Abstract
The formation of oligodendrocytes (oligodendrogenesis) and myelin is regulated by several neurotrophic factors. Strategies to increase the level of these trophic molecules may facilitate repair in demyelinating conditions, such as multiple sclerosis (MS). Because leukocytes are a source of neurotrophic factors, and as glatiramer acetate (GA) generates T helper 2 (Th2) lymphocytes that are not known to be harmful, we tested the hypothesis that GA regulates oligodendrogenesis and myelin formation. First, we generated GA-reactive Th2 cells and determined that they produced transcripts for neurotrophic factors, including insulin-like growth factor-1 (IGF-1). The conditioned medium from GA-reactive T cells elevated IGF-1 protein and promoted the formation of oligodendrocyte precursor cells (OPCs) from embryonic brainderived forebrain cells in culture. We next subjected mice to lysolecithin-induced demyelination of the spinal cord. At 7 days after the insult, the number of OPCs in the demyelinated dorsal column was higher than that in uninjured controls, and was further increased by the daily s.c. injection with GA. Increased OPC generation by GA was associated temporally with the elevation of IGF-1 and brain-derived neurotrophic factor (BDNF) in the spinal cord. Finally, the resultant remyelination at 28 days was higher in mice treated with GA during the first 7 days of injury compared with vehicle controls. These results indicate that GA promotes oligodendrogenesis and remyelination through mechanisms that involve the elevation of growth factors conducive for repair.
Keywords: beneficial inflammation, neurotrophic factors, oligodendrocyte, regeneration, remyelination
Multiple sclerosis (MS) is characterized by inflammation and demyelination in the CNS. The demyelinated lesions can be repaired and, indeed, the extent of remyelination is substantial in some patients (1). There is increasing interest in facilitating remyelination as its benefits extend beyond restoration of nerve impulse conduction to preventing axonal degeneration, since axon and myelin units have dynamic interactions involving survival signaling.
Much has been learned about the process of remyelination (2) and key steps include the proliferation and maturation of oligodendrocyte precursor cells (OPCs) and the appropriate interactions of oligodendrocyte processes with axons to form myelin. A large number of molecules are critically involved in the proliferation, maturation and survival of OPCs, and among these are neurotrophic factors such as platelet-derived growth factor (PDGF), insulin-like growth factor-1 (IGF-1) and brain-derived neurotrophic factor (BDNF) (3–6). Accordingly, strategies that have been used to induce remyelination in animals have included the provision of neurotrophic factors through infusion or gene therapy.
Another means to deliver growth factors to the nervous system takes advantage of the observation that leukocytes are rich sources of neurotrophic factors. Indeed, even the disease-promoting inflammatory cells of perivascular cuffs in MS lesions are immunoreactive for neurotrophic factors such as BDNF (7). However, any approach to use leukocytes to deliver neurotrophic factors for repair must balance their potential detriments in exacerbating the pathology of MS.
Glatiramer acetate (GA), a medication used in relapsing-remitting MS (8), generates GA-reactive T lymphocytes that are of the T helper 2 (Th2) anti-inflammatory bias (9, 10). GA-reactive Th2 cells accumulate in the CNS, where they have been shown to produce not only anti-inflammatory cytokines but also neurotrophic factors (11). Importantly, Th2 cells have not been found to be neurotoxic, unlike the proinflammatory Th1 or Th17 lymphocytes that destroy neurons in tissue culture (12). More recently, GA has also been found to modulate monocytoid cells into those that express anti-inflammatory cytokines (13).
Here, we have tested the hypothesis that T cells exposed to GA elevate neurotrophic factors that are important for oligodendrogenesis in culture. Furthermore, we have used mice with lysolecithin-induced demyelination to address whether treatment with GA would increase OPC numbers and remyelination within the lesioned spinal cord. These studies have relevance to the biology of harnessing the benefits of inflammation to evoke repair.
Results
T Cells Exposed to GA Elevate Their Production of Growth Factors in Vitro.
