INTRODUCTION
The development of neurons and virtually all other cell types in the organism depends upon interactions with molecules in their environment. Studics of individual cell types have revealed tremendous diversity in the molecules that regulate the development of cells in the nervous system. These include chemotropic and trophic factors [e.g. nerve growth-factor (NGF) and brain derived neurotrophic factor (BDNF)], cell adhesion molecules [e.g. the neural cell adhesion molecule (NCAM) and N-cadherin], and molecules secreted into the extracellular matrix (ECM) [e.g. laminin (LN) and fibronectin (FN)]. Each class of molecule has now been shown to influence major steps in the development of the nervous system, including neuronal survival, determination, and migration; axonal growth and guidance; synapse formation; and glial differentiation. As molecules in the ECM influence all of these events and can be used to illustrate many of the principles derived from studies of the other classes of molecules, this review focuses upon constituents of the ECM and their receptors. The role of the ECM in neural development has recently been reviewed in this series (Sanes 1989). This review focuses on cellular and molecular themes not emphasized in the previous one and includes examples of recent work on nonneural systems that illustrate probable future directions for research in the nervous system.
Recent reviews on the composition and function of the ECM and its receptors include those of Hynes (1990), Hemler (1990), Kishimoto et al (1989), Plow & Ginsberg (1989), Burgeson (1988), Buck & Horowitz (1987), McDonald (1988), Ruoslahti (1988, 1989), Fessler & Fessler (1989), and Erickson & Bourdon (1989). Reviews focusing on aspects of ECM function in neural development include those by Lander (1989), Sanes (1989), and Edgar (1989). Because of space limitations, only representative examples and references are cited in this review.
COMPOSITION AND FUNCTION OF THE EXTRACELLULAR MATRIX
The ECM was originally defined morphologically as a cellular material with structure visible in the electron microscope (e.g. fibrils or basal lamina). It is now defined more broadly to include secreted molecules that are immobilized outside cells, even if they lack organization that is detectable in the microscope. Major constituents identified initially in the ECM included collagens, noncollagenous glycoproteins, and proteoglycans. Recent work has revealed a surprising diversity in these molecules. The number of distinct collagens is now greater than 12; the number of adhesive glycoproteins is constantly growing. Multiple genes and differential splicing further add to diversity in both classes of ECM glycoproteins (cf. Ehrig et al, sequences of the core proteins for several proteoglycans has shown that this class of molecules is best considered as a group of diverse glycoproteins with functions mediated both by their protein cores and by their carbohydrate side chains (cf. Ruoslahti 1989, Saunders et al 1989, Clement et al 1989). Recent work also indicates that several cell-cell adhesion molecules interact with or have forms that are immobilized in the ECM (e.g. NCAM, myelin-associated glycoprotein) (Sanes et al 1986, Fahrig et al 1987). Finally, the ECM has also been shown to bind to and regulate the activity and stability of several growth factors, most notably basic-fibroblast growth factor (FGF) and transforming growth factor (TGF)-β (cf. Rifkin & Moscatelli 1989, McCaffrey et al 1989).
DOMAINS IN EXTRACELLULAR MATRIX PROTEINS
The collagens and other ECM glycoproteins are molecular giants, in some cases spanning distances of several hundred nanometers. Primary sequence analysis has revealed that they are chaemeric proteins, assembled by exon shuffling. Multiple functional domains have been identified in individual ECM glycoproteins such as FN or LN (Figure 1). These include binding sites for cells, other ECM glycoproteins, glycosaminoglycans, and glycolipids (e.g. Mann et al 1989). Some ECM glycoproteins and proteoglycans have also been shown to bind growth factors and proteases (cf. Rifkin & Moscatelli 1989, Silverstein et al 1986). With most of the ECM glycoproteins, it has been possible to localize specific functions, such as cell binding, to specific domains, sometimes as short as 3–20 amino acids (cf. Pierschbacher & Ruoslahti 1984, Wayner et al 1989, Guan & Hynes 1990, Tashiro et al 1989). One of these short peptide sequences, RGD, functions as a cell attachment site in several different ECM glycoproteins, including FN (see Table 1). The demonstration of a specific function for a particular structure in one protein has raised the possibility that this structure functions similarly wherever it is found. By this criterion, many of the domain structures in those ECM proteins that have been sequenced are potentially adhesive. Most prominent among these domains is the fibronectin type III repeat, one of which contains the sequence RGDS as the major cell attachment site in fibronectin (Pierschbacher & Ruoslahti 1984). Multiple copies of this motif are found in FN, tenascin, and thrombospondin (Figure 1). Currently, two of the 18 such domains in FN, one of seven in tenascin, and one of six in thrombospondin have been shown to include cell attachment sites (Wayner et al 1989, Guan & Hynes 1990, Spring et al 1989, Lawler et al 1988). It seems likely that more of these domains will prove to have adhesive functions when tested with appropriate cells. FN type III domains are differentially spliced in both FN and tenascin (cf. Hynes 1985, Spring et al 1989). In the case of FN, one of the differentially spliced type III domains has been shown to contain a cell attachment site recognized by both neural crest cells and sensory neurons (cf. Dufour et al 1988, Humphries et al 1986, 1988).
Figure 1.
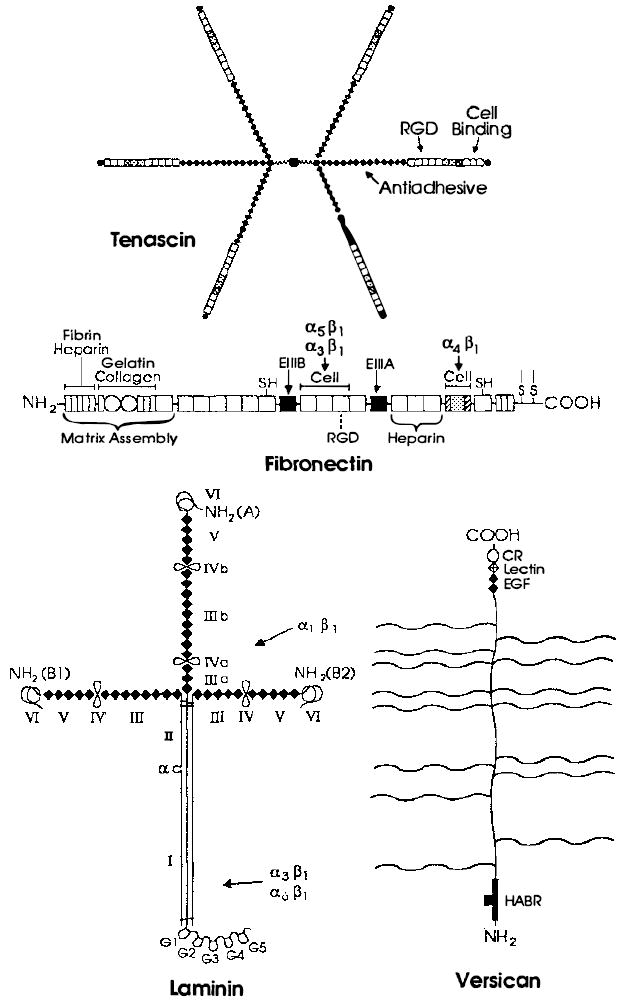
Examples of extracellular matrix glycoproteins and proteoglycans. Schematic structures for the adhesive glycoproteins tenascin, fibronectin, and laminin and for the proteoglycan versican arc presented. Fibroncctin is a dimer, but only one subunit is depicted. The sulfhydryl residues near its carboxyl terminus mediate its dimerization. The positions of FN type I [ ] FN type II [○], and FN type III [□] repeats are indicated in fibronectin. FN type III repeats are also found in tenascin, where their positions are marked by the same symbol [□]. Shaded FN type III repeats in fibronectin and tenascin are encoded by differentially spliced exons and are thus missing from some forms of these glycoproteins. The positions of domains homologous to those in the EOF-precursor, the EOF repeats [◆], are indicated in tenascin, laminin, and versican. The positions of domains homologous to functional domains in lectins [
] FN type II [○], and FN type III [□] repeats are indicated in fibronectin. FN type III repeats are also found in tenascin, where their positions are marked by the same symbol [□]. Shaded FN type III repeats in fibronectin and tenascin are encoded by differentially spliced exons and are thus missing from some forms of these glycoproteins. The positions of domains homologous to those in the EOF-precursor, the EOF repeats [◆], are indicated in tenascin, laminin, and versican. The positions of domains homologous to functional domains in lectins [ ], complement regulatory proteins [
], complement regulatory proteins [ ], and hyaluronic acid-binding proteins [
], and hyaluronic acid-binding proteins [ ] are indicated in versican. Note that many of these domains are also found in receptors, some of which are illustrated in Figure 2. In tenascin, the positions of the ROD sequence, the major cell-binding domain, and the region implicated in the anti-adhesive activity of this protein are shown. Similarly, in fibronectin, the positions of the RGD sequence and domains mediating interactions with fibrin, heparin, gelatin, collagen, and cells are indicated. Both the N-terminal region and the major cell attachment site containing the RGD sequence are required for assembly of fibronectin into the extracellular matrix. The regions of fibronectin recognized by the integrins α5β1, α3β1 and α4β1 are indicated by arrows. Note that the integrin α4β1 recognizes a sequence located in a differentially spliced exon that is not present in all forms of fibronectin. In laminin, homologous domains present in the A, B1, and B2 chains are indicated by roman numerals. Loops indicate the locations of putative globular domains, many of which have been visualized in the electron microscope. Domains I and II in the long arm of laminin’s cruciform structure represent regions in which the three subunits associate in a triple coiled rod. The major proteolytic fragments of laminin E3, E8, E1, and E1-4 described in the text contain approximately the following domains: E3 (G4 and G5), E8 (G1, G2, G3, and most of I), E1 (part of II, III, IV, IIIa, IVa, IIIb, IVb), EI-4 (part of II, III, IIIa, IIIb, IV, IV’, IVa, IVb, V, and VI). As indicated, the binding domains of the integrins α1β1, α3β1 and α6β1 have been roughly mapped by using these fragments. The site utilized by the integrin α2β1 has not yet been defined. The wavy lines in versican indicate the positions of several chondroitin sulfate side chains. All of these glycoproteins contain N-linked carbohydrate, but this is not shown.
] are indicated in versican. Note that many of these domains are also found in receptors, some of which are illustrated in Figure 2. In tenascin, the positions of the ROD sequence, the major cell-binding domain, and the region implicated in the anti-adhesive activity of this protein are shown. Similarly, in fibronectin, the positions of the RGD sequence and domains mediating interactions with fibrin, heparin, gelatin, collagen, and cells are indicated. Both the N-terminal region and the major cell attachment site containing the RGD sequence are required for assembly of fibronectin into the extracellular matrix. The regions of fibronectin recognized by the integrins α5β1, α3β1 and α4β1 are indicated by arrows. Note that the integrin α4β1 recognizes a sequence located in a differentially spliced exon that is not present in all forms of fibronectin. In laminin, homologous domains present in the A, B1, and B2 chains are indicated by roman numerals. Loops indicate the locations of putative globular domains, many of which have been visualized in the electron microscope. Domains I and II in the long arm of laminin’s cruciform structure represent regions in which the three subunits associate in a triple coiled rod. The major proteolytic fragments of laminin E3, E8, E1, and E1-4 described in the text contain approximately the following domains: E3 (G4 and G5), E8 (G1, G2, G3, and most of I), E1 (part of II, III, IV, IIIa, IVa, IIIb, IVb), EI-4 (part of II, III, IIIa, IIIb, IV, IV’, IVa, IVb, V, and VI). As indicated, the binding domains of the integrins α1β1, α3β1 and α6β1 have been roughly mapped by using these fragments. The site utilized by the integrin α2β1 has not yet been defined. The wavy lines in versican indicate the positions of several chondroitin sulfate side chains. All of these glycoproteins contain N-linked carbohydrate, but this is not shown.
Table 1.
Summary of integrin heterodimers and their ligands
| Class | Mr | α Subunit | Mr | I domain | Ca/Mg sites | Cleaved | Ligands | References |
|---|---|---|---|---|---|---|---|---|
| β1 | 115K | α1 | 200K | + | 3 | − | col, LN (E1) | a |
| α2 | 150K | + | 3 | − | col, LN1 | b, c | ||
| α3 | 105K | 32 | + | LN (E8), FN4, col1 | c, d | |||
| α4 | 140K | − | 3 | +/−3 | FN (CS-1), VCAM-1 | e | ||
| α5 | 150K | − | 4 | + | FN (RGD) | f | ||
| α6 | 150K | − | 3 | + | LN (E8) | g | ||
| α7 | 130K | ?2 | ?2 | + | LN | h | ||
| αVN | 150K | − | 4 | + | FN4, VN1, 4 | i | ||
| β2 | 90K | αLFA-1 | l70K | + | 3 | − | I-CAM-1, I-CAM-2 | j |
| αMac-1 | 180K | + | 3 | − | C3bi4, FG | k | ||
| αp150 | l50K | + | 3 | − | ? | |||
| β3 | 95K | αIIb | 136K | 4 | + | FN4, VN4, vWF4, FG4 | l | |
| αVN | 150K | 4 | + | VN4, TS4, vWF4, FG4 | m, n | |||
| β4 | 205K | α6 | 150K | 3 | + | ? | o | |
| β5 | 90K | αVN | 150K | 4 | + | VN4, FN4 | n | |
| βP | 95K | α4 | l40K | − | 3 | +/−3 | ? | p |
Six integrin classes, distinguished by distinct but homologous β subunits, are shown, Each β subunit can associate noncovalently with a subgroup of homologous α suhunits. Tntegrin nomenclature is described in Hemler (1990). The numerical designations of α1-6 correspond to the human T cell VLA antigen α subunit nomenclature. The indicated subunit Mrs were determined by SDS-PAGE in nonredueing conditions. Note, some a/β heterodimers recognize more than one ligand. In some cases a ligand is recognized only by a subset of the cells expressing a heterodimer. Additional ligands may yet be discovered for those receptor heterodimers listed, Additional α and β subunits and additional associations between listed α and β subunits are also being discovered. The reference list below is selective and does not always list original reports because of limited space.
Abbreviations: col, collagen; FG, fibrinogen; FN, fibronectin; ICAM, intercellular adhesion molecule: LN, laminin; TN, tenascin; TS, thrombospondin; VCAM, vascular cell adhesion molecule; VN, vitronectin; vWF, von Willebrand factor; (RGD), RGDS site in FN; (CS-1), CS-1 site in FN; (E1) and (E8), elastase fragments of LN.
Indicates ligands recognized in some, but not all cells.
The sequence was not completed at the time of this review.
Cleavage of α4 is variable.
The RGD sequence is on this ligand and appears to be used by this receptor.
