is faster and more accurate for RVF than for LVF presentation (Jordan et al., 2003). Accordingly, in an experiment with lateral study words and central test words, accuracy of response to the test probes could be affected if the initial study words were misread (which happens more often in the LVF/RH: Fontenot & Benton, 1972; Pirozzolo & Rayner, 1977) or disappeared before processing was completed (which is more likely to happen for the slower LVF/RH: Day, 1979). In other words, if RH word processing difficulties prevent participants from accurately perceiving LVF/RH study words, the participants will not be able to recognize these poorly read words when repeated at test.
This problem can be circumvented by examining the responses to correct trials, so that comparisons are made between words that were likely adequately encoded in both VFs. Using this approach, Federmeier and Benjamin (2005) observed lag-related changes in the RT data for hits: RTs to words repeated at short lags favored RVF/LH encoding, but correct responses at the longer lags were faster for words that had been studied in the LVF/RH. In the present experiment, a similar RT pattern was obtained (though smaller and nonsignificant, probably due to the change in instructions emphasizing accuracy over speed). Furthermore, lag-related VF effects were evident in the ERP old/new effect, which has been linked to explicit recognition processes subserved primarily by anterior temporal lobe structures (Guillem et al., 1999; see Friedman & Johnson, 2000, for review). Words tested at short and medium lags elicited comparable old/new effects across the two VF conditions, whereas for repetitions at long lags, the old/new effect elicited to words initially studied in the LVF/RH was stronger than that to RVF/LH-studied words, paralleling the RT advantages observed by Federmeier and Benjamin (and weakly replicated in the RT data here). It is striking that the old/new effect at short and medium lags did not manifest the RVF/LH advantage seen here and previously (Federmeier & Benjamin, 2005) in the speed of overt responses to these items, given that modulations of the old/new effect did mirror other aspects of the behavior (e.g., the amplitude of the old/new effect decreased incrementally with increasing lag, mimicking the corresponding increase in RTs, and this was true for words that had been studied in either VF). This suggests that the observed RVF/LH behavioral advantages may not reflect memory asymmetries as such, but may instead be driven by perceptual or attentional biases favoring RVF/LH word apprehension (consistent with the appearance of these behavioral effects with immediate repetition, when memory demands are low). In contrast, long lag advantages were seen for LVF/RH studied words in both measures – the RT crossover and the emergence of a stronger old/new effect – pointing to a memory signal that decays more gradually in the RH and eventually balances or even overcomes the standing RVF/LH perceptual advantages. Thus, something about how the RH encodes words seems to afford it an advantage for retaining verbal information over long time intervals, across increasing amounts of interference, or a combination of both factors.
Federmeier and Benjamin (2005) speculated that the LVF/RH advantage for retrieval at long lags may arise because the RH tends to encode verbal material more veridically than the LH does, as has been shown in a number of priming and perceptual studies (e.g., Marsolek, 1999; Marsolek et al., 1992) and as is also consistent with the results of some memory experiments (e.g., Fabiani et al., 2001; Metcalfe et al., 1995). In line with the conclusion that the RH retains stimulus information that the LH does not, the present experiment showed a repetition effect (amplitude changes on the P2) that occurred only in response to correctly recognized words that had been studied in the LVF/RH. An increase in P2 amplitude has been reported in a variety of tasks involving either implicit or explicit perceptual matching processes (explicit verbal memory: Curran & Dien, 2003; feature extraction: Luck & Hillyard, 1994; repetition priming: Misra & Holcombe, 2003; semantic predictions: Federmeier et al., 2005). If P2 changes are sensitive to perceptual matching, then in the context of a memory experiment, the size of P2 repetition effects might index the process of comparing perceptual aspects of the test stimulus with stored memory traces. Such processes would be expected to be particularly strong in the RH, given its retention of form-based information. Indeed, at test, LVF/RH-studied words elicited P2 responses that were enhanced relative to the P2 seen to new test words, and this effect was maintained across the lag spectrum. That this effect remains for LVF/RH-studied items even at repetition lags of 20–50 suggests that form-based information associated with LVF/RH-encoded words is relatively robust to interference and decay, and thus can be retained over long time intervals. Furthermore, at longer lags, when explicit memory for information encoded by either hemisphere is weak (as seen in the reduction of old/new effect amplitude and response accuracy), the support provided by this relatively rapid, perceptual facilitation may become particularly important, and may contribute to the long lag advantages seen for LVF/RH-studied words in other measures (i.e., RTs and the old/new effect). In contrast, repeated words studied in the RVF/LH produced P2 responses that did not differ from those to new test words at any lag. The absence of such an effect for RVF/LH-encoded words suggests that even when the LH encodes words well enough to recognize them at test, it either retains little information about their visual form or fails to use this information during the early stages of processing repeated words, even when words are repeated almost immediately.
