Abstract
Attaining the full therapeutic utility of antisense and siRNA oligonucleotides will require understanding of the biological barriers that stand between initial administration of these drugs and their final actions within cells. This article examines some of the key barriers that affect the biodistribution of oligonucleotides both in molecular form and when they are associated with nanocarriers. An understanding of the biological processes underlying these barriers will aid in the design of more effective delivery systems.
Introduction
There is currently enormous interest in the possibility of using oligonucleotides, including siRNA, antisense, and aptamers, as therapeutic agents. Both antisense and nucleic acid aptamers have reached clinical practice 1-3, while numerous clinical trials of siRNA are underway 4. However, there is a widespread sense in the field that the full potential of oligonucleotides as therapeutic agents will not be attained until better methodologies for targeted delivery to cells and tissues are developed. Over the last few years many laboratories have addressed this issue by chemical modification of oligonucleotides, via use of various nanocarriers, or by some combination thereof. Our own approach has involved chemical conjugation of oligonucleotides with receptor-specific targeting ligands 5, 6. However, many other strategies have been devised and these have been the subject of multiple articles and reviews, only a few of which can be cited here 7-12.
In this article we will take a different approach to the issue. We will seek to succinctly describe some of the major biological barriers to efficient delivery of therapeutic oligonucleotides and in some cases we will attempt to project means for overcoming these barriers. Hopefully this discussion will be of some value to investigators already active in the field, but it may be of more value to those who are considering entering the challenging area of nucleic acid therapeutics. It is important to note that the barriers encountered by ‘monomeric’ oligonucleotides (that is single molecules of antisense, siRNA, or aptamers) will be quite different from those encountered by oligonucleotides associated with various nanoparticles or other nanocarriers. In both cases rapid clearance will be an issue, but the mechanisms and locales involved will be very different, as discussed below. Since antisense oligonucleotides predate siRNA, there is considerably more known about the pharmacokinetics and biodistribution of the former than the later. However, information on how the body handles various forms of siRNA is rapidly increasing.
Nuclease Stability
One of the first biological barriers encountered by administered siRNA and antisense oligonucleotides is presented by the nuclease activity in plasma and tissues. The major activity in plasma is a 3′ exonuclease, however, cleavage of internucleotide bonds can also take place. Chemical modification can drastically improve the stability of oligonucleotides in the biological milieu, as well as allowing improvements in selectivity and reduced toxicity. There have been several recent reviews that provide good overviews of chemical modification strategies for siRNA 12, 13 and antisense 12, 14, 15. Below we will highlight a few key aspects.
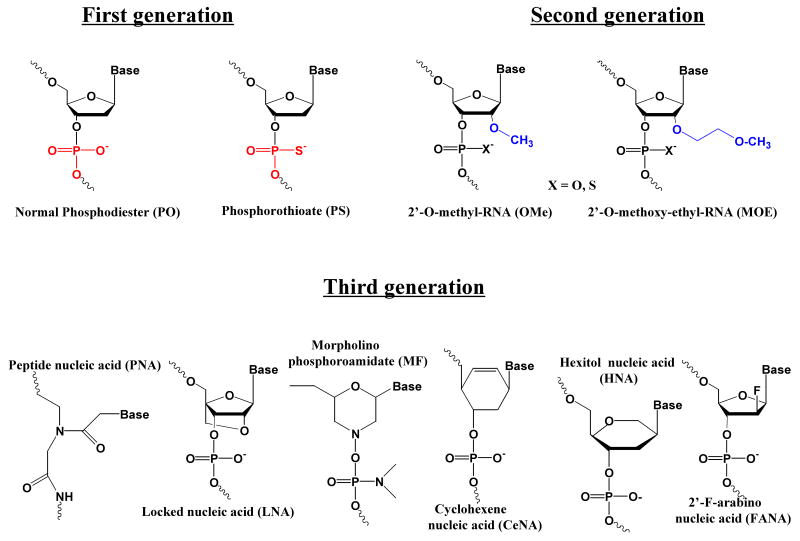
Simple phosphodiester oligonucleotides are quite unstable. Substitution of sulfur for oxygen forms phosphorothioate (PS) oligonucleotides, the most common stabilizing modification for both antisense and siRNA. However, PS oligonucleotides tend to bind non-specifically to proteins thus causing toxicities 16. Other highly improved oligonucleotide chemistries have been developed including 2′-OH modifications, locked nucleic acids (LNAs), peptide nucleic acids (PNAs), morpholino compounds, and hexitol nucleic acids (HNAs) 17, See Fig 1. In terms of antisense use, oligonucleotides including these entities have high affinities for mRNA and are more stable to nucleases; however, they do not support RNase H activity. Thus oligomers with every residue modified cannot be used as typical antisense agents (although they may be very effective for modification of splicing or translation arrest). The placement of several central phosphorothioate residues, thereby creating ‘gapmers’, results in oligonucleotides that activate RNase H while retaining many of the valuable properties of the parent compounds. This is exemplified in a recent study using HNA modified antisense gapmers 18.
Figure 1.
Chemical Modifications. The structures of various forms of chemically modified oligonucleotide residues discussed in the text are illustrated.
The nuclease stability and other properties of siRNA can also be enhanced by appropriate chemical modifications. However, these have to be placed in the context of an overall design strategy for the siRNA 19. A very common form of well-tolerated modification involves the utilization of 2′-F or 2′-OMe residues 20-22. Another recent approach has involved use of LNA modifications; in general limited LNA inclusion can improve stability and effectiveness, whereas saturation of the oligonucleotide with LNA residues generally reduces efficacy 12, 23. Similarly, judicious placement of residues containing 6-carbon sugars instead of ribose can result in siRNAs with improved stability and more persistent pharmacological actions 24, 25.
Thus the barrier presented by blood and tissue nucleases is rapidly being overcome by the provision of a variety of chemically modified oligonucleotide building blocks. At this time there does not seem to be one type of chemical modification that is superior in all situations. It may be that different forms of oligonucleotides work best in different tissues, as has been suggested by some recent studies 26.
