Abstract
Background and objectives: GFR is scaled to body surface area (S), whereas hemodialysis dosage is scaled to total body water (V). Scaling to metabolic rate (M) or liver size (L) has also been proposed.
Design, setting, participants, & measurements: In 1551 potential kidney donors (662 men and 889 women) for whom GFR had been estimated from 125I-iothalamate clearance (iGFR) between the years 1973 and 2005, iGFR scaling was examined. Scaling was to estimates of S, V, M, or L. The study looked at the variation of iGFR by gender, age, S, V, M, and L within the study population.
Results: In multiple regression analysis, neither gender nor race was significantly associated with iGFR after controlling for height, weight, and age. Raw iGFR averaged 122 ± 23 ml/min in men and 106 ± 21 ml/min in women (P < 0.001). In an adjusted analysis, iGFR scaled to S or L was similar for men and women (NS), whereas iGFR scaled to either V or M was substantially different between the genders (P < 0.001). When the patients by gender were divided into five quintiles of V or S, the iGFR-V ratio varied more with body size than iGFR scaled to the other measures.
Conclusions: iGFR scaled to S or L was similar in men and women. Scaling to either M or V resulted in a sizeable gender difference, whereas scaling to V led to markedly different values of iGFR across body size.
Renal GFR customarily is scaled to body surface area (S) in clinical practice (1,2). S can be derived by any of a variety of equations, the most popular of which is the equation by Dubois and Dubois (3). The generalizability of scaling GFR to S has been questioned: Scaling to extracellular fluid volume may provide more uniform values in children (4). Scaling to M also has been proposed (5,6). A novel concept may be to scale GFR to liver size (L), given that the liver and the kidney act as tandem organs of detoxification (7,8).
Dialysis therapy is commonly scaled to the urea distribution volume (V), which is approximately equal to total body water (V) (9). Concern has been raised about the relatively low amounts of dialysis clearance (Kt) provided to smaller patients and, in particular, to women, who have relatively low values for V for a given amount of S (10,11). Because the identity of the clinically relevant uremic toxins is unknown and because dialyzer clearance can be thought of as a surrogate for renal clearance, we attempted to gain some insight into optimal scaling of dialysis dosage by examining how GFR scales to various estimates of body size and function, specifically, S, V, M, and estimated L. We were particularly interested in how each scaling parameter would affect values of GFR measured by 125I-iothalamate clearance (iGFR) between the genders and also wanted to know how scaled iGFR would vary across a range of body sizes. We further examined how each method of scaling would be reflected in the change of GFR observed with age.
Materials and Methods
This study involved 1551 potential kidney donors who had their GFR measured at the Cleveland Clinic Renal Function Laboratory between 1973 and 2005. iGFR was measured as described by Israelit et al. (12). A detailed description of the procedure has been previously reported (13). In brief, after hydration and blockage of the thyroid uptake of 125I-iothalamate, 25 μCi (Glofil; Questor Pharmaceuticals, Union City, CA) of radioisotope were injected subcutaneously. A voluntarily voided urine sample (minimum 250 ml) was discarded, followed by two timed clearance urine collections. Blood samples were drawn before and after each urine collection. Isotope activity was determined by gamma counting of 0.5-ml samples on a Packard Minaxi 5000 series counter (PerkinElmer Life Sciences, Downers Grove, IL). The serum counts were taken as the average of the bracketed blood samples for each clearance period. The mean iGFR was calculated as the average of the two individual-period clearance estimates.
Mean iGFR results were then corrected to S as estimated from the Dubois and Dubois equation (3) and multiplying by 1.73 or, alternatively, were corrected to V using the equations proposed by Watson et al. (14), to M computed using the Mifflin equation (15), or to L estimated by an equation proposed by Johnson et al. (16) (Table 1).
Table 1.
Equations used for adjustment of iGFR
| Surface area (3) |
| S (m2) = 0.007184 × weight0.425 × height0.725 |
| Total body water (14) |
| men: V (liters) = 2.447 + 0.3362 × weight + 0.1074 × height − 0.09516 × age (yr) |
| women: V = −2.097 + 0.2466 × weight + 0.1069 × height |
| Resting energy expenditure (metabolic rate) (15) |
| M (kcal/d) = 9.99 × weight + 6.25 × height − 4.92 × age + 166 × gender (men, 1; women, 0) − 161 |
| Liver size (16) |
| L (ml) = 722 × S1.176 |
Weight is in kg, and height is in cm.
Variables were summarized by mean and SD or frequency and percentage where appropriate. Linear regression was used to assess the relationship between adjusted iGFR and several demographic characteristics. A linear spline was used to allow for the rate of change in adjusted iGFR to change at age 45.
The stability of raw iGFR and of ratios of iGFR to S, V, M, and L across subgroups defined by gender, age, and measures of body size was assessed by summarizing the mean and SD of the iGFR, iGFR/S, iGFR/V, iGFR/M, and iGFR/L levels across the subgroups. The age subgroups were defined by subdividing patients into five age quintiles. The body size subgroups were first defined by separately subdividing men and women into gender-specific quintiles for Watson volume. For ensuring that results were not an artifact of the specific size index used, the analysis was repeated with the body size subgroups defined by gender-specific quintiles for Dubois S. The dependence of the iGFR/S, iGFR/V, iGFR/M, and iGFR/L indices on gender was also assessed by performing multiple regression analyses to compare these indices between men and women after statistically controlling for age quintile and race.
