Abstract
Hyperpolarization-activated ion channels, encoded by four mammalian genes (HCN1-4), contribute in an important way to the cardiac pacemaker current If. Here, we describe the transcription profiles of the four HCN genes, the NRSF, KCNE2 and Kir2.1 genes from embryonic stage E9.5 dpc to postnatal day 120 in the mouse. Embryonic atrium and ventricle revealed abundant HCN4 transcription but other HCN transcripts were almost absent. Towards birth, HCN4 was downregulated in the atrium and almost vanished from the ventricle. After birth, however, HCN isotype transcription changed remarkably, showing increased levels of HCN1, HCN2 and HCN4 in the atrium and of HCN2 and HCN4 in the ventricle. HCN3 showed highest transcription at early embryonic stages and was hardly detectable thereafter. At postnatal day 10, HCN4 was highest in the sinoatrial node, being twofold higher than HCN1 and fivefold higher than HCN2. In the atrium, HCN4 was similar to HCN1 and sevenfold higher than HCN2. In the ventricle, in contrast, HCN2 was sixfold higher than HCN4, while HCN1 was absent. Subsequently all HCN isotype transcripts declined to lower adult levels, while ratios of HCN isotypes remained stable. In conclusion, substantial changes of HCN isotype transcription throughout cardiac development suggest that a regulated pattern of HCN isotypes is required to establish and ensure a stable heart rhythm. Furthermore, constantly low HCN transcription in adult myocardium may be required to prevent atrial and ventricular arrhythmogenesis.
Electronic supplementary material
The online version of this article (doi:10.1007/s00395-009-0031-5) contains supplementary material, which is available to authorized users.
Keywords: Hyperpolarization-activated ion channels, Pacemaker channels, Gene expression, Sinoatrial node, Transcription, Developmental biology
Introduction
The mammalian genome encodes four HCN channels (HCN1-4), which in response to hyperpolarization activate an inward current, termed If, obviously important in generation and regulation of the heart rate [5]. Initially, If was thought to be restricted to the cardiac pacemaker regions, but HCN channels appeared throughout the heart [5, 16, 35]. It has been difficult to show whether the functional heterogeneity at the sinoatrial node (SAN), atrium or ventricle correlates with the appearance of various HCN isotypes and how individual isotypes contribute to the pacemaker function [10, 17, 20, 27, 28]. Yasui et al. [34] showed that an If current, similar to the If of adult SAN, is present in early embryonic mouse ventricular myocytes. They related this current to extensive expression of the pacemaker channel HCN4, which declined later on during the second half of embryonic development. In the adult SAN of rabbit, mouse and dog, HCN4 is the dominant HCN isotype, accounting for the majority of the total HCN transcripts [11, 17, 27, 37], while in rat SAN, in contrast, HCN2 appeared at a higher level than HCN4 [10]. In healthy human hearts HCN1, 2, and 4 were detected in atrium, ventricle and Purkinje fibres with increased levels of HCN1 and HCN4 in the atrium [7] and highest HCN4 level in the human SAN [4]. Consistent with this, HCN4 mutations were associated with inherited sinus node bradycardia, indicating that, in adult humans, HCN4 activity strongly influences the heart rate [19, 21, 26, 30]. Recent evidence suggested that the formation of HCN heteromers [1] or interaction of HCN subunits with auxiliary β-subunits such as KCNE2 [36] might modulate If properties. Interestingly, If was increasingly detected in failing infant and adult hearts [2, 29, 38], suggesting that during remodelling in pathophysiological processes embryonic patterns may reappear and support arrhythmogenesis.
Unfortunately, the current view of HCN isotype transcription is based on the data obtained from different cardiac regions and species. In order to examine whether HCN isotype transcription pattern might reflect features of If currents at different regions of the myocardium, we measured transcript levels in mouse atrium and ventricle over a life span from embryonic stage E9.5 dpc to postnatal day 120. In addition, we studied cardiac transcription of the neural restrictive silencer factor (NRSF) which is thought to repress HCN4 gene activity in cardiomyocytes [12], of KCNE2, which may co-assemble and interact with HCN isotypes [36], and of Kir2.1, shown to stabilize the resting potential of cardiomyocytes [18, 23].
Methods
Tissue and RNA preparation
All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication number 85–23, revised 1996) and the European Community guidelines for the use of experimental animals.
Hearts used in this study came from C57BL/6 mice (Charles River, Sulzfeld, Germany) donors of either sex, aged E9,5 dpc; E13,5 dpc; E15,5 dpc; E18,5 dpc; P2; P4; P10; P20; P34; P60; P90; P120. Hearts were excised, immediately prepared using a stereomicroscope and frozen in liquid nitrogen. Atria including left and right atrium, interatrial septum and the SAN region were separated from whole ventricular probes including left and right ventricle and interventricular septum, AV-region was excluded from the samples. Early embryonic hearts were dissected according to the section planes indicated (Supplement, Fig. 1). To isolate the SAN of P10 animals, an area at the junction downwards from the ventral surface of the superior vena cava with the right atrium next to the crista terminalis was excised, spanning approximately 2 × 1 mm [33]. The left atrial auricle (LAA), left (LV) and right (RV) ventricle, and interventricular septum (Se) were carefully prepared and frozen in N2. For each sample, total RNA from two pools of 5–6 mice was prepared using TRIzol-Reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions.
cDNA Synthesis
Total RNA was reverse transcribed in 100 μl containing 5× First Strand Buffer (Invitrogen), 30 U RNAguard (GE Healthcare Europe, Freiburg, Germany), 1.1 mM dNTP (Invitrogen), 11.3 mM DTT (Invitrogen), 5 μg Pd(N)6 random hexamer primers (GE Healthcare) and 1,000 U Superscript II Reverse Transcriptase (Invitrogen). 15 μg total RNA was added to the mixture after being incubated for 5 min at 68°C, put 2 min on ice and incubated for 1 h at 37°C.