Pharmacokinetics and Biodistribution
In this section we discuss the pharmacokinetic and biodistribution behavior of antisense and siRNA oligonucleotides when administered as individual molecules. As we will see below, the kidney plays a key role in oligonucleotide clearance in this case. The liver also plays an important role, but that role is reinforced when the oligonucleotides are associated with nanocarriers of various types, as discussed in another section.
Extensive studies of the pharmacokinetics and biodistribution of systemically administered antisense oligonucleotides were undertaken in the 1990s and have been extensively reviewed 14, 27. Most of the early in vivo observations dealt with molecules having a phosphorothioate backbone. Some important generalizations to emerge from these studies include the following. (i) The biphasic plasma half-lives of oligonucleotides are in the range of minutes for t1/2α (distributional phase) and many minutes to several hours for t1/2β (elimination phase). (ii) Oligonucleotides are accumulated in most tissues, particularly the kidney and liver, but not the central nervous system. (iii) Although phosphorothioate oligonucleotides are significantly protein bound, the major route of elimination is via the kidneys. (iv) While the most detailed studies have been done in rodents, in general, the pharmacokinetic behavior of phosphorothioate oligonucleotides in humans is similar to that seen in lower animals.
More recently there have been numerous studies of the pharmacokinetics and biodistribution of various chemically modified antisense molecules. For example, a very comprehensive study examined the fate of 2′-O-(2-methoxyethyl) (2′-MOE) modified antisense molecules in rodents, monkeys and humans 28. This highly protein bound oligonucleotide had a longer circulation lifetime than would be expected of a simple phosphodiester. However, at high doses protein binding capacity was exceeded and more rapid renal excretion took place. Tissue uptake was most extensive in liver and kidney, similar to older forms of antisense. Once again, the behavior of the molecule in humans was predictable based on animal studies. Similarly, a study using LNA (locked nucleic acid) modified antisense oligonucleotides in rodents found extensive kidney uptake and excretion and a pharmacokinetic profile similar to standard oligonucelotides 29.
The pharmacokinetics and biodistribution of siRNA has also received much recent attention. Several studies in animals report that the biodistribution of siRNA duplexes is similar to that of single stranded antisense molecules, with highest uptake in kidney followed by liver 30-32. Interestingly, the high renal uptake has been associated with strong effects of the siRNA in ‘knocking down’ target molecules in that tissue 32. The technology for PK and biodistribution studies of siRNA does not seem to be as sophisticated as that for antisense molecules. For example, recent studies of siRNA biodistribution in animals have relied on radioactive tagging 31, or RNase protection assays 33, or simple HPLC 34, while some recent antisense studies have used advanced capillary electrophoresis methods 35.
An interesting variation involves the use of cholesterol-conjugated oligonucleotides. This approach was originated in the antisense literature 36 but has been extensively applied to modifying the behavior of siRNA. Conjugation of cholesterol 33, tocopherol 37, or of other lipid moieties 38, enhances binding of the oligonucleotide to serum lipoproteins and/or albumin. This results in enhanced circulation lifetimes and, more importantly, in enhanced hepatic uptake via the low-density lipoprotein receptor.
An important recent approach involves use of imaging technology to allow real time analysis of oligonucleotide biodistribution and pharmacokinetics 30, 32, 39. Micro SPECT or other radioimaging techniques can provide very detailed information on how an oligonucleotide is distributed and cleared. However, like all methods that depend on radiolabeling, there is always the underlying issue of separation of the label from the molecule being studied.
While it is clearly important to understand the pharmacokinetics and biodistribution of antisense and siRNA oligonucleotides intended for therapeutic use, one should observe the caution that this type of data may not be predictive of pharmacological effects. For example, in a study of LNA antisense oligonucleotides designed to cause an alteration of mRNA splicing, the major effects were observed in liver, colon, and small intestine; however the major site of accumulation of the LNA was the kidney 26. Thus, there may be a disconnect between physical biodistribution and functional biodistribution, with the latter determined not only by the amount of oligonucleotide present in a tissue, but also by how effectively it interacts with the biochemical machinery of the specific tissue.
Clearance via the Reticuloendothelial System / Role of the Liver and Spleen
In addition to circulating nucleases and renal clearance, a major barrier to effective in vivo delivery of antisense drugs is clearance by the reticuloendothelial system (RES). The RES is comprised of phagocytic cells, including circulating monocytes and tissue macrophages, whose physiological function is to clear the body of foreign pathogens, remove cellular debris that is generated during tissue remodeling, and to clear cells that have undergone apoptosis 40. Phagocytic cells of the RES, particularly the abundant Kupffer cells in the liver and splenic macrophages, also detect and phagocytose antisense antisense and siRNA oligonucleotide, as well as nanoparticle carriers 41 that may be used to enhance their delivery.
Macrophages differentiate from circulating monocytes, which migrate into tissue in the steady state or in response to inflammation. During monocyte development, myeloid progenitor cells sequentially give rise to monoblasts, pro-monocytes and finally monocytes, which exit the bone marrow and enter the bloodstream. Monocytes migrate from the blood into tissue to replenish specialized, tissue-specific macrophages 40.
Pathogens and particles in circulation are bound by opsonins, which include immnoglobulins, complement components, and other serum proteins. Opsonized particles are recognized by a variety of receptors present on the cell surface of macrophages. Immunoglobulin IgG-opsonized particles are recognized by Fc receptors, activating a network of signaling pathways that involve Src family kinases, PI3-kinase, phospholipase C, protein kinase C and Rho GTPases. Fc receptor-mediated internalization is characterized by cytoskeletal rearrangement, pseudopodia extension and engulfment of the opsonized particle 42.
Complement-opsonized particles are internalized by complement receptors. CR1 is a single-chain transmembrane receptor, while CR3 and CR4 are members of the integrin family of heterodimeric membrane proteins (CR3: αMβ2; CR4: αxβ2). CR1 is involved in particle binding while CR3 and CR4 mediate internalization; all three require additional signals in order to mediate internalization 42, 43. CRIg is a recently-identified complement receptor of the immunoglobulin superfamily. Once an opsonized particle contacts the macrophage cell surface, CRIg-expressing enosomes are recruited to the site of contact to replenish the plasma membrane, where CRIg is thought to ensure improved binding of the particle to the macrophage surface 43.
A third class of macrophage receptor is comprised of the scavenger receptors, which bind diverse ligands such as LPS and modified lipoproteins. While they are involved in particle binding, scavenger receptors require coreceptors to facilitate internalization. Importantly, binding and uptake of monomolecular oligonucleotides by Kupffer cells in the liver 44, 45 and proximal tubular cells in the kidney 46, 47 has been suggested to be mediated by scavenger receptors; however, there have been some contrary reports 48.
Following particle internalization, the phagosome fuses with lysosomal compartments and its contents are subject to enzymatic degradation by proteases and hydrolases that operate efficiently in the low-pH lyosomal environment. Factors controlling the kinetics of phagosome-lysosome fusion include the number and interaction of receptors in the phagosome and their ligands on the particle surface, as well as a host of complex signaling pathways 42.
Tissues macrophages are most abundant in the liver (Kupffer cells) and spleen, tissues that also receive high blood flow and exhibit fenestrated vasculature (see below). Thus it is not surprising that these organs accumulate the highest concentrations of antisense and siRNA oligonucleotides following systemic administration. Strategies to avoid uptake and clearance by the RES following systemic administration include the use of nanoparticle carriers, targeting ligands and conjugation of PEG 12, 49. In addition to its affect on biodistribution, uptake by the RES has toxicological relevance, as in vivo toxicity often correlates with the capture and long-term deposition of antisense drugs in RES organs, causing harmful side effect such as renal tubule degeneration, splenomegaly and elevation of liver transaminases 50. Of note, rodents are generally more sensitive to oligonucleotide-induced immune stimulation than primates 51.
Clearly uptake by the RES plays a critical role in the biodistribution of oligonucleotides associated with nanocarriers. However, as outlined above, uptake into RES-rich organs, particularly the liver, also plays a major role in the biodistribution of many types of oligonucleotides in monomolecular form.
The Endothelial Barrier
The endothelial cells lining the vascular lumen present both a barrier and an opportunity for oligonucleotide-based therapeutics. On the opportunity side they constitute a key cell type that is readily accessed by systemically administered materials. Since endothelial cells play essential roles in pathogenic processes as diverse as tumor angiogenesis, control of blood pressure, and regulation of hemostasis, they offer a tempting therapeutic target. However, in many cases nucleic acids need to pass across the endothelium and be delivered to tissue parenchymal cells, and thus the endothelium becomes a major barrier. This is particularly true when the oligonucleotides are part of a nanoparticle or other nanocarrier system, but it also applies to some extent to ‘free’ oligonucleotides as well.
Endothelial cells line the vasculature, adhering tightly to the underlying extracellular matrix largely via integrins, and forming junctions with each other via several types of cell-cell adhesion molecules including VE-cadherin, JAM, occludins, claudins and PECAM. The integrity of the endothelial junctions is influenced by complex signal transduction processes 52 that respond to a variety of mediators. For example, microvascular transport of macromolecules increases during inflammation 53. In particular, there are a number of mediators that increase vascular permeability including thrombin, histamine, VEGF, TNFα, and reactive oxygen species 52. Therefore sites of inflammation are also potentially areas where increased movement of oligonucleotides from blood to tissue may take place.
The permeation of both large and small molecules across normal endothelium is often described in terms of a model that postulates abundant small pores of about 45 angstroms diameter accompanied by relatively scarce large pores of about 250 angstroms 54. A significant component of the small pore transport likely to take place via paracellular routes involving imperfections in the junctions between the endothelial cells. Based on their molecular weights, antisense and siRNA oligonucleotides should readily traverse the endothelial barrier via the small pore system. However, it is important to keep in mind that the apparent hydrodynamic radii of highly charged polynucleotides are larger than those of either globular proteins or random coil uncharged polymers (eg dextrans) of similar molecular mass (Kang & Juliano, unpublished observations). Thus the blood to tissue permeation rates may be slower than expected based on molecular mass.
There is some controversy regarding the identity of the large pore system. Some endothelial cells contain an abundance of small endosomal vesicles derived from calveolae, lipid rich membrane structures containing the protein calveolin. One theory suggests that large pore transport involves ‘transcytosis’, that is, entry of material into caveolae on the luminal side followed by active, energy requiring movement of the calveolar vesicles across the cell, and release on the abluminal side. However, other investigators suggest that the large pores represent rare large defects in the endothelial junctions, or that multiple calveolar vesicles fuse to form channels that cross the cell, in either case providing passive pathways for transport. One argument for the vesicular model of large pore transport is that, while vascular permeability declines dramatically with molecular size up to about 50 angstroms (the small pore limit), it is only weakly dependent on molecular size above that. Unfortunately key experiments testing energy dependence of large pore transport, or even the role of calveolin in this process have provided contradictory results 55, 56.
In certain specialized tissues the organization of the endothelium permits the ready egress of larger moieties. For example, the microvessels of both liver and spleen have relatively large ‘fenestrations’ of 100-200 nm diameter between endothelial cells 57. As mentioned above, the liver is one of the major sites of oligonucleotide accumulation, both as ‘free’ molecules and when associated with liposomes or other nanoparticulate carriers.
Tumors are another instance where microvasculature abnormalities may potentially enhance delivery of therapeutic oligonucleotides either as ‘free’ agents or when associated with nanocarriers. Thus, the overall architecture of the vasculature differs between tumors and adjacent normal tissue. In addition, there are often regional differences within the tumor and between the primary tumor and its metastases. These differences include the number, length, degree of branching of microvessels, and the velocity of blood flow 58. While small tumors may be relatively homogeneous, larger ones usually have several distinct regions including: (i) a necrotic, hypoxic core with almost no blood flow, (ii) a semi-necrotic region with relatively poor flow in un-branched vessels, (iii) a stable region with branched vessels and relatively good flow, (iv) and advancing front where active angiogenesis is taking place 58, 59. Transport of macromolecules across the tumor endothelium involves diffusion, convection and possibly transcytosis, with convective and active transport playing a greater role as molecular size increases. The transvascular permeability of tumors is generally higher than that of normal tissue, likely due to an increased number and size of the ‘large pore’ component 58. Some of this is presumably associated with fenestrations between the endothelial cells that result from rapid and disorganized angiogenesis in the tumor. Some studies have found pores or fenestrations in tumor vessels ranging from 100 to over 700 nm 60; however, as always, tumors display great heterogeneity in this regard.
A factor that works against the transvascular permeation of both molecules and nanocarriers is the existence of a high interstitial fluid pressure in tumors. This obtunds the convective component of transport, which relies on the differences in osmotic and hydrostatic pressure between the blood and the interstitial fluid. The existence of a high interstitial pressure in tumors has been ascribed to limited development of a lymphatic system in tumor tissue.
The relatively leaky character of the tumor endothelium, along with poor lymphatic drainage, results in the so-called “EPR” effect (EPR, enhanced permeability and retention) that sometimes allows selective accumulation of macromolecules or nanocarriers in tumors. The larger pore size due to fenestrations of the endothelial lining of microvessels provides opportunities for egress of relatively large entities, and the poor lymph flow means that the extravasated material persists in the tumor longer than it would in normal tissue 60. Some studies have suggested that it is the retention component due to limited lymphatic drainage that is most important in the EPR effect 61. Therapeutic oligonucleotides associated with nanocarriers such as lipoplexes, polymers, or various nanoparticles would be likely to demonstrate some degree of EPR effect. By contrast, ‘free’ oligonucleotides may behave more like small molecule drugs and not show a major EPR effect 29. However, free oligonucleotides may offer some therapeutic advantages in cancer in cases where the tumor does not have a grossly disturbed vascular architecture, or when, as is becoming common, anti-angiogenic agents are being used as part of combination chemotherapy thus ‘normalizing’ the tumor vasculature 62.
The fact that the endothelial lining in many tissues restricts egress of materials larger than 4 or 5 nanometers has implications for therapeutic use of oligonucleotides. It suggests that many nanocarrier formulations will not be able to deliver to these sites in the body. Thus, while nanoocarriers may be useful for delivery to certain types of tumors and to normal tissues having fenestrated endothelia, other types of formulations may achieve broader tissue distribution. For example, molecular conjugates of oligonucleotides with targeting ligands or with ‘cell penetrating peptides’ may have advantages in terms of delivery to many types of tissues12.
Cellular Uptake and Subcellular Distribution
Therapeutic oligonucleotides encounter their pharmacological targets within mammalian cells63. To affect gene expression by RNaseH-mediated degradation of complementary mRNA, or by splicing correction, antisense oligonucleotides need to enter the nucleus of cells. In the cytoplasm, antisense oligonucleotides can function by translation arrest. Generally, siRNA has to approach target mRNA that is associated with cytosolic RNA processing machinery 64. However, RNAi was also observed to occur in the nucleus of mammalian cells 65. Evidence was further provided for a mechanism orchestrating the localization of the siRNA in the cytoplasm or the nucleus, depending on where the target RNA resides 66.
Local exposure of oligonucleotides to their intracellular targets, and thereafter, the magnitude and duration of pharmacologic response, is affected substantially by changes in cellular uptake and subcellular distribution of oligonucleotides. Like other biological macromolecules that are large, polar, and sometimes charged, oligonucleotides reach their pharmacological targets from extracellular space by some form of endocytosis 63, which is subdivided into five major classes: (i) the ‘classic’ clathrin-coated pit pathway; (ii) the caveolar pathway; (iii) one or more noncaveolar, clathrin-independent pathways (CLIC pathways); (iv) phagocytosis (that mainly takes place in ‘professional phagocytes’ such as macrophages and granulocytes); and (v) macropinocytosis (in which macromolecules being internalized are simply dissolved in the ambient medium) 67.
The first four classes often involve a cell surface receptor and are collectively termed receptor-mediated endocytosis. It is these pathways that are mainly utilized in cellular delivery of macromolecular drugs such as oligonucleotides. In general, receptor-mediated endocytosis includes three major steps:
Receptor binding and internalization facilitate the initial entry of oligonucleotides into the cell as the primary barrier for oligonucleotide transport. After the ligand binds to the receptor, the ligand-receptor complex enters vesicles that bud from the cytosolic face of the plasma membrane and then pinch off with the assistance of the dynamin GTPase 68. Ligand-receptor binding determines which target cells and tissues oligonucleotides are delivered to. In addition, the precise mechanism of entry can affect the ultimate fate and function of the ligand-receptor complex. For example, using the same initial clathrin-coated pit pathway, LDL-receptor binding ends up with the degradation of LDL in lysosomes 69 whereas the transferrin-receptor complex recycles the ligand to plasma membrane 70.
Initial uptake is followed by sequential intracellular trafficking into a variety of low pH endomembrane compartments, including early/sorting endosomes, late endosomes/multi-vesicular bodies, and lysosomes. In some cases, receptors/ligands can traffic to the Golgi complex. In many instances, receptor and ligand are dissociated in the low pH endosome environment, and in some cases the receptor can recycle back to the cell surface via the recycling endosomes. The complex flow of endomembrane traffic is regulated by the Rab family of small GTPases and by tethering complexes, while vesicular fusion events are controlled by SNARE proteins 71-73. The SNX (sorting nexin) proteins also are important in sorting and cargo retrieval from endosomes 74. Vesicular trafficking can be rate-limiting in that many nonproductive pathways can circumvent the transport of oligonucleotides to the target, for example, sorting to secretory or lysosomal vesicles may lead to export of oligonucleotides out of cells or degradation in the lysosomes.
