Abstract
Background and objectives: The aim of the investigation presented here was to compare the rates, causes, and timing of cardiovascular (CV) death in incident peritoneal dialysis (PD) and hemodialysis (HD) patients.
Design, setting, participants, & measurements: The study included all adult Australian and New Zealand patients commencing dialysis between January 1, 1997 and December 31, 2007. Rates of and times to CV death were compared by incident rate ratios, cumulative incidence, and multivariable Cox proportional hazards model analyses. Dialysis modality was included in the model as a time-varying covariate, and a competing risks approach was used to obtain cause-specific hazard ratios.
Results: Of the 24,587 patients who commenced dialysis (first treatment PD n = 6521; HD n = 18,066) during the study, 5669 (21%) died from CV causes [PD 2044 (28%) versus HD 3625 (21%)]. The incidence rates of CV mortality in PD and HD patients were 9.99 and 7.96 per 100 patient-years, respectively (incidence rate ratio PD versus HD, 1.25; 95% confidence interval 1.12 to 1.32). PD was consistently associated with an increased hazard of CV death compared with HD after 1 yr of treatment. This increased risk in PD patients was largely accounted for by an increased risk of death due to myocardial infarction.
Conclusions: Dialysis modality is significantly associated with the risk, causes, and timing of CV death experienced by ESRD patients in Australia and New Zealand.
Cardiovascular disease (CVD) represents the leading cause of death in dialysis patients, accounting for up to 40% of deaths in Australia, New Zealand, and the United States (1,2). Individuals with chronic kidney disease (CKD) have up to a 10- to 20-fold greater risk of cardiac death than age- and sex-matched controls without CKD (3,4). Once dialysis patients develop cardiac events, they are significantly less likely to receive important interventions and are far more likely to die than patients without CKD (5).
The increased incidence of CVD in dialysis patients is only partially explained by an increased prevalence of traditional risk factors, such as hypertension, diabetes mellitus, dyslipidemia, smoking, obesity, and physical inactivity (6,7). Additional risk may be conferred by nontraditional factors that are frequently observed in advanced CKD, such as hyperhomocysteinemia, anemia, abnormal calcium/phosphate metabolism, inflammation, malnutrition, oxidative stress, and elevated lipoprotein(s) (6,8–11).
There is limited evidence that dialysis modality may also influence CVD risk. Bleyer et al. (12) observed an increased risk of cardiac death in hemodialysis (HD) patients immediately after weekends, possibly related to the more frequent occurrence of hyperkalemia and fluid overload at this time. In contrast, the continuous nature of peritoneal dialysis (PD) may potentially minimize cardiovascular risk related to fluctuations in body fluid and electrolyte compositions. HD patients may also be exposed to a greater risk of CVD compared with PD patients as a result of more rapid loss of residual renal function (13,14) and more hyperdynamic circulation conferred by the presence of an arteriovenous fistula and extracorporeal circulation (15). On the other hand, PD patients are exposed to greater amounts of glucose in dialysate, leading to a much higher prevalence of insulin resistance, dyslipidemia, and metabolic syndrome (16). There is also evidence that PD patients exhibit greater coagulability, possibly related to dyslipidemia (17). Despite observed differences in known CVD risk factors between PD and HD patients, there has been little study to date of the influence of dialysis modality on CVD risk. Kennedy et al. (6) observed that PD was of borderline statistical significance (P = 0.06) as an independent predictor of carotid atherosclerosis in stage 5 CKD. Previous registry studies have shown conflicting results with respect to the influence of dialysis modality on all-cause mortality (reviewed in reference 18), but none have specifically examined cardiovascular mortality. Moreover, many of these studies have suffered from serious limitations, such as inclusion of prevalent patients, use of proportional versus nonproportional hazards models, single-center design, data coding ambiguity, use of outdated data, dialysis modality selection bias, lack of adjustment for demographic and clinical variables, and residual confounding (18). The aim of the study presented here was to evaluate the effects of dialysis modality on the frequency, types, and causes of cardiovascular mortality in a large, incident, ESRD population.
Patients and Methods
The study included all adult ESRD patients in Australia and New Zealand who were older than 18 yr and commenced dialysis between January 1, 1997 and December 31, 2007. Follow-up continued until December 31, 2007. Complete details of the structure and methods of the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry have been reported elsewhere (19).
The primary outcome measure was cardiovascular death after commencing dialysis. Cause of cardiovascular death was reported to the registry by the patient's attending nephrologist according to the following categories: myocardial ischemia/infarction, pulmonary edema, hypertensive cardiac failure, other cause of cardiac failure, cardiac arrest (cause uncertain), cerebrovascular accident, aortic aneurysm rupture, and bowel infarction. No information was collected regarding laboratory parameters, prescribed medications, percutaneous or surgical revascularizations, or other treatments used.