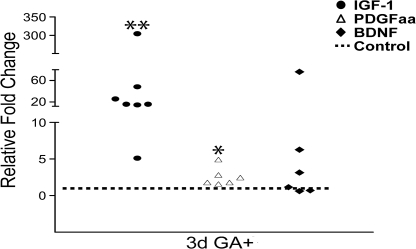
Cells obtained from the lymph nodes of GA-pretreated mice (Fig. S1) were incubated with APCs and GA, resulting in a proliferative response (Fig. S2) that suggested the presence of T cells reactive to GA. Cell conditioned medium from these GA-reactive cells was then examined for the accumulation of IFN-γ, a Th1 cytokine, and for IL-5, a Th2 cytokine. Increasing culture periods with GA resulted in loss of detectable IFN-γ and steady accumulation of IL-5 (Fig. S2), indicating that the GA-reactive T cells were of the Th2 phenotype, as reported by others (9–11). With this confirmation, we examined the capacity of GA-reactive T cells to produce growth factors. From several mouse donors previously treated with GA, lymph node cells were restimulated with GA and RNA was harvested. Fig. 1 shows that increases in transcripts encoding IGF-1 and PDGFaa were evident after 3 days of GA treatment. Transcripts for BDNF was variable, with three cultures elevating this trophic factor in response to GA whereas three did not.
Fig. 1.
GA-reactive T cells in tissue culture produce growth factors. Real-time PCR analyses and GADPH-normalized levels show that compared with non-GA exposed cells (control), treatment of T cells with GA resulted in increase in levels of IGF-1 and PDGFaa. Each circle, square, or triangle within a given growth factor dataset represents a separate culture. *, P < 0.05; **, P < 0.01, compared with non-GA exposed controls (t test).
The cell-conditioned medium collected from T cells over 3 days of culture was analyzed for IGF-1 protein content. IGF-1 protein was significantly elevated in T-cell cultures treated with GA, either collected at first in vitro restimulation with GA (day 3), or after restimulation a week after (day 10) (Fig. S2).
Conditioned Medium from GA-Reactive T Cells Increases the Number of OPCs in Culture.
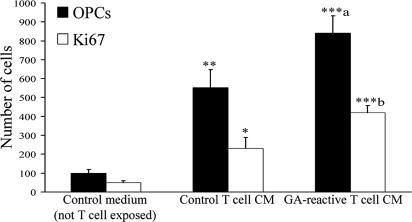
We evaluated whether growth factors produced by GA-reactive T cells (Fig. 1) were sufficient to regulate the formation of OPCs from neural precursor cells. Single cells from the anterior entopenduncular area (AEP) of embryonic day 15 mice were exposed to conditioned medium collected from GA-reactive T cells for 72 h. Fig. 2 and Fig. S3 show that there were significantly more OPCs and Ki67-positive cells when the embryonic brain cells were incubated with medium from GA-reactive T cells, compared with medium from control T cells (not restimulated with GA). These results show that GA-reactive T cells secrete factors conducive for oligodendrogenesis.
Fig. 2.
Conditioned medium (CM) from GA-reactive T cells promotes the development of OPCs in vitro. OPCs were identified by PDGFRα labeling whereas cycling cells were tagged by Ki67. Values are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with control non-T-cell-exposed medium. a, P < 0.05; b, P < 0.01, comparing GA T-cell medium data to control T-cell CM (one-way ANOVA with Bonferroni multiple comparisons).
GA Treatment of Mice with Lysolecithin Injury Increases the Number of OPCs in the Lesioned Spinal Cord.