Ultimately, the oligonucleotide must exit from the endosome to reach its site of action in the cytoplasm or nucleus. Endosomal trapping represents an important barrier for oligonucleotide delivery; however, nuclear entry may not be the rate-limiting step for free oligonucleotides. Studies have shown that oligonucleotides are able to continuously shuttle between the nucleus and the cytoplasm. A portion of the nuclear uptake of oligonucleotides and their complexes is an active process mediated by nuclear pore structures, however, it does not require classical nuclear localization signals 75, 76. When oligonucleotides are bound to a nanocarrier, nuclear entry may become rate-limiting due to the significant increase in size.
Efficient endocytosis requires functional synergy of cellular uptake, vesicular trafficking, and endosomal release. These steps determine the magnitude and duration of oligonucleotide exposure to its target, and ultimately govern pharmacological response. Depending on their relative efficiency and capacity, cellular uptake, vesicular trafficking, or endosomal release may control the cellular response of oligonucleotides when the corresponding transport step becomes rate-limiting. In that case, identifying the rate-limiting step by mechanistic studies can help design a specific strategy to overcome this barrier. For example, conjugating oligonucleotides with endosomal release signal peptide 77 or nuclear localization signal peptide linked to a nanocarrier 78 has been utilized to overcome the endosomal trapping or enhance nuclear entry of oligonucleotides. On the other hand, the results of these peptide conjugations also help understand the real barrier(s) in cellular delivery of oligonucleotides.
Although attention has been focused on the initial step of cellular uptake, studies have recently emerged exploring intracellular trafficking and endosomal release of oligonucleotides into the cytoplasm. Recently, a caveosomal trafficking pathway was proposed to deliver surprisingly large amounts of naked siRNA to perinuclear sites in mammalian cells under stimulation of single stranded phosphorothioate oligonulelotides 79, leading to measurable though moderate target suppression 80. By attributing limited efficacy to intracellular sequestration of siRNA, several strategies have been applied to improve the endosomal escape, including a photochemical methodology 81 and peptide conjugation 77. In the latter study, conjugating an endosomal release signal peptide from bacterial protein toxins did not enhance the cellular uptake of siRNA, but nonetheless improved target inhibition dramatically 77, highlighting that endosomal release is the rate-limiting step of intracellular delivery of naked siRNA to its site of action in cytoplasm.
Similar lessons were learned from a study on intracellular delivery of oligonucleotides 5, in which a bivalent RGD peptide was conjugated to an antisense oligonucleotide (termed 623) that corrects splicing of a firefly luciferase gene mutant. The RGD-623 accumulated in cells 2-fold higher than free 623, however, it achieved a 7-fold higher effect on luciferase induction 5. The uptake and trafficking pathway involved an initial caveolar-mediated uptake followed by trafficking to the trans-Golgi, indicating that the pharmacological effect of an antisense oligonucleotide depends not only on total cell uptake but also on the intracellular trafficking.
These examples also provide evidence that DNA and RNA oligonucleotides are able to enter the cells from the extracellular milieu without the need of a transfection agent, and certain cell types may be equipped with pre-existing uptake and trafficking systems for that are favorable for oligonucleotide effects. Elucidation of the cellular uptake, vesicular trafficking, and endosomal release pathways of oligonucleotides can help optimize the chemistry of nucleic acid drugs for greater biological effectiveness. Previous studies of oligonucleotide uptake have extensively used several drugs as endocytotic pathway blockers. However, such inhibitors often affect multiple uptake and trafficking pathways. Molecular inhibitors of endocytotic pathways, such as dominant-negative Rab proteins to interfere selectively with trafficking patterns 82, can provide additional mechanistic information.
Conclusions
Multiple barriers still stand in the way of effective oligonucleotide therapeutics. Issues of stability and efficacy have largely been overcome. However the matter of effective intracellular delivery remains a key problem despite many clever attempts to surmount it. Overly rapid renal clearance of monomolecular oligonucleotides remains an issue, as does unwanted RES sequestration of siRNA or antisense nanoparticles. Steady progress is being made on all of these aspects and hopefully oligonucleotide based therapeutics will soon become a valued part of the pharmacological armamentarium.
Figure 2.
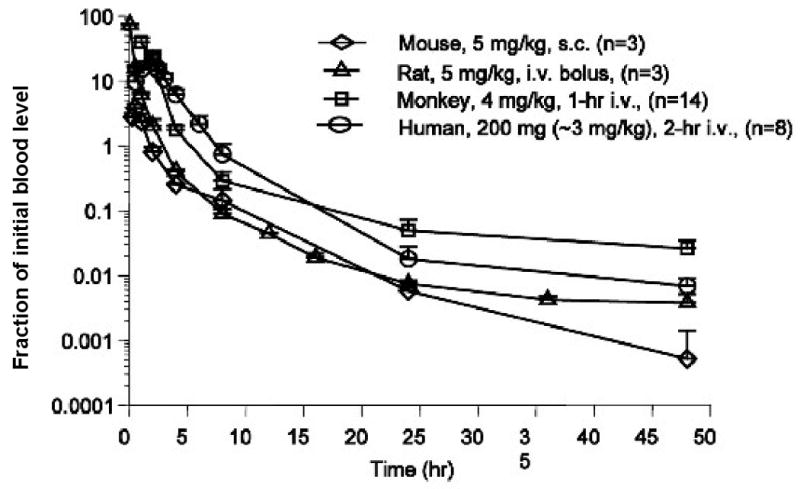
Oligonucleotide Pharmacokinetics. The normalized clearance rates of a chemically modified antisense oligonucleotide are illustrated in several species including man. Adapted from reference 28 with permission.
Fig 3.
