Abstract
Vascular disrupting agents (VDAs) cause acute shutdown of abnormal established tumor vasculature, followed by massive intratumoral hypoxia and necrosis. However, a viable rim of tumor tissue invariably remains from which tumor regrowth rapidly resumes. We have recently shown that an acute systemic mobilization and homing of bone marrow derived circulating endothelial precursor cells (CEPs) can promote tumor regrowth following treatment with either a VDA or certain chemotherapy drugs. The molecular mediators of this systemic reactive host process are unknown. Here we show that following treatment of mice with OXi-4503, a second generation potent pro-drug derivative of combretastatin-A 4 phosphate (CA4P), rapid increases in circulating plasma VEGF, SDF-1, and G-CSF levels are detected. With the aim of determining whether G-CSF is involved in VDA-induced CEP mobilization, mutant G-CSF-R−/− mice were treated with OXI-4503. We found that as opposed to wildtype controls, G-CSF-R−/− mice failed to mobilize CEPs or show induction of SDF-1 plasma levels. Furthermore, Lewis lung carcinomas grown in such mice treated with OXi-4503 showed greater levels of necrosis compared to tumors treated in wildtype mice. Evidence for rapid elevations in circulating plasma G-CSF, VEGF, and SDF-1 were also observed in VDA (CA4P) treated cancer patients. These results highlight the possible impact of drug-induced G-CSF on tumor re-growth following certain cytotoxic drug therapies, in this case using a VDA, and hence G-CSF as a possible therapeutic target.
INTRODUCTION
The growth of new blood vessel capillaries in tumors – therapeutic inhibition of which is now a clinically validated strategy – appears to be mediated not only by the local division of pre-existing differentiated vascular endothelial cells (“sprouting angiogenesis”)(1) but also by a systemic process involving the mobilization and homing of a variety of bone marrow-derived cell (BMDC) populations(2). These include circulating endothelial progenitor cells (CEPs), which can incorporate into the lumens of growing blood vessels and differentiate into endothelial cells(2, 3).
We recently reported that exposure of tumor-bearing mice to cytotoxic-like vascular disrupting agents (VDAs) can cause a rapid (i.e. within hours) mobilization of BMDCs, some of which are CEPs, followed by their homing to the viable rim of tumor tissue(4)which characteristically remains after treatment with VDAs, and surrounds a sometimes massive necrotic tumor center(4, 5). This acute reactive host response contributes to the rapid regrowth of the drug treated tumors and thus compromises the durability of the initial anti-tumor effect induced by VDA treatment. Furthermore, we recently reported that certain cytotoxic chemotherapy drugs (administered at maximum tolerated doses) such as taxanes or 5-FU, can also cause acute mobilization of CEPs (6). In both cases, combination treatment with an antiangiogenic drug, e.g., DC101, a VEGFR-2 neutralizing antibody(4, 6, 7), blocks CEP mobilization and thus enhances the anti tumor activity of the cytotoxic therapy used(4, 6). Given the remarkably acute and robust nature of the BMDC response induced by VDAs, and its consequences for tumor response and impact on therapeutic benefit, we decided to investigate possible molecular mediators of this therapy induced mobilization of proangiogenic BMDCs, including CEPs. To do so, we used a potent VDA, namely the microtubule-inhibiting agent, OXi-4503, a second generation pro-drug derivative of the VDA combretastatin A4 phosphate (CA4P)(5).
MATERIALS AND METHODS
Human blood samples
Patients with advanced solid tumors who had given consent to participate in a phase I study evaluating escalating doses of CA4P given in combination with bevacizumab(8) participated in this study(n=15). Patients entered as one of 3 cohorts and received 45mg/m2, 54mg/m2 or 63mg/m2 CA4P intravenously (i.v.) in the first treatment cycle without bevacizumab. For the purpose of this study, blood was collected at baseline (pretreatment), and4 hours after CA4P treatment. The protocol was approved by the ethics committee.
Tumor and animal models
All animal studies were conducted in accordance to the animal care guidelines at Sunnybrook Health Sciences Centre (Toronto, Canada), and at Washington University (St. Louis, MO), and described in detail in supplementary online material.
Evaluation of circulating endothelial prognitor cells by flow cytomtery
Blood was obtained from anaesthetized mice by retro-orbital sinus bleeding. Viable CEPs were quantitated using flow cytometry, as described previously(3), and in supplementary online material.
