Abstract
Purpose
Recent literature comparing the effectiveness of above-elbow and below-elbow plaster casts appears to suggest that either cast type offers adequate immobilization for distal radius and ulna fractures. The idea that an appropriate mold placed on the cast is the most significant determinant of successful immobilization and, thereby, patient outcome has also been elucidated. The purpose of this study was to compare the effectiveness of above-elbow versus below-elbow fiberglass casts in maintaining distal radius/ulna fracture reduction and to identify factors associated with treatment failures.
Methods
We reviewed the radiographs and clinical data of 253 children with distal third forearm fractures requiring reduction under conscious sedation or a hematoma block. Outcome measures included rates of re-manipulation, loss of reduction, and cast complications.
Results
One hundred and nineteen children were treated with below-elbow fiberglass casts and 134 were treated with above-elbow fiberglass casts based on a clinical pathway created before the study period. There were no differences between the two groups in age, weight, fracture pattern, percentage of both-bone fractures, and initial fracture angulation. Of the 253 fractures in the study, 38 (15%) were considered to have less than ideal outcomes. There were no differences between the ‘ideal’ and ‘non-ideal’ groups in age, fracture pattern, presence of ulna fracture, cast index, or cast type. All immediate post-reduction measures (anterior-posterior [AP] and lateral displacement/angulation) were significantly correlated with treatment outcome, except angulation on AP films. The magnitude of reduction as measured by a newly described variable, the angle between the second metacarpal and long axis of the radius in the AP projection, was significantly correlated with treatment failure (r = −0.139, P = 0.027). Binary logistic regression was performed and demonstrated that the success of the reduction, as determined by the AP radiograph second metacarpal-radius angle, was a significant predictor of treatment success (odds ratio 1.6, P < 0.001). Also, the change in lateral view angulation post-reduction was a significant predictor of treatment failure based on regression (odds ratio 1.2, P = 0.004). The above-elbow cast group had a slightly greater cast index (0.80) compared to the below-elbow cast group (0.77) (P = 0.003). Whereas below-elbow fiberglass casts appear to be equally effective in immobilizing pediatric distal third forearm fractures as above-elbow fiberglass casts, it seems that they have an increased risk for poor molding, particularly with regards to ulnar deviation. We did not find an association between the treatment ‘failure’ and cast index, likely because the number of poor molds (cast index >0.8) was nearly equal in each group (above-elbow with 61 and below-elbow with 45). However, the mold seen on the AP radiograph as determined by the second metacarpal-radius angle was a reproducible radiographic predictor of treatment success. If molded with ulnar deviation (second metacarpal-radius angle >0°), the outcome was considered to be ideal in 86.7% of cases compared to only 74.4% when it was <0°.
Conclusion
We agree with prior studies suggesting the equal efficacy of below-elbow versus above-elbow casts in distal radius and ulna fracture treatment using either plaster or fiberglass, but wish to emphasize the importance of not only the cast index, but also the ulnar deviation mold (for most dorsally displaced fractures), as measured by the second metacarpal-radius angle.
Keywords: Distal radius fractures, Above-elbow cast, Below-elbow cast, Second metacarpal-radius angle
Introduction
Forearm fractures are among the most common injuries in children. In Blount’s series, 75% of forearm fractures involved the distal one-third [1]. In addition, the distal radial physis is the most commonly fractured growth plate, accounting for 40 to 58% of all physeal injuries. Distal radius physeal injuries alone make up 15% of forearm fractures in children [2–5].
Following the closed reduction of displaced distal one-third forearm fractures in children, most authors recommend immobilization using an above-elbow cast [2, 3, 6]. Other series have reported good results when treating these injuries with below-elbow casts. Chess et al. [7] described excellent results using below-elbow casts to maintain reduction in pediatric distal third forearm fractures, provided the casts were ‘well-molded.’ Other studies noted no difference in outcome between above-elbow and below-elbow plaster casts in immobilizing these injuries, including two relatively small prospective randomized studies [8–10].