Finally, this was the first experiment to look at subsequent memory (Dm effects) for lateralized study words. The Dm effect is believed to reflect activity in medial temporal lobe structures, which are more active during successful encoding, that is, encoding that later leads to correct recognition (Fernández et al., 1999; see Friedman & Johnson, 2000, for review). As seen in prior studies (e.g., Paller et al., 1987), words that were later remembered were associated with more positive responses at encoding than words that were later forgotten. This was true for words studied in either VF, and the size of this effect was statistically comparable across the two (though numerically bigger and statistically more robust for LVF words). The similarity of this encoding activity across VF conditions suggests that despite encoding biases, some of the processes that are engaged during LH-biased and RH-biased encoding are similar. This is particularly important given evidence that the presence of a Dm effect is contingent upon deep semantic processing (Van Petten & Senkfor, 1996); if encoding processes must involve semantic elaboration in order to be predictive of subsequent recognition, then the current results imply that both RVF/LH and LVF/RH study words initiate such processing. Thus, although the RH seems to retain veridical traces that include form-based information, it also seems to engage in higher-order semantic processing and to make this information available at later decision-making stages. It is important to note, however, that because the current analyses were restricted to study words that were later repeated at long lags (because other lag conditions did not have enough miss trials), it is possible that stronger VF biases in the Dm effect could be obtained to study words repeated at short or medium lags, as such judgments may rely on slightly different information from the encoding experience.
5. Conclusions
Overall, the pattern of similarities and differences between the behavioral and electrophysiological measures for this task highlights the importance of investigating cognitive questions with multiple, converging measures; this seems particularly relevant for VF designs, in which asymmetries may arise from attentional or perceptual biases that preempt the higher-order functions under investigation. In the present study, analyses performed on three ERP memory effects failed to find RVF/LH advantages commensurate with those seen in behavioral performance for the same stimuli and task. In contrast, analyses of both the old/new memory effect and the P2 repetition effect found stronger memory signals for words studied in the LVF/RH. The perceptual facilitation indexed by the P2 effect was seen for LVF/RH-studied test words at all lags and may reflect a RH bias for form-based encoding. In contrast, the old/new effect advantage for the LVF/RH-studied items was seen only at the longest repetition lags, where Federmeier and Benjamin had previously seen RT advantages for these items. Taken together, these data suggest that memory for items studied in the LVF/RH is less disrupted by intervening words than memory for RVF/LH-studied items, and this may reflect RH-specific encoding strategies.
The results from the current experiment and from Federmeier and Benjamin (2005) are in line with a growing number of studies showing that the two cerebral hemispheres make important and unique contributions to various aspects of verbal processing. Furthermore, these studies provide an important link between the literature on language processing, which has pointed to a privileged role for the RH in processing discourse and other sorts of higher-level language structures that require verbal information to be retained and revised (see Brownell & Martino, 1998, for review), and the literature on memory for single words, which has suggested that the RH’s verbal representations are impoverished and that its processing limitations become exacerbated as memory demands increase (Day, 1977, 1979; Hannay & Malone, 1976; Hines, 1976; Hines et al., 1973). On the surface, these results seem to be incompatible: If the RH has difficulties encoding and retaining the information associated with a single word, how can it play an important role in maintaining, integrating, and updating material associated with multiple words? Using measures that minimize the influence of biases that favor the RVF/LH for the basic apprehension of words, this study and the work by Federmeier and Benjamin (2005) found advantages for RH encoding in RTs, the old/new effect, and the P2 repetition effect, especially with long study-test retrieval lags. Hence, though further work is clearly needed, these findings recast verbal memory asymmetries in a way that connects the processing and retention of single words to the RH’s vital role in processing various complex language structures that unfold over time, and therefore rely, to some extent, on the retention of verbal information.