A second major domain implicated in cell adhesion, the immunoglobulin domain, has recently been identified in the major HeS-proteoglycan, percalin, which contains multiple copies of this structural motif (Noonan et al 1988). Similar domains have been found in several Ca2+-independent neural cell adhesion molecules, including NCAM, L1, contactin, neuroglian, and fasciclin II (cf. Harrelson & Goodman 1988). Intriguingly, most of these cell adhesion molecules also contain several FN type III repeat motifs. Even an ECM receptor subunit, integrin β4 contains three FN type III repeats in its cytoplasmic domain (Suzuki & Naitoh 1990). Therefore neither the immunoglobulin nor FN type III domains distinguish cell membrane-associated adhesion molecules from adhesive glycoproteins found in the ECM.
Individual proteoglycans have recently been found to contain several other domains that suggest adhesive or mitogenic functions, including putative hyaluronic acid binding domains, lectin binding domains, leucine-rich repeats, and epidermal growth factor (EGF) repeats (cf. Ruoslahti 1989). For example, the very large (2389 amino acids) chondroitin sulfate proteoglycan named versican, identified originally as a secretory product of fibroblasts, is identical to glial hyaluronic acid-binding protein, which has been isolated from brain white matter (Perides et al 1989, Zimmerman & Ruoslahti 1989). In addition to a functional hyaluronate-binding domain, versican contains two EGF repeats, a lectin-like domain, and a complement-regulatory protein-like repeat (Figure 1). All of these domains are also found on one or more transmembrane cell surface glycoproteins that function as receptors (cf. Ruoslahti 1989). Thus, structural analyses detect few motifs that distinguish cell-surface-associated and ECM-associated adhesive glycoproteins.
Not all domains in ECM constituents are believed to function in cell adhesion. In particular, domains homologous to the EGF precursor, named EGF repeats, are found in LN, entactin, thrombospondin, and tenascin, thereby raising the possibility that ECM molecules may have interactions with cells similar to those mediated by EGF and its receptor (cf. Engel 1989).
STRUCTURE AND FUNCTION OF ECM RECEPTORS: INTEGRINS
Progress has been so rapid in identifying receptors for the ECM that the field is very fluid. Many receptor classes identified on nonneural cells have not yet been found in the nervous system, although it seems likely that they will eventually be found there. At this point, neurons appear to use the same general classes of receptors as nonneural cells, so studies on the latter are useful in understanding the functions of these receptors in the nervous system. In this section, we first consider the major superfamily of ECM receptors, the integrins (listed in Table 1), and then consider other receptors likely to be important in mediating interactions with the ECM.
Integrin receptors are noncovalently associated heterodimeric glycoprotein complexes expressed on the surfaces of neurons and nonneural cells that appear to be the primary cellular receptors for many ECM constituents, including LN, FN, thrombospondin, and several collagens (see Table 1; cf. Hemler 1990). With assays of attachment or neurite outgrowth in vitro, antibodies to the integrins, notably the CSAT antibody that binds to the chicken integrin β1 subunit, appear sufficient to disrupt virtually all interactions of neurons with both purified ECM glycoproteins and complex extracellular matrices (cf. Bozyczko & Horwitz 1986, Cohen et al 1986, Tomaselli et al 1986). Thus there is direct evidence that functional integrins are required for binding of neurons to the ECM. Injections into developing avian embryos of antibodies to the integrin β1 subunit have dramatic inhibitory effects on migration of several cell types, including cranial neural crest cells and myoblasts populating limb masses (Bronner-Fraser 1986, Jaffredo et al 1988). Thus this class of receptors also has important functions in vivo.
As illustrated in Table 1, the integrins are a large receptor family (see also Hemler 1990). As shown in Figure 2, integrin receptors are transmembrane heterodimers composed of one α chain noncovalently associated with a β subunit. Multiple genes encode families of both α and β subunits. Ligands for almost all of the individual heterodimers have now been identified with function-blocking monoclonal antibodies and affinity chromatography on purified ECM glycoproteins (see Table 1). At present, there are six (and probably more) distinct, but homologous, β subunits (βl–β5, βp), each of which can associate with one or more of at least 12 α subunits. By convention (Hynes 1987), integrins have been classified into subfamilies according to the β subunit that is shared among several different heterodimers. Originally, groups of α subunits were thought to associate with one particular β subunit. It has recently become clear, however, that some α subunits can interact with more than one β subunit (cf. Cheresh et al 1989b, Kajiji et al 1989, Hemler et al 1989, Holzmann & Weissman 1989). Nevertheless, it is still useful to refer to integrin β-class subfamilies.
Figure 2.
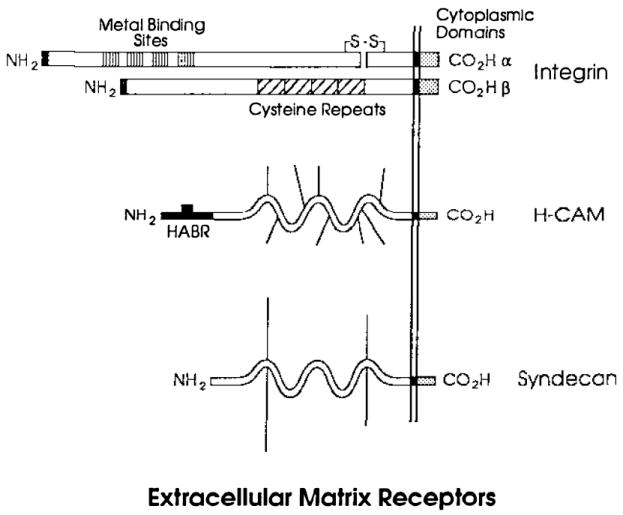
Extracellular matrix receptors. Examples of three classes of integral membrane glycoprotein receptors are illustrated in this figure. An integrin similar to the fibronectin receptor α5βl is shown above. The α subunit contains four putative metal-binding sites, a site at which it is cleaved into large and small fragments during maturation, a transmembrane domain, and a cytoplasmic domain (Argraves et al 1987). Some integrin α subunits contain I domains inserted at approximately the position of the first metal-binding site, and lack protease cleavage sites. The β subunit contains four repeats of a cysteine-rich motif, a transmembrane, and a cytoplasmic domain. H-CAM, also named the Hermes antigen or CD44, is illustrated in the middle with positions of a hyaluronic acid–binding domain [ ]. The positions of several carbohydrate chains are indicated by thin lines. The structure of syndecan, a proteoglycan that mediates binding of several cell types to several collagens and fibronectin is illustrated at the bottom. Positions of glycosaminoglycan chains are indicated by thin lines.
]. The positions of several carbohydrate chains are indicated by thin lines. The structure of syndecan, a proteoglycan that mediates binding of several cell types to several collagens and fibronectin is illustrated at the bottom. Positions of glycosaminoglycan chains are indicated by thin lines.
Integrins are expressed by neurons and glial cells in both the central and peripheral nervous systems (cf. Bozyczko & Horwitz 1986, Cohen et al 1987, Tomaselli et al 1986, Hall et al 1987, Pesheva et al 1988, Letourneau & Shattuck 1989, Tawil et al 1990). In particular, β1-class integrins have been shown to mediate neuronal attachment and process outgrowth in response to several ECM proteins, including LN, FN, and several collagens. Of the eight known β1 integrins (Table 1), five (α1, α3, α5, α6, αVN) have been identified in either primary neurons or neuronal cell lines (Tomaselli et al 1988a,b, 1989, and unpublished, Turner et al 1989, Ignatius & Reichardt 1988, Ignatius et al 1990, Toyota et al 1990, Vogel et al 1990). Strong indirect evidence for the expression of α4 by peripheral neurons comes from studies of their interactions with the alternatively spliced IIICS domain in fibronectin (Humphries et al 1988, Guan & Hynes 1990). β3 integrins have also been described on retinal neurons (K. M. Neugebauer and L. F. Reichardt, unpublished). Members of the β2 family, the lymphocyte integrins, have yet to be identified on neural cells. β2 integrins are unlikely to play a prominent role in the nervous system, however, since mutations in the β2 subunit have no obvious effects on the development or function of the nervous system (cf. Kishimoto et al 1989). Although which of the recently discovered β subunits (β4, β5, βp) are expressed in the nervous system is not yet clear, those found on epithelial cells, β4 and β5, seem likely to be present there. A major challenge for the future is to catalog the integrin α and β subunits expressed in different neural cells and to identify the functions of individual receptors in mediating the interactions of these different cells with ECM-bound and other ligands.
The integrins have several important features to consider in understanding their demonstrated and potential functions in the nervous system. First, as shown in Table 1, their ligands include not only virtually all described ECM constituents, but also cell surface molecules. In the β1 family, α4β1 has been shown to interact directly with FN and with an integral membrane glycoprotein ligand, VCAM-1, which contains several extracellular immunoglobulin-like domains (Elices et al 1990). Based upon their distributions, it has been suggested that other members of this family, notably α2β1 and α3β1, may also have cell surface ligands. Such ligands seem likely to be particularly important in the central nervous system, where many ECM constituents that are present in the peripheral nervous system are largely absent. Secondly, both the α and β subunits have been shown to be important in determining ligand binding properties of integrin heterodimers (cf. Cheresh et al 1989). As a consequence, tremendous potential functional diversity is encoded by the integrin family. Since many subunits have only been discovered in the past year, additional subunits will very likely be discovered in coming years, adding further to diversity of these receptors. Third, the integrins include both heterodimcrs that recognize many ligands, e.g. α3β1, which binds LN, FN, and collagen, and heterodimers that bind only one ligand, e.g. α6β1 and α5β1, which are specific for LN and FN, respectively. Thus, some heterodimers are likely to play very general roles whereas others play much more specific roles in regulating development of neurons and other cells in the nervous system. Finally, cell-specific factors have recently been shown to modify the ligand-binding specificities of two integrins. Both α2β1 and α3β1 heterodimers exhibit different ligand-binding specificities in different cell types (Languino et al 1989, Elices & Hemler 1989, Gehlsen et al 1989). The α2β1 receptor mediates binding to collagen wherever it is expressed but mediates binding to LN in only a subset of cells. The ability of the α3β1 receptor to bind collagen is also regulated by cell-specific factors. The molecular bases for these surprising results are not yet understood.
Structurally, both integrin α and β subunits are transmembrane glycoproteins. Primary sequence analysis of the five sequenced β subunits indicates that each has a large N-terminal extracellular domain, containing a fourfold repeat of a 40-amino-acid cysteine-rich segment, a single transmembrane domain, and, except for β4, a short cytoplasmic domain of ca. 40 amino acids (Figure 2; cf. Tamkun et al 1986, Fitzgerald et al 1987, Suzuki et al 1990). The integrin β4 subunit has a very large cytoplasmic domain containing more than 1000 amino acids (Suzuki & Naitoh 1990). The β-subunit cytoplasmic domains contain potential phosphorylation sites for tyrosine and serine/threonine kinases. Differential splicing has been shown to result in β3 and β4 subunits with two different cytoplasmic domains (van Kuppevelt et al 1989, Suzuki & Naitoh 1990). It is not yet clear whether these are both functionally significant. As is discussed below, the cytoplasmic domains appear to mediate interactions with cytoskeletal elements and are required for the adhesive functions of integrins on cells (Solowska et al 1989, Hayashi et al 1990).
Primary sequence analysis of the 11 sequenced α subunits indicates that each also has a large N-terminal extracellular domain, a single transmembrane domain, and a short cytoplasmic domain (see Figure 2; cf. Hemler 1990). The N-terminal half of each α sequence encodes seven repeats of an approximately 50-amino-acid domain, three or four of which, depending on the subunit, contain putative divalent cation binding sites. Cross linking with small peptide ligands has shown that the ligand-binding site is located in the vicinity of the first three divalent cation-binding sites in the vitronectin receptor, αVNβ3 (Smith & Cheresh 1990). Insertions of ca. 200 amino acids are present in the leukocyte family of integrins (αLFA-1, αMac-1, αgp150) and at least two members of the β1 family (α1 and α2) (cf. Kishimoto et al 1989, Takada & Hemler 1989, Ignatius et al 1990). These “interactive” or I domains are inserted between the second and third repeats within a putative ligand binding site defined by inferred homology to αVNβ3. These I domains are similar to I domains in several collagen-binding ECM proteins, such as von Willebrand’s factor, thereby suggesting the possibility that they mediate interactions of the α1β1 and α2β1 receptors with collagen. Differential splicing has been shown to insert an exon in the putative ligand-binding region of the Drosophila integrin α subunit, PS2 (Brown et al 1989). To date, there is no evidence for differential splicing of vertebrate integrin α subunits.
In the electron microscope, integrins appear as long, double rods extending ca. 20 nm from the membrane with globular domains at their extracellular termini that appear to mediate subunit association (Carrell et al 1985, Nermut et al 1988). Association of the two subunits is required for surface exprcssion and ligand binding (cf. Bodary et al 1989). Cross-linking studies with small peptide ligands have shown that segments of both subunits form the ligand binding site in the integrins αIIbβ3 and αVNβ3 (D’Souza et al 1988, Smith & Cheresh 1988, 1990). Subunit association of some integrins requires low concentrations of a divalent cation (Fitzgerald & Phillips 1985). The ligand-binding activity of all integrins requires much higher, millimolar concentrations of Mg2+ or Ca2+ (cf. Marlin & Springer 1987, Gailit & Ruoslahti 1988). The divalent cation dependence of integrin function has been exploited recently to isolate several individual integrin heterodimers by affinity chromatography on ECM ligands.
Integrin functions have been shown to be regulated on several levels. Both FN and LN receptors are dynamically regulated on neurons. Rat sensory neurons have been shown to lose functional FN receptors between E16 and P4 (Kawasaki et al 1986). Chick ciliary ganglion neurons lose functional LN receptors between E8 and E14 (Tomaselli & Reichardt 1988). Chick retinal neurons have been shown to lose functional FN and LN receptors between E6 and E12 (Cohen et al 1986, Hall et al 1987). All of these decreases in functional receptors correlate with the establishment of functional contacts with the targets of these diverse neuronal populations. Ablation of the optic tectum reduces the loss of functional LN receptors on retinal ganglion cells, thus suggesting that target contact is responsible for loss of receptor function (Cohen et al 1989). Although these changes seem likely partly to reflect changes in gene expression, unambiguous evidence for loss of individual subunits has not yet been obtained (see e.g. Hall et al 1987). In addition to the postulated loss of integrin subunits, evidence suggests that the affinity of LN receptors on retinal ganglion cells is reduced upon target contact (Cohen et al 1989). Whatever the detailed mechanisms, the dynamic regulation of integrin receptor function in several distinct classes of neurons seems likely to be important in regulating changes in neuronal behavior in vivo.