The purpose of our investigation was to compare the effectiveness of above-elbow and below-elbow fiberglass casts in immobilizing pediatric distal one-fourth forearm fractures requiring closed reduction with manipulation in a children’s hospital with an active teaching service. In addition, we attempted to identify patient, fracture, or treatment characteristics associated with less than ideal outcomes.
Materials and methods
We retrospectively reviewed the clinical data and radiographs of all patients with closed distal one-fourth forearm fractures requiring closed reduction under either conscious sedation or hematoma block treated at our institution during the study period from January 2005 to November 2006. Inclusion criteria included an open distal radial physis, no neurovascular compromise, the absence of ipsilateral extremity injury affecting the choice of cast, use of conscious sedation or hematoma block, initial reduction and casting performed at our institution, and satisfactory availability of clinical and radiographic data from initial injury to cast removal. Following closed reduction, patients were placed in either above-elbow or below-elbow fiberglass casts, according to a clinical pathway that was created to ease the decision of cast type based on the pre-selected preference of the attending surgeon on-call on the day of presentation. The casts were split with spacers placed to maintain a gap of 6 mm in the cast. The reductions were performed in the emergency department by the on-call orthopedic resident or mid-level provider (nurse practitioner or physician assistant). Exclusion criteria included patients sustaining non-displaced or pathologic fractures, forearm fracture-dislocations, those patients with incomplete data sets (missing clinical or radiographic data), or those violating the clinical pathway (attending surgeon preference of cast type).
Hospital records and office charts were reviewed for patient age, weight, side of injury, mechanism of injury, and type of sedation. Anterior-posterior (AP) and lateral radiographs were analyzed for fracture displacement and angulation at initial injury, post-reduction, and cast removal. Other parameters recorded were fracture pattern, direction of deformity (flexion vs. extension injury), presence of an associated ulna fracture, and casting duration.
The quality of cast molding was measured by two methods. The first method utilized was the cast index. This radiographic variable was determined according to the method described by Chess et al. [7] as a ratio of the inner cast dimensions on AP and lateral radiographs, and was felt to represent the quality of cast molding on the lateral view. Chess et al. described the ‘ideal’ cast index as <0.8. The second method utilized was coined the second metacarpal-radius angle as measured on the AP radiograph. Clinically, this measurement relates to the degree of wrist ulnar deviation relative to the long axis of the forearm. The cast mold in ulnar deviation is based on the method of reduction proposed by Cotton [11]. Ideal ulnar deviation using this method would place the first metacarpal parallel to the radius. However, since the first metacarpal can be positioned out of plane with the forearm, the more reproducible radiographic parameter to evaluate this AP projection molding parameter is the angle created by bisection of the long axis of the second metacarpal and long axis of the radius on AP radiographs (Fig. 1).
Fig. 1.
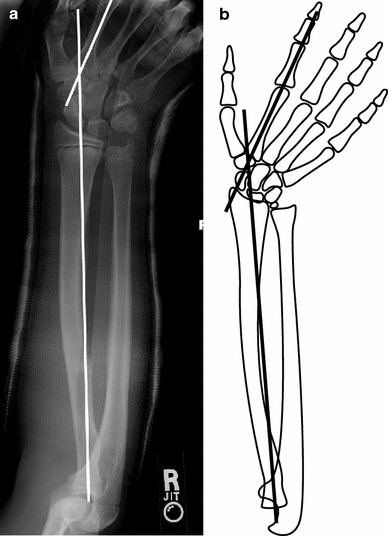
The second metacarpal-radius angle: a radiographic parameter to aide in the assessment of adequate cast molding in the lateral plane on anterior–posterior (AP) radiographs.a AP radiograph, andb associated line drawing to facilitate understanding of newly defined radiographic measure
A treatment failure of the initial casting was defined as patients requiring re-manipulation for any cause. These causes included: progression of fracture angulation (in AP or lateral planes) greater than 10° during cast wear, cast changes for any reason, or the need for cast wedging.