Acknowledgements
This work was supported by NIA grant AG26308 to KDF, and NIH training grant T32 MH-1819990 to KME. We thank Aaron Benjamin for contributing to the experimental design and commenting on earlier versions of this paper, and we also thank the undergraduate assistants in the Cognition and Brain Lab for their help with data collection, especially Caterina Gratton, Shruti Gupta, Angela Kaplan, and Matthew Rambert.
Footnotes
Participants who were unable to suppress saccadic eye movements to the lateralized study items, resulting in fewer than 18 out of a possible 48 trials in one or more experimental conditions, were dropped from the study
Accuracy, rather than speed, was emphasized in order to reduce contamination of the ERPs by response-related activity.
Distributional analyses thus used a total of 16 scalp sites: left lateral, left medial, right medial, and right lateral electrodes across each of prefrontal, frontal, central, and occipital regions.
Words presented in a single visual half field, such as the study words in this experiment, yield a contralateral selection negativity in this time-window (e.g., Federmeier & Kutas, 1999;Neville, Kutas, & Schmidt, 1982), seen here as a main effect of Study VF (F(1,23) = 10.36, p = .004), and interactions between Study VF and distributional factors. Because these effects are expected for lateralized stimuli, and do not interact with the critical factors under investigation (here, Memory), they will not be reported in detail.
References
- Berrini R, Capitani E, Della Sala S, Spinnler H. Interaction between lateralization of memory and probe stimulus in the recognition of verbal and spatial visual stimuli. Neuropsychologia. 1984;22:517–520. doi: 10.1016/0028-3932(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Berrini R, Della Sala S, Spinnler H, Sterzi R, Vallar G. In eliciting hemisphere asymmetries which is more important: The stimulus input side or the recognition side? A tachistoscopic study on normals. Neuropsychologia. 1982;20:91–94. doi: 10.1016/0028-3932(82)90091-4. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Capitani E, Luzzatti C, Spinnler HR. Does the hemisphere stimulated play a specific role in delayed recognition of complex abstract patterns? A tachistoscopic study. Neuropsychologia. 1979;17:93–97. doi: 10.1016/0028-3932(79)90027-7. [DOI] [PubMed] [Google Scholar]
- Bihrle AM, Brownell HH, Powelson JA, Gardner H. Comprehension of humorous and nonhumorous materials by left and right brain-damaged patients. Brain and Cognition. 1986;5:399–411. doi: 10.1016/0278-2626(86)90042-4. [DOI] [PubMed] [Google Scholar]
- Blanchet S, Desgranges B, Denise P, Lechevalier B, Eustache F, Faure S. New questions on the hemispheric encoding/retrieval asymmetry (HERA) model assessed by divided visual-field tachistoscopy in normal subjects. Neuropsychologia. 2001;39:502–509. doi: 10.1016/s0028-3932(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Brownell H, Martino G. Deficits in interference and social cognition: The effects of right hemisphere brain damage on discourse. In: Beeman M, Chiarello C, editors. Right hemisphere language comprehension: Perspectives from cognitive neuroscience. Mahwah, NJ: Lawrence Erlbaum Associates; 1998. pp. 309–328. [Google Scholar]
- Brownell HH, Simpson TL, Bihrle AM, Potter HH, Gardner H. Appreciation of metaphoric alternative word meanings by left and right brain-damaged patients. Neuropsychologia. 1990;28:375–383. doi: 10.1016/0028-3932(90)90063-t. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Marsolek CJ. Letter-case-specific priming in the right cerebral hemisphere with a form-specific perceptual identification task. Brain and Cognition. 1997;35:239–258. doi: 10.1006/brcg.1997.0940. [DOI] [PubMed] [Google Scholar]
- Chiarello C. Lateralization of lexical processes in the normal brain: A review of visual half-field F research. In: Whitaker HA, editor. Contemporary reviews in neuropsychology. New York: Springer-Verlag; 1988. pp. 36–76. [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Quarterly Journal of Experimental Psychology A. 1981;33:497–505. [Google Scholar]
- Coney J, MacDonald S. The effect of retention interval upon hemispheric processes in recognition memory. Neuropsychologia. 1988;26:287–295. doi: 10.1016/0028-3932(88)90081-4. [DOI] [PubMed] [Google Scholar]
