Summary
To understand mechanisms resulting in the absence of two-thirds of spinal sensory neurons in mice lacking NT-3, we have compared dorsal root ganglia development in normal and mutant embryos. The reduction in neurons, achieved by E13, results from several deficits: first, elevated neuronal apoptosis significantly reduces neuronal numbers; second, elevated neurogenesis between E11 and E12, without changes in rates of precursor proliferation or apoptosis, depletes the precursor pool; consequently, the reduced precursor pool prevents increases in neuronal numbers between E12 and E13, when most neurons are born in normal animals. Although deficits occur before final target innervation, we show that NT-3 is expressed at all stages in regions accessible to these neurons or their axons and is only restricted to final targets after innervation.
Introduction
During development of the nervous system, the final size of a neuronal population is largely determined by the number of neurons generated from their embryonic precursors and those neurons subsequently lost owing to cell death. Little is known about the regulation of the process of neurogenesis including the mechanisms that determine the size of precursor and neuronal populations as well as their temporal relationships. However, much more is known about the regulation of neuronal number by cell loss. This knowledge comes largely from the identification of molecules such as the neurotrophins, a family of structurally and functionally related proteins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), NT-4/5 and NT-6, capable of promoting the survival of specific neuronal populations through their interaction with tyrosine kinase receptors named trkA, trkB, and trkC (see Lewin and Barde, 1996). The observation that different neurotrophins promote in vitro the survival of specific populations of postmitotic neurons has recently been supported in vivo by the phenotype of mice carrying targeted mutations in any of the genes coding for neurotrophins or their receptors (reviewed by Fariñas and Reichardt, 1996). In particular, dorsal root ganglia (DRG), which are comprised of several subpopulations of primary sensory neurons distinguished by their physiological properties, connections, and transmitter content (Scott, 1992), represent a very valuable model for the analysis of the neurotrophin requirements of selective neuronal populations. In neonatal mice lacking NGF (Crowley et al., 1994) or its receptor trkA (Smeyne et al., 1994; Silos-Santiago et al., 1995; Minichiello et al., 1995), all DRG nociceptive neurons, which mediate pain perception, are lost. Additionally, NT-3/trkC signaling is required for the survival of all proprioceptive neurons, which convey information about the degree of muscle stretch and tension (Ernfors et al., 1994; Fariñas et al., 1994; Klein et al., 1994; Tessarollo et al., 1994; Tojo et al., 1995; Kucera et al., 1995). NT-3 seems to be also necessary in vivo for the survival of low threshold cutaneous mechanoreceptors innervating hair follicles and Merkel cells (Airaksinen et al., 1996). However, it is still unclear how these deficits are achieved during development.
The most accepted model postulates that targets for developing neurons produce limiting amounts of these survival molecules such that, following target innervation, only those neurons successful in obtaining the factors survive (see Barde, 1989). However, recent evidence from in vitro studies indicates that neurotrophins, and NT-3 in particular, could also act at earlier stages, to regulate events that precede target encounter in the PNS. For instance, NT-3 has been shown to promote survival of trigeminal neurons prior to target innervation (Buchman and Davies, 1993) and to accelerate differentiation of newly born spinal sensory neurons (Wright et al., 1992). Moreover, this neurotrophin seems capable of inducing survival, differentiation, and/or proliferation of neural precursor populations (Kalcheim et al., 1992; Pinco et al., 1993; DiCicco-Bloom et al., 1993; Verdi and Anderson, 1994; Chalazonitis et al., 1994; Henion et al., 1995; Karavanov et al., 1995; Memberg and Hall, 1995). This is in agreement with the widespread distribution of NT-3, and its major receptor trkC, during early embryogenesis (e.g., Schecterson and Bothwell, 1992; Tessarollo et al., 1993; Lamballe et al., 1994; White et al., 1996). Moreover, injection of antibodies to either NT-3 (Gaese et al., 1994) or trkC (Lefcort et al., 1996) into chick embryos very early in development, before target innervation takes place, resulted in a cell reduction in DRGs of ∼40%. These results indicate that NT-3 is likely to be playing a role in events occurring prior to target innervation also in vivo.
Because of these potential activities of NT-3 during embryonic development, it seems interesting to carefully analyze the developing PNS in animals lacking NT-3 and accurately determine how the deficits in the final number of neurons are produced. Previous studies of mouse embryos deficient in NT-3 have indicated that the deficits in the number of neurons are produced before embryonic day E13 (Tessarollo et al., 1994; Kucera et al., 1995; ElShamy and Ernfors, 1996). In the present study, we have identified the steps in DRG development in which NT-3 is involved by independently analyzing the dynamics of the population of precursor cells and the neuronal population. We have uncovered two different effects of the absence of NT-3. The first effect is reduced survival of postmitotic neurons, and the second is premature differentiation of precursor cells toward the neuronal phenotype resulting in neurogenesis and, hence, early depletion of the pool of precusor cells. These two effects, death and reduced generation of neurons from a diminished progenitor population, lead to the final deficit found at E13, before most sensory neurons actually reach their targets. At times when the deficits are being produced, we find that expression of NT-3, as assessed by the expression of a lacZ reporter gene present in the targeting construct, is very high around the DRGs and the growing sensory axons.
Results
Lack of NT-3 Causes Loss of Neurons in Early Stages of DRG Development
To determine mechanisms underlying the dramatic deficiency in spinal sensory neurons observed at birth in NT-3-deficient animals, we carefully characterized the prenatal development of DRGs in both normal and mutant animals. In the present study, we have determined the total number of cells, number of neurons, and number of precursors in several DRGs. Because there is a rostro-caudal gradient of development (see for instance Lawson and Biscoe, 1979), we have analyzed the cellular dynamics of four independent DRGs along the anterior-posterior axis: thoracic 1 (T1), thoracic 6 (T6), lumbar 1 (L1), and lumbar 4 (L4). The overall dynamics were similar in the different ganglia, but in the thoracic ganglia, which are located more rostrally, some aspects developed more rapidly. Since precursor cells can not be distinguished from neurons before embryonic day (E)15 using conventional stainings, we have considered the number of neurons between E10 and E15 to be the number of cells immunoreactive for the 150 KDa neurofilament subunit (Figures 1D, 1I, and 1J), a marker that distinguishes early postmitotic neurons from neuronal precursors (Cochard and Paulin, 1984). From E15 to birth, neurons were identified by their morphology and counted in Nissl stained sections. To determine the number of precursor cells, we counted the total number of cells present in the different ganglia from E10 to E13 in Nissl stained sections (Table 2) and subtracted the corresponding number of neurofilament positive cells. To provide independent evidence that all cells not expressing neurofilament are indeed precursor cells, we injected pregnant wild-type females bearing E11 embryos with repeated pulses (five injections over 12 hr) of the nucleotide analogue 2′-bromo-5′-deoxyuridine (BrdU) and determined the complete fraction of proliferating cells (Nowakowski et al., 1989). The number determined by this procedure in the T1 ganglion (4196 ± 228) is not significantly different from the calculated number of neurofilament-negative cells (3714 ± 300). This result suggests that all precursors found in early developing ganglia are cycling and indicates that our procedure to calculate the number of precursor cells is accurate.
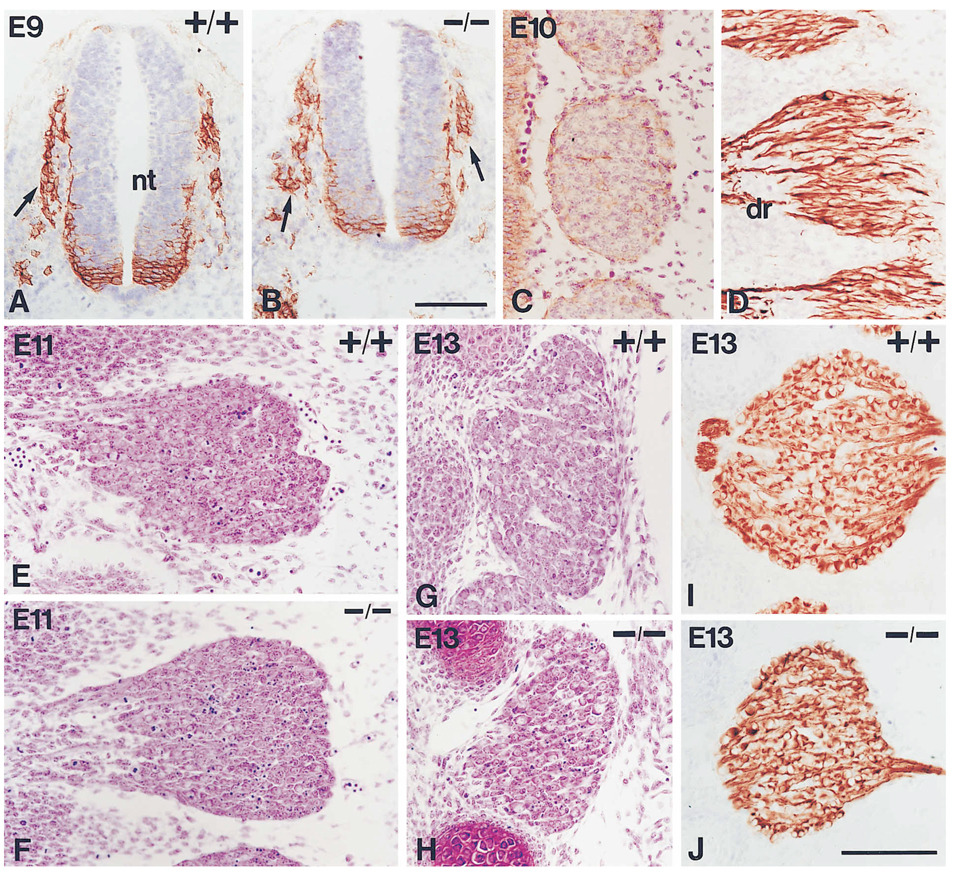
Figure 1.
Analysis and Phenotype of Early DRG Development in Wild-Type and NT-3 Mutant Mice
(A and B) Transverse sections through a wild-type ([A], +/+) and a mutant ([B], −/−) embryo at embryonic day (E) 9 stained with antibodies to the low affinity neurotrophin receptor p75NTR to label neural crest cells (arrows) migrating out of the neural tube (nt). Neural crest migration is apparently normal in embryos deficient for NT-3 (cells are also labeled within the ventral neural tube).