Results were expressed as frequencies and percentages for categorical variables and median (interquartile range) for continuous non-normally distributed variables. Baseline analyses were carried out separately for presence or absence of CVD (coronary artery disease and/or cerebrovascular disease and/or peripheral vascular disease) at baseline. Differences between patients receiving PD or HD at first treatment (day 0) were analyzed by χ2 test for categorical data and Mann–Whitney test for continuous non-normally distributed data. Comparison of the rates of cardiovascular mortality between PD and HD patients were calculated by dividing the number of deaths by patient-years at risk and presented as an incidence rate ratio (IRR) [95% confidence interval (CI)]. Time to cardiovascular death was evaluated by multivariate Cox proportional hazards survival analyses using a competing risks approach. This involved fitting the Cox model on an augmented data set stratified by cause of death (cardiovascular or other), as described by Lunn and McNeil (20). Dialysis modality was included in the model as a time-varying covariate. A delay of 60 d was allowed after a change of dialysis modality so that deaths occurring within 60 d of a change were attributed to the previous modality. The covariates included in the model were age, sex, racial origin (nonindigenous, Australian Aboriginal or Torres Strait Islander, and Maori or Pacific Islander), body mass index (BMI), late referral (referral to nephrologist within 3 mo of commencing renal replacement therapy), smoking status (never/former or current), chronic lung disease, coronary artery disease, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, country of treatment (Australia or New Zealand), and center size (based on quartiles of numbers of patients). Complete data were available on all covariates. Dialysis era was included as a stratification variable to allow different baseline hazards in each time period of dialysis commencement. Data were censored for renal transplantation, recovery of renal function, loss to follow-up, and end of study (December 31, 2007). Proportional hazards assumptions were checked by Schoenfeld residuals and scaled Schoenfeld residuals, examined graphically and by formal hypothesis test. First-order interaction terms between the significant covariates were examined for all models. There was no evidence of an interaction between modality and dialysis era, age, diabetes, country, or CVD. However there were significant interactions between modality and BMI and racial origin. Cumulative incidence was used to estimate probabilities of specific types of cardiovascular death, because this approach takes into account the presence of competing risks with deaths from other types of CVD. Nonoverlapping 95% confidence bands indicate a significant difference (at the 5% level) in cumulative incidence between the two treatment groups. Data were analyzed using Stata/IC 10 (Stata Corporation, College Station, TX). P values less than 0.05 were considered statistically significant.
Results
Baseline Characteristics
During the study period, a total of 24,587 adult patients commenced dialysis in Australia and New Zealand (first treatment PD n = 6521; HD n = 18,066). The total study follow-up was 66,005 person-years (PD 20,466 person-years; HD 45,539 person-years). Compared with patients starting dialysis on HD, patients starting on PD were more likely to be older, female, nonobese, treated in New Zealand, treated in larger centers, referred to a nephrologist more than 3 mo before commencing renal replacement therapy, and commenced on dialysis before 2002 (Table 1). PD patients were also less likely to be a current smoker, have chronic lung disease, or be of Australian Aboriginal or Torres Strait Islander racial origin. Although PD patients were more likely to change modality at least once (Table 1), patients commencing HD were more likely to change modality within the first 6 mo of treatment (19.5% versus 8.7%). Statistically significant differences were observed in comorbid illness burden between the two groups, although the magnitudes of these differences were small. The prevalence of automated PD use in PD patients during the study period was 16.1%.
Table 1.