- Coulson S, Williams RF. Hemispheric asymmetries and joke comprehension. Neuropsychologia. 2005;43:128–141. doi: 10.1016/j.neuropsychologia.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Curran T, Dien J. Differentiating amodal familiarity from modality-specific memory processes: An ERP study. Psychophysiology. 2003;40:979–988. doi: 10.1111/1469-8986.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Unpublished doctoral dissertation. San Diego, La Jolla: University of California; 1994. Source localization and spatial discriminant analysis of event-related potentials: Linear approaches. [Google Scholar]
- Day J. Right-hemisphere language processing in normal right-handers. Journal of Experimental Psychology: Human Perception and Performance. 1977;3:518–528. doi: 10.1037//0096-1523.3.3.518. [DOI] [PubMed] [Google Scholar]
- Day J. Visual half-field word recognition as a function of syntactic class and imageability. Neuropsychologia. 1979;17:515–519. doi: 10.1016/0028-3932(79)90059-9. [DOI] [PubMed] [Google Scholar]
- Dee H, Fontenot DJ. Cerebral dominance and lateral differences in perception and memory. Neuropsychologia. 1973;11:167–173. doi: 10.1016/0028-3932(73)90004-3. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Kroll NEA, Tulving E, Knight RT, Gazzaniga MS. Unilateral medial temporal lobe memory impairment: Type deficit, function deficit, or both? Neuropsychologia. 1998;36:115–127. doi: 10.1016/s0028-3932(97)00094-8. [DOI] [PubMed] [Google Scholar]
- Elger CE, Grunwald T, Lehnhertz K, Kutas M, Helmstaedter C, Brockhaus A, Van Roost D, Heinze HJ. Human temporal lobe potentials in verbal learning and memory processes. Neuropsychologia. 1997;35:657–667. doi: 10.1016/s0028-3932(96)00110-8. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Stadler MA, Wessels PM. True but not false memories produce a sensory signature in human lateralized brain potentials. Journal of Cognitive Neuroscience. 2000;12:941–949. doi: 10.1162/08989290051137486. [DOI] [PubMed] [Google Scholar]
- Faust M, Bar-lev A, Chiarello C. Sentence priming effects in the two cerebral hemispheres: Influences of lexical relatedness, word order, and sentence anomaly. Neuropsychologia. 2003;41:480–492. doi: 10.1016/s0028-3932(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Faust M, Chiarello C. Sentence context and lexical ambiguity resolution by the two hemispheres. Neuropsychologia. 1998;36:827–836. doi: 10.1016/s0028-3932(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Faust M, Kravetz S, Babkoff H. Hemisphericity and top-down processing of language. Brain and Language. 1993;44:1–18. doi: 10.1006/brln.1993.1001. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Benjamin AS. Hemispheric asymmetries in the timecourse of recognition memory. Psychonomic Bulletin and Review. 2005;12:993–998. doi: 10.3758/bf03206434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M. Right words and left words: Electrophysiological evidence for hemispheric differences in meaning processing. Cognitive Brain Research. 1999;8:373–392. doi: 10.1016/s0926-6410(99)00036-1. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Mai H, Kutas M. Both sides get the point: Hemispheric sensitivities to sentential constraint. Memory and Cognition. 2005;33:871–886. doi: 10.3758/bf03193082. [DOI] [PubMed] [Google Scholar]
- Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, Van Roost D, Elger CE. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–1585. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Finnigan S, Humphreys MS, Dennis S, Geffen G. ERP ‘old/new’ effects: Memory strength and decisional factor(s) Neuropsychologia. 2002;40:2288–2304. doi: 10.1016/s0028-3932(02)00113-6. [DOI] [PubMed] [Google Scholar]
- Fontenot DJ, Benton AL. Perception of direction in the right and left visual fields. Neuropsychologia. 1972;10:447–452. doi: 10.1016/0028-3932(72)90007-3. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage. Boston: Houghton Mifflin Company; 1982. [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gardner H, Brownell HH, Wopner W, Michelow D. Missing the point? The role of the right hemisphere in the processing of complex linguistic materials. In: Perecman E, editor. Cognitive processing in the right hemisphere. New York: Academic Press; 1983. pp. 169–191. [Google Scholar]
- Geffen G, Bradshaw JL, Nettleton NC. Hemispheric asymmetry: Verbal and spatial encoding of visual stimuli. Journal of Experimental Psychology. 1972;95:25–31. doi: 10.1037/h0033265. [DOI] [PubMed] [Google Scholar]
