Abstract
D-Glucosamine has been widely reported to have immunosuppressive actions on neutrophils, lymphocytes and other cells of the immune system. However, under conditions used in biological experiments (e.g., neutral pH and phosphate buffers), we have found that D-glucosamine self-reacts to form 2,5-deoxyfructosazine [2-(D-arabino-tetrahydroxybutyl)-5-(D-erythro-2,3,4-trihydroxybutyl)pyrazine] (1) and 2,5-fructosazine [2,5-bis(D-arabino-tetrahydroxybutyl)pyrazine] (2). When tested for bioactivity at nontoxic concentrations, these D-glucosamine derivatives were more effective inhibitors of IL-2 release from PHA-activated T cells than D-glucosamine. Hence, fructosazines constitute a novel class of immunomodulators.
Keywords: immune cell, Cytokine release, D-Glucosamine, Fructosazine
1. Introduction
D-glucosamine, 2-amino-2-deoxy-D-glucose (D-GlcN), is an important multipurpose cell metabolite. This amino sugar is a precursor of glycolipids and glycoproteins, a regulator of glucose transport, and a substrate for the O-GlcNAc (N-acetyl-D-glucosamine) signaling pathway.1, 2 Recent studies have shown that D-GlcN has immunomodulatory properties. D-GlcN diminishes neutrophil oxidant production and migratory properties.3 D-GlcN has also been shown to influence lymphocyte activation and proliferation.4,5 Moreover, D-GlcN reduces IL-2 production by activated lymphocytes, apparently by diminishing the translocation of transcription factors to the nucleus.6 In vivo, D-GlcN has been reported to diminish allograft rejection and the acute phase of an animal model of multiple sclerosis.4,7 Hence, D-GlcN and its biological mechanisms of action are of considerable importance.
Under certain conditions such as neutral pH, D-GlcN can react under surprisingly mild conditions. Moreover, due to the presence of both amino and aldehyde groups, D-GlcN can undergo a variety of decomposition reactions. Consequently, it is unclear if the reported bioeffects of D-GlcN are due solely to D-GlcN’s effect on cells, or if a reaction product also possess biological activity. In the present study we find that cyclocondensation products of D-GlcN have greater biological activity than native D-GlcN.
2. Results and discussion
2.1 Chemistry
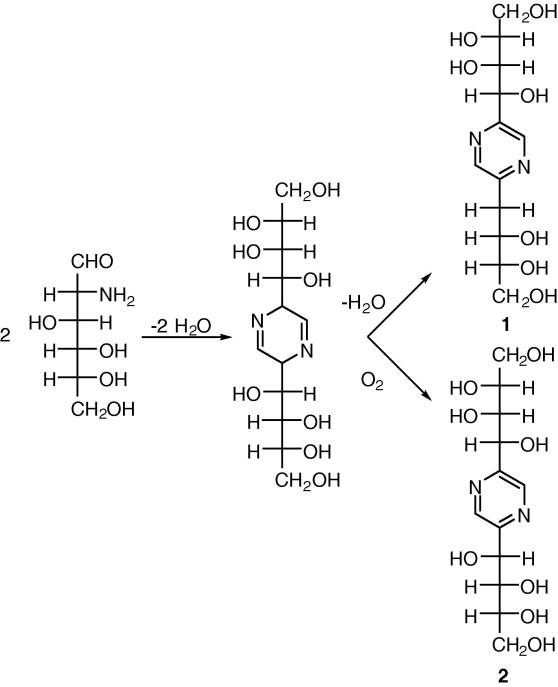
In aqueous solution above pH 7.0, D-GlcN undergoes a cyclocondensation reaction that results in complex mixtures of reaction products. It has been reported that 2,5-deoxyfructosazine [2-(D-arabino-tetrahydroxybutyl)-5-(D-erythro-2,3,4-trihydroxybutyl)pyrazine] (1) and 2,5-fructosazine [2,5-bis(D-arabino-tetrahydroxybutyl)pyrazine] (2) are major components (Scheme 1).8 As compounds 1 and 2 are closely related, conventional reversed phase HPLC columns do not provide a satisfactory separation. Consequently, we developed a simple LC–ESIMS method using a porous graphitic carbon column for separation and identification of reaction products.
