Abstract
Objective
Although partial meniscectomy is a risk factor for the development of knee osteoarthritis (OA), there is a lack of evidence that meniscal damage that is not treated with surgery would also lead to OA, suggesting that surgery itself may cause joint damage. Furthermore, meniscal damage is common. The aim of this study was to evaluate the association between meniscal damage in knees without surgery and the development of radiographic tibiofemoral OA.
Methods
We conducted a prospective case–control study nested within the observational Multicenter Osteoarthritis Study, which included a sample of men and women ages 50–79 years at high risk of knee OA who were recruited from the community. Patients who had no baseline radiographic knee OA but in whom tibiofemoral OA developed during the 30-month followup period were cases (n = 121). Control subjects (n = 294) were drawn randomly from the same source population as cases but had no knee OA after 30 months of followup. Individuals whose knees had previously undergone surgery were excluded. Meniscal damage was defined as the presence of any medial or lateral meniscal tearing, maceration, or destruction.
Results
Meniscal damage at baseline was more common in case knees than in control knees (54% versus 18%; P < 0.001). The model comparing any meniscal damage with no meniscal damage (adjusted for baseline age, sex, body mass index, physical activity, and mechanical knee alignment) yielded an odds ratio of 5.7 (95% confidence interval 3.4–9.4).
Conclusion
In knees without surgery, meniscal damage is a potent risk factor for the development of radiographic OA. These results highlight the need for better understanding, prevention, and treatment of meniscal damage.
Osteoarthritis (OA) is listed among the top 10 conditions representing a global disease burden, according to the World Health Organization, with the knee being one of the most frequently affected joints (1,2). After surgical removal of the damaged parts of meniscal tissue, knees are at high risk for the later development of OA (3–5). A torn meniscus combined with surgical resection leads to increased joint cartilage contact stress through altered load transmission, decreased shock absorption, and decreased joint stability (6–8). The correlation between the amount of meniscus removed and the risk of later OA implies that large resections may worsen the long-term prognosis (4,9).
Population-based data suggest that meniscal damage is present in at least one-third of the knees of middle-aged or elderly persons (10), and reports based on cohorts comprised mostly of patients with OA reveal a remarkably high frequency of meniscal pathology (11–15). Paradoxically, very little is known of the consequence of meniscal damage without surgical intervention. This is because, until now, studies of meniscal damage and the development of radiographic OA have focused on patients who have undergone meniscal surgery. Noninvasive magnetic resonance imaging (MRI) offers indirect visualization of the menisci, with sensitivity and specificity for detecting a meniscal tear of 82–96% (16). Based on cross-sectional studies (11,14,17) and longitudinal MRI studies of cartilage loss in knees with preexisting OA (18–20), it is plausible to assume that meniscal damage without surgical intervention is associated with the development of radiographic knee OA. Until now, however, no studies have evaluated this assumption. If meniscal damage represents a strong risk factor for knee OA in the absence of surgery, it would constitute a problem on a public health level in an aging population and justify increased efforts toward better understanding of etiology, prevention, and better treatment.
Therefore, in a prospective case–control trial nested within the Multicenter Osteoarthritis Study (MOST), we investigated the association between meniscal damage in knees without surgery and the development of radiographic tibiofemoral OA over 30 months in middle-aged and elderly persons.
Patients and Methods
The Multicenter Osteoarthritis Study
MOST was a large, prospective epidemiologic cohort study of individuals ages 50–79 years, in which the goal was to identify risk factors for incident symptomatic knee OA and progressive knee OA. Study subjects either had symptomatic knee OA at baseline or were at high risk of developing the disease. Factors considered to contribute to a high risk of knee OA included being overweight or meeting the criteria for obesity, frequent knee pain, aching, or stiffness on most of the preceding 30 days, a history of major knee injury, and previous knee surgery. All 3,026 subjects were recruited from 2 communities in the US (Birmingham, Alabama and Iowa City, Iowa) through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns.
Subjects were excluded if they screened positive for rheumatoid arthritis (21), had ankylosing spondylitis, psoriatic arthritis, chronic reactive arthritis, a severe medical condition that made continued participation in the study unlikely, bilateral knee replacement surgery, inability to walk without the help of another person or walker, or were planning to move out of the area during the next 3 years. Both knees were included in all except 102 persons (3.4%), who participated with only 1 knee (typically due to contralateral knee replacement surgery).
The baseline and 30-month assessments followed a similar protocol, and each included a telephone interview and clinic visit. Subjects completed surveys on physical activity (Physical Activity Scale for the Elderly [22]), knee symptoms, a history of knee injury (leading to a limited ability to walk for ≥2 days), and/or a history of knee surgery. All participants were weighed and had their height measured.
Acquisition and grading of knee radiographs
At the baseline and 30-month clinic visits, subjects underwent weight-bearing posteroanterior knee radiography, using a fixed-flexion protocol (23,24). One musculoskeletal radiologist (PA) and 1 of 2 rheumatologists (BS or DF) graded all films according to the Kellgren/Lawrence (K/L) scale (25); discrepancies were adjudicated by a panel of 3 readers (PA, BS, and DF), all of whom were experienced in reading study films. Readers were blinded to MRI findings and clinical data. Films were read paired, and the readers were not blinded to the sequence. The interrater reliability for determining K/L grade was κ = 0.80 for PA versus BS and κ = 0.77 for PA versus DF. Radiographic tibiofemoral OA was considered present if the knee had K/L grade ≥2 on the posteroanterior film, and patellofemoral OA was determined (via the lateral film) as the presence of either a grade 2 osteophyte or grade 1 joint space narrowing with the co-occurrence of a bony feature (26).
Full-limb radiographs of both legs were obtained at baseline. The mechanical axis was defined as the angle formed by the intersection of a line from the center of the head of the femur to the center of the tibial spines, and a second line from the center of the talus to the center of the tibial spines. The interobserver intraclass correlation coefficient (ICC) for determining the mechanical axis was 0.99 (P < 0.001).
Case and control knees
For the present study, we selected case and control knees within MOST for MRI readings (Figure 1). Of 2,713 participants who had completed their 30-month followup visit, 2,661 persons had their paired radiographs read for knee OA development or progression (51 persons did not consent to knee radiography at the 30-month followup, and films for 1 subject were lost). Knees eligible for the development of radiographic OA at 30 months were those with no definite tibiofemoral OA (K/L grade 0 or 1) or patellofemoral OA at the baseline examination (3,111 knees in 1,863 subjects). Case knees were defined as those in which either tibiofemoral OA (K/L grade ≥2) or patellofemoral OA (or both) was present on the 30-month radiograph. To satisfy the definition for development of tibiofemoral OA, we required the visual presence of loss of joint space (we did not measure this quantitatively) in addition to osteophyte development or enlargement (Figure 2). We did not characterize any knee as having developed OA if the only change observed on the followup radiograph was enlargement of an incipient osteophyte.
Figure 1.
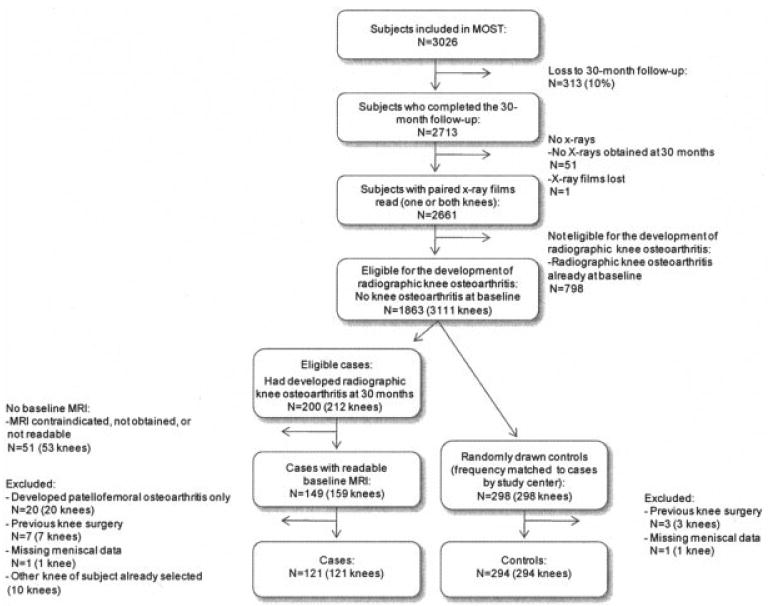
Study sample selection procedure. MOST = Multicenter Osteoarthritis Study; MRI = magnetic resonance imaging.
Figure 2.
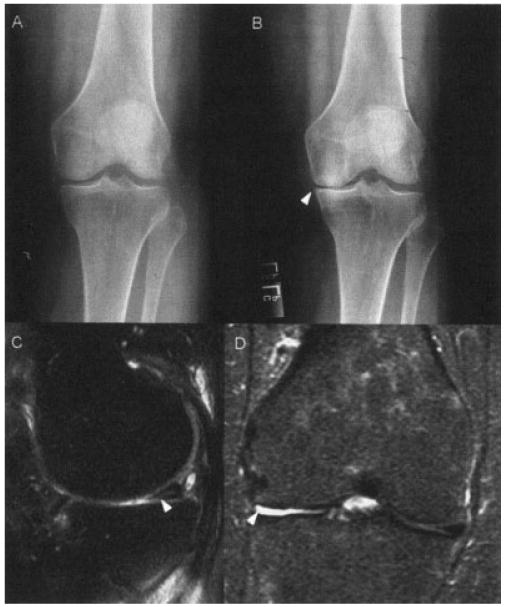
A and B, Radiographs of the knee of a subject in the Multicenter Osteoarthritis Study (MOST) who did not have osteoarthritis (OA) at baseline (A) but in whom OA had developed at the 30-month visit (B). At 30 months, the subject had joint space narrowing and an enlarging marginal tibial osteophyte in the medial compartment (arrowhead). C and D, Examples of meniscal damage in 2 other MOST subjects. Proton-density magnetic resonance imaging scans show a grade 1 meniscal tear (arrowhead) extending into the inferior surface of the posterior horn of the medial meniscus (sagittal view) in 1 subject at baseline (C), and complete maceration/destruction (grade 4; arrowhead) of the body of the lateral meniscus (coronal view) in the other subject at 30 months (D). In addition, the well-defined hyperintense area posterior to the torn meniscus (C) represents part of a Baker's cyst.
We identified 212 case knees with incident OA in 200 subjects. Of these, 149 subjects had at least 1 case knee for which a readable baseline MRI scan was available. Among the 10 subjects who had 2 case knees with readable MRI scans, 1 was selected for reading, in a random manner. We randomly selected 298 control knees, 1 per subject and in a 2:1 ratio to case knees, frequency-matched to cases by study center, from among all subjects eligible for incident knee OA in whom it did not develop and for whom a readable baseline MRI scan was available. We did not match for age and sex. For the present analysis, we excluded 20 case knees in which only incident patellofemoral, not tibiofemoral, OA developed and 7 case knees and 3 control knees with any type of previous knee surgery according to the individual's self-report. Also, 1 case knee and 1 control knee were excluded due to missing data for meniscus status on MRI. Therefore, 121 case knees and 294 control knees with baseline MRI scans were available for analysis.
Acquisition and grading of knee MRI scans
At baseline, knee MRI scans of all MOST participants who were willing and eligible were obtained with a 1.0T MR system (OrthOne; Oni, Wilmington, MA) with a circumferential transmit–receive extremity coil. MRIs were performed using sagittal and axial fat-suppressed fast spin-echo proton density–weighted sequences (repetition time [TR] 5,800/2,500 msec, time to echo [TE] 35 msec, slice thickness 3 mm, field of view [FOV] 14 cm, matrix 288 × 192 pixels), and coronal STIR sequence (TR 7,820 msec, TE 15 msec, slice thickness 3 mm, FOV 14 cm, matrix 256 × 256 pixels) (27). Using the same protocol, we also obtained readable 30-month followup MRI scans of 112 case knees (93%) and 274 control knees (93%).
Two musculoskeletal radiologists (AG and FR), who were blinded to case/control status and clinical and radiographic data, read the paired images, with knowledge of the time sequence. The distribution of case and control knees was even between the 2 readers. Meniscal tear, maceration, and/or destruction, which in this study are referred to as meniscal damage, were graded according to the Whole-Organ MRI Score (WORMS) method (28). The anterior horn, body segment, and the posterior horn of the medial and lateral menisci were graded separately on a scale of 0–4, where 0 = intact, 1 = minor radial or parrot-beak tear, 2 = nondisplaced tear (breaking 2 or more surfaces), 3 = displaced tear or partial maceration or destruction, and 4 = complete maceration or destruction (interobserver weighted κ = 0.80). The readers regarded an increased intrameniscal signal (often a linear signal within the meniscus) as a meniscal tear when it communicated with the inferior or superior margin of the meniscus on at least 2 slices (Figure 2).
For analyses of associations of meniscal damage, we used a collapsed categorical scale, where 0 = no damage, 1 = minor tear (extending to the surface, as described above), and 2 = nondisplaced tear, displaced tear, maceration, or destruction. The highest grade from any region on the medial or lateral side was used for analyses. For a dichotomized predictor variable, we defined meniscal damage as having a meniscal grade of ≥1. The meniscal damage detected is typical of damage that also would have been identified by a radiologist on a routine knee MRI scan in a clinical setting.
Medial and lateral meniscal extrusion were graded as 0 = absent, 1 = ≤50%, and 2 = >50% from the midposterior coronal slice where the medial tibial spine was of maximal volume. The point of reference for meniscal extrusion was the tibial plateau osteochondral junction at the joint margin (excluding osteophytes) (29). Cartilage status was also graded semiquantitatively according to the WORMS method (28) (ICC = 0.64). We defined a WORMS cartilage grade of 2 (partial-thickness focal defect <1 cm in greatest width) or higher as a cartilage lesion in any of the following 10 tibiofemoral regions: anterior, posterior, and central surface of the medial and lateral tibial plateau and the central and posterior articular surface of the medial and lateral femur.
Statistical analysis
We calculated means for continuous variables and proportions for categorical variables, then compared the difference in means and proportions between cases and controls using a t-test or chi-square/Fisher's test as appropriate. To evaluate the association between meniscal damage and developing radiographic tibiofemoral OA, we used multivariable logistic regression models. In addition to the primary model, we also evaluated the estimate of association in models stratified by sex. We also performed stratified analyses according to radiographic K/L grade or cartilage status on MRI as part of a sensitivity analysis, because a K/L grade of 1 can be considered as early-stage radiographic OA (30). We tested for interaction between the meniscal variable and mechanical knee alignment on the development of radiographic OA but observed no significant interaction (P = 0.13). All P values (2-tailed) were calculated using SAS for Windows, version 9.1 (SAS Institute, Cary, NC). P values less than or equal to 0.05 were considered significant.
Results
The age and sex distributions of patients (n = 121) were similar to those of control subjects (n = 294) (Table 1). Fifty-one eligible case subjects were excluded due to having no MRI at baseline (because an MRI was contraindicated, not obtained, or not readable); these subjects did not differ by age, sex, ethnicity, or clinical investigation site from the case subjects in the final sample but were slightly more obese (mean body mass index [BMI] 33.3 versus 31.0 kg/m2; P = 0.02).
Table 1.
Baseline characteristics of the cases and control subjects*
| Characteristic | Cases (n = 121) |
Controls (n = 294) |
P |
|---|---|---|---|
| Age, mean ± SD years | 61.6 ± 7.9 | 61.6 ± 7.7 | 0.99 |
| Female sex | 75 (62) | 175 (60) | 0.64 |
| Body mass index, mean ± SD kg/m2 | 31.0 ± 5.3 | 28.9 ± 4.4 | <0.001 |
| Physical activity scale score, mean ± SD | 181 ± 87 | 186 ± 94 | 0.63 |
| Self-report of knee injury | 26 (21) | 50 (17) | 0.46 |
| Frequent knee pain, aching, or stiffness | 36 (30) | 54 (18) | <0.001 |
| Mechanical axis† | |||
| Neutral (179–181°) | 35 (29) | 117 (40) | Reference |
| Valgus (>181°) | 19 (16) | 50 (17) | 0.47 |
| Varus (<179°) | 65 (55) | 126 (43) | 0.03 |
| Kellgren/Lawrence grade | |||
| 0 | 36 (30) | 231 (79) | Reference |
| 1 | 85 (70) | 63 (21) | <0.001 |
| Meniscal integrity‡ | |||
| No damage | 56 (46) | 240 (82) | Reference |
| Minor radial tear or parrot-beak tear | 15 (12) | 25 (9) | 0.01 |
| Nondisplaced or displaced tear, or partial maceration or destruction | 50 (41) | 29 (10) | <0.001 |
| Meniscal extrusion§ | |||
| None | 57 (48) | 209 (71) | Reference |
| ≤50% | 57 (48) | 81 (28) | <0.001 |
| >50% | 6 (5) | 4 (1) | 0.01 |
| Tibiofemoral cartilage lesion¶ | |||
| No | 29 (24) | 161 (55) | Reference |
| Yes | 92 (76) | 131 (45) | <0.001 |
Except where indicated otherwise, values are the number (%). Cases were designated as individuals in whom radiographic tibiofemoral osteoarthritis had developed at 30 months.
Values were missing for 2 cases and 1 control.
Highest damage grade from any region of the medial or lateral meniscus.
Values were missing for 1 case.
Whole-Organ Magnetic Resonance Imaging Score ≥2 (see Patients and Methods for further details).
Values were missing for 2 controls.
In case knees, i.e., knees in which radiographic tibiofemoral OA (K/L grade ≥2) had developed at the 30-month followup visit, the frequency of K/L grade 1 radiographic changes was greater than that in control knees (70% versus 21%; P < 0.001), and case knees had more cartilage lesions on MRI at baseline (76% versus 45%; P < 0.001). Also, case subjects had a higher BMI (31.0 versus 28.9 kg/m2; P < 0.001) and more often reported frequent knee pain and aching or stiffness for that knee (30% versus 18%; P < 0.001) (Table 1).
Meniscal damage (meniscal grade ≥1) at baseline was more frequent in case knees than control knees (54% versus 18%; P < 0.001), as was any meniscal extrusion (52% versus 29%; P < 0.001) (Table 1). No case or control knees had complete maceration or destruction (WORMS grade 4) of any meniscal region at baseline.
The crude and adjusted odds for the development of radiographic tibiofemoral OA were greater for knees with more severe meniscal damage at baseline (P for trend < 0.001). After controlling for age, sex, BMI, physical activity, and mechanical knee alignment in the primary model, the odds ratio (OR) for a minor meniscal tear versus no meniscal damage was 3.0 (95% confidence interval [95% CI] 1.4–6.4); for more severe meniscal damage versus no damage, the adjusted OR was 7.9 (95% CI 4.4–14) (Table 2). The c statistic for the adjusted model was 0.762. After removing the meniscus variable, the c statistic was reduced to 0.632.
Table 2.
Association between meniscal damage at baseline and the development of radiographic tibiofemoral OA at 30 months*
| Meniscus status† | No. (%) of knees in which radiographic OA developed | OR (95% CI) |
Adjusted OR (95% CI)‡ |
|---|---|---|---|
| No damage (n = 296) | 56 (19) | Reference | Reference |
| Minor radial tear or parrot-beak tear (n = 40) | 15 (38) | 2.6 (1.3–5.2) | 3.0 (1.4–6.4) |
| Nondisplaced or displaced tear, or partial maceration or destruction (n = 79) | 50 (63) | 7.4 (4.3–13) | 7.9 (4.4–14) |
OA = osteoarthritis; OR = odds ratio; 95% CI = 95% confidence interval.
Highest damage grade from any region of the medial or lateral meniscus.
Adjusted for baseline age, sex, body mass index, physical activity level, and mechanical knee alignment.
The adjusted model comparing any meniscal damage versus no meniscal damage yielded an OR of 5.7 (95% CI 3.4–9.4). The ORs for men and women with meniscal damage were essentially the same (6.5 [95% CI 2.9–14] and 4.9 [95% CI 2.5–9.5], respectively). All estimates remained essentially the same if additional adjustment for previous knee injury, clinical site, and MRI reader was performed (data not shown).
Importantly, evidence can be provided that K/L grade 1 radiographic changes represent “early” tibiofemoral OA. Thus, many knees—particularly case knees—would be misclassified as not having disease at baseline (i.e., not being “truly” incident cases of OA). Therefore, we performed an additional adjusted multivariable analysis excluding both case and control knees with K/L grade 1 at baseline. Meniscal damage (versus no meniscal damage) in the K/L grade 0 stratum was still strongly associated with the development of tibiofemoral OA (OR 7.4 [95% CI 3.2–17]).
We also evaluated the association between meniscal damage and the development of radiographic OA in knees stratified by tibiofemoral cartilage status on MRI scans. The crude analysis still suggested an association between meniscal damage and incident radiographic tibiofemoral OA in the stratum without focal cartilage lesions at baseline (Table 3), with the adjusted multivariable model yielding an OR of 10 (95% CI 3.5–29).
Table 3.
Association between meniscal damage and the development of radiographic tibiofemoral OA in knees with and those without tibiofemoral cartilage lesions at baseline*
| Tibiofemoral cartilage status at baseline | ||||
|---|---|---|---|---|
| Normal or increased cartilage signal only but no focal lesion | Partial- or full-thickness lesion(s) | |||
| Meniscus status | Cases (n = 29) |
Controls (n = 161) |
Cases (n = 92) |
Controls (n = 131) |
| No damage | 14 | 143 | 42 | 95 |
| Damage† | 15 | 18 | 50 | 36 |
| Crude OR (95% CI) | 8.5 (3.5–20) | 3.1 (1.8–5.5) | ||
| Adjusted OR (95% CI)‡ | 10 (3.5–29) | 3.5 (1.9–6.3) | ||
A cartilage lesion was defined as cartilage grade ≥2 according to the Whole-Organ Magnetic Resonance Imaging Score. Values for baseline cartilage status were missing for 2 control knees. Case knees were those in which radiographic tibiofemoral osteoarthritis (OA) had developed at 30 months. OR = odds ratio; 95% CI = 95% confidence interval.
Any region of the medial or lateral meniscus.
Adjusted for baseline age, sex, body mass index, physical activity level, and mechanical knee alignment.
Although the focus of our investigation was the relationship between meniscal damage at baseline and the development of OA in knees without meniscal damage at baseline, such damage developed in 29 case knees (57%) and 13 control knees (6%) during the 30-month followup period (P < 0.001). Fifty-four percent of case knees and 7% of control knees had an increase in any regional WORMS meniscal damage grade (P < 0.001). Meniscal extrusion developed or progressed in 40% of case knees compared with 2% of control knees (P < 0.001) (Table 4). In case knees at followup, 72 (86%) of the 84 extruded menisci had coexistent meniscal damage (i.e., meniscal damage in the same meniscus as was extruded). Among the subset of knees without tibiofemoral cartilage lesion on MRI at baseline, a cartilage lesion had developed in 21 case knees (84%) and 16 control knees (11%) at 30 months (P < 0.001).
Table 4.
Change in meniscus status from baseline to 30 months*
| Case knees | Control knees | P | |
|---|---|---|---|
| Meniscal grade | |||
| No change | 52 (46) | 254 (93) | Reference |
| Increase in any region/compartment† | 60 (54) | 20 (7) | <0.001 |
| Meniscal extrusion | |||
| No change | 65 (59) | 264 (98) | Reference |
| Increase in any compartment of the medial or lateral meniscus | 44 (40) | 5 (2) | <0.001 |
| Decrease in any compartment of the medial or lateral meniscus | 1 (1) | 1 (0.4) | 0.36 |
Except where indicated otherwise, values are the number (%). Cases were designated as individuals in whom radiographic tibiofemoral osteoarthritis had developed at 30 months. Among cases, values for meniscal grade were missing for 9 knees, and values for meniscal extrusion were missing for 11 knees. Among controls, values for meniscal grade were missing for 20 knees, and values for meniscal extrusion were missing for 24 knees.
Includes the anterior horn, body, or posterior horn of the medial or lateral meniscus, according to the Whole-Organ Magnetic Resonance Imaging Score.
Discussion
In this prospective case–control study, which was nested in an observational study of middle-aged and elderly community-dwelling persons, we examined the association between meniscal damage in knees without surgery and the development of radiographic OA. The results strongly suggest that meniscal tear, rather than only damage associated with meniscectomy, is a potent structural risk factor for the development of radiographic tibiofemoral OA.
To our knowledge, this is the first longitudinal study to demonstrate this association in knees without previous knee surgery, using the gold standard definition of radiographic OA as outcome. A nested case–control study design was planned due to the high cost and practical limitations of reading MRI scans of all knees in which radiographic OA did not develop. Both exposure status (meniscal damage) and outcome (the development of radiographic OA) were prospectively ascertained. Importantly, even though this is the largest sample of cases of newly developed radiographic knee OA yet assembled and is drawn from a very large number of knees at risk, estimates of associations must be interpreted cautiously. The relationship between the site of meniscal tear, type of lesion, or coexistent meniscal extrusion and the development of knee OA will require further study, but the sample size needed and cost may become major hurdles.
For a complex disorder such as OA, with its wide spectrum of signs and features, there are no easy solutions for defining the disease for epidemiologic study purposes. The widely used gold standard definition of K/L grade ≥2 has high specificity. Still, it is a blunt cutoff, probably yielding several false-negative results when considering knees that “truly” have OA disease activity, resulting in misclassification of disease (30).
However, as part of a sensitivity analysis, we showed that meniscal tear is strongly associated with incident OA even in knees with K/L grade 0. Naturally, at baseline, even a knee with K/L grade 0 may harbor cartilage degradation and other degenerative knee joint changes typical of early OA that are not captured by radiography. Nonetheless, in knees without any focal tibiofemoral cartilage lesion seen on MRI scans at baseline, the association was also strong, suggesting that a meniscal tear is an early and prominent predictor of “classic” radiographic knee OA. In other words, what differentiated those in whom radiographic OA developed from those in whom radiographic OA did not develop was the presence of a meniscal tear at baseline. This was true even for knees with normal cartilage at baseline, as suggested by MRI results. In a knee that already has “preradiographic” OA changes, it is possible that further disease development is also determined by other structural predictors such as the presence of cartilage lesions, which would explain the slightly weaker estimate of the effect of a meniscal tear in that stratum. We did not adjust for meniscal extrusion, bone marrow lesions, effusion, or synovitis, because we consider these MRI features as possible intermediate factors on the causal pathway between meniscal damage and knee OA.
During followup, meniscal extrusion developed in several of our cases, probably as a result of a meniscal tear or other degenerative changes that disrupt the hoop tension normally generated by the circumferentially oriented meniscal matrix fibers. Importantly, meniscal maceration, destruction, resection, or extrusion may contribute to the joint space narrowing seen on radiographs, in addition to loss of joint cartilage (29). However, by using the cutoff of K/L grade ≥2, which requires the presence of a definite osteophyte, our cases displayed not only joint space narrowing but also bony features of OA. Not surprisingly, cartilage lesions also developed more frequently in case knees than in control knees, suggesting that the radiography-based case definition had parallels in MRI-based cartilage loss.
The subjects included in this study were all drawn from the general population of persons with common risk factors for knee OA, such as being overweight or having frequent knee pain, aching, or stiffness. Due to the high strength of the associations obtained, the suggestion of a dose-response relationship, and convincing biologic plausibility, we conclude that the evidence for causality is strong and likely to be valid in the general population of middle-aged and elderly individuals without other known risk factors for OA (6–8,18–20). The natural course of meniscal damage in young individuals without knee surgery and its effect on OA development remains to be studied.
Interestingly, in a large population-based sample from Framingham, Massachusetts, the frequency of meniscal damage was higher among middle-aged men compared with middle-aged women (10). Still, knee OA is more common in elderly women than in elderly men, a paradox that probably illustrates the complex and multifactorial origin of a disorder in which women carry a greater inherent risk of OA.
The meniscus may tear due to knee trauma or may tear spontaneously due to aging and degenerative processes (31,32). In middle-aged and elderly persons, in whom degenerative damage is the most prevalent, meniscal lesions per se often cause little or no symptoms (10–12). Still, we advocate that meniscal damage represents a problem on a public health level due to its high prevalence in the general population and the present evidence of its detrimental role in the chain of events in early OA disease (10,33). This chain of events may in turn lead to “classic” knee OA, and pain may be elicited by structures or processes other than the damaged meniscus (e.g., synovium or bone marrow lesions) (34,35). Unfortunately, modern techniques of meniscal repair or transplant aimed at restoring meniscal function have still not been shown to reduce the risk of OA development (36,37). Even so, our data provide evidence that meniscal tears per se would otherwise lead to radiographic OA, and it is conceivable that treatments aimed at restoring meniscal function may lower this risk. Future challenges will include the disentanglement of distinct knee trauma in an otherwise healthy joint versus more slowly occurring degradation of meniscal tissue as the principal etiologic factor in meniscal damage. We suggest that the finding of a degenerative meniscal tear could be regarded as a signal of early OA disease, and that patients with a degenerative meniscal tear should probably be treated accordingly (38–40).
This study found that a meniscal tear in middle-aged and elderly persons without previous knee surgery, but who otherwise are at risk of OA, is common and precedes and is strongly associated with the development of radiographic tibiofemoral OA. Our study supports an increased effort to understand the role of damage to the menisci in early OA disease and prevent such damage from occurring. In addition, high-quality, long-term research is needed to assess treatments when meniscal damage has already occurred.
Acknowledgments
We thank all MOST staff and study participants in Birmingham, Alabama and Iowa City, Iowa. We also thank all of the involved personnel at the University of California, San Francisco MOST coordinating center, particularly Jean Hietpas and Irina Tolstykh, and those at the MOST analytic center at Boston University, Boston, Massachusetts.
Dr. Englund's work was supported by the Arthritis Foundation. Drs. Lewis, Torner, Nevitt, and Felson's work was supported by the NIH (National Institute on Aging grants U01-AG-18947, U01-AG-18832, U01-AG-19069, and U01-AG-18820, respectively). The Multicenter Osteoarthritis Study is a cooperative epidemiologic study of knee osteoarthritis funded by the NIH/National Institute on Aging.
Footnotes
Author Contributions: Dr. Englund had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Englund, Lewis, Torner, Nevitt, Felson.
Acquisition of data. Guermazi, Roemer, Aliabadi, Lewis, Torner, Nevitt, Sack.
Analysis and interpretation of data. Englund, Guermazi, Roemer, Yang, Lewis, Nevitt, Felson.
Manuscript preparation. Englund, Guermazi, Roemer, Lewis, Nevitt, Felson.
Statistical analysis. Englund, Yang.
Critical review of manuscript drafts. Lewis, Torner.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008;34:515–29. doi: 10.1016/j.rdc.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–6. [PubMed] [Google Scholar]
- 4.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–9. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 5.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30:164–70. [PubMed] [Google Scholar]
- 6.Seedhom BB, Hargreaves DJ. Transmission of the load in the knee joint with special reference to the role of the meniscus. Part I+II Eng Med. 1979;4:207–28. [Google Scholar]
- 7.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–90. [PubMed] [Google Scholar]
- 8.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975:184–92. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Hede A, Larsen E, Sandberg H. The long term outcome of open total and partial meniscectomy related to the quantity and site of the meniscus removed. Int Orthop. 1992;16:122–5. doi: 10.1007/BF00180200. [DOI] [PubMed] [Google Scholar]
- 10.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85-A:4–9. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007;56:4048–54. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 13.Davies-Tuck ML, Martel-Pelletier J, Wluka AE, Pelletier JP, Ding C, Jones G, et al. Meniscal tear and increased tibial plateau bone area in healthy post-menopausal women. Osteoarthritis Cartilage. 2008;16:268–71. doi: 10.1016/j.joca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, Martel-Pelletier J, Pelletier JP, Abram F, Raynaud JP, Cicuttini F, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34:776–84. [PubMed] [Google Scholar]
- 15.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239:811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 16.Fox MG. MR imaging of the meniscus: review, current trends, and clinical implications. Radiol Clin North Am. 2007;45:1033–53. vii. doi: 10.1016/j.rcl.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7:526–32. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 18.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–63. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–73. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 24.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 25.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3 A:3–70. [PubMed] [Google Scholar]
- 27.Roemer FW, Guermazi A, Lynch JA, Peterfy CG, Nevitt MC, Webb N, et al. Short tau inversion recovery and proton density-weighted fat suppressed sequences for the evaluation of osteoarthritis of the knee with a 1.0 T dedicated extremity MRI: development of a time-efficient sequence protocol. Eur Radiol. 2005;15:978–87. doi: 10.1007/s00330-004-2608-6. [DOI] [PubMed] [Google Scholar]
- 28.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Hunter DJ, Zhang YQ, Tu X, LaValley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–95. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 30.Hart DJ, Spector TD. Kellgren & Lawrence grade 1 osteophytes in the knee: doubtful or definite? Osteoarthritis Cartilage. 2003;11:149–50. doi: 10.1053/joca.2002.0853. [DOI] [PubMed] [Google Scholar]
- 31.Poehling GG, Ruch DS, Chabon SJ. The landscape of meniscal injuries. Clin Sports Med. 1990;9:539–49. [PubMed] [Google Scholar]
- 32.Noble J, Hamblen DL. The pathology of the degenerate meniscus lesion. J Bone Joint Surg Br. 1975;57:180–6. [PubMed] [Google Scholar]
- 33.Englund M. The role of the meniscus in osteoarthritis genesis. Rheum Dis Clin North Am. 2008;34:573–9. doi: 10.1016/j.rdc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Englund M, Guermazi A, Roemer FW, Lynch JA, Yang M, Lewis CE, et al. Meniscal damage or extrusion on MRI is associated with incident or enlarging bone marrow lesions—results from the MOST 30-month follow-up. Arthritis Rheum. 2008;58 9:S236. abstract. [Google Scholar]
- 35.Felson DT, Niu J, Guermazi A, Roemer F, Alibadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 36.Howell JR, Handboll HH. Surgical treatment for meniscal injuries of the knee in adults. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD001353. CD001353. [DOI] [PubMed] [Google Scholar]
- 37.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 38.Herrlin S, Hallander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393–401. doi: 10.1007/s00167-006-0243-2. [DOI] [PubMed] [Google Scholar]
- 39.Kirkley A, Birmingham TB, Litchfield RB, Giffin JR, Willits KR, Wong CJ, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359:1097–107. doi: 10.1056/NEJMoa0708333. [DOI] [PubMed] [Google Scholar]
- 40.Moseley JB, O'Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–8. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]