Tissue processing and imaging
Tissue processing and immunohistochemistry were performed as described previously(4, 9), and in supplementary online material.
Evaluation of circulating G-CSF, VEGF and SDF-1 plasma levels by ELISAs
Tumor-free or 500mm3 MeWo human melanoma bearing nude mice, as well as tumor free G-CSF-R−/− and wt control mice were treated with an i.p. injection of 100mg/kg OXi-4503, or PBS as a control. Four hours later, mice were sacrificed and blood was drawn by cardiac puncture. In patients, blood samples were obtained by intravenous puncture. Plasma was separated using heparin and subsequently stored at −70°C until assayed. Levels of mouse or human VEGF, SDF-1 and G-CSF were assessed by using commercially available sandwich ELISAs (R&D Systems).
Statistical Analysis
Data are expressed as mean ± S.D. and the statistical significance of differences in mean values was assessed by two-tailed Student’st-test. Differences between means of experimental/treated groups compared to control-untreated or baseline (pretreatment) groups (unless indicated otherwise) were considered significant at values of P < 0.01 (**) or 0.05 < P < 0.01 (*).
RESULTS
Rapidly elevated plasma levels of predominantly host derived G-CSF, VEGF and SDF-1 are induced by OXi-4503
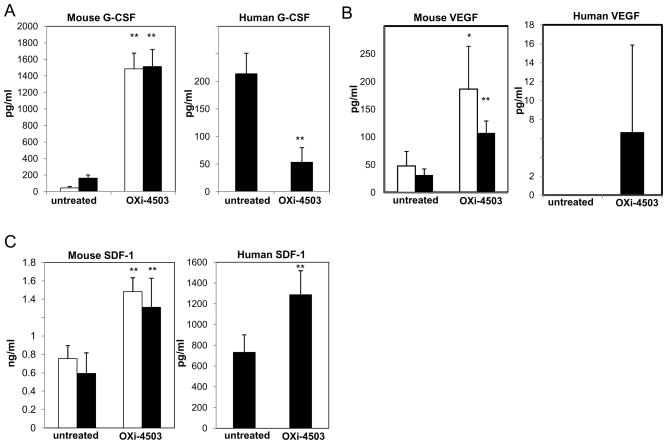
To investigate whether induction of certain growth factors may be involved in the acute mobilization of CEPs by VDA treatment, we analyzed plasma from non-tumor or 500mm3 MeWo human melanoma bearing nude mice obtained 4 hours after treatment with OXi-4503 for human and mouse G-CSF, VEGF, and SDF-1 using specific ELISAs. Emphasis was placed on these particular factors since all are known to induce CEP mobilization(10–12). The results in Figure 1A–C show acute increases in both normal and tumor-bearing mice of mouse G-CSF, VEGF and SDF-1 (10–20, 4–5, 2 fold increases, respectively). Interestingly, levels of human G-CSF were significantly lower 4 hours after OXi-4503 treatment. Human VEGF levels were not detectable in untreated tumor bearing mice, but a relatively small amount (15–18.32pg/ml)was detected in 2out of 5 mice, 4 hours after OXi-4503 treatment. Of note, we recently reported marked elevations of plasma SDF-1 in non-tumor bearing mice treated with maximum tolerated dose paclitaxel, which contributed to the rapid CEP spike observed similar in nature to the VDA induced effect(6). Overall, these results suggest that several factors known to mediate mobilization of CEPs are acutely increased by OXi-4503 treatment and are, in the main, derived from the host.
Figure 1. Circulating G-CSF, VEGF and SDF-1 plasma levels in tumor-free and MeWo tumor-bearing nude mice 4 hours after treatment with OXi-4503.
Plasma levels of mouse and human (A) G-CSF, (B) VEGF, and (C) SDF-1 were obtained from tumor-free (open bars), or tumor-bearing mice (filled bars), 4 hours after treatment with OXi-4503.
Elevated G-CSF contributes to the rapid CEP mobilization induced by VDA treatment
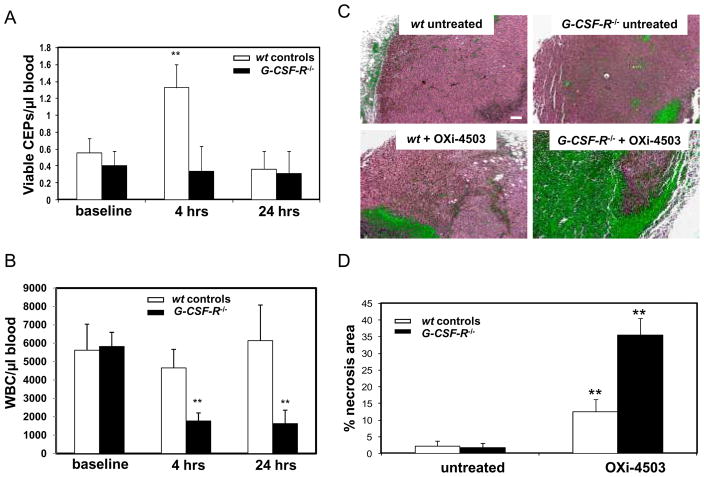
G-CSF is well known as a mobilizing factor for hematopoietic stem cells (HSC), as well as CEPs(12, 13). Given the pronounced elevation of G-CSF induced by OXi-4503 we asked whether it has a direct effect on CEP mobilization following VDA treatment. To do so, tumor-free BALB/c mice were infused with 5μg/kg human recombinant G-CSF, and blood was drawn 4 and 24 hours later for the evaluation of CEPs. The results in Supplementary Figure 1 show that G-CSF induced a 3–4 fold spike in CEPs levels, similar to what we observed using OXi-4503. Similar results were obtained using C57Bl/6 mice (data not shown). Next, we evaluated levels of CEPs and relative tumor response as measured by necrosis in mutant G-CSF receptor (G-CSF-R−/−)mice(14)after OXi-4503 treatment. Figure 2A-B and Supplementary Figure 2 show that levels of CEPs in G-CSF-R−/− mice do not significantly change within the first 24 hours, in contrast to their wildtype controls. In addition, transplanted LLC tumors grown in G-CSFR−/− mice or wildype control mice were allowed to grow until the tumors reached 500mm3, at which point the mice were treated with OXi-4503. Three days later tumors were removed and evaluated for tumor response by measuring necrosis. The results in Figure 2C–D show that whereas tumors grown in wildtype mice, which exhibited a prominent necrotic center exceeding ~15% of total tumor area, tumors treated in G-CSF-R−/− mice had a significantly greater degree of tumor necrosis (~35%). These results suggest a direct effect of G-CSF on promoting CEP mobilization, which contributes, at least in part, to the rapid re-growth of tumors from the viable tumor rim, after VDA treatment.
Figure 2. WBC and CEP levels and relative tumor necrosis detected in G-CSF-R−/− mice following treatment with OXi-4503.
(A) CEP levels, and (B) WBC counts were evaluated at baseline, 4 and 24 hours after OXi-4503 treatment, in non-tumor bearing G-CSF-R−/− mice (filled bars) and their respective wt controls (open bars). (C) Relative necrosis in tumor sections from both G-CSF-R−/− and wt control mice following treatment with OXi-4503 (bar=100μm). (D) Quantitative data on the percentage of necrosis area from all tumor area.
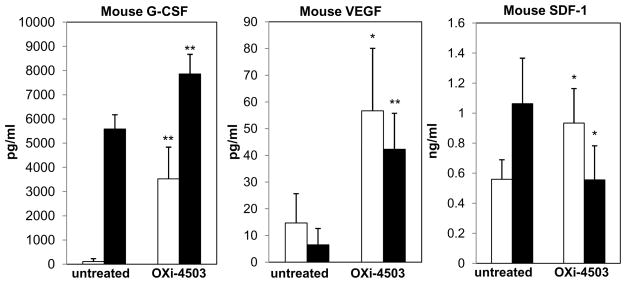
To further assess the contribution of G-CSF to CEP mobilization, plasma levels of SDF-1, VEGF, and G-CSF were evaluated in G-CSF-R−/− mice, 4 hours after they were treated with OXi-4503. The results in Figure 3 show that both VEGF and G-CSF plasma levels were substantially elevated, although the baseline levels of G-CSF in G-CSF-R−/− mice were remarkably higher in comparison to their levels in wild type control mice. Interestingly, SDF-1 levels were downregulated in G-CSF-R−/− mice which suggests that SDF-1 may also be secondarily involved in G-CSF-induced CEP mobilization following OXi-4503 treatment.
Figure 3. Circulating G-CSF, VEGF, and SDF-1 plasma levels in G-CSF-R−/− mice and their wt control after treatment with OXi-4503.