Growth factors and oncogenes have been shown to regulate integrin subunit expression in nonneural systems. At least one integrin subunit, α1, is strongly induced by NGF on pC12 pheochromocytoma cells (Rossino et al 1990). The cell adhesion molecules L1 and NCAM are also induced by NGF (cf. Prentice et al 1987). If similar inductions of integrins and CAMs occur on primary neurons, they are likely to facilitate collateral sprouting that is induced, at least partly, by increases in NGF levels in denervated fields (cf. Diamond et al 1987). Since axons of severed peripheral and central neurons have been shown to be capable of regenerating on ECM substrates (cf. Davis et al 1987), it seems very likely that they re-express functional integrin receptors. Understanding the factors that regulate expression of these receptors is potentially useful for developing conditions that promote maximal nerve regeneration.
FUNCTIONS OF OTHER ECM RECEPTOR FAMILIES
Precedents from nonneural systems and preliminary studies on neural cells suggest that nonintegrin receptor systems may also be important in mediating interactions of cells in the nervous system with several ECM constituents. Laminin is an intriguing example of a glycoprotein for which there is now evidence for interactions mediated by both integrins and other receptors. Several adhesive sites have been identified on the classic laminin isoform that contain the A, B1, and B2 chains (summarized in Kleinman & Weeks 1989, Beck et al 1990). These include RGD-containing and IKVAV-containing sites in the A chain, both of which appear capable of promoting neurite outgrowth by PC12 cells (Tashiro et al 1989). Antibodies to the IKVAV peptide inhibit PC12 interactions with intact LN, thereby indicating that this site is functional. Similar experiments need to be done by using antibodies to the RGD-containing peptide to determine whether the RGD site is also functional in intact LN. Interactions of PC12 cells with LN are mediated by two integrins, α1β1 and α3β1 either or both of which may recognize these sites in LN (Tomaselli et al 1990). Recent work has identified proteolytic fragments of LN that are utilized by individual integrin heterodimers. The α3β1 and α6β1 dimers have been shown to bind sites in the long arm of LN present in the fragment E8 (Gehlsen et al 1989, Hall et al 1990). The α1β1 receptor has been shown to interact with a site near the center of LN’s cruciform structure, present in the fragment E1 (Tomaselli et al 1990, Hall et al 1990). Based on their locations in the structure of LN, the RGD and IKVAV sites are candidates to interact with the integrins α1β1 and α3β1 respectively.
In addition to the integrins, a set of antigenically related proteins with MrS of 32K, 45K, and 67K bind LN with high affinity and have been found on many cells and tissues, including chick brain (see Kleinman & Weeks 1989, Douville et al 1988). The 67K protein is reported to utilize a sequence YIGSR located in an EGF repeat in the B1 chain of LN (Graf et al 1987). The 32K protein is identical to the galactose-specific lectin CBP 35 (Woo et al 1990). Although antibodies to the 67K protein do not inhibit LN binding by neuronal cell lines (Kleinman et al 1988), they are reported to inhibit the binding of some other cell types (Graf et al 1987). Evidence that a cell surface enzyme, galactosyltransferase, functions as a LN receptor has recently been obtained (Runyon et al 1986). Antibodies to or modifiers of this enzyme, and modifications of carbohydrate substrates on LN for this enzyme, partially inhibit both initiation and extension of neurites by a neuronal cell line on LN without significantly affecting cell attachment (Begovac & Shur 1990). Similar results have been obtained in assays of avian ncural crcst ccll migration on LN (Runyon et al 1986). These intriguing observations suggest roles for both a neuronal cell surfase enzyme and LN-linked oligosaccharides in regulating growth cone motility. Finally, gangliosides and sulfatides are present on neural cells and have been shown to bind LN (cf. Roberts et al 1985). Exogenous brain gangliosides GD1A and GT1B can inhibit neurite outgrowth on LN by neuroblastoma cells (Laitinen et al 1987). Still uncertain, though, is whether these gangliosides compete with receptors or have less direct effects.
The receptors that mediate neuronal interactions with the two most prominent ECM glycoproteins in the CNS—tenascin and thrombospondin—have not yet been unambiguously identified. A neuronal proteoglycan has been shown to interact with tenascin and is consequently one candidate to be a tenascin receptor (Hoffman et al 1988). Though associated with the neuronal surface, this proteoglycan is not an intrinsic membrane constituent, and thus would presumably function indirectly in attaching neurons to tenascin. A second candidate, described in nonneural cells, is an apparently novel integrin, purified by affinity chromatography on tenascin (Bourdon & Ruoslahti 1989). This receptor’s function is inhibited by RGD peptides, though, and it is not certain that cell interactions with tenascin are mediated by an RGD site (cf. Spring et al 1989). Although avian tenascin contains an RGD site, this site is not conserved in all species. Moreover, mapping both adhesive and anti-adhesive sites to individual domains of tenascin has been possible by using fusion proteins and antibodies (Spring et al 1989). The most prominent adhesive site in tenascin has been mapped to a FN type III repeat distant from the repeat containing the RGD sequence; the site on tenascin that inhibits cell flattening and motility has been mapped to EGF repeats, again far from the RGD-containing site. As discussed by Spring et al (1989), convincing identification of any receptor, integrin or otherwise, that mediates either of these two activities is still lacking. Identifying the receptor that mediates the anti-adhesive activity of tenascin will be particularly interesting, because this is a novel activity for an ECM macromolecule. In the case of thrombospondin, both the integrin αVNβ3 and an 88 kDa intrinsic membrane glycoprotein named GPIV have been shown to function as receptors in nonneural cells (Lawler et al 1988, Tandon et al 1989). Though neural cells clearly interact with thrombospondin (O’Shea et al 1990, Boyne et al 1989), the question of which, if either, of these receptors mediates the observed interactions remains obscure.
Although their neural functions can only be addressed speculatively, several other ECM receptor classes seem likely to be important in the nervous system. Prominent among these are hyaluronic acid receptors. Hyaluronic acid is located in pathways utilized by many cells, including neural crest cells, for migration. Ligands that bind cell surface–anchored hyaluronate dramatically inhibit migration of neural crest cells (Perris & Johansson 1990). The Hermes antigen (CD44) is a transmembrane receptor that functions in lymphocyte binding and has a domain with strong homology to several hyaluronic acid-binding proteins (Goldstein et al 1989). This receptor has been shown to interact with both ECM and cell surface-associated ligands (cf. St. John et al 1990). Whether these are exclusively hyaluronic acid is not yet clear. This protein has recently been localized on both eNS glia and Schwann cells (Picker et al 1989). Although it appears not to be expressed by adult neurons, its distribution in the developing nervous system merits attention.
Proteoglycans function as both ECM constituents and receptors (cf. Ruoslahti 1989). Individual proteoglycans have important functions in cell adhesion, proliferation, and differentiation. Specific examples document that heparan-sulfate proteoglycans can function as receptors, binding cells to ECM glycoproteins, including several collagens, FN, and thrombospondin (cf. LeBaron et al 1989, Saunders et al 1989). In addition, proteoglycans have been shown to modulate the binding of other receptors to ECM proteins. As one example, a chondroitin sulfate proteoglycan with weak affinity for FN has been shown to inhibit binding of a purified integrin to FN, probably by steric hindrance (Hautanen et al 1989). Proteoglycans are widely distributed in the nervous system. Despite appreciation of their presence and probable importance, they have received comparatively little attention. With the exception of versican, which of the comparatively well-characterized nonneuronal proteoglycans are also found in the nervous system is not yet clear. Recently, new, more powerful methods for characterizing proteoglycans have been applied to analyze proteoglycans in the brain. The results suggest that there are very large numbers of distinct proteoglycans in the mammalian brain (Herndon & Lander 1990). Perhaps the most interesting proteoglycan that has been identified in the brain is a chondroitin sulfate proteoglycan defined by the monoclonal antibody Cat-301 (cf. Zaremba et al 1989). This is an extracellular proteoglycan, present on subsets of neurons in the brain, whose expression is regulated by synaptic activity. Although no function is known for this proteoglycan, its expression pattern suggests that it may be involved in synaptogenesis.
Finally, gangliosides and other glycolipids are differentially expressed macromolecules that are prominent neuronal surface constituents. These interact directly with many ECM glycoproteins, including FN, LN, and thrombospondin (cf. Roberts et al 1985). Thus they may function directly as receptors. In addition, gangliosides have been shown to modulate the functions of integrins (cf. Stallcup 1988, Santoro 1989). The integrin heterodimer—αVNβ3—has been purfied in tight association with a ganglioside (Cheresh et al 1987). Gangliosides have been shown to be essential for functional reconstitution of this integrin. Hence, gangliosides may act on neurons in part by interacting with integrins.
ECM REGULATION OF CELL MIGRATION AND AXONAL GUIDANCE
The importance of extrinsic cues in guiding migrating cells and motile growth cones is now well established in both invertebrate and vertebrate model systems (cf. Harrelson & Goodman 1988, Dodd & Jessell 1988). In several systems, cues localized on cells and within the extracellular matrix appear to provide the major guides for cell movements. In the developing grasshopper limb, for example, growth cones of pioneer neurons follow trajectories restricted on one side by a basal lamina and on the other by epithelial cells (cf. Bentley & Caudy 1983). Developing limbs can be isolated, opened, stripped of mesoderm, and cultured as a flat explant (Lefcort & Bentley 1987). In these conditions, designed to eliminate the effects of electric fields, gradients of diffusible molecules, and tissues extrinsic to the limb, growth cones of pioneer neurons still follow normal trajectories. Thus the limb epithelium and its associated ECM appear to provide adequate guidance cues. Retraction of growth cones as a consequence of removal of the basal lamina by proteolysis implies that there are adhesive interactions between the growth cones and basal lamina (Condic & Bentley 1989a). In such preparations, the polarity of initial outgrowth and selective interactions with limb segment boundaries and neuronal cells still occur normally, thereby indicating that the intact basal lamina is not required in this system for normal guidance (Condic & Bentley 1989b). Oriented axon outgrowth has also been examined in explants of embryonic chick and quail retinae (cf. Halfter & Deiss 1984). In these cultures, also designed to eliminate guidance by diffusible molecules, already existing cues are followed by axons in developmentally older portions of the retina, but these cues cannot be established in the developing retinal rim. When tested on the isolated ECM, growth cones grow at normal rates but are not oriented normally, thus implying that necessary guidance cues are not contained in the retinal ECM (Halfter et al 1987). To summarize, both of these examples indicate that ECM molecules in vivo can provide permissive substrates for growth cone motility. Neither suggests the presence of information in the ECM that can steer them.
A mechanism by which ECM fibrils can guide cell migration is provided by recent studies on neural crest cells (Newgreen 1989). Fibrils of ECM are easily aligned by a variety of forces. On a major substrate for crest cell migration, the medial face of the somites, fibrils of matrix are aligned parallel to the direction of neural crest cell migration. In vitro, crest cells migrate preferentially along the axis of alignment of the matrix fibrils, thus suggesting that this directional cue is utilized in vivo. Thus, structural cues may promote preferential migration in one direction (Boocock 1989).
Substantial progress in identifying individual ECM glycoproteins implicated in cell migration has come from studies on the developing neural crest and cerebellum. FN, LN, tenascin, thrombospondin, and hyaluronic acid are each found in pathways followed by neural crest cells (cf. Perris & Bronner-Fraser 1989, Epperlein et al 1988). Several reagents that inhibit interactions of neural crest cells with ECM constituents in vitro dramatically impair the migration of chick cranial neural crest cells. These include RGDS-containing peptides, modeled as competitors for a cell-binding site in FN; the INO antibody, which prevents cell interactions with an isoform of LN; tenascin antibodies; and an antibody that blocks the functions of β1 subunit-containing integrins (Boucaut et al 1984, Bronner-Fraser & Lalier 1988, Bronner-Fraser 1986, 1988). These results suggest that integrins are important receptors on neural crest cells and that an RGD-containing ECM glycoprotein (e.g. FN, thrombospondin, or vitronectin), a LN isoform, and tenascin are essential substrates for these cells. Since neural crest cells can migrate efficiently on substrates coated with individual ligands in vitro, such as FN or LN, it is not clear why interfering with the function of a single ligand in vivo has such dramatic effects. In analogous in vitro systems in which cells or growth cones move on substrates with multiple ligands, blocking the function of a single molecule usually has only a partial inhibitory effect (cf. Bixby et al 1988). One possibility is that some of the peptides and antibodies used in the in vivo studies do not inhibit migration directly, but instead inhibit steps in neural crest cell differentiation prior to migration. This can now be addressed more directly by visualizing individual cells during migration via confocal microscopy.
The ability to study cerebellar granule cell migration in tissue slices in vitro has provided an opportunity to assess the role of ECM proteins in the migration of central neurons. During postnatal development, cerebellar granule cells proliferate in the external granule cell layer and then migrate through the molecular layer to reach their final positions in the internal granule cell layer. Several ECM components and CAMs have been identified in the developing cerebellar cortex, including tenascin (cytotactin), thrombospondin, chondroitin sulfate proteoglycan, hyaluronic acid, and L1 (Hoffman et al 1988, Chuong et al 1987, O’Shea et al 1990, Ripellino et al 1989). Perturbation experiments implicate several of these molecules in granule cell development. Antibodies to the CAM, L1, strongly inhibit granule cell migration (Chuong et al 1987). Cells accumulate in the pre-migratory zone of the external granule cell layer. Since these cells may not have initiated migration, these antibodies may inhibit differentiation of premigratory granule cells instead of interfering directly with migration. Tenascin surrounds granule cells in the external granule layer and is also expressed in the molecular layer, primarily on the surfaces of Bergmann glia (Chuong et al 1987). In the presence of tenascin antibodies, granule cells accumulate in the molecular layer, thus indicating that they start, but do not complete, migration. Both observations suggest that tenascin functions as an important substrate for granule cell migration. Thrombospondin is associated with granule cells in the premigratory zone of the external granule cell layer and in the molecular layer (O’Shea et al 1990). In contrast to tenascin, thrombospondin is not associated with the surfaces of the Bergmann glia, but is instead concentrated at the leading edges of the migrating granule cells. Thrombospondin antibodies prevent the migration of granule cells into the molecular layer. Although thrombospondin may stimulate migration as a substrate, its ability to bind and activate plasminogen and tissue plasminogen activator (Silverstein et al 1986) suggests that it may function instead by localizing and activating proteases released by the granule cells (Verrall & Seeds 1989, O’Shea et al 1990). Consistent with this idea, inhibitors of the tissue plasminogen activator/plasminogen system can also inhibit cerebellar granule cell migration from the external granule cell layer into the molecular layer (Moonen et al 1982). To summarize, L1, tenascin, and thrombospondin have been shown to promote cell attachment in vitro and may therefore also act as substrates in vivo. Differences in their distributions, activities, and effects of antibody injection suggest, though, that each also has a very specific, nonredundant function.