- Geffen G, Bradshaw JL, Wallace G. Interhemispheric effects on reaction time to verbal and nonverbal visual stimuli. Journal of Experimental Psychology. 1971;87:415–442. doi: 10.1037/h0030525. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Dimond SJ, Gazzaniga MS. Left field superiority for word matching. Neuropsychologia. 1972;10:463–466. doi: 10.1016/0028-3932(72)90009-7. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, Gabrieli JDE. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- Gonsalves B, Paller KA. Neural events that underlie remembering something that never happened. Nature Neuroscience. 2000;3:1316–1321. doi: 10.1038/81851. [DOI] [PubMed] [Google Scholar]
- Guillem F, Rougier A, Claverie B. Short- and long-delay intracranial ERP repetition effects dissociate memory systems in the human brain. Journal of Cognitive Neuroscience. 1999;11:437–458. doi: 10.1162/089892999563526. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Malone DR. Visual field effects and short-term memory for verbal material. Neuropsychologia. 1976;14:203–209. doi: 10.1016/0028-3932(76)90049-x. [DOI] [PubMed] [Google Scholar]
- Hellige JB. Effects of perceptual quality and visual field of probe stimulus presentation on memory search for letters. Journal of Experimental Psychology: Human Perception and Performance. 1980;6:639–651. doi: 10.1037//0096-1523.6.4.639. [DOI] [PubMed] [Google Scholar]
- Hellige JB. What’s right and what’s left: Hemispheric asymmetries. Cambridge, MA: University Press; 1993. [Google Scholar]
- Hellige JB, Webster R. Right hemisphere superiority for initial stages of letter processing. Neuropsychologia. 1979;17:653–660. doi: 10.1016/0028-3932(79)90040-x. [DOI] [PubMed] [Google Scholar]
- Hines D. Recognition of verbs, abstract nouns and concrete nouns from the left and right visual half-fields. Neuropsychologia. 1976;14:211–216. doi: 10.1016/0028-3932(76)90050-6. [DOI] [PubMed] [Google Scholar]
- Hines D, Satz P, Clementino T. Perceptual and memory components of the superior recall of letters from the right visual field. Neuropsychologia. 1973;11:175–180. doi: 10.1016/0028-3932(73)90005-5. [DOI] [PubMed] [Google Scholar]
- Hines D, Satz P, Schell B, Schmidlin S. Differential recall of digits in the left and right visual half-fields under free and fixed order of recall. Neuropsychologia. 1969;7:13–22. [Google Scholar]
- Johnson MK, Nolde SF, Mather M, Kounios J, Schacter DL, Curran T. The similarity of brain activity associated with true and false recognition memory depends on test format. Psychological Science. 1997;8:250–257. [Google Scholar]
- Jordan TR, Patching GR, Thomas SM. Assessing the role of hemispheric specialization, serial-position processing, and retinal eccentricity in lateralized word recognition. Cognitive Neuropsychology. 2003;20:49–71. doi: 10.1080/02643290244000185. [DOI] [PubMed] [Google Scholar]
- Kaplan JA, Brownell HH, Jacobs JR, Gardner H. The effects of right hemisphere damage on the pragmatic interpretation of conversational remarks. Brain and Language. 1990;38:315–333. doi: 10.1016/0093-934x(90)90117-y. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezen FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Peterson SE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim J, Kwon JS. The effect of immediate and delayed word repetition on event-related potential in a continuous recognition task. Cognitive Brain Research. 2001;11:387–396. doi: 10.1016/s0926-6410(01)00011-8. [DOI] [PubMed] [Google Scholar]
- Kimura D. Dual functional asymmetry of the brain in visual perception. Neuropsychologia. 1966;4:275–285. [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Leehey SC, Cahn A. Lateral asymmetries in the recognition of words, familiar faces, and unfamiliar faces. Neuropsychologia. 1979;17:619–635. doi: 10.1016/0028-3932(79)90036-8. [DOI] [PubMed] [Google Scholar]
- Leiber L. Interhemispheric effects in short-term recognition memory for single words. Cortex. 1982;18:113–124. doi: 10.1016/s0010-9452(82)80023-3. [DOI] [PubMed] [Google Scholar]
- Luck S, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ. Dissociable neural subsystems underlie abstract and specific object recognition. Psychological Science. 1999;10:111–118. [Google Scholar]