Plasma samples obtained from G-CSF-R−/− (filled bars) or wt control mice (open bars) at baseline and 4 hours after treatment with OXi-4503, were applied on ELISAs for the evaluation of mouse G-CSF, VEGF, and SDF-1 levels.
Increases in circulating G-CSF, VEGF and SDF-1 plasma levels in cancers treated with a VDA
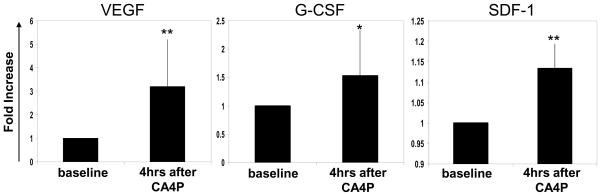
To evaluate whether some of our preclinical results could be reproduced in the clinical setting, blood was collected from patients with advanced solid tumors enrolled in a phase I clinical study(8)at baseline (pretreatment) and 4 hours after treatment with CA4P. Plasma was separated for the evaluation of G-CSF, VEGF and SDF-1 levels using specific ELISAs. The results in Figure 4 show that the levels of all three cytokines were substantially increased within 4 hours of CA4P treatment, similar to the results we observed in mice using OXi-4503. Overall, these preliminary clinical results are remarkably consistent with the respective preclinical findings.
Figure 4. Evaluation of circulating G-CSF, VEGF and SDF-1 plasma levels in VDA (CA4P)treated cancer patients.
Blood collected from 15 cancer patients at baseline, and 4 hours after CA4P treatment was evaluated for G-CSF (n=12), VEGF (n=12) and SDF-1 (n=6) plasma levels. The results obtained were plotted as fold increase from the baseline level of each patient.
DISCUSSION
Cytotoxic anti-cancer drugs, or treatments, including chemotherapy, radiation and VDA scan often cause major tumor responses manifested by tumor cell death subsequently leading to various degrees of tumor shrinkage. However, the benefit of such responses, at least in terms of increased survival times, is often limited because of rapid tumor repopulation(15). Consequently developing and optimizing strategies to slow down such rapid tumor repopulation are of considerable therapeutic interest(15). Some possible strategies include closer spacing of cytotoxic therapies such as ‘dose-dense’ chemotherapy, or hyperfractioned radiation(15), or the combined use of biologic therapies that target cell proliferative mechanisms, including antiangiogenic drugs, during the treatment-free intervals in between successive cycles of cytotoxic therapy(4, 6, 15–17).
Tumor cell repopulation/regrowth has generally been viewed as an intrinsic tumor cell-driven property(15). However, our previous studies implicated that a number of cytotoxic drugs can rapidly activate various host processes which can contribute significantly to tumor repopulation, one of which involves acute mobilization and tumor homing of pro-angiogenic BMDCs, including CEPs(4, 6). In our recent study we reported that SDF-1 is one of the molecular mediators of CEP mobilization following paclitaxel treatment, as neutralizing anti-SDF-1 antibodies that were administered in combination with chemotherapy resulted in failure of induction of CEP spikes(6). Here, using a VDA as the cytotoxic-(like) agent, we have implicated another molecular mediator responsible for CEP mobilization. The increase in circulating G-CSF was remarkable, being in the range of one order of magnitude, and moreover, it occurred within 4 hours of VDA treatment. However G-CSF-R−/− mice failed to induce elevated levels of both CEPs and SDF-1. Eash et.al., reported that G-CSF mobilizes neutrophils within hours, probably by directly downregulating CXCR4 expression(18). Moreover, AMD3100, a CXCR4 antagonist, was also found to mobilize CEPs and other HSCs within 4 hours(19). Whether G-CSF acts in a similar fashion to AMD-3100by downregulating CXCR4 expression on CEPs remains to be determined in future studies. In addition, we also observed a significant and rapid reduction in levels of WBC counts, following treatment with OXi-4503, in G-CSF-R−/− mice but not in wt controls, which is consistent with recent findings by Petit et.al.,(20)implicating a direct effect of VDAs on hematopoietic cells. However, how G-CSF acts to maintain normal WBC counts in wt mice following VDA treatment remains unknown.
Also noteworthy with respect to our results was the observation of rapid elevation of G-CSF, VEGF and SDF-1 plasma levels observed in the peripheral blood of cancer patients treated with CA4P, results which are obviously similar in some respects to the rapid elevations of the three cytokines in our preclinical study. We would note that although we did not evaluate levels of CEPs in clinical samples, recent studies have shown that cancer patients treated with either MTD paclitaxel-based chemotherapy or a VDA (known as AVE8062) combined with cisplatinum, exhibited a rapid elevation in CEP levels (6, 21).