(C and D) Thoracic DRGs as seen in sagittal sections through E10 embryos stained either with p75NTR antibodies and counterstained with cresyl violet (C) or with antibodies to the 150 KDa neurofilament subunit (D). Neurofilament antibodies label the cell bodies and the projections of neurons present in the ganglia. dr, dorsal root. At this stage there is no difference between wild-type and mutant ganglia, and only mutants are shown. Note that neurons (neurofilament-positive cells) can not be distinguished from other cells in Nissl stained material.
(E and F) L1 DRGs from wild-type (+/+) and mutant (−/−) embryos at E11 stained with cresyl violet. Note that the mutant ganglion is not significantly reduced in size but shows more pyknotic figures.
(G and H) Nissl stained L1 DRGs from wild-type (+/+) and mutant (−/−) embryos at E13. The mutant ganglion shows a clear reduction in volume and more pyknotic figures.
(I and J) L1 DRGs from wild-type (+/+) and mutant (−/−) embryos at E13 stained with anti-neurofilament antibodies, showing the same reduction in size for the mutant as in (G) and (H). Bars, 100 µm (A and B); 100 µm (C–J).
Table 2.
Total Numbers of Cells in the DRGs of Normal and Mutant Mice during Sensory Gangliogenesis
| Mice | E10 | E11 | E12 | E13 | |
|---|---|---|---|---|---|
| T1 | +/+ | 2728 ± 420 (2) | 5383 ± 148 (4) | 5217 ± 304 (5) | 6478 ± 350 (5) |
| −/− | 2480 ± 232 (2)n | 3100 ± 260 (2)b | 3160 ± 539 (5)a | 3193 ± 161 (5)c | |
| T6 | +/+ | 1444 ± 152 (2) | 4502 ± 631 (3) | 4735 ± 282 (6) | 5364 ± 264 (4) |
| −/− | 1616 ± 84 (2)n | 2666 ± 245 (3)a | 3206 ± 307 (4)b | 2962 ± 306 (3)b | |
| L1 | +/+ | NG | 5384 ± 421 (4) | 6865 ± 139 (5) | 8472 ± 487 (4) |
| −/− | NG | 4776 ± 456 (3)n | 5228 ± 186 (4)c | 4768 ± 356 (5)c | |
| L4 | +/+ | NG | 6538 ± 138 (2) | 8292 ± 359 (4) | 11357 ± 722 (5) |
| −/− | NG | 5828 ± 385 (3)n | 5556 ± 494 (4)b | 5853 ± 692 (2)b | |
The numbers of cells in DRGs in wild-type (+/+) and mutant (−/−) E10 to E13 embryos were determined by counting all cells in Nissl stained sections through four different ganglia: thoracic 1 (T1), thoracic6 (T6), lumbar 1 (L1), and lumbar 4 (L4). Numbers are represented as mean ± SEM. The number of individual embryos analyzed in each case is indicated in parentheses. NG, not discernible ganglia. Differences in cell numbers in mutant vs. wild-type ganglia were tested using a one-way Student’s t-test.
p < 0.05,
p < 0.005,
p < 0.001,
not significant. Compared with wild-type values, the relative numbers of cells in ganglia of mutant embryos progressively decrease, up to 40%–50%.
As illustrated in Figures 1A and 1B, migrating neural crest cells of the trunk, some of which give rise to the DRGs, are clearly detectable at E9, before any spinal ganglion is discernible. Qualitative inspection of sections through E9 embryos stained with antibodies to the low affinity neurotrophin receptor p75NTR (Weskamp and Reichardt, 1991), a marker for migratory crest cells, revealed no apparent abnormalities in NT-3 mutant animals, even though murine neural crest cells express the NT-3 receptor trkC (Tessarollo et al., 1993). The thoracic ganglia were easily visible at E10 and accurate determinations of the number of total cells (Figure 1C) and neurons (Figure 1D) could be made (see Table 1; see Table 2). The counts at this age revealed no differences between wild-type and mutant animals either in the number of neurons or in the total number of cells, further indicating that previous stages, such as migration and coalescence, are not affected in the NT-3-deficient embryos. Consequently, we conclude that early steps in gangliogenesis proceed normally in the absence of NT-3.
Table 1.
Number of Neurons in DRGs of Normal and Mutant Mice during Embryonic Development
| Mice | E10 | E11 | E12 | E13 | E15 | E17 | P0 | |
|---|---|---|---|---|---|---|---|---|
| T1 | +/+ | 720 ± 252 (2) | 1669 ± 174 (5) | 2123 ± 198 (4) | 4599 ± 219 (3) | 4729 ± 140 (7) | 4913 ± 155 (4) | 5583 ± 167 (4) |
| −/− | 638 ± 140 (3)n | 921 ± 114 (4)b | 1864 ± 205 (4)n | 2152 ± 58 (4)c | 2004 ± 113 (9)c | 1825 ± 269 (4)c | 1588 ± 48 (2)c | |
| T6 | +/+ | 298 ± 2 (2) | 1385 ± 113 (3) | 1680 ± 62 (5) | 3373 ± 98 (3) | 2988 ± 178 (4) | ND | ND |
| −/− | 320 ± 56 (2)n | 723 ± 25 (3)b | 1986 ± 113 (4)n | 2066 ± 141 (4)c | 1769 ± 130 (4)b | ND | ND | |
| L1 | +/+ | NG | 2109 ± 150 (3) | 2506 ± 299 (5) | 4808 ± 192 (3) | 5444 ± 264 (8) | 5595 ± 490 (6) | 5189 ± 286 (3) |
| −/− | NG | 1378 ± 154 (2)a | 2309 ± 256 (3)n | 2277 ± 184 (4)c | 2241 ± 134 (9)c | 2449 ± 182 (5)c | 2236 ± 257 (3)b | |
| L4 | +/+ | NG | 2592 ± 24 (2) | 2816 ± 449 (5) | 6830 ± 343 (3) | 6332 ± 607 (3) | ND | 5745 ± 530 (3) |
| −/− | NG | 1676 ± 44 (2)b | 2883 ± 415 (3)n | 2691 ± 264 (4)b | 1848 ± 325 (3)b | ND | 1530 ± 378 (2)a | |
Numbers of neurons were counted in four different DRGs: thoracic 1 (T1), thoracic 6 (T6), lumbar 1 (L1), and lumbar 4 (L4) of wild-type (+/+) and mutant (−/−) mice at the indicated embryonic (E) or postnatal (P) stages. Between E10 and E13, the number of neurons was calculated by counting the number of neurofilament-positive cells present in each ganglion. After E15, neurons were quantitated in Nissl stained sections. At E15, the number of DRG neurons was obtained in both Nissl stained embryos and embryos stained for neurofilament. Because both determinations yielded identical numbers, they have been pooled together in the table. Numbers are expressed as mean ± SEM. The numbers in parenthesis indicate the number of individual embryos analyzed in each case. Abbreviations, ND, not determined; NG, no discernible ganglia. Differences between the two genotypes were tested using a one-way Student’s t-test.
p < 0.01,
p < 0.005,
p < 0.001,
not significant. Notice that there are significantly fewer neurons at E11 in each ganglion in mutant vs. wild-type animals, but there are no statistically significant differences at E12.
After coalescence in normal embryos, DRG neuron numbers increase progressively until E13, when the final number of neurons present at birth is reached (Figure 2A; Table 1). Thus, developmental processes taking place prior to E13 in normal mice are the primary determinants of the final size of the spinal ganglia. In the NT-3 mutant embryos, the final deficit in neuronal number found at birth develops between E10 and E13 (Tables 1 and 2; see also, Figures 1E–1J and 2A) and follows a rather complex pattern, indicating that NT-3 is involved in more than one aspect of the development of these ganglia. First, at E11 there is a significant loss in the number of neurons in mutant embryos (Figure 2 and Table 1). In the lumbar area, where DRGs develop more slowly, it is clear that this reduction happens in absence of any effects on the precursor population (Figure 2B) and, therefore, it is likely to represent a specific defect on the neurofilament-positive cells. At E12, however, mutant embryos have at least as many neurons as wild-type embryos, but fewer precursor cells (Figure 2B), suggesting that neurogenesis occurs at an abnormally high rate in mutant embryos during the E11–12 interval. Finally, between E12 and E13, there is a remarkable increase in the number of neurons in the ganglia of wild-type but not mutant embryos. This difference is large enough to account for the total deficit finally observed in mutant animals.
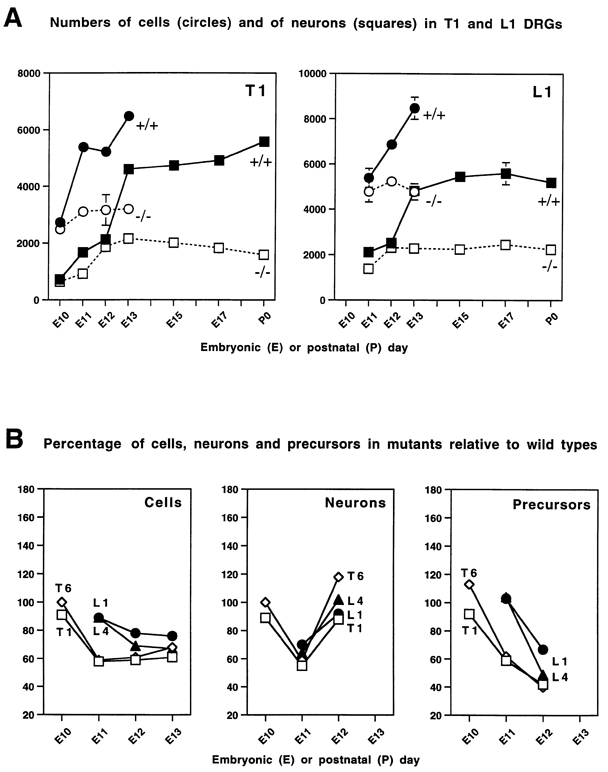
Figure 2.
Graphical Representations of Numbers of Cells, Neurons, and Precursors in Normal and Mutant DRGs
(A) Quantitation of the number of total cells and neurons at different embryonic (E) and postnatal (P) stages in thoracic 1 (T1) and lumbar 1 (L1) DRGs in wild types and NT-3 mutants. Total number of cells (circles) and of neurons (squares) in wild-type (filled symbols, continuous line) and mutant (empty symbols, dotted line) ganglia. Numerical data used to construct these graphs is presented in Tables 1 and 2. Since the normal development and phenotype of the mutation are similar in all ganglia analyzed, the data for only two of the four ganglia are presented graphically here. At E10 there are no differences between wild-type and mutant embryos in the number of cells or neurons, as seen in the T1 DRG (lumbar ganglia are not well defined at this stage). At E13, the final number of neurons in normal embryos and the complete deficit found in mutant embryos at birth are achieved in both T1 and L1 DRGs. Note that the number of neurons is significantly reduced in mutant ganglia at E11 (see Table 1 for statistical significance), but that between E11 and E12 higher than normal neurogenesis in the mutant eliminates the deficits in total neuronal numbers by E12. Between E12 and E13, the number of neurons increases very rapidly in wild-type but not in mutant animals. (B) Relative percentages of cells, neurons, and precursors (total number of cells minus number of neurofilament positive cells) present in mutant compared with wild-type ganglia from E10 to E13. Data from the four different ganglia analyzed are plotted: T1 (squares), T6 (diamonds), L1 (circles), and L4 (triangles). There is an initial significant (see Table 1) deficit in the number of neurons in all ganglia at E11 that is compensated at E12. In lumbar ganglia, a deficit in neurons is not accompanied by a deficit in precursors. Notice also that the increased numbers of neurons appearing in mutant ganglia between E11 and E12 is accompanied by equally dramatic reductions in the numbers of precursors, suggesting that neurogenesis may be accelerated in the absence of NT-3.
As illustrated in Table 1 and Figure 2, during the E10–11 interval many more neurons accumulate in wild-type than mutant DRGs. To investigate whether increased apoptosis could explain the reduced numbers of neurons present in ganglia of mutant embryos, we quantitated the number of pyknotic profiles seen in our sections (Figures 1E–1H and Figures 3C and 3D). Figure 3G shows the proportion of dying cells in ganglia of wild-type and mutant E11 embryos relative to the total number of cells present in these DRGs. In the NT-3 mutants, there is a significant increase in the number of dying cells (between 2.8- and 7-fold for the different ganglia).To confirm this, we applied the TUNEL (terminal deoxynucleotide transferase [TdT]-mediated dUTP nick end labeling) technique to E11 embryos (Figures 3E and 3F) to visualize all cells undergoing DNA fragmentation (Gavrieli et al., 1992). We counted all labeled cells in individual sections of either thoracic or lumbar ganglia and divided each number by the total number of cells found in the same sections. The percentages (mean ± SEM) of TUNEL-positive cells found per section were as follows: (+/+) 4.2 ± 1.5 and (−/−) 26.6 ± 5.1 for thoracic and (+/+) 5.4 ± 0.9 and (−/−) 34.2 ± 8.8 for lumbar ganglia. Consequently, each method of analysis indicates that NT-3 deficiency increases the rate of apoptosis in E11 embryos. This suggests that the neuron-specific deficits found in mutant DRGs at this stage are due to cell death. In agreement with this, we could also observe colocalization of neurofilament immunoreactivity and TUNEL labeling in the same cells in E11 mutant ganglia (Figure 4A). However, quantitation is not possible when the TUNEL technique is combined with immunocytochemistry for cytoplasmic antigens: a negative result in this kind of approach is very difficult to interpret because the combination relies heavily on the integrity of the cytoplasm in cells that are actually undergoing degeneration. Results indicating that precursors are not a major cell type undergoing apoptosis will be presented below. Cell death was also significantly elevated in the DRGs of mutant embryos at E12 and E13 (Figure 3H), indicating that neuronal death also occurs after E11.
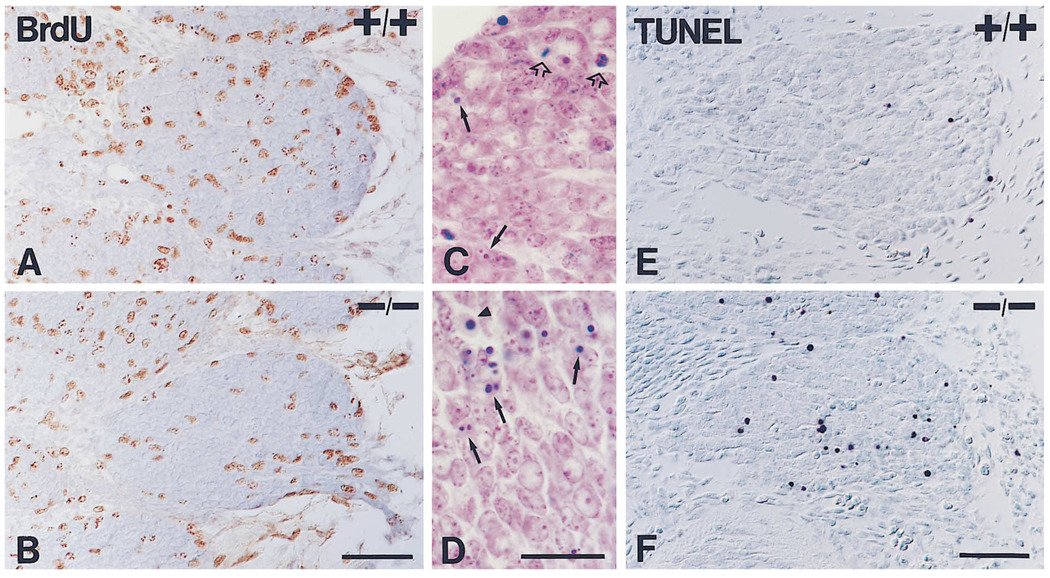
Figure 3.
Proliferation and Cell Death in Wild-Type (+/+) and Mutant (−/−) DRG
(A–F) Illustrations of assays of proliferation and apoptosis in T1 DRGs of E11 embryos.
(A and B) Immunodetection of BrdU after a 2 hr pulse in E11 wild-type (A) and mutant (B) embryos. Notice that BrdU-positive nuclei are not pyknotic.
(C and D) High magnification micrographs of Nissl stained sections through ganglia of E11 wild-type (C) and mutant (D) embryos. At this stage, pyknotic figures (arrows) and mitotic figures (empty arrows) can be observed. Notice that pyknotic profiles are more frequent in the mutant ganglion. Care was taken to exclude red blood cells (arrowhead) from the quantitation.
(E and F) Sections of ganglia of E11 wild-type (E) and mutant (F) embryos stained with the TUNEL method for the detection of cells undergoing apoptosis. In agreement with an increase in pyknotic figures, the mutant ganglion shows more cells stained by this method. Bars, 100 µm (A and B); 50 µm (C and D); 100 µm (E and F).
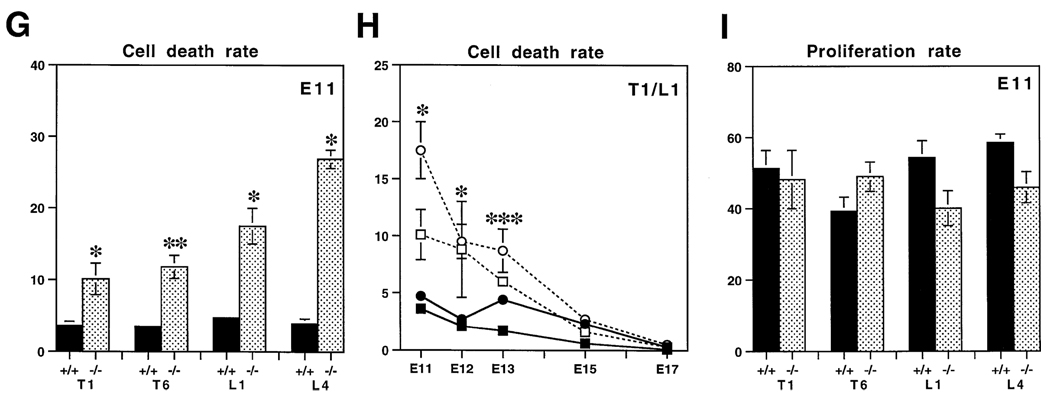
(G–I) Quantification of apoptosis and proliferation in wild-type and mutant DRGs.
(G) Quantitation of the rate of cell death in wild-type (+/+, solid bars) and mutant (−/−, shaded bars) ganglia at stage E11 for thoracic 1 (T1), thoracic 6 (T6), lumbar 1 (L1), and lumbar 4 (L4) DRGs. Dying cells were calculated by counting all pyknotic figures in each of the different ganglia. The numbers are expressed as the relative number of dying cells per total number of cells present in each ganglion. The number of embryos analyzed in each case are the same as in Table 2.
(H) Cell death rates between E11 and E17 in wild-type (filled symbols, continuous line) and mutant (unfilled symbols, dotted line) embryos, obtained by dividing the number of pyknotic figures either by the total number of cells (E11-E13) or by the total number of neurons (E15-E17) present in the ganglia (see Tables 1 and 2). At E10 there are no pyknotic figures. Represented are the values for T1 (squares) and L1 (circles) DRGs as examples. Very similar data was obtained for T6 and L4 DRGs, but are not presented for simplicity. Notice that cell death is significantly elevated in the mutant ganglia at all stages between E11 and E13. At E15 and E17, cell death in the ganglia of both wild-type and mutant animals is very low and is not elevated significantly in mutant ganglia.
(I) Quantitation of the rate of BrdU incorporation in wild-type (+/+, solid bars) and mutant (−/−, shaded bars) ganglia at E11. The rate of BrdU incorporation or labeling index was calculated by dividing the number of BrdU-positive nuclei in each ganglion by the calculated number of precursors (see also Table 3). No significant differences in labeling index were found comparing wild-type and mutant ganglia. In each case, values are represented as mean ± SEM. Statistical significance was tested using a one-tailed Student’s t-test: * p < 0.05, ** p < 0.01, *** p < 0.001.
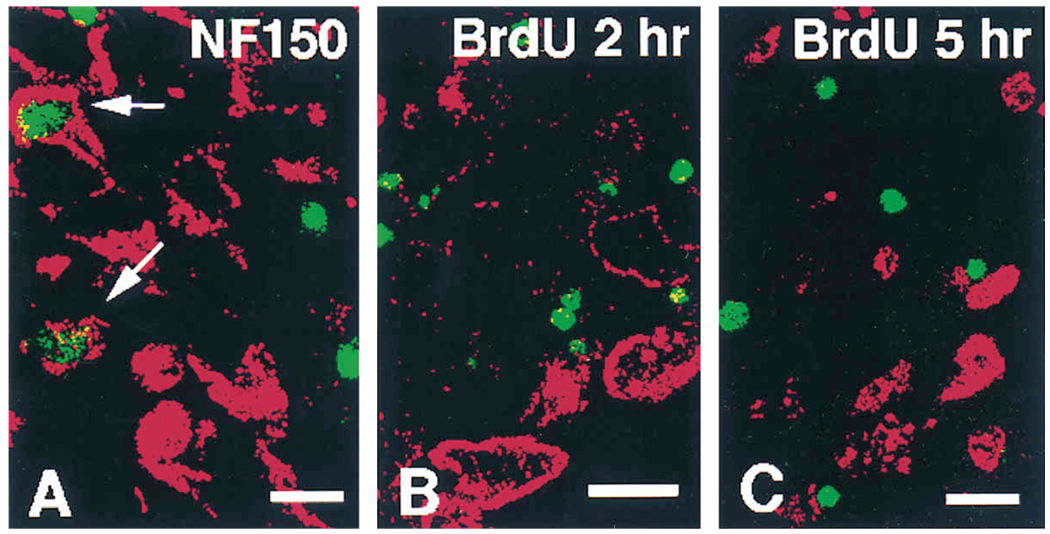
Figure 4.
Attempts to Detect Apoptosis in Neurons and Neuronal Precursors
(A) Double immunofluorescence labeling for TUNEL (green) and neurofilament (NF150, red) in a thoracic ganglion of an E11 mutant embryo. In this figure, there are two immunopositive cells for neurofilament in the field whose nuclei are labeled by the apoptosis detection method (arrows). This suggests that neurons die at this stage.
(B and C) Double labeling for TUNEL (green) and BrdU (red) after either 2 or 5 hr injection pulse of BrdU at E11 in thoracic ganglia, respectively. Colocalization was never observed, indicating that precursors are not dying in the mutant animals, but instead are lost through more rapid differentiation into cells with a neuronal phenotype. Bars, 10 µm (A and C); 10 µm (B).
Lack of NT-3 Accelerates Neurogenesis at E11–E12
Changes in the dynamics of precursor populations may result in profound quantitative alterations in resultant neuronal populations. Therefore, we evaluated the size and activity of the precursor population in the mutant ganglia with particular attention to changes found between E10 and E12. By E13, the deficit in the number of neurons is already complete and gliogenesis is at its peak (Lawson and Biscoe, 1979), suggesting that any estimation of the number of precursors at this stage would probably include significant numbers of actively dividing glial progenitors. Estimations of the number of precursors in the ganglia of mutants relative to wild-type embryos at E12 (Figure 2B) show that this population is significantly reduced in all mutant ganglia, indicating that precursor cells are depleted in the absence of NT-3. This decrease is concomitant with the increase in the number of neurofilament-positive cells observed at E12. One likely explanation for these findings is that progenitor cells deprived of NT-3 are abandoning the cell cycle and differentiating into neurons. However, the precursor cell deficit in NT-3 mutant embryos could also reflect changes in proliferation or survival of these cells and, thus, we decided to study whether any of these parameters were affected by the mutation.
To study proliferation, we first established the parameters of the cell cycle for the DRG precursor population in normal embryos. For these studies, pregnant females at 11 days of gestation received cumulative injections of BrdU every 3 hr during a 12 hr period, and calculations of cell cycle parameters were done following the method described by Nowakoski et al. (1989). We found that dividing cells in normal DRGs (calculations done in C1 and T1 DRGs) at E11 have a cell cycle of 11.5 ± 2.8 hr with an S-phase that lasts 3.1 ± 0.4 hr (data not shown). Next, we study the proliferative activity of the precursor cells in mutant and wild-type embryos, by injecting heterozygous pregnant females at 11 days of gestation with 50 mg of BrdU per kg of body weight, 2 hr before sacrifice. According to the analysis of the cell cycle parameters, this labeling window and concentration, standard in this kind of experiment, labels ∼50% of the DRG precursor population (see also Figure 3I). We concentrated our proliferation analysis on E11 embryos because: 1) at this stage reduced numbers of precursors are seen in thoracic, but not lumbar ganglia in mutant embryos (Figure 2B), permitting comparative studies on mitotic activity in ganglia with normal and reduced precursor populations; 2) at E11, only sensory precursors are found in the ganglion; gliogenesis starts after this stage and peaks at E13 (Lawson and Biscoe, 1979). Results are illustrated in Figures 3A and 3B and quantified in Table 3. In thoracic ganglia of NT-3 mutants, there is a reduction in the total number of BrdU-positive cells, approximately proportional to the decrease in the total number of precursors. In lumbar DRGs, where normal numbers of precursors are present, approximately normal numbers of BrdU-positive cells are also seen in mutant embryos. To determine whether the proliferative activity of the precursors was affected by the mutation, we calculated the labeling index or rate of BrdU incorporation by dividing the absolute counts by the calculated number of precursors present in each ganglion. As illustrated in Figure 3I, no significant differences were found between normal and NT-3-deficient embryos. We also determined the rate of proliferation at E13, during the gliogenesis peak, and also failed to detect consistent differences (data not shown). We conclude from these data that the rate of proliferation is apparently normal in these ganglia in the absence of NT-3.
Table 3.
Proliferative Activity in the DRGs of Normal and Mutant Embryos at E11
| Mice | Measurement | T1 | T6 | L1 | L4 |
|---|---|---|---|---|---|
| +/+ | Total # LI. | 1902 ± 192 (3) | 1281 ± 132 (3) | 1788 ± 152(3) | 2310 ± 94(2) |
| 51.3 ± 5.1 | 39.2 ± 4.1 | 54.4 ± 4.7 | 58.5 ± 3.4 | ||
| −/− | Total # LI. | 1050 ± 178(2)a | 953 ± 81 (3)a | 1368 ± 162 (2)n | 1909 ± 184(3)n |
| 48.2 ± 8.2n | 49.0 ± 4.1n | 40.1 ± 4.9n | 45.9 ± 4.4n |
After a 2 hr pulse injection at E11, the total number (total #) and percentage (labeling index, LI.) of BrdU-positive nuclei were determined in four different DRGs: thoracic 1 (T1), thoracic 6 (T6), lumbar 1 (L1), and lumber 4 (L4) of wild-type (+/+) and mutant (−/−) mice. All immunoreactive nuclei were counted in every fourth section through each ganglion to calculate the total numbers. Numbers are represented as mean ± SEM. To calculate the labeling index, total counts were divided by the number of precursors present in each ganglion. This index is expressed as the number of labeled cells per 100 precursors (mean ± SEM). In parentheses are the numbers of individual embryos analyzed in each case.
p < 0.05,
not significant, one-way Student’s t-test. The conclusion is that the labeling index is not reduced in mutant embryos either in thoracic ganglia, where the number of precursors and, hence, of BrdU incorporating cells, is reduced or in lumbar ganglia, where no deficits in the precursor population are apparent at this stage.
To determine directly whether absence of NT-3 compromises the survival of DRG precursor cells, we combined TUNEL staining with BrdU labeling. This combination does not have the limitations of a combination of TUNEL labeling with cytoplasmic markers detection, since both BrdU and TUNEL labels are aimed at the DNA. Two sets of BrdU injections were performed in E11 embryos, either 2 hr or 5 hr before sacrifice, which, according to our previously described calculations of cell cycle dynamics, should label ∼50% or 80%–90% of the precursor cells, respectively. Figures 4B and 4C show the combination of the TUNEL staining (green) with the detection of the injected BrdU (red) after either 2 hr or 5 hr in mutant embryos. A total of ∼300 BrdU-and 140 TUNEL-positive cells were analyzed. No colocalization of the two labels was ever observed, either in thoracic or lumbar ganglia. The same results were found in normal embryos (data not shown). Moreover, BrdU immunopositive pyknotic figures were not seen in our proliferation counts, which scored around 6,500 cells in wild-type specimens and ∼4,700 cells in mutants (Figures 3A and 3B; Figures 4B and 4C). Together, these observations indicate that precursor cells are not dying in the absence of NT-3.
As described above (see Figure 2), during the E11–E12 interval of development, there is a dramatic increase in the accumulation of neurons in the ganglia of NT-3 mutant compared with wild-type embryos. Over the same interval, the precursor pool is greatly depleted in the NT-3 mutants. The data demonstrate that the proliferation rate and survival of the precursor cells are not altered in the ganglia of mutant embryos, suggesting that an accelerated transformation of precursors into neurons occurs instead in the absence of NT-3. This results in an increase in the number of neurons in the ganglia of mutant embryos at E12, but also in a depletion of the precursor pool. It follows that this depletion is a major reason for the failure of mutant ganglia to accumulate neurons between E12 and E13, the period during which more than half of the final complement of neurons is generated in wild-type embryos. However, apoptosis remains ∼2-fold higher in mutant versus wild-type ganglia during this interval (Figure 3H) and also undoubtedly contributes to the complete absence of neuronal accumulation in mutant animals.
NT-3 Expression Is Consistent with the Deficits Found in Mutant DRGs
Results described above predict that NT-3 is expressed in the vicinity of developing DRGs or sensory neuron growing axons during the critical interval of development between E10 and E13. The presence of a lacZ gene in the targeting construct used to generate the NT-3 mutation has enabled us to determine the pattern of lacZ expression as a reporter for the endogenous expression of NT-3 (see also Tojo et al., 1996). Histochemical staining of whole embryos provides useful spatial information that can be related to the development of whole structures. In parallel, the staining in sections allows for a better cellular resolution than that achieved by in situ hybridization. In all instances where NT-3 expression has been previously analyzed by in situ hybridization (e.g., Ernfors and Persson, 1991; Ernfors et al., 1992; Schecterson and Bothwell, 1992; White et al., 1996), the overall expression pattern revealed by the histochemical reaction is in complete agreement, suggesting that the transcriptional regulation of the reporter gene follows that of the endogenous gene. In addition, we have observed that the pattern of expression is identical in both homozygous and heterozygous NT-3 mutant mice (data not shown), indicating that NT-3 is not required for its own expression.
We concentrated our analysis of the expression pattern in those stages and structures most relevant to the early development of the DRGs. At E9, when the majority of neural crest cells are still migrating, most of the expression is found in the head, particularly the first branchial arch and the mesencephalic vesicle, with no detectable expression in the trunk region (data not shown). Starting at E10, intense expression is visible both in the anterior part of the trunk and in the head. Expression in the head is found in the branchial arches, midbrain, otic vesicle, and developing eye. In the trunk, the blue product of the histochemical reaction is detected along the sides of the embryo, close to the nascent DRGs. Additional staining is seen in the most proximal third of the forelimb and along the dorsal aorta (Figure 5A). A similar pattern is observed at E11, but the expression is more intense and extends more caudally (Figure 5B). Dorsally, two longitudinal stripes of staining, corresponding to the lateral motor columns, are seen in the spinal cord, with a peak of expression in the rostro-caudal axis at the level of the limbs (Figure 5C).
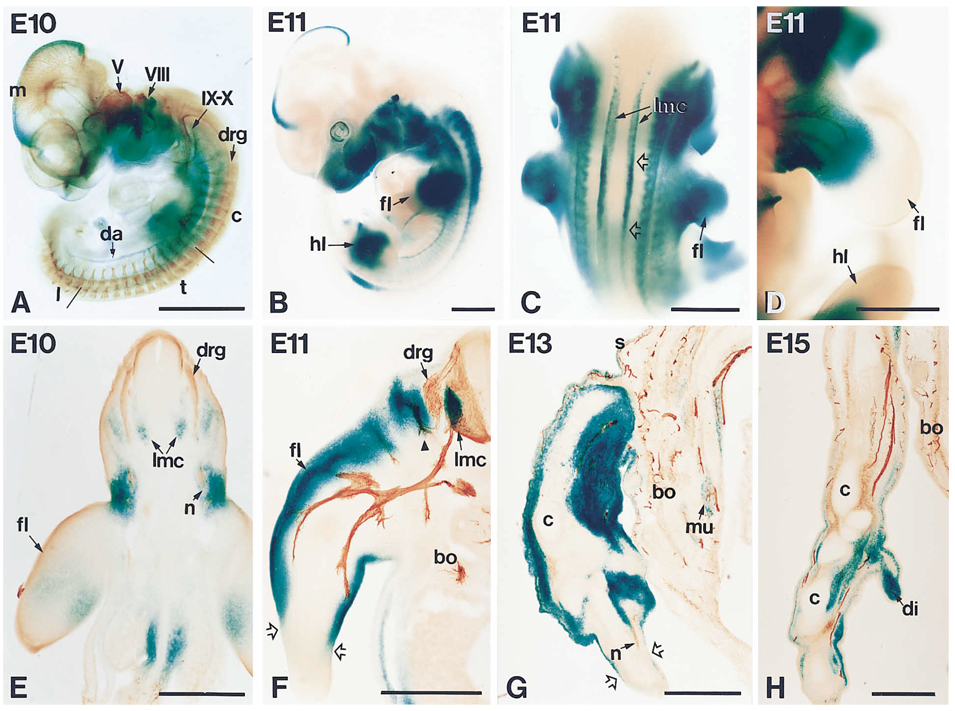
Figure 5.
Development of NT-3 Expression and Axonal Projections in E10-E15 Embryos as Assessed by the Expression of the Reporter Gene lacZ Introduced into the NT-3 Locus and β-III-Tubulin
Heterozygous mice were histochemically stained for β-galactosidase activity either in whole mounts or in sections through the thoracic region.
(A) E10 embryo double-stained for β-galactosidase (blue) and β-III-tubulin (brown) to show the relationship between the expression of the reporter and the developing nervous system. LacZ expression is observed in the head, including the top of the mesencephalon (m), the branchial arches, the developing ear and eye, and in the anterior part of the trunk. Staining is also observed in the most proximal part of the forelimb and, more caudally, in the dorsal aorta (da). Antibodies to tubulin label all neurons and their projections, including all cranial ganglia (V, VIII, IX–X) and all formed DRGs (drg). Notice that DRGs are more developed rostrally, the caudal DRGs being barely discernible at this stage. c, cervical; t, thoracic; l, lumbar. Note also that the expression of the lacZ reporter is not within the DRGs themselves but in the surrounding area, especially around the projecting axons.
(B) At E11 the expression becomes more intense and extends caudally. Conspicuous staining is now seen in the proximal part of both forelimb (fl) and hindlimb (hl).
(C) Dorsal view of an E11 embryo showing two longitudinal stripes of intense staining that correspond to the lateral motor columns (lmc). The staining is particularly strong at the level of the forelimb (fl) (empty arrows).
(D) Higher magnification of a forelimb (fl) of an E11 embryo double-labeled for β-galactosidase and β-III-tubulin, showing that the growing peripheral projection is exposed to high levels of NT-3. hl, hindlimb.
(E) Transverse vibratome section through the thoracic region of the E10 embryo shown in (A). Expression of the lacZ reporter is seen lateral to the DRGs (drg) and strongly around the tip of the projecting nerve (n). Additional staining is seen in the developing lateral motor column (lmc) and in the forelimb (fl).
(F) Transverse vibrotome section of an E11 embryo containing one side of the spinal cord and body (bo) and one forelimb (fl). The histochemical reaction was combined with immunocytochemistry with antibodies to neurofilament to label the DRGs (drg) and growing axons. Intense staining for β-galactosidase is seen in areas where sensory and motor axons are actively growing. Especially strong is the expression surrounding the branch projecting dorsally toward the skin (arrowhead), close to the DRG. The most distal part of the limb, devoid of axons at this time, does not express NT-3 (delineated by open arrows). The staining in the lateral motor column (lmc) is particularly strong at this stage. bo, body.
(G) Double-stained cryosection through the forelimb of an embryo at E13 showing that both axons (n) and lacZ expression have extended more distally (as far as broken arrows). Simultaneously, expression of the reporter is downregulated in the body (bo) becoming restricted to some muscles (mu) and to the skin (s). c, cartilage.
(H) Double-stained cryosection through the most distal part of a forelimb of an E15 embryo showing that simultaneous labeling for axonal projections and reporter expression occurs at the tip of the limb. More proximally, expression is strongly down-regulated. bo, body; c, cartilage; di, digit. Bars, 1 mm (A–D); 0.5 mm (E and H).
To study the relationship between reporter expression and axonogenesis in the developing PNS, histochemical staining for β-galactosidase, which delineates NT-3 expression, was combined with the immunocytochemical detection of the neuron-specific class III β-tubulin, which labels neurons and their projections (Easter et al., 1993). Results show that, at E10 and E11, NT-3 is strongly expressed around the DRGs and their projecting axons (Figures 5A and 5D). Transverse sections of the thoracic region, including the forelimbs, reveal that the lacZ expression seen at E10 and E11 corresponds to the expression of NT-3 by the mesenchyme immediately adjacent to the ganglia and proximal part of the limb. No expression has been detected within the ganglia prior to E15 (Figure 6C; see also Schecterson and Bothwell, 1992), that is, long after the deficit in neuronal number has developed in the NT-3 mutant. Expression of NT-3 in the limbs, as assessed using the lacZ reporter, is always seen in association with the growing tips of the sensory-motor projection (Figures 5E–5H; Figures 6A and 6B). As the projection moves distally, so does the expression of the neurotrophin. Simultaneously, expression is reduced and becomes restricted to specific target tissues in more proximal areas. Thus, at E13, the expression extends distally into the limb, although expression in the body is largely restricted to a few developing muscles (Figure 5G). At E15, the highest expression of NT-3 in the embryo is at the tip of the limb, where axons are actively growing (Figure 5H).
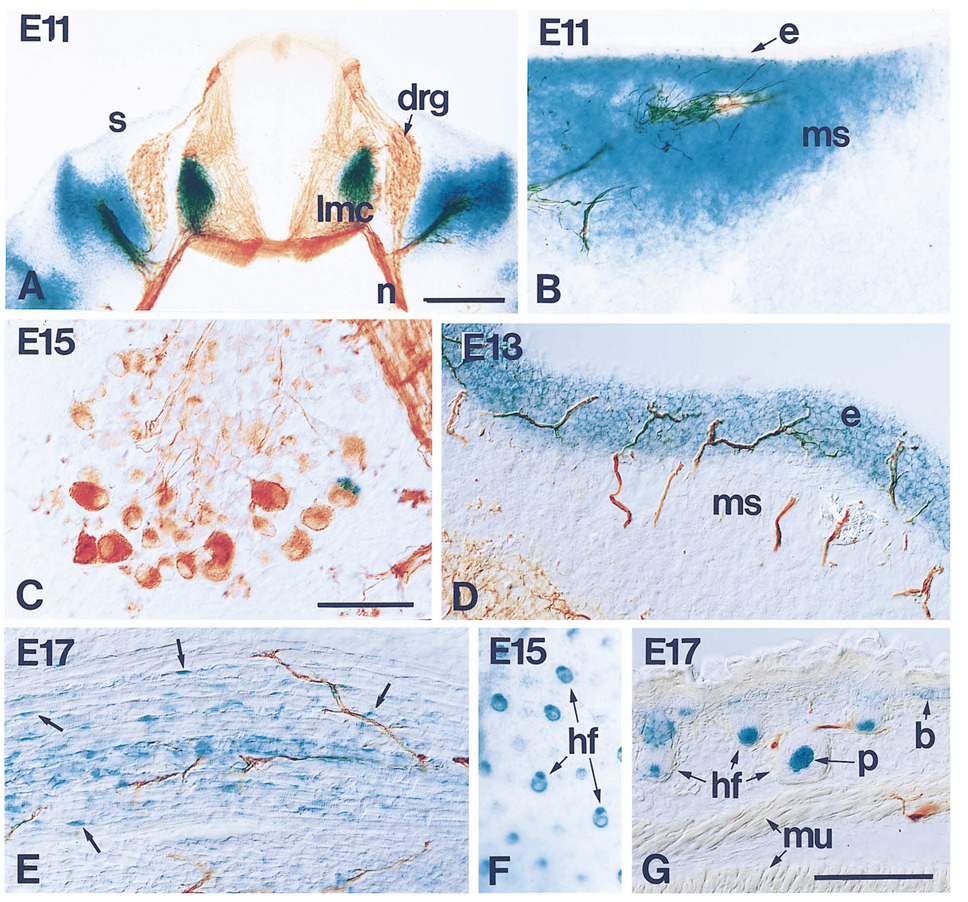
Figure 6.
Expression of lacZ and Neurofilament in Sections of Developing Heterozygous Embryos at the Stages Indicated to Show the Relationship between Growing Peripheral Axons and NT-3 Expression
(A) Transverse section through the thoracic region of an E11 embryo showing the spinal cord, DRGs (drg), and the peripheral nerves (n). Strong expression of the reporter is found in the ventral horn of the spinal cord, where the lateral motor column (lmc) is developing, and around the DRG and sensory-motor projection. The skin (s) expresses low, if detectable NT-3 at this stage. Notice that there is no expression of NT-3 within the DRGs themselves.
(B) Peripheral axons at the mid part of the forelimb. Axons growing through areas of mesenchymal/pre-muscle cells (ms) are surrounded by cells expressing NT-3, as assessed by lacZ expression, while the expression decreases dramatically toward the distal end of the limb (to the right), where axons have not reached. The epidermis (e), also devoid of any innervation at this time, does not express NT-3.
(C) Expression of NT-3, as assessed by lacZ, by neurons within the DRGs is not seen until E15 when a small number of cells express this factor.
(D) At E13, when sensory axons invade profusely the epidermis (e), NT-3 expression is strong there, but disappears from the mesenchyme (ms).
(E) Expression of the reporter in formed skeletal muscle as seen at E17. The neurotrophin appears to be produced in fetal myoblasts (arrows), but not in muscle fibers.
(F and G) Late expression in the skin becomes restricted to hair follicles (hf), as can be seen either in whole mounts of skin (F) or in sections (G). Expression is also seen in the basal layer of the epidermis (b). p, papilla; mu, muscle. Bars, 20 µm (A); 100 µm (C); 100 µm (B, D, E, and F).
Sensory neurons known to be sensitive to NT-3 in vivo include muscle-innervating proprioceptive neurons (Fariñas and Reichardt, 1996) and some cutaneous mechanoreceptors, including hair afferents (Airaksinen et al., 1996). Expression of NT-3 at E11 is restricted to the mesenchyme surrounding the traveling axons destined to innervate the skin (Figure 6B). When, at E13, sensory axons invade and ramify within the epidermis, NT-3 expression switches from the underlying mesenchyme to the epidermis (Figure 6D). This expression becomes restricted to hair follicles and to the basal layer of the epidermis at E15 (Figures 6F and 6G). NT-3 expression is high in mesenchymal/premuscle cells in areas of axonal growth, but is dramatically down-regulated in innervated muscles. Additionally, NT-3 expression appears to be restricted to a subset of innervated muscles (Figures 6E and 6G). LacZ staining did not label muscle fibers themselves, but revealed staining of small cells likely to be fetal myoblasts (Figure 6E).
Discussion
Mice carrying a targeted mutation in the NT-3 gene develop with severe sensory deficits, including the loss of 60%–70% of the neurons present in the DRGs (Ernfors et al., 1994; Fariñas et al., 1994). In the present study, we have carefully analyzed the developmental dynamics of both neuronal and progenitor populations in the DRGs to determine how this remarkable deficit is produced. With this approach, we have shown that the deficit found in the DRGs of newborn NT-3 mutant mice is due to two direct and distinct effects of the mutation: first, neuronal survival is specifically compromised, and second, progenitor cells abandon prematurely the cell cycle. Both effects, elevated neuronal apoptosis and fewer rounds of precursor cell division, cause the final deficit in cell and neuron number, as early as embryonic day 13, in NT-3 mutant mice. We have also shown that in normal animals NT-3 expression is initially found around DRGs and subsequently around their projections.
When neuronal numbers are quantified, a significant reduction in the number of neurofilament-positive cells is already seen at E11, a time when cell death is significantly increased in the mutant embryos (see also White et al., 1996). This deficit is likely to involve cell death of postmitotic neurons, because the present study shows that it happens in the absence of any detectable effects in the precursor population. We propose that these neurons are the proprioceptive neurons that have been shown to be missing in postnatal NT-3-deficient mice (Ernfors et al., 1994; Fariñas et al., 1994; Tessarollo et al., 1994; Tojo et al., 1995; Kucera et al., 1995). Large cells, including proprioceptive neurons, are the earliest-born neurons in the DRGs and are present between E10 and E11 in lumbar areas (Lawson and Biscoe, 1979). The axons of these neurons are the first ones to exit the ganglia and are likely to be exposed to NT-3 at the time of the deficit, as shown by our lacZ reporter studies in combination with axonal labeling. Proprioceptive neurons are also missing in newborn mice lacking the kinase domain of trkC (Klein et al., 1994). The finding that trkC mutants also have elevated cell death in their lumbar DRGs at E11 (White et al., 1996) suggests that the loss of proprioceptive neurons occurs at the same developmental stage in both mutants. Since NT-3 is the only identified ligand for trkC, these results indicate that NT-3 action through a signaling kinase–containing trkC receptor is required for the survival of proprioceptive neurons soon after they are born, long before these cells have approached their final muscle targets (see also Kucera et al., 1995). Proprioceptive neurons also need NT-3 for survival at later stages of development, after they innervate the muscle, as shown by injections of anti-NT-3 antibodies into chick embryos during the period of cell death following final target innervation (Oakley et al., 1995). Consistent with this requirement, these neurons expressed trkC all throughout prenatal development into postnatal life (Mu et al., 1993; McMahon et al., 1994). Moreover, NT-3 has been shown to be expressed in innervated muscle (see also White et al., 1996) and muscle spindles (Copray and Brouwer, 1994). Thus, several lines of evidence indicate that proprioceptive neurons need NT-3 for early survival, before target encounter, and for later target-dependent survival and maintenance.
Our conclusion that neurons, not precursors, are undergoing apoptosis in dorsal root ganglia in the absence of NT-3 was derived initially from our analyses of the dynamics of these two cell populations. Specifically, in lumbar ganglia, neuronal numbers are dramatically reduced at a time when precursor numbers remain normal. Consistent with this conclusion, an analysis of the kinetics of cell populations in the trigeminal ganglion indicates that neurons, not precursors, are also the major population undergoing apoptosis in the absence of NT-3 (Wilkinson et al., 1996). In agreement with counts of neurons and precursor numbers, we could not detect apoptosis in cells that had gone through S-phase in the previous 2 hr or 5 hr. Our observations and conclusions appear to be inconsistent with a recent report indicating that precursors, not neurons, are the population that is undergoing apoptosis in the NT-3 mutant (ElShamy and Ernfors, 1996). In this paper, neurons and precursors were not counted separately. However, the authors observed that almost the entire complement of precursors, defined by a 5 hr BrdU pulse, exhibited positive labeling with TUNEL, suggesting that many or all of these cells were undergoing apoptosis. As one possible explanation, we have noticed that in the study by ElShamy and Ernfors (1996), the dose of BrdU used to label proliferating cells was at least four times higher than the concentration normally used in these kind of experiments (e.g., Yoshida et al., 1987; Nowakowski et al., 1989). The effects that unusually high concentrations of the halogenated nucleotide analog BrdU could have on the cellular behavior of DRG progenitor cells are unclear. However, high concentrations of BrdU have been shown to be cytotoxic in other developmental systems (e.g., Yoshida et al., 1987 and references therein). Other differences in methodology could potentially explain the differences with our observations. Whatever the explanation, our results indicate that there is not elevated apoptosis of precursors in NT-3 mutants. We also believe that this discrepancy is unlikely to be due to a difference in genetic background, because the phenotypes of these two strains of mice appear to be identical at birth (Ernfors et al., 1994; Fariñas et al., 1994).
Our results show that premature differentiation of sensory precursors is taking place between E11 and E12 in NT-3-deficient mice, since the reduction in the number of precursors in the mutant embryos at this time is accompanied by an increase in the number of neurons. The premature differentiation of precursor cells in mutant ganglia found in this study is likely to be a direct effect of the lack of the neurotrophin and not just a consequence of the neuronal degeneration taking place in the ganglia. Embryos lacking the kinase domain of the trkC receptor probably have a similar initial neuronal loss of proprioceptive neurons (see above); however, they do not appear to have any additional deficits. While newborn mice lacking the kinase domain of trkC have been shown to lose around 20% of all DRG neurons (Klein et al., 1994; Minichiello et al., 1995), a percentage likely to include exclusively the population of proprioceptive neurons, NT-3 mutant mice are missing at least 60% of the normal complement of DRG neurons at birth (Ernfors et al., 1994; Fariñas et al., 1994). These observations indicate that the precursor population is likely to behave normally in the trkC mutants and that the effect on precursors reported here is directly dependent on NT-3, but is not mediated by a kinase-containing trkC isoform. This may reflect the involvement of different splice variants of trkC (Tsoulfas et al., 1993; Valenzuela et al., 1993) or of other trk receptors, such as trkA or trkB, each of which is known to be responsive to NT-3 in at least some cellular contexts (e.g., Ip et al., 1993).
The possible roles of neurotrophins in cell cycle regulation and neuronal differentiation are not completely understood. Interestingly, NT-3 appears to promote the proliferation in culture of embryonic rat DRG precursor cells (Memberg and Hall, 1995). In agreement with these results, we show that in vivo a deficiency in NT-3 expression has a striking effect on cell cycle regulation of embryonic murine DRG precursor cells, reducing the number of precursors at E12. Our data is consistent with the possibility that NT-3 acts to keep precursors in the proliferative state as an inhibitor of their differentiation. Other reports, however, have shown that the addition of NT-3 in vitro enhances differentiation of precursor cells in the PNS, including sensory, sympathetic, renal, and enteric neural progenitor populations (Pinco et al., 1993; Verdi and Anderson, 1994; Karavanov et al., 1995; Chalazonitis et al., 1994). These observations indicate that cell-specific factors may regulate the nature of cellular responses to this neurotrophin. Alternatively, it has also been shown that different levels of NT-3 have distinct effects on the behavior of precursor populations. Specifically, low concentrations of NT-3 have been shown to promote the survival of cultured sympathetic neuroblasts, while high concentrations induce the differentiation of these cells (Verdi and Anderson, 1994).
Experiments done in vivo using chick embryos have shown similar complex effects of increasing or decreasing NT-3 levels. Consistent with our observations in murine embryos, injection of anti-NT-3 into chick embryos at early developmental times, before the period of naturally occurring cell death, leads to a reduction in the number of cells in sensory ganglia of ∼40% by an unknown mechanism (Gaese et al., 1994). Surprisingly, though, injections of NT-3 at the same early developmental stages cause a similar decrease in the number of cells along with a reduction in the number of proliferating cells in these same ganglia (Ockel et al., 1996), suggesting that NT-3 can act as a proliferation stop signal. Thus, different levels of NT-3 may have distinct effects on the behavior of avian sensory precursor populations in vivo.
The action of NT-3 on precursor cells predicts that this population is directly exposed to the neurotrophin during normal development. We have not detected NT-3 expression within the ganglia prior to E15, in agreement with previous in situ hybridization studies (Schecterson and Bothwell, 1992; Ernfors et al., 1992; White et al., 1996), despite the fact that our lacZ reporter expression seems extremely sensitive. However, we have found high levels of expression of NT-3, as assessed by the expression of the reporter lacZ, immediately adjacent to the ganglia. It is thus possible that the surrounding neurotrophin gains access to the ganglion by diffusion and exerts its action directly on the precursor cells. The apparent expression of NT-3 detected by RNAse protection assay in isolated E11 DRGs (ElShamy and Ernfors, 1996) is likely to come from contaminating mesenchyme surrounding the DRGs, which are very difficult to individualize at this early stage.
In normal embryos, more than 50% of the final complement of neurons is produced very rapidly, between E12 and E13. Neurogenesis experiments have indicated that many neurons in lumbar DRGs are actually born at E12 (Lawson and Biscoe, 1979). Additional data supporting a remarkable second wave of neuron formation comes from the characterization of mutant mice deficient in the retinoblastoma gene (Rb) protein (Lee et al., 1994), in which cells can not withdraw from the cell cycle and, therefore, die by apoptosis. In the DRGs of mice deficient in Rb protein there is a highly significant elevation in the number of apoptotic cells at E12, indicating that, at this time, many precursor cells in normal spinal ganglia leave the cell cycle and become postmitotic. Accordingly, this rapid increase in the number of neurons is accompanied by a parallel reduction in the number of precursors. Because in the NT-3 mutant embryos the pool of precursors has been prematurely depleted, this last increase in the number of neurons can not occur. However, much of this deficit in neuronal accumulation appears to be not a direct effect of the lack of NT-3 but a consequence of the diminished progenitor pool. Expression of NT-3 around the ganglion decreases by E12, becoming more prominent in distal areas. It is tempting to speculate that in normal animals the presence of NT-3 in the vicinity of the ganglion keeps the precursors dividing and that when the neurotrophin levels drop the precursor population differentiates. In embryos lacking NT-3, precursor cells would prematurely leave the cell cycle and differentiate into neurons.
The neuronal population in DRGs of NT-3 mutant neonatal mice is reduced more than 60% (Ernfors et al., 1994; Fariñas et al., 1994). Our results indicate that the defect in the number of neurons, other than proprioceptive, could be mostly due to reduced generation of these neurons. A detailed analysis of the modalities present in the DRGs of these NT-3 mutant mice will be necessary to determine which kinds of neurons are produced in the NT-3 mutant mice during early development. It is also possible that compensatory mechanisms exist to ensure adequate proportions of those different populations in the ganglion. Consistent with this hypothesis, DRGs of NT-3-deficient newborn mice have a reduced number of trkA-positive cells, but the proportion of trkA-positive neurons relative to the total number of neurons is kept approximately constant (I. F. and L. F. R., unpublished data). Nevertheless, we have found increased apoptosis at times later than E11 (see also White et al., 1996) that could also contribute to the final deficit in the number of neurons. Further studies will be necessary to determine which neurons are dying after E11 in the absence of NT-3.
In the present study, analysis of NT-3 expression, as assessed using a lacZ reporter inserted in the NT-3 gene, has indicated that there is a remarkable association between regions of high NT-3 expression and the positions of growing axons in many peripheral tissues. In addition, NT-3 expression becomes rapidly down-regulated as the axons move distally and finally are restricted to final targets. These observations suggest the exciting possibility that the growing tips of sensory axons might regulate NT-3 production. Recent studies have demonstrated that neuregulins, expressed and secreted by many types of neurons, are capable of inducing NT-3 expression in undifferentiated mesenchymal cells surrounding sympathetic neurons (Verdi et al., 1996). Our observations on the correlation between NT-3 expression and sensory axonogenesis suggest that this regulatory circuit could be important in many areas of the PNS. Another possible regulatory mechanism that could explain the dynamic pattern of expression would be that as mesenchymal cells differentiate, many of their derivatives can no longer respond to neuregulins or other nerve-derived inducers.
In conclusion, the multiplicity of actions of NT-3 in the developing DRGs indicates that NT-3 plays a fundamental role in determining the final size of these ganglia. This role is exerted very early in development, long before spinal neurons reach their final targets. Mice carrying targeted mutations have been analyzed in an attempt to determine the neurotrophin requirements of specific groups of neurons. In many instances, it is clear that neurons conveying sensory information of a given modality rely on the presence of a particular neurotrophin for their survival. However, we present evidence here that the reduction in a neuronal population can also be due to reduced generation of neurons, indicating that neurotrophins can play various different roles during the development of the PNS.
Experimental Procedures
Animals
The generation of the NT-3 mutant mice used in the present study has been described elsewhere (Fariñas et al., 1994). Timed embryos were obtained by overnight mating of heterozygotes. The morning when the vaginal plug was observed has been considered embryonic (E) day 0. Pregnant females at different stages of gestation (E9, 10, 11,12, 13, 15, 17) were sacrificed by cervical dislocation and embryos dissected out and fixed in either 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3) for about 1 hr or Carnoy’s fixative (60% ethanol, 30% chloroform, 10% acetic acid) overnight. Neonate animals were perfused with saline solution (0.9% sodium chloride in distilled water) and fixed in Carnoy’s fixative. All animals employed in this study were genotyped by DNA blot analysis as described (Fariñas et al., 1994).
Cell Counts
Embryos and neonates fixed in Carnoy’s solution were embedded in paraffin and serially sectioned at 7 µm. Between stage 10 and 13, complete series through the embryos were either stained with cresyl violet (Nissl), for total cell counts, or immunostained with antibodies to 150 KDa neurofilament subunit, for neuronal counts. In each case, the number of precursors was estimated as the total number of cells minus the number of neurofilament-positive cells. At stage E15, the number of neurons was quantitated in embryos either stained with cresyl violet or immunoreacted with neurofilament antibodies. The numbers obtained at this stage using the two different stainings were identical and, thus, they have been pooled together in the graphs. After E15, only neurons characterized by morphological criteria were quantitated in Nissl stained sections. Individual ganglia were identified first in the sections using a dissecting scope. From E12 to birth, ribs were used as landmarks. Before E12, the entire ganglion chain was visually reconstructed and each ganglion mapped. In each case cells were counted in every fourth section to calculate the total number in the ganglion, and numbers were not corrected. Pyknotic figures were counted in cresyl violet stained sections being careful not to include red blood cells (Coggeshall et al., 1994).
Immunocytochemistry
Endogenous peroxidases were quenched in 10% methanol, 3% hydrogen peroxide in Tris buffered saline (TBS; 10 mM Tris-HCl [pH 7.5], 150 mM sodium chloride) and nonspecific interactions blocked with TBS containing 0.4% Triton X-100, 3% bovine serum albumin, and 10% normal goat serum. All antibody dilutions and washes were done in the same blocking buffer. Primary antibodies included rabbit polyclonal antibodies to the neurofilament subunit of relative molecular mass 150,000 (Chemicon, Temecula, CA; dilution 1:2,000) and p75 (Weskamp and Reichardt, 1991; 1:1,000) and mouse monoclonals to β-tubulin (Easter et al., 1993; 1:1,000). For bright field immunocytochemistry, sections were incubated with the appropriate biotinylated secondary antibody followed by avidin–biotin–peroxidase complex (Vector Labs, Burlingame, CA) according to the instructions of the manufacturer. Sections were developed in 0.05% 3-3′-diaminobenzidine tetrahydrochloride and 0.003% hydrogen peroxide in 0.1 M Tris–HCl (pH 7.5), dehydrated, and mounted with DPX. The same method was used for whole mounts, except that incubations were much longer and dimethylsulfoxide was added to the blocking buffer at a 1:4 ratio. For immunofluorescence, Texas red conjugated secondary antibodies (Jackson Immunochemicals, West Grove, PA) were used at a 1:1000 dilution, and sections were mounted with FICTguard (Testog, Chicago, IL) and analyzed with a confocal microscope.
Proliferation and Cell Cycle Studies
Pregnant females were injected with 50 mg/kg of body weight 2′-bromo-5′-deoxyuridine (100–150 µl of a 10 mg/ml stock solution in 0.1 M Tris–HCl buffer [pH 7.5]), 2 hr before sacrifice. Embryos were fixed in Carnoy’s and embedded in paraffin. Sections were treated with 2 N HCl for 30 min at 37°C and neutralized in 0.1 M sodium borate (pH 8.5), for 5–10 min. Immunocytochemical detection of the nucleotide was performed as described above using a monoclonal antibody from Novocastra (New Castle upon Tyne, UK) at a 1:100 dilution. Sections were counterstained with hematoxylin. All immunopositive nuclei were counted in every fourth section through the ganglia of E11 and E13 embryos. Cell cycle measurements were done according to Nowakowski et al. (1989).
TUNEL Staining
Sections were processed for end-labeling of nicked DNA using the ApoTag kit (Oncor, Gaithersburg, MD) following the instructions of the manufacturer. Alkaline phosphatase conjugated anti-digoxigenin antibodies (Boehringer-Manheim) were used at a 1:1000 dilution, and a mixture of NBT/BCIP was used as substrate for detection. Sections were counterstained with methyl green (Zymed, So. San Francisco, CA) and mounted in PBS-glycerol. Labeled nuclei and total number of cells were counted in ten independent sections of thoracic and lumbar ganglia from three different wild-type and three mutant embryos at E11, and the percentage of apoptotic cells calculated. For combinations of TUNEL staining with immunological detection of BrdU or neurofilament, sections from two wild-type and two mutant E11 embryos were reacted according to the kit protocol and later incubated with fluorescein conjugated anti-digoxigenin antibodies along with the primary antibody for the desired cellular antigen. When combined with BrdU detection, acid treatment was carried out immediately before application of the antibodies to digoxigenin and to BrdU.
β-Galactosidase Histochemistry
Histochemical detection of β-galactosidase was performed either in whole-mount embryos or in sections. In every case, the embryos were fixed for 1–2 hr with 4% paraformaldehyde in 0.1 M phosphate buffer. For whole-mount staining, embryos were permeabilized in 0.02% Nonidet P-40 and stained overnight at 37°C in X-gal solution containing 0.02% Nonidet P-40, 0.01% sodium deoxycholate, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, and 1 mg/ ml X-gal in phosphate buffered saline (PBS) (pH 7.3). Embryos were then washed, cleared in a 1:1 PBS-glycerol mixture, and photographed. In some cases, stained embryos were subsequently immunostained using antibodies to tubulin as explained before. For section staining, embryos were either cryoprotected, frozen, and cryosectioned at 30 µm or directly vibratome sectioned at 100 µm and then stained overnight at 37°C in the same X-gal solution. Sections were fixed again in 4% paraformaldehyde and stained with neurofilament antibodies as described above. Double-stained sections were mounted in PBS-glycerol.
Acknowledgments
We would like to thank A. Frankfurter for the generous gift of anti-βIII-tubulin antibodies and P. Gamp and E. Grady for their help with the confocal microscope. We also thank R. Kleiman and A. Patapoutian for very helpful comments on the manuscript. This work was supported by research grants from the NIH (MH 48200) and the Howard Hughes Medical Institute (L. F. R.); I. F. is the recipient of a long-term fellowship from the Human Frontier Science Program Organization; L. F. R. is an Investigator of the Howard Hughes Medical Institute.
References
- Airaksinen MS, Koltzenburg M, Lewin GR, Masu Y, Helbig C, Wolf E, Brem G, Toyka KV, Thoenen H, Meyer M. Specific subtypes of cutaneous mechano-receptors require neurotrophin-3 following peripheral target innervation. Neuron. 1996;16:287–295. doi: 10.1016/s0896-6273(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Barde Y-A. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Rothman TP, Chen J, Lamballe F, Barbacid M, Gershon MD. Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J. Neurosci. 1994;14:6571–6584. doi: 10.1523/JNEUROSCI.14-11-06571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard P, Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J. Neurosci. 1984;4:2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Pover CM, Fitzgerald M. Dorsal root ganglion cell death and surviving cell numbers in relation to the development of sensory innervation in the rat hindlimb. Dev. Brain Res. 1994;82:193–212. doi: 10.1016/0165-3806(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Copray JCVM, Brouwer N. Selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience. 1994;63:1125–1135. doi: 10.1016/0306-4522(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton L, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Friedman WJ, Black IB. NT-3 stimulates sympathetic neuroblast proliferation by promoting precursor survival. Neuron. 1993;11:1101–1111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Ross LS, Frankfurter A. Initial tract formation in the mouse brain. J. Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElShamy WM, Ernfors P. A local action of neurotrophin-3 prevents the death of proliferating sensory neuron precursor cells. Neuron. 1996;16:963–972. doi: 10.1016/s0896-6273(00)80119-1. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Persson H. Developmentally regulated expression of HDNF/NT-3 mRNA in rat spinal cord motoneurons and expression of BDNF mRNA in embryonic dorsal rot ganglion. Eur. J. Neurosci. 1991;3:953–961. doi: 10.1111/j.1460-9568.1991.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio J-P, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur. J. Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Reichardt LF. Neurotrophic factors and their receptors: implications of genetic studies. Semin. Neurosci. 1996;8:133–143. [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang X-Y, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Gaese F, Kolbeck R, Barde YA. Sensory ganglia require neurotrophin-3 early in development. Development. 1994;120:1613–1619. doi: 10.1242/dev.120.6.1613. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henion PD, Garner AS, Large TH, Weston JA. trkC-mediated NT-3 signaling is required for the early development of a subpopulation of neurogenic neural crest cells. Dev. Biol. 1995;172:602–613. doi: 10.1006/dbio.1995.8054. [DOI] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, Barbacid M, Yancopoulos GD. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Carmeli C, Rosenthal A. Neurotrophin 3 is a mitogen for cultured neural crest cells. Proc. Natl. Acad. Sci. USA. 1992;89:1661–1665. doi: 10.1073/pnas.89.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanov A, Sainio K, Palgi J, Saarma M, Saxen L, Sariola H. Neurotrophin 3 rescues neuronal precursors from apoptosis and promotes neuronal differentiation in the embryonic metanephric kidney. Proc. Natl. Acad. Sci. USA. 1995;92:11279–11283. doi: 10.1073/pnas.92.24.11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Kucera J, Fan G, Jaenisch R, Linnarsson S, Ernfors P. Dependence of developing group Ia afferents on neurotrophin-3. J. Comp. Neurol. 1995;363:307–320. doi: 10.1002/cne.903630211. [DOI] [PubMed] [Google Scholar]
- Lamballe F, Smeyne RJ, Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J. Neurosci. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Biscoe TJ. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J. Neurocytol. 1979;8:265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- Lee EY, Hu N, Yuan SS, Cox LA, Bradley A, Lee W-H, Herrup K. Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes and Dev. 1994;8:2008–2021. doi: 10.1101/gad.8.17.2008. [DOI] [PubMed] [Google Scholar]
- Lefcort F, Clary DO, Rusoff AC, Reichardt LF. Inhibition of the NT-3 receptor trkC, early in chick embryogenesis, results in severe reduction in multiple neuronal subpopulations in the dorsal root ganglia. J. Neurosci. 1996;16:3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde Y-A. Physiology of the neurotrophins. Ann. Rev. Neurosci. 1996;19:289–318. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Proliferation, differentiation, and survival of rat sensory neuron precursors in vitro require specific trophic factors. Mol. Cell. Neurosci. 1995;6:323–335. doi: 10.1006/mcne.1995.1025. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Piehl F, Vazquez E, Scimmang T, Hökfelt T, Represa J, Klein R. Differential effects of combined trk receptor mutations on dorsal root ganglion and inner ear sensory neurons. Development. 1995;121:4067–4075. doi: 10.1242/dev.121.12.4067. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Garner AS, Large TH, Frank E. Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal cell death. Development. 1995;121:1341–1350. doi: 10.1242/dev.121.5.1341. [DOI] [PubMed] [Google Scholar]
- Ockel M, Lewin GR, Barde Y-A. In vivo effects of neurotrophin-3 during sensory neurogenesis. Development. 1996;122:301–307. doi: 10.1242/dev.122.1.301. [DOI] [PubMed] [Google Scholar]
- Pinco O, Carmeli C, Rosenthal A, Kalcheim C. Neurotrophin-3 affects proliferation and differentiation of distinct neural crest cells and is present in the early neural tube of avian embryos. J. Neurobiol. 1993;24:1626–1641. doi: 10.1002/neu.480241207. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Scott SA. New York: Oxford University Press; 1992. Sensory Neurons: Diversity, Development, and Plasticity. [Google Scholar]
- Silos-Santiago A, Molliver DC, Ozaki S, Smeyne RJ, Fagan AM, Barbacid M, Snider WD. Non-trkA-expressing small DRG neurons are lost in trkA deficient mice. J. Neurosci. 1995;15:5929–5942. doi: 10.1523/JNEUROSCI.15-09-05929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF. Nature. 1994;368:246–248. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Martin-Zanca D, Gilbert DJ, Jenkins NA, Copeland NG, Parada LF. trkC, a receptor for neurotrophin-3, is widely expressed in the developing nervous system and in non-neuronal tissues. Development. 1993;118:463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Vogel KS, Palko ME, Reid SW, Parada LF. Targeted mutation in the neurotrophin-3 gene results in loss of muscle sensory neurons. Proc. Natl. Acad. Sci. USA. 1994;91:11844–11848. doi: 10.1073/pnas.91.25.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo H, Kaisho Y, Nakata M, Matsuoka K, Kitagawa M, Abe T, Takami K, Yamamoto M, Shino A, Igarashi K, Aizawa S, Shiho O. Targeted disruption of the neurotrophin-3 gene with lacZ induces loss of trkC-positive neurons in sensory ganglia but not in spinal cords. Brain Res. 1995;669:163–175. doi: 10.1016/0006-8993(94)01219-8. [DOI] [PubMed] [Google Scholar]
- Tojo H, Takami K, Kaisho Y, Nakata M, Abe T, Shiho O, Igarashi K. Analysis of neurotrophin-3 expression using the lacZ reporter gene suggests its local mode of neurotrophic activity. Neuroscience. 1996;71:221–230. doi: 10.1016/0306-4522(95)00445-9. [DOI] [PubMed] [Google Scholar]
- Tsoulfas P, Soppet D, Escandon E, Tessarollo L, Mendoza-Ramirez J-L, Rosenthal A, Nikolics K, Parada LF. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993;10:975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- Valenzuela DM, Maisonpierre PC, Glass DJ, Rojas E, Nuñez L, Kong Y, Gies DR, Stitt TN, Ip NY, Yancopoulos GD. Alternative forms of rat TrkC with different functional capabilities. Neuron. 1993;10:963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Anderson DJ. Neurotrophins regulate sequential changes in neurotrophin receptor expression by sympathetic neuroblasts. Neuron. 1994;13:1359–1372. doi: 10.1016/0896-6273(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Groves AK, Fariñas I, Jones K, Marchionni MA, Reichardt LF, Anderson DJ. A reciprocal cell-cell interaction mediated by NT-3 and neuregulins controls the early survival and development of sympathetic neuroblasts. Neuron. 1996;16:515–527. doi: 10.1016/s0896-6273(00)80071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- White FA, Silos-Santiago I, Molliver DC, Nishimura M, Phillips H, Barbacid M, Snider WD. Synchronous onset of NGF and trkA survival dependence in developing dorsal root ganglia. J. Neurosci. 1996;16:4662–4672. doi: 10.1523/JNEUROSCI.16-15-04662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GA, Fariñas I, Backus C, Yoshida CK, Reichardt LF. Neurotrophin-3 is a survival factor in vivo for early mouse trigeminal neurons. J. Neurosci. 1996;16:7661–7669. doi: 10.1523/JNEUROSCI.16-23-07661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM, Vogel KS, Davies AM. Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron. 1992;9:139–150. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Yamada M, Wakabayashi K, Ikuta F. Immunohistochemical detection of DNA replicating cells in the developing nervous system: use of bromodeoxyuridine and its monoclonal antibody to rat fetuses. Biomed. Res. 1987;8:431–444. [Google Scholar]