- Marsolek CJ, Kosslyn SM, Squire LR. Form-specific visual priming in the right cerebral hemisphere. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:492–508. doi: 10.1037//0278-7393.18.3.492. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ, Nicholas CD, Andresen DR. Interhemispheric communication of abstract and specific visual-form information. Neuropsychologia. 2002;40:1983–1999. doi: 10.1016/s0028-3932(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ, Schacter DL, Nicholas CD. Form-specific visual priming for new associations in the right cerebral hemisphere. Memory and Cognition. 1996;24:539–556. doi: 10.3758/bf03201082. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Funnell M, Gazzaniga MS. Right-hemisphere memory superiority: Studies of a split-brain patient. Psychological Science. 1995;6:157–164. [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clinical Neurosurgery. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Misra M, Holcolmbe PJ. Event-related potential indices of masked repetition priming. Psychophysiology. 2003;40:115–130. doi: 10.1111/1469-8986.00012. [DOI] [PubMed] [Google Scholar]
- Müunte TF, Urbach TP, Düzel E, Kutas M. Event-related brain potentials in the study of human cognition and neuropsychology. In: Boller F, Grafman J, Rizzolatti G, editors. Handbook of neuropsychology. Amsterdam: Elsevier Science; 2000. pp. 1–97. [Google Scholar]
- Nagy ME, Rugg MD. Modulation of event-related potentials by word repetition: The effects of inter-item lag. Psychophysiology. 1989;26:431–436. doi: 10.1111/j.1469-8986.1989.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Kutas M, Schmidt A. Event-related potential studies of cerebral specialization during reading. I. Studies of normal adults. Brain and Language. 1982;16:300–315. doi: 10.1016/0093-934x(82)90088-8. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olichney JM, van Petten C, Paller KA, Salmon DP, Iragui VJ, Kutas M. Word repetition in amnesia: Electrophysiological measures of impaired and spared memory. Brain. 2000;123:1948–1963. doi: 10.1093/brain/123.9.1948. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalography and Clinical Neurophysiology. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pillon B, Bazin B, Deweer B, Ehrle N, Baulac M, Dubois B. Specificity of memory deficits after right or left temporal lobectomy. Cortex. 1999;35:561–571. doi: 10.1016/s0010-9452(08)70819-0. [DOI] [PubMed] [Google Scholar]
- Pirozzolo FJ, Rayner K. Hemispheric specialization in reading and word recognition. Brain and Language. 1977;4:248–261. doi: 10.1016/0093-934x(77)90021-9. [DOI] [PubMed] [Google Scholar]
- Rugg MD. Event-related potentials dissociate repetition effects of high and low frequency words. Memory and Cognition. 1990;18:367–379. doi: 10.3758/bf03197126. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Cox CJ, Doyle MC, Wells T. Event-related potentials and the recollection of low and high frequency words. Neuropsychologia. 1995;33:471–484. doi: 10.1016/0028-3932(94)00132-9. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Doyle . Event-related potentials and stimulus repetition in direct and indirect tests of memory. In: Heinze HJ, Münte TF, Mangun GR, editors. Cognitive electrophysiology. Boston: Birkhauser; 1994. pp. 124–148. [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shapiro BE, Danly M. The role of the right hemisphere in the control of speech prosody in propositional and affective contexts. Brain and Language. 1985;25:19–36. doi: 10.1016/0093-934x(85)90118-x. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ. Memory for words and novel visual patterns: Repetition, recognition, and encoding effects in the event-related brain potential. Psychophysiology. 1996;33:491–506. doi: 10.1111/j.1469-8986.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Vilkki J. Incidental and deliberate memory for words and faces after focal cerebral lesions. Neuropsychologia. 1987;25:221–230. doi: 10.1016/0028-3932(87)90133-3. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Marsolek CJ. Hemisphere asymmetries in memory processes as measured in a false recognition paradigm. Cortex. 2003;39:627–642. doi: 10.1016/s0010-9452(08)70857-8. [DOI] [PubMed] [Google Scholar]
- Zaidel E. Disconnection syndrome as a model for laterality effects in the normal brain. In: Hellige JB, editor. Cerebral hemisphere asymmetry: Methods, theory and application. New York: Praeger; 1983. pp. 95–151. [Google Scholar]