Abstract
Immunocytochemistry has been used to examine the location of trkA, the high-affinity receptor for nerve growth factor, in adult rat dorsal root ganglia, trigeminal ganglia and spinal cord. TrkA immunoreactivity was observed in small and medium sized ganglion cells and in the dorsal horn of the spinal cord. In lumbar L4 and L5 ganglia trkA-immunoreactive cells constitute 40% of dorsal root ganglion cells and range in size from 15 to 45 μm in diameter. Double labelling using markers for various dorsal root ganglion subpopulations revealed that virtually all (92%) trkA-immunoreactive cells express calcitonin gene-related peptide (CGRP) immunoreactivity. In contrast only 4 and 13% of trkA-immunoreactive cells are labelled by the monoclonal antibody LA4 or the lectin Griffonia simplicifolia IB4, markers for small non-peptide-containing cells. Eighteen percent of trkA-immunoreactive cells belong to the ‘large light’ subpopulation, identified by their strong immunostaining by the neurofilament antibody RT97. TrkA immunoreactivity in the dorsal horn is heaviest in laminae I and II outer, has a similar distribution to CGRP, and is depleted by dorsal rhizotomy. Our results show that trkA-expressing cells in dorsal root ganglia correspond almost exactly with the CGRP, peptide-producing population. The receptor is present not only on cell bodies but also on central terminals. Non-peptide-containing small cells, which constitute 30% of dorsal root ganglion cells, are not trkA-immunoreactive and therefore most probably are functionally independent of nerve growth factor.
Keywords: rat, NGF, neurotrophin, dorsal root ganglion, spinal cord
Introduction
Nerve growth factor (NGF) is the best characterized of a small family of molecules termed neurotrophins, the other known members being brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5. The biological role of NGF has been most intensively studied in development, where there is evidence to suggest it functions as a target-derived neurotrophic factor, regulating the density of innervation of a number of peripheral targets (for review see Davies, 1992). In the adult animal, NGF continues to be produced by peripheral targets, and there are clear physiological consequences of alterations in its availability. These are most marked for sensory and sympathetic neurons of the peripheral nervous system. For instance, NGF deprivation, produced by the administration of neutralizing antibodies to NGF or by autoimmunization procedures, leads to down-regulation of tyrosine hydroxylase activity in sympathetic neurons (Gorin and Johnson, 1980) and reduced expression of the neuropeptide substance P (Schwartz et al., 1982) in sensory neurons. Administration of exogenous NGF increases substance P levels (Goedert et al., 1981). Additionally, exogenous NGF can alter pain-related behaviour, a large single systemic dose producing hyperalgesia which lasts days (Lewin et al., 1994). A smaller subcutaneous dose results in local hyperalgesia lasting hours (Andreev et al., 1994). There are also recent reports that NGF deprivation (with neutralizing antibodies) can prevent the hyperalgesia that normally develops after several forms of inflammatory stimulus, such as Freund’s adjuvant or carrageenan (Lewin et al., 1994).
Since NGF exerts these clear effects on sensory systems, it is natural to ask about the peripheral expression of receptors to NGF. Two such receptors have been described. The first is known as the p75 receptor or the low-affinity NGF receptor (LNGFR). It is widely expressed throughout the nervous system but its role remains enigmatic since most of the biological effects of NGF are observed in the absence of this receptor, and conversely the expression of this receptor alone in a number of cell lines does not confer sensitivity to NGF (for review see Chao, 1992). There remains the possibility that, while not essential, the p75 receptor contributes to the responses to NGF in some cells. Such a suggestion is supported by observations on a transgenic mouse lacking the ability to produce p75. In these animals, some peptidergic sensory neurons appear to be lost (Lee et al., 1992). Furthermore, developing sensory neurons from these animals grown in culture show reduced sensitivity to NGF in a survival assay (Davies et al., 1993).
The second type of NGF receptor has been recognized relatively recently (Chao, 1992; Lindsay et al., 1994). It is the product of the TRK140 proto-oncogene, and is referred to as the trkA receptor (since there are other trk receptors responsive to the other members of the neurotrophin family) or, more simply, trk. This receptor is capable of forming homodimers. It is a membrane-spanning molecule with an extracellular domain (with an NGF binding site) and an intracellular domain with tyrosine kinase activity, presumed to be responsible for signal transduction. There is a plethora of evidence that trkA is essential for the mediation of the normal biological effects of NGF (Lindsay et al., 1994).
The expression of neurotrophin receptors on sensory neurons has been studied using a number of techniques, including in situ hybridization for trkA mRNA (Carroll et al., 1992; Verge et al., 1992; Mu et al., 1993; McMahon et al., 1994; Wright and Snider, 1994), high-affinity binding of labelled NGF (Richardson et al., 1986; Verge et al., 1989a. 1989b, 1990, 1992) or the retrograde transport from peripheral nerve to dorsal root ganglia of iodinated NGF (Richardson and Riopelle, 1984; DiStefano et al., 1992). These studies have generally shown that trkA expression in adult dorsal root ganglion (DRG) cells is restricted to a subpopulation of cells. However with the exception of Verge et al. (1989a). the techniques employed did not allow more detailed neurochemical characterization. In the present study we have therefore used double labelling immunocytochemistry and a polyclonal antiserum raised against recombinant rat truncated trkA receptor (Clary et al., 1994) in order to examine the expression of trkA by various DRG subpopulations. Markers employed for DRG subpopulations were RT97 (large cell marker), calcitonin gene-related peptide (CGRP, peptide marker) and two markers for small non-peptide cells (monoclonal antibody LA4 and lectin GrifSonia simplicifolia IB4) (for review see Lawson, 1992). We have also examined the distribution of trkA in the dorsal horn of the spinal cord. A preliminary account of some of this work has appeared in abstract form (Averill et al., 1994).