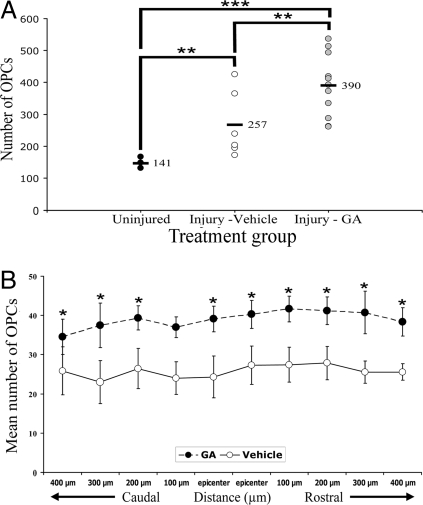
We next investigated whether GA could increase OPC numbers in vivo. To better simulate conditions in patients, we injected mice daily with s.c. GA. Previous studies have shown that significant demyelination (14, 15) and newly generated OPCs (16) could be found by day 7 after lysolecithin administration, so we chose this time point for analysis. Fig. S4 of MBP immunoreactivity shows that demyelination of the dorsal column of the spinal cord was extensive at day 7. In the area of demyelination, a dense accumulation of Iba1+ microglia/macrophages was readily observed. Moreover, OPCs identified by PDGFRα immuno-labeling and as discrete cells could be discerned (Fig. S4). We blindly enumerated the number of OPCs in the dorsal column across 10 sections of the spinal cord, each spaced 100 μm apart, and centered around the epicenter of injury. Fig. 3 shows that in mice subjected to lysolecithin injury and treated s.c. with PBS vehicle, the average total number of OPCs in the dorsal column summed across 10 sections of individual mice averaged 257. This represents an increase from uninjured mice (average sum of 141 OPCs across 10 sections). Impressively, daily treatment with s.c. GA for 7 days after lysolecithin injury resulted in a further increase of OPC numbers, where an average of 390 OPCs across 10 sections was found per mouse (Fig. 3).
Fig. 3.
GA treatment increases injury-induced rise of OPC numbers. (A) The number of PDGRα-positive OPCs were counted in the dorsal column of 10 sections, each spaced 100 μm apart and centered around the lesion epicenter. The sum per mouse was then plotted in A, where each value is from a separate mouse. There were 7 uninjured mice, 8 lysolecithin-vehicle mice, and 11 lysolecithin-GA animals. **, P < 0.01; ***, P < 0.001 (one way ANOVA with Bonferroni multiple comparison test). (B) Data of OPC numbers (mean ± SEM) in discrete sections around the lesion epicenter are displayed, to indicate that the increase in OPCs induced by GA is widespread across several sections containing demyelination. *, P < 0.05, one way ANOVA with Bonferroni multiple comparison test. All analyses were blinded.
The increase of OPC numbers in the dorsal column induced by GA compared with vehicle occurred not only at the lesion epicenter, but also rostrally or caudally across 300–400 μm on either side (Fig. 3) containing a demyelinating lesion.
Overall, these results demonstrate that the daily s.c. injection of GA for 7 days after lysolecithin injury results in an elevation of the injury-induced increase of OPCs in the spinal cord and that this increase occurs locally in the dorsal column that contains a demyelinating injury.
Alterations of Cytokine and Growth Factor Levels in Spinal Cords of GA-Treated Mice.
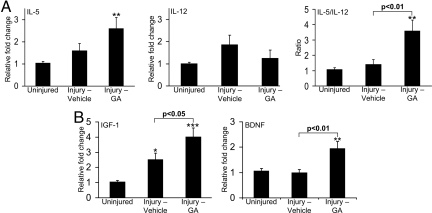
We determined whether GA treatment of lysolecithin-injured mice led to alterations of inflammatory cytokines or growth factors in vivo. Coronal spinal cord blocks from around the injury site were harvested from mice treated s.c. with GA or vehicle daily for 7 days after lysolecithin injury, and RNA was collected and subjected to real-time PCR. Fig. 4 shows that lysolecithin injury led to rise of levels of the Th2 cytokine, IL-5; and IL-12, a proinflammatory cytokine expressed by APCs including macrophages and important in the generation of Th1 cells (IFN-γ was not detected). Analysis of the ratio of IL-5 to IL-12 from individual mice showed that this ratio was highly elevated by GA compared with vehicle-treatment.
Fig. 4.