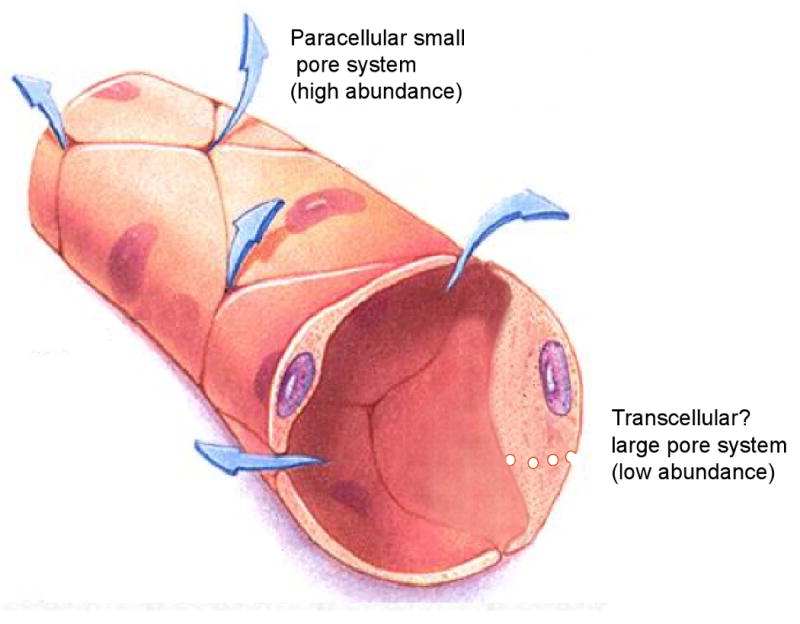
The Endothelial Lining. An idealized version of the endothelial lining is illustrated with indications of the large pore and small pore systems. Adapted with permission from reference 83.
Figure 4.
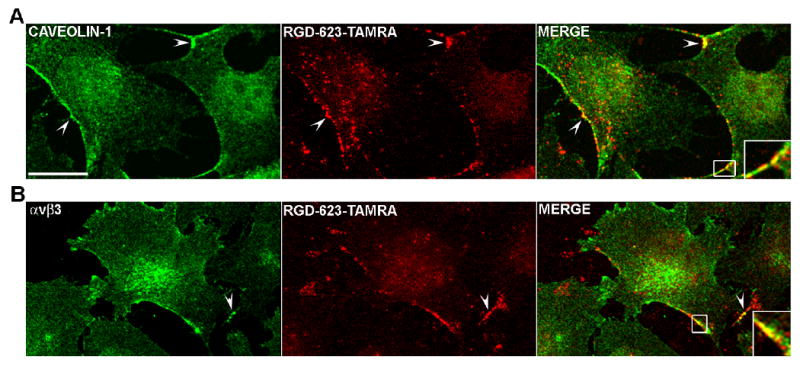
Intracellular Trafficking of Oligonucleotides. RGD-623-Tamra is a 2′-OMe-PS antisense oligonucelotide that has a 5′-RGD targeting ligand and a 3′-Tamra fluorophore. Its distribution in cells is compared to that of caveolin (A) or the avb3 integrin (B) (its receptor) as visualized by immunostaining. Fletchings indicate areas of overlap. Adapted from reference 5 with permission.
References
- 1.Chan JH, Lim S, Wong WS. Antisense oligonucleotides: from design to therapeutic application. Clin Exp Pharmacol Physiol. 2006;33:533–40. doi: 10.1111/j.1440-1681.2006.04403.x. [DOI] [PubMed] [Google Scholar]
- 2.Vinores SA. Pegaptanib in the treatment of wet, age-related macular degeneration. Int J Nanomedicine. 2006;1:263–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Tamm I, Dorken B, Hartmann G. Antisense therapy in oncology: new hope for an old idea? Lancet. 2001;358:489–97. doi: 10.1016/S0140-6736(01)05629-X. [DOI] [PubMed] [Google Scholar]
- 4.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6:443–53. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam MR, Dixit V, Kang H, Li ZB, Chen X, Trejo J, Fisher M, Juliano RL. Intracellular delivery of an anionic antisense oligonucleotide via receptor-mediated endocytosis. Nucleic Acids Res. 2008;36:2764–76. doi: 10.1093/nar/gkn115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang H, Alam MR, Dixit V, Fisher M, Juliano RL. Cellular delivery and biological activity of antisense oligonucleotides conjugated to a targeted protein carrier. Bioconjug Chem. 2008;19:2182–8. doi: 10.1021/bc800270w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean NM, Bennett CF. Antisense oligonucleotide-based therapeutics for cancer. Oncogene. 2003;22:9087–96. doi: 10.1038/sj.onc.1207231. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–84. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 9.Manoharan M. Oligonucleotide conjugates as potential antisense drugs with improved uptake, biodistribution, targeted delivery, and mechanism of action. Antisense Nucleic Acid Drug Dev. 2002;12:103–28. doi: 10.1089/108729002760070849. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz AM. On future's doorstep: RNA interference and the pharmacopeia of tomorrow. J Clin Invest. 2007;117:3612–4. doi: 10.1172/JCI34274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Paula D, Bentley MV, Mahato RI. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA. 2007;13:431–56. doi: 10.1261/rna.459807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–71. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–19. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- 14.Crooke ST. Progress in antisense technology. Annu Rev Med. 2004;55:61–95. doi: 10.1146/annurev.med.55.091902.104408. [DOI] [PubMed] [Google Scholar]
- 15.Juliano RL, Dixit VR, Kang H, Kim TY, Miyamoto Y, Xu D. Epigenetic manipulation of gene expression: a toolkit for cell biologists. J Cell Biol. 2005;169:847–57. doi: 10.1083/jcb.200501053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg AM, Stein CA. Phosphorothioate oligodeoxynucleotides: antisense or anti-protein? Antisense Res Dev. 1995;5:241. doi: 10.1089/ard.1995.5.241. [DOI] [PubMed] [Google Scholar]
- 17.Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628–44. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang H, Fisher MH, Xu D, Miyamoto YJ, Marchand A, Van Aerschot A, Herdewijn P, Juliano RL. Inhibition of MDR1 gene expression by chimeric HNA antisense oligonucleotides. Nucleic Acids Res. 2004;32:4411–9. doi: 10.1093/nar/gkh775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, Wanders L, Griffey RH, Swayze EE, Bhat B. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem. 2005;48:901–4. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- 21.Prakash TP, Allerson CR, Dande P, Vickers TA, Sioufi N, Jarres R, Baker BF, Swayze EE, Griffey RH, Bhat B. Positional effect of chemical modifications on short interference RNA activity in mammalian cells. J Med Chem. 2005;48:4247–53. doi: 10.1021/jm050044o. [DOI] [PubMed] [Google Scholar]
- 22.Choung S, Kim YJ, Kim S, Park HO, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342:919–27. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42:7967–75. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 24.Fisher M, Abramov M, Van Aerschot A, Xu D, Juliano RL, Herdewijn P. Inhibition of MDR1 expression with altritol-modified siRNAs. Nucleic Acids Res. 2007;35:1064–74. doi: 10.1093/nar/gkl1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikhailov SN, Bobkov GV, Brilliantov KV, Rozenski J, Van Aerschot A, Herdewijn P, Fisher MH, Juliano RL. 2′-O-hydroxyalkoxymethylribonucleosides and their incorporation into oligoribonucleotides. Nucleosides Nucleotides Nucleic Acids. 2007;26:1509–12. doi: 10.1080/15257770701543007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts J, Palma E, Sazani P, Orum H, Cho M, Kole R. Efficient and persistent splice switching by systemically delivered LNA oligonucleotides in mice. Mol Ther. 2006;14:471–5. doi: 10.1016/j.ymthe.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Juliano RL, Alahari S, Yoo H, Kole R, Cho M. Antisense pharmacodynamics: critical issues in the transport and delivery of antisense oligonucleotides. Pharm Res. 1999;16:494–502. doi: 10.1023/a:1011958726518. [DOI] [PubMed] [Google Scholar]