Materials and methods
Immunofluorescence
Eight adult male Wistar rats (250–400 g body weight) were anaesthetized with sodium pentobarbital (Sagatal, RMB, UK; 6 mg/100 g body weight) and perfused through the ascending aorta with 30 ml vascular rinse followed by 300 ml 4% paraformaldehyde in 0.1 M phosphate buffer. After 2.5–3 h postfixation, tissue blocks were cryoprotected in 15% sucrose and then 8–10 μm sections of dorsal root ganglia, trigeminal ganglia and spinal cord were cut on a cryostat. Sections were then stained for single- and dual-colour immunofluorescence light microscopy using standard procedures.
Incubations consisted of 1 h in 10% normal serum followed by 18–24 h in primary antibody and 3 h in secondary antibody. For single labelling a rabbit primary antibody directed against the extracellular domain of trkA (code labelled RTA) was used at 2.5 μg/ ml. For double labelling this antibody was combined with one of the following: RT97 mouse monoclonal antibody, which recognizes the phosphorylated form of the 200 kDa neurofilament subunit (1:50 dilution); LA4 mouse IgM monoclonal antibody, which recognizes α-galactose (α-fucose)-asialo-GM1 glycolipid (undiluted supernatant); CGRP sheep polyclonal antiserum (Affiniti, Nottingham, UK) raised against synthetic rat α-CGRP (1:2000); and Griffonia simplicifolia IB4 lectin (Sigma, UK), which recognizes terminal α-galactose residues (12.5 μg/ml biotinylated IB4). The full characteristics and staining specificity of most of these markers have been reported previously (RTA: Clary et al., 1994; Sobreviela et al., 1994; RT97: Lawson et al., 1984; LA4: Chou et al., 1989; Alvarez et al., 1991; IB4: Silverman and Kmger, 1990). Regarding the CGRP antiserum, staining in the spinal cord is abolished by preincubation of the diluted antiserum with 10 nmol/ml synthetic CGRP but is unaffected by 10 nmol/ml substance P or galanin. Secondary reagents used included both fluorescein isothiocyanate (FITC)- and TRITC-labelled anti-rabbit IgG, anti-sheep IgG, anti-mouse IgG and anti-mouse IgM affinity-purified antisera (Jackson Immuno Research, PA, USA; 1:100 dilution) and 1:200 ExtrAvidin–FITC (for IB4 localization; Sigma). For double labelling the two sets of reagents were applied together. Antisera were diluted in phosphate-buffered saline (PBS) containing 0.2% Triton X-100 and incubations were carried out at room temperature. For IB4 staining the PBS also contained 0.1 mM CaCl2, 0.1 mM MgCl2 and 0.1 mM MnCl2.
After incubation in secondary antibody, sections were washed briefly in PBS and then mounted in PBS/glycerol (1:3) containing 2.5% 1,4 diazobicyclo-(2,2,2)-octane (antifading agent; Sigma). Sections were viewed on a Leica epifluorescence microscope using N2 (TRITC) and L3 (FITC) filter blocks. Immunostaining was documented either by photography using Ilford Pan F and T-MAX film or by imaging using a Grundig FA87 digital camera coupled to a Sony UP-860CE graphics printer.
Immunoperoxidase
Three animals were perfused with 4% paraformaldehyde, 0.2% glutaraldehyde in 0.1 M phosphate buffer and processed for peroxidase–antiperoxidase or avidin–biotin peroxidase immunocytochemistry. The spinal cord and brain were dissected out, postfixed for 2.5 h in the same fixative and immersed in PBS. Sections (40 μm) were cut using a vibratome (Oxford) and were pretreated with 1% sodium borohydride in PBS for 30 min before immunostaining. Sections were incubated for 30 min in 10% normal goat serum and then transferred to RTA polyclonal antibody (2.5 μg/ml) for 12 h at 4°C. Primary antibody was subsequently revealed using either 1:50 goat anti-rabbit IgG (Sigma) and 1:100 rabbit peroxidase–antiperoxidase (Sigma) or 1:400 biotinylated goat anti-rabbit IgG (Vector Laboratories, UK) and Vectorstain standard peroxidase reagent (Vector Laboratories). Sections were then developed with a solution containing 0.05% 3,3’-diaminobenzidine, 0.04% (NH4)2SO4.NiSO4 and 0.01 % H2O2 in 0.1 M phosphate buffer, pH 7.3. Incubations were carried out at room temperature and antisera were diluted in PBS containing 0.2% Triton X-100. Stained sections were then contrasted in OsO4 (1%) and uranyl acetate (1%), dehydrated and flat-embedded in Durcupan (Fluka, UK).
TrkA immunostaining controls
To test the specificity of the trkA immunostaining in rat spinal cord and dorsal root ganglia, the immunofluorescence protocol described above was employed but using the RTA antiserum preadsorbed with recombinant rat truncated trkA protein (RTA antigen; Clary et al., 1994). Preadsorption was carried out by incubating 1 μg stock RTA antiserum (100 μg/ml) with 20 μg RTA antigen (167 μl) for 4 h at room temperature. The preadsorbed RTA antiserum was then used at its working dilution (2.5 μg/ml).
Surgery
In addition to control animals, two adult male Wistar rats had a unilateral L5 dorsal root section 7 days prior to perfusion. They were anaesthetized with pentobarbitone (40 mgkg, i.p.) and, with sterile precautions, a laminectomy of the L3 vertebra was made. The dura was incised and the L5 dorsal root cut and tied unilaterally. The wound was closed in layers and the animals allowed to recover; recovery was uneventful in both cases. The animals were perfused with 4% paraformaldehyde and cryostat sections of lumbar spinal cord were processed using immunofluorescence and immunoperoxidase procedures basically as described above.
Quantitation
The proportion of trkA-immunoreactive DRG cells was determined by counting the number of immunoreactive neuronal profiles in sections of dorsal root ganglia taken from six animals and also the total number of neuronal profiles visible in the same sections. Neuronal profiles were visualized using either a fluorogold counterstain (100 μg/ml, Leica filter block A) or dark-field illumination. In double-labelled sections the percentage of immunoreactive cells expressing a second marker was assessed by switching between FITC and TRITC/Texas red filter blocks. At least 250 immunoreactive DRG cells were examined for each marker. Cells were counted on randomly chosen DRG sections from at least four animals.