Inflammatory and growth factor profiles at day 7 after lysolecithin injury. (A) Displays data from real-time PCR for IL-5 and IL-12; the IL-5:IL-12 ratio of individual mice shows that GA biased the cytokine profile within the spinal cord toward one that is less proinflammatory. Each histogram is of 12–15 mice. (B) GA treatment is found to increase IGF-1 and BDNF transcripts. Each histogram represents 9–12 mice. All values are mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with uninjured controls, one way ANOVA with Bonferroni multiple comparison test.
For growth factors (Fig. 4), we found that IGF-1 transcript increased after lysolecithin injury in vehicle treated mice compared with uninjured controls, and this was elevated further after injury by GA treatment. BDNF did not rise after injury from uninjured controls, but GA treatment significantly raised BDNF levels.
GA Treatment Increases Indices of Remyelination 28 Days After Lysolecithin Injury.
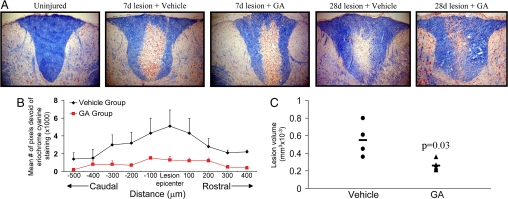
In correspondence with the documented remyelination that occurs after lysolecithin-induced demyelination (14–16), we found that the extent of eriochrome cyanine staining was greater in the dorsal column at 28 days of vehicle-treated mice compared with that at 7 days after lysolecithin injury (Fig. 5). An initial extensive demyelination at 7 days, which resulted in a large area of the dorsal column being devoid of intact myelin, was progressively repaired by day 28. Impressively, in mice treated with daily s.c. GA for the first 7 days of injury, eriochrome cyanine staining at 28 days indicated that an even greater degree of remyelination had occurred compared with the profile in vehicle-treated mice (Fig. 5). Much of the initially demyelinated area of the dorsal column in the GA-treatment group contained patches of blue profiles (Fig. S5), reminiscent of “shadow plaques” that have been used to describe remyelination in the human CNS.
Fig. 5.
Evolution of de- and remyelination and impact of GA. (A) Displays representative eriochrome cyanine staining whereby demyelination is very prominent and comparable in both the vehicle and GA-treated mice at 7 days after lysolecithin injury. Progressive remyelination indicated by increased eriochrome cyanine profiles in the dorsal column is evident at day 28 after injury, and this is enhanced further in mice treated with GA. Ten times original magnification. (B) The area of demyelination (mean ± SEM) within the dorsal column of sections caudal and rostral to the lesion epicenter is displayed; demyelination is calculated based on pixel intensity devoid of eriochrome cyanine staining, and was determined using Image-Pro Plus software. (C) The volume of remaining demyelination encompassing 1 mm of the spinal cord shows a smaller remaining lesion (t test) in GA-treated mice compared with that in the vehicle group.
We used the pixel intensity of eriochrome cyanine staining to document the area of each dorsal funiculus that was devoid of myelin. Such analyses were conducted across 10 sections spanning 1 mm and centered around the lesion epicenter. Fig. 5 demonstrates that the remaining demyelination at day 28 was more extensive in vehicle-treated mice compared with that in the GA-treated group, and this was evident not only around the lesion epicenter, but also rostrally and caudally.
Finally, we documented the area of demyelination per section and summed these across 10 sections spanning 1 mm per mice to obtain an index of the lesion volume. Fig. 5 reveals that the volume of remaining demyelination at day 28 was significantly less in the GA-treatment group (average of 0.26 ± 0.03 mm3 × 10−3) (mean ± SEM) compared with vehicle controls (average of 0.55 ± 0.10 mm3 × 10−3) (P < 0.05, Mann–Whitney unpaired T test). This was not the result of differing lesion volumes at day 7 after the lysolecithin insult, which was found to be similar in both groups (average of 0.98 ± 0.21 mm3 × 10−3 vs. 0.72 ± 0.20, vehicle vs. GA, mean ± SEM, n of 7 each, P > 0.05). Comparing across time points, the lesion volume at day 28 in the vehicle group was 56% of that at day 7 whereas that in the GA group at day 28 was 36% of that at day 7.