The characterization of genes that guide circumferential migrations of mesodermal cells and pioneer axons in C. elegans has provided convincing evidence for a role for an ECM constituent in providing directional information (Hedgecock et al 1990). In C. elegans, pioneer axons migrate between the epidermis and basal lamina. Mesodermal cells migrate on the opposite side of the basal lamina. Three genes—unc-5, unc-6, and unc-40—are required for guiding both classes of cells along the dorsoventral but not anterior-posterior axis. Unc-6, which affects migrations in both dorsal and ventral directions, has been shown to be a homologue of the LN B2 chain (cited in Hedgecock et al 1990). Considering the importance of LN in regulating epithelial cell development (cf. Klein et al 1988), it seems likely that some isoforms of LN are still present in unc-6 mutants. The genetic analyses of migration-deficient mutants in C. elegans has provided the most specific role for an ECM molecule in cell guidance that has been identified thus far. That guidance and motility are not affected along the anterior-posterior axis is particularly striking. There is persuasive genetic evidence for interaction of two other gene products—unc-5 and unc-40—with unc-6 (Hedgecock et al 1990). Unc-5 has been cloned and has properties consistent with its being a receptor, including a predicted external Ig domain and a putative transmembrane domain (cited in Hedgecock et al 1990). Existence of opposing adhesive gradients have been proposed as a model to explain guidance in C. elegans. One gradient could potentially be formed by a LN isoform containing the unc-6 product. In vertebrate systems, though, neurons in vitro do not seem able to follow gradients of LN (McKenna & Raper 1988).
Many ECM molecules stimulate neuronal process outgrowth in vitro. These include LN, FN, several collagens, thrombospondin, and tenascin (cf. Manthorpe et al 1983, Rogers et al 1983, O’Shea et al 1990, Chiquet 1990). Some of these glycoproteins are also capable of guiding axons in vitro (cf. Gunderson 1987). Essentially all of these molecules are localized in vertebrate embryos where they could potentially serve as permissive substrates or provide guidance cues for growth cones (reviewed in Sanes 1989). LN isoforms, for example, have been detected in both the vertebrate peripheral and central nervous systems in locations that would suggest a role in axonal growth: in developing sensory and autonomic ganglia and spinal nerve roots (Rogers et al 1986), in the pathway of trigeminal nerve fibers (Riggott & Moody 1987), in developing muscle prior to innervation (Sanes & Chiu 1983), in the spinal ventral longitudinal pathway (Letourneau et al 1988), and in the optic nerve (cf. Cohen et al 1987). In almost all cases, LN expression appears to be transient, occurring at high levels during periods of axonal growth and, at least, in the central nervous system, diminishing later in development. Two notable exceptions are the continued expression of LN isoforms in the basal lamina of peripheral nerve (Chiu et al 1986) and the optic nerves of goldfish (Hopkins et al 1985, Liesi 1985), two favorable sites in the adult for either continued axonal growth or nerve regeneration. In contrast to studies on cell migration, surprisingly few attempts have been made to use antibodies or other reagents to interfere with axonal growth. In one of these, the INO antibody, which prevents cell interactions with an isoform of LN, was shown to slow, but not prevent reinnervation of the iris by sympathetic neurons (Sandrock & Matthew 1987).
Although experimental evidence is largely missing, it seems likely that ECM constituents function as permissive substrates for axonal growth in many areas of the developing nervous system. The ability of many ECM constituents to guide growth cones in vitro has suggested that they might also guide growth cone movements in vivo (cf. Gunderson 1987). In principle, observed heterogeneities in distribution of ECM proteins or changes in their concentrations could direct axonal growth. Recent results indicate that heterogeneity in both the number and function of ECM glycoproteins has been underestimated. Most strikingly, there are many more genes for ECM glycoproteins than was previously appreciated. The collagens, for example, have proliferated in both number of new collagens and number of genes encoding new isoforms of existing collagens (Burgeson 1988). Some of these have quite restricted distributions (cf. Hostikka et al 1990). The recently described heterogeneity in LN is of particular interest to neurobiologists, because LN has many dramatic effects on neuronal differentiation. In addition to its effects on neurite outgrowth, LN also modulates the activities of neurotrophic factors, regulates expression of transmitter enzymes, and has many other striking effects on the differentiation of neurons, not mimicked by other ECM glycoproteins (cf. Edgar et al 1984, Kalcheim et al 1987, Acheson et al 1986). Although it has been appreciated for several years that 1aminins from different sources have different structures and antigenic properties (cf. Lander et al 1985, Edgar et al 1988), only in the past year, with the discovery of new genes encoding LN homologues, has a definite advance been made in understanding the causes of this heterogeneity.
Merosin is an A-chain homologue that associates with the B1 and B2 chains to form a novel isoform of LN (Ehrig et al 1990). Merosin-containing LN generally appears late in development and is the major LN in adult skeletal muscle, cardiac muscle, and peripheral nerve (Leivo & Engvall 1988, Sanes et al 1990). Specific functions relevant for cell differentiation appear to be differentially encoded by different A chain genes. For example, expression of the classic A chain appears necessary for normal development of kidney epithelial cells in organ cultures of embryonic kidney anlage (Klein et al 1989, Sorokin et al 1990). Consistent with the possibility that A chain genes differentially encode sites relevant for axon growth, recent work has demonstrated that sensory neurons utilize different combinations of integrins to extend neurites on merosin-containing and A-chain containing isoforms of LN (K. Tomaselli et al 1991, unpublished).
S-laminin was originally identified as a synapse-specific component of muscle basal lamina (cf. Sanes & Chiu 1983). Recently shown by sequencing to be a homologue of the LN B1 chain (Hunter et al 1989a), it has not yet been shown to associate with other LN subunits. S-laminin has a much more restricted distribution than LN in vivo (Hunter et al 1989a). Only a subset of neurons, at this point only motoneurons from the ciliary ganglion, appear to interact with it (Hunter et al 1989b). It is associated with sites at which axons cease elongation, suggesting that it effects on neuronal differentiation are quite different from those of other LN isoforms (Covault et al 1987). Recent work has implicated a peptide sequence LRE as the attachment site for motoneurons in S-laminin fusion proteins (Hunter et al 1989b). Since S-laminin is likely to associate with A and B2 chain homologues, the function of this sequence needs to be confirmed by using native isoforms of LN containing this subunit.
The discoveries of merosin and S-laminin make it possible in theory to assemble at least four different LN isoforms. In addition, there is evidence suggesting additional diversity in LN isoforms, not attributable to different genes. The INO antibody was isolated as an inhibitor of the activity of LN-containing neurite outgrowth factors (Matthew & Patterson 1983). It clearly binds an epitope associated with LN-heparan sulfate complexes (Chiu et al 1986). It also inhibits axonal extension on sections of the peripheral nerve where merosin, B1, and B2 are the major visible LN subunits (Sandrock & Matthew 1987b, Sanes et al 1990). Even though this suggests that INO recognizes LN isoforms containing merosin, its distribution in vivo is quite distinct from that of merosin or any other identified LN subunit (Chiu et al 1986, Sanes et al 1990). This suggests that the structure and possibly biological activities of LN can be modified by associating with other molecules, such as proteoglycans.
FN, which promotes axonal outgrowth by peripheral neurons, illustrates how differential splicing may contribute to diversification of the ECM (Hynes 1990). Embryonic chick sensory and sympathetic neurons extend neurites on two non-overlapping fragments of FN, a 75 kDa fragment containing the RGDS sequence and a 33 kDa fragment containing an alternatively spliced FN type III repeat (cf. Humphries et al 1988, Rogers et al 1987). Spinal cord neurons interact poorly with the RGDS-containing fragment, but interact efficiently with the 33 kDa fragment (Rogers et al 1987). These differences in behavior almost certainly reflect differences in integrin subunit expression by the different populations of neurons. The integrin α4β1 interacts with the alternatively spliced CS-1 domain in the 37 kDa fragment (cf. Guan & Hynes 1990) and is thus probably present on both central and peripheral neurons. The integrins α5β1 and α3β1 both interact with the RGDS-containing fragment of FN (cf. Hemler 1990) and are consequently not likely to be expressed by spinal cord neurons. As mentioned previously, expression of specific domains in other ECM glycoproteins, including tenascin, is also regulated by differential splicing. In principle, this could generate tremendous diversity in the temporal and spatial patterns of ECM protein expression during development of the nervous system.
Tenascin is a particularly intriguing ECM glycoprotein because it is expressed at high levels in the nervous system, has already been implicated by perturbation experiments as a mediator of neural crest and granule cell migration, and has an anti-adhesive as well as adhesive domain (Spring et al 1989; for review, see Erickson & Bourdon 1989). The anti-adhesive domain actually prevents responsive cells from attaching strongly to permissive substrates such as FN (cf. Lotz et al 1989). Tenascin’s anti-adhesive activity in vitro suggests that it might function in vivo to inhibit the growth of responsive axons, similar to intrinsic membrane proteins isolated recently from myelin and posterior somite, two tissues avoided by axons in vivo (Caroni & Schwab 1988, Davies et al 1990). In particular, since tenascin is found in boundaries of vibrissae-related barrel fields in the somatosensory cortex of mice (Steindler et al 1989), it has been suggested that its anti-adhesive activity may function in vivo to separate the fields of different afferent inputs. Recent work indicates that there is a family of tenascins and tenascin-like glycoproteins. First, there are several isoforms of tenascin derived from one gene by differential splicing (cf. Spring et al 1989). These differ in the number of FN type III repeats included in the translation product. Still more diversity is provided by a second gene encoding an isoform of tenascin that was discovered recently by virtue of its proximity to the gene encoding complement factor IV (Morel et al 1989). Further heterogeneity in tenascin-like proteins has been discovered by characterization of the J1 family of proteins (cf. Pesheva et al 1989). ECM proteins recognized by J1 antibodies include the major isoforms of tenascin and, in addition, two smaller proteins, J1-160 and J1-180. When examined in the EM, these smaller forms are dimeric and trimeric kinked arm rods, respectively, whereas tenascin is a hexamer of kinked arm rods (Pesheva et al 1989). J1-160 and J1-180 thus have striking structural similarities with tenascin. In contrast to tenascin, which is primarily synthesized by astroglia and fibroblasts (ffrench-Constant et al 1986, Gatchalian et al 1989), the J1-160 and J1-180 are synthesized by oligodendrocytes (Pesheva et al 1989). Like tenascin, J1-160 and J1-180 are nonpermissive substrates for attachment of some cells, including cerebellar neurons and astroglia. Similar to tenascin, J1 isoforms also have anti-adhesive activities on mixed substrates (cf. Pesheva et al 1989, Lotz et al 1989). To summarize, tenascin and its relatives are expressed at high levels in the nervous system. In different parts of the nervous system, evidence suggests that tenascin may have quite different functions, e.g. promoting migration of cerebellar granule cells in the cerebellum but limiting the extent of afferent branching in the barrel fields of the somatosensory cortex (Chuong et al 1987, Steindler et al 1989). The recent discoveries of new genes, differential splicing, and related glycoproteins increase its potential functions and the interest in identifying TN receptors.
To summarize this section, there is comparatively strong evidence that ECM constituents function as permissive substrates for cell and growth cone migration. Except for studies in C. elegans, there is little evidence that ECM constituents provide directional cues. Recent discoveries of new genes encoding ECM glycoprotein homologues and differential splicing of transcripts encoding these molecules indicate that the ECM in the developing nervous system may well be much more heterogeneous spatially than was previously appreciated. Thus there is at least the potential for the ECM to provide some of the guidance cues required for establishing migratory routes and axon tracts in the nervous system. At this point, though, ECM proteins have not yet been shown to distinguish between individual fiber pathways, as has been shown for some cell adhesion molecules, e.g. fasciclins I, II, and III in the insect nervous system (Harrelson & Goodman 1988). On balance, these results suggest that much of the information specifying direction and pathway choice is likely to be provided by other molecules, such as cell adhesion molecules and chemotropic factors (cf. Harrelson & Goodman 1988, Dodd & Jessell 1988).
ECM REGULATION OF SYNAPSE FORMATION
Studies on regeneration of amphibian neuromuscular junctions have demonstrated a critical role for the basal lamina in inducing formation of both pre- and postsynaptic specializations (see Nitkin et al 1983, Sanes & Chiu 1983). The basal lamina at the neuromuscular synapse has been shown to be molecularly distinguishable from non-synaptic basal lamina. ECM glycoproteins present only in synaptic basal lamina include two LN subunits (A and S) and two collagen IV chains [α3(IV) and α4(IV)] (Sanes et al 1990); agrin, an apparently novel ECM glycoprotein (Nitkin et al 1987); and, in Torpedo, a large proteoglycan named TAP-1 (Carlson & Wight 1987). Studies of these molecules imply that synaptic basal lamina acquires its unique molecular constituents from both neurons and myotubes. Thus, S-laminin is a biosynthetic product of myotubes (Sanes & Chiu 1983). Although its function in the synapse is unknown, its effects on peripheral motoneurons described above suggest that it may function to induce one or more steps in differentiation of the presynaptic terminals of motoneurons. The glycoprotein agrin was identified initially as a factor that induces clustering of acetylcholine receptors on skeletal myotubes (cf. Nitkin et al 1983). Purified agrin has been shown to induce concentrations of both ECM-associated and membrane-associated synaptic molecules on skeletal myotubes. Specifically it clusters acetylcholine receptors, acetylcholine esterase, and butyrylcholine esterase (Nitkin et al 1987, Wallace 1986). Agrin antigen is specifically localized at neuromuscular junctions in vivo (cf. Reist et al 1987), making it a strong candidate to induce clustering of the same molecules in vivo. Agrin is transported anterogradely by motoneurons (Magill-Solc & McMahan 1988). Agrin can also be detected in uninnervated muscle masses (Fallon & Gelfman 1989). Thus, it seems likely that it is synthesized by both neurons and skeletal myotubes. TAP-1 is a large synaptic vesicle–associated proteoglycan that appears to be synthesized by motoneurons in Torpedo (Carlson & Wight 1987). An isoform of this proteoglycan is associated with the synaptic basal lamina in the Torpedo electric organ. Since it also has properties of an integral membrane protein, it is a good candidate to function as a receptor that mediates binding of the nerve terminal plasma membrane to the synaptic basal lamina.
Although synapse-specific constituents of the ECM have been identified, very little is known about the intracellular signaling mechanisms involved in induction of pre- and postsynaptic differentiation. Extracellular Ca2+ is required for the formation of agrin-induced aggregates of acetylcholine receptor (Wallace 1988). Inhibitors of energy metabolism and activators of kinase C both inhibit clustering of this receptor. Future studies extending these promising initial results should be informative.
At this point, it is possible to propose how molecular differences between synaptic and nonsynaptic basal lamina are established. In part, this must reflect the contributions to the synaptic basal lamina of constituents synthesized by motoneurons, such as TAP-1 and agrin and, in turn, must reflect localization in the synaptic basal lamina of muscle-specific products, such as S-laminin. Mechanisms by which ECM constituents synthesized by skeletal myotubes become localized to synaptic basal lamina are not well understood. Preferential transcription of genes encoding synaptic molecules in myotube nuclei located near the synapse is one potential mechanism. The genes encoding the acetylcholine receptor appear to be regulated in this fashion (cf. Goldman & Staple 1989).