Using a Sight Systems (UK) image analysis system, the cell size distribution of trkA-immunoreactive cells and the grey level of trkA and CGRP immunofluorescence in double-labelled cells were quantified. Images were captured directly off the microscope at 25X magnification using a Grundig FA87 digital camera with integrating framestore. Immunoreactive cells were then outlined manually using a computer mouse and the computer measured area and mean grey level. Areas were subsequently converted into diameters on the assumption that DRG cells are approximately circular. At least 100 cells from four animals were counted.
Results
TrkA immunostaining in dorsal root ganglia
TrkA immunostaining with the RTA antiserum was analysed in L4 and L5 dorsal root ganglia. Many immunofluorescent DRG cells were visible, with cells apparently distributed throughout the ganglion (Fig. 1A). The staining appeared to be predominantly intracellular, with staining of Golgi complexes plus a more diffuse cytoplasmic staining (Fig. 1B). Immunostained cells showed a range of staining intensity, but the subcellular characteristics of the staining meant that even lightly stained cells could be distinguished from background. In addition to DRG cells, immunofluorescent axons derived from cells and also coursing through the ganglion were visible in well stained preparations (Fig. 1B). TrkA immunofluorescence was also present on axons in the dorsal root and peripheral nerve attached to the ganglion (Fig. 1C). In addition, some preparations showed a bright speckled surface staining of immunoreactive axons (Fig. 1C) and cell bodies. A broadly similar pattern of immunostaining was seen in cervical and trigeminal ganglia. Control sections stained with the RTA antiserum preadsorbed with RTA antigen (see Materials and methods) displayed only background staining, with none of the specific features described above.
Fig. 1.
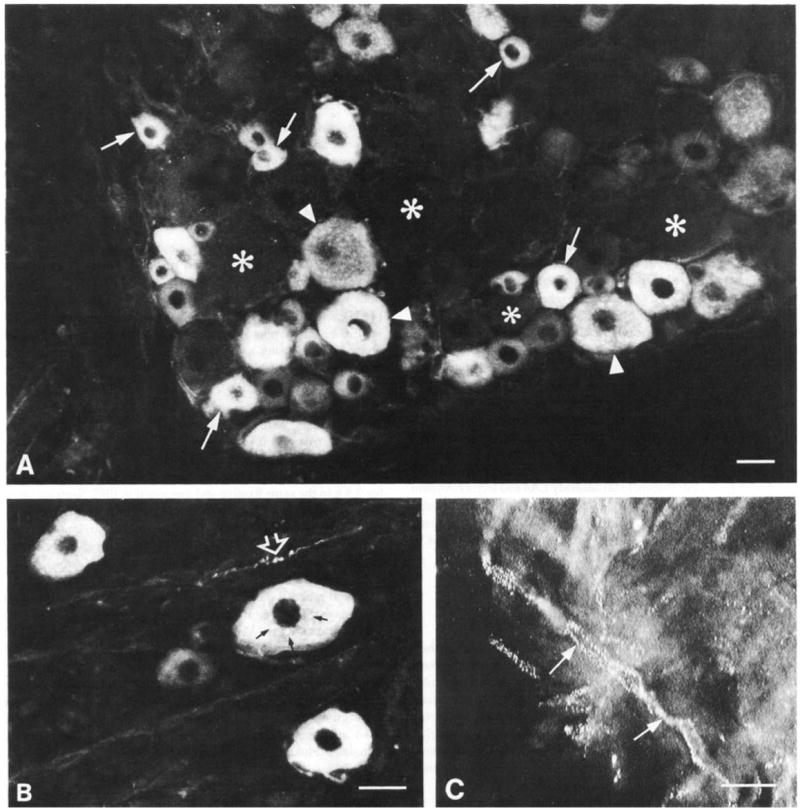
Immunofluorescence micrographs showing trkA-immunoreactive cells (A, B) and axons (B, C) in L5 dorsal root ganglia. (A) TrkA-immunoreactive cells are mainly small (arrows) but medium sized cells (arrowheads) are also present. Asterisks indicate neighbouring non-immunoreactive cells. (B) Immunostained cells show diffuse immunostaining plus granular staining (small arrows) which corresponds to Golgi complexes. The open arrowhead indicates a trkA-immunoreactive axon coursing between the cells. (C) Immunostained axons (arrows) in a section of nerve adjoining the ganglion. In some preparations the trkA immunoreactivity had this characteristic speckled appearance. Scale bars = 25 μm.
Using fluorogold counterstaining or dark-field illumination, non-immunostained DRG cells could be observed in addition to immunostained ones. Counts of the percentage of trkA-immunoreactive L4 and L5 DRG cells in different preparations varied from 37.2 to 45.6% with a mean of 40.4% (Table 1). Most of the cells appeared small, although an appreciable minority were medium in diameter (e.g. Fig. 1A). Few large cells were seen. This impression was confirmed by the quantitative analysis of the distribution of cell profiles (Fig. 2). The trkA-immunostained cells represented 55% of small cells (diameters <30 μm) but only 19%of cells with diameter >30 μm.
TABLE 1.
Percentage of single-and-double labelled trkA-immunoreactive cells in L4 and L5 ganglia
| trkA single labeling trkA (32, 5) | trkA +ve DRG cells 448 | all DRG cells 1108 | % DRG cells expressing trkA |
||
|---|---|---|---|---|---|
| 40.4% | |||||
| trkA double labelling | trkA +ve and other +ve (n) | trkA +ve and other −ve (n) | trkA −ve and other +ve (n) | % of trkA expressing other | % of other expressing trkA |
| trkA + CGRP (24, 4) | 347 | 30 | 42 | 92% | 89.2% |
| trkA + RT97 (30, 4) | 74 | 333 | 173 | 18.2% | 27.8% |
| trkA + IB4 (38, 4) | 80 | 532 | 612 | 13.1% | 11.6% |
| trkA + LA4 (20, 4) | 14 | 348 | 162 | 3.9% | 8.0% |
+ve, immunoreactive, −ve, non-immunoreactive. Data from LA and L5 ganglia were pooled. n, number of cell profiles counted. The numbers in parentheses in column 1 indicate the number of sections analysed, and the number of animals the sections were taken from.
Fig. 2.

Size distribution of trkA-immunostained cells in L4 and L5 dorsal root ganglia. 230 cell profiles were counted in 12 sections taken from three animals. Results from L4 and L5 were pooled.
Colocalization of trkA with other neuronal markers
Double labelling of L5 ganglia allowed analysis of the neurochemical characteristics of trkA-immunoreactive cells. Very clear correlations were apparent. More than 90% of trkA-immunoreactive cells were also immunostained for CGRP (Fig. 3A, B; Table 1). In contrast, few trkA-immunoreactive cells were positive for the other neuronal markers. Eighteen percent of trkA-immunoreactive cells were immunostained for RT97, and conversely 28% of RT97-immunoreactive cells showed trkA immunostaining (Fig. 4A, B). Although a large number of DRG cells were labelled for either trkA or the lectin IB4 (Fig. 4C, D), very few were double-labelled (≤13%, Table 1). Even fewer cells were immunoreactive for both trkA and LA4 (≤8%, Fig. 4E, F; Table 1).
Fig. 3.

Immunofluorescence double labelling for CGRP (A) and trkA (B) in L5 dorsal root ganglia. Most of the cells in this field of view express immunoreactivity for both antigens. Numbers indicate pairs of double-labelled cells. In addition, a few CGRP single-labelled cells are present (asterisks). Arrows in A indicate CGRP-immunoreactive axons. Scale bars = 25 μm.
Fig. 4.

Pairs of micrographs showing immunofluorescence double labelling for trkA (A, C, E) and RT97 (B), IB4 (D) and LA4 (F). L5 dorsal root ganglia. (A, B) Several medium to large sized cells are visible which express both trkA and RT97 immunoreactivity (numbers indicate pairs of double-labelled cells) but many small trkA single-labelled cells (arrows) are also present. Open arrows in B indicate large-diameter RT97-immunoreactive axons. (C, D) TrkA single-labelled cells (arrows in C) and IB4 single-labelled cells (open arrow in D) are visible together with a single double-labelled cell (I). (E, F) Three trkA single-labelled cells are visible in E (arrows) and adjoin a group of LA4 single-labelled cells (open arrows in F). The cell indicated by an asterisk is also single-labelled for trkA but in panel F shows some background non-specific staining. Scale bars = 25 μm.
The correlation between trkA and CGRP expression was also apparent in the intensity of immunostaining observed in individual neurons (Fig. 5). Quantitation revealed that the immunofluorescence grey level of the two antigens was well correlated (r2 = 0.42 and 0.63 in two separate experiments), and this correlation was statistically highly significant (P < 0.01 in both cases).
Fig. 5.

Correlation between CGRP and trkA immunostaining intensities (mean grey levels, arbitrary units), r2 = 0.63.
TrhA immunostaining in spinal cord
In lumbar spinal cord, a dense band of immunostaining was present in the superficial dorsal horn (Fig. 6A). High-magnification analysis revealed that this consisted of immunoreactive fibres and varicosities (Fig. 6B) which were confined mainly to laminae I and II outer (IIo) and to patches in the deeper dorsal horn (Fig. 6B). Double staining revealed that areas of trkA and CGRP immunoreactivity were largely coincident, with double labelling apparent both in superficial (Fig. 7) and deep dorsal horn. In animals with a unilateral L5 dorsal rhizotomy, a clear depletion of CGRP immunostaining was evident in the lateral region of lamina II on the side of the lesion (Fig. 7C). TrkA immunoreactivity was depleted in exactly the same region as the CGRP (Fig. 7D).
Fig. 6.
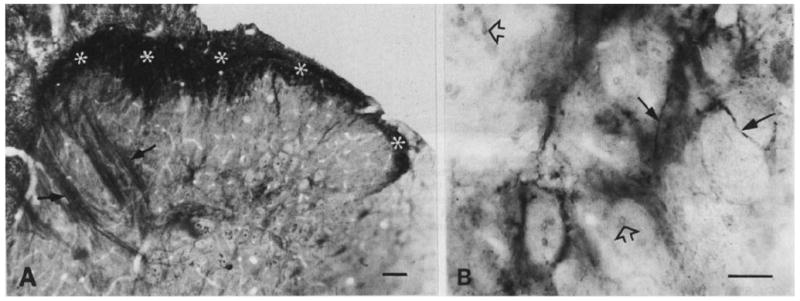
Low (A) and high (B) magnification micrographs showing trkA-immunoreactive terminals in the dorsal horn of lumbar spinal cord. Avidin–biotin peroxidase immunostaining with OsO4 contrasting. (A) TrkA immunostaining is present mainly in the superficial layers (asterisks) of the dorsal horn. Arrows indicate non-immunostained but osmium-contrasted myelinated fibre bundles which penetrate deep dorsal horn. (B) A patch of trkA immunoreactivity in lamina V. The staining consists of fine varicose axons (arrows). Open arrowheads indicate neighbouring non-immunostained but osmium-contrasted myelinated axons. Scale bars = 50 μm (A), 25 μm (B).
Fig. 7.
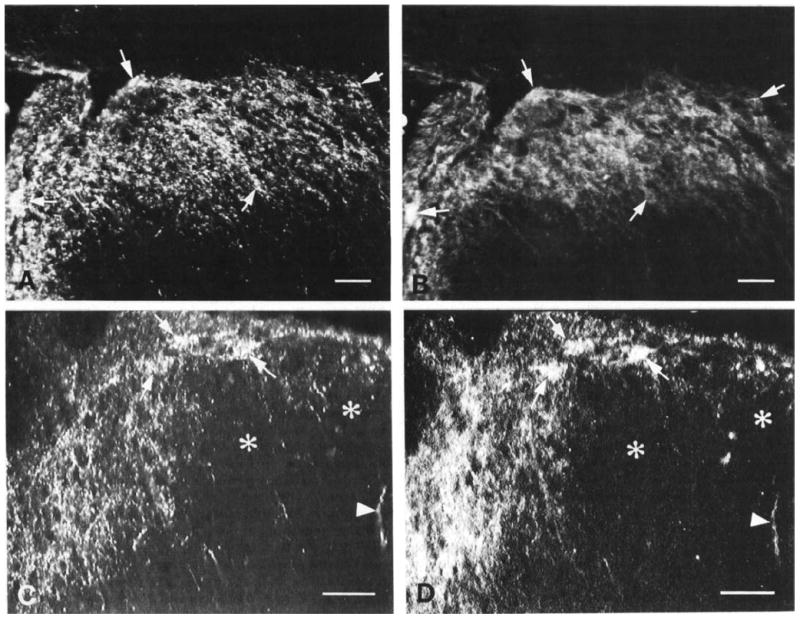
Pairs of micrographs showing immunofluorescence double labelling for CGRP (A, C) and trkA (B, D) in laminae I and II of lumbar dorsal horn. (A. B) Normal animal. Immunostaining for CGRP (A) and trkA (B) is largely coincident in laminae I and II. Arrows indicate double-labelled patches of axons and terminals. (C, D) Animal with a unilateral L5 dorsal rhizotomy. Ipsilateral dorsal horn. Marked depletion of both CGRP (A) and trkA (B) immunofluorescence is apparent in the lateral dorsal horn (asterisks). Arrowheads indicate an isolated spared fibre which is double-labelled for CGRP and trkA. Arrows indicate remaining double-labelled fibre bundles in the adjoining intact medial dorsal horn. Scale bars = 50 μm.
Discussion
TrkA expression by dorsal root ganglion cells
In these experiments we have observed that a polyclonal antibody (RTA) directed against the extracellular domain of the trkA receptor (Clary et al, 1994) provides robust staining of a major subpopulation of DRG cell bodies and central terminals. We found that 40% of L5 DRG neurons are trkA-immunoreactive. This value is in close agreement with the number of rat lumbar DRG neurons expressing trkA mRNA (cf. 40% found by Verge et al., 1992, and 44% by McMahon et al., 1994) and those showing high-affinity binding of iodinated NGF (40% reported by Verge et al., 1992). This suggests that the antibody recognizes essentially all trkA-producing cells, a conclusion supported by our adsorption controls and by other immunological controls. Immunoprecipitation, immunoblot and inhibition of NGF binding indicate that RTA recognizes trkA but not LNGFR, trkB or trkC (Clary et al., 1994). The specificity of the antiserum is also supported by the fact that the RTA antiserum labels cholinergic cells in the basal forebrain (Sobreviela et al., 1994), in agreement with the results of workers using other antisera (Steininger et al., 1993).
We observed that trkA-immunostained cells are mostly of small diameter, but with a significant number of medium size. Immunostained terminals are abundant in laminae I and IIo and in occasional patches in deeper dorsal horn. The cell size distribution of trkA-immunoreactive DRG cells agrees closely with results from other methods of studying trkA expression. For example, using in situ hybridization one of us (McMahon et al., 1994) recently reported that about three-quarters of lumbar DRG cells that express trkA mRNA are <30 μm in diameter, but that the very largest cells are unstained. Other studies using in situ hybridization (Mu et al., 1993), high-affinity NGF binding (Verge et al., 1989a, b) and retrograde transport of iodinated NGF (DiStefano et al., 1992) also report trkA expression in small and some medium sized cells.
The depletion following dorsal rhizotomy, and the codistribution with CGRP, indicate that trkA immunoreactivity in the superficial dorsal horn is derived predominantly from primary afferents. TrkA immunoreactivity has not been reported previously in the spinal cord. Indeed Arvidsson and colleagues (1994) recently reported that another trkA antibody (antibody sc-118; Santa Cruz Biotechnology, Santa Cruz, CA) failed to give specific staining in the spinal cord, although the same antibody has been observed to stain small DRG cells (Risling et al., 1994). However, high-affinity binding sites for NGF have been described in laminae I and II and in patches in deep dorsal horn (Richardson et al., 1986; Verge et al., 1989b) and have a distribution that is strikingly similar to that we observe for trkA. A very similar distribution has also been reported for LNGFR (Yip and Johnson, 1987), LNGFR immunoreactivity in the spinal and medullary dorsal horn showing a high degree of colocalization with CGRP (Fried et al., 1990). It thus appears clear that a group of small and medium sized DRG cells express trkA both in their cell bodies and central terminals. The neurochemical features of this group, and the functional implications of trkA expression, will now be discussed in turn.
Coexpression of trkA with other neumnal markers
In this study we employed various neurochemical markers in order to further characterize the trkA-expressing DRG cells. DRG cells can be divided into three minimally overlapping subgroups (Fig. 8). Firstly, the population traditionally described as ‘large light’ can be identified by the anti-neurofilament antibody RT97 (Lawson et al., 1984). RT97-immunoreactive cells constitute ~40% of lumbar DRG cells, have mostly myelinated axons, and in the periphery are presumably connected to mechanosensitive endings such as Pacinian corpuscles, hair follicle afferents and muscle spindles (for review see Lawson, 1992). A second population of DRG cells contain cell surface glycoconjugates with terminal d-galactose residues and can be identified with markers such as the monoclonal antibody LA4 (Alvarez et al., 1991) and the lectin Griflonia simplicifolia IB4 (Silverman and Kruger, 1990). This population appears to correspond more or less to the cell group expressing enzyme activity for fluoride- resistant acid phosphatase (FRAP) and thiamine monophosphatase (Silverman and Kruger, 1990). LA4-immunoreactive cells constitute ~30% of trigeminal and dorsal root ganglion cells (Alvarez et al., l991). have unmyelinated axons (Alvarez et al., 1989) and do not show RT97 immunoreactivity (Averill, S. and Priestley, J. V., unpublished observations). They thus represent about half of the classical ‘small dark’ cell population which innervates predominantly nociceptors and thermoreceptors. The third population of DRG cells consists of those that constitutively synthesize neuropeptides. The best marker for this group is the neuropeptide that is expressed by the largest number of DRG cells, namely CGRP. About 45% of lumbar DRG cells contain CGRP immunoreactivity, and peptides such as substance P, somatostatin and galanin coexist with CGRP (Lawson, 1992). CGRP-immunoreactive cells are predominantly small with unmyelinated axons and hence form the other half of the ‘small dark’ population. However, there is also a significant number of medium sized CGRP-immunoreactive cells, such that about one-third of CGRP cells show RT97 immunoreactivity (Lawson, 1992). Of the three markers LA4, IB4 and FRAP, LA4 shows the least overlap with CGRP and FRAP shows the most (as many as 50% of FRAP-positive cells contain CGRP immunoreactivity; Carr et al., 1990). Hence LA4 appears to be the most reliable marker for non-peptide-producing small DRG cells (Alvarez et al., 1991). The three main populations described above are summarized in Figure 8. However, note that this represents the normal, constitutive state of DRG cells. Following nerve or tissue damage the expression of all these markers changes (reviewed in Hökfelt et al., 1994).
Fig. 8.

Pie chart showing neurochemically defined dorsal root ganglion subpopulations.
A striking result of our study is that trkA expression corresponds almost perfectly with the CGRP population. Ninety-two percent of trkA-immunoreactive cells contain CGRP. In contrast the non-peptide LA4nB4 population is largely trkA-negative, with only 6% of LA4-immunoreactive cells showing trkA immunoreactivity. The overlap observed between trkA and the markers RT97, IB4 and LA4 corresponds closely to the known overlap between CGRP and these markers (described above). The close correlation between trkA and CGRP is further indicated by the highly significant correlation in immunostaining intensity in individual cells. This was not simply a consequence of some ganglia staining more poorly than others, since the correlation was clear in individual sections. The correlation between trkA and CGRP extends also to the central terminals of DRG cells. TrkA and CGRP are coincident in lamina I, lamina IIo and in patches of deep dorsal horn but are both largely absent from lamina II inner, the site of termination of the LA4 population (Alvarez et al., 1991).
These findings agree closely with the results of an earlier NGF binding study by Verge and colleagues (1989a), who concluded that CGRP and substance P cells possess high-affinity NGF receptors but somatostatin and thiamine monophosphatase-positive cells do not possess these receptors. Our results extend this study by using a cleaner marker for the non-peptide small cell population, by showing that it is CGRP content and not merely cell size or neurofilament content that correlates with trkA expression, and by examining central terminals. The absence of NGF binding sites reported by Verge et al. (1989a) for somatostatin-immunoreactive cells is also consistent with our results. The small number of somatostatin-immunoreactive DRG cells represent the one peptide group known to show LA4 and IB4 staining (e.g. Ambalavanar and Morris, 1992). It is possible that these cells lie at the interface between the CGRP and LA4 populations (Fig. 8) and correspond to the small number of CGRP-immunoreactive cells observed by us and by Verge et al. (1989a) to lack trkA.
Functional significance of trkA expression by the CGRP subpopulation
NGF appears to have quite different effects on somatosensory neurons depending on their stage of development (for review see Lewin and Mendell, 1993). Although adult DRG cells are not dependent on NGF for survival (e.g. Gorin and Johnson, 1980; Schwartz et al., 1982), NGF in adults does appear to modulate functions such as the expression of certain neuropeptides (reviewed by Lindsay, 1992). sensitivity of nociceptors (Lewin and Mendell, 1993). cell survival and regeneration following axotomy (Rich et al., 1987). and sprouting by intact collaterals into denervated targets (reviewed by Diamond et al., 1992). For each of these functions there is evidence that not all DRG cells are affected equally and that it is the small-cell, CGRP/substance P-producing, nociceptive population that is modulated by NGF. Thus the expression of substance P and CGRP is selectively regulated by NGF (Lindsay, 1992): NGF sensitizes C and Aδ mechanoreceptors but does not affect low-threshold mechanoreceptors (Lewin and Mendell, 1993; Dmitrieva and McMahon, 1994). NGF is more effective at preventing axotomy-produced atrophy in small cells than in large cells (Rich et al., 1987), and Aδ and C fibres, but not Aα/β fibres, show NGF-dependent collateral sprouting (Diamond et al., 1992). Our demonstration that trkA is expressed by the CGRP-immunoreactive DRG cell population but not by the majority of the RT97, large-cell population is entirely consistent with these selective effects of NGF. As regards regeneration and sprouting, it is also interesting to note that the CGRP/trkA population, but not the RT97 or LA4 populations, constitutively expresses GAP-43 (Verge et al., 1990; Averill et al., 1993).
The presence of trkA on the central terminals of the CGRP-immunoreactive cells may simply reflect the fact that DRG anterograde axonal transport does not distinguish between central and peripheral terminals. However several early studies suggest that the central trkA terminals may be functional. Iodinated NGF injected into the adult spinal cord is retrogradely transported to DRG cell bodies (Richardson and Riopelle, 1984), and it has been proposed that under certain circumstances DRG neurons require central support from NGF along with other trophic factors (Johnson and Yip, 1985). The presence of NGF mRNA in the spinal cord (Shelton and Reichardt, 1986) has been disputed (Korsching and Thoenen, 1985), but recently several groups have described NGF or pro-NGF immunoreactivity in cell bodies in the spinal and medullary dorsal horn (Senut et al., 1990; Nishio et al., 1994). Our results suggest that the location and function of spinal cord-derived NGF deserves closer study.