Quantitative RT-PCR
Quantification was performed using an ABS 7500 “real-time” PCR system and 96 well optical detection plates (Applera Corp., Norwalk, CT, USA). Wells were loaded to a total volume of 25 μl consisting of 10–50 ng cDNA, 1× TaqMan Universal Master Mix and pre-designed TaqMan probes and primers (TaqMan Gene Expression Assays, Applera) specific to mouse HCN1-4 (Mm00468832_m1; Mm00468538_m1; Mm00468543_m1; Mm0117086_m1), NRSF (Mm00803268_m1), Kir2.1 (Mm00434616_m1), KCNE2 (Mm00506492_m1) as well as to the housekeeping gene GAPDH (Mm999 99915_g1), used for normalization. Probes were labelled with the fluorescent reporter dye 6-carboxyfluorescein (FAM, Applera) at the 5′ end and with the non-fluorescent quencher at the 3′ end. Cycling conditions comprised an initial denaturing step at 95°C (10 min), and 45 cycles with 95°C (15 s) and 60°C (40 s). Data were analysed using the threshold cycle (CT) relative quantification method [13]. All PCR reactions were performed in triplicate, and data are expressed as an average of the triplicates.
Data analysis
The ΔΔCT method [13] was used for data analysis, where the CT value of a target transcript is expressed as a relative change between the two experimental conditions. We calculated target gene expression relative to that of the housekeeping gene GAPDH, which was shown to be expressed relatively stable throughout mouse cardiac development, between different cardiac tissues and in cardiomyocytes under various conditions [32; supplement Fig. 2]. In some diagrams of this study (Figs. 2, 4) we describe developmental and regional stages of target transcripts relative to their expression in P120 ventricular probes (=1) using the ΔΔCT method. In other diagrams we used the ΔCT method to display gene expression relative to GAPDH.
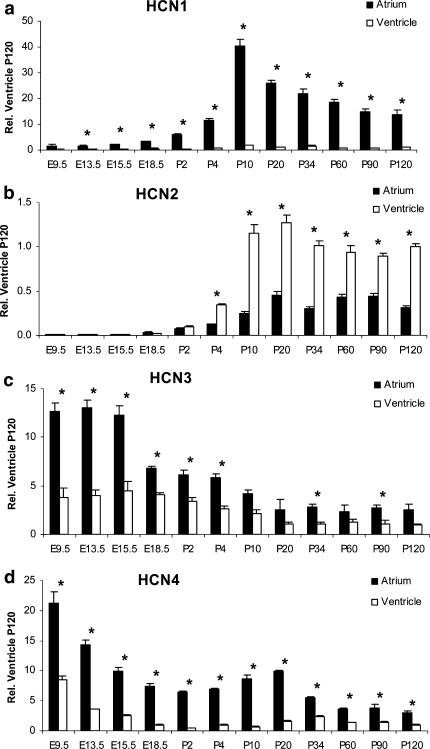
Fig. 2.
Comparative analysis of atrial and ventricular HCN isotype transcription in relation to their ventricular levels at P120. a HCN1 transcripts, low at embryonic myocardium, rise in the atrium after birth, reaching a maximum level at P10 that slowly declines to adult levels. In the ventricle, HCN1 transcription is negligible. b HCN2 transcripts, almost absent from the embryonic myocardium, appear postnatally with ventricular levels always higher than atrial levels. c HCN3 transcription, although very low at all, shows highest levels in the atrium and at early embryonic stages that gradually decrease to adult levels. d HCN4 transcription is highest at E9.5 dpc, then declines towards birth. A moderate rise after birth falls to stable adult levels after P20. Atrial HCN4 levels always dominate ventricular levels. Statistical analysis: *P < 0.05, atrium versus ventricle compared (n = 3)
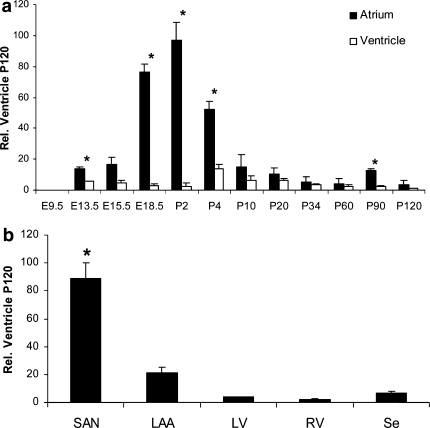
Fig. 4.