One implication of our results relates to the outcome of cytotoxic ‘dose-dense’ chemotherapy regimens which are made possible by the use of recombinant G-CSF growth factor support to accelerate host recovery from myelosuppression. While there is no clinical evidence at present to suggest administration of recombinant G-CSF worsens clinical outcomes, it is conceivable that the anti-tumor benefits obtained by using such dose-dense (and hence more intensive) chemotherapy regimens are less than would otherwise be the case. Moreover, regarding the possibility of G-CSF-induced mobilization of BMDCs possibly facilitating tumor angiogenesis, we would also note the recent findings by Shojaei et.al. who reported that mobilization of proangiogenic Gr1+CD11b+ myeloid suppressor cells can be induced indirectly by G-CSF through upregulation of Bv8/prokineticin(22).
In summary, our results suggest a host reactive molecular mechanism contributing to the rapid tumor re-growth following cytotoxic anti-cancer drug therapy using VDAs, and suggest G-CSF could conceivably be exploited transiently as a therapeutic target under selected circumstances, e.g. as a means of enhancing the efficacy of VDA therapy. Such targeting may be particularly suitable in situations where the cytotoxic drug treatment is not associated with myelosuppression. In this regard VDAs are not associated with high grade myelosuppression(5, 8, 21).
Supplementary Material
Acknowledgments
We thank Cassandra Cheng for her excellent secretarial assistance. This work was supported by grants from the National Institutes of Health (NIH), and the National Cancer Institute of Canada and Canadian Institutes of Health Research to RSK; NIH (R01 HL073762) to DCL; National Cancer Institute (NCI R01 CA 90722) to JMA; the Associazione Italiana per la Ricerca sul Cancro (AIRC), Istituto Superiore di Sanita (ISS), and the sixth EU Framework Programme (Integrated Project “Angiotargeting” contract no. 504743) in the area of “Life sciences, genomics and biotechnology for health” to FB; and sponsored research agreement with ImClone Systems New-York and OXiGENE Inc, Boston (to RSK); Cancer Research UK to AH; and Dr. Saal van Zwanenberg Stichting fellowship to LD. The human study was sponsored and fully funded by OXiGENE Inc.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: from promiscuity to surrogate marker and target identification. Nature Rev Cancer. 2006;6:835–45. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 4.Shaked Y, Ciarrocchi A, Franco M, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–7. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 5.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–35. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 6.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–73. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaked Y, Bertolini F, Man S, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–11. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PD, Judson I, Padhani A, et al. A phase I study of combretastatin A4 phosphate (CA4P) and bevacizumab in subjects with advanced solid tumors. Journal of Clinical Oncology. 2008;26:5–20. [15S, Abstract No. 3550] [Google Scholar]
- 9.Franco M, Man S, Chen L, et al. Targeted anti-VEGFR-2 therapy leads to short and long term impairment of vascular function and increases in tumor hypoxia. Cancer Res. 2006;66:3639–48. doi: 10.1158/0008-5472.CAN-05-3295. [DOI] [PubMed] [Google Scholar]
- 10.De Falco E, Porcelli D, Torella AR, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–82. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell TM, Paul JD, Hill JM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, Wu HY, Wesselschmidt R, Kornaga T, Link DC. Impaired production and increased apoptosis of neutrophils in granulocyte colony-stimulating factor receptor-deficient mice. Immunity. 1996;5:491–501. doi: 10.1016/s1074-7613(00)80504-x. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–25. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA. Clinical implications of antiangiogenic therapies. Oncology (Williston Park) 2005;19:26–31. [PubMed] [Google Scholar]
- 17.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–5. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 18.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009 doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Petit I, Karajannis MA, Vincent L, et al. The microtubule-targeting agent CA4P regresses leukemic xenografts by disrupting interaction with vascular cells and mitochondrial-dependent cell death. Blood. 2008;111:1951–61. doi: 10.1182/blood-2007-05-089219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farace F, Massard C, Borghi E, Bidart JM, Soria JC. Vascular disrupting therapy-induced mobilization of circulating endothelial progenitor cells. Ann Oncol. 2007;18:1421–2. doi: 10.1093/annonc/mdm367. [DOI] [PubMed] [Google Scholar]
- 22.Shojaei F, Ferrara N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 2008;68:5501–4. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.