The lack of expression of trkA in the LA4/IB4 group is striking and also worthy of further investigation. Most studies on the role of NGF in the adult have not examined this cell population, although there is evidence that this group is more vulnerable to nerve damage than the CGRP/substance P population (Baranowski et al., 1991). Paradoxically, there are several reports indicating that NGF can modulate FRAP expression. Thus Csillik et al. (1985) and Fitzgerald et al. (1985) observed in vivo that, after sciatic nerve section, the expected down-regulation of FRAP could be reversed by administration of NGF to the damaged nerve. Further, in vitro, Winter and colleagues (1988) reported that capsaicin sensitivity is regulated by levels of NGF, and it has long been known that capsaicin treatment in vivo diminishes FRAP activity (Jancsó and Knyihár, 1975). These types of study deserve to be repeated using a cleaner marker for the non-peptide population (see discussion above). Another possibility is that the effects of NGF on the FRAP population are actually indirect, mediated by local paracrine release of BDNF or NT-3, which subsequently act on trkB or trkC receptors (Eriksson et al., 1994). However, in situ hybridization studies indicate that a significant percentage of DRG cells do not express trkA, trkB or trkC receptors (34%, McMahon et al., 1994; 26%, Wright and Snider, 1994). and it seems likely that this group corresponds to the LA4 population. It is therefore possible that a novel high-affinity neurotrophin receptor, with some sensitivity to NGF, is specifically expressed by these neurons. Alternatively this cell group may indeed be insensitive to any of the known neurotrophins. Why small, predominantly nociceptive neurons should be divided into peptidehrk-positive and non-peptide/trk-negative populations is presently not known.
Acknowledgments
This work was supported by the Medical Research Council (UK) and the Wellcome Trust. Dr J. Wood, and Drs T. M. Jessell and J. Dodd are gratefully thanked for provision of RT97 and LA4 antibodies respectively. Thanks also to Dr M. Wessendorf for providing the recipe for fluorogold counterstaining.
Abbreviations
- ABC
avidin–biotin peroxidase
- BDNF
brain-derived neurotrophic factor
- CGRP
calcitonin gene-related peptide
- DAB
3,3’ -diaminobenzidine
- DRG
dorsal root ganglion
- FRAP
fluoride-resistant acid phosphatase
- LNGFR
low-affinity NGF receptor
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- PBS
phosphate-buffered saline
References
- Alvarez FJ, Rodrigo J, Jessell TM, Dodd J, Priestley JV. Ultrastructure of primary afferent fibres and terminals expressing alpha-galactose extended oligosaccharides in the spinal cord and brainstem of the rat. J Neurocytol. 1989;18:631–645. doi: 10.1007/BF01187083. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Moms HR, Priestley JV. Sub-populations of smaller diameter trigeminal primary afferent neurons defined by expression of calcitonin gene-related peptide and the cell surface oligosaccharide recognized by monoclonal antibody LA4. J Neurocytol. 1991;20:716–731. doi: 10.1007/BF01187846. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Morris R. The distribution of binding by isolectin I-B4 from Griflonia simplicifolia in the trigeminal ganglion and brainstem trigeminal nuclei in the rat. Neuroscience. 1992;47:421–429. doi: 10.1016/0306-4522(92)90256-2. [DOI] [PubMed] [Google Scholar]
- Andreev N, Inuishin M, McMahon SB. Nerve growth factor acutely enhances the responsiveness of dorsal horn neurones to noxious heat. Soc Neurosci Abstr. 1994;20:455.16. [Google Scholar]
- Arvidsson U, Risling M, Frisen J, Piehl F, Fried K, Hökfelt T, Cullheim S. TrkC-like immunoreactivity in the primate descending serotoninergic system. Eur J Neurosci. 1994;6:230–236. doi: 10.1111/j.1460-9568.1994.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Averill S, Ching Y, Wilkin G, Lopez Costa J, Priestley JV. The growth associated protein GAP-43 in adult rat dorsal root ganglion cells is confined to specific neurochemical subpopulations. Proc XXXII Congress IUPS. 1993:51.20. abstract. [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. TrkA immunohistochemistry in adult rat dorsal root ganglia. Soc Neurosci Abstr. 1994;20:23.2. [Google Scholar]
- Baranowski AP, McMahon SB, Ng GYT, Priestley JV, Wall PD. Differential response of markers for small diameter dorsal root ganglion cells projecting into blind nerve stumps. Proceedings, International Symposium on Pain and Neuroplasticity, Montreal. 1991 abstract. [Google Scholar]
- Carr PA, Yamamoto T, Nagy JI. Calcitonin gene-related peptide in primary afferent neurons of rat: co-existence with fluoride- resistant acid phosphatase and depletion by neonatal capsaicin. Neuroscience. 1990;36:751–760. doi: 10.1016/0306-4522(90)90017-x. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992;9:779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Chou DK, Dodd J, Jessell TM, Costello CE, Jungalwala FB. Identification of alpha-galactose (alpha-fucose)-asialo-GM1 glycolipid expressed by subsets of rat dorsal root ganglion neurons. J Biol Chem. 1989;264:3409–3415. [PubMed] [Google Scholar]
- Clary DO, Weskamp G, Austin LAR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Bid Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csillik B, Schwab ME, Thoenen H. Transganglionic regulation of central terminals of dorsal root ganglion cells by nerve growth factor (NGF) Brain Res. 1985;331:11–16. doi: 10.1016/0006-8993(85)90709-7. [DOI] [PubMed] [Google Scholar]
- Davies AM. Cell death and the trophic requirements of developing sensory neurons. In: Scott SA, editor. Sensory Neurons Diversity, Development, and Plasticity. Oxford University Press; New York: 1992. pp. 194–214. [Google Scholar]
- Davies AM, Lee KF, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- Diamond J, Gloster A, Kitchener P. Regulation of the sensory innervation of skin: trophic control of collateral sprouting. In: Scott SA, editor. Sensory Neurons Diversity, Development, and Plasticity. Oxford University Press; New York: 1992. pp. 309–332. [Google Scholar]
- DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, McMahon SB. NGF acutely sensitises primary sensory neurones. Soc Neurosci Abstr. 1994;20:109.10. [Google Scholar]
- Eriksson NP, Lindsay RM, Aldskogius HNA. BDNF and NT-3 rescue sensory but not motoneurones following axotomy in the neonate. NeuroReport. 1994;5:1445–1448. [PubMed] [Google Scholar]
- Fitzgerald M, Wall PD, Goedert M, Emson PC. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 1985;332:131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Fried K, Risling M, Arvidsson U, Paulie S. Nerve growth factor receptor-like immunoreactivity in nerve fibers in the spinal and medullary dorsal horn of the adult monkey and cat: correlation with calcitonin gene-related peptide-like immunoreactivity. Brain Res. 1990;536:321–326. doi: 10.1016/0006-8993(90)90043-b. [DOI] [PubMed] [Google Scholar]
- Goedert M, Stoeckel K, Otten U. Biological importance of the retrograde axonal transport of nerve growth factor in sensory neurons. Proc Nail Acad Sci USA. 1981;78:5895–5898. doi: 10.1073/pnas.78.9.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin PD, Johnson EM. Effects of long-term nerve growth factor deprivation on the nervous system of the adult rat: an experimental autoimmune approach. Brain Res. 1980;198:27–42. doi: 10.1016/0006-8993(80)90341-8. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17:22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Knyihár E. Functional linkage between nociception and fluoride-resistant acid phosphatase activity in the Rolando substance. Neurobiology. 1975;5:42–43. [PubMed] [Google Scholar]
- Johnson EM, Yip HK. Central nervous system and peripheral nerve growth factor provide trophic support critical to mature sensory neuronal survival. Nature. 1985;314:751–752. doi: 10.1038/314751a0. [DOI] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor supply for sensory neurones: site of origin and competition with the sympathetic nervous system. Neurosci Lett. 1985;54:201–205. doi: 10.1016/s0304-3940(85)80079-3. [DOI] [PubMed] [Google Scholar]
- Lawson S, Harper AA, Harper EI, Garson JA, Anderton RH. Monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984;228:263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Morphological and biochemical cell types of sensory neurons. In: Scott SA, editor. Sensory Neurons Diversity, Development, and Plasticity. Oxford University Press; New York: 1992. pp. 27–59. [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. The role of neurotrophic factors in functional maintenance of mature sensory neurons. In: Scott SA, editor. Sensory Neurons Development, Diversity, and Plasticity. Oxford University Press; New York: 1992. pp. 404–420. [Google Scholar]
- Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS. Neurotrophic factors—from molecule to man. Trends Neurosci. 1994;17:182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T, Furukawa S, Akiguchi I, Oka N, Ohnishi K, Tomimoto H, Nakamura S, Kimura J. Cellular localization of nerve growth factor-like immunoreactivity in adult rat brain—quantitative and immunohistochemical study. Neuroscience. 1994;60:67–84. doi: 10.1016/0306-4522(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Rich KM, Luszczynski JR, Osborne PA, Johnson EM., Jr Nerve growth factor protects adult sensory neurons from cell death and atrophy caused by nerve injury. J Neurocytol. 1987;16:261–268. doi: 10.1007/BF01795309. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Riopelle RJ. Uptake of nerve growth factor along peripheral and spinal axons of primary sensory neurons. J Neurosci. 1984;4:1683–1689. doi: 10.1523/JNEUROSCI.04-07-01683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Riopelle RJ. Distribution of neuronal receptors for nerve growth factor in the rat. J Neurosci. 1986;6:2312–2321. doi: 10.1523/JNEUROSCI.06-08-02312.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risling M, Dalsgaard CJ, Frisen J, Sjogren AM, Fried K. Substance P-, calcitonin gene-related peptide, growth-associated protein-43, and neurotrophin receptor-like immunoreactivity associated with unmyelinated axons in feline ventral roots and pia mater. J Comp Neurol. 1994;339:365–386. doi: 10.1002/cne.903390306. [DOI] [PubMed] [Google Scholar]
- Schwartz JP, Pearson J, Johnson EM. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res. 1982;244:378–381. doi: 10.1016/0006-8993(82)90102-0. [DOI] [PubMed] [Google Scholar]
- Senut MC, Lamour Y, Lee J, Brachet P, Dicou E. Neuronal localization of the nerve growth-factor precursor-like immunoreactivity in the rat brain. Inr J Dev Neurosci. 1990;8:65–80. doi: 10.1016/0736-5748(90)90024-v. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Reichardt LF. Studies on the expression of the beta nerve growth factor (NGF) gene in the central nervous system: level and regional distribution of NGF mRNA suggest that NGF functions as a trophic factor for several distinct populations of neurons. Proc Natl Acad Sci USA. 1986;83:2714–2718. doi: 10.1073/pnas.83.8.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kardower JH, Mufson EJ. TrkA imunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase and serotonin. J Comp Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Wainer BH, Klein R, Barbacid M, Palfrey HC. High-affinity nerve growth factor receptor (Trk)immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat brain. Brain Res. 1993;612:330–335. doi: 10.1016/0006-8993(93)91681-h. [DOI] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Benoit R, Riopelle RJ. Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor. J Neurocytol. 1989a;18:583–591. doi: 10.1007/BF01187079. [DOI] [PubMed] [Google Scholar]
- Verge VM, Riopelle RJ, Richardson PM. Nerve growth factor receptors on normal and injured sensory neurons. J Neurosci. 1989b;9:914–922. doi: 10.1523/JNEUROSCI.09-03-00914.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Tetzlaff W, Richardson PM, Bisby MA. Correlation between GAP43 and nerve growth factor receptors in rat sensory neurons. J Neurosci. 1990;10:926–934. doi: 10.1523/JNEUROSCI.10-03-00926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Merlio JP, Grondin J, Ernfors P, Persson H, Riopelle RJ, Hökfelt T, Richardson PM. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J Neurosci. 1992;12:4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Forbes CA, Sternberg J, Lindsay RM. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to the excitotoxin capsaicin. Neuron. 1988;1:973–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor expression defines distinct subpopulations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Yip HK, Johnson EM. Nerve growth factor receptors in rat spinal cord: an autoradiographic and immunohistochemical study. Neuroscience. 1987;22:267–279. doi: 10.1016/0306-4522(87)90217-x. [DOI] [PubMed] [Google Scholar]