To address the possibility that the mean levels might be affected by relatively small subsets of patients with extreme values of iGFR, we conducted sensitivity analyses to compare the median values of the same indices between the same demographic and body size subgroups. Additional sensitivity analyses were performed to compare adjusted mean levels of iGFR, iGFR/S, iGFR/V, iGFR/M, and iGFR/L between the subgroups indicated in the previous paragraph after controlling for race and the calendar year of the GFR to assess whether comparisons between subgroups might be affected by variations as a result of race or secular trends over calendar time. The results of both of these sets of sensitivity analyses did not differ materially from the main summaries on the basis of means and SD and are not reported in here.
A multiple linear regression analysis was also performed to relate jointly raw iGFR to gender, age quintile, race (coded as black or nonblack), height, and weight. Throughout this article, the expression “adjusted GFR” is used to refer to adjustment of GFR by factoring the raw GFR by S, V, M, or L. The expression “statistical adjustment” is used to refer to the statistical adjustment of iGFR for specific covariates in the multiple regression analysis.
Results
Summary of Selected Measures by Gender
The sample group included 662 men and 889 women. The mean ages are as shown in Table 2. Raw iGFR was substantially higher in the men (122 ± 23) than in women (106 ± 21; P < 0.001); however, once iGFR was normalized to S (per 1.73/m2 Dubois S), there was no longer a significant difference between the genders, as S was also proportionately higher in the men. In contrast, iGFR/V and iGFR/M were markedly lower in men than in women (<0.001), with iGFR/V being 20% lower and iGFR/M being 13% lower. iGFR/L was slightly lower in men also, but the magnitude of the difference was small (approximately 4%). Dividing unadjusted iGFR by body weight (kg), height (cm), or body mass index (BMI) also resulted in marked gender differences between men and women, although dividing iGFR by body weight to the 0.75 power (a putative scaling factor for GFR) reduced the mean between gender difference markedly, from approximately 8% for iGFR/weight to approximately 2.5% for iGFR/weight0.75 (Table 2).
Table 2.
Continuous variables for patients studied
| Variable | Men (Mean [SD]; N = 662) | Women (Mean [SD]; N = 885) | P |
|---|---|---|---|
| Age | 38.1 (11.0) | 40.8 (11.0) | <0.001 |
| BMI (w/h2) × 1000 | 27.2 (4.1) | 26.3 (4.8) | <0.001 |
| S (m2) | 2.02 (0.18) | 1.74 (0.70) | <0.001a |
| V (liters) | 46.6 (5.3) | 32.5 (3.7) | <0.001 |
| M (kcal/d) | 1779 (170) | 1351 (160) | <0.001a |
| L (ml) | 1771 (190) | 1484 (170) | <0.001a |
| Raw iGFR (ml/min) | 122 (23) | 107 (21) | <0.001a |
| Unadjusted iGFR × 1.73/S | 104 (18) | 106 (20) | 0.082a |
| Unadjusted iGFR/V | 2.63 (0.44) | 3.30 (0.62) | <0.001 |
| Unadjusted iGFR/ M | 0.069 (0.011) | 0.079 (0.014) | <0.001 |
| Unadjusted iGFR/L | 0.069 (0.012) | 0.072 (0.014) | <0.001a |
| Unadjusted iGFR/weight | 1.451 (0.281) | 1.567 (0.344) | <0.001 |
| Unadjusted iGFR/weight0.75 | 4.382 (0.773) | 4.486 (0.887) | 0.014a |
| Unadjusted iGFR/height | 0.689 (0.123) | 0.655 (0.126) | <0.001 |
| Unadjusted iGFR/BMI | 4.549 (0.900) | 4.148 (0.937) | <0.001 |
| V/S | 23.00 (0.75) | 18.60 (0.36) | <0.001 |
| V/M | 0.026 (0.001) | 0.024 (0.001) | <0.001 |
| V/L | 0.026 (0.001) | 0.022 (0.000) | <0.001 |
| M/S | 879 (27) | 774 (33) | <0.001 |
| L/S | 874 (14) | 851 (14) | <0.001 |
t tests were used for all comparisons unless otherwise indicated.
A t test for unequal variance.
Categorical variables are shown in Table 3. Most of the donors were nonblack, and the percentage of black donors was similar for men and women. The age breakdown was similar between men and women. The percentage of men and women drawn from the various time points is also shown. As expected, when stratifying the entire group by body size using Watson V, most of the smaller patients were women and most of the larger patients were men.
Table 3.
Patient characteristics
| Factor | N | Male (n [%]) | Female (n [%]) | P |
|---|---|---|---|---|
| Race | 0.12 | |||
| nonblack | 1323 | 577 (86.8) | 746 (83.9) | |
| black | 231 | 88 (13.2) | 143 (16.1) | |
| Age (yr) | <0.001 | |||
| ≤45 | 1050 | 493 (74.1) | 557 (62.7) | |
| >45 | 504 | 172 (25.9) | 332 (37.3) | |
| Decades | 0.24 | |||
| 1970 | 78 | 41 (6.2) | 37 (4.2) | |
| 1980 | 355 | 157 (23.6) | 198 (22.3) | |
| 1990 | 445 | 190 (28.6) | 255 (28.7) | |
| 2000 | 676 | 277 (41.7) | 399 (44.9) | |
| Rank for V (Watson) | <0.001 | |||
| first quintile (>23.80, ≤30.70) | 310 | 0 (0.00) | 310 (34.90) | |
| second quintile (>30.70, ≤34.12) | 311 | 4 (0.60) | 307 (34.50) | |
| third quintile (>34.12, ≤40.30) | 311 | 68 (10.20) | 243 (27.30) | |
| fourth quintile (>40.30, ≤46.72) | 311 | 283 (42.60) | 28 (3.20) | |
| fifth quintile (>46.72, ≤69.80) | 311 | 310 (46.60) | 1 (0.11) |
χ2 tests were used to test for differences by gender.
Relationship of the Ratio of V, M or L to S versus S by Gender
For better understanding how the different size denominators for iGFR might be affected by gender and body size, the ratios of V/S, M/S, and L/S were plotted against S separately for men and women. The results are shown in Figures 1, 2, and 3, respectively. These anthropometric body size measurements were highly correlated with one another, with the highest correlation being between S and L.
Figure 1.
Scatterplot of the ratio of V (Watson) to S (Dubois) plotted against S. Note that this ratio is substantially higher in men than in women at the same level of S. Note also the positive slope to the scatterplots for both men and women.
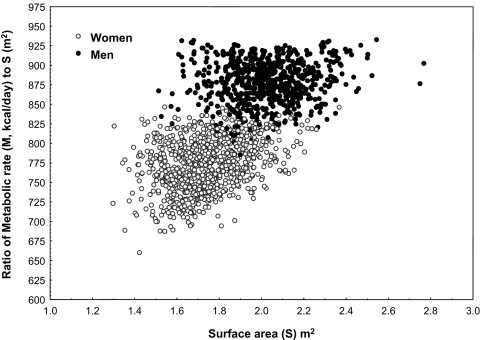
Figure 2.
Scatterplot of the ratio of M (Mifflin) to S (Dubois) plotted against S. Note that, similar to Figure 1 for V, this ratio is substantially higher in men than in women at the same level of S.
Figure 3.
Scatterplot of the ratio of estimate L (Johnson) to S (Dubois) plotted against S. As S decreases, this ratio also decreases, suggesting that across body size, L scaling will not be the same as that for S, but in contrast to Figures 1 and 2, there is no gender offset as the equation used to estimate L is a simple power function of S.
Effect of Anthropometric Variables on iGFR
Because the various formulas for S, V, M, and L may themselves be incorrect and/or hold a gender bias (in the case of M or V), we began by examining the predictive power of the constituent elements of these anthropometric equations in terms of iGFR in a multiple regression analysis. In a joint model that included gender, height, weight, age quintile, and race, at fixed levels of the other predictor variables, mean raw iGFR increased by 6.07 ml/min for each 20-cm increase in height and by 5.60 ml/min for each 10-kg increment in weight. At fixed levels of the remaining factors, mean raw iGFR did not differ between men and women or between black and nonblack donors but was 18 ml/min lower in potential donors aged 50 to 65 than those aged 14 to 29 yr (Table 4).
Table 4.
Effect of height, weight, age, race, and gender on iGFR
| Variable | Parameter Estimate | 95% CI | P > t |
|---|---|---|---|
| Intercept | 26.120 | 2.05 to 50.17 | 0.03 |
| Male versus female | 0.762 | −2.06 to 3.58 | 0.60 |
| Height (20-cm increments) | 6.070 | 2.93 to 9.21 | <0.001 |
| Weight (10-kg increments) | 5.600 | 4.84 to 6.35 | <0.001 |
| Age quintile 2 versus 1 | −2.620 | −5.62 to 0.37 | 0.09 |
| Age quintile 3 versus 1 | −5.000 | −8.05 to −1.95 | 0.001 |
| Age quintile 4 versus 1 | −9.590 | −12.64 to −6.54 | <0.001 |
| Age quintile 5 versus 1 | −18.300 | −21.31 to −15.28 | <0.001 |
| Black versus nonblack | −0.682 | −3.39 to 2.03 | 0.62 |
R2 = 0.33. Age quintiles: 1, 14 to 29; 2, 30 to 36; 3, 37 to 42; 4, 43 to 49; 5, 50 to 65. CI, confidence interval.