Analysis of variance (ANOVA) was performed to compare pre-treatment, immediate post-treatment, and cast removal radiographic variables between the above-elbow and below-elbow cast groups. A similar analysis was then done to compare these same variables between treatment success and treatment failure patients. Following this, Spearman’s rho non-parametric correlation coefficient was calculated to identify possible relationships and the direction of that relationship between independent variables (demographic, radiographic, cast type) and the dependent variable of an initial cast treatment failure. The variables that were found to be significantly correlated with less than ideal treatment were entered into a binary logistic regression as possible predictors of treatment failure (yes/no = the binary dependent variable) to obtain the odds ratio and estimates of R2.
Results
During the study period, 438 children/fractures were identified (193 treated with above-elbow casts and 245 with below-elbow casts), but only 253 patients met the inclusion criteria. Patients were excluded for inadequate follow-up (n = 56), missing radiographs (n = 91), or a violation in the on-call orthopedic surgeon’s cast preference (n = 38). The average age was 10 ± 3 years. There were 60 females and 193 males included in the study. Ipsilateral ulna fractures occurred in 173 patients. One hundred and nineteen children were treated with below-elbow casts and 134 were treated with above-elbow casts. The duration of casting was similar between both groups, with the average being 7 weeks (and a similar standard deviation of 2 weeks in both groups). Of the 253 fractures in the study, 38 (15%) were considered to be initial cast treatment ‘failures.’
The pre-operative radiographic variables were similar in the above-elbow and below-elbow cast groups (Table 1). The AP view displacement and angulation, as well as the lateral view displacement and angulation, were not significantly different (P > 0.05). Immediately following reduction, these radiographic variables continued to be similar between the two cast groups (P > 0.05). The angle between the second metacarpal and radius was similar in both cast groups, on average deviated ulnarly 2° (P = 0.44). The cast index was found to be significantly different (P < 0.003, Table 1). On average, the above-elbow group index was 0.80 compared to 0.77 in the below-elbow cast group. At cast removal, the two cast groups were not significantly different in terms of the four radiographic measures (AP view displacement, AP view angulation, lateral view displacement, and lateral view angulation) (P > 0.05). There were no significant differences in the proportion of patients who experienced a treatment failure between the two cast groups (15.1% failure in the above-elbow cast group, 14.9% failure in the below-elbow cast group, P = 0.96).
Table 1.
Radiographic values for the above- and below-elbow cast groups
| Above-elbow | Below-elbow | P-value | |
|---|---|---|---|
| Pre-treatment | |||
| AP view displacement | 2 ± 2 | 2 ± 2 | 0.22 |
| AP view angulation | 4 ± 8 | 9 ± 9 | 0.87 |
| Lateral view displacement | 3 ± 4 | 3 ± 4 | 0.56 |
| Lateral view angulation | 23 ± 11 | 21 ± 10 | 0.18 |
| Immediately post-reduction | |||
| AP view displacement | 1 ± 1 | 1 ± 1 | 0.91 |
| AP view angulation | 2 ± 3 | 1 ± 3 | 0.67 |
| Lateral view displacement | 1 ± 1 | 1 ± 1 | 0.22 |
| Lateral view angulation | 4 ± 4 | 3 ± 4 | 0.10 |
| Cast index | 0.80 ± 0.01 | 0.77 ± 0.01 | 0.003 |
| Second metacarpal-radius angle | 2 ± 10 | 2 ± 10 | 0.44 |
| At cast removal | |||
| AP view displacement | 0.1 ± 0.5 | 0.1 ± 0.5 | 0.94 |
| AP view angulation | 3 ± 4 | 2 ± 3 | 0.09 |
| Lateral view displacement | 0.2 ± 0.7 | 0.1 ± 0.4 | 0.27 |
| Lateral view angulation | 6 ± 6 | 6 ± 6 | 0.72 |
As the cast type did not appear to be associated with treatment failure, the radiographic variables for the 215 treatment successes were compared to the 38 treatment failures. The pre-operative radiographic variables were similar in the treatment success and failure groups (P > 0.05, Table 2). On immediate post-reduction radiographs, the failure group had significantly greater AP view displacement (1.3 ± 1 mm), lateral view displacement (1.2 ± 1 mm), and lateral view angulation (5° ± 5°) compared to the successfully treated patients (0.7 ± 1 mm, 0.8 ± 1 mm, 3° ± 4°, respectively) (P < 0.01). The AP view angulation and cast index were similar between the treatment success and failure groups (Table 2, P > 0.05). Patients who failed treatment had a cast mold in which the second metacarpal-radius angle was deviated, on average, toward the radius (−1° ± 9°) as compared to an ulna deviated angle (3° ± 10°) in the patients who experienced successful treatment (P = 0.03). At cast removal, the two cast groups were not significantly different in terms of the four radiographic measures (AP view displacement, AP view angulation, lateral view displacement, and lateral view angulation) (P > 0.05).