Overall, studies of indices of remyelination suggest that GA treatment in the first 7 days after lysolecithin administration favors a more pronounced repair over the ensuing 28 days.
Discussion
Summary of Results.
We have determined that in culture, GA-reactive T cells produced growth factors described for oligodendrogenesis, and that the conditioned medium from GA-reactive T cells increased the number of OPCs that were formed from embryonic brain cells. After demyelination of the spinal cord in mice, the daily treatment for 7 days with s.c. GA elevated the injury-induced rise of OPCs correspondent with an mRNA profile in the injured tissue that was anti-inflammatory and proreparative. Although the size of the demyelination at day 7 was comparable between the two groups, suggesting that GA did not modulate grossly the initial demyelinating events, determinations at day 28 show increased remyelination when mice were exposed to GA during the first 7 days of injury.
Growth Factors Regulate the Generation of OPCs in Culture, in Development, and in Remyelination.
Recovery after demyelination requires the presence of OPCs and the ability of these cells to divide, migrate, mature, and enwrap axons in response to signals in demyelinated lesions. The generation and maturation of OPCs are dependent on several growth factors. In tissue culture studies, the proliferation of OPCs is stimulated by PDGF, among others. BDNF increases the proliferation of OPCs and their maturation into oligodendrocytes (3, 4). IGF-1 has strong capacity to facilitate both OPC proliferation and maturation (5), and is a survival factor for oligodendrocytes.
Developmental studies have also highlighted the importance of growth factors. Transgenic mice overexpressing PGDF presented with hyperproliferation of precursor cells whereas PDGF null mice had severely reduced number of OPCs (6). IGF-1 transgenic mice have larger brain and higher myelin content than controls (17). Conversely, IGF-1 null mice have lower number of OPCs at 1–3 weeks of age, and mice with deficient IGF receptor 1 signaling have retarded developmental myelination (18).
Growth factors are also critical during remyelination. The increase of several growth factors within the demyelinated CNS has been reported (19). IGF-1 transgenic mice remyelinated more readily compared with wildtype after demyelination of the corpus callosum; moreover, type 1 IGF receptor null mice did not remyelinate adequately after insult, corresponding with the failure of OPCs to accumulate, proliferate or survive in mutants (20). It must be noted that although we have focused discussions to PDGF, BDNF, and IGF-1, a myriad of molecules has been described to influence myelin formation, including the chemokine GRO-α, hormones, notch signaling and LINGO.
Specific growth factors have been infused or genetically expressed in animal models of demyelination in attempts to promote remyelination. IGF-1 treatment of rats subjected to EAE increased the numbers of proliferating oligodendrocyte precursors (21). For BDNF, a single dose in rabbits subjected to cervical spinal cord ventral root avulsion and replantation promoted remyelination of axons that regenerated from the replantation (22).
The provision of a particular growth factor may sometimes result in proliferation of cells but not their subsequent maturation. This is highlighted by Woodruff et al. (23) where the transgenic increase of PDGFaa led to a rise in numbers of OPCs after lysolecithin demyelination; however, there was a trend toward lower remyelination in the PDGF transgenics, which the authors speculated to be due to the prolonged mitogenic stimulus.
These results indicate that although single growth factor treatment may be adequate to affect repair, this may be suboptimal or counterproductive in some cases. The concomitant provision of multiple growth factors, to include those with proliferative and/or differentiating capacity, would appear to be required to enhance repair.
Beneficial Aspects of Neuroinflammation.
One means to deliver multiple growth factors into the CNS takes advantage of the observation that leukocytes have the capacity to generate a range of growth factors (7). For example, activated T cells, B cells, and macrophages express nerve growth factor and BDNF in vitro, and inflammatory infiltrates of T cells, NK cells, and macrophages in the CNS of animals afflicted with experimental autoimmune encephalomyelitis (EAE) were immunoreactive for neurotrophin-3 and BDNF (24). These reports highlight that there are beneficial aspects to neuroinflammation, which should not be unexpected since a main role for an inflammatory response in other tissues is to enhance repair. In accordance, Schwartz and colleagues (25) have noted that even autoreactive T cells could prevent neuronal death in neurotrauma.