Adjusted Values of iGFR Scaled to S, V, M, or L by Gender
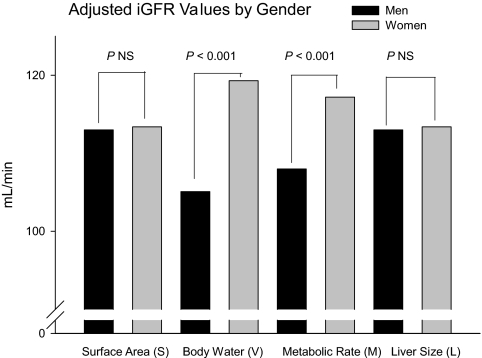
Values for iGFR adjusted for S, V, M, or L are shown in Table 5 and in Figure 4. There was no significant gender difference when iGFR was adjusted for body size. A moderate gender difference was present when the adjustment was for M (resting energy expenditure), and the largest gender difference was when the adjustment was for V.
Table 5.
Mean iGFR factored by S, V, M, or L, statistically controlling for age and race
| Mean iGFR Factored by | Men (Adjusted Mean [95% CI]) | Women (Adjusted Mean [95% CI]) | P |
|---|---|---|---|
| S | 113.0 (111.3 to 114.7) | 113.4 (112.0 to 114.8) | 0.74 |
| V | 105.1 (102.8 to 107.4) | 119.3 (117.5 to 121.1) | < 0.001 |
| M | 108.0 (105.9 to 109.9) | 117.2 (115.5 to 118.8) | < 0.001 |
| L | 113.0 (111.3 to 114.7) | 113.4 (111.9 to 114.8) | 0.74 |
CI, confidence interval.
Figure 4.
Adjusted iGFR values factored by S, V, M, or L and statistically adjusted for age quintiles and race.
Differently Scaled Measures of iGFR by Age
We examined how iGFR/S, iGFR/V, iGFR/M, and iGFR/L would vary by age in women and men separately. The results are shown in Table 6. Mean unadjusted iGFR was inversely associated with age in both genders, as were iGFR/S, iGFR/L, and iGFR/V. The inverse relationship of these quantities with age was most pronounced for the highest age quintile. Scaling to M attenuated the decrease in iGFR with advancing age. We also examined how the ratio of the scaling parameters V/S, V/M, and V/L varied with age.
Table 6.
Effect of age
| Factor | Age Quintile |
||||
|---|---|---|---|---|---|
| 1 (14 to 29; Mean [SD]; n = 166) | 2 (30 to 36; Mean [SD]; n = 142) | 3 (37 to 42; Mean [SD]; n = 130) | 4 (43 to 49; Mean [SD]; n = 109) | 5 (50 to 65; Mean [SD]; n = 115) | |
| Women | |||||
| unadjusted iGFR | 113 (22) | 112 (19) | 110 (21) | 105 (19) | 96 (20) |
| iGFR ×1.73/S | 114 (19) | 112 (18) | 109 (19) | 103 (18) | 95.6 (18) |
| iGFR/V | 3.56 (0.60) | 3.50 (0.58) | 3.37 (0.58) | 3.21 (0.56) | 2.97 (0.58) |
| GFR/M | 0.081 (0.013) | 0.082 (0.014) | 0.081 (0.014) | 0.079 (0.014) | 0.075 (0.015) |
| GFR/L | 0.078 (0.013) | 0.077 (0.013) | 0.074 (0.013) | 0.070 (0.012) | 0.065 (0.013) |
| V/S | 18.50 (0.36) | 18.60 (0.34) | 18.70 (0.39) | 18.60 (0.37) | 18.60 (0.32) |
| V/M | 0.023 (0.000) | 0.023 (0.000) | 0.024 (0.000) | 0.025 (0.000) | 0.025 (0.000) |
| M/S | 817.0 (13.5) | 796.0 (13.8) | 779.0 (15.8) | 760.0 (16.2) | 733.0 (19.0) |
| S (m2) | 1.720 (0.160) | 1.730 (0.180) | 1.760 (0.180) | 1.760 (0.170) | 1.740 (0.154) |
| V (liters) | 31.800 (3.500) | 32.200 (3.900) | 32.900 (3.900) | 32.800 (3.700) | 32.500 (3.329) |
| M (kcal/d) | 1403 (145) | 1380 (167) | 1373 (165) | 1338 (156) | 1279 (142) |
| L (ml) | 1457 (160) | 1473 (182) | 1501 (177) | 1500 (170) | 1483 (154) |
| Men | |||||
| unadjusted iGFR | 127 (23) | 124 (22) | 124 (22) | 121 (20) | 111 (23) |
| iGFR × 1.73/S | 110 (17) | 107 (17) | 105 (16) | 103 (15) | 94 (18) |
| iGFR/V | 2.71 (0.42) | 2.66 (0.42) | 2.66 (0.42) | 2.62 (0.40) | 2.43 (0.47) |
| GFR/M | 0.070 (0.011) | 0.069 (0.011) | 0.070 (0.011) | 0.069 (0.010) | 0.065 (0.012) |
| GFR/L | 0.073 (0.011) | 0.071 (0.011) | 0.070 (0.011) | 0.068 (0.010) | 0.062 (0.012) |
| V/S | 23.50 (0.62) | 23.20 (0.66) | 23.00 (0.67) | 22.70 (0.64) | 22.30 (0.65) |
| V/M | 0.026 (0.001) | 0.026 (0.001) | 0.026 (0.001) | 0.026 (0.000) | 0.027 (0.000) |
| M/S | 911.00 (9.43) | 891.00 (8.71) | 875.00 (8.64) | 861.00 (9.64) | 838.00 (13.30) |
| S (m2) | 2.00 (0.20) | 2.02 (0.17) | 2.04 (0.19) | 2.05 (0.20) | 2.04 (0.16) |
| V (liters) | 46.9 (5.5) | 46.8 (5.1) | 46.9 (5.4) | 46.6 (5.7) | 45.5 (4.5) |
| M (kcal/d) | 1818 (174) | 1797 (163) | 1787 (175) | 1762 (188) | 1708 (146) |
| L (ml) | 1743 (192) | 1762 (175) | 1787 (192) | 1793 (208) | 1783 (161) |