KCNE2 transcription related to ventricular level at P120. a At embryonic stage E9.5 dpc, KCNE2 is undetectable in atrium and ventricle. KCNE2 transcription, first detected at E13.5 dpc, rises in the atrium to attain maximum level at P2 but remains low in the ventricle. After P2, atrial KCNE2 declines rapidly to adult levels in atrium and ventricle. Statistical analysis: *P < 0.05, atrium versus ventricle compared. b At P10, the majority of KCNE2 appears in the sinoatrial node (SAN), levels are significantly higher than in the whole atrium, intermediate amounts in the left atrial auricle (LAA), and low levels in the right (RV) and left ventricle (LV) and the interventricular septum (Se). Statistical analysis: *P < 0.05, SAN and LAA, respectively, are compared pairwise to whole atrium at P10. #P < 0.05, LV, RV and Se, respectively, are compared pairwise to whole ventricle at P10 (n = 3)
Statistical analysis
The average of the relative transcript levels of each group of samples was characterized by calculating the arithmetic means as well as the standard errors (SE) of the individual reactions. Groups were compared by ANOVA, subregional versus regional as well as atrial versus ventricular transcription levels were compared pairwise by a Student’s t test. Differences between two samples were judged significant at confidence levels >95% (P < 0.05).
Results
In order to characterize transcript levels of mouse cardiac HCN subunits, quantitative RT-PCR analysis of HCN isotypes 1–4 was performed separately on dissected atrial and ventricular tissue. Furthermore, transcript levels of the neural-restrictive silencer factor (NRSF), [12], the potassium channel β-subunit KCNE2 [36], and the inward rectifier channel Kir2.1 [18] were determined using TaqMan assays.
Atrial transcription of HCN isotypes
Comparing transcript levels of HCN isotypes 1–4 throughout cardiac development revealed that HCN4, with a level of 2.4 ± 0.22% of the GAPDH level, was the sole HCN transcript in the atrium at E9.5 dpc (Fig. 1a). During progress, HCN4 levels declined to below 1% of GAPDH at birth (Fig. 1a). After birth, HCN4 temporary rose to a maximum of 1.3 ± 0.02% of the GAPDH level at P20 and then fell to adult levels of about 0.4% of GAPDH after P60 (Fig. 1a). HCN1 transcripts, absent from early embryonic atrium, slowly emerged towards birth, reaching a maximum of 0.7 ± 0.04% of the GAPDH level at P10 (Fig. 1a) that declined to adult levels of about 0.3% of GAPDH after P60 (Fig. 1a). Absent from early embryonic atrium, HCN2 transcription rose to a maximum at P20 (0.3 ± 0.07% of GAPDH) that slowly declined to adult levels of about 0.2% of GAPDH after P60 (Fig. 1a). HCN3 transcription, compared to GAPDH, almost was absent.
Fig. 1.
Transcription profiling of HCN isotypes throughout mouse cardiac development. a Atrial HCN isotype transcription. In the early embryonic atrium, HCN4 transcripts are the sole source of If current. HCN1-3 is absent. From birth to P34, HCN transcription is reorganized and achieves constant adult levels at P60. b Ventricular HCN isotype transcription. Only HCN4 transcripts appear in the early embryonic ventricle. Postnatally, HCN transcription is changed to predominant HCN2 and moderate HCN4 levels while HCN1 and HCN3 transcripts are virtually missing. c Regional differences at P10. In the sinoatrial node (SAN), HCN4 is expressed at a significantly higher level than in the whole atrial tissue (P < 0.05) and clearly dominates HCN1 and HCN2 transcription. In the left atrial auricle (LAA), HCN4 is reduced to one-third of the SAN level, being expressed significantly lower than in whole atrium (P < 0.05). In the left (LV) and right (RV) ventricle and in the interventricular septum (Se) HCN2 clearly dominates HCN4 while HCN1 like HCN3 is negligible. Interestingly, HCN4 is significantly higher in the Se and significantly lower in LV and RV than in the whole ventricular tissue, respectively. Statistical analysis: *P < 0.05, SAN and LAA, respectively, are compared pairwise with whole atrium at P10. #P < 0.05, LV, RV and Se, respectively, are compared pairwise with whole ventricle at P10 (n = 3)
Ventricular transcription of HCN isotypes
HCN4, abundantly transcribed at embryonic stage E9.5 dpc (1.0 ± 0.06% of GAPDH), continuously declined and was almost undetectable at birth (Fig. 1b). Postnatally, a slow and weak increase was observed to adult HCN4 levels of about 0.2% of the GAPDH level (Fig. 1b). HCN2 transcription, in contrast, almost absent from embryonic ventricle, rose to a maximum at P20 (0.7 ± 0.04% of GAPDH) which then declined to adult levels (0.5% of GAPDH; Fig. 1b). Compared to GAPDH, the HCN1 and HCN3 transcripts were lacking from embryonic and adult ventricle (Fig. 1b).
Regional distribution of cardiac HCN transcripts at postnatal day 10
After birth, HCN isotype transcription strikingly changed (Fig. 1a, b). In order to correlate these changes to local alteration in particular cardiac regions, we dissected mouse hearts at P10 when changes were high. In the SAN, HCN4 was the major HCN transcript with a level of 1.8 ± 0.11% of GAPDH, about twice that of HCN1 (P < 0.05) and about fivefold that of HCN2 (P < 0.05) (Fig. 1c). In LAA, by contrast, clearly separated from the SAN, the ratio of HCN transcripts diverged remarkably to HCN4 level of 0.6 ± 0.02% of GAPDH, similar to HCN1 (0.7 ± 0.03% of GAPDH), and to much lower HCN2 level (0.1 ± 0.02%; Fig. 1c). A quite different pattern appeared in the ventricle with HCN2 levels (0.5% of GAPDH) that clearly outbalanced HCN4. HCN1 and HCN3 transcripts virtually were absent (Fig. 1c). In the interventricular septum, a small increase of HCN2 (0.7 ± 0.02% of GAPDH) and HCN4 (0.2 ± 0.003% of GAPDH) and an almost negligible rise of HCN1 were observed (Fig. 1c).
HCN isotype transcription related to their ventricular levels at P120
Comparing atrial and ventricular transcript levels of each of the four HCN isotypes in relation to their ventricular levels at P120 revealed an isotype-specific behaviour. HCN1 and HCN2 transcripts virtually were undetectable at embryonic stages. After birth, HCN1 predominantly appeared in the atrium (Fig. 2a), while HCN2 preferentially appeared in the ventricle (Fig. 2b). A different course of transcription was observed for HCN3 and HCN4 (Fig. 2c, d). Although absent compared to GAPDH, HCN3, preferentially transcribed in the atrium, appeared on highest level at early embryonic stages (Fig. 2c). HCN4, however, predominant in the atrium, appeared abundantly at E9.5 dpc, declined towards birth, and temporarily reappeared after birth (P < 0.05) (Fig. 2d).
NRSF, a potential regulator of HCN4 gene activity
Based on the hypothesis that NRSF negatively regulates HCN4 gene activity in cardiomyocytes [12], we asked how NRSF is regulated during mouse cardiac development. A constant rise of NRSF transcription was observed that attained a maximum of 0.1 ± 0.003% of the GAPDH level at P10, then rapidly declined to 0.05 ± 0.001% of GAPDH at P20 and slowly reached adult levels (Fig. 3a). From E9.5 dpc to P10, atrial levels invariantly exceeded ventricular levels (P < 0.05; Fig. 3a). At P10, when the NRSF level was highest, a possible relationship of NRSF to HCN4 was considered. We observed low NRSF transcription in the SAN that was accompanied by a high HCN4 level and, conversely, high NRSF transcription in the atrium that was accompanied by a low HCN4 level. This correlation, however, was absent from the ventricle, where intermediate NRSF transcription in LV, RV and Se was accompanied by very low transcription levels of HCN4 (Fig. 3b, c).
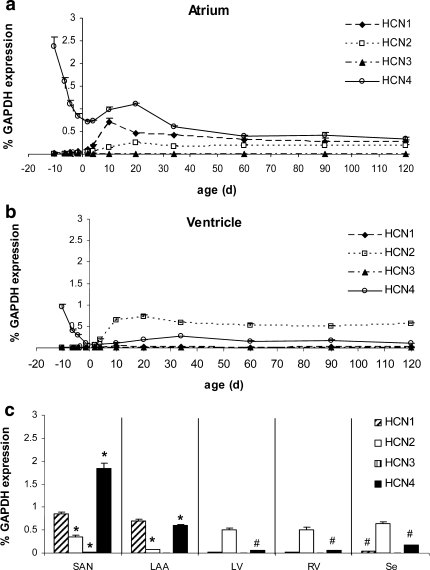
Fig. 3.
Cardiac NRSF transcription. a Although higher in atrium than in ventricle, NRSF transcription reaches a maximum level at P10 and declines thereafter to adult levels. Statistical analysis: *P < 0.05, atrium versus ventricle compared. b At P10, NRSF level is significantly lower in the sinoatrial node (SAN) in comparison to whole atrial tissue. Intermediate levels are detected in the right ventricle (RV), left ventricle (LV) and interventricular septum (Se). c Correspondingly, minor HCN4 levels in LAA, RV, LV and Se indicate inverse relations to NRSF. Statistical analysis: *P < 0.05, SAN and LAA, respectively, are compared pairwise to whole atrium at P10. #P < 0.05, LV, RV and Se, respectively, are compared pairwise to whole ventricle at P10 (n = 3)
Cardiac KCNE2 transcription
In an attempt to study whether KCNE2 transcription is correlated to HCN isotype transcription in the mouse, KCNE2 levels were determined in relation to their ventricular level at P120. Absent at embryonic stage E9.5 dpc, atrial KCNE2 level strikingly increased after E15.5 dpc, reached a maximum at P2 and rapidly declined to low adult levels (Fig. 4a). A much lower KCNE2 level appeared in the ventricle (Fig. 4a). At P10, the vast majority of the KCNE2 transcripts was observed in the SAN, low levels in LAA and interventricular septum, and almost undetectable levels in the ventricle (RV, LV; Fig. 4b).
Cardiac Kir2.1 transcription
Almost undetectable at embryonic stage 9.5 dpc (Fig. 5a), Kir2.1 transcription continuously increased, reaching a maximum of 1.3 ± 0.09% of the GAPDH level at P10 in the atrium and of 0.9 ± 0.09% of GAPDH in the ventricle at P4 (Fig. 5a). During progress, Kir2.1 levels slowly decreased to adult levels of about 0.7% of GAPDH in atrium and ventricle at P60 (Fig. 5a). When compared in different cardiac regions at P10, the lowest Kir2.1 levels appeared in the SAN where heart beating is initiated (Fig. 5b). Much higher Kir2.1 levels appeared in LAA and intermediate levels in the ventricle (Fig. 5b).