There is increasing evidence that inflammation aids remyelination. The depletion of macrophages (26) or T cells (27) impairs remyelination after lysolecithin-induced demyelination. Conversely, the promotion of an inflammatory response by creating a stab injury to the spinal cord (28) or by injecting zymosan along with OPC transplants into the retina (29) increased remyelination.
The benefits of inflammatory cells are attributed in part to their production of neurotrophic factors described above but other aspects of an inflammatory response may also help. For instance, proteases released by inflammatory cells may help remove nonpermissive molecules, such as proteoglycans that hinder recovery (14). The challenge of harnessing beneficial neuroinflammation is how to avoid its potential detriments, given the extensive literature that persistent neuroinflammation can harm the CNS.
Harnessing the Benefits of Inflammation with GA.
GA has been in clinical use to treat MS for over 15 years where it has been shown to be safe, to reduce relapse rate and to produce longterm stabilization of disease in a significant proportion of patients (8). The mechanisms of GA in MS are multiple, and include the generation of Th2 cells and Type 2 monocytes (9, 13). These cells are not known to harm the CNS and may elicit CNS outcomes differently from other inflammatory cell types. When Th2 cells were contrasted with proinflammatory Th1 cells, it was found that Th2 cells interacted with glia to elevate neurotrophins; in contrast, the interaction of Th1 cells with glia did not affect neurotrophins but enhanced the levels of proinflammatory molecules (30). In our study, we were unable to reproducibly detect T cells at sites of demyelination by immunohistochemistry or flow cytometry (data not shown), even though the PCR data (Fig. 4) suggested a Th2:Th1 bias in GA-treated mice. Future studies will need to examine representation of Th2 versus Th1 T cells, or Type 2 monocytes, in detail in the demyelinated spinal cord after GA-treatment.
In accordance with potentially useful outcomes of GA for the CNS, GA has been found to confer neuroprotection in animal models of optic nerve injury, EAE, ALS, Parkinson's, and Alzheimer's disease (31). GA has also been studied in the context of neural repair. Immunoglobulins harvested from GA-primed mice and injected into mice with viral-induced demyelination of the spinal cord promoted remyelination (32); whether similar outcome could result from daily s.c. injections to mimic the mode of treatment of MS patients with GA was not addressed. Treatment of EAE mice using GA has been reported to increase the extent of neurogenesis (33) and the number of OPCs (34) in the CNS. Overall, these studies support the repair potential of GA that is suggested by our study.
Conclusion
We have determined that the MS medication, GA, promotes oligodendrogenesis and indices of remyelination in mice subjected to lysolecithin-induced demyelination. This demonstration of the myelination capacity of GA injections in a noninflammatory animal model, suggests the direct effect on myelin formation is not solely due to the reduction of a harmful inflammatory milieu. The results herein indicate that it is possible to employ GA to harness the benefits of neuroinflammation for repair. The data also suggest that the long term benefits observed in patients on GA treatment (8) may in part be due to remyelination, and this deserves further evaluation.
Materials and Methods
Assessment of Neurotrophic Factors Produced by GA-reactive T Cells in Vitro.
Responder and APC populations (see SI Text) freshly harvested from mice were cocultured (3 million each, in 3 mL medium) in 6-well plates in the absence or presence of 10 μg/mL GA. Three days after, total RNA was extracted from cells with 1 mL of TRIzol solution (Life Technologies BRL). The mRNA expression of IGF-1, BDNF, PDGFaa, and the housekeeping gene, 18s rRNA, was analyzed by real-time PCR. All PCR primer sets were purchased from SA Biosciences (IGF-1, PPM03387A; BDNF, PPM03006A; and PDGFaa, PPM03103A). For 18s rRNA the following forward (F) and reverse (R) primers were used, F; GTAACCCGTTGAACCCCATT, R; CCATCCAATCGGTAGTAGