Abstract
Background and objectives: As a major component of uremic syndrome, cardiovascular disease is largely responsible for the high mortality observed in chronic kidney disease (CKD). Preclinical studies have evidenced an association between serum levels of indoxyl sulfate (IS, a protein-bound uremic toxin) and vascular alterations. The aim of this study is to investigate the association between serum IS, vascular calcification, vascular stiffness, and mortality in a cohort of CKD patients.
Design, setting, participants, & measurements: One-hundred and thirty-nine patients (mean ± SD age: 67 ± 12; 60% male) at different stages of CKD (8% at stage 2, 26.5% at stage 3, 26.5% at stage 4, 7% at stage 5, and 32% at stage 5D) were enrolled.
Results: Baseline IS levels presented an inverse relationship with renal function and a direct relationship with aortic calcification and pulse wave velocity. During the follow-up period (605 ± 217 d), 25 patients died, mostly because of cardiovascular events (n = 18). In crude survival analyses, the highest IS tertile was a powerful predictor of overall and cardiovascular mortality (P = 0.001 and 0.012, respectively). The predictive power of IS for death was maintained after adjustment for age, gender, diabetes, albumin, hemoglobin, phosphate, and aortic calcification.
Conclusions: The study presented here indicates that IS may have a significant role in the vascular disease and higher mortality observed in CKD patients.
Cardiovascular disease (CVD) mortality is significantly higher in patients with chronic kidney disease (CKD) than in the general population (1,2). Vascular calcification and arterial stiffness are considered to be major contributors to this elevated mortality (3). The pathophysiology of CVD in a CKD setting is not completely understood; in addition to the traditional CVD risk factors, uremia-related elements play an important role. Although these elements may differ from one individual to another, the accumulation of toxic compounds is a common scenario in CKD. These compounds are classified according to their molecular weight and protein binding ability and several have been shown to exert adverse biologic effects and thus enhance the potential for cardiovascular damage (4). Interestingly, we recently observed that cardiac and aortic abnormalities were independently associated with the extent of endothelial dysfunction and renal failure in a mouse model of CKD (5), corroborating the hypothesis whereby the accumulation of uremic toxins is involved in the development of CKD-related cardiovascular abnormalities.
Indoxyl sulfate (IS) is a protein-bound uremic toxin that results from the metabolism of dietary tryptophan. Briefly, tryptophan is metabolized into indole by intestinal bacteria and after intestinal absorption is further converted to IS in the liver. The kidneys excrete the toxin via proximal tubular secretion. Consequently, IS accumulates in the blood of patients with impaired renal function. Moreover, IS cannot be efficiently removed by conventional hemodialysis because of its high binding affinity for albumin (6). The role of IS as a uremic toxin was first revealed by its accelerating effects on the progression of CKD (7). Recently, it has been reported that IS may also act as a vascular toxin. In endothelial cells, IS has been shown to (1) induce oxidative stress by modifying the balance between pro- and antioxidant mechanisms (8), (2) stimulate the release of endothelial microparticles (9), and (3) blunt endothelial healing ability (10). Furthermore, IS directly stimulates rat vascular smooth muscular cell proliferation in a concentration-dependent manner (11). Accordingly, increased aortic wall thickness and severe aortic calcification with colocalization of osteoblast-specific proteins (e.g., Cbfa-1, osteopontin, and alkaline phosphatase) were observed in Dahl salt-sensitive, hypertensive rats to which IS was administered (12). These findings suggest that IS may play a role in vascular dysfunction in CKD patients.
To investigate this hypothesis, we assessed serum IS levels, aortic calcification, and vascular stiffness (as measured by pulse wave velocity, PWV) in a cohort of CKD patients. We further examined whether IS levels might predict mortality in this population.
Materials and Methods
Patient Selection
Over an 18-mo period (from January 2006 to June 2007), a total of 150 Caucasian prevalent CKD patients were recruited from the Nephrology Department's outpatient clinic at Amiens University Hospital. All patients gave their informed, written consent. The study was approved by the local investigational review board and performed in accordance with the ethical principles of the Declaration of Helsinki.