- 28.Yu RZ, Kim TW, Hong A, Watanabe TA, Gaus HJ, Geary RS. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab Dispos. 2007;35:460–8. doi: 10.1124/dmd.106.012401. [DOI] [PubMed] [Google Scholar]
- 29.Fluiter K, ten Asbroek AL, de Wissel MB, Jakobs ME, Wissenbach M, Olsson H, Olsen O, Oerum H, Baas F. In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res. 2003;31:953–62. doi: 10.1093/nar/gkg185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Behe M, Kissel T. In vivo SPECT and real-time gamma camera imaging of biodistribution and pharmacokinetics of siRNA delivery using an optimized radiolabeling and purification procedure. Bioconjug Chem. 2009;20:174–82. doi: 10.1021/bc800408g. [DOI] [PubMed] [Google Scholar]
- 31.Braasch DA, Paroo Z, Constantinescu A, Ren G, Oz OK, Mason RP, Corey DR. Biodistribution of phosphodiester and phosphorothioate siRNA. Bioorg Med Chem Lett. 2004;14:1139–43. doi: 10.1016/j.bmcl.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 32.van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34:1393–7. doi: 10.1124/dmd.106.009555. [DOI] [PubMed] [Google Scholar]
- 33.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–4. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 35.Yu RZ, Lemonidis KM, Graham MJ, Matson JE, Crooke RM, Tribble DL, Wedel MK, Levin AA, Geary RS. Cross-species comparison of in vivo PK/PD relationships for second-generation antisense oligonucleotides targeting apolipoprotein B-100. Biochem Pharmacol. 2009;77:910–9. doi: 10.1016/j.bcp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Bijsterbosch MK, Rump ET, De Vrueh RL, Dorland R, van Veghel R, Tivel KL, Biessen EA, van Berkel TJ, Manoharan M. Modulation of plasma protein binding and in vivo liver cell uptake of phosphorothioate oligodeoxynucleotides by cholesterol conjugation. Nucleic Acids Res. 2000;28:2717–25. doi: 10.1093/nar/28.14.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, Yokota T. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol Ther. 2008;16:734–40. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 38.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 39.Jia F, Figueroa SD, Gallazzi F, Balaji BS, Hannink M, Lever SZ, Hoffman TJ, Lewis MR. Molecular imaging of bcl-2 expression in small lymphocytic lymphoma using 111In-labeled PNA-peptide conjugates. J Nucl Med. 2008;49:430–8. doi: 10.2967/jnumed.107.045138. [DOI] [PubMed] [Google Scholar]
- 40.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–52. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 43.van Lookeren Campagne M, Wiesmann C, Brown EJ. Macrophage complement receptors and pathogen clearance. Cell Microbiol. 2007;9:2095–102. doi: 10.1111/j.1462-5822.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 44.Bijsterbosch MK, Manoharan M, Rump ET, De Vrueh RL, van Veghel R, Tivel KL, Biessen EA, Bennett CF, Cook PD, van Berkel TJ. In vivo fate of phosphorothioate antisense oligodeoxynucleotides: predominant uptake by scavenger receptors on endothelial liver cells. Nucleic Acids Res. 1997;25:3290–6. doi: 10.1093/nar/25.16.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biessen EA, Vietsch H, Kuiper J, Bijsterbosch MK, Berkel TJ. Liver uptake of phosphodiester oligodeoxynucleotides is mediated by scavenger receptors. Mol Pharmacol. 1998;53:262–9. doi: 10.1124/mol.53.2.262. [DOI] [PubMed] [Google Scholar]
- 46.Carome MA, Kang YH, Bohen EM, Nicholson DE, Carr FE, Kiandoli LC, Brummel SE, Yuan CM. Distribution of the cellular uptake of phosphorothioate oligodeoxynucleotides in the rat kidney in vivo. Nephron. 1997;75:82–7. doi: 10.1159/000189504. [DOI] [PubMed] [Google Scholar]
- 47.Sawai K, Mahato RI, Oka Y, Takakura Y, Hashida M. Disposition of oligonucleotides in isolated perfused rat kidney: involvement of scavenger receptors in their renal uptake. J Pharmacol Exp Ther. 1996;279:284–90. [PubMed] [Google Scholar]
- 48.Butler M, Crooke RM, Graham MJ, Lemonidis KM, Lougheed M, Murray SF, Witchell D, Steinbrecher U, Bennett CF. Phosphorothioate oligodeoxynucleotides distribute similarly in class A scavenger receptor knockout and wild-type mice. J Pharmacol Exp Ther. 2000;292:489–96. [PubMed] [Google Scholar]
- 49.Lee WC, Berry R, Hohenstein P, Davies J. siRNA as a tool for investigating organogenesis: The pitfalls and the promises. Organogenesis. 2008;4:176–81. doi: 10.4161/org.4.3.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi F, Hoekstra D. Effective intracellular delivery of oligonucleotides in order to make sense of antisense. J Control Release. 2004;97:189–209. doi: 10.1016/j.jconrel.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Jason TL, Koropatnick J, Berg RW. Toxicology of antisense therapeutics. Toxicol Appl Pharmacol. 2004;201:66–83. doi: 10.1016/j.taap.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 53.Szekanecz Z, Koch AE. Vascular endothelium and immune responses: implications for inflammation and angiogenesis. Rheum Dis Clin North Am. 2004;30:97–114. doi: 10.1016/S0889-857X(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 54.Rippe B, Rosengren BI, Carlsson O, Venturoli D. Transendothelial transport: the vesicle controversy. J Vasc Res. 2002;39:375–90. doi: 10.1159/000064521. [DOI] [PubMed] [Google Scholar]
- 55.Schubert W, Frank PG, Razani B, Park DS, Chow CW, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–22. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 56.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 57.Scherphof GL. In vivo behavior of liposomes: Interactions with the mononuclear phagocyte system and implications for drug targeting. In: Juliano RL, editor. Targeted Drug Delivery. Springer; Berlin, New York: 1991. pp. 285–300. [Google Scholar]
- 58.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 59.Rubin P, Casarett G. Microcirculation of tumors. I. Anatomy, function, and necrosis. Clin Radiol. 1966;17:220–9. doi: 10.1016/s0009-9260(66)80027-2. [DOI] [PubMed] [Google Scholar]
- 60.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20:1337–50. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 61.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res. 1998;89:307–14. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 63.Opalinska JB, Gewirtz AM. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–14. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- 64.Pontes O, Pikaard CS. siRNA and miRNA processing: new functions for Cajal bodies. Curr Opin Genet Dev. 2008;18:197–203. doi: 10.1016/j.gde.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robb GB, Brown KM, Khurana J, Rana TM. Specific and potent RNAi in the nucleus of human cells. Nat Struct Mol Biol. 2005;12:133–7. doi: 10.1038/nsmb886. [DOI] [PubMed] [Google Scholar]
- 66.Berezhna SY, Supekova L, Supek F, Schultz PG, Deniz AA. siRNA in human cells selectively localizes to target RNA sites. Proc Natl Acad Sci U S A. 2006;103:7682–7. doi: 10.1073/pnas.0600148103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–12. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 68.Damke H. Dynamin and receptor-mediated endocytosis. FEBS Lett. 1996;389:48–51. doi: 10.1016/0014-5793(96)00517-0. [DOI] [PubMed] [Google Scholar]
- 69.Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- 70.Dautry-Varsat A. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie. 1986;68:375–81. doi: 10.1016/s0300-9084(86)80004-9. [DOI] [PubMed] [Google Scholar]
- 71.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–82. doi: 10.1016/j.devcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 72.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–21. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–7. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins--unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 75.Lorenz P, Misteli T, Baker BF, Bennett CF, Spector DL. Nucleocytoplasmic shuttling: a novel in vivo property of antisense phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 2000;28:582–92. doi: 10.1093/nar/28.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartig R, Shoeman RL, Janetzko A, Grub S, Traub P. Active nuclear import of single-stranded oligonucleotides and their complexes with non-karyophilic macromolecules. Biol Cell. 1998;90:407–26. [PubMed] [Google Scholar]
- 77.Detzer A, Overhoff M, Wunsche W, Rompf M, Turner JJ, Ivanova GD, Gait MJ, Sczakiel G. Increased RNAi is related to intracellular release of siRNA via a covalently attached signal peptide. Rna. 2009 doi: 10.1261/rna.1305209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Franzen S. Factors determining the efficacy of nuclear delivery of antisense oligonucleotides by gold nanoparticles. Bioconjug Chem. 2008;19:1009–16. doi: 10.1021/bc700421u. [DOI] [PubMed] [Google Scholar]
- 79.Overhoff M, Sczakiel G. Phosphorothioate-stimulated uptake of short interfering RNA by human cells. EMBO Rep. 2005;6:1176–81. doi: 10.1038/sj.embor.7400535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mescalchin A, Detzer A, Wecke M, Overhoff M, Wunsche W, Sczakiel G. Cellular uptake and intracellular release are major obstacles to the therapeutic application of siRNA: novel options by phosphorothioate-stimulated delivery. Expert Opin Biol Ther. 2007;7:1531–8. doi: 10.1517/14712598.7.10.1531. [DOI] [PubMed] [Google Scholar]
- 81.Oliveira S, Hogset A, Storm G, Schiffelers RM. Delivery of siRNA to the target cell cytoplasm: photochemical internalization facilitates endosomal escape and improves silencing efficiency, in vitro and in vivo. Curr Pharm Des. 2008;14:3686–97. doi: 10.2174/138161208786898789. [DOI] [PubMed] [Google Scholar]
- 82.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–17. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 83.Juliano R. Biological barriers to nanocarrier mediated delivery of therapeutic and imaging agents. In: Mirkin CA, Niemeyer CM, editors. Nanobiotechnology II. Wiley-VCH; Weinheim: 2007. [Google Scholar]