Baseline characteristics of the study population by presence of CVD (coronary artery disease and/or cerebrovascular disease and/or peripheral vascular disease) at baselinea
| Characteristic | No CVD |
CVD |
||||
|---|---|---|---|---|---|---|
| PD (n = 3323) | HD (n = 8902) | P Value | PD (n = 3198) | HD (n = 9164) | P Value | |
| Age (yr) | 57.2 (44.6 to 68.5) | 53.7 (41.4 to 65.9) | <0.001 | 67.4 (58.8 to 74.0) | 67.2 (57.4 to 74.7) | 0.79 |
| Women | 1701 (51%) | 3751 (42%) | <0.001 | 1305 (41%) | 3239 (35%) | <0.001 |
| Race | ||||||
| nonindigenous | 2828 (85%) | 7277 (82%) | <0.001 | 2627 (82%) | 7406 (81%) | <0.001 |
| Aboriginal/Torres Strait Islander | 138 (4%) | 717 (8%) | 146 (5%) | 783 (9%) | ||
| Maori/Pacific Islander | 357 (11%) | 908 (10%) | 425 (13%) | 975 (11%) | ||
| BMI (kg/m2) | ||||||
| underweight (<18.5) | 139 (4%) | 409 (5%) | <0.001 | 86 (3%) | 309 (3%) | <0.001 |
| normal (18.5 to <25) | 1392 (42%) | 3498 (39%) | 1192 (37%) | 3276 (36%) | ||
| overweight (25 to <30) | 1105 (33%) | 2746 (31%) | 1192 (37%) | 2931 (32%) | ||
| obese (≥30) | 685 (21%) | 2221 (25%) | 716 (22%) | 2574 (28%) | ||
| Late referral | 512 (15%) | 2553 (29%) | <0.001 | 520 (16%) | 2627 (29%) | <0.001 |
| Current smoker | 446 (13%) | 1269 (14%) | 0.23 | 378 (12%) | 1246 (14%) | 0.01 |
| Chronic lung disease | 260 (8%) | 840 (9%) | 0.01 | 646 (20%) | 2212 (24%) | <0.001 |
| Coronary artery disease | – | – | – | 2548 (80%) | 7417 (81%) | 0.12 |
| Peripheral vascular disease | – | – | – | 1742 (54%) | 4796 (52%) | 0.04 |
| Cerebrovascular disease | – | – | – | 1039 (33%) | 2753 (30%) | 0.01 |
| Diabetes mellitus | 912 (27%) | 2259 (25%) | 0.02 | 1769 (55%) | 4944 (54%) | 0.18 |
| New Zealand residence | 779 (23%) | 1491 (17%) | <0.001 | 843 (26%) | 1358 (15%) | <0.001 |
| Era | ||||||
| 1997 to 1999 | 763 (23%) | 2106 (24%) | 0.36 | 840 (26%) | 1965 (21%) | <0.001 |
| 2000 to 2002 | 885 (27%) | 2408 (27%) | 924(29%) | 2371 (26%) | ||
| 2003 to 2005 | 984 (27%) | 2664 (30%) | 843 (26%) | 2769 (30%) | ||
| 2006 to 2007 | 691 (21%) | 1724 (19%) | 591 (18%) | 2059 (22%) | ||
| Center size (number of patients) | ||||||
| small (<360) | 611 (18%) | 2380 (27%) | <0.001 | 644 (20%) | 2514 (27%) | <0.001 |
| small to medium (360 to 699) | 757 (23%) | 2136 (24%) | 744 (23%) | 2364 (26%) | ||
| medium to large (700 to 839) | 853 (26%) | 1902 (21%) | 823 (26%) | 1926 (21%) | ||
| large (≥840) | 1102 (33%) | 2484 (28%) | 987 (31%) | 2360 (26%) | ||
| Never change modality | 2203 (66%) | 7028 (79%) | <0.001 | 2185 (68%) | 7054 (77%) | <0.001 |
Results are expressed as median (interquartile range) or number (%).
Cardiovascular Mortality
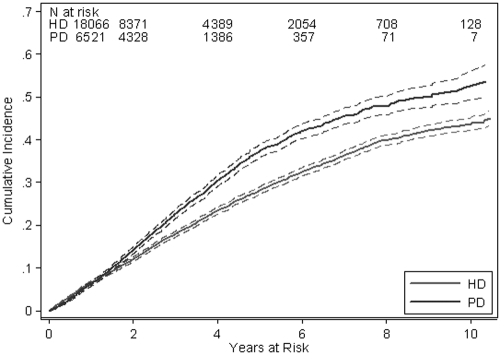
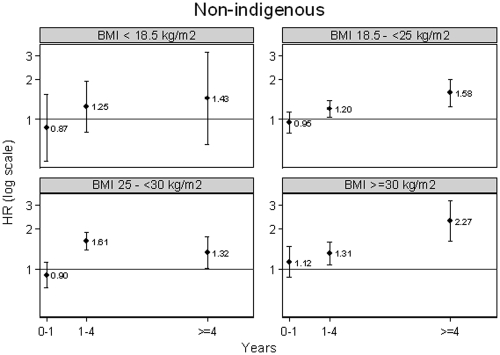
A total of 10,298 patients (42%) died during the study period [PD 3578 (55%) versus HD 6720 (37%)], including 5669 (23%) deaths from cardiovascular causes [PD 2044 (31%) versus HD 3625 (20%)] (Table 2) and 4629 (19%) deaths from other causes (PD 1534 [24%] versus HD 3095 [17%]). The incidence rates of cardiovascular mortality in PD and HD patients were 9.99 and 7.96 per 100 patient-years, respectively [unadjusted IRR PD versus HD 1.25, 95% CI 1.12 to 1.32]. The incidence rates of deaths from noncardiovascular causes in PD and HD patients were 7.50 and 6.80 per 100 patient-years, respectively (unadjusted IRR PD versus HD 1.10, 95% CI 1.04 to 1.17). When cumulative incidence functions were calculated for cardiovascular deaths, 95% confidence bands did not overlap after 2 yr, confirming that PD was associated with an increased risk of death from CVD after 2 yr of treatment (Figure 1). On competing risks multivariable Cox