Table 2.
Radiographic values for successful treatment versus failures
| Success | Failure | P-value | |
|---|---|---|---|
| Pre-treatment | |||
| AP view displacement | 1.5 ± 2 | 2.4 ± 2 | 0.11 |
| AP view angulation | 9 ± 8 | 8 ± 7 | 0.89 |
| Lateral view displacement | 3 ± 4 | 3 ± 3 | 0.74 |
| Lateral view angulation | 22 ± 11 | 19 ± 7 | 0.11 |
| Immediately post-reduction | |||
| AP view displacement | 0.7 ± 1 | 1.3 ± 1 | 0.014 |
| AP view angulation | 2 ± 3 | 2 ± 2 | 0.73 |
| Lateral view displacement | 0.8 ± 1 | 1.2 ± 1 | 0.02 |
| Lateral view angulation | 3 ± 4 | 5 ± 5 | 0.002 |
| Cast index | 0.78 ± 0.01 | 0.79 ± 0.01 | 0.66 |
| Second metacarpal-radius angle | 3 ± 10 | –1 ± 9 | 0.03 |
| At cast removal | |||
| AP view displacement | 0.1 ± 0.4 | 0.3 ± 0.9 | 0.008 |
| AP view angulation | 2 ± 3 | 5 ± 5 | ≤0.001 |
| Lateral view displacement | 0.1 ± 0.5 | 0.2 ± 0.9 | 0.35 |
| Lateral view angulation | 5 ± 5 | 11 ± 8 | ≤0.001 |
In the correlation analysis, none of the pre-treatment radiographic measures correlated with the initial cast treatment success/failure (Table 3). All of the immediate post-reduction radiographic measures of fracture alignment were correlated with the treatment outcome, except the AP view angulation (Table 3). The cast index, presence of an ulna fracture, a completely displaced fracture, and cast type were not significantly correlated with initial cast treatment failure (P > 0.10). The magnitude of reduction as measured by the change in degree of lateral radiograph angulation was significantly correlated with treatment failure (r = 0.172, P = 0.009). A greater reduction in this plane was associated with initial cast treatment success. The angle between the second metacarpal and radius after casting was also significantly correlated with treatment outcome (r = −0.139, P = 0.027). The greater the ulnar deviation was, the more likely the success of the initial cast.
Table 3.
Spearman correlation values for all potential predictors’ relationships with treatment failure/success
| Cast type | Correlation coefficient | −0.003 |
| Sig. (2-tailed) | 0.965 | |
| Age (year) | Correlation coefficient | −0.075 |
| Sig. (2-tailed) | 0.239 | |
| Pre-treatment X-rays AP displacement (mm) | Correlation coefficient | 0.154 |
| Sig. (2-tailed) | 0.055 | |
| Pre-treatment X-rays AP angulation (°) | Correlation coefficient | 0.009 |
| Sig. (2-tailed) | 0.888 | |
| Pre-treatment lateral displacement (mm) | Correlation coefficient | 0.057 |
| Sig. (2-tailed) | 0.495 | |
| Pre-treatment lateral angulation (°) | Correlation coefficient | −0.087 |
| Sig. (2-tailed) | 0.187 | |
| Post AP displacement (mm) | Correlation coefficient | 0.179 |
| Sig. (2-tailed) | 0.004 | |
| Post AP angulation (°) | Correlation coefficient | 0.064 |
| Sig. (2-tailed) | 0.313 | |
| Post lateral displacement (mm) | Correlation coefficient | 0.183 |
| Sig. (2-tailed) | 0.003 | |
| Post lateral angulation (°) | Correlation coefficient | 0.159 |
| Sig. (2-tailed) | 0.011 | |
| Cast index | Correlation coefficient | 0.023 |
| Sig. (2-tailed) | 0.710 | |
| Second metacarpal-radius angle rd− ud+ | Correlation coefficient | −0.139 |
| Sig. (2-tailed) | 0.027 | |
| Ulna fracture | Correlation coefficient | 0.094 |
| Sig. (2-tailed) | 0.139 | |
| Completely displaced fracture | Correlation coefficient | 0.082 |
| Sig. (2-tailed) | 0.196 | |
| Change AP view angulation | Correlation coefficient | 0.160 |
| Sig. (2-tailed) | 0.100 | |
