Abstract
Background and objectives: Mineral metabolism abnormalities and inflammation are concerns in chronic kidney disease (CKD). Interrelationships among these parameters have not been analyzed.
Design, setting, participants, & measurements: The study included 133 patients with CKD not on dialysis and not receiving calcium (Ca) supplements, phosphate binders, or vitamin D. Estimated GFR (eGFR) was 34.1 ± 6.8 ml/min/1.73 m2; 107 participants had stage 3 CKD, and 26 had stage 4.
Results: Patients were classified by tertiles of Ca, phosphorus (P), Ca-P product (Ca x P), and parathyroid hormone (PTH). After adjustment for age, gender, and eGFR, the levels of C-reactive protein (CRP) and IL-6 (IL-6) of the third tertile of P, Ca x P, and PTH were significantly higher than those of the first and second tertiles. Serum P and Ca x P directly correlated with CRP and IL-6, whereas HDL-cholesterol and eGFR inversely correlated with the levels of the inflammatory parameters. After partial correlation analysis, the previous associations between CRP and eGFR, and serum P, as well as the relationship between IL-6 and eGFR, and serum P, remained significant. Multiple regression analysis demonstrated that eGFR and serum P were independently associated with CRP and IL-6. Finally, logistic regression analysis using the presence/absence of an inflammatory state as the dependent variable showed that eGFR was a protective factor, whereas serum P was an independent risk factor for the presence of an inflammatory state.
Conclusions: Elevated serum P might play a role in the development of inflammation in CKD.
Alteration of mineral metabolism is a prevalent condition in chronic kidney disease (CKD). Large epidemiologic studies have shown a strong relationship between elevated levels of calcium (Ca), phosphorus (P), Ca-P product (Ca x P), and parathyroid hormone (PTH) with cardiovascular morbidity and mortality (1–3). Among abnormalities of mineral metabolism, one of the most prominent and relevant is hyperphosphatemia. Elevated serum P has been related to cardiovascular morbidity and mortality in both hemodialysis and predialysis patients. Vascular and coronary artery calcification have been suggested as the link between abnormal mineral metabolism in general, and hyperphosphatemia in particular, and cardiovascular events in this population (4–6). Hyperphosphatemia has been pointed out as the primary culprit in the process of cardiovascular calcification (4,8–10), an event already present in the early phases of renal failure (7,8). More interesting, a significant association between the progression of coronary artery calcification and serum P concentration was observed in CKD patients, despite serum P being in the normal range. Faster progression was found in patients with a high-normal serum P, which was accompanied by more frequent cardiovascular events (11). However, despite these previous findings, the mechanisms by which serum P contributes to cardiovascular disease are not completely known.
Vascular and coronary artery calcification are markers of atherosclerotic disease. In the last decades, intensive investigations have led to a paradigm shift in the interpretation of atherosclerosis, from a purely metabolic process (i.e., mainly driven by hypercholesterolemia) to a disease where inflammation is the dominant pathophysiological and biochemical alteration (12). Cardiovascular disease is up to 20-fold more frequent in ESRD patients and accounts for up to 50% of all deaths, with accelerated atherosclerosis being consistently implicated in this process (13). Traditional cardiovascular risk factors cannot completely explain the prevalence of atherosclerosis, the elevated cardiovascular risk, and the disproportional predisposition for adverse cardiovascular outcomes in this population. Therefore, novel emergent cardiovascular risk factors are suggested to contribute to atherogenesis and have been associated with cardiovascular risk. Among these, it is now known that inflammation is a highly prevalent condition in CKD patients, with diverse studies having documented increased concentrations of inflammatory mediators in dialysis patients as well as subjects with advanced renal failure (14,15). More importantly, it has been demonstrated that several inflammatory parameters, mainly C-reactive protein (CRP) and IL-6 (IL-6), are strong and independent predictors of all-cause and cardiovascular mortality (16–19).
In spite of the important prevalence and relevance of alterations of mineral metabolism and inflammation in CKD patients, the interrelationships among these factors have been scarcely analyzed, especially in predialysis patients. Most previous studies have been performed in hemodialysis, with a limited number of subjects, and with the potentially confounding effect of concomitant treatment with Ca supplements, phosphate binders, or vitamin D derivates (20,21). The aim of this study was to examine the interrelationships among the main parameters of mineral metabolism (Ca, P, Ca x P, and PTH) and inflammation (CRP and IL-6) in patients with advanced CKD.
Materials and Methods
Participants
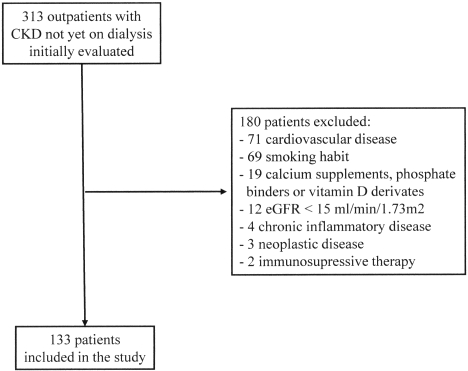
A cohort of 313 outpatients with CKD not yet on dialysis was evaluated. Finally, based on the inclusion/exclusion criteria, this cross-sectional study involved 133 patients (Figure 1), with 41 of them (30.8%) suffering from diabetes. Entry criteria included age older than 18 yr; ability to provide informed consent for participation; CKD stages 3 to 4 according to the National Kidney Foundation criteria (22) (with an estimated GFR [eGFR] lower than 45 ml/min/1.73m2, calculated using the four-variable Modification of Diet in Renal Disease Study equation) (23); and no treatment with Ca supplements, phosphate binders, or vitamin D derivates. Exclusion criteria included current smoking habit (defined as patients smoking at least one cigarette per day during the previous 12 mo), history of any clinical manifestations of cardiovascular disease (coronary artery disease, cerebral vascular disease, and/or peripheral vascular disease) or chronic inflammatory disease, an acute inflammatory or infectious disease, institutionalized subjects, and patients on immunotherapy or immunosuppressive treatment. Before the definitive inclusion, the possible existence of immunologic diseases, malignancy, and infections was investigated. White blood cell count was lower than 10,000/mm3 in all cases. Determination of tumoral markers including carcino-embryonic antigen, α-fetoprotein, cancer antigen 125, and prostate-specific antigen were negative. Serologic tests for antinuclear antibodies, antineutrophil cytoplasmic antibodies, rheumatoid factor, inmunoglobulins, and complement were negative or within the normal range. Urine cultures and serology to hepatitis B, hepatitis C, and HIV were also negative. Weight and height data were collected from each individual to calculate body mass index (BMI) as body weight divided by height squared. The protocol was in accordance with the Declaration of Helsinki and was approved by the local committee, and all subjects gave their informed consent.
Figure 1.
Flow diagram for enrolment in the study.
Laboratory Analyses
Blood samples were drawn from each patient before breakfast in the morning (between 8 and 11 a.m.), after an 8-hr to 12-h overnight fast. Samples were collected in sterile tubes, centrifuged at 3000 g for 10 min at 4°C, and then stored at −80°C until assayed. Serum concentrations of Ca, P, intact PTH, triglycerides, total cholesterol, and HDL- and LDL-cholesterol were measured. The concentration of high-sensitive C-reactive protein (CRP) was determined by ultrasensitive competitive immunoassay (Calbiochem, La Jolla, CA), with an interassay coefficient of variation of 8.4%. Mean concentration in healthy individuals is 0.63 mg/L. Serum concentrations of IL-6 were measured by a solid-phase, chemiluminescent immunometric assay. The analytical sensitivity was 2 pg/ml, and the intra- and interassay coefficients of variability were 6.2% and 7.5%, respectively. Mean concentration in healthy individuals is 2.1 pg/ml. Urinary albumin excretion (UAE) was determined as albumin-to-creatinine ratio in the first morning urine sample.
Statistics
Results are expressed as means and SD (SD), except for CRP and IL-6, which are presented as geometric means and ranges. The Shapiro-Wilk W test was used in testing for normality. Due to non-normal distribution, CRP and IL-6 were logarithmically transformed for analyses and then back-transformed to their natural units for presentation in tables and figures. Mineral metabolism parameters (Ca, P, and PTH) were analyzed by dividing the distribution into tertiles. For comparisons among the Ca, P, and PTH tertiles, the ANOVA post hoc Scheffé test and the chi-square test were used, as appropriate. Correlation analysis was performed to evaluate the associations between the inflammatory parameters and the other variables. Partial correlation analysis was performed to determine the extent to which a relationship was altered after adjusting for the other variables. A forward stepwise multiple regression analysis was performed to determine the independent association between diverse independent variables (age; gender; presence or absence of diabetes; BMI; serum total and LDL- and HDL-cholesterol; albumin; eGFR; UAE; and serum concentrations of Ca, P, and PTH) and CRP and IL-6 as the dependent variables. Polynomial regression analysis was used to test for potential nonlinearity. Finally, multiple logistic regression analysis was performed to determine risk factors for the presence of an inflammatory state in the patients. The study sample was divided into two groups based on level of CRP (high CRP ≥3 mg/L versus low CRP <3 mg/L) as defined in a recent guideline from the Centers for Disease Control and Prevention, and the American Heart Association (24). A p-value <0.05 was considered to be statistically significant. SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL) was used to analyze the data.
Results
Three hundred thirteen patients initially were evaluated for study participation, and 133 were finally included in the study. The main characteristics of the 133 participants are shown in Table 1. One hundred seven participants (80.4%) had stage 3 CKD, and 26 (19.5%) had stage 4. For the purposes of this study, the presence of an inflammatory state in a patient was defined when the serum concentration of CRP was ≥3 mg/L. Thus, 26 patients (19.5%) presented an inflammatory state.
Table 1.
Clinical characteristics of 133 study participants
| Age in years | 61.8 ± 11.2 |
| Gender (M/F) | 75/58 |
| Body mass index (BMI) (Kg/m2) | 26.1 ± 2.7 |
| Diabetes | 41 (30.8%) |
| Estimated glomerular filtration rate (eGFR) (ml/min per 1.73m2) | 34.1 ± 6.8 |
| Total cholesterol (mg/dl) | 180.3 ± 34.3 |
| LDL-cholesterol (mg/dl) | 101.1 ± 26.2 |
| HDL-cholesterol (mg/dl) | 44.8 ± 7.6 |
| Triglycerides (mg/dl) | 169.1 ± 53.9 |
| Albumin (g/dl) | 4.04 ± 0.31 |
| Calcium (Ca) (mg/dl) | 9.4 ± 0.6 |
| Phosphorus (P) (mg/dl) | 4.4 ± 1.1 |
| Ca-P product (Ca x P) | 41.9 ± 11.3 |
| Intact parathyroid hormone (PTH) (pg/ml) | 177.5 ± 96.6 |
| Urinary albumin excretion (UAE) (mg/g) | 1439 ± 1124 |
| C-reactive protein (mg/l) | 1.18 (0.30-9.7) |
| IL-6 (pg/ml) | 6.31 (1.1-25) |
Characteristics of participants by tertiles of Ca, P, Ca x P, and PTH are showed in Tables 2, 3, 4, and 5, respectively. The only difference among patients classified by Ca tertile was the higher value of Ca x P observed in patients in the highest tertile. No differences were observed in the levels of CRP and IL-6. Regarding P, patients in the highest tertile exhibited a significantly lower eGFR, with a higher proportion of patients in CKD stage 4. Serum PTH and Ca x P, as well as inflammatory parameters, were also higher in patients in the highest tertile. Similar results were found when patients were classified according to Ca x P tertiles. Finally, patients in the highest tertile of PTH showed higher concentrations of P, Ca x P, CRP, and IL-6. In this group, the proportion of patients with CKD stage 4 was higher, and the mean eGFR lower, than was observed in patients in the first and second tertiles. After adjustement for age, gender, and eGFR, the levels of CRP and IL-6 of the third tertile of P and PTH were significantly higher than CRP and IL-6 of the first and second tertiles.
Table 2.
Clinical characteristics of participants stratified by tertile of calcium
| Characteristic | 1: ≤ 9.19 mg/dl n = 44 | 2: 9.20-9.59 mg/dl n = 4 | 3: ≥ 9.60 mg/dl n = 40 | P |
|---|---|---|---|---|
| Age in years | 63.3 ± 9.6 | 61.5 ± 13 | 60.5 ± 10.4 | NS |
| Gender (M/F) | 28/16 | 24/25 | 23/17 | NS |
| Chronic kidney disease (CKD) stage 3/4 | 36/8 | 41/8 | 30/10 | NS |
| BMI (Kg/m2) | 26.2 ± 3 | 26 ± 2.7 | 26.1 ± 2.4 | NS |
| Diabetes | 13 (29.5%) | 15 (30.6%) | 13 (32.5%) | NS |
| span lang=ITeGFR (ml/min per 1.73m2) | 34.4 ± 6.4 | 34.4 ± 6.5 | 33.3 ± 7.6 | NS |
| Albumin (g/dl) | 4.03 ± 0.29 | 3.97 ± 0.31 | 4.12 ± 0.33 | NS |
| Total cholesterol (mg/dl) | 176 ± 34 | 176 ± 30 | 189 ± 38 | NS |
| LDL-cholesterol (mg/dl) | 102 ± 28 | 96 ± 24 | 105 ± 26 | NS |
| HDL-cholesterol (mg/dl) | 44 ± 7 | 44 ± 7 | 45 ± 8 | NS |
| Triglycerides (mg/dl) | 166 ± 59 | 166 ± 49 | 175 ± 54 | NS |
| P (mg/dl) | 4.4 ± 1.2 | 4.4 ± 1.2 | 4.4 ± 1 | NS |
| C x P | 39.4 ± 10.5 | 41.6 ± 11.8 | 44.3 ± 10.8 | <0.001 |
| Intact PTH (pg/ml) | 177 ± 89 | 178 ± 94 | 176 ± 108 | NS |
| UAE (mg/g) | 1456 ± 1074 | 1332 ± 981 | 1552 ± 806 | NS |
| C-reactive protein (mg/l) | 1.14 (0.30-9.7) | 1.32 (0.3-7.2) | 1.08 (0.3-8.8) | NS |
| IL-6 (pg/ml) | 6.31 (2-22) | 6.21 (1.1-20) | 6.43 (1.1-25) | NS |
Table 3.
Clinical characteristics of participants stratified by tertile of phosphorus
| Characteristic | 1: ≤ 3.89 mg/dl n = 44 | 2: 3.90-4.99 mg/dl n = 46 | 3: ≥ 5 mg/dl n = 43 | P |
|---|---|---|---|---|
| Age in years | 62.1 ± 12.5 | 63.2 ± 9.9 | 60 ± 10.9 | NS |
| Gender (M/F) | 26/18 | 27/19 | 22 21 | NS |
| CKD stage 3/4 | 41/3 | 41/5 | 25/18 | <0.05 |
| BMI (Kg/m2) | 26.6 ± 2.9 | 26.2 ± 2.5 | 26.1 ± 2.4 | NS |
| Diabetes | 14 (31.8%) | 14 (30.4%) | 13 (30.2%) | NS |
| eGFR (ml/min per 1.73m2) | 37.9 ± 6 | 34.8 ± 5.2 | 29.4 ± 6.4 | <0.01 |
| Albumin (g/dl) | 3.98 ± 0.35 | 4.05 ± 0.26 | 4.08 ± 0.33 | NS |
| Total cholesterol (mg/dl) | 185 ± 34 | 177 ± 38 | 177 ± 28 | NS |
| LDL-cholesterol (mg/dl) | 107 ± 23 | 99 ± 26 | 96 ± 27 | NS |
| HDL-cholesterol (mg/dl) | 46 ± 8 | 44 ± 8 | 43 ± 5 | NS |
| Triglycerides (mg/dl) | 172 ± 56 | 163 ± 43 | 171 ± 61 | NS |
| Ca (mg/dl) | 9.3 ± 0.4 | 9.3 ± 0.6 | 9.4 ± 0.5 | NS |
| C x P | 30.2 ± 4.9 | 40.8 ± 3.85 | 55.1 ± 5.5 | <0.001 |
| Intact PTH (pg/ml) | 126.8 ± 71.5 | 171.9 ± 84.6 | 235.3 ± 101.3 | <0.001 |
| UAE (mg/g) | 1524 ± 1104 | 1612 ± 1358 | 1147 ± 898 | NS |
| C-reactive protein (mg/l) | 0.72 (0.30-7.2) | 0.90 (0.3-4.6) | 2.6 (0.3-9.7) | <0.001 |
| IL-6 (pg/ml) | 4.2 (1.1-17) | 5.35 (1.1-12) | 11.3 (2.1-25) | <0.001 |
Table 4.
Clinical characteristics of participants stratified by tertile of calcium x phosphorus product
| Characteristic | 1: ≤ 35.99n = 45 | 2:36-46.10n = 44 | 3: ≥ 46.11n = 44 | P |
|---|---|---|---|---|
| Age in years | 63.2 ± 13 | 62.6 ± 9.0 | 59.5 ± 10.9 | NS |
| Gender (M/F) | 27/18 | 27/17 | 21/23 | NS |
| CKD stage 3/4 | 40/5 | 40/4 | 27/17 | <0.05 |
| BMI (Kg/m2) | 26.4 ± 3.0 | 26.2 ± 2.6 | 25.7 ± 2.5 | NS |
| Diabetes | 14 (31.1%) | 14 (31.8%) | 13 (29.5%) | NS |
| eGFR (ml/min per 1.73m2) | 37.1 ± 6.5 | 35.2 ± 5.0 | 29.9 ± 6.5 | <0.001 |
| Albumin (g/dl) | 4.00 ± 0.35 | 4.04 ± 0.26 | 4.08 ± 0.32 | NS |
| Total cholesterol (mg/dl) | 188 ± 35 | 182 ± 36 | 185 ± 29 | NS |
| LDL-cholesterol (mg/dl) | 111 ± 24 | 104 ± 23 | 105 ± 25 | NS |
| HDL-cholesterol (mg/dl) | 45 ± 8 | 46 ± 8 | 42 ± 6 | NS |
| Triglycerides (mg/dl) | 174 ± 55 | 165 ± 56 | 167 ± 50 | NS |
| Ca (mg/dl) | 9.3 ± 0.5 | 9.4 ± 0.7 | 9.5 ± 0.5 | NS |
| P | 3.2 ± 0.5 | 4.3 ± 0.3 | 5.8 ± 0.6 | <0.001 |
| Intact PTH (pg/ml) | 126.7 ± 73.3 | 171.9 ± 83.9 | 234 ± 100 | <0.001 |
| UAE (mg/g) | 1412 ± 1132 | 1625 ± 1305 | 1174 ± 854 | NS |
| C-reactive protein (mg/l) | 0.76 (0.30-7.20) | 0.90 (0.3-4.6) | 2.42 (0.30-9.70) | <0.001 |
| IL-6 (pg/ml) | 4.4 (1.1-17) | 5.34 (1.1-12) | 10.7 (2.1-25) | <0.0001 |
Table 5.
Clinical characteristics of participants stratified by tertile of parathyroid hormone
| Characteristic | 1: ≤ 123.9 mg/dln = 48 | 2: 124-214.9 mg/dln = 47 | 3:≥ 215 mg/dln = 38 | P |
|---|---|---|---|---|
| Age in years | 61.3 ± 13.2 | 62.2 ± 9.6 | 61.9 ± 10.6 | NS |
| Gender (M/F) | 29/19 | 27/20 | 24/14 | NS |
| CKD stage 3/4 | 44/4 | 41/6 | 22/16 | <0.05 |
| BMI (Kg/m2) | 26.4 ± 2.8 | 26.3 ± 2.4 | 25.6 ± 2.9 | NS |
| Diabetes | 15 (31.2%) | 14 (29.7%) | 12 (31.5%) | NS |
| eGFR (ml/min per 1.73m2) | 34.8 ± 6.6 | 35.8 ± 5.2 | 31.6 ± 7.6 | <0.01 |
| Albumin (g/dl) | 4.02 ± 0.33 | 4.01 ± 0.28 | 4.08 ± 0.33 | NS |
| Total cholesterol (mg/dl) | 191 ± 37 | 178 ± 27 | 186 ± 35 | NS |
| LDL-cholesterol (mg/dl) | 112 ± 24 | 101 ± 22 | 107 ± 25 | NS |
| HDL-cholesterol (mg/dl) | 45 ± 8 | 44 ± 7 | 44 ± 7 | NS |
| Triglycerides (mg/dl) | 175 ± 56 | 158 ± 46 | 173 ± 57 | NS |
| Ca (mg/dl) | 9.5 ± 0.5 | 9.4 ± 0.5 | 9.4 ± 0.6 | NS |
| P (mg/dl) | 4 ± 1.1 | 4.2 ± 1.1 | 5 ± 0.9 | <0.01 |
| C x P | 38 ± 10.9 | 39.9 ± 11 | 47.9 ± 9.6 | <0.01 |
| UAE (mg/g) | 1563 ± 1302 | 1487 ± 854 | 1325 ± 1102 | NS |
| C-reactive protein (mg/l) | 0.95 (0.30-7.2) | 1.05 (0.3-6) | 1.67 (0.3-9.7) | <0.05 |
| IL-6 (pg/ml) | 5.8 (2-20) | 5.41 (1.1-20) | 9.93 (1.1-25) | <0.01 |
Table 6 lists findings from the bivariate correlation analysis examining CPR, IL-6, and potential correlates. Serum P and Ca x P most strongly directly correlated with CRP and IL-6, followed by PTH. On the contrary, HDL-cholesterol and eGFR inversely correlated with the levels of the inflammatory parameters. However, after adjusting for the effect of other variables by partial correlation analysis, only the previous associations between CRP and eGFR (r = −0.43, P < 0.0001), and serum P (r = 0.35, P < 0.01) remained significant. Likewise, the relationship between IL-6 and eGFR (r = −0.70, P < 0.0001), and serum P (r = 0.38, P < 0.0001) remained significant.
Table 6.
Correlation coefficients (r) between inflammatory parameters and clinical characteristics
| Variables | C-reactive protein |
IL-6 |
||
|---|---|---|---|---|
| r | P | r | P | |
| Age | 0.03 | 0.65 | 0.03 | 0.91 |
| BMI | 0.05 | 0.49 | 0.04 | 0.59 |
| eGFR | −0.57 | <0.001 | −0.78 | 0.001 |
| Albumin | 0.12 | 0.15 | 0.13 | 0.11 |
| Total cholesterol | 0.09 | 0.27 | 0.16 | 0.85 |
| LDL-cholesterol | 0.12 | 0.14 | 0.03 | 0.96 |
| HDL-cholesterol | −0.19 | <0.05 | −0.22 | <0.01 |
| Triglycerides | 0.06 | 0.66 | 0.06 | 0.42 |
| Ca | 0.01 | 0.89 | 0.08 | 0.31 |
| P | 0.53 | <0.001 | 0.59 | <0.001 |
| C x P | 0.52 | <0.001 | 0.57 | <0.001 |
| PTH | 0.29 | 0.001 | 0.21 | 0.01 |
| UAE | 0.25 | 0.08 | 0.12 | 0.40 |
To test the hypothesis of an independent association between variables of mineral metabolism and inflammatory parameters, forward stepwise multiple regression analysis was performed with CRP and IL-6 as the dependent variables. Age; gender; the presence or absence of diabetes; BMI; serum total and LDL- and HDL-cholesterol; albumin; eGFR; UAE; and serum concentrations of Ca, P, and PTH were considered as possible predictors of inflammatory parameters. The result showed that from the different independent variables, eGFR and serum P were independently associated with CRP (adjusted R2 = 0.41, P < 0.0001) and IL-6 (adjusted R2 = 0.66, P < 0.001) (Table 7). A marginal association was present between CRP and serum LDL-cholesterol (P = 0.14), and between IL-6 and age (P = 0.18), serum total and HDL-cholesterol (P = 0.25 and P = 0.19, respectively), and BMI (P = 0.30). No significant associations were observed for serum levels of Ca or PTH. There was NS collinearity between the independent predictors. Finally, analysis for potential nonlinearity of the associations was performed, which did not show any significant relationship between inflammatory and mineral metabolism parameters.
Table 7.
Multiple stepwise regression analysis for C-reactive protein and IL-6 as dependent variablesa
| Independent variable | Beta-regression coefficient | SE of regression coefficient | t | p-value |
|---|---|---|---|---|
| C-reactive protein | ||||
| eGFR | −0.41 | 0.07 | −5.43 | <0.001 |
| Serum P | 0.32 | 0.07 | 4.25 | <0.001 |
| LDL-cholesterol | 0.09 | 0.06 | 1.44 | 0.149 |
| IL-6 | ||||
| eGFR | −0.65 | 0.05 | −11.14 | <0.0001 |
| Serum P | 0.27 | 0.05 | 4.69 | <0.0001 |
| HDL-cholesterol | −0.06 | 0.05 | −1.30 | 0.194 |
| Total cholesterol | 0.05 | 0.05 | 1.13 | 0.258 |
| Age | −0.06 | 0.05 | −1.32 | 0.188 |
| BMI | 0.05 | 0.05 | 1.03 | 0.303 |
Age; gender; the presence or absence of diabetes; BMI; serum total and LDL- and HDL-cholesterol; albumin; eGFR; UAE; and serum concentrations of Ca, P, Ca x P, and PTH were considered as possible predictors of inflammatory parameters.
For logistic regression analysis, patients with a serum concentration of CRP ≥3 mg/L were classified as having an inflammatory state. Therefore, the presence/absence of this inflammatory state was used as the dependent variable. The logistic regression analysis showed that higher eGFR (0.80 [0.70 to 0.91], P = 0.001) was a protective factor, whereas higher serum P (1.86 [1.08 to 3.19], P < 0.05) was an independent risk factor for the presence of a significant inflammatory state (Table 8).
Table 8.
Multivariable logistic regression analysis for the presence of an inflammatory state
| Variables | Odds Ratio | 95% Confidence Interval | P |
|---|---|---|---|
| Age | 1.00 | 0.83–1.03 | 0.99 |
| Diabetes status (no/yes) | 1.09 | 1.01–1.16 | 0.30 |
| BMI | 1.06 | 0.81–1.38 | 0.66 |
| eGFR | 0.80 | 0.70–0.91 | 0.001 |
| Albumin | 2.84 | 0.30–6.70 | 0.36 |
| Total cholesterol | 1.01 | 0.97–1.04 | 0.61 |
| LDL-cholesterol | 0.97 | 0.92–1.02 | 0.29 |
| HDL-cholesterol | 0.92 | 0.83–1.03 | 0.16 |
| Triglycerides | 0.99 | 0.98–1.01 | 0.55 |
| Ca | 0.85 | 0.29–2.90 | 0.54 |
| P | 1.86 | 1.08–3.19 | 0.02 |
| C x P | 0.82 | 0.63–1.06 | 0.39 |
| PTH | 0.99 | 0.98–1.00 | 0.34 |
Discussion
In this cross-sectional study, we explored the associations of markers of mineral metabolism (Ca, P, and PTH) and parameters of inflammation (CRP and IL-6). Numerous studies have documented the importance of abnormal mineral metabolism (1–3) and inflammation (16–19) as pivotal factors for the increased cardiovascular risk in CKD patients. However, the inter-relationship between both factors has been scarcely analyzed. Two previous studies in hemodialysis, with a limited number of patients, showed that a high Ca x P was associated with high CRP concentrations (20,21). Consequently, there is a potential basis to speculate that alterations of mineral metabolism may be associated with inflammation in CKD.
The main finding of the present study was that, in this sample of patients with CKD stages 3 to 4, without a history or symptoms of cardiovascular disease, and with no previous treatment with Ca supplements, phosphate binders, or vitamin D derivates, the level of the inflammatory parameters CRP and IL-6 was significantly higher in the highest serum P tertile even after adjustment for age, gender, and eGFR. Moreover, serum P concentration was positive, significant, and independently associated with CRP and IL-6, and it was an independent risk factor for the presence of an inflammatory state. In addition, our results show that eGFR is a protective factor for the presence of this inflammatory condition.
Inflammation is recognized in up to 50% of CKD patients, being a common feature of advanced renal disease (25,26). The most extensively studied biomarkers of inflammation in cardiovascular disease are CRP and IL-6. The Cardiovascular Health Study reported that levels of CRP and IL-6 were significantly higher in patients with renal insufficiency compared with patients with normal kidney function (27), whereas several studies have associated changes in GFR with biomarkers of inflammation, particularly in patients with advanced disease (28,29). Diverse factors, including genetic background, volume overload, co-morbidity, advanced glycation end products, reactive oxygen species, autonomic dysfunction, peripheral polymorphonuclear leukocyte priming, and reduced clearance of pro-inflammatory cytokines as the GFR declines, have been involved as causes of the highly prevalent state of inflammation in CKD (30–32). The results of the present study indicate that elevated serum P is an independent predictor of increased levels of inflammatory parameters in these patients, suggesting that hyperphosphatemia may promote and/or facilitate the development of inflammation in CKD patients. In addition, and interestingly, numerous studies in the last years have demonstrated that P and inflammation run in parallel with similar points regarding deleterious consequences in CKD. Therefore, both elevated serum P levels and increased serum inflammatory biomarkers are considered important factors in the progression of CKD (33–35), abnormalities of cardiac structure and function (36–38), vascular calcification (39–42), as well as cardiovascular events and mortality (1,43–45). On the other hand, interesting data derive from studies reporting potential anti-inflammatory properties of phosphate binders, specifically sevelamer. In a randomized prospective study of 108 chronic hemodialysis patients, treatment with sevelamer resulted in significant reductions in serum CRP levels, as well as in serum P and total and LDL-cholesterol (46,47). In those studies, no data are available about a potential correlation between reduction of P and decrease of CRP. However, a more recent study by Yamada et al. (48) in hemodialysis subjects showed that CRP experienced a significant decrease during sevelamer therapy, and that the reduction rate of CRP was significantly correlated with the change of P. In this study, a titration protocol was used, and it is possible that patients with better P control and higher P reduction received a higher sevelamer dosage. Therefore, the effects on CRP could be related to more of the pleiotropic instead of the P lowering effects of sevelamer.
The mechanisms potentially involved in the stimulation of inflammation by P are unknown. However, since important steps of inflammatory pathways are regulated by phosphorylation events, it is possible to speculate that increased P concentration may trigger phosphorylation-driven signaling inflammatory cascades (49–52). In addition, interesting data regarding basic Ca phosphate (BCP) crystals are known from the study of degenerative arthritis. In this setting, BCP crystals have been shown to activate synovial fibroblasts, inducing the activation of intracellular signaling pathways, including protein kinase C, MAP kinase, and NFκB (53). Furthermore, in vitro studies have shown the ability of macrophages to interact with BCP crystals, resulting in cytokine production (54). Finally, Nadra et al. (55) elegantly demonstrated that BCP crystals are able to interact with and activate human macrophages in a pro-inflammatory manner. In this study, the interaction of human macrophages with BCP crystals results in the secretion of biologically significant quantities of pro-inflammatory cytokines, which were capable of stimulating the activation of endothelial cells and recruitment of mononuclear cells. Therefore, increase of serum P may induce or favor the formation of BCP crystals, which may act similarly to antigens that activate the main cell types participating in the chronic inflammatory response— namely monocytes and macrophages.
A relevant strength in the present study is the lack of interference of potential confounders such as smoking habit, presence of clinical cardiovascular disease, and, importantly, the effect of treatments affecting mineral metabolism parameters (Ca supplements, phosphate binders, or vitamin D analogues). On the other hand, several limitations need also to be taken into account: the relatively small sample size, which may have prevented some of the detected associations from being statistically significant at the P < 0.05 level; and the cross-sectional design and the potential influence of unmeasured confounders. Therefore, our study does not indicate certainty about a direct causality relationship between elevated serum P and the development of inflammation, but we believe, conceptually, that it is hypothesis-generating. Despite these limitations, to the best of our knowledge, this is the first study that investigates the potential association between mineral metabolism and inflammation in patients with advanced CKD who were not undergoing dialysis, showing that serum P is a risk factor for the presence of an inflammatory state in these subjects. Our observation on the association between serum P and pro-inflammatory cytokines requires further exploration and confirmation by longitudinal prospective studies.
Disclosures
None.
Acknowledgments
This study was supported by ACINEF.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie WG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208– 2218, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788– 1793, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179– 1187, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Blacher J, Guerin AP, Pannier B, Marchais SG, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938– 942, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK, Chertow GM: Cardiac calcification in adult hemodialyisis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695– 701, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith D, Ritz E, Covic A: Vascular calcification: A stiff challenge for the nephrologist. Kidney Int 66: 1315– 1323, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreuci VE: Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis 44: 1024– 1030, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Tomiyama C, Higa A, Dalboni MA, Cendoroglo M, Draibe SA, Cuppari L, Carvalho AB, Neto EM, Canziani ME: The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrol Dial Transplant 21: 2464– 2471, 2006 [DOI] [PubMed] [Google Scholar]
- 9.London GM, Guérin AP, Marchais SJ, Metivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731– 1740, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P: Control of hyperphosphatemia beyond phosphate. Kidney Int 71: 376– 379, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, Brancaccio D, Cozzolino M, Biondi ML, Andreucci VE: Progression of coronary artery calcification in predialysis patients. Am J Nephrol 27: 152– 158, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Libby P: Inflammation in atherosclerosis. Nature 420: 868– 874, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Amann K, Tyralla K, Gross ML, Eifert T, Adamczak M, Ritz E: Special characteristics of atherosclerosis in chronic renal failure. Clin Nephrol 60[ Suppl 6]: S13– S21, 2003 [PubMed] [Google Scholar]
- 14.Menon V, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Relationship between C-reactive protein, albumin and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis 42: 44– 52, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P: Inflammation in end-stage renal disease: The hidden enemy. Nephrology (Carlton) 11: 36– 41, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Tripepi G, Mallamachi F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17 [ Suppl 3]: S169– S173, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kessler M, Zannad F, Lehert P, Gründfeld JP, Thuilliez C, Leizorovicz A, Lechat P; FOSIDIAL Investigators: Predictors of cardiovascular events in patients with end-stage renal disease: An analysis from the Fosinopril in dialysis study. Nephrol Dial Transplant 22: 3573– 3579, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335– 1342, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, Rindi P, Donati G, Antonelli A, Panicucci E, Tripepi G, Tetta C, Palla R; RISCAVID Study Group: Chronic inflammation and mortality in haemodialysis: Effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant 23: 2337– 2343, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Movilli E, Feliciano A, Camerini C, Brunori G, Zubani R, Scolari F, Parrinello G, Cancarini GC: A high calcium-phosphate product is associated with high C-reactive protein concentrations in hemodialysis patients. Nephron Clin Pract 101: c161– c167, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Nasri H: Linkage of elevated CaxPO4 product with inflammation in maintenance hemodialysis patients. Minerva Urol Nephrol 58: 339– 345, 2006 [PubMed] [Google Scholar]
- 22.National Kidney Foundation: K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, classification, and stratification. Am J Kidney Dis 39 [ Suppl 1]: S1– S266, 2004 [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461– 470, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: Application to clinic and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107: 499– 511, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Stenvinkel P: Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Renal Nutr 23: 1295– 1301, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmerfalb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009– 1016, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Shlipak MG, Fried LF, Crump E, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 107: 87– 92, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P: Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 41: 1212– 1218, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Panichi V, Migliori M, De Pietro S, Taccola D, Bianchi AM, Giovannini L, Norpoth M, Metelli MR, Cristofani R, Bertelli AA, Sbragia G, Tetta C, Palla R, Colombo R: C-reactive protein and interleukin-6 levels are related to renal function in predialytic chronic renal failure. Nephron 91: 594– 600, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Sela S, Shurtz-Swiriski R, Cohen-Mazor M, Mazor R, Chezar J, Shapiro G, Hassan K, Shkolnik G, Geron R, Kristal B: Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol 16: 2431– 2438, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Stenvinkel P: New insights on inflammation in chronic kidney disease—genetic and non-genetic factors. Nephrol Ther 2: 111– 119, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz MI, Carrero JJ, Axelson J, Lindholm B, Stenvinkel P: Low-grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: Sources and consequences. Clin Nephrol 68: 1– 9, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP: Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1: 825– 831, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW; PREPARE Study Group: High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909– 2916, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G; Cholesterol and Recurrent Events (CARE) Trial Investigators: Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int 68: 237– 245, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Galleta F, Cupisti A, Franzoni F, Femia FR, Rossi M, Barsotti G, Santoro G: Left ventricular function and calcium phosphate plasma levels in uraemic patients. J Intern Med 258: 378– 384, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Achinger SG, Ayus JC: Left ventricular hypertrophy: Is hyperphosphatemia among dialyisis patients a risk factor. J Am Soc Nephrol 17 [ Suppl 3]: S255– S261, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Erten Y, Tulmac M, Derici U, Pasaoglu H, Altok Reis K, Bali M, Arinsoy T, Cengel A, Sindel S: An association between inflammatory state and left ventricular hypertrophy in hemodialysis patients. Ren Fail 27: 581– 589, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Cannata-Andíia J, Rodríiguez-Garciía M, Carrillo-López N, Naves-Diíaz M, Daz-López B: Vascular calcifications: Pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol 17[ Suppl 3]: S267– S273, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Jung HH, Kim SW, Han H: Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant 21: 1915– 1920, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Moe SM, Chen NX: Inflammation and vascular calcification. Blood Purif 23: 64– 71, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Baber U, de Lemos JA, Khera A, McGuire DK, Omland T, Toto RT, Hedayati H: Non-traditional risk factors predict coronary calcification in chronic kidney disease in a population-based cohort. Kidney Int 73: 615– 621, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520– 528, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Knight EL, Rimm EB, Pai JK, Rexrode KM, Cannuscio CC, Manson JE, Stampfer MJ, Curhan GC: Kidney dysfunction, inflammation, and coronary events: A prospective study. J Am Soc Nephrol 15: 1897– 1903, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Panichi V, Maggiore U, Taccola D, Migliori M, Rizza GM, Consani C, Bertini A, Sposini S, Perez-Garcia R, Rindi P, Palla R, Tetta C: Interleukin-6 is a stronger predictor of total and cardiovascular mortality that C-reactive protein in haemodialysis patients. Nephrol Dial Transplant 19: 1154– 1160, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Chertow GM, Raggi P, McCarthy JT, Schulman G, Silberzweig J, Kuhlik A, Goodman WG, Boulay A, Burke SK, Toto RD: The effects of sevelamer and calcium acetate on proxies of atherosclerotic and arteriosclerotic vascular disease in hemodialysis patients. Am J Nephrol 23: 307– 314, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Ferramosca E, Burke S, Chasan-Taber S, Ratti C, Chertow GM, Raggi P: Potential antiatherogenic and anti-inflammatory properties of sevelamer in maintenance hemodialysis patients. Am Heart J 149: 820– 825, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Yamada K, Tokura T, Fukudome K, Ochiai H, Komatsu H, Sato Y, Hara S, Eto T: Effect of sevelamer on dyslipidemia and chronic inflammation in maintenance hemodialysis patients. Ren Fail 27: 361– 365, 2005 [PubMed] [Google Scholar]
- 49.Russello SV: Assessing cellular protein phosphorylation: High throughput drug discovery technologies. Assay Drug Dev Technol 2: 225– 235, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Viatour P, Merville MP, Bours V, Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem Sci 30: 43– 52, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Ihnatko R, Kubes M: TNF signalling: Early events and phosphorylation. Gen Physiol Biophys 26: 159– 167, 2007 [PubMed] [Google Scholar]
- 52.Shanley TP: Phosphatases: Counterregulatory role in inflammatory cell signalling. Crit Care Med 30[ Suppl 1]: S80– S88, 2002 [PubMed] [Google Scholar]
- 53.McCarthy GM, Augustine JA, Baldwin AS, Christopherson PA, Cheung HS, Westfall PR, Scheinman RI: Molecular mechanism of basic calcium phosphate crystal-induced activation of human fibroblasts. Role of nuclear factor κB, activator protein I, and protein kinase C. J Biol Chem 273: 35161– 35169, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Meng ZH, Hudson AP, Schumacher HRJ, Baker JF, Baker DJ: Monosodium urate, hydroxyapatite, and calcium pyrophosphate crystals induce tumor necrosis factor-alpha expression in a mononuclear cell line. J Rheumatol 24: 2385– 2388, 1997 [PubMed] [Google Scholar]
- 55.Nadra I, Mason JC, Philippidis P, Florey O, Smythe CD, McCarthy GM, Landis RC, Haskard DO: Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways. A vicious cycle of inflammation and arterial calcification? Cir Res 96: 1248– 1256, 2005 [DOI] [PubMed] [Google Scholar]