ECM REGULATION OF CELL PROLIFERATION AND DIFFERENTIATION
At early stages of development of the CNS, shortly after neural tube closure, the neuroepithelium is a single cell layer in thickness. At this time, germinal neuroepithelial cells in many areas of the nervous system contact at their basal surfaces an ECM that is rich in fibronectin, laminin, thrombospondin, and entactin (e.g. Tuckett & Morris-Kay 1986, O’Shea & Dixit 1988). At later stages, in some parts of the CNS that have undergone considerable cell proliferation (e.g. the retina) contact between germinal cells and overlying basement membrane is maintained. Recent studies on retinal neurogenesis in frogs provide evidence for a role of ECM molecules in controlling the extent of proliferation of retinal germinal neuroepithelial cells. When slices of tadpole retinae are maintained in culture with the surrounding basement membrane intact, neuroepithelial cells in the proliferative zone continue to divide for up to 3 weeks. Removal of the basement membrane by collagenase treatment, however, leads to a significant decline in germinal cell proliferation, and this deficit can be partially ameliorated with a basement membrane extract that is rich in laminin, collagen, and heparan sulfate proteoglycan (Reh & Radke 1988). Therefore, at least in some regions of the CNS, cell proliferation apparently may be stimulated by the presence of proteins within a basal lamina. In regions of the CNS where proliferating cells are not in contact with the basement membrane (e.g. the cerebral cortex), other ECM molecules, including FN, thrombospondin, and tenascin, are often in the proliferative zone (cf. Chun & Schatz 1988, O’Shea & Dixit 1988).
Although the mechanisms by which ECM constituents regulate proliferation of neuroepithelial cells are not well understood, studies on non-neural systems provide evidence for two roles of the ECM. First, the ECM localizes growth factors. FGF, for example, binds heparan sulfate on proteoglycans in the ECM (cf. Saksela et al 1988). This has been shown to protect FGF from proteolysis. As a result, the ECM serves as a reservoir of FGF (cf. Rifkin & Moscatelli 1989). Since FGF is both a potent mitogen and a differentiation factor for embryonic neuroepithelial cells (cf. Bartlett et al 1988), these observations are very relevant for understanding early development of the nervous system. Other growth factors, including a mitogen for Schwann cells, also bind to heparin (e.g. Ratner et al 1988). The activity of TGF-β is regulated in two distinct ways by proteoglycans. First, heparin activates it by dissociating it from an inactive complex with another protein (McCaffrey et al 1989). Second, a cell surface proteoglycan named decorin seems to function as a low-affinity receptor, binding and concentrating TGF-β on the cell surface, where it can then be bound by the high-affinity TGF-β receptor (Boyd & Massague 1989, Ruoslahti 1989). In addition to indirect effects mediated by growth factors, the ECM contains major constituents, such as LN, tenascin, and thrombospondin, that can directly promote cell proliferation. In vitro, LN has been shown to promote proliferation of several cell types, including fibroblasts and Schwann cells (cf. Panayatou et al 1989, McGarvey et al 1984). Thrombospondin and tenascin also promote the proliferation of specific cell types, including smooth muscle cells and fibroblasts, respectively (see Engel 1989). All three proteins contain domains with homology to EGF. In the case of soluble LN, mitogenic activity has been localized within a fragment containing multiple EGF repeats that does not contain the major cell attachment or neurite outgrowth-promoting sites (Panayatou et al 1989). A cell line lacking EOF receptors does not respond to soluble LN, thus making it attractive to imagine that the EOF receptor mediates the mitogenic response. Soluble LN does not compete with EGF for its receptor, however, so the mechanisms of LN’s action are unclear. Whatever the mechanisms, the effectiveness of soluble LN suggests that the mitogenic action of LN is due to direct modulation of intracellular second messengers rather than effects on cell adhesion.
ECM REGULATION OF CELL SURVIVAL AND DIFFERENTIATION
The effects of the ECM on neuronal survival and differentiation also seem likely to reflect modulations of cytoplasmic second messenger systems. In particular, LN has striking effects on the survival of early embryonic neurons and on the responsiveness of older neurons to neurotrophic factors. Early embryonic sympathetic neurons require an LN substrate but not a trophic factor to survive in vitro (Ernsberger et al 1989). For initial survival in vitro, embryonic sensory neuron precursors have a similar requirement for an ECM-coated substrate, but not a trophic factor (Ernsberger & Rohrer 1988). At later stages, both sympathetic and sensory neurons become dependent on trophic factors. Some sympathetic neurons remain dependent on a LN substrate (cf. Edgar et al 1984). Those capable of surviving without LN can be maintained on LN by much lower concentrations of NGF than would otherwise be effective. As LN is a prominent constituent of embryonic sympathetic and sensory ganglia at these stages of development, the ECM is likely to be critical in regulating proliferation and differentiation of these cells in vivo. Consistent with this view, embryonic sensory neuron precursors, when separated from the neural tube in vivo, can be rescued from cell death by the trophic factor BDNF only in combination with LN (Kalcheim et al 1987).
The effects in the nervous system of ECM molecules are not limited to neurons. Studies on Schwann cells, the myelin-forming cells of the peripheral nervous system, indicate that constituents of the basal lamina are required for myelination. Agents that disrupt Schwann cell basal lamina production in vitro—e.g. inhibitors of collagen or proteoglycan biosynthesis—prevent myelination of axons in vitro (Carey et al 1987, Eldridge et al 1987). Basal lamina–deficient Schwann cells can be induced to mye1inate axons by the addition of LN. Eldridge et al (1989) have proposed that LN promotes assembly of a normal basal lamina that in turn enables Schwann cells to myelinate the available axons. Schwann cells thus provide an interesting example of an autocrine role for the ECM in inducing expression of a highly differentiated phenotype. The mechanisms by which the ECM promotes Schwann cell differentiation are not understood. Both adhesive interactions that polarize the Schwann cells and modulation of second messenger systems may be important.
ECM components have been shown to regulate differentiation of neural precursor cells into neurons and to regulate the expression of transmitter enzymes in neural cells. Thus, LN has been shown to induce amphibian retinal pigment epithelial cells to express a neuronal phenotype (Reh et al 1987). A substrate-attached factor from conditioned medium, which is probably an isoform of LN, dramatically enhances the ability of NGF to convert neonatal adrenal chromaffin cells to sympathetic neurons (Doupe et al 1985). The same factor is absolutely required for NGF-induced conversion of adult adrenal chromaffin cells. ECM components have also been shown to induce expression of an adrenergic phenotype by neural crest cells and adrenal chromaffin cells (Maxwell & Forbes 1987, Acheson et al 1986). When used as a substrate for adrenal chromaffin cell growth, LN increases the amount of tyrosine hydroxylase over a period of days, but is also able to increase the activity of tyrosine hydroxylase within a few hours (Acheson et al 1986). The latter result suggests that LN acts on tyrosine hydroxylase by regulating the activity of one of the many protein kinases known to modulate its activity (cf. McTigue et al 1985).
MECHANISMS OF ACTION OF ECM CONSTITUENTS AND THEIR RECEPTORS
ECM constituents obviously influence many aspects of cell behavior during development of the nervous system. Knowledge of the mechanisms by which ECM components exert their effects is still sketchy; however, in the case of integrin-mediated interactions with the ECM, a picture has begun to emerge. Upon binding of ligand, integrins appear to transduce signals across the plasma membrane in two ways: by interacting directly with cytoskeletal proteins, and by regulating the levels of second messengers. Some integrin-mediated actions may, in principle, reflect simply effects of interactions with the cytoskeleton. The functions of macromolecules such as FN in modulating migration of neural crest celis, for example, seem potentially understandable in thisconceptual framework. Other effects of ECM constituents, though, such as effects upon cell survival, mitosis, or trans-determination, are difficult to explain as simple consequences of adhesion. Instead, they seem much more easily explicable if ECM macromolecules also influence the intracellular second messenger systems of the cells. Studies on integrins, particularly in lymphocytes and platelets, provide evidence that they can indeed transfer signals across the membrane that influence both the cytoskeleton and intracellular second messenger systems. There is also convincing evidence that intracellular metabolism can alter the ligand-binding functions of several integrin receptors.
An important effector of integrin-mediated signaling is likely to be a change in integrin structure, mediated by proteins inside and outside the cell. With regard to extracellular mediators, both binding of ligands and of divalent cations have been shown to alter integrin conformation (Parise et al 1987). Regulated conformation-dependent epitopes have been identified on the platelet integrin αIIb/β3 and on the lymphocyte integrins by using monoclonal antibodies (Frelinger et al 1988, Dransfield & Hogg 1989). The epitope on glycoprotein αIIb/β3 is located in the αIIb subunit sequence at a site that is well separated in the linear sequence from the ligand and cation-binding sites, a position suggesting that it is a reporter for a significant structural change (Frelinger et al 1988).
Binding of antibodies and ligands to external domains of integrin receptors has been shown to regulate several cytoplasmic events. Among these, binding to an ECM protein-coated substrate promotes the formation of focal contacts in which integrins are associated with the termini of F-actin filaments and several other proteins, most notably talin, vinculin, and α-actinin (cf. Burridge et al 1988, Fath et al 1989). Each of these proteins has been detected in growth cones (cf. Letourneau & Shattuck 1989). Purified integrins have been shown to bind talin, which can in turn bind vinculin (Horwitz et al 1986, Tapley et al 1989). Based on observed interactions of these proteins in vitro, it has been proposed that talin, vinculin, and α-actinin link the integrins to the cytoskeleton (reviewed in Burridge et al 1988). Analysis of early events in cell attachment has demonstrated that binding to ligand induces integrin-talin coaggregation at sites of membrane-ECM contact, which is followed by association of other cytoskeletal proteins (Mueller et al 1989). Agents that disrupt adhesion, such as RGD-containing peptides, have been shown to dissociate these cytoskeletal proteins from integrins. In addition to regulation by adhesion, colocalization can also be regulated by intracellular messengers and protein phosphorylation (cf. Tapley et al 1989).
The cytoplasmic domain of the integrin β1 subunit has been shown to be important for its colocalization with the cytoskeleton and for its function in cell adhesion (Solowska et al 1989, Hayashi et al 1990). When expressed in heterologous cells, integrin β1 subunits lacking the cytoplasmic domain associate with α subunits to form receptors that can still bind ligands in biochemical assays. Within the cell, though, they do not localize to focal contacts and are not effective at promoting cell adhesion. This indicates that integrins must interact with the cytoskeleton to promote cell adhesion (cf. Lotz et al 1989). The cytoplasmic domains of several integrin subunits are substrates in vivo for tyrosine or serine-threonine protein kinases (cf. Tapley et al 1989). Some evidence suggests that phosphorylation of these domains regulates associations of integrins with the cytoskeleton.
Regulation of integrin interactions with the cytoskeleton is important for controlling the motility of cells and growth cones. In stationary cells, ligand binding by integrins leads to their accumulation in stable focal adhesions. Integrin associations with adhesive zones are comparatively stable (Duband et al 1988). In motile cells, however, integrins have becn shown to diffuse rapidly in the membrane, thereby indicating that associations with the ECM and cytoskeleton are transient. The importance of actin dynamics in growth cone motility and guidance is well established (cf. Smith 1988, Mitchison & Kirschner 1988). Growth cone motility is driven in part by the behavior of the actin-based cytoskeleton. The flow of actin depends on actin polymerization at the leading edge of cells, energy-dependent retrograde movement of actin filaments, and depolymerization of these filaments in the cell interior. Integrin-mediated interactions with F-actin can in principle regulate any of these steps. Lateral interactions have the potential to slow retrograde movements, generating traction against the substrate. Interactions at filament ends may regulate capping, polymerization, or depolymerization. Receptors interacting with moving filaments of actin will generate tension inside the growth cone, which can potentially regulate the shape and dynamics of all cytoskeletal elements in the growth cone. In addition, stretch-activated and stretch-inactivated K+ channels have recently been discovered in growth cones (cf. Sigurdson & Morris 1989). By regulating membrane potential, these channels may modulate voltage-dependent Ca2+ channels and cytoplasmic Ca2+ levels, which in turn may affect several other second messengers. As Ca2+ has been shown to be an important modulator of growth cone motility (cf. McCobb et al 1988), critical examination of the functions of these channels should prove interesting.
Binding to integrin receptors of antibodies and ligands has been shown to regulate other cytoplasmic events, at least some of which are not related to cell shape or adhesion. Some anti-integrin monoclonal antibodies can initiate signaling cascades; for example, binding of some monoclonal antibodies to the platelet integrin αIIbβ3 induces platelet activation (Jennings et al 1985). During platelet activation, tyrosine protein kinases phosphorylate proteins in three waves. The function of the platelet integrin glycoprotein αIIbβ3 is required for the third wave of phosphorylation, which does not occur in platelets lacking this integrin or in platelets activated in the presence of RGDS peptides (Ferrell & Martin 1989). Similarly, in fibroblasts, interactions of the fibronectin receptor with fragments of fibronectin or with antibodies capable of crosslinking this receptor induce expression of genes encoding ECM-degrading proteases (Werb et al 1989). These effects are not related to visible changes in cell shape or cytoskeletal organization. Consistent with this evidence implicating integrins as modulators of intracellular signaling, the functions of integrins have been shown to be required for differentiation of several cell types (e.g. myoblasts) on substrates for which they are not essential for cell adhesion (cf. Menko & Boettiger 1987).
Mechanisms by which integrins regulate signal cascades are not understood. In platelets, there is evidence that the integrin αIIbβ3 can associate with an additional membrane-associated protein, CD9, which itself is involved in platelet activation (Slupsky et al 1989). Similar associations of other proteins with integrins may potentially be involved in transducing signals in other cells. Intriguingly, the cytoplasmic domain of an individual integrin subunit has typically proven to be highly conserved among species (cf. Marcantonio & Hynes 1988), but α and β subunits within a species have quite different cytoplasmic domains (cf. Takada & Hemler 1989). It seems possible, therefore, that these domains interact with additional, unknown proteins, and that different subunits have significant differences in their interactions and signaling capabilities. This may provide at least a partial explanation for the existence of multiple integrin receptors that interact with a single ECM glycoprotein (Table 1).
Intracellular metabolism can alter both the structure and function of the extracellular domains of the integrins. The ligand-binding functions of several integrins, most strikingly the platelet integrin (αIIbβ3 and the lymphocyte integrins αLFA-1β2 and αMac-1β2, have been shown-to be regulated by ligand binding to other receptors, by physiological stimuli, and by agonists or activators of second messenger systems (cf. Frelinger et al 1988, Dustin & Springer 1989). Appearance of a conformation-dependent epitope shared by each of the lymphocyte integrins (αLFA-1 β2, αMac-1 β2,and αgp150 β2) has been shown to require not only Mg2+ binding but also cellular metabolism (Dransfield & Hogg 1989). Phosphorylation of cytoplasmic domains of integrin subunits seems likely to be one mechanism by which integrin-mediated cell adhesion is regulated (Tapley et al 1989, Danilov & Juliano 1989). This may be one mechanism by which trophic factors and chemotropic factors influence neuronal growth cone interactions with the ECM.
Additional mechanisms for rapid regulation of integrin function have been described in lymphoid cells and may be useful paradigms for understanding effects of substrates, growth factors, and chemotropic agents on growth cone behavior. Large fractions of the integrins(αMac-1 β2 and αgpl50β2 are sequestered in intracellular vesicles in neutrophils (Bainton et al 1987). Physiological stimuli induce exocytosis of these granules, transferring them to the cell surface. Factors that induce aggregation of these receptors or their ligands have also been shown to enhance their activity (cf. Hermanowski-Vosatka et al 1988). In a series of ingenious experiments in which a membrane-impermeant, cleavable crosslinking agent was used, integrins have been shown to cycle rapidly between intracellular and surface compartments in fibroblasts, being inserted preferentially at leading edges of motile cells (Bretscher 1989). By similar mechanisms, chemotropic agents and trophic factors could potentially regulate growth cone behavior by regulating the cycling or by targeting the insertions of integrins and other adhesion molecules.
Contributor Information
Louis F. Reichardt, Program in Neuroscience, Department of Physiology and Howard Hughes Medical Institute, University of California School of Medicine, San Francisco, California 94143-0724
Kevin J. Tomaselli, Athena Neurosciences, South San Francisco, California 94080
Literature Cited
- Acheson A, Edgar D, Timpl R, Thoenen H. Laminin increases both levels and activity of tyrosine hydroxylase in calf adrenal chromaffin cells. J Cell Biol. 1986;102:151–59. doi: 10.1083/jcb.102.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argraves WS, Suzuki S, Arai H, Thompson K, Pierschbacher MD, Ruoslahti E. Amino acid sequence of the human fibronectic receptor. J Cell Biol. 1987;105:1183–90. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton DF, Miller LJ, Kishimoto TK, Springer RA. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987;166:1641–53. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett PF, Reid HH, Bailey KA, Bernard O. Immortalization of mouse neural precursor cells by the c-myconcogene. Proc Natl Acad Sci USA. 1988;85:3255–59. doi: 10.1073/pnas.85.9.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K, Hunter I, Engel J. Structure and function of laminin: Anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–60. doi: 10.1096/fasebj.4.2.2404817. [DOI] [PubMed] [Google Scholar]
- Begovac PC, Shur BD. Cell surface galactosyltransferase mediates the initiation of neurite outgrowth from PC12 cells on laminin. J Cell Biol. 1990;110:461–70. doi: 10.1083/jcb.110.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D, Caudy M. Navigational substrates for peripheral pioneer growth cones: Limb-axis polarity cues, limb-segment boundaries, and guidepost neurons. Cold Spring Harbor Symp Quant Biol. 1983;48:573–85. doi: 10.1101/sqb.1983.048.01.062. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Lilien J, Reichardt LF. Identification of the major proteins that promote neuronal process outgrowth on Schwann cells in vitro. J Cell Biol. 1988;107:353–61. doi: 10.1083/jcb.107.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary SC, Napier MA, McLean JW. Expression of recombinant platelet glycoprotein IIbIIIa results in a functional fibrinogen-binding complex. J Biol Chem. 1989;264:18859–62. [PubMed] [Google Scholar]
- Boocock CA. Unidirectional displacement of cells in fibrillar matrices. Development. 1989;107:881–90. doi: 10.1242/dev.107.4.881. [DOI] [PubMed] [Google Scholar]
- Boucaut J-C, Darriberre T, Poole TJ, Aoyama H, Yamada KM, Thiery J-P. Biologically active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984;99:1822–30. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon MA, Ruoslahti E. Tenascin mediates cell attachment through an RGD-dependent receptor. J Cell Biol. 1989;108:1149–55. doi: 10.1083/jcb.108.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd FT, Massague J. Transforming growth factor-β inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989;264:2272–78. [PubMed] [Google Scholar]
- Boyne LJ, O’Shea KS, Dixit VM. Neural crest migration on an extracellular matrix protein thrombospondin. J Cell Biol. 1989;109:112a. doi: 10.1083/jcb.111.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko D, Horwitz The participation of a putative cell surface receptor for laminin and fibronectin in peripheral neurite extension. J Neurosci. 1986;6:1241–51. doi: 10.1523/JNEUROSCI.06-05-01241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CRO cells. EABO J. 1989;8:1341–48. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. An antibody to a receptor for fibronectin and laminin perturbs cranial neural crest development in vivo. Dev Biol. 1986;117:528–36. doi: 10.1016/0012-1606(86)90320-9. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Distribution and function of tenascin during cranial neural crest developmental in the chick. J Neurosci Res. 1988;21:135–47. doi: 10.1002/jnr.490210206. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Lallier T. A monoclonal antibody against a laminin-heparan sulfate proteoglycan complex perturbs cranial neural crest migration in vivo. J Cell Biol. 1988;106:1321–29. doi: 10.1083/jcb.106.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NH, King DL, Wilcox M, Kafatos FC. Developmentally regulated alternative splicing of Drosophila integrin PS2 α transcripts. Cell. 1989;59:185–95. doi: 10.1016/0092-8674(89)90880-5. [DOI] [PubMed] [Google Scholar]
- Buck CA, Horwitz AF. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–206. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burgeson RE. New collagens, new concepts. Annu Rev Cell Biol. 1988;4:551–77. doi: 10.1146/annurev.cb.04.110188.003003. [DOI] [PubMed] [Google Scholar]
- Burridge J, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesion: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Carey DJ, Rafferty CM, Todd MS. Effects of inhibition of protcoglycan synthesis on the differentiation of cultured rat Schwann cells. J Cell Biol. 1987;105:1013–21. doi: 10.1083/jcb.105.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SS, Wight TN. Nerve terminal anchorage protein 1 (TAP-1) is a chondroitin sulfate proteoglycan: Biochemical and electron microscopic characterization. J Cell Biol. 1987;105:3075–86. doi: 10.1083/jcb.105.6.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–88. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell NA, Fitzgerald LA, Steiner B, Erickson HP, Phillips DR. Structure of human platelet membrane glycoprotein IIb and IIIa as determined by electron microscopy. J Biol Chem. 1985;260:1743–49. [PubMed] [Google Scholar]
- Cheresh DA, Smith JW, Cooper HM, Quaranta V. A novel vitronectin receptor integrin (αvβx) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989;57:59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Pytela R, Pierschbacher MD, Klier GF, Ruoslahti E, Reisfeld RA. An arg-gly-asp-directed receptor on the surface of human melanoma cells exists as a divalent cation-dependent functional complex with the disialoganglioside GD2. J Cell Biol. 1987;105:1163–73. doi: 10.1083/jcb.105.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, Matthew WD, Patterson PH. A monoclonal antibody that blocks the activity of a neurite regeneration-promoting factor: Studies on the binding site and its localization in vivo. J Cell Biol. 1986;103:1383–98. doi: 10.1083/jcb.103.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJM, Shatz CJ. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988;106:857–72. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Crossin KL, Edelman GM. Sequential expression and differential function of multiple adhesion molecules during the formation of cerebellar cortical layers. J Cell Biol. 1987;104:331–42. doi: 10.1083/jcb.104.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement B, Sequi-Real B, Hassell JR, Martin GR, Yamada Y. Identification of a cell surface-binding protein for the core protein of the basement membrane proteoglycan. J Biol Chem. 1989;264:12467–71. [PubMed] [Google Scholar]
- Cohen J, Burne JF, McKinlay C, Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev Biol. 1987;122:407–18. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- Cohen J, Burne JF, Winter J, Bartlett P. Retinal ganglion cells lose response to laminin with maturation. Nature. 1986;322:465–67. doi: 10.1038/322465a0. [DOI] [PubMed] [Google Scholar]
- Cohen J, Nurcombe V, Jeffrey P, Edgar D. Developmental loss of functional laminin receptors on retinal ganglion cells is regulated by their target tissue, the optic tectum. Development. 1989;107:381–87. doi: 10.1242/dev.107.2.381. [DOI] [PubMed] [Google Scholar]
- Condic ML, Bentley D. Removal of the basal lamina in vivo reveals growth cone-basal lamina adhesive interactions and axonal tension in grasshopper embryos. J Neurosci. 1989a;9:2678–86. doi: 10.1523/JNEUROSCI.09-08-02678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic ML, Bentley D. Pioneer neuron pathfinding from normal and ectopic locations in vivo after removal of the basal lamina. Neuron. 1989b;3:427–39. doi: 10.1016/0896-6273(89)90202-x. [DOI] [PubMed] [Google Scholar]
- Covault J, Cunningham JM, Sanes JR. Neurite outgrowth on cryostat sections of innervated and denervated skeletal muscle. J Cell Biol. 1987;105:2479–88. doi: 10.1083/jcb.105.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov YN, Juliano RL. Phorbol ester modulation of integrin-mediated cell adhesion: A postreceptor event. J Cell Biol. 1989;108:1925–33. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JA, Cook GMW, Stern CD, Keynes RJ. Isolation from chick somites of a glycoprotein fraction that causes collapse of dorsal root ganglion growth cones. Neuron. 1990;2:11–20. doi: 10.1016/0896-6273(90)90439-m. [DOI] [PubMed] [Google Scholar]
- Davis GE, Blaker SN, Engvall E, Varon S, Manthorpe M, Gage FH. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo. Science. 1987;236:1106–9. doi: 10.1126/science.3576223. [DOI] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous β nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Natl Acad Sci USA. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–99. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Landis SC, Patterson PH. Environmental influences in the development of neural crest derivatives: Glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985;5:2119–42. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douville PJ, Harvey WJ, Carbonetto S. Isolation and partial characterization of high affinity laminin receptors in neural cells. J Biol Chem. 1988;263:14964–69. [PubMed] [Google Scholar]
- Dransfield I, Hogg N. Regulated expression of Mg2+ binding epitope on leukocyte integrin α subunits. EMBO J. 1989;8:3759–65. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza SE, Ginsberg MH, Burke TA, Lam SC-T, Plow EF. Localization of an Arg-Gly-Asp recognition site within an integrin adhesion receptor. Science. 1988;242:91–93. doi: 10.1126/science.3262922. [DOI] [PubMed] [Google Scholar]
- Duband J-L, Nuckolls GH, Ishihara A, Hasegawa T, Yamada KM, et al. Fibronectin receptor exhibits high lateral mobility in embryonic locomoting cells but is immobile in focal contacts and fibrillar streaks in stationary cells. J Cell Biol. 1988;107:1385–96. doi: 10.1083/jcb.107.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour S, Duband J-L, Humphries MJ, Obara M, Yamada KM. Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988;7:2662–71. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–24. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Edgar D. Neuronal laminin receptors. Trends Neurosci. 1989;12:248–51. doi: 10.1016/0166-2236(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Edgar D, Timpl R, Thoenen H. The heparin binding domain of laminin is responsible for its effects on neurite outgrowth and neuronal survival. EMBO J. 1984;3:1463–68. doi: 10.1002/j.1460-2075.1984.tb01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar D, Timpl R, Thoenen H. Structural requirements for the stimulation of neurite outgrowth by two variants of laminin and their inhibition by antibodies. J Cell Biol. 1988;106:1299–1306. doi: 10.1083/jcb.106.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Angvall E. The tissue-specific basement membrane protein merosin is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264–68. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–34. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP. Differentiation of axon-related Schwann cells in vitro: II. Control of myelin formation by basal lamina. J Neurosci. 1989;9:625–38. doi: 10.1523/JNEUROSCI.09-02-00625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Hemler ME. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci USA. 1989;86:9906–10. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, et al. VCAM-1 on activated endothelium inter-8-:ts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Engel J. EGF-like domains in extracellular matrix proteins: Localized signals for growth and differentiation? FEBS Lett. 1989;251:1–7. doi: 10.1016/0014-5793(89)81417-6. [DOI] [PubMed] [Google Scholar]
- Epperlein H-W, Halfter W, Tucker RP. The distribution of fibronectin and tenascin along migratory pathways of the neural crest in the trunk of amphibian embryos. Development. 1988;103:743–56. doi: 10.1242/dev.103.4.743. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Bourdon MA. Tenascin: An extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Edgar D, Rohrer H. The survival of early chick sympathetic neurons in vitro is dependent on a suitable substrate but independent on NGF. Dev Biol. 1989;135:250–62. doi: 10.1016/0012-1606(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Rohrer H. Neuronal precursor cells in chick dorsal root ganglia: Differentiation and survival in vitro. Dev Biol. 1988;126:420–32. doi: 10.1016/0012-1606(88)90151-0. [DOI] [PubMed] [Google Scholar]
- Fahrig T, Landa C, Pesheva P, Kuhn K, Schachner M. Characterization of binding properties of the myelin-association glycoprotein to extracellular matrix constituents. EMBO J. 1987;6:2875–83. doi: 10.1002/j.1460-2075.1987.tb02590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JR, Gelfman CE. Agrin-related molecules are concentrated at acetylcholine receptor clusters in normal and aneural developing muscle. J Cell Biol. 1989;108:1527–35. doi: 10.1083/jcb.108.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Edgell C-JS, Burridge K. The distribution of distinct integrins in focal contacts is determined by the substratum composition. J Cell Sci. 1989;92:67–75. doi: 10.1242/jcs.92.1.67. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Martin GS. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci USA. 1989;86:2234–38. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler JH, Fessler LI. Drosophila extracellular matrix. Annu Rev Cell Biol. 1989;5:309–39. doi: 10.1146/annurev.cb.05.110189.001521. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Miller RH, Kruse J, Schachner M, Raff MC. Molecular specialization of astrocyte processes at nodes of Ranvier in rat optic nerve. J Cell Biol. 1986;102:844–52. doi: 10.1083/jcb.102.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LA, Phillips DR. Calcium regulation of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985;260:11366–74. [PubMed] [Google Scholar]
- Fitzgerald LA, Steiner B, Rail SC, Jr, Lo S-S, Phillips DR. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. J Biol Chem. 1987;262:3936–39. [PubMed] [Google Scholar]
- Frelinger AL, Lam SC, Plow EF, Smith MA, Loftus JC, Ginsberg MH. Occupancy of an adhesive glycoprotein receptor modulates expression of an antigenic site involved in cell adhesion. J Biol Chem. 1988;263:12397–12402. [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. J Biol Chem. 1988;263:12927–32. [PubMed] [Google Scholar]
- Gatchalian CL, Schachner M, Sanes JR. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin (J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol. 1989;108:1873–90. doi: 10.1083/jcb.108.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K, Dickerson K, Argraves WS, Engvall E, Ruoslahti E. Subunit structure of a laminin-binding integrin and localization of its binding site on laminino. J Biol Chem. 1989;264:19034–38. [PubMed] [Google Scholar]
- Goldman D, Staple J. Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron. 1989;3:219–28. doi: 10.1016/0896-6273(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Zhou DFH, Picker LJ, Minty CN, Bargatze RF, et al. A human lymphocyte homing receptor, the Hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989;56:1063–72. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Graf J, Iwamoto Y, Sasaki M, Martin GR, Kleinman HK, et al. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis and receptor binding. Cell. 1987;48:989–96. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Guan J-L, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor α4β1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Gunderson RW. Response of sensory neurites and growth cones to patterned substrata of laminin and fibronectin in vitro. Dev Biol. 1987;121:423–31. doi: 10.1016/0012-1606(87)90179-5. [DOI] [PubMed] [Google Scholar]
- Halfter W, Deiss S. Axon growth in embryonic chick and quail retinal whole mounts in vitro. Dev Biol. 1984;102:344–55. doi: 10.1016/0012-1606(84)90199-4. [DOI] [PubMed] [Google Scholar]
- Halfter W, Reckhaus W, Kroger S. Nondirected axonal growth on basal lamina from avian embryonic neural retina. J Neurosci. 1987;7:3712–22. doi: 10.1523/JNEUROSCI.07-11-03712.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Neugebauer K, Reichardt L. Embryonic neural retina cell response to extracellular matrix proteins: Developmental changes and effects of the cell substratum attachment antibody, CSAT. J Cell Biol. 1987;104:623–34. doi: 10.1083/jcb.104.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, et al. The α1/β1 and α6/β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–84. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrelson AL, Goodman CS. Growth cone guidance in insects: Fasciclin II is a member of the immunoglobulin superfamily. Science. 1988;242:700–8. doi: 10.1126/science.3187519. [DOI] [PubMed] [Google Scholar]
- Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264:1437–42. [PubMed] [Google Scholar]
- Hayashi Y, Haimovich B, Reszka A, Boettiger D, Horwitz A. Expression and function of chicken integrin β1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990;110:175–84. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 gencs guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Crouse C, Sonnenberg A. Associations of the VLA α6 subunit with a novel protein. J Biol Chem. 1989;264:6529–35. [PubMed] [Google Scholar]
- Hemler ME. VLA proteins in the integrin family: Structure, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hermanowski-Vosatka A, Detmers PA, Gotze O, Silverstein SC, Wright SD. Clustering of ligand on the surface of a particle enhances adhesion to receptor-bearing cells. J Biol Chem. 1988;263:17822–27. [PubMed] [Google Scholar]
- Herndon ME, Lander AE. A diverse set of developmentally regulated proteogiyeans is expressed in rat central nervous system. Neuron. 1990;4:949–61. doi: 10.1016/0896-6273(90)90148-9. [DOI] [PubMed] [Google Scholar]
- Hoffman S, Crossin KL, Edelman GM. Molecular forms, binding functions, and developmental expression patterns of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules. J Cell Biol. 1988;106:519–32. doi: 10.1083/jcb.106.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B, McIntyre BW, Weissman IL. Identification of a murine Peyer’s patch-specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA-4α. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Holzmann B, Weissman IL. Peyer’s patch-specific lymphocyte homing receptors consist of a VLA-4-like α chain associated with either of two integrin β chains, one of which is novel. EMBO J. 1989;8:1735–41. doi: 10.1002/j.1460-2075.1989.tb03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins JM, Ford-Holevinski TS, McCoy JP, Agranoff BW. Laminin and optic nerve regeneration in the goldfish. J Neurosci. 1985;5:3030–38. doi: 10.1523/JNEUROSCI.05-11-03030.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Buck C, Beckerie M, Burridge K. Interaction of plasma membrane fibronectin with talin: A transmembrane lineage. Nature. 1986;320:531–33. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hostikka SL, Eddy RL, Byers MG, Hoyhtya M, Shows TB, Tryggvason K. Identification of a distinct type IV collagen α chain with restricted kidney distribution and assignment of its gene to the locus of X chromosome-linked Alport syndrome. Proc Natl Acad Sci USA. 1990;87:1606–10. doi: 10.1073/pnas.87.4.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986;103:2637–47. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Neurite extension of chicken peripheral nervous system neurons on fibronectin: Relative importance of specific adhesion sites in the central cell-binding domain and the alternatively spliced type III connecting segment. J Cell Biol. 1988;106:1289–98. doi: 10.1083/jcb.106.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D, Shah V, Merlie J, Sanes J. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989a;338:229–34. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Porter BE, Bulock JW, Adams SP, Merlie JP, Sanes JR. Primary sequence of a motor neuron-selective adhesive site in the synaptic Basal lamina protein S-laminin. Cell. 1989b;59:905–13. doi: 10.1016/0092-8674(89)90613-2. [DOI] [PubMed] [Google Scholar]
- Hynes R. Integrins: A family of cell surface receptors. Cell. 1987;48:549–54. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Ignatius MJ, Reichardt LF. Identification of a neuronal laminin receptor: An Mr 200/120 kD integrin heterodimer that binds laminin in a divalent cation-dependent manner. Neuron. 1988;1:713–25. doi: 10.1016/0896-6273(88)90170-5. [DOI] [PubMed] [Google Scholar]
- Ignatius MJ, Large TH, Houde M, Tawil JW, Barton A, Esch F, Carbonetto S, Reichardt LF. Molecular cloning of the rat integrin α1-subunit: A receptor for laminin and collagen. J Cell Biol. 1990;111:709–20. doi: 10.1083/jcb.111.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffredo T, Horwitz AF, Buck CA, Rong PM, Dieterlen-Lievre F. Myoblast migration specifically inhibited in the chick embryo by grafted CSAT hybridoma cells secreting an anti-integrin antibody. Development. 1988;103:431–46. doi: 10.1242/dev.103.3.431. [DOI] [PubMed] [Google Scholar]
- Jennings L, Phillips DR, Walker WS. Monoclonal antibodies to human platelet glycoprotein lIb that initiate distinct platelet responses. Blood. 1985;65:1112–19. [PubMed] [Google Scholar]
- Kajiji S, Tamura RN, Quaranta V. A novel integrin (αEβ4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989;8:673–80. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheim C, Barde Y-A, Thoene H, LeDouarin NM. In vivo effect of brain-derived neurotrophic factor on the survival of developing dorsal root ganglion cells. EMBO J. 1987;6:2871–73. doi: 10.1002/j.1460-2075.1987.tb02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Horie H, Takenaka T. The maturation-dependent change in fibronectin receptor density of mouse dorsal root ganglion neurons. Brain Res. 1986;397:185–88. doi: 10.1016/0006-8993(86)91384-3. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, O’Conner K, Springer TA. Leukocyte adhesion deficiency: Aberrant splicing of a conserved integrin sequence causes a moderate deficiency phenotype. J Biol Chem. 1989;264:3588–95. [PubMed] [Google Scholar]
- Kishimoto TK, Larson RS, Corbi AL, Dustin ML, Staunton DE, Springer TA. The leukocyte integrins. Adv Immunol. 1989;46:149–81. doi: 10.1016/s0065-2776(08)60653-7. [DOI] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–41. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Ogle RC, Cannon FB, Little CD, Sweeney TM, Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci USA. 1988;85:1282–86. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Weeks BS. Laminin: Structure, functions, and receptors. Curr Opinion Cell Biol. 1989;1:964–67. doi: 10.1016/0955-0674(89)90066-5. [DOI] [PubMed] [Google Scholar]
- Kramer RH, McDonald KA, Vu MP. Human melanoma cells express a novel integrin receptor for laminin. J Biol Chem. 1989;264:15642–49. [PubMed] [Google Scholar]
- Krotoski DM, Domingo C, Bronner-Fraser M. Distribution of a putative cell surface receptor for fibronectin and laminin in the avian embryo. J Cell Biol. 1986;103:1061–71. doi: 10.1083/jcb.103.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen J, Loppenen R, Merenmies J, Rauvala H. Binding of laminin to brain gangliosides and inhibition of laminin-neuron interaction by the gangliosides. FBBS Lett. 1987;217:94–100. doi: 10.1016/0014-5793(87)81250-4. [DOI] [PubMed] [Google Scholar]
- Lander AD. Understanding the molecu1es of neural cell contacts: Emerging patterns of structure and function. Trends Neurosci. 1989;12:189–95. doi: 10.1016/0166-2236(89)90070-2. [DOI] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF. Purification of a factor that promotes neurite outgrowth: Isolation of 1aminin and associated molecules. J Cell Biol. 1985;101:898–913. doi: 10.1083/jcb.101.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino RR, Gehlsen KR, Wayner E, Carter WG, Engvall E, Ruoslahti E. Endothelial cells use α2β1 integrin as a laminin receptor. J Cell Biol. 1989;109:2455–62. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: The role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988;107:2341–61. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron RG, Hook A, Esko JD, Gay S, Hook M. Binding of heparan sulfate to type V collagen. J Biol Chem. 1989;264:7950–56. [PubMed] [Google Scholar]
- Lefcort F, Bentley D. Pathfinding by pioneer neurons in isolated, opened and mesoderm-free limb bunds of embryonic grasshoppers. Dev Biol. 1987;119:466–80. doi: 10.1016/0012-1606(87)90050-9. [DOI] [PubMed] [Google Scholar]
- Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle and trophoblasts, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544–48. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Madsen AM, Palm SL, Furcht LT. Immunoreactivity for laminin in the developing ventral longitudinal pathway of the brain. Dev Biol. 1988;125:135–44. doi: 10.1016/0012-1606(88)90066-8. [DOI] [PubMed] [Google Scholar]
- Letourneau PC, Shattuck TA. Distribution and possible interactions of actin-associated proteins and cell adhesion molecules of nerve growth cones. Development. 1989;105:505–19. doi: 10.1242/dev.105.3.505. [DOI] [PubMed] [Google Scholar]
- Liesi P. Laminin-immunoreactive glia distinguish regenerative adu1t CNS systems from non-regenerative ones. EMBO J. 1985;4:2505–11. doi: 10.1002/j.1460-2075.1985.tb03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz MM, Burdsal CA, Erickson HP, McClay DR. Cell adhesion to fibronectin and tenascin: Quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989;109:1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill-Solc C, McMahan UJ. Motor neurons contain agrin-like molecules. J Cell Biol. 1988;107:1825–33. doi: 10.1083/jcb.107.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Deutzrnann R, Aumailley M, Timpl R, Raimondi L, et al. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J. 1989;8:65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M, Engvall E, Ruoslahti E, Longo FM, Davis GE, Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983;97:1882–90. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcantonio EE, Hynes RO. Antibodies to the conserved cytoplasmic domain of the integrin β1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988;106:1765–72. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin SD, Springer TA. Purified intercellular adhesion molecule (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–19. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Matthew WD, Patterson PH. The production of a monoclonal antibody that blocks the action of a neurite outgrowth-promoting factor. Cold Spring Harbor Syrnp Quant Biol. 1983;48:625–31. doi: 10.1101/sqb.1983.048.01.066. [DOI] [PubMed] [Google Scholar]
- Maxwell GD, Forbes ME. Exogenous basement-membrane-like matrix stimulates adrenergic development in avian neural crest cultures. Developrnent. 1987;101:767–76. doi: 10.1242/dev.101.4.767. [DOI] [PubMed] [Google Scholar]
- McCaffrey TA, Falcone DJ, Brayton CF, Agarwal LA, Welt FGP, Weksler BB. Transforming growth factor-β activity is potentiated by heparin via dissociation of the transforming growth factor-β/α2-macroglobulin inactive complex. J Cell Biol. 1989;109:441–48. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb DP, Cohan CS, Connor JA, Kater SB. Interactive effects of serotonin and acetylcholine on neurite elongation. Neuron. 1988;1:377–85. doi: 10.1016/0896-6273(88)90187-0. [DOI] [PubMed] [Google Scholar]
- McDonald JA. Extracellular matrix assembly. Annu Rev Cell Biol. 1988;4:183–207. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- McGarvey ML, Baron-Van Evercooren A, Kleinman HK, Dubois-Dalcq M. Synthesis and effects of basement membrane components in cultured rat Schwann cells. Dev Biol. 1984;105:18–28. doi: 10.1016/0012-1606(84)90257-4. [DOI] [PubMed] [Google Scholar]
- McKenna MP, Raper JA. Growth cone behavior on gradients of substratum bound laminin. Dev Biol. 1988;130:232–36. doi: 10.1016/0012-1606(88)90429-0. [DOI] [PubMed] [Google Scholar]
- McTigue M, Cremins J, Halegoua S. Nerve growth factor and other agents mediate phosphorylation and activation of tyrosine hydroxylase. J Biol Chem. 1985;260:9047–56. [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–72. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Moonen G, Grau-Wagemans MP, Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982;298:753–55. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- Morel Y, Bristow J, Gitelman SE, Miller WL. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci USA. 1989;86:6582–86. doi: 10.1073/pnas.86.17.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Kelly T, Dai M, Dai H, Chen W-T. Dynamic cytoskeleton-integrin associations induced by cell binding to immobilized fibronectin. J Cell Biol. 1989;109:3455–64. doi: 10.1083/jcb.109.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut MV, Green NM, Eason P, Yamada SS, Yamada KM. Electron microscopy and structural model of human fibronectin receptor. EMBO J. 1988;7:4093–99. doi: 10.1002/j.1460-2075.1988.tb03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen DF. Physical influences on neural crest cell migration in avian embryos: Contact guidance and spatial restriction. Dev Biol. 1989;131:136–48. doi: 10.1016/s0012-1606(89)80045-4. [DOI] [PubMed] [Google Scholar]
- Nitkin RM, Smith MA, Magill C, Fallon JR, Yao Y-MM, Wallace BG, McMahan UJ. Identification of agrin, a synaptic organizing protein from Torpedo election organ. J Cell Biol. 1987;105:2471–78. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkin RM, Wallace BG, Spira ME, Godfrey EW, McMahan UJ. Molecular components of the synaptic basal lamina that direct differentiation of regenerating neuromuscular junctions. Cold Spring Harbor Syrnp Quant Biol. 1983;48:653–65. doi: 10.1101/sqb.1983.048.01.069. [DOI] [PubMed] [Google Scholar]
- Noonan DM, Horigan EA, Ledbetter SR, Vogeli G, Sasaki M, et al. Identification of cDNA clones encoding different domains of the basement membrane heparan sulfate proteoglycan. J Biol Chem. 1988;263:16379–87. [PubMed] [Google Scholar]
- O’Shea KS, Rheinheimer JST, Dixit VM. Deposition and role of throm-bospondin in the histogenesis of the cerebellar cortex. J Cell Biol. 1990;110:1275–84. doi: 10.1083/jcb.110.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea KS, Dixit VM. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988;107:2737–48. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotou G, End P, Aumailley M, Timpl R, Engel J. Domains of laminin with growth-factor activity. Cell. 1989;56:93–101. doi: 10.1016/0092-8674(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Parise LV, Helgerson SL, Steiner B, Nannizzi L, Phillips DR. Synthetic peptides derived from fibrinogen and fibronectin change the conformation of purified platelet glycoprotein IIb-IIIa. J Biol Chem. 1987;262:12597–12602. [PubMed] [Google Scholar]
- Perides G, Lane WS, Andrews D, Dahl D, Bignami A. Isolation and partial characterization of a glial hyaluronate-binding protein. J Biol Chem. 1989;264:5981–87. [PubMed] [Google Scholar]
- Perris R, Johansson S. Inhibition of neural crest cell migration by aggregating chondroitin sulfate proteoglycans is mediated by their hyaluronan-binding region. Dev Biol. 1990;137:1–12. doi: 10.1016/0012-1606(90)90002-z. [DOI] [PubMed] [Google Scholar]
- Perris R, Bronner-Fraser M. Recent advances in defining the role of the extracellular matrix in neural crest development. Comments Dev Neurobiol. 1989;1:61–83. [Google Scholar]
- Pesheva P, Juliano RL, Schachner M. Expression and localization of the fibronectin receptor in the mouse nervous system. J Neurosci Res. 1988;20:420–30. doi: 10.1002/jnr.490200404. [DOI] [PubMed] [Google Scholar]
- Pesheva P, Spiess E, Schachner M. J1-160 and J1-180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol. 1989;109:1765–78. doi: 10.1083/jcb.109.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;303:31–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Plow EF, Ginsberg MH. Cellular adhesion: GPIIb/IIIa as a prototypic adhesion receptor. Prog Hemost Thromb. 1989;9:117. [PubMed] [Google Scholar]
- Prentice HM, Moore SE, Dickson JG, Doherty P, Walsh FS. Nerve growth factor-induced changes in neural cell adhesion molecule (N-CAM) in PC12 cells. EMBO J. 1987;6:1859–63. doi: 10.1002/j.1460-2075.1987.tb02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher M, Ruoslahti E. Identification and isolation of a 140 kD cell surface glycoprotein with properties of a fibronectin receptor. Cell. 1985a;40:191–98. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschacher MD, Ruoslahti E. A 125/115 kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci USA. 1985b;82:5766–70. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher M, Ginsburg M, Plow E, Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: A member of a family of ARG-GLY-ASP specific adhesion receptors. Science. 1986;231:1559–67. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Ratner N, Hong D, Lieberman MA, Bunge RP, Glaser L. The neuronal cell-surface molecule mitogenic for Schwann cells is a heparin-binding protein. Proc Natl Acad Sci USA. 1988;85:6992–96. doi: 10.1073/pnas.85.18.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Radke K. A role for the extracellular matrix in retinal neuro-genesis in vitro. Dev Biol. 1988;129:283–93. doi: 10.1016/0012-1606(88)90375-2. [DOI] [PubMed] [Google Scholar]
- Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- Reist N, Magill C, McMahan UJ. Agrin-like molecules at synaptic sites in normal, denervated, and damaged skeletal muscles. J Cell Biol. 1987;105:2457–69. doi: 10.1083/jcb.105.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin DB, Moscatelli D. Recent development in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989;109:1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggott MJ, Moody SA. Distribution of laminin and fibronectin along peripheral trigeminal axon pathways in the developing chick. J Comp Neurol. 1987;258:580–96. doi: 10.1002/cne.902580408. [DOI] [PubMed] [Google Scholar]
- Ripellino JA, Margolis RY, Margolis RK. Immunoelectron microscopic localization of hyaluronic acid binding region and link protein epitopes in brain. J Cell Biol. 1989;108:1899–1907. doi: 10.1083/jcb.108.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Rao CN, Magnani JL, Spoitalnik SL, Liotta LA, Ginsburg V. Laminin binds specifically to sulfated glycolipids. Proc Natl Acad Sci USA. 1985;82:1306–11. doi: 10.1073/pnas.82.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Edson KJ, Letourneau PC, McLoon SC. Distribution of laminin in the developing peripheral nervous system of the chick. Dev Biol. 1986;113:429–35. doi: 10.1016/0012-1606(86)90177-6. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Letourneau PC, Peterson BA, Furcht LT, McCarthy JB. Selective interaction of peripheral and central nervous system cells with two distinct cell-binding domains of fibronectin. J Cell Biol. 1987;105:1435–42. doi: 10.1083/jcb.105.3.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Letourneau PC, Palm SL, McCarthy J, Furcht LT. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev Biol. 1983;98:212–20. doi: 10.1016/0012-1606(83)90350-0. [DOI] [PubMed] [Google Scholar]
- Rossino P, Gavazzi IF, Timpl R, Aumailley M, Abbadini M, Giancotti F, et al. Nerve growth factor induces increased expression of a laminin-binding integrin in rat pheochromocytoma PC12 cells. Exp Cell Res. 1990;189:100–8. doi: 10.1016/0014-4827(90)90262-9. [DOI] [PubMed] [Google Scholar]
- Runyan RB, Maxwell GD, Shur BD. Evidence for a novel enzymatic mechanism of neural crest cell migration on extracellular glycoconjugate matrices. J Cell Biol. 1986;102:434–41. doi: 10.1083/jcb.102.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–414. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989;264:13369–72. [PubMed] [Google Scholar]
- Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988;107:743–51. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock AW, Matthew WD. An in vitro neurite-promoting antigen functions in axonal regeneration in vivo. Science. 1987;237:1605–8. doi: 10.1126/science.3306923. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Schachner M, Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle. J Cell Biol. 1986;102:420–31. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Chiu AY. The basal lamina of the neuromuscular junction. Cold Spring Harbor Symp Quant Biol. 1983;48:667–78. doi: 10.1101/sqb.1983.048.01.070. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: Isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111:1685–99. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro SA. Inhibition of platelet adhesion to fibronectin, fibrinogen,and von Willebrand factor substrates by complex gangliosides. Blood. 1989;73:484–89. [PubMed] [Google Scholar]
- Saunders S, Jalkanen M, O’Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547–56. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson WJ, Morris CE. Stretch-activated ion channels in growth cones of snail neurons. J Neurosci. 1989;9:2801–8. doi: 10.1523/JNEUROSCI.09-08-02801.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Harpel PC, Nachman RL. Tissue plasminogen activator and urokinase enhance the binding of plasminogen to thrombospondin. J Biol Chem. 1986;261:9959–65. [PubMed] [Google Scholar]
- Slupsky JR, Seehafer JG, Tang S-C, Masellis-Smith A, Shaw ARE. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989;264:12289–93. [PubMed] [Google Scholar]
- Smith JW, Cheresh DA. The Arg-Gly-Asp binding domain of the vitronectin receptor. J Biol Chem. 1988;263:18726–31. [PubMed] [Google Scholar]
- Smith JW, Cheresh DA. Integrin (αvβ3) ligand interaction: Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990;265:2168–72. [PubMed] [Google Scholar]
- Smith SJ. Neuronal cytomechanics: The actin-based motility of growth cones. Science. 1988;242:708–15. doi: 10.1126/science.3055292. [DOI] [PubMed] [Google Scholar]
- Solowska J, Guan J-L, Marcantonio EE, Trevithick JE, Buck CA, Hynes RO. Expression of normal and mutant avian integrin subunits in rodent cells. J Cell Biol. 1989;109:853–61. doi: 10.1083/jcb.109.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the α6 subunit is essential for epithelial polarization in developing kidney tubules. J Biol Chem. 1990;111:1265–73. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring J, Beck H, Chiquet-Ehrismann R. Two contrary functions of tenascin: Dissection of the active sites by recombinant tenascin fragments. Cell. 1989;59:325–34. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Staatz WC, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg+ +-dependent adhesion of platelets to collagen. J Cell Biol. 1989;108:1917–24. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB. Involvement of gangliosides and glycoprotein fibronectin receptors in cellular adhesion to fibronectin. Exp Cell Res. 1988;177:90–102. doi: 10.1016/0014-4827(88)90027-4. [DOI] [PubMed] [Google Scholar]
- Staunton DE, Dustin ML, Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61–64. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- Steindler DA, Cooper NGF, Faissner A, Schachner M. Boundaries defined by adhesion molecules during development of the cerebral cortex: The J1/tenascin glycoprotein in the mouse somatosensory cortical barrel field. Dev Biol. 1989;131:243–60. doi: 10.1016/s0012-1606(89)80056-9. [DOI] [PubMed] [Google Scholar]
- St. John T, Meyer J, Idzerda R, Gallatin WM. Expression of CD44 confers a new adhesive phenotype on transfected cells. Cell. 1990;60:45–52. doi: 10.1016/0092-8674(90)90714-p. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Oldberg A, Hayman EG, Pierschbacher MD, Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985;4:2519–24. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin β4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990;9:757–63. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Huang Z-S, Tanihara H. Cloning of an integrin β subunit exhibiting high homology with integrin β3 subunit. Proc Natl Acad Sci USA. 1990;87:5354–58. doi: 10.1073/pnas.87.14.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Elices MJ, Crouse C, Hemler ME. The primary structure of the α4 subunit of VLA-4: Homology to other integrins and a possible cell-cell adhesion function. EMBO J. 1989;8:1361–68. doi: 10.1002/j.1460-2075.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Hemler ME. The primary structure of the VLA-2/collagen receptor α2 subunit (platelet GPIa): Homology to other integrins and the presence of a possible collagen-binding domain. J Cell Biol. 1989;109:397–407. doi: 10.1083/jcb.109.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, et al. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–82. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Tamura RN, Rozzo C, Starr L, Chambers J, Reichardt LF, et al. Epithelial integrin α6β4: Complete primary structure of α6 and variant forms of β4. J Biol Chem. 1990;111:1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon MM, Lipsky RH, Burgess WH, Jamieson GA. Isolation and characterization of platelet glycoprotein IV (CD36) J Biol Chem. 1989;264:7570–75. [PubMed] [Google Scholar]
- Tapley P, Horwitz A, Buck C, Duggan K, Rohrschneider L. Integrins isolated from Rous sarcoma virus-transformed chicken embryo fibroblasts. Oncogene. 1989;4:325–33. [PubMed] [Google Scholar]
- Tashiro K, Sephel GC, Weeks B, Sasaki M, Martin GR, et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264:16174–82. [PubMed] [Google Scholar]
- Tawil NJ, Houde M, Blacher R, Esch F, Reichardt LF, et al. α1β1, integrin heterodimer functions as a dual laminin/collagen receptor in neural cells. Biochemistry. 1990;29:6540–44. doi: 10.1021/bi00479a028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Reichardt LF, Bixby JL. Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J Cell Biol. 1986;103:2659–72. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: Two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988a;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Tomaselli K, Damsky C, Reichardt L. Purification and characterization of mammalian integrins expressed by a rat neuronal cell line (PC12): Evidence that they function as α/β heterodimeric receptors for collagen IV and laminin. J Cell Biol. 1988b;107:1241–52. doi: 10.1083/jcb.107.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Reichardt LF. Peripheral motoneuron interactions with laminin and Schwann cell-derived neurite-promoting molecules: Developmental regulation of laminin receptor furiction. J Neurosci Res. 1988;21:275–85. doi: 10.1002/jnr.490210220. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, et al. A neuronal cell line (PC12) expresses two β1-class integrins—α1β1 and α3β1—that recognize different neurite outgrowth-promoting domains in laminin. Neuron. 1990;5:651–62. doi: 10.1016/0896-6273(90)90219-6. [DOI] [PubMed] [Google Scholar]
- Toyota B, Carbonetto S, David S. A dual laminin-collagen receptor acts in peripheral nerve regeneration. Proc Natl Acad Sci USA. 1990;87:1319–22. doi: 10.1073/pnas.87.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Yamamoto F, Miura Y, Takio K, Titani K, et al. Characterization through eDNA cloning of galactoprotein b3 (Gap b3), a cell surface membrane glycoprotein showing enhanced expression on oncogenic transformation. J Biol Chem. 1990;265:7016–21. [PubMed] [Google Scholar]
- Tuckett S, Morris-Kay GM. The distribution of fibronectin, laminin and entactin in the neurulating rat embryo studied by indirect immuno-fluorescence. J Embryol Exp Morphol. 1986;94:95–112. [PubMed] [Google Scholar]
- Turner DC, Flier LA, Carbonetto S. Identification of a cell-surface protein involved in PC12 cell-substratum adhesion and neurite growth on laminin and collagen. J Neurosci. 1989;9:3287–96. doi: 10.1523/JNEUROSCI.09-09-03287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuppevelt TH, Languino LR, Gailit JO, Suzuki S, Ruoslahti E. An alternative cytoplasmic domain of the integrin beta 3 subunit. Proc Natl Acad Sci USA. 1989;86:5415–18. doi: 10.1073/pnas.86.14.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall S, Seeds NW. Characterization of 125I-tissue plasminogen activator binding to cerebellar granule neurons. J Cell Biol. 1989;109:265–71. doi: 10.1083/jcb.109.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (αvβ1) J Biol Chem. 1990;265:5934–37. [PubMed] [Google Scholar]
- Wallace BG. Aggregating factor from Torpedo electric organ induces patches containing acetylcholine receptors, acetylcholinesterase, and butyrylcholinesterase on cultured myotubes. J Cell Biol. 1986;102:783–94. doi: 10.1083/jcb.102.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BG. Regulation of agrin-induced acetylcholine receptor aggregation by Ca+ + and phorbol ester. J Cell Biol. 1988;107:267–78. doi: 10.1083/jcb.107.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner EA, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique α and common β subunits. J Cell Biol. 1987;105:1873–84. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner EA, Garcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternativecell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989;109:1321–30. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle B, Chiquet M. Tenascin is accumulated along developing peripheral nerves and allows neurite outgrowth in vitro. Development. 1990;110:401–15. doi: 10.1242/dev.110.2.401. [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–89. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H-J, Shaw LM, Messier JM, Mercurio AM. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2) J Biol Chem. 1990;265:7097–99. [PubMed] [Google Scholar]
- Wright SD, Reddy PA, Jong MTC, Erickson BW. C3bi receptor (complement receptor type 3) recognizes a region of complement protein C3 containing the sequence Arg-Gly-Asp. Proc Natl Acad Sci USA. 1987;84:1965–68. doi: 10.1073/pnas.84.7.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba S, Guimaraes A, Kalb RG, Hockfield S. Characterization of an activity-dependent, neuronal surface proteoglycan identified with monoclonal antibody Cat-301. Neuron. 1989;2:1207–19. doi: 10.1016/0896-6273(89)90305-x. [DOI] [PubMed] [Google Scholar]
- Zimmerman DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan versican. EMBO J. 1989;8:2975–81. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