| Change AP view displacement | Correlation coefficient | −0.035 |
| Sig. (2-tailed) | 0.662 | |
| Change lateral view angle | Correlation coefficient | 0.211 |
| Sig. (2-tailed) | 0.009 | |
| Change lateral view displacement | Correlation coefficient | −0.005 |
| Sig. (2-tailed) | 0.949 |
Bold denotes measures with statistical significance
The variables significantly correlating with outcome were then entered into a binary logistic regression (Table 4). The success of the reduction, as determined by the change in lateral view (dorsal) angulation, was a significant predictor of treatment success (odds ratio 1.2, P = 0.004). Further examination of this variable showed that patients who failed treatment had a 13° ± 8° decrease in dorsal angulation, compared to an 18° ± 11° decrease in patients who had successful treatment of the fracture. The only other significant predictor was the second metacarpal-radius angle (odds ratio 1.6, P < 0.001); treatment failures, on average, deviated radially (−1° ± 9°) compared to the treatment successes deviated ulnarly (3° ± 10°) (P = 0.034).
Table 4.
Significant predictors of treatment failure based on regression
| Odds ratio | P-value | |
|---|---|---|
| Change in lateral view angulation | 1.2 | 0.004 |
| Second metacarpal-radius angle | 1.6 | <0.001 |
Discussion
Distal forearm fractures are exceedingly common injuries in children. Following closed manipulation, most authors recommend the immobilization of displaced fractures in an above-elbow cast [2, 3, 6, 12]. Chess et al. reported success with treating distal one-third forearm fractures in a below-elbow cast, provided the cast was well-molded [7]. More recent studies noted equal outcomes when treating these injuries in both below-elbow and above-elbow plaster casts [9, 10]. Our data corroborated these conclusions for fiberglass casts, showing no advantage of above-elbow casts over below-elbow casts in preventing fracture displacement during the casting period or in reducing the incidence of fracture re-manipulation.
In separate investigations, both Chess et al. and Webb et al. noted the importance of a well-molded below-elbow plaster cast to prevent fracture displacement [7, 10]. The adequacy of cast molding has been quantified by the cast index, which is the ratio of the AP inner diameter of the cast divided by the lateral inner diameter of the cast. The normal index of a child’s forearm, based on anthropometric studies by Chess et al. [7], is 0.7, and this cast index is, therefore, considered to be ideal. Both authors noted that re-manipulation was not required on any patient with a cast index less than 0.7. A poor cast index as been historically defined as greater than 0.79. While the quality of cast molding as seen on the lateral radiograph is quite important, we were unable to show a relationship between a higher cast index and a higher rate of initial cast treatment failure. We had 106 poorly molded casts (based on the cast index), of which 61 were in the above-elbow cast group and 45 were in the below-elbow cast group. For all casts with a poor cast index (0.8 or greater), there were 90 failures (85%) and 16 successful treatments. For all casts with a good cast index (0.7 or less), there were 24 without failure (86%) and four with failure. No true threshold value of the cast index predicted casting success, as one of the failures had a cast index of 0.64, which was one of the better molds placed in this study group.