Included patients had to be over the age of 40 with a confirmed diagnosis of CKD (defined as being on hemodialysis or having two previous estimated creatinine clearances—calculated according to the Cockcroft and Gault formula (13)—with an interval of 3 to 6 mo and values <90 ml/min/1.73 m2). Stage 5D CKD patients had been receiving thrice-weekly hemodialysis for at least 3 mo. Exclusion criteria included the presence of chronic inflammatory disease, atrial fibrillation, complete heart block, abdominal aorta aneurysm, aortic and/or femoral artery prosthesis, primary hyperparathyroidism, kidney transplantation, and any acute cardiovascular event in the 3 mo before screening for inclusion. The 139 patients who met all inclusion criteria and had available IS serum level measurements were included in these analyses.
Study Protocol
All patients were hospitalized for the day to perform laboratory blood tests, blood pressure measurement, PWV determination, a lateral lumbar x-ray, and multislice spiral computed tomography (MSCT) scanning. Hemodialysis patients were preferentially seen on a dialysis-free day or the morning before the dialysis session. A patient interview focused on comorbidities and the personal disease history. The patients' medical charts were reviewed to identify and record any concomitant medications. For descriptive purposes, patients who reported active or historical use of insulin and/or orally administered hypoglycemic drugs were considered as diabetics. Previous CVD was defined as a history of any of the following events: myocardial infarction, stroke, heart failure, angina pectoris, surgical procedures for angina, or coronary/peripheral artery disease (including percutaneous transluminal angioplasty).
Laboratory
Blood samples were collected the same morning, before the other investigations were undertaken. Selected variables were measured after the samples had been frozen and stored at −80°C. Serum calcium, phosphate, albumin, cholesterol, hemoglobin, creatinine (Scr), and C-reactive protein levels were assayed in an on-site biochemistry laboratory using standard autoanalyzer techniques (the Modular IIP system, Roche Diagnostics, Basel, Switzerland). Serum intact parathyroid hormone (iPTH) was determined in a chemiluminometric immunoassay (Liaison N-tact PTH CLIA, Diasorin, Stillwater, OK). For determination of IS serum levels, samples were deproteinized by heat denaturation and the analysis was performed by reversed-phase HPLC. The serum concentrations were then determined by fluorescence detection (excitation 280 nm, emission 340 nm) using a reference value for healthy controls of 0.113 ± 0.06 mg/100 ml. Serum cystatin C (CysC) levels were determined by immunonephelometry (N Latex Cystatin C, Dade Behring, Marburg, Germany). To describe the true GFR as closely as possible, the estimated GFR combining Scr and CysC measurements (CKD-epi) was calculated for all nondialyzed patients according to the following recently published (14) “CKD-epi” equation: 177.6 × Scr−0.65 × CysC−0.57 × age−0.20 × (0.82 if female). For descriptive purposes, patients were then classified into CKD stages according to the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative guidelines (15).
PWV Evaluation
Carotid-femoral PWV was determined automatically with a dedicated, validated device (Complior Colson, Createch Industrie, Massy, France) as described previously (16). Detailed information is provided elsewhere (16,17).
Abdominal Aorta Imaging with Plain Radiography
A technique similar to that described by Kauppila et al. (18) was used to obtain images of the lower abdominal aorta and generate an aortic calcification score. Two investigators reviewed all x-rays and a consensus on the interpretation was reached for all patients (17).
MSCT Scan
To quantify the presence and extent of aortic calcifications, each patient underwent a MSCT scan. All examinations were performed with a 64-detector computed tomography scanner (Lightspeed VCT, GE Healthcare, Milwaukee, WI). Detailed technical information on the procedure is provided elsewhere (17).
Survival
Death records were made prospectively by considering all patients included at least 1 yr before the study end date (June 30, 2008). Each medical chart was reviewed and a physician assigned the cause of death on the basis of all of the available clinical information. For out-of-hospital deaths, the patient's general practitioner was interviewed to gain pertinent information on the cause. Cardiovascular mortality was defined as any death directly related to cardiovascular system dysfunction (stroke, myocardial infarction, congestive heart failure, or sudden death).
Statistical Analyses
Data are expressed as the mean ± SD, median, and range or frequency, as appropriate. For analytical purposes, patients were divided according to IS tertiles (1st tertile <0.26 mg/100 ml, 2nd tertile ≥0.26 mg/100 ml and ≤0.76 mg/100 ml, and 3rd tertile >0.76 mg/100 ml). Intergroup comparisons were performed using a χ2 test for categorical variables and the one-way ANOVA or the Kruskal–Wallis test for continuous variables. Pearson's correlation coefficient or Spearman's rank correlation were used to assess the relationships between serum IS levels and selected clinical or biochemical variables. A multiple linear regression analysis was performed to identify factors independently associated with serum IS. Because the latter parameter had a non-Gaussian distribution, logarithmic normalized values of IS concentrations were used to replace tests that assume normally distributed variables. A Kaplan–Meyer actuarial method was used to estimate overall survival for the IS tertiles. The log-rank test was used to compare survival curves. Univariate and multivariate analyses of mortality were performed by using a Cox proportional hazards model of death as a function of serum IS. In the multivariate analysis, the predefined noncumulative models included those variables significantly associated with death in univariate analyses and those of prognostic importance from published observations. The limited number of events (n = 25) did not allow us to include more than three variables for each model. To overcome this problem, supplementary Cox regression analyses were performed including a propensity score adjustment, which considers the probability of exposure for each individual for measured confounding variables (i.e., hemoglobin and aortic calcification score), as detailed elsewhere (19). A P ≤ 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS software 9.1 (SAS Institute Inc., Cary, NC).
Results
Table 1 shows the demographic, clinical, and biochemical characteristics of the 139 analyzed patients. Concerning CKD, 8% of the patients were classified as stage 2, 26.5% as stage 3, 26.5% as stage 4, 7% as stage 5, and 32% as stage 5D.
Table 1.
Clinical and demographic characteristics of the study population
| Characteristic | All (n = 139) | Serum IS |
P Value | ||
|---|---|---|---|---|---|
| 1st Tertile (n = 46) | 2nd Tertile (n = 46) | 3rd Tertile (n = 47) | |||
| Age (yr) | 67 ± 12 | 68 ± 10 | 66 ± 14 | 67 ± 13 | 0.798 |
| Male gender [n (%)] | 84 (60) | 26 (56) | 31 (67) | 27 (57) | 0.496 |
| Body mass index (kg/m2) | 28 ± 6 | 28 ± 6 | 30 ± 7 | 27 ± 5 | 0.107 |
| Diabetes mellitus [n (%)] | 58 (42) | 23 (50) | 21 (46) | 14 (30) | 0.114 |
| Smoking habit [n (%)] | 55 (40) | 15 (33) | 24 (54) | 16 (34) | 0.068 |
| Presence of CVD [n (%)] | 45 (32) | 16 (35) | 10 (22) | 19 (40) | 0.143 |
| Systolic arterial pressure (mmHg) | 153 ± 26 | 148 ± 25 | 156 ± 28 | 155 ± 26 | 0.298 |
| Diastolic arterial pressure (mmHg) | 81 ± 12 | 80 ± 12 | 83 ± 12 | 80 ± 13 | 0.278 |
| CKD stage [n (%)] | <0.0001 | ||||
| 2 | 11 (8) | 11 (24) | 0 (0) | 0 (0) | |
| 3 | 37 (26.5) | 24 (52.5) | 13 (28) | 0 (0) | |
| 4 | 37 (26.5) | 6 (13) | 27 (59) | 4 (8) | |
| 5 | 10 (7) | 2 (4) | 3 (6.5) | 5 (11) | |
| 5D | 44 (32) | 3 (6.5) | 3 (6.5) | 38 (81) | |
Data are expressed as mean ± SD or, for binary variables, number (frequency). Tertiles are ordered from low serum IS levels to high levels: 1st tertile <0.26 mg/100 ml; 2nd tertile ≥0.26 mg/100 ml and <0.76 mg/100 ml; and 3rd tertile ≥0.76 mg/100 ml.
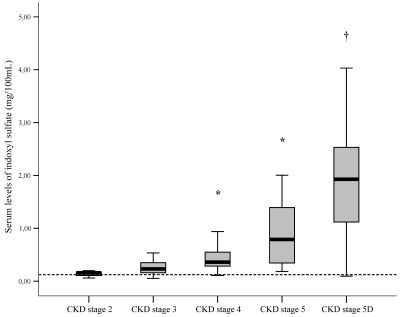
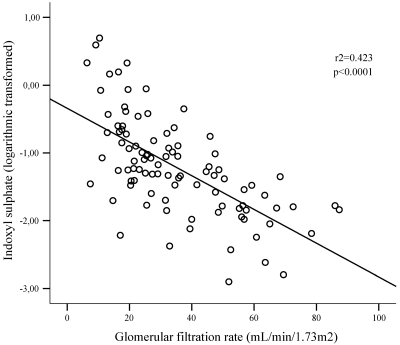
Figure 1 shows the distribution of IS serum levels as a function of the CKD stage. Serum IS increased significantly and progressively with increasing CKD stages. When considering only predialysis CKD patients (n = 95), there was still a significant relationship between the serum IS and the estimated GFR, as illustrated by Figure 2.
Figure 1.
Serum levels of IS as a function of CKD stages. *P < 0.05 versus CKD stages 2, 3 or 5D; †P < 0.05 versus CKD stages 2, 3, 4, or 5. The dotted line indicates the reference value for healthy controls.
Figure 2.
Linear regression curve. Relationship between serum levels of IS and the estimated GFR for patients at CKD stages 2 to 5 (n = 95).
When comparing patients divided by IS tertiles, there were no intergroup differences concerning age, gender, body mass index, diabetes mellitus status, smoking habits, systolic and diastolic arterial pressure values, or a positive history for cardiovascular events. Concerning CKD stages, the 3rd IS tertile contained a higher proportion of patients at later stages (i.e., 5 and 5D). Serum levels of phosphate were significantly higher in patients in the 3rd IS tertile when compared with the 1st IS tertile. Similarly, iPTH levels were significantly higher in the 3rd IS tertile, compared with both the 1st and 2nd IS tertiles. Conversely, the hemoglobin levels and the estimated GFR were significantly higher for patients from the 1st IS tertile in comparison with the 2nd and 3rd IS tertiles (Table 2).
Table 2.
Biochemical characteristics of the study population
| Biochemical Marker | All (n = 139) | Serum IS |
P Value | ||
|---|---|---|---|---|---|
| 1st Tertile (n = 46) | 2nd Tertile (n = 46) | 3rd Tertile (n = 47) | |||
| Calcium (mMol/L) | 2.29 ± 0.18 | 2.30 ± 0.18 | 2.32 ± 0.16 | 2.25 ± 0.21 | 0.142 |
| Phosphate (mMol/L) | 1.28 ± 0.44 | 1.11 ± 0.36a | 1.31 ± 0.33 | 1.41 ± 0.55 | 0.003 |
| iPTH (pg/ml) (median) | 137 ± 138 (80) | 66 ± 52ab (46) | 128 ± 99c (112) | 213 ± 183 (161) | <0.0001 |
| Albumin (g/L) | 37 ± 6 | 38 ± 7 | 38 ± 6 | 36 ± 5 | 0.135 |
| C-reactive protein (mg/L) (median) | 10.7 ± 23 (3.4) | 8.7 ± 19 (2.1) | 9.1 ± 12 (4.1) | 15 ± 33 (4.0) | 0.146 |
| Hemoglobin (g/L) | 12 ± 1.7 | 12.7 ± 1.6a | 12 ± 1.7 | 11.6 ± 1.7 | 0.005 |
| GFR-epid (ml/min/1.73m2) | 37 ± 22 | 47 ± 19ab | 26 ± 10 | 14 ± 6 | <0.0001 |
| Total cholesterol (mMol/L) | 4.9 ± 1.1 | 5.0 ± 1.1 | 5.0 ± 1.1 | 5.0 ± 1.3 | 0.844 |
| LDL cholesterol (mMol/L) | 2.7 ± 0.9 | 2.7 ± 0.8 | 2.7 ± 0.8 | 2.6 ± 1.0 | 0.814 |
| Triglycerides (mMol/L) | 2.0 ± 1.3 | 1.74 ± 0.78 | 2.08 ± 1.69 | 2.32 ± 1.28 | 0.114 |
| IS (mg/100 ml) (median) | 0.88 ± 0.98 (0.37) | 0.17 ± 0.06 (0.17) | 0.42 ± 0.13 (0.38) | 2.11 ± 0.93 (1.90) | – |
Data are expressed as means ± SD and sometimes (median) for variables with a non-Gaussian distribution.
P < 0.05 for 1st tertile versus 3rd tertile.
P < 0.05 for 1st tertile versus 2nd tertile.
P < 0.05 for 2nd tertile versus 3rd tertile.
Calculated for patients at CKD stages 2 to 5 (n = 95).
The univariate correlations between log-normalized serum IS levels and the clinical and biochemical characteristics of the study population are shown in Table 3. There was a positive correlation between serum IS levels and phosphate, iPTH, and triglyceride levels, whereas a negative correlation was observed for hemoglobin and the GFR-epi. In multivariate linear regression analysis, albumin, iPTH, and GFR-epi were independently associated with IS serum levels (coefficient Beta = 0.178, P = 0.022; coefficient Beta = 0.240, P = 0.006; coefficient Beta = −0.583, P < 0.0001, for albumin, iPTH, and GFR-epi, respectively, model r2 = 0.52).
Table 3.
Correlations between log-normalized serum IS level and baseline clinical and biochemical characteristics
| Characteristic | r | P |
|---|---|---|
| Age | −0.019 | 0.825 |
| Systolic arterial pressure | 0.108 | 0.206 |
| Body mass index | −0.099 | 0.245 |
| Albumin | −0.148 | 0.085 |
| C-reactive protein | 0.150 | 0.077 |
| LDL cholesterol | −0.049 | 0.578 |
| Triglycerides | 0.193 | 0.026 |
| Hemoglobin | −0.247 | 0.003 |
| Calcium | −0.072 | 0.401 |
| Phosphate | 0.298 | <0.0001 |
| IPTH | 0.438 | <0.0001 |
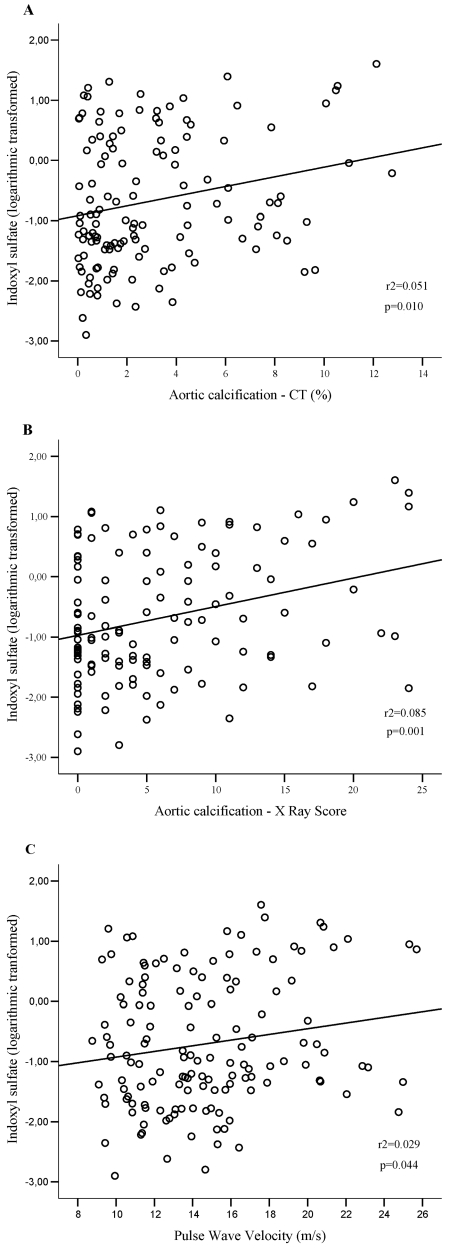
There was a positive and linear relationship between log-normalized serum IS and vascular stiffness, as well as with aortic calcification measures, as illustrated in Figure 3.
Figure 3.
Linear regression curves. (A) The relationship between serum IS and the aortic calcification score as quantified by MSCT (n = 129). (B) The relationship between serum IS and the aortic calcification score as quantified on a plain, abdominal x-ray (n = 122). (C) The relationship between serum IS and PWV (n = 139).
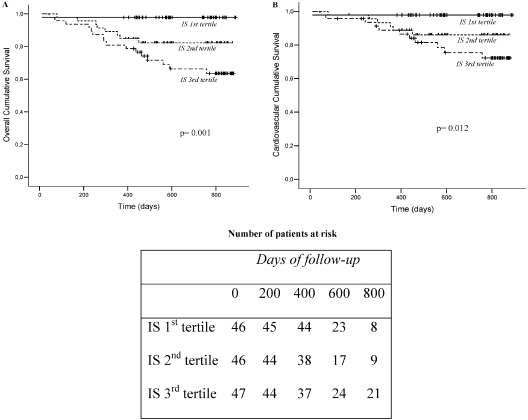
During the study period (mean follow-up period for survivors: 668 ± 165 d; median: 743 d; range: 382 to 889 d), 25 patients died (18 from cardiovascular events, 5 from infectious diseases, and 2 from other causes). In crude analyses (Figure 4), the serum IS level was a predictor of overall and cardiovascular mortality (P = 0.001 and P = 0.012, respectively). Table 4 shows the results of univariate and multivariate Cox analyses of the risk factors for overall death. Patients in the 2nd and 3rd IS tertiles were at a significantly increased risk of death. After adjustment for selected univariate predictors of death (age, albumin and hemoglobin levels, and aortic calcification) and forced variables of prognostic importance in the CKD population (gender, diabetes mellitus status, phosphate levels, and PWV), the patients at the 3rd IS tertile were still found to have a significant increased risk of death (models 1 to 4). To further elucidate the contribution of elevated IS serum levels, we performed the univariate and multivariate Cox analyses with the log-normalized IS serum level as a continuous variable (Table 5). These analyses confirmed that higher IS serum levels were indeed associated with an increased mortality risk, even after adjustment for multiple variables. In fact, for each 0.1-mg/ml increment in the IS serum level, there is a significant, 5% increase in the risk of death [an increase that remains constant across all models tested (data not shown)]. A supplementary Cox regression model including age, albumin, and the calculated propensity score (by quartiles), to better adjust for confounders as detailed in the methodology section, confirmed that the patients in the 3rd IS tertile were at increased risk of death (risk ratio = 13.18; P = 0.013). Similar Cox regression analyses were performed for cardiovascular mortality, with comparable results in univariate models and multivariate models (1 to 4) considering IS as a continuous variable. In contrast, multivariate adjusted models (2 to 4) considering IS as a categorical variable showed limited significance.
Figure 4.
Kaplan–Meyer estimates of (A) overall mortality and (B) cardiovascular mortality for patients as a function of tertiles for serum IS levels.
Table 4.
Multivariate Cox regression analysis of risk factors at baseline for all-cause mortality—IS entered as tertiles.
| Models of Patient Survival (event n = 25) | RR | 95% CI | P |
|---|---|---|---|
| Unadjusted (GF = 17; P < 0.0001) | |||
| IS 1st tertile | 1.00e | 0.011 | |
| IS 2nd tertile | 8.735 | 1.092 to 69.860 | 0.041 |
| IS 3rd tertile | 17.112 | 2.268 to 129.091 | 0.006 |
| Model 1a(GF = 31; P < 0.0001) | |||
| IS 1st tertile | 1.00e | 0.004 | |
| IS 2nd tertile | 7.389 | 0.910 to 59.912 | 0.061 |
| IS 3rd tertile | 19.875 | 2.594 to 152.265 | 0.004 |
| Model 2b (GF = 25; P < 0.0001) | |||
| 1st tertile | 1.00e | 0.030 | |
| 2nd tertile | 8.366 | 1.030 to 67.930 | 0.047 |
| 3rd tertile | 13.767 | 1.797 to 105.483 | 0.012 |
| Model 3c (GF = 29; P < 0.0001) | |||
| 1st tertile | 1.00e | 0.025 | |
| 2nd tertile | 6.595 | 0.809 to 53.760 | 0.078 |
| 3rd tertile | 12.934 | 1.701 to 98.336 | 0.013 |
| Model 4d (GF = 18; P < 0.0001) | |||
| 1st tertile | 1.00e | 0.035 | |
| 2nd tertile | 7.087 | 0.871 to 57.664 | 0.067 |
| 3rd tertile | 12.584 | 1.646 to 96.236 | 0.015 |
GF, goodness of fit; RR, risk ratio; CI, confidence interval. Tertiles are ordered from low serum IS levels to high levels. Please refer to Table 1 for cut-off values.
Model 1 was adjusted for age (in decades), diabetes status, and male gender.
Model 2 was adjusted for serum albumin (in 1g/L increments), hemoglobin (in 1-g/L increments), and phosphate (in 1-mMol/L increments) levels.
Model 3 was adjusted for the aortic calcification score on CT (in 1% increments) and PWV (in 1-m/s increments).
Model 4 was adjusted for the aortic calcification score on x-ray (in 1-unit increments).
Reference value; P values for trend across categories.
Table 5.
Multivariate Cox regression analysis of risk factors at baseline for all-cause mortality—log-normalized serum IS level entered as a continuous variable
| Models of Patient Survival (event n = 25) | RRa | 95% CI | P |
|---|---|---|---|
| Unadjusted (GF = 18; P < 0.0001) | 2.391 | 1.551 to 3.684 | <0.0001 |
| Model 1a (GF = 32; P < 0.0001) | 2.468 | 1.617 to 3.767 | <0.0001 |
| Model 2b (GF = 25; P < 0.0001) | 2.105 | 1.373 to 3.226 | 0.001 |
| Model 3c (GF = 29; P < 0.0001) | 2.165 | 1.377 to 3.402 | 0.001 |
| Model 4d (GF = 19; P < 0.0001) | 2.226 | 1.363 to 3.637 | 0.001 |
Summarizing the risk of a 1-SD (1.076) increment in log-normalized serum IS level for unadjusted model and for noncumulative models adjusted for multiple variables.
Model 1 was adjusted for age (in decades), diabetes status, and male gender.
Model 2 was adjusted for serum albumin (in 1-g/L increments), hemoglobin (in 1-g/L increments), and phosphate (in 1-mMol/L increments) levels.
Model 3 was adjusted for the aortic calcification score on computed tomography (in 1% increments) and PWV (in 1-m/s increments).
Model 4 was adjusted for the aortic calcification score on x-ray (in 1-unit increments).
Discussion
In the study presented here, we have demonstrated for the first time that there is a gradual rise in serum IS with the severity of CKD from the very earliest stages of the disease. Moreover, serum IS was directly associated with aortic calcification and vascular stiffness. Most importantly, higher serum IS was associated with an increased overall and cardiovascular mortality risk within the study cohort. This effect was independent of age, gender, diabetes mellitus, phosphate, albumin and hemoglobin levels, vascular stiffness, and aortic calcification.
Niwa et al. have already reported that IS accumulates markedly in the serum of chronic hemodialysis patients, as a consequence of renal dysfunction or the ineffective removal of IS by conventional hemodialysis (6). The same group found that the blood concentrations of IS were significantly higher in predialysis CKD patients, in comparison with healthy subjects (20). However, these observations were made in a small cohort of patients with insufficient information on the CKD stage. In the study presented here, we have demonstrated that there is a gradual and significant increase in serum IS as the CKD stage worsens.
Second, we demonstrated that serum IS concentrations were directly associated with increased aortic calcification and vascular stiffness in the study cohort. Recent research has revealed how IS produces its toxic biologic effects. In renal tubular cells, it has been shown that IS reduces cell viability (21), upregulates the expression of genes related to tubulointerstitial fibrosis in rats (22), and induces free radical production (23)—factors that may well contribute to the progression of renal failure (24). It has also been reported that IS (at similar concentrations as those found in CKD patients) is capable of inhibiting endothelial cell proliferation and thus impairing the endothelium self-healing ability (10). In fact, it has been demonstrated that IS induces oxidative stress by modifying the balance between pro- and antioxidant mechanisms (8). Additionally, Yamamoto et al. showed that IS can stimulate the proliferation of rat vascular smooth muscular cells in vitro (11). It was subsequently demonstrated that IS induces aortic calcification in vivo in a rat model of hypertension, with aortic wall thickening and the expression of osteoblast-specific proteins (12). It is noteworthy that the oral charcoal adsorbent AST-120 (Kremezin, Kureha Chemical Industry, Tokyo, Japan)—which adsorbs hydrophobic uremic toxins such as IS and attenuates the oxidative stress generated by the latter—has been shown to favorably influence CKD and the related cardiovascular outcomes in animal and clinical studies. Accordingly, in a CKD rat model in which oxidative stress was induced by adriamycin, Fujii et al. demonstrated that the rats treated with AST-120 had lower levels of IS, smaller heart and left ventricular volumes, cardiac fibrosis, and lower histologic expression and urinary excretion of oxidative stress markers than nontreated controls, despite having similar renal function (25). In line with these findings, it had been reported that AST-120 given to CKD patients before dialysis initiation improved their overall survival rates in comparison with CKD patients to whom AST-120 was not administered (26). Lastly, in a recent study in which AST-120 was administered to predialysis CKD patients for 2 yr, a significant reduction in the carotid intima-media thickness and PWV was reported in the AST-120 group when compared with those not receiving AST-120 (27).
Interestingly (and despite the observed association between IS and both aortic calcification and PWV), the effect of IS in increasing the risk of overall and cardiovascular mortality in our study appears to have been at least partially independent of these vascular parameters. Given that CVD was the main cause of death, this partial independence suggests that IS may also affect the cardiovascular system in other ways than by increasing vascular calcification and stiffness. Moreover, it is important to bear in mind that the cardiovascular parameters studied here may not have necessarily revealed other important cardiovascular alterations present in CKD patients (e.g., left ventricular hypertrophy) that can affect mortality (1). Furthermore, one can speculate that the effect of IS on mortality might also have noncardiovascular components. In this regard, Kawashima et al. have demonstrated that at a concentration similar to that found in the uremic milieu, IS strongly suppressed lymphocyte blast formation and IL-2 production (28), which might denote a role for IS in modulating the inflammatory response. However, this hypothesis requires further investigation.
The major limitation of the study presented here is that its design does not enable one to determine whether IS actively alters the course of CKD or, in contrast, whether it is simply a marker of renal function. Kidney dysfunction probably contributes to the lower survival observed in patients with the highest serum IS. Nevertheless, evidence from both in vitro and in vivo studies shows that IS can indeed play a part in the progression of CKD and associated cardiovascular comorbidities. The above-mentioned observations whereby administration of an oral adsorbent of IS reduced arterial stiffness in CKD patients (27) and (in a rat model) improved cardiovascular parameters (25) corroborate the latter hypothesis.
In conclusion, these studies emphasized that IS may be involved in vascular disease and in the higher mortality observed in CKD patients. Further clinical studies are needed to confirm our findings, together with laboratory studies capable of elucidating the biologic activity of IS (in conjunction with or independently of the uremic syndrome). The possibility that the detrimental effects of IS may be exerted in general population cohorts with high cardiovascular risk and moderate renal dysfunction needs to be investigated.
Disclosures
This study was funded by a grant from Amiens University Hospital [PHRC: 2006/0100 (March 27, 2006)]. D.V.B. and F.C.B. received postdoctoral grants from the Picardy Regional Council/University of Picardy, Jules Verne and postdoctoral scholarships from CNPq, Brazil. F.C.B. and D.V.B. contributed equally to this article.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Levin A, Foley RN: Cardiovascular disease in chronic renal insufficiency. Am J Kidney Dis 36: S24– S30, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Vanholder R, Massy Z, Argiles A, Spasovski G, Verbeke F, Lameire N: Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 20: 1048– 1056, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938– 942, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J: A bench to bedside view of uremic toxins. J Am Soc Nephrol 19: 863– 870, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Maizel J, Six I, Slama M, Tribouilloy C, Sevestre H, Poirot S, Giummelly P, Atkinson J, Choukroun G, Andrejak M, Kamel S, Maziere JC, Massy ZA. Mechanisms of aortic and cardiac dysfunction in uremic mice with aortic calcification. Circulation 119: 306– 313, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Niwa T, Takeda N, Tatematsu A, Maeda K: Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem 34: 2264– 2267, 1988 [PubMed] [Google Scholar]
- 7.Niwa T, Ise M: Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med 124: 96– 104, 1994 [PubMed] [Google Scholar]
- 8.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P: The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302– 1308, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F: Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost 4: 566– 573, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P: The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442– 451, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, Kusano E: Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 69: 1780– 1785, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T: Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant 23: 1892– 1901, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31– 41, 1976 [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395– 406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1– S266, 2002 [PubMed] [Google Scholar]
- 16.Zureik M, Temmar M, Adamopoulos C, Bureau JM, Courbon D, Thomas F, Bean K, Touboul PJ, Ducimetiere P, Benetos A: Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens 20: 85– 93, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Boitte F, Choukron G, Fournier A, Massy ZA: Vitamin D affects survival independently of vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol 4: 1128– 1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW: New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 132: 245– 250, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Fitzmaurice G: Confounding: propensity score adjustment. Nutrition 22: 1214– 1216, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S: The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl 62: S23– S28, 1997 [PubMed] [Google Scholar]
- 21.Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, Takayama F, Aoyama I, Nakamura S, Endou H, Niwa T: Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol 13: 1711– 1720, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki T, Ise M, Seo H, Niwa T: Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1, and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl 62: S15– S22, 1997 [PubMed] [Google Scholar]
- 23.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T: Uraemic toxins induce proximal tubular injury via organic anion transporter 1-mediated uptake. Br J Pharmacol 135: 555– 563, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T: Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int 63: 1671– 1680, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Fujii H, Nishijima F, Goto S, Sugano M, Yamato H, Kitazawa R, Kitazawa S, Fukagawa M. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant 24: 2089– 2095, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Ueda H, Shibahara N, Takagi S, Inoue T, Katsuoka Y: AST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren Fail 30: 856– 860, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Kawagoe Y, Matsuda T, Ueda Y, Shimada N, Ebihara I, Koide H: Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res 27: 121– 126, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kawashima Y: [Study on the uremic protein binding inhibitors as uremic toxin: Toxic effect on erythroid colony formation, lymphocyte blast formation and renal function]. Nippon Jinzo Gakkai Shi 31: 1151– 1161, 1989 [PubMed] [Google Scholar]