Scheme 1.
Structures of D-GlcN reaction products.
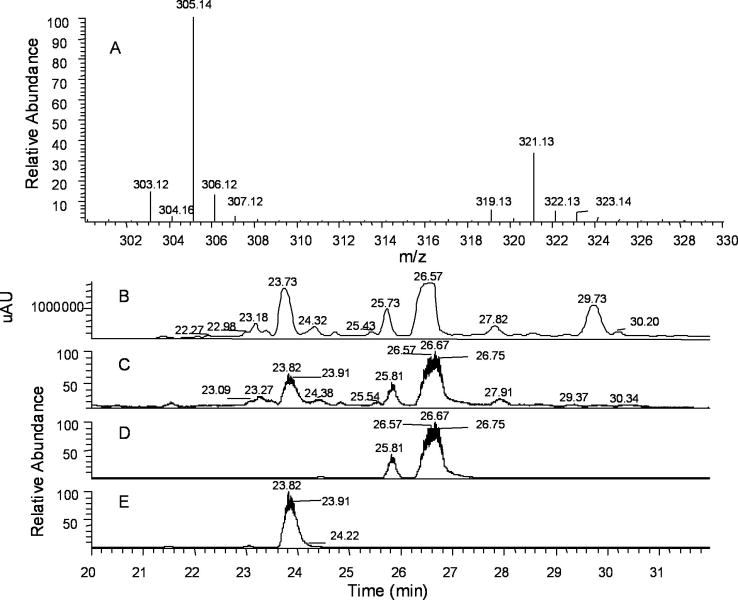
Porous graphitic carbon (PGC) exhibits exceptional physical and chemical stability, the ability to purify both hydrophobic and polar compounds, and the capacity to resolve isomers. PGC columns have been successfully applied to the separation polar phenolic compounds,9 polyethoxylated alcohols,10 lipids,11 and carbohydrates.12 The overall retention on PGC columns is a combination of multiple mechanisms including molecular shape, dispersive interactions with the matrix and charge-induced interactions with PCG’s polarizable surface.13 As demonstrated in Fig. 1, the PGC column provides good separation and resolution for D-GlcN products (panel B). As illustrated in panel B, there are three major products in the solution. Unreacted D-GlcN is not observed as it has no absorption at 290–310 nm. The ratio of compound 1 and 2 (Scheme 1) is estimated to be 3:1 from m/z intensity and HPLC peaks. It is important to note that pyrazine compounds easily form complexes with anions in solution. Hence, the mass spectra recorded in the negative-ion mode are m/z of complexes, not free molecules as observed for the positive-ion mode studies. The third major product is retained on the PCG column for longer periods of time. Unfortunately, it was not ionized well (panel C) under these conditions. Further analyses showed that the m/z 305.1 peak corresponded to the chromatography peak at 26.67 min, whereas the m/z 321.1 peak was associated with the major peak at 23.82 min. The separation and identification of the third compound is under investigation.
Fig. 1.
Chemical analysis of D-GlcN reaction products. (A) Porous graphitic carbon–LCMS spectrum of D-GlcN reaction products in PBS using positive-ion mode analysis. Mass spectrum of major total ion current (TIC) peaks (23.75–27.94 min); (B) Elution profile from the PGC column (absorption at 300 nm); (C) TIC trace (15–45 min) showing that the peak at 29.73 min. in panel B did not ionize well; (D) extracted base peak of m/z 305.1 showing its correspondence to the major peak at 26.67 min.; (E) extracted base peak of m/z 321.1 showing its correspondence to the major peak at 23.82 min.
2.2 Biology
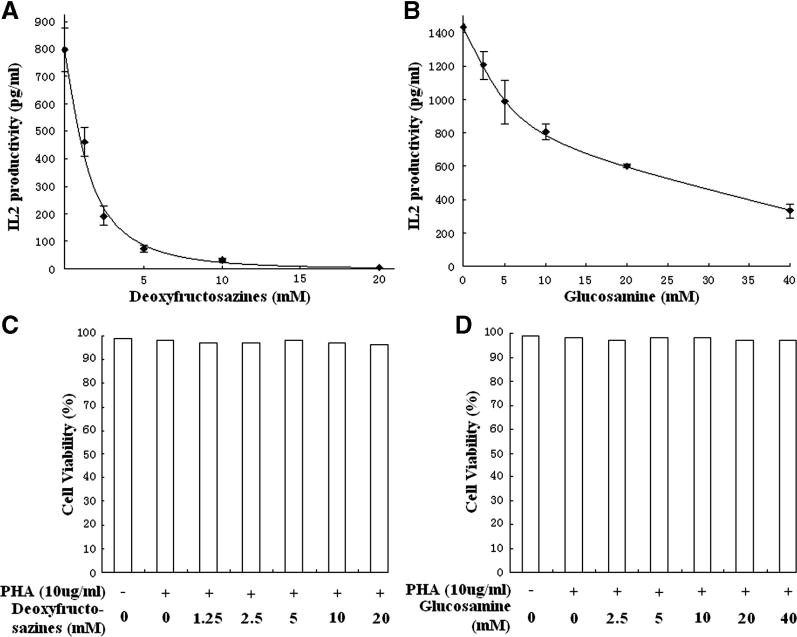
We next examined the biological properties of fructosazines (a mixture of compounds 1 and 2). As the starting material, D-GlcN, effects lymphocyte cytokine production,6 we examined the ability of fructosazines to influence this important biological parameter. We examined the effect of fructosazines on IL-2 production by Jurkat cells, a leading in vitro model of this physiological process. Jurkat cells were stimulated with 10 μg/mL phytohemagglutinin (PHA) overnight in the presence of fructosazines or D-GlcN in a humidified incubator. Supernates were then measured for IL-2 content using standard assays. A dramatic reduction in IL-2 production was found (Fig. 2A) in the presence of fructosazines, with a half-maximal inhibition at roughly 1.25 mM. To compare this D-GlcN derivative with D-GlcN, we next measured IL-2 production in the presence of various concentrations of D-GlcN (Fig. 2B). Although D-GlcN reduced IL-2 production, as previously noted,6 it was substantially less effective at reducing IL-2 release than fructosazine. This effectiveness of D-GlcN was not likely to be due to the formation of deoxyfructosazine during the incubation period, as D-GlcN was freshly prepared and diluted before experiments and no discoloration of the samples was noted at the end of experiments. The half-maximal D-GlcN dose was roughly 15 mM. Moreover, IL-2 was not completely blocked at any of the D-GlcN concentrations tested. As this reagent included a mixture of 1 and 2, we prepared 1 using the clean conversion method described by Rohivec et al.14 A similar inhibition of IL-2 production was obtained. These data strongly suggest that fructosazine is a better inhibitor of IL-2 production than D-GlcN.
Fig. 2.
Effect of fructosazines on cytokine release by Jurkat cells.
Fructosazines (A) and D-GlcN (B) reduce IL-2 production by Jurkat cells in a dose-dependent fashion. Cells were activated with PHA in the presence of various doses of fructosazines or D-GlcN. Fructosazines caused a dramatic reduction in IL-2 production by cells. As these data illustrate, fructosazines are substantially more effective in blocking IL-2 release than its parent compound, D-GlcN. Cell viability was also measured in the presence of fructosazines (C) and D-GlcN (D). In all cases, there was no significant effect of these compounds or of the stimulatory PHA molecules on the viability of Jurkat cells.
Several additional experiments were conducted to rigorously exclude other possible explanations for the decrease in cytokine production. One possible counter-argument is that this reagent was toxic to cells. To check cell viability, Trypan Blue experiments were conducted as described in Section 4.4. Experiments were conducted in the presence and absence of PHA and in the presence of PHA and various concentrations of fructosazine or D-GlcN (Fig. 2C and D, respectively). In all cases cell viability was >96% with no statistically significant differences found among all trials with both reagents. Another possible artifactual explanation of our findings is that fructosazine displaced PHA from the cell surface, thus causing a reduction in IL-2 release. This is not an unreasonable idea because PHA is a lectin. To test this possibility, cells were incubated with Alexa-488-labeled PHA in the presence or absence of 20 mM fructosazine at room temperature. When cells were evaluated by fluorescence microscopy, we observed no changes in the intensity of PHA binding or in its pattern of binding. Hence, non-physiological pathways of cytokine inhibition such as cell death and competition with the stimulating agent cannot explain our findings. Therefore, fructosazines are having a direct role in the inhibition of IL-2 production.
3. Conclusions
In the present study we have shown that D-GlcN reacts with itself under conditions used in biological studies to form fructosazines. One might suppose that the physiological effects would disappear after cyclocondensation. However, the biological effects were substantially enhanced by fructosazine formation. D-GlcN is purported to have beneficial effects in certain inflammatory diseases, such as arthritis,3 although the concentrations achieved in peripheral blood are likely to be too low to manifest effects on lymphocytes. As fructosazines may be present in many in vitro and in vivo studies with D-GlcN, they may contribute to some of the reported bioactivities of D-GlcN preparations. Fructosazines are one class of molecules containing a pyrazine structure, which can found in certain pharmaceuticals. Fructosazines may therefore constitute an important new class of lead compounds in the treatment of immunological and inflammatory disease.
4. Experimental section
4.1 Sample preparation
D-GlcN hydrochloride (0.86g, 4 mmol) (Sigma Chemical Co., St. Louis, MO) was dissolved in 6 mL of 10× PBS, adjusted pH to 7.4 and then diluted to 10 mL. This solution was incubated at room temperature for 24 h. The brown solution was filtered with a 0.2-μm syringe filter (Nalge Nunc, Rochester, NY) for LC–ESIMS analysis.
4.2 LC–ESIMS analysis
LC separation was carried out on a ThermoElectron Surveyor HPLC system using a Hypercarb column (5μ, 100×2.1 mm, Thermo) with a drop-in guard cartridge (10×2.1 mm). A 10-μL sample was injected. A photodiode array detector was used to monitor the absorption at 290–310 nm. Mobile phase A was 5 mM ammonium acetate (pH 8.5), whereas mobile phase B was acetonitrile. The gradient was as follows: 0–5 min, 0%B; 35–40 min, 30% B; 45min, 0% B. A 150-μL/min flow was directed into the source of the mass spectrometer after the first 15 min was diverted to waste.
ESIMS analysis was recorded on a ThermoElectron Finnigan LTQ linear ion trap mass spectrometer equipped with an electrospray ionization source. Full scan mass range m/z was 100–1000. The ESI voltage was set at 3.5 kV and the capillary temperature was 250 °C. The sheath gas and auxiliary gas were 15 units and 5 units, respectively.
4.3 Cells and cell stimulation
Jurkat cells (ATCC, Manassas, VA) were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA) containing 10% FCS and 1% antibiotics. To activate the Jurkat cells, they were treated with PHA (10 μg/mL) (Invitrogen) in the presence of D-GlcN or fructosazines at 37 °C. Alexa-488-conjugated PHA was obtained from Molecular Probes. Fluorescence microscopy was carried out as previously described.6
4.4 Bioassays
IL-2 production was measured using an ELISA kit from R & D Systems (Minneapolis, MN). Cells were incubated overnight under control or stimulated (10 μg/mL PHA) conditions. Cell viability was assessed by Trypan Blue exclusion. To allow precise assessment of cell viability, Jurkat cells were distributed on a Cell-Tak (BD Biosciences, San Jose, CA) coated surface then treated with the various reagents. After 4 hours the cells were stained with Trypan Blue.
Fig. 3.
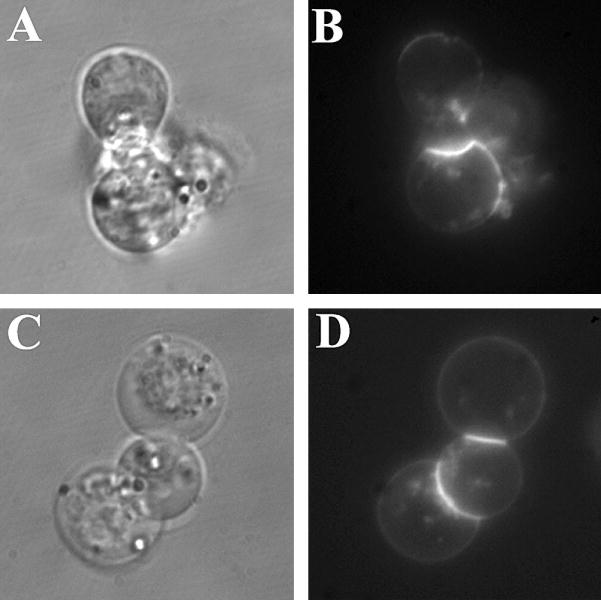
Fluorescence microscopy of PHA binding to the Jurkat cell surface. (A) and (C) are differential interference contrast images of cells, whereas (B) and (D) are fluorescence micrographs. Alexa-488-conjugated PHA was added to cells with buffer alone (A) and (B) or fructosazines (C) and (D). No differences in binding were found.
Acknowledgments
This research was supported, in part, by the Intramural Program of the National Institute of Child Health and Human Development, NIH, DHHS and by NIAID grant 51789.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Love DC, Hanover JA. Science’s STKE. 2005;312:1–14. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 2.Filippis A, Clark S, Proietto J. Biochem J. 1997;324:981–985. doi: 10.1042/bj3240981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hua J, Sakamoto K, Nagoaka I. J Leuk Biol. 2002;71:632–640. [PubMed] [Google Scholar]
- 4.Ma L, Rudert WA, Harnaha J, Wright M, Machen J, Lakomy R, Qian S, Lu L, Robbins PD, Trucco M, Giannoukakis N. J Biol Chem. 2002;277:39343–39349. doi: 10.1074/jbc.M204924200. [DOI] [PubMed] [Google Scholar]
- 5.Forchhammer L, Thorn M, Met O, Gad M, Weidner MS, Claesson MH. Scand J Immunol. 2003;58:404–411. doi: 10.1046/j.1365-3083.2003.01313.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang JB, Clark AJ, Petty HR. Cell Immunol. 2007 doi: 10.1016/j.cellimm.2007.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang GX, Yu S, Gran B, Rostami A. J Immunol. 2005;175:7202–7208. doi: 10.4049/jimmunol.175.11.7202. [DOI] [PubMed] [Google Scholar]
- 8.Sumoto K, Irie M, Mibu N, Miyano S, Nakashima Y, Watanabe K, Yamaguchi T. Chem Pharm Bull. 1991;39:792–794. doi: 10.1248/cpb.39.792. [DOI] [PubMed] [Google Scholar]
- 9.Vial J, Hennion MC, Fernando-Alba A, Agüera A. J Chromatogr A. 2001;937:21–29. doi: 10.1016/s0021-9673(01)01309-7. [DOI] [PubMed] [Google Scholar]
- 10.Chaimbault P, Elfakir C, Lafosse M. J Chromatogr A. 1988;797:83–91. [Google Scholar]
- 11.Gaudin K, Chaminade P, Baillet A. J Chromatogr A. 2002;973:69–83. doi: 10.1016/s0021-9673(02)01120-2. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kawasaki N, Itoh S, Ohta M, Hayakawa T. Anal Biochem. 2003;316:15–22. doi: 10.1016/s0003-2697(03)00031-9. [DOI] [PubMed] [Google Scholar]; (b) Schulz BL, Packer NH, Karlsson NG. Anal Chem. 2002;74:6088–6097. doi: 10.1021/ac025890a. [DOI] [PubMed] [Google Scholar]; (c) Barroso B, Dijkstra R, Geerts M, Lagerwerf F, van Veelen P, de Ru A. Rapid Commun Mass Spectrom. 2002;16:1320–1329. doi: 10.1002/rcm.723. [DOI] [PubMed] [Google Scholar]
- 13.Thermo Hypercarb HPLC Columns Technical Guide. http://www.interscience.nl/promotiesites/hypersil/topics/promotiesites/hypersil/nieuws/hypercarb_technical.pdf.
- 14.Rohovec J, Kotek J, Peters JA, Maschmeyer T. Eur J Org Chem. 2001:3899–3901. [Google Scholar]