Size Effects: iGFR Scaled to Various Parameters by Quintiles of V or S
We did further analyses by body size within each gender, given that most of the lower size quintiles would be populated by women and the upper size quintiles by men. The results are shown in Tables 7 and 8 (V) and Tables 9 and 10 (S). It can be seen that for both women and men, unadjusted iGFR values increased as body size increased. For women, iGFR/S decreased by approximately 2 and 5%, respectively, in the two highest quintiles of V, but iGFR/S was stable across all five V quintiles in men (Tables 7 and 8). In contrast, iGFR/V decreased by approximately 10% from the lowest to the highest size quintile in women and 6% in men. The variation of iGFR/M and iGFR/L with body size was similar to that with iGFR/S, especially in men; iGFR scaled to either M or L varied little with body size. In a sensitivity analysis, we repeated this analysis with the same patients divided by quintiles of S (Tables 9 and 10). The results were quite similar to those described when V was used as the size parameter. We also examined how iGFR divided by height, weight, weight0.75, and BMI varied by size quintile of S. The results are shown in Tables 9 and 10. There was large variation in iGFR scaled by weight, between the smallest and largest S quintiles (approximately 20%), which was reduced considerably when iGFR was scaled by weight0.75, but none of these scaling factors approached the relative uniformity of iGFR/S across the S quintiles.
Table 7.
Continuous measures by Watson's V quintiles: Women
| Factor | Q1 (≥23.76, <29.35; Mean [SD]; n = 176) | Q2 (≥29.35, <31.23; Mean [SD]; n = 178) | Q3 (≥31.23, <32.98; Mean [SD]; n = 177) | Q4 (≥32.98, <35.55; Mean [SD]; n = 177) | Q5 (≥35.55, <48.34; Mean [SD]; n = 177) |
|---|---|---|---|---|---|
| Age | 38.2 (11.0) | 41.7 (11.0) | 41.8 (11.0) | 40.6 (10.0) | 41.6 (10.0) |
| BMI | 21.5 (2.3) | 23.5 (2.6) | 25.8 (3.0) | 28.7 (3.0) | 32.0 (4.0) |
| S | 1.52 (0.06) | 1.65 (0.03) | 1.73 (0.03) | 1.82 (0.04) | 1.99 (0.10) |
| V | 27.80 (1.20) | 30.30 (0.55) | 32.10 (0.52) | 34.20 (0.75) | 38.00 (2.30) |
| L (ml) | 1263 (61) | 1388 (30) | 1472 (29) | 1566 (39) | 1733 (98) |
| M (kcal/d) | 1165 (73) | 1255 (63) | 1330 (62) | 1424 (64) | 1578 (106) |
| Unadjusted iGFR | 95.1 (17.0) | 102.0 (19.0) | 108.0 (19.0) | 111.0 (21.0) | 117.0 (23.0) |
| iGFR × 1.73/S | 108 (19) | 107 (19) | 108 (19) | 105 (19) | 102 (20) |
| iGFR/V | 3.42 (0.61) | 3.38 (0.61) | 3.36 (0.59) | 3.23 (0.60) | 3.09 (0.63) |
| iGFR/M | 0.082 (0.014) | 0.081 (0.014) | 0.081 (0.013) | 0.078 (0.013) | 0.074 (0.014) |
| iGFR/L | 0.075 (0.014) | 0.074 (0.013) | 0.073 (0.013) | 0.071 (0.013) | 0.068 (0.014) |
Table 8.
Continuous measures by Watson's V quintiles: Men
| Factor | Q1 (≥32.26, <42.45; Mean [SD]; n = 132) | Q2 (≥42.45, <45.16; Mean [SD]; n = 132) | Q3 (≥45.16, <47.53; Mean [SD]; n = 133) | Q4 (≥47.53, <50.73; Mean [SD]; n = 133) | Q5 (≥50.73, <69.18; Mean [SD]; n = 132) |
|---|---|---|---|---|---|
| Age | 39.3 (12.0) | 37.7 (11.0) | 37.4 (11.0) | 38.9 (11.0) | 36.9 (10.0) |
| BMI | 23.1 (2.6) | 25.3 (2.8) | 27.1 (2.5) | 28.6 (2.6) | 32.0 (3.0) |
| S (m2) | 1.78 (0.09) | 1.93 (0.05) | 2.02 (0.04) | 2.11 (0.05) | 2.28 (0.11) |
| V (liters) | 39.70 (2.10) | 43.70 (0.80) | 46.40 (0.66) | 49.00 (0.91) | 54.30 (3.30) |
| L (ml) | 1525 (92) | 1672 (51) | 1766 (46) | 1863 (51) | 2031 (116) |
| M (kcal/d) | 1551 (78) | 1688 (42) | 1775 (34) | 1855 (39) | 2028 (112) |
| Unadjusted iGFR | 107 (20) | 116 (19) | 123 (19) | 126 (21) | 138 (23) |
| iGFR × 1.73/S | 104 (19) | 104 (18) | 106 (16) | 103 (18) | 105 (17) |
| iGFR/V | 2.71 (0.48) | 2.66 (0.45) | 2.66 (0.41) | 2.58 (0.42) | 2.54 (0.40) |
| iGFR/M | 0.069 (0.012) | 0.069 (0.012) | 0.069 (0.010) | 0.068 (0.011) | 0.068 (0.011) |
| iGFR/L | 0.071 (0.013) | 0.069 (0.012) | 0.070 (0.011) | 0.068 (0.012) | 0.068 (0.011) |
Table 9.
Continuous measures by Dubois S quintiles: Women
| Factor | Q1 (≥1.30, <1.60; Mean [SD]; n = 177) | Q2 (≥1.60, <1.69; Mean [SD]; n = 177) | Q3 (≥1.69, <1.77; Mean [SD]; n = 177) | Q4 (≥1.77, <1.88; Mean [SD]; n = 177) | Q5 (≥1.88, <2.37; Mean [SD]; n = 177) |
|---|---|---|---|---|---|
| Age | 38.8 (11.0) | 41.9 (11.0) | 41.6 (11.0) | 40.0 (10.0) | 41.7 (10.0) |
| BMI | 21.7 (2.6) | 23.9 (3.0) | 25.9 (3.5) | 28.5 (3.5) | 31.5 (4.1) |
| S (m2) | 1.52 (0.06) | 1.65 (0.03) | 1.73 (0.02) | 1.82 (0.03) | 1.99 (0.09) |
| V (liters) | 27.80 (1.20) | 30.30 (0.62) | 32.10 (0.65) | 34.20 (0.88) | 37.90 (2.30) |
| L (ml) | 1262 (61) | 1387 (26) | 1471 (23) | 1566 (33) | 1735 (96) |
| M (kcal/d) | 1161 (70) | 1254 (59) | 1331 (58) | 1427 (61) | 1578 (107) |
| Unadjusted iGFR | 94.8 (17.0) | 101.0 (18.0) | 108.0 (19.0) | 113.0 (21.0) | 116.0 (22.0) |
| iGFR × 1.73/S | 108 (20) | 107 (19) | 108 (19) | 107 (20) | 101 (20) |
| iGFR/V | 3.41 (0.62) | 3.35 (0.61) | 3.36 (0.60) | 3.30 (0.61) | 3.08 (0.60) |
| iGFR/M | 0.082 (0.014) | 0.080 (0.014) | 0.081 (0.014) | 0.079 (0.014) | 0.074 (0.014) |
| iGFR/L | 0.075 (0.014) | 0.073 (0.013) | 0.073 (0.013) | 0.072 (0.013) | 0.067 (0.013) |
| iGFR/weight | 1.79 (0.34) | 1.65 (0.32) | 1.59 (0.31) | 1.49 (0.28) | 1.31 (0.27) |
| iGFR/weight0.75 | 4.82 (0.89) | 4.63 (0.86) | 4.56 (0.86) | 4.39 (0.82) | 4.03 (0.81) |
| iGFR/height | 0.604 (0.110) | 0.630 (0.110) | 0.661 (0.110) | 0.689 (0.130) | 0.691 (0.130) |
| iGFR/BMI | 4.42 (0.90) | 4.31 (0.94) | 4.25 (0.99) | 4.01 (0.86) | 3.74 (0.84) |
Table 10.
Continuous measures by Dubois S quintiles: Men
| Factor | Q1 (≥1.51, <1.87; Mean [SD]; n = 132) | Q2 (≥1.87, <1.98; Mean [SD]; n = 132) | Q3 (≥1.98, <2.06; Mean [SD]; n = 133) | Q4 (≥2.06, <2.17; Mean [SD]; n = 133) | Q5 (≥2.17, <2.77; Mean [SD]; n = 132) |
|---|---|---|---|---|---|
| Age | 35.6 (11.0) | 38.5 (11.0) | 37.3 (9.8) | 39.5 (11.0) | 39.4 (10.0) |
| BMI | 23.2 (2.8) | 25.5 (2.7) | 26.9 (2.8) | 28.8 (2.9) | 31.6 (3.3) |
| S (m2) | 1.78 (0.08) | 1.93 (0.03) | 2.02 (0.02) | 2.12 (0.03) | 2.28 (0.10) |
| V (liters) | 39.9 (2.4) | 43.6 (1.4) | 46.4 (1.1) | 49.0 (1.5) | 54.1 (3.6) |
| L (ml) | 1518 (84) | 1670 (32) | 1766 (25) | 1864 (32) | 2038 (109) |
| M (kcal/d) | 1564 (90) | 1683 (63) | 1775 (47) | 1855 (60) | 2020 (120) |
| Unadjusted iGFR | 110 (20) | 115 (20) | 124 (20) | 126 (20) | 137 (24) |
| iGFR × 1.73/S | 107 (19) | 103 (18) | 106 (17) | 103 (16) | 104 (18) |
| iGFR/V | 2.74 (0.46) | 2.63 (0.45) | 2.66 (0.42) | 2.56 (0.40) | 2.54 (0.42) |
| iGFR/M | 0.070 (0.012) | 0.068 (0.012) | 0.069 (0.011) | 0.068 (0.010) | 0.068 (0.011) |
| iGFR/L | 0.072 (0.013) | 0.069 (0.012) | 0.070 (0.011) | 0.067 (0.011) | 0.067 (0.011) |
| iGFR/weight | 1.64 (0.30) | 1.48 (0.27) | 1.46 (0.25) | 1.36 (0.23) | 1.30 (0.23) |
| iGFR/weight0.75 | 4.69 (0.82) | 4.40 (0.78) | 4.44 (0.73) | 4.23 (0.71) | 4.16 (0.71) |
| iGFR/height | 0.644 (0.120) | 0.658 (0.120) | 0.695 (0.110) | 0.700 (0.110) | 0.747 (0.130) |
| iGFR/BMI | 4.76 (0.93) | 4.55 (0.92) | 4.63 (0.89) | 4.41 (0.87) | 4.38 (0.83) |
Discussion
In this relatively large and diverse population of potential kidney donors, our results suggest that scaling iGFR to S estimated from the Dubois and Dubois equation results in comparable values in men versus women, whereas scaling iGFR to either V or M does not. The differences in adjusted mean iGFR between the genders was approximately 14% when scaling to V and 7% when scaling to M, whereas there was no gender difference when scaling to S. If the assumption is made that a constant GFR versus size measure across a patient population reflects the most appropriate scaling method, then our results support the existing tradition of scaling iGFR to S, because iGFR/S was similar in men and women across a large range of body sizes.
We did not measure any of the scaling parameters directly (S, V, L, or M) but used estimating equations for them. It would be of interest to extend these findings in a separate study in which M, V, and L would be measured directly. It might be argued that these estimating equations themselves might be incorrect and have a gender bias; however, if one considers a man and a woman of the same height and weight, then the man will have a larger amount of muscle mass. Because both M and V are closely related to muscle mass, both of these measures would be expected to be somewhat lower in a woman compared with a similarly sized man, regardless of the estimating equation used. To determine whether a similar gender-related variation occurred in GFR independent of any equation, we performed a multiple regression analysis directly relating raw iGFR to gender, race, age, height, and weight. In this analysis, once age, height, and weight were controlled for, neither gender nor race accounted for any residual variance in iGFR.
One trend of iGFR was not neutralized by normalizing to S: The progressive decrease in iGFR with age. The decrease in raw iGFR with age was substantial, with mean iGFR being approximately 13 to 14% less in the highest versus lowest age quintiles. Scaling to S did nothing to diminish the effect of age on iGFR; however, scaling to M completely abolished the association of GFR with age in the first four quintiles for both men and women. Analysis of the data does not permit one to separate change in GFR with age because of variation with age in S, V, or M versus pathologic (physiologic) decreases in GFR associated with normal aging. Thus, despite that both GFR and M fall with age, this alone does not suggest that M is a more appropriate scaling parameter.
A third area of variance examined was body size. Analysis here was somewhat complicated by the use of common parameters to scale iGFR and to define body size: For example, when dividing into quintiles by V, one might expect the analysis to overstate the effect of scaling iGFR by V compared with scaling iGFR by S. Similarly, when separating the population by size quintiles defined by S, one might expect the analysis to overstate the effect of scaling by S compared with scaling by V; however, in fact, both S and V are dependent on the same size parameters (weight and height), and the results were found to be very similar between the two scaling approaches.
For women, iGFR scaled by S or M varied less across different levels of body size than when iGFR was scaled by V. The same was true for men. Whether it was more consistent to scale by S or by M across different quintiles of body size was not clear: In men, iGFR/S had less of a size effect than iGFR/M, whereas, in women, there was only a small size effect with either iGFR/S or iGFR/M, with a slight advantage in favor of iGFR/S.
The question of how GFR should be scaled most commonly has been approached across species, comparing mice with rodents with elephants (5,6). The broad consensus of such analyses has been that scaling of GFR across species is approximately mass to the 0.75 power, which is similar to M (5,6); however, scaling to S, which varies to the 0.67 power of body weight (5,6), has traditionally been applied to scaling of iGFR within humans (1,2). In humans, scaling of iGFR to S seems to work quite well, the one exception being in children, for whom scaling iGFR to extracellular fluid volume may give more consistent results (4).
We also examined scaling of iGFR to estimated L, given the speculation that perhaps the visceral organs generate the bulk of uremic toxins and that kidney and liver function are tied as a paired set of excretory organs (7,8). A number of equations have been devised to predict L, and one based on a meta-analysis, by Johnson et al. (16), is simply a power function of S with no gender term, so it is not surprising that using L to scale iGFR resulted in a similar lack of gender difference as when scaling to S. The Johnson equation does not include an age term, whereas L has been found to decrease with age (17). Tandem decreases in L and GFR during middle age may also be physiologically linked.