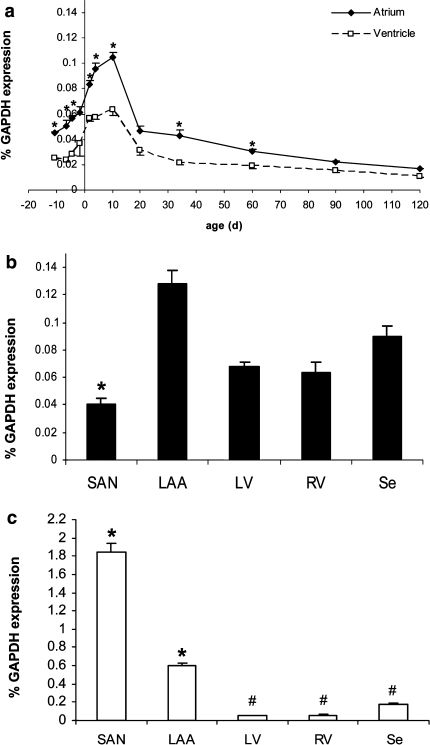
Fig. 5.
Cardiac Kir2.1 transcription. a From barely detectable levels at E9.5 dpc, Kir2.1 levels rise similarly in atrium and ventricle reaching maximum shortly after birth and slowly decline to adult levels. Statistical analysis: *P < 0.05, atrium versus ventricle compared. b Kir2.1 transcription is lowest in the sinoatrial node (SAN), highest in the left atrial auricle (LAA) and shows intermediate levels in the right (RV) and left ventricle (LV) and interventricular septum (Se). Statistical analysis: *P < 0.05, SAN and LAA, respectively, are compared pairwise to whole atrium at P10. #P < 0.05, LV, RV and Se, respectively, are compared pairwise to whole ventricle at P10 (n = 3)
Discussion
In mammals, cardiac pacemaker activity controlling cardiac excitability is located in a restricted area of the heart, termed the SAN, which among other features is characterized by abundant expression of the hyperpolarization-activated and nucleotide-gated ion channel HCN4 [14, 20, 27; for review see 16]. Even though HCN4 accounts for the majority of the total HCN messages in the SAN, different HCN isotypes were observed in the SAN of rabbit, canine, rat, and mouse [10, 17, 20, 27, 37]. Thus, an important question in understanding the molecular basis of pacemaker activity is the determination of the spatial and temporal appearance of ion channels underlying the electrical activity that regulates heart beating [25, 31]. Expression profiling of the ion channels HCN1-4, Kir2.1 and KCNE2, as well as of the transcription factor NRSF, was performed using quantitative RT-PCR of total RNA isolated from complete and dissected mouse hearts from embryonic stage 9.5 dpc to postnatal day 120.
We found remarkable differences in the HCN isotype transcription throughout mouse cardiac development that may contribute significantly to changes that determine the properties of pacemaker channels and thereby influence the heart rate. HCN4, previously identified as the main source of HCN transcripts in embryonic atrium and SAN [27, 28], was abundantly transcribed at E9.5 dpc with atrial level twofold higher than ventricular level. Until birth, HCN4 transcripts substantially declined in the atrium and almost disappeared from the ventricle; this profile is consistent with the findings of Yasui et al. [34] who measured prominent If currents in the ventricle at E9.5 dpc that almost vanished towards birth. The importance of HCN4 in cardiac development was underscored strikingly by the premature death of mice lacking the pacemaker channel HCN4 [9, 28] or expressing HCN4 channels unable to bind cAMP [8]. On the other hand, the absence of HCN1 and HCN2 from embryonic myocardium is underlined by the lack of cardiac symptoms in mice deficient for HCN1 [22] and the normal embryonic development of HCN2 deficient mice which showed sinus dysrhythmia at adult stages [15].
After birth, HCN isotype transcription changed remarkably. HCN1, 2 and 4 levels increased in the atrium, leaving HCN4 predominant while HCN3 almost disappeared. In the ventricle, in contrast, HCN2 and HCN4 levels increased, leading to predominant HCN2 levels while HCN1 like HCN3 were almost absent [14, 17, 27].
In human atrium, as in the mouse, elevated levels of HCN1, HCN2, and HCN4 were detected [7] and HCN4 was abundant in human SAN [4]. In human ventricle, unlike the mouse, HCN4 appeared at a higher level than HCN2, indicating differences in HCN isotype patterning among the species [8, 9, 19, 21, 26, 29, 30]. However, the functional role of If in the ventricle is still unclear, even though increasing If might contribute to arrhythmogenesis in heart failure [2, 29, 38]. Comparative analysis of atrial and ventricular HCN transcription revealed remarkable differences, believed to underlie significant aspects in the generation of If currents. For pairwise comparison of single HCN isotypes between atrium and ventricle, HCN transcription was monitored in relation to their ventricular level at P120 after normalization to GAPDH (Fig. 2). Almost absent from embryonic myocardium, HCN1 appeared restricted to the atrium, whereas HCN2 obtained the majority in the ventricle. On the other hand, HCN3 and HCN4 preferentially transcribed in the atrium showed highest levels in early embryonic myocardium.
After birth, HCN4 transcription rose again in the atrium while ventricular levels remained constant and HCN3 continued to decline in atrium and ventricle. A recent study [24] investigating the molecular basis of spontaneous activity of mouse embryonic stem cells (mESC) demonstrated that If in these cells was consistent with the expression of HCN2 and HCN3. Interestingly, undifferentiated mESC strongly expressed HCN3. In the course of cardiac differentiation of mESC, HCN3 transcription declined, identifying HCN3 as a very early HCN isotype of cardiac development, which is consistent with the declining transcription kinetic of HCN3 in our study. The fact that HCN4 (Fig. 1) occurred alone throughout embryonic development, followed by substantial changes in HCN transcription after birth, caused us to examine HCN isotypes in dissected mouse hearts at P10. The ratio between HCN isotypes, at P10, turned out to be similar to P70 [17] and HCN4 was predominant in the SAN, similar to HCN1 and higher than HCN2 in the atrium and lower than HCN2 in the ventricle while HCN1 and HCN3 were absent from the ventricle.
However, HCN isotypes, abundantly transcribed at P10, declined to lower adult levels thereafter, which also was observed in the rat [10]. Lower adult HCN levels may ensure a physiological rhythmogenic state, considering that increased transcription of HCN2 and HCN4 was observed in cardiomyopathy and may support arrhythmogenesis [6]. Undoubtedly, in mammals, the SAN as the primary pacemaker of the heart dominates the heart rate control. Interestingly, based on the Cre-mediated ablation of HCN4, it has been suggested that the HCN4 channel is not of critical importance for acceleration of the heart rate in adult mice. Moreover, the study showed that lack of If caused a cardiac arrhythmia which was characterized not by bradycardia but by recurrent sinus pauses, questioning the role of If in the cardiac pacemaking process in adult mice [8, 9]. In adult humans, in contrast, HCN4 mutations were associated with inherited sinus node bradycardia, and sinus node inhibitors like ivabradine reduce the heart rate in a dose-dependent manner, indicating that HCN4 activity in human strongly influences the heart rate [19, 21, 26, 30]. Further studies are needed to elucidate the differences in HCN isotype patterning and the role of If throughout the developmental stages between different species to uncover the complexity of heart rate regulation [25, 31].
In another set of experiments, we examined cardiac expression of the NRSF, which may repress HCN4 gene activity in cardiomyocytes [12]. Rising NRSF levels throughout embryonic development were accompanied by a reduction of HCN4 transcripts. After birth, at P10, NRSF and HCN4 appeared inversely related in atrium and SAN. However, in contrast to the suggested regulation, transcription of both NRSF and HCN4 was down-regulated in the ventricle.
KCNE2 subunits, appeared in the atrium of embryonic and adult myocardium [3, 36]. They may modulate If currents by interacting with HCN subunits. At P10, the vast majority of KCNE2 transcripts were assigned to the SAN, where co-assembling might occur; low levels appeared in the atrium and negligible levels in the ventricle and septum.
An additional component, shown to stabilize the resting potential of cardiomyocytes, is the inward-rectifier potassium channel Kir2.1 [18, 23]. In early heart development, when excitability is high, Kir2.1 transcripts were almost undetectable. However, with differentiation, Kir2.1 levels increased until P10 in the atrium and until P4 in the ventricle, indicating that shortly after birth control of myocardial excitability by Ik1 may be of particular importance. At P10, consistent with results from adult mice [17], Kir2.1 level was lowest in the SAN.
The aim of our study was to determine the temporal and spatial appearance of HCN isotype transcripts as the molecular basis of cardiac pacemaker If currents throughout cardiac development in a single species. Quantitative RT-PCR of total RNA isolated from complete and dissected mouse hearts from embryonic stage E9.5 dpc to P120 was performed.
Our results suggest that a specific pattern of HCN isotypes is required to establish and to stabilize heart rhythm, considering that increased transcription of HCN isotypes points to heart failure and may support arrhythmogenesis [2, 29, 38].
Limitations of the study
It should be noted that transcript levels do not perfectly match the level of functional channels; however, the correlated decline of HCN4 transcripts and If currents from E9.5 dpc towards birth [34] as well as the large differences in HCN transcript levels between developmental stages and at different cardiac regions implied that at least some of the regulation of If properties occurs on the transcriptional level. Interestingly, If currents, observed in adult ventricular myocytes of rat, guinea pig, dog [34] and human [29], were absent from rabbit [27]. In adult mouse ventricular myocytes, however, evidence of If currents remained unclear and further studies are needed to elucidate the functional presence of If under physiological and pathological conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1. Preparation of early embryonic hearts (E9.5 dpc) under the stereo microscope. A: indicated are section planes for atrial and B: ventricular bounds. SV, Sinus venosus;A, Atrium; V, Ventricle; BC, Bulbous cordis; AR, Aortic roots. (JPG 5970 kb)
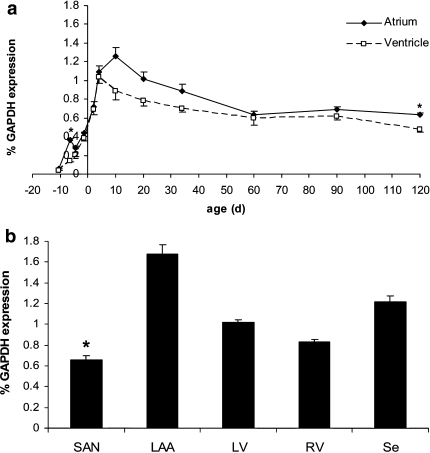
Fig. 2. Logarithmic diagram of GAPDH expression at four representative developmental stages (E13,5; E18,5; P10 and P120) in atrium an ventricle relative to the housekeeping gene 18S rRNA. We found no significant differences between atrial and ventricular GAPDH expression at the investigated time points after normalization to 18S rRNA; statistical analysis: pairwise comparison by Student t test, P < 0.05 was considered significant. Furthermore, no significant age-related changes were detected in terms of GAPDH expression normalized to 18S rRNA, along the investigated time points of atrium and ventricle, respectively; statistical analysis: here ANOVA was followed by a Tukey post hoc test for individual age group comparisons, P < 0.05 was considered significant. (JPG 383 kb)
Acknowledgments
The excellent technical assistance of Michaela Bauer and Simone Bauer is gratefully acknowledged. We thank Dr. J. Wray, Department of Biomolecular Mechanisms, Max-Planck-Institute for Medical Research, Germany for critically reading the manuscript, and Dr. L. Edler, Department of Biostatistics, German Cancer Research Center, Germany for kind help with our statistical analysis. The work was supported by the “Young Investigator Award” of the Faculty of Medicine, University of Heidelberg (PAS).
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00395-009-0031-5) contains supplementary material, which is available to authorized users.
References
- 1.Altomare C, Terragni B, Brioschi C, Milanesi R, Pagliuca C, Viscomi C, Moroni A, Baruscotti M, DiFrancesco D (2003) Heteromeric HCN1-HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol 549:347–359 [DOI] [PMC free article] [PubMed]
- 2.Borlak J, Thum T (2003) Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J 17:1592–1608 [DOI] [PubMed]
- 3.Chun KRJ, Koenen M, Katus HA, Zehelein J (2004) Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp Mol Med 36:367–371 [DOI] [PubMed]
- 4.Dobrzynski H, Boyett MR, Anderson RH (2007) New insights into pacemaker activity promoting understanding of sick sinus syndrome. Circulation 115:1921–1932 [DOI] [PubMed]
- 5.DiFrancesco D (1996) The hyperpolarization-activated (If) current: autonomic regulation and the control of pacing. In: Morad M, Ebashi S, Trautwein W, Kurachi Y (eds) Molecular physiology and pharmacology of cardiac ion channels and transporters. Kluwer Academic Publishers, Dordrecht, pp 31–37
- 6.Fernández-Velasco M, Goren N, Benito G, Blanco-Rivero J, Boscá L, Delgado C (2003) Regional distribution of hyperpolarization-activated current (If) and hyperpolarization-activated cyclic nucleotide-gated channel mRNA expression in ventricular cells from control and hypertrophied rat hearts. J Physiol 553.2:395–405 [DOI] [PMC free article] [PubMed]
- 7.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S (2007) Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol 582.2:675–693 [DOI] [PMC free article] [PubMed]
- 8.Harzheim D, Pfeiffer KH, Fabritz L, Kremmer E, Buch T, Waisman A, Kirchhof P, Kaupp UB, Seifert R (2008) Cardiac pacemaker function of HCN4 channels in mice is confined to embryonic development and requires cyclic AMP. EMBO J 27:692–703 [DOI] [PMC free article] [PubMed]
- 9.Herrman S, Stieber J, Stöckl G, Hofmann F, Ludwig A (2007) HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J 26:4423–4432 [DOI] [PMC free article] [PubMed]
- 10.Huang X, Yang P, Du Y, Zhang J, Ma A (2007) Age-related down-regulation of HCN channels in rat sinoatrial node. Basic Res Cardiol 102:429–435 [DOI] [PubMed]
- 11.Ishii TM, Takano M, Xie LH, Noma A, Ohmori H (1999) Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J Biol Chem 274:12835–12839 [DOI] [PubMed]
- 12.Kuratomi S, Kuratomi A, Kuwahara K, Ishii TM, Nakao K, Saito Y, Takano M (2007) NRSF regulates the developmental and hypertrophic changes of HCN4 transcription in rat cardiac myocytes. Biochem and Biophys Res Comm 353:67–73 [DOI] [PubMed]
- 13.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2T−ΔΔC method. Methods 25:402–408 [DOI] [PubMed]
- 14.Liu J, Dobrzynski H, Yanni J, Boyett MR, Lei M (1999) Organisation of the mouse sinoatrial node: structure and expression of HCN channels. Cardiovasc Res 73:729–738 [DOI] [PubMed]
- 15.Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Lanenabartels A, Wotjak C, Munsch T, Zong X, Feil S, Feil R, Lancel M, Chien KR, Konnerth A, Pape HC, Biel M, Hofmann F (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 22:216–224 [DOI] [PMC free article] [PubMed]
- 16.Mangoni ME, Nargeot JL (2008) Genesis and regulation of the heart automaticity. Physiol Rev 88:919–982 [DOI] [PubMed]
- 17.Marionneau C, Couette B, Liu J, Mangoni ME, Nargeot J, Lei M, Escande D, Demolombe S (2005) Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol 562.1:223–234 [DOI] [PMC free article] [PubMed]
- 18.Miake J, Marbán E, Nuss HB (2002) Biological pacemaker created by gene transfer. Nature 419:132–133 [DOI] [PubMed]
- 19.Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D (2006) Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 354:151–157 [DOI] [PubMed]
- 20.Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A (2001) Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem 268:1646–1652 [DOI] [PubMed]
- 21.Nof E, Luria D, Brass D, Marek D, Lahat H, Reznik-Wolf H, Pras E, Dascal N, Eldar M, Glikson M (2007) Point mutation in the HCN4 cardiac ion channel pore affecting synthesis, trafficking, and functional expression is associated with familial asymptomatic sinus bradycardia. Circulation 116:463–470 [DOI] [PubMed]
- 22.Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A (2003) The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar purkinje cells. Cell 115:551–564 [DOI] [PubMed]
- 23.Piao L, Li J, McLerie M, Lopatin AN (2007) Transgenic upregulation of IK1 in the mouse heart is proarrhythmic. Basic Res Cardiol 102:416–428 [DOI] [PubMed]
- 24.Qu Y, Whitaker GM, Hove-Madsen L, Tibbits GF, Accili EA (2008) Hyperpolarization-activated cyclic nucleotide-modulated ‘HCN’ channels confer regular and faster rhythmicity to beating mouse embryonic stem cells. J Physiol 586.3:701–716 [DOI] [PMC free article] [PubMed]
- 25.Richards AG, Simonson E, Visscher MB (1953) Electrocardiogram and phonogram of adult and newborn mice in normal conditions and under the effect of cooling, hypoxia and potassium. Am J Physiol 174:293–298 [DOI] [PubMed]
- 26.Schulze-Bahr E, Neu A, Friederich P, Kaupp UB, Breithardt G, Pongs O, Isbrandt D (2003) Pacemaker channel dysfunction in a patient with sinus node disease. J Clin Invest 111:1537–1545 [DOI] [PMC free article] [PubMed]
- 27.Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS (1999) Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res 85:1–6 [DOI] [PubMed]
- 28.Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, Ludwig A (2003) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 100:15235–15240 [DOI] [PMC free article] [PubMed]
- 29.Stillitano F, Lonardo G, Zicha S, Varro A, Cerbai E, Mugelli A, Nattel S (2008) Molecular basis of funny current (If) in normal and failing human heart. J Mol Cell Cardiol 45:289–299 [DOI] [PubMed]
- 30.Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, Morita H, Higashiuesato Y, Hirano Y, Yasunami M, Takishita S, Yamashina A, Ohe T, Sunamori M, Hiraoka M, Kimura A (2004) Functional characterization of a trafficking-defective HCN4 mutation, D553 N, associated with cardiac arrhythmia. J Biol Chem 279:27194–27198 [DOI] [PubMed]
- 31.Wang L, Swirp S, Duff H (2000) Age-dependent response of the electrocardiogram to K+ channel blockers in mice. Am J Physiol Cell Physiol 278:C73–C80 [DOI] [PubMed]
- 32.Winer J, Jung CK, Shackel I, Williams PM (1999) Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270:41–49 [DOI] [PubMed]
- 33.Yamamoto M, Dobrzynski H, Tellez J, Niwa R, Billeter R, Honjo H, Kodama I, Boyett MR (2006) Extended atrial conduction system characterised by the expression of the HCN4 channel and connexin 45. Cardiovasc Res 72:271–278 [DOI] [PubMed]
- 34.Yasui K, Liu W, Opthof T, Kada K, Lee JK, Kamiya K, Kodama I (2001) If Current and spontaneous activity in mouse embryonic ventricular myocytes. Circ Res 88:536–542 [DOI] [PubMed]
- 35.Yu H, Chang F, Cohen IS (1993) Pacemaker current exists in ventricular myocytes. Circ Res 72:232–236 [DOI] [PubMed]
- 36.Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R (2001) MinK-related peptide 1: A subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res 88:e84 [DOI] [PubMed]
- 37.Zicha S, Fernandez-Velasco M, Lonardo G, L’Heureux N, Nattel S (2005) Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res 66:472–481 [DOI] [PubMed]
- 38.Zorn-Pauly K, Schaffer P, Pelzmann B, Bernhart E, Lang P, Zink M, Mächler H, Rigler B, Koidl B (2003) A hyperpolarization activated inward current (If) is present in infant ventricular myocytes. Basic Res Cardiol 98:362–366 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Fig. 1. Preparation of early embryonic hearts (E9.5 dpc) under the stereo microscope. A: indicated are section planes for atrial and B: ventricular bounds. SV, Sinus venosus;A, Atrium; V, Ventricle; BC, Bulbous cordis; AR, Aortic roots. (JPG 5970 kb)
Fig. 2. Logarithmic diagram of GAPDH expression at four representative developmental stages (E13,5; E18,5; P10 and P120) in atrium an ventricle relative to the housekeeping gene 18S rRNA. We found no significant differences between atrial and ventricular GAPDH expression at the investigated time points after normalization to 18S rRNA; statistical analysis: pairwise comparison by Student t test, P < 0.05 was considered significant. Furthermore, no significant age-related changes were detected in terms of GAPDH expression normalized to 18S rRNA, along the investigated time points of atrium and ventricle, respectively; statistical analysis: here ANOVA was followed by a Tukey post hoc test for individual age group comparisons, P < 0.05 was considered significant. (JPG 383 kb)