proportional hazards model analysis, the cause-specific hazard ratios of cardiovascular death and death from other causes between PD and HD were found to be nonconstant over time (i.e., nonproportional hazards). The proportional hazards assumption was also violated for presence of coronary artery disease, presence of cerebrovascular disease, late referral to a nephrologist, and current smoking status. To deal with nonproportional hazards, the follow-up period was divided into survival over the first year of treatment, between 1 and 4 yr of treatment, and after 4 yr of treatment. In each of these time periods, the proportional hazards assumption was verified and interaction terms between time on study and each of the covariates exhibiting nonproportional hazards were included in the model to obtain hazard ratios for each period of time. There were statistically significant interactions between modality and BMI and racial origin, so hazard ratios were calculated separately for each BMI and racial origin combination. Figure 2 shows the hazard ratios with 95% CI by BMI for nonindigenous patients. The hazard of death from CVD in PD patients was not significantly different from that in HD patients in the first year of treatment or with a BMI less than 18.5 kg/m2, but was consistently and significantly increased in PD patients compared with HD patients with a BMI of 18.5 kg/m2 or more after 1 yr of treatment (Figure 2). The interaction was quantitative in nature so the degree of increased risk varied by BMI category and racial origin. Hence, similar results were obtained for Australian Aboriginal and Torres Strait Islanders and for Maori and Pacific Islanders (data not shown).
Table 2.
Outcomes of incident HD and PD patients in Australian and New Zealand 1991 to 2007 by presence of CVD at baselinea
| Parameter | No CVD |
CVD |
||
|---|---|---|---|---|
| PD (n = 3323) | HD (n = 8902) | PD (n = 3198) | HD (n = 9164) | |
| Follow-up duration (patient-years) | 10,822 | 24,096 | 9643 | 21,444 |
| Total deaths | 1124 (10.39) | 2237 (9.28) | 2454 (25.45) | 4483 (20.91) |
| Cardiovascular deaths | ||||
| myocardial ischemia/infarction | 241 (2.23) | 332 (1.38) | 709 (7.35) | 1011 (4.71) |
| pulmonary edema | 8 (0.07) | 21 (0.09) | 14 (0.15) | 38 (0.18) |
| hypertensive cardiac failure | 5 (0.05) | 9 (0.04) | 8 (0.08) | 21 (0.10) |
| other cause of cardiac failure | 149 (1.38) | 322 (1.34) | 352 (3.65) | 664 (3.10) |
| cardiac arrest (cause uncertain) | 20 (0.18) | 45 (0.19) | 53 (0.55) | 63 (0.29) |
| cerebrovascular accident | 66 (0.61) | 107 (0.44) | 140 (1.45) | 257 (1.20) |
| aortic aneurysm rupture | 0 (0.00) | 10 (0.04) | 12 (0.12) | 29 (0.14) |
| bowel infarction | 24 (0.22) | 45 (0.19) | 29 (0.30) | 55 (0.26) |
| withdrawal because of cardiac disease | 11 (0.10) | 43 (0.18) | 59 (0.61) | 195 (0.91) |
| withdrawal because of CVD | 20 (0.18) | 34 (0.14) | 44 (0.46) | 123 (0.57) |
| withdrawal because of peripheral vascular disease | 13 (0.12) | 30 (0.12) | 67 (0.69) | 171 (0.80) |
| total | 557 (5.15) | 998 (4.14) | 1487 (15.42) | 2627 (12.25) |
| Modality change | 1120 (34%) | 1874 (21%) | 1013 (32%) | 2110 (23%) |
| Renal transplantation | 969 (29%) | 2469 (28%) | 191 (6%) | 451 (5%) |
| Lost to follow-up | 2 (0.1%) | 6 (0.1%) | 3 (0.1%) | 8 (0.1%) |
| Recovery of renal function | 2 (0.1%) | 73 (0.8%) | 4 (0.1%) | 74 (0.8%) |
Death outcomes are expressed as number (incidence rate per 100 patient-years).
Figure 1.
Cumulative incidence with 95% confidence bands of deaths in PD and HD patients from CVD.
Figure 2.
Hazard ratios (PD versus HD) with 95% confidence bars of cardiovascular deaths in nonindigenous patients by BMI.
Causes of Cardiovascular Mortality
The causes of cardiovascular death are shown in Table 2. In patients with CVD at baseline, cardiac failure and cardiac arrest were significantly more common causes of death in PD patients compared with HD patients (Table 3). Death resulting from withdrawal from treatment due to cardiac disease was significantly less common in PD patients. Myocardial infarction/ischemia was significantly more common in PD patients regardless of the presence of CVD at baseline (Table 3). There were no significant differences between PD and HD patients' incidence rates of other forms of cardiovascular death (Table 3).
Table 3.
Unadjusted incident rate ratios (IRRs) for different causes of cardiovascular mortality in incident PD and HD patients in Australian and New Zealand 1991 to 2007 by presence of CVD at baselinea
| Cause of Cardiovascular Mortality | No CVD (IRR (95% CI)) | CVD (IRR (95% CI)) |
|---|---|---|
| Myocardial ischemia/infarction | 1.62 (1.36 to 1.91) | 1.56 (1.41 to 1.72) |
| Cardiac arrest (cause uncertain) | 0.99 (0.55 to 1.71) | 1.87 (1.27 to 2.74) |
| Cerebrovascular accident | 1.37 (0.99 to 1.88) | 1.21 (0.98 to 1.49) |
| Bowel infarction | 1.19 (0.69 to 1.99) | 1.17 (0.72 to 1.87) |
| Cardiac failure | 1.04 (0.85 to 1.26) | 1.17 (1.03 to 1.33) |
| Withdrawal because of cerebrovascular disease | 1.31 (0.71 to 2.34) | 0.80 (0.55 to 1.13) |
| Withdrawal because of peripheral vascular disease | 0.96 (0.46 to 1.91) | 0.87 (0.65 to 1.16) |
| Pulmonary edema | 0.85 (0.32 to 1.99) | 0.82 (0.41 to 1.55) |
| Aortic aneurysm rupture | 0.00 (0.00 to 0.99) | 0.92 (0.43 to 1.86) |
| Withdrawal because of cardiac disease | 0.57 (0.26 to 1.12) | 0.67 (0.49 to 0.90) |
| All-cause cardiovascular mortality | 1.24 (1.12 to 1.38) | 1.26 (1.18 to 1.34) |
An IRR > 1 signifies an increased risk in PD patients relative to HD patients.
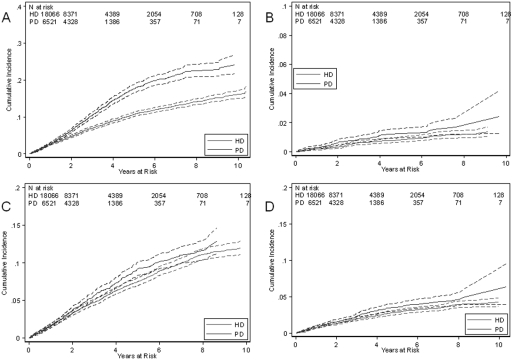
The excess cardiovascular-specific mortality in PD patients was explained by an augmented risk of myocardial ischemia/infarction (Figure 3A). Cumulative incidence of myocardial ischemia/infarction was increased in PD patients after 1 yr of treatment. The cumulative incidences of deaths from myocardial ischemia/infarction in PD and HD patients were 0.03 and 0.03 at 1 yr and 0.18 and 0.11 at 5 yr, respectively. There were no significant differences in cumulative incidences of cardiac arrest (Figure 3B); nonhypertensive cardiac failure (Figure 3C); cerebrovascular accident (Figure 3D); pulmonary edema; hypertensive cardiac failure; aortic aneurysm rupture; bowel infarction; or withdrawal from treatment due to cardiac, cerebrovascular, or peripheral vascular disease (data not shown).
Figure 3.
Cumulative incidence with 95% confidence bands of deaths in PD and HD patients from (A) ischemic heart disease, (B) sudden cardiac death, (C) cardiac failure, and (D) cerebrovascular disease.
Discussion
The study presented here demonstrated that the risk of death from CVD was significantly increased in PD patients compared with HD patients after the first year of treatment. Our findings are in keeping with those of previous publications (21–24), which have suggested poorer cardiovascular outcomes in PD patients. Bloembergen et al. (21,22) analyzed the U.S. Renal Data System for prevalent dialysis patients (1987 to 1989) and observed a significantly increased mortality risk for PD compared with HD for all cause-of-death categories, except malignancy, for which there was a higher mortality risk for HD. The excess all-cause mortality observed in PD-treated patients was accounted for, in decreasing order, by infection (35%), acute myocardial infarction (24%), other cardiac causes (16%), cerebrovascular disease (8%), and withdrawal (8%). However, this study was significantly limited by the inclusion of prevalent patients, thereby raising the possibility of selective survival bias.
More recently, Ganesh et al. (23) used nonproportional Cox regression models to estimate the relative risk of death for U.S. patients with and without coronary artery disease by dialysis modality using intention to treat and as-treated approaches. They observed that patients with established coronary artery disease treated with PD had significantly poorer survival compared with HD. Using the same population of patients and the same analytical methods, Stack et al. (24) reported that new ESRD patients with a clinical history of congestive heart failure experienced poorer survival on PD compared with HD. Vonesh et al. (25) subsequently performed a U.S. Renal Data System analysis using a more recent cohort of patients with stratification for age and a general level of comorbidity rather than a specific comorbid condition. They observed that survival differences varied substantially over time between PD and HD according to age and baseline comorbidity. Nevertheless, all of the U.S. studies have been potentially limited by underreporting of comorbidity and other data commonly associated with Medical Evidence Form 2728 (18), which may potentially vary according to dialysis modality and by the relatively low penetration of PD compared with HD in the United States.
In contrast, Locatelli et al. (26) observed no difference in the risk of death [risk ratio (RR) 0.91, 95% CI 0.79 to 1.06] or de novo CVD (RR 1.06, 95% CI 0.79 to 1.43) between PD and HD in 3120 patients without established CVD who commenced renal replacement therapy in Lombardy between 1994 and 1997. Similarly, Foley and coworkers (27) found no difference between PD and HD in the proportions of 433 incident dialysis patients who went on to develop new ischemic heart disease or cardiac failure while on dialysis treatment. Both of these studies were limited by lack of statistical power, lack of consideration of nonproportional hazards and competing risks, and uncertain relevance of outcomes observed over a decade ago to contemporary dialysis practice. The relevance to current practice is particularly important in view of observations that outcomes in PD patients have improved over time relative to those in HD patients (18,28).
Several other registry (29–35) and prospective observational cohort studies (36–39) have examined the effect of dialysis modality on patient outcomes, with generally conflicting results. These studies have been reviewed by Vonesh et al. (18) and have primarily focused on all-cause mortality rather than cardiovascular mortality. To date, most investigations have observed that PD patient outcomes are equivalent to or better than those for HD patients in the first 1 to 2 yr, but progressively worsen relative to HD thereafter. Our group has also observed this phenomenon for all-cause mortality (40) and infectious mortality (41). A novel finding of the study presented here is that we have demonstrated for the first time that the increasing risk of all-cause mortality in PD versus HD over time also applies to cardiovascular mortality. The reasons for this observation are uncertain but may relate to the differential rate of residual renal function decline between PD and HD (13,14,42); the declining rates of dialysis catheter usage in HD patients after the first year (43); the increasing rate of infectious complications in PD patients relative to HD patients over time (41); or the greater propensity for PD patients to develop insulin resistance, dyslipidemia, and metabolic syndrome over time as a result of peritoneal exposure to glucose (16,44).
Because CVD is the major cause of death in dialysis populations, further studies are warranted to confirm whether cardiovascular mortality differs between PD and HD. The ideal vehicle for comparison is a randomized controlled trial. However, two previous attempts at such trials have been complicated by lack of statistical power (45) and poor recruitment (46). Given that the feasibility of a definitive randomized controlled trial in this area is highly questionable, large observational studies are likely to offer the best available evidence, although they do not directly offer evidence of causality.
The strengths of our study include its very large sample size, use of contemporary incident patients, high PD penetration (41%), and robustness of findings across different statistical methodologies. We included all patients who began dialysis therapy in Australia or New Zealand over the time period; thus, various centers were included with varying approaches to use of dialysis modalities and varying rates of transplantation. This greatly enhanced the external validity of our findings.
Nevertheless, the study has several limitations. ANZDATA does not collect detailed information on PD or HD prescription, medication use (including vitamin D), patient compliance, nonfatal CVD, HD catheter use, hospitalizations, individual unit management protocols, laboratory values, residual urine output, or the severity of comorbidities, therefore unidentified associations cannot be entirely excluded. Although we adjusted for many patient characteristics, the possibility of residual confounding also cannot be excluded. Moreover, time-updated covariate data were not available for analysis. The results may also have been influenced by selection bias because the choice of dialysis modality was nonrandom. In common with other registries, ANZDATA is a voluntary registry and there is no external audit of data accuracy (including coding of the cause of death). Consequently, the possibility of coding/classification bias cannot be excluded (47). Although we included all patients who commenced dialysis therapy as their first RRT modality, overall treated ESRD incidence rates in Australia are currently around 100 per million per year, substantially lower than the United States and slightly lower than many European countries, although broadly comparable with rates in other countries, such as Sweden, The Netherlands, and Poland (48). Differences in the characteristics of patients might explain the differing results; alternatively, it might be that there are systematic differences in the treatment outcomes between countries. Although there are no published data that allow comparisons of PD mortality rates, there is substantial variation in HD mortality rates, which is not explained by measured comorbidities (49).
The study presented here demonstrates that PD is associated with an increased risk of cardiovascular mortality compared with HD after the first year. This excess risk is due primarily to a heightened risk of myocardial ischemia/infarction, which increases with increasing time on dialysis. Although the increased risk of cardiovascular-related mortality in PD patients in our study should not be interpreted as an endorsement of HD over PD, these study findings should form part of the basis for informing shared decision-making about dialysis modality selection in conjunction with patient preference, individual circumstances, and the other well characterized pros and cons of selecting PD versus HD. Further attention to cardiovascular risk factor management in PD would also appear warranted.
Disclosures
Professor David Johnson is a consultant for Baxter Healthcare Pty Ltd. and has previously received research funds from this company. He has also received speakers' honoraria and research grants from Fresenius Medical Care. Dr Kym Bannister is a consultant for Baxter Healthcare Pty Ltd. Dr McDonald has received speaking honoraria from Fresenius Australia and Baxter Australia. All other authors have no competing interests.
Acknowledgments
The authors gratefully acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the ANZDATA registry database.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.McDonald S, Excell L: ANZDATA Registry Report 2005. Adelaide, South Australia, Australian and New Zealand Dialysis and Transplant Registry, 2006 [Google Scholar]
- 2.Annual Data Report Minneapolis, MN, U.S. Renal Data Systems, 2006 [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112– S119, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307– 1315, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Keough-Ryan TM, Kiberd BA, Dipchand CS, Cox JL, Rose CL, Thompson KJ, Clase CM: Outcomes of acute coronary syndrome in a large Canadian cohort: Impact of chronic renal insufficiency, cardiac interventions, and anemia. Am J Kidney Dis 46: 845– 855, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy R, Case C, Fathi R, Johnson D, Isbel N, Marwick TH: Does renal failure cause an atherosclerotic milieu in patients with end-stage renal disease? Am J Med 110: 198– 204, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Isbel NM, Haluska B, Johnson DW, Beller E, Hawley C, Marwick TH: Increased targeting of cardiovascular risk factors in patients with chronic kidney disease does not improve atheroma burden or cardiovascular function. Am Heart J 151: 745– 753, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz FM, Akay H, Duranay M, Yilmaz G, Oztekin PS, Kosar U, Tekeli N, Altay M, Parpucu H, Yucel D: Carotid atherosclerosis and cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Clin Biochem 40: 1361– 1366, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Zoccali C: Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int 70: 26– 33, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Kaisar M, Isbel N, Johnson DW: Cardiovascular disease in patients with chronic kidney disease. A clinical review. Minerva Urol Nefrol 59: 281– 297, 2007 [PubMed] [Google Scholar]
- 11.Kaisar MO, Isbel NM, Johnson DW: Recent clinical trials of pharmacologic cardiovascular interventions in patients with chronic kidney disease. Rev Recent Clin Trials 3: 79– 88, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553– 1559, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert Shearon T, Jones CA, Bloembergen WE: Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 11: 556– 564, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, Nolph KD, Pollock CA, Prowant B, Farrell PC: The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 37: 598– 604, 1991 [PubMed] [Google Scholar]
- 15.Dikow R, Schwenger V, Zeier M, Ritz E: Do AV fistulas contribute to cardiac mortality in hemodialysis patients? Semin Dial 15: 14– 17, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Johnson DW, Armstrong K, Campbell SB, Mudge DW, Hawley CM, Coombes JS, Prins JB, Isbel NM: Metabolic syndrome in severe chronic kidney disease: Prevalence, predictors, prognostic significance and effects of risk factor modification. Nephrology (Carlton) 12: 391– 398, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Yorioka N, Yamakido M: Hypercoagulability and secondary hyperfibrinolysis may be related to abnormal lipid metabolism in patients treated with continuous ambulatory peritoneal dialysis. Nephron 76: 56– 61, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney Int Suppl S3– S11, 2006 [DOI] [PubMed] [Google Scholar]
- 19.McDonald SP, Chang S, Excell L: ANZDATA Registry Report 2007 Adelaide, Australia, Australian and New Zealand Dialysis and Transplant Registry, 2007 [Google Scholar]
- 20.Lunn M, McNeil D: Applying Cox regression to competing risks. Biometrics 51: 524– 532, 1995 [PubMed] [Google Scholar]
- 21.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: A comparison of cause of death between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 6: 184– 191, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Bloembergen WE, Port FK, Mauger EA, Wolfe RA: A comparison of mortality between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 6: 177– 183, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG: Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 14: 415– 424, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B: Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 64: 1071– 1079, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int 66: 2389– 2401, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Locatelli F, Marcelli D, Conte F, D'Amico M, Del Vecchio L, Limido A, Malberti F, Spotti D: Survival and development of cardiovascular disease by modality of treatment in patients with end-stage renal disease. J Am Soc Nephrol 12: 2411– 2417, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Foley RN, Parfrey PS, Harnett JD, Kent GM, O'Dea R, Murray DC, Barre PE: Mode of dialysis therapy and mortality in end-stage renal disease. J Am Soc Nephrol 9: 267– 276, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R: Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol 2: 1317– 1328, 2007 [DOI] [PubMed] [Google Scholar]
- 29.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155– 163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fenton SS, Schaubel DE, Desmeules M, Morrison HI, Mao Y, Copleston P, Jeffery JR, Kjellstrand CM: Hemodialysis versus peritoneal dialysis: A comparison of adjusted mortality rates [see comments]. Am J Kidney Dis 30: 334– 342, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Serkes KD, Blagg CR, Nolph KD, Vonesh EF, Shapiro F: Comparison of patient and technique survival in continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis: A multicenter study. Perit Dial Int 10: 15– 19, 1990 [PubMed] [Google Scholar]
- 32.Schaubel DE, Morrison HI, Fenton SS: Comparing mortality rates on CAPD/CCPD and hemodialysis. The Canadian experience: Fact or fiction? Perit Dial Int 18: 478– 484, 1998 [PubMed] [Google Scholar]
- 33.Heaf JG, Lokkegaard H, Madsen M: Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 17: 112– 117, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Nelson CB, Port FK, Wolfe RA, Guire KE: Comparison of continuous ambulatory peritoneal dialysis and hemodialysis patient survival with evaluation of trends during the 1980s. J Am Soc Nephrol 3: 1147– 1155, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Held PJ, Port FK, Turenne MN, Gaylin DS, Hamburger RJ, Wolfe RA: Continuous ambulatory peritoneal dialysis and hemodialysis: Comparison of patient mortality with adjustment for comorbid conditions. Kidney Int 45: 1163– 1169, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Termorshuizen F, Korevaar JC, Dekker FW, Van Manen JG, Boeschoten EW, Krediet RT: Hemodialysis and peritoneal dialysis: Comparison of adjusted mortality rates according to the duration of dialysis: Analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol 14: 2851– 2860, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Jaar BG, Coresh J, Plantinga LC, Fink NE, Klag MJ, Levey AS, Levin NW, Sadler JH, Kliger A, Powe NR: Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med 143: 174– 183, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Murphy SW, Foley RN, Barrett BJ, Kent GM, Morgan J, Barre P, Campbell P, Fine A, Goldstein MB, Handa SP, Jindal KK, Levin A, Mandin H, Muirhead N, Richardson RM, Parfrey PS: Comparative mortality of hemodialysis and peritoneal dialysis in Canada. Kidney Int 57: 1720– 1726, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Maiorca R, Vonesh EF, Cavalli P, De Vecchi A, Giangrande A, La Greca G, Scarpioni LL, Bragantini L, Cancarini GC, Cantaluppi A. A multicenter, selection-adjusted comparison of patient and technique survivals on CAPD and hemodialysis. Perit Dial Int 11: 118– 127, 1991 [PubMed] [Google Scholar]
- 40.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155– 163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown F, Bannister K, Wiggins KJ: Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kid Dis 53: 290– 297, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, Hollett P: Predictors of residual renal function decline in new peritoneal dialysis patients. Perit Dial Int 23: 276– 283, 2003 [PubMed] [Google Scholar]
- 43.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG: Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol 15: 477– 486, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Kronenberg F, Lingenhel A, Neyer U, Lhotta K, Konig P, Auinger M, Wiesholzer M, Andersson H, Dieplinger H: Prevalence of dyslipidemic risk factors in hemodialysis and CAPD patients. Kidney Int Suppl S113– S116, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Gutman RA, Blumenkrantz MJ, Chan YK, Barbour GL, Gandhi VC, Shen FH, Tucker T, Murawski BJ, Coburn JW, Curtis FK: Controlled comparison of hemodialysis and peritoneal dialysis: Veterans Administration multicenter study. Kidney Int 26: 459– 470, 1984 [DOI] [PubMed] [Google Scholar]
- 46.Korevaar JC, Feith GW, Dekker FW, Van Manen JG, Boeschoten EW, Bossuyt PM, Krediet RT: Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: A randomized controlled trial. Kidney Int 64: 2222– 2228, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Li SQ, Cass A, Cunningham J: Cause of death in patients with end-stage renal disease: Assessing concordance of death certificates with registry reports. Aust N Z J Public Health 27: 419– 424, 2003 [DOI] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services: USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006 [Google Scholar]
- 49.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: The Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol 14: 3270– 3277, 2003 [DOI] [PubMed] [Google Scholar]