What is the physiologic link between GFR and these various scaling parameters? The rationale for scaling GFR to V might be that soluble toxins are dissolved in the V. Scaling to M has been proposed on the basis of cross-species comparisons (5). The arguments against scaling to M have been described in a separate review article and are not repeated here (8). L, which scales to S, is an interesting way to look at scaling of GFR. One might consider the liver and kidney as together, being a set of detoxifying organs, with the liver first converting fat-soluble waste products to their water-soluble form for excretion by the kidney. In the final analysis, because all of these scaling parameters are highly correlated among patients, the differences based on choice of scaling parameter are quite small in patients other than those in stage 5 chronic kidney disease (CKD; GFR <15 ml/min/m2).
One of the reasons that we were interested in examining this issue is the current controversy about the appropriateness of scaling dialysis dosage to V and that V-based dialysis dosing leads to giving less dialysis to women and smaller patients than if dialysis dosing were scaled to S (8). Another area of interest might be in staging CKD according to GFR. Our data suggest that the current practice of staging CKD on the basis of GFR scaled to S might be altered if GFR were to be rescaled to M, as has been suggested by others (5), although this change would probably be of minor clinical importance.
An intrinsic assumption of our analysis has been that a constant scaling factor–adjusted GFR across a population might be evidence that such a scaling factor is perhaps a particularly appropriate one to use. This may not be correct if there are physiologic reasons that, for example, GFR should be truly different in men versus women of similar body size. For example, GFR might be related to protein intake, and one would expect more protein intake in a man versus a similarly sized woman. Furthermore, the observation that iGFR divided by S is relatively similar across quintiles of body size (as defined by V or S) also does not mean that dividing GFR by S is the most correct approach for scaling GFR, because there may be functional differences in toxin generation between small and large people that might necessitate different amounts of renal function to remove. Nonetheless, the data do show a relative constancy of GFR scaled to S between the two genders and when examined in patients of different body size.
Disclosures
None.
Acknowledgments
This study was presented at the annual meeting of the American Society of Nephrology; November 4 through 9, 2008; Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.White AJ, Strydom WJ: Normalisation of glomerular filtration rate measurements. Eur J Nucl Med 18: 385– 390, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Blake GM, Grewal GS: An evaluation of the body surface area correction for 51Cr-EDTA measurements of glomerular filtration rate. Nucl Med Commun 26: 447– 451, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Dubois D, Dubois EF: A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17: 863– 871, 1916 [Google Scholar]
- 4.Peters AM, Henderson BL, Lui D: Indexed glomerular filtration rate as a function of age and body size. Clin Sci (Lond) 98: 439– 444, 2000 [PubMed] [Google Scholar]
- 5.Singer MA: Of mice and men and elephants: Metabolic rate sets glomerular filtration rate. Am J Kidney Dis 37: 164– 178, 2001 [DOI] [PubMed] [Google Scholar]
- 6.McKnab BK: Scaling of metabolism and thermal relations. Part 2, Chapter 3. In: The Physiological Ecology of Vertebrates: A View from Energetics, Ithaca, NY, Cornell University Press, 2002, pp. 31– 43 [Google Scholar]
- 7.Kotanko P, Levin NW: The impact of visceral mass on survival in chronic hemodialysis patients. Int J Artif Organs 30: 993– 999, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Daugirdas JT, Levin NW, Kotanko P, Depner TA, Kulhmann M, Chertow GM, Rocco MV: Comparison of proposed alternative methods for rescaling dialysis dose: Resting energy expenditure, high metabolic rate organ mass, liver size, and body surface area. Semin Dial 5: 377– 384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotch FA: Kinetics of solute removal in hemodialysis. Adv Exp Med Biol 223: 227– 237, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Owen WF, Jr, Chertow GM, Lazarus JM, Lowrie EG: Dose of hemodialysis and survival: Differences by race and sex. JAMA 280: 1764– 1768, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Depner T, Daugirdas J, Greene T, Allon M, Beck G, Chumlea C, Delmez J, Gotch F, Kusek J, Levin N, Macon E, Milford E, Owen W, Star R, Toto R, Eknoyan GHemodialysis Study Group: Dialysis dose and the effect of gender and body size on outcome in the HEMO Study. Kidney Int 65: 1386– 1394, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Israelit AH, Long DL, White MG, Hull AR: Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int 4: 346– 349, 1973 [DOI] [PubMed] [Google Scholar]
- 13.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations in the estimation of IGFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459– 466, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27– 39, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Mifflin MD, St. Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO: A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 51: 241– 247, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A: Changes in liver volume from birth to adulthood: A meta-analysis. Liver Transpl 11: 1481– 1493, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Choukèr A, Martignoni A, Dugas M, Eisenmenger W, Schauer R, Kaufmann I, Schelling G, Löhe F, Jauch KW, Peter K, Thiel M: Estimation of liver size for liver transplantation: The impact of age and gender. Liver Transpl 10: 678– 685, 2004 [DOI] [PubMed] [Google Scholar]