Abstract
We report a child with a severe choreadystonic movement disorder, bilateral periventricular nodular heterotopia (BPNH), and secondary microcephaly based on compound heterozygosity for two new ARFGEF2 mutations (c.2031_2038dup and c.3798_3802del), changing the limited knowledge about the phenotype. The brain MRI shows bilateral hyperintensity of the putamen, BPNH, and generalized atrophy. Loss of ARFGEF2 function affects vesicle trafficking, proliferation/apoptosis, and neurotransmitter receptor function. This can explain BPNH and microcephaly. We hypothesize that the movement disorder and the preferential damage to the basal ganglia, specifically to the putamen, may be caused by an increased sensitivity to degeneration, a dynamic dysfunction due to neurotransmitter receptor mislocalization or a combination of both.
Electronic supplementary material
The online version of this article (doi:10.1007/s10048-009-0192-2) contains supplementary material, which is available to authorized users.
Keywords: Bilateral periventricular nodular heterotopia, ARFGEF2, BIG2, FLNA, Microcephaly, Child, Basal ganglia, Extrapyramidal movement disorder
Introduction
In 2004 mutations in the gene ARFGEF2 encoding ADP-ribosylation factor guanine nucleotide exchange factor 2, were found to cause autosomal recessive bilateral periventricular nodular heterotopia (BPNH) in four patients from two Turkish pedigrees [1]. Clinical features reported were microcephaly, feeding difficulties, severe mental retardation, quadriparesis, and epilepsy. More details on the phenotype were not reported and since then, to our knowledge, no additional patients have been published. We discuss a patient with a different phenotype caused by two new ARFGEF2 mutations.
Case
Our patient is the only child of non-consanguineous parents from Dutch descent. Prenatal ultrasounds showed growth retardation. She was born at 38 weeks of gestation with an Apgar score of 10 after 5 min, birth weight 2,790 g, and length 46 cm. Occipito-frontal circumference was 34.2 cm at the age of 3 weeks (−1.5 SD). During the first year developmental delay became apparent. She showed social smiling at 2 months and grasping at 5 months, and rolled over at the age of 8 months. There were excessive extension movements. She had persistent feeding problems, severe drooling, and frequent vomiting. At the age of 9 months a pediatrician noted dystonic-spastic paraplegia with axial hypotonia. With physical therapy she showed some progression, but did not reach normal milestones. At the age of 4 years feeding difficulties prompted a gastrostomy. Despite adequate caloric intake, growth is unsatisfactory with height progressing at 2.5 SD below normal. Occipito-frontal circumference growth went from the –1.5 SD to 0.5 cm below the –2.5 SD curve. She never had seizures. Now, at the age of 7 years, she makes eye contact, smiles, and understands some words. She vocalizes, but does not produce any words. Pupillary responses are normal. There is no nystagmus. Axial muscle tone is low and variable in the extremities. Muscle strength is normal. She shows a severe extrapyramidal movement disorder with irregular repetitive jerking movements of all extremities and the face (chorea) and sustained abnormal movements and posturing (dystonia). This is exacerbated by emotions and during infections when large proximal jerking movements may resemble ballism. There is no rigidity or ataxia. Tendon reflexes are lively and symmetric, with extensor plantar responses (Babinski sign). She can sit with support, but cannot stand. The movement unrest can be so severe that she becomes exhausted and is partially responsive to treatment with high doses of benzodiazepines.
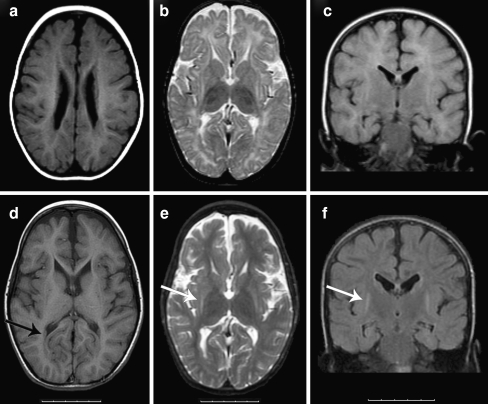
Brain MRI at the age of 13 months showed BPNH, myelination delay, and generalized atrophy, a second MRI at age 4 years and 10 months showed improved myelination, stable or minimally progressive generalized atrophy but also symmetric atrophy of the basal ganglia, specifically of the globus pallidus and the putamen with focal T2-hyperintensity of the putamen (Fig. 1). In retrospect, inhomogeneous abnormalities of the putamen can also be seen on the first MRI and suggest early degeneration resulting in gliosis. EEGs show delta-activity without differentiation or epileptiform activity. Metabolic testing of urine and plasma, including amino acids, lactate, pyruvate, and creatine kinase was normal. The family history is unremarkable.
Fig. 1.
Brain MRI made at the age of 13 months (images A, B, and C) and at the age of 4 years and 10 months (images D, E, and F). A and D T1-weighted images showing bilateral periventricular nodular heterotopia with the intensity of gray matter (black arrow in image D). B and E T2-weighted images showing hyperintensity of the putamen and atrophy of the putamen and globus pallidus (white arrow in image E). C and F Flair images, coronal view, showing bilateral hyperintensity of the putamen, more clearly visible on image F made at a later age (white arrow), and mild atrophy of the hippocampi
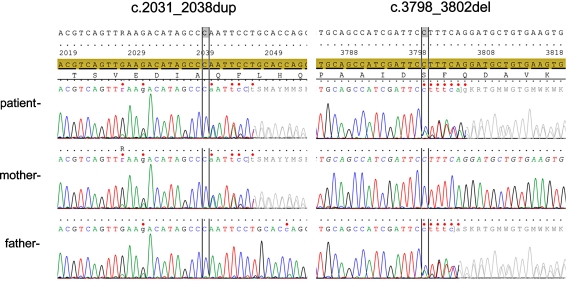
Sequence analysis of all 39 coding exons and intron–exon boundaries of the ARFGEF2 gene on chromosome 20 shows compound heterozygosity for two variations that have not been previously reported. At the DNA level c.2031_2038dup and c.3798_3802del using reference sequence NM_006420.2 and at protein level p.Gln680ProfsX2 and p.Phe1267GlyfsX17, respectively. Both these variations lead to frame shift followed by a premature stop-codon and are therefore considered pathogenic mutations. Parents are heterozygote for found mutations (Fig. 2).
Fig. 2.
The sequences of the relevant areas of the ARFGEF2 gene are depicted showing the maternal origin of c.2031_2038dup and the paternal origin of c.3798_3802del
Discussion
Mutations in ARFGEF2 can cause autosomal recessive bilateral periventricular nodular heterotopia (BPNH) with microcephaly [1]. Our patient shows that a choreadystonic extrapyramidal movement disorder can be part of the phenotype. The movement disorder is reflected in MRI abnormalities of the basal ganglia, specifically of the putamen, in addition to generalized atrophy and BPNH. Anatomically, putamen and caudate nucleus form the dorsal striatum and are the major afferent center of the basal ganglia, receiving excitatory input from the cerebral cortex, thalamus, and brain stem. The putamen receives projections from the cortical motor and somatosensory areas, the midbrain, and the raphe nuclei. The striatum projects to the globus pallidus and substantia nigra, mainly with GABA-ergic neurons. Output is mediated by local inhibitory interneurons [2]. Similar choreadystonic movement disorders and putamen degeneration on MRI are seen in Huntington’s disease, Wilson’s disease and Leigh syndrome.
ARFGEF proteins are guanine nucleotide exchange factors (GEFs) that activate ADP-ribosylation factors (ARFs). ARFGEF2 protein, also known as BIG2, is one of three GEFs expressed in the brain during the period of neural proliferation and migration with high expression in the cortical ventricular zone and ganglionic eminences, the future basal ganglia [1]. ARFs regulate the formation of coated vesicles from the Golgi, trans-Golgi network (TGN), and endosomes. In recycling endosomes BIG2 loss of function result in failure to deliver proteins to the cell membrane [3–5]. This was shown for the GABA(A) receptor, but also for the transferrin receptor and the tumor necrosis factor receptor [1, 4, 6, 7]. Other cell surface receptors are likely to be also affected.
To understand our patient’s phenotype, different consequences of ARFGEF2 loss of function need to be considered. The BPNH are similar to those seen in patients with loss-of-function mutations in the X-linked filamin A gene (FLNA). FLNA is the most commonly identified genetic cause for BPNH, found in approximately one third of patients [8]. In heterozygous females BPNH is associated with epilepsy and/or developmental delay or may be an accidental finding. In males FLNA mutations are often fatal, but may result in a similar phenotype to affected females [8, 9]. In both ARFGEF2 and FLNA patients the neuro-epithelium lining the ventricles is disrupted leading to failed migration of later-born neurons [9]. The similarity may be solely due to failed transport of the filamin A protein to the cell membrane by BIG2 dysfunction or to a final common pathway disrupting vesicle trafficking [9, 10]. However, despite the similar brain phenotype, FLNA patients do not have extrapyramidal movement disorders or microcephaly. Speculatively, in ARFGEF2 neuronal migration may be more severely affected due to disruption in GABA(A) receptor function in the embryonic period, as GABA(A) antagonists have been shown to impair neuronal motility in rodents in vitro and in vivo [11, 12].
Microcephaly can be due to diminished neuronal proliferation and BIG2 inhibition in vitro experiments seem to suggest this occurs [1]. Increased apoptosis can also cause microcephaly and atrophy. Other BIG2 inhibition experiments show some signs of endoplasmic reticulum stress in cultured cells [13]. Possibly, ER stress increases susceptibility to apoptosis in specific neurons. Our patient’s normal occipito-frontal circumference at birth shows that congenital microcephaly is not obligatory. Why the putamen should show a specific sensitivity for neuronal loss is unknown.
Finally, ARFGEF2 is expressed postnatally and neurotransmitter receptor function can be impaired by defective exosome function [3, 6]. Disruptions in the recycling of receptors also hinder dendritic arborization [9]. This may have contributed to the movement disorder.
Our patient is compound heterozygote for two new mutations and new patients are needed to determine if a genotype–phenotype relation exists for ARFGEF2 mutations.
BPNH caused by autosomal recessive ARFGEF2 mutations is more than an anatomical malformation. We suggest that the clinical features of the choreadystonic movement disorder, BPNH, and microcephaly of our patient can be understood in the light of abnormal vesicle transport resulting in neuroependyma disruption, decreased proliferation of neurons, and/or increased sensitivity to apoptosis and lifelong exosome dysfunction affecting neurotransmitter receptor function and neuronal development.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
Conflicts of interest
This study was supported by the Revolving Fund of the Erasmus MC. The authors have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Sheen VL, Ganesh VS, Topcu M, Sebire G, Bodell A, Hill RS, et al. Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex. Nat Genet. 2004;36(1):69–76. doi: 10.1038/ng1276. [DOI] [PubMed] [Google Scholar]
- 2.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science 4th ed. New York: McGraw-Hill; 2000. [Google Scholar]
- 3.Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ARFs in membrane traffic between the TGN and endosomes. Mol Biol Cell. 2008;19(6):2650–2660. doi: 10.1091/mbc.E07-10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen X, Xu KF, Fan Q, Pacheco-Rodriguez G, Moss J, Vaughan M. Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake. Proc Natl Acad Sci U S A. 2006;103(8):2635–2640. doi: 10.1073/pnas.0510599103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, et al. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004;90(1):173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100(2):279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- 7.Islam A, Shen X, Hiroi T, Moss J, Vaughan M, Levine SJ. The brefeldin A-inhibited guanine nucleotide-exchange protein, BIG2, regulates the constitutive release of TNFR1 exosome-like vesicles. J Biol Chem. 2007;282(13):9591–9599. doi: 10.1074/jbc.M607122200. [DOI] [PubMed] [Google Scholar]
- 8.Parrini E, Ramazzotti A, Dobyns WB, Mei D, Moro F, Veggiotti P, et al. Periventricular heterotopia: phenotypic heterogeneity and correlation with Filamin A mutations. Brain. 2006;129(Pt 7):1892–1906. doi: 10.1093/brain/awl125. [DOI] [PubMed] [Google Scholar]
- 9.Ferland RJ, Batiz LF, Neal J, Lian G, Bundock E, Lu J, et al. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009;18(3):497–516. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Tiao G, Folkerth R, Hecht J, Walsh C, Sheen V. Overlapping expression of ARFGEF2 and Filamin A in the neuroependymal lining of the lateral ventricles: insights into the cause of periventricular heterotopia. J Comp Neurol. 2006;494(3):476–484. doi: 10.1002/cne.20806. [DOI] [PubMed] [Google Scholar]
- 11.Manent JB, Demarque M, Jorquera PC, Ben-Ari Y, Aniksztejn L, et al. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25(19):4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heck N, Kilb W, Reiprich P, Kubota H, Furukawa T, Fukuda A, Luhmann HJ. GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb Cortex. 2007;17(1):138–148. doi: 10.1093/cercor/bhj135. [DOI] [PubMed] [Google Scholar]
- 13.Citterio C, Vichi A, Pacheco-Rodriguez G, Aponte AM, Moss J, Vaughan M. Unfolded protein response and cell death after depletion of brefeldin A-inhibited guanine nucleotide-exchange protein GBF1. Proc Natl Acad Sci U S A. 2008;105(8):2877–2882. doi: 10.1073/pnas.0712224105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.