Previous investigators have attempted to identify factors associated with the risk of loss of reduction. Voto et al. implicated a loose cast at the fracture site, loss of three-point fixation, improper initial molding, and initial fracture mal-reduction [6]. Proctor et al. found that the presence of complete initial displacement and failure to achieve a perfect reduction increased the chance of re-displacement of forearm fractures in children [13]. In a review of 86 children, Haddad and Williams concluded similarly, reporting that the best predictors of re-displacement were complete fracture displacement at the time of presentation and the lack of anatomical reduction on the immediate post-reduction radiograph [14]. In contrast, after reviewing 346 children with displaced metaphyseal fractures treated with closed reduction and casting, Younger et al. concluded that well-reduced fractures are more likely to lose reduction. They surmised that fractures may be inherently more stable in a position of deformity than in a reduced position due to periosteal and soft-tissue disruption [8]. In contrast to these previous findings, we found that our failure group had significantly greater post-reduction residual angulation and displacement on the lateral radiographic views when compared to the non-failure group. Therefore, a fracture that was poorly or under-reduced was more likely to fail initial treatment. Also, in contrast to the previous studies, we found no correlation to failure with those fractures that were completely displaced at presentation.
Beyond using previously described measures to evaluate our fracture reductions, we developed a second radiographic parameter to help assess the cast mold in the ulnar direction, as visualized on AP radiographs by measuring the angle created by the long axes of the second metacarpal and radius. This parameter demonstrated a statistically significant difference between the failure and non-failure groups, and was a successful predictor of failure by regression analysis. We believe the optimal second metacarpal-radius angle to be 10–20° of ulnar deviation, which correlates roughly with a parallel position of the first metacarpal (thumb) long axis to the forearm long axis. Furthermore, this direction of wrist deviation is only optimal if the fracture pattern (dorsal–radial displacement) remains consistent with the anatomic findings of Hughston [15], when the muscles of the brachioradialis, pronator quadratus, and extensors and abductors of the thumb pull the distal fragment toward the ulna, in a pronated and radially deviated position at the time of displacement. For other less common volar-ulnar displaced fractures, this position of molding clearly does not apply. Our study did not separate these less common fracture displacement types to evaluate only those fractures that presented with initial radial deviation on the lateral radiographs.
The most significant limitations to this study are those associated with retrospective design. A more specific limitation to this study was our inability to perform intra- and inter-observer reliability testing for the second metacarpal-radius angle; however, we feel that the specific angle is not as important as the actual molding of the cast that places about 10–20° of ulnar deviation.
Moreover, due to the retrospective nature of our study, we had a large drop-out rate due to the exclusion criteria of incomplete data sets. This was most commonly related to poor radiographs (as defined by collimated images that left the metacarpals unexposed and limited the ability to make all of our radiographic measurements). We also attempted to exclude those cases that violated the clinical protocol that was selected by each attending surgeon prior to the study collection period. The residents placing the casts during the study period were instructed to place casts according to the pre-determined attending surgeon preferences, and, occasionally, a cast was placed that did not match with the clinical pathway and, therefore, was excluded, since this might have created a selection bias for the type of cast utilized.
It is interesting to note in our study group, that the above-elbow casts appear to have had worse cast molding than the below-elbow casts, at least when comparing the cast index. The most probable explanation for this phenomenon is that the orthopedic residents performing the reductions and applying the casts have less variables to consider when applying a below-elbow cast. The ability to place a good mold on a below-elbow cast is facilitated by not dividing the casting physician’s attention between the mold at the fracture and the antecubital crease in the above-elbow casts. Although a difference between 0.8 and 0.77 (that found between the above-elbow cast and below-elbow cast groups, respectively) is statistically significant, the clinical significance may be argued. However, because the standard deviation for both groups was less than 1%, it implies that a mean of 0.8 would place at least half of the above-elbow cast group in a poor mold based on historic controls, whereas a mean of 0.77 implies that many more in the below-elbow cast group have at least an acceptable mold. The inability to keep the cast index statistically similar between the two cast type groups (although not enough to predict treatment failure) may diminish our ability to confirm that below-elbow casts can maintain reductions equal to above-elbow casts, since the former appear to have been better molded than the latter.
From our analysis of distal one-fourth forearm fractures in children, it is clear that the success of closed reduction and fiberglass cast application is dependent on many variables. As with plaster casts, we have found that adequately reduced fractures placed in well-molded below-elbow fiberglass casts are as effective as above-elbow fiberglass casts. Both cast types immobilized these injuries effectively, preventing re-displacement during cast treatment and the need for re-manipulation. Furthermore, our data supports the utilization of the second metacarpal-radius angle as another radiographic parameter to define the degree of ulnar deviation that will help predict final outcomes in pediatric distal radius fractures, both with and without associated ulna fractures.
Acknowledgments
This project was supported by the Children’s Specialists of the San Diego Orthopedic Research and Education Fund. None of the authors received financial support for this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Blount WP. Fractures in children. Baltimore: Williams & Wilkins; 1955. [Google Scholar]
- 2.Armstrong PF, Joughin VE, Clarke HM. Pediatric fractures of the forearm, wrist, and hand. In: Green NE, Swiontkowski MF, editors. Skeletal trauma in children. Philadelphia: WB Saunders; 1998. pp. 161–257. [Google Scholar]
- 3.Chambers HG, De La Garza JF, O’Brien E, Price CT, Stanley E, Wilkins KE. Fractures of the radius and ulna. In: Rockwood CA, Wilkins KE, Beaty JH, editors. Fractures in children. Philadelphia: Lippincott, Williams & Wilkins; 1996. pp. 449–651. [Google Scholar]
- 4.Dicke TE, Nunley JA. Distal forearm fractures in children. Complications and surgical indications. Orthop Clin North Am. 1993;24:333–340. [PubMed] [Google Scholar]
- 5.Lee BS, Esterhai JL, Jr, Das M. Fracture of the distal radial epiphysis. Characteristics and surgical treatment of premature, post-traumatic epiphyseal closure. Clin Orthop Relat Res. 1984;185:90–96. [PubMed] [Google Scholar]
- 6.Voto SJ, Weiner DS, Leighley B. Redisplacement after closed reduction of forearm fractures in children. J Pediatr Orthop. 1990;10:79–84. doi: 10.1097/01241398-199001000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Chess DG, Hyndman JC, Leahey JL, Brown DC, Sinclair AM. Short arm plaster cast for distal pediatric forearm fractures. J Pediatr Orthop. 1994;14:211–213. doi: 10.1097/01241398-199403000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Younger ASE, Tredwell SJ, Mackenzie WG. Factors affecting fracture position at cast removal after pediatric forearm fracture. J Pediatr Orthop. 1997;17:332–336. [PubMed] [Google Scholar]
- 9.Bohm ER, Bubbar V, Yong-Hing K, Dzus A. Above and below-the-elbow plaster casts for distal forearm fractures in children. A randomized controlled trial. J Bone Joint Surg Am. 2006;88(1):1–8. doi: 10.2106/JBJS.E.00320. [DOI] [PubMed] [Google Scholar]
- 10.Webb GR, Galpin RD, Armstrong DG. Comparison of short and long arm plaster casts for displaced fractures in the distal third of the forearm in children. J Bone Joint Surg Am. 2006;88(1):9–17. doi: 10.2106/JBJS.E.00131. [DOI] [PubMed] [Google Scholar]
- 11.Cotton FJ. Dislocations and joint-fractures. Chapter 16: the wrist. Philadelphia: WB Saunders; 1910. pp. 348–351. [Google Scholar]
- 12.Thomas EM, Tuson KWR, Browne PSH. Fractures of the radius and ulna in children. Injury. 1975;7:120–124. doi: 10.1016/0020-1383(75)90009-1. [DOI] [PubMed] [Google Scholar]
- 13.Proctor MT, Moore DJ, Paterson JMH. Redisplacement after manipulation of distal radial fractures in children. J Bone Joint Surg Br. 1993;75:453–454. doi: 10.1302/0301-620X.75B3.8496221. [DOI] [PubMed] [Google Scholar]
- 14.Haddad FS, Williams RL. Forearm fractures in children: avoiding redisplacement. Injury. 1995;26(10):691–692. doi: 10.1016/0020-1383(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 15.Hughston JC. Fractures of the forearm: anatomical considerations. J Bone Joint Surg Am. 1962;44:1664–1667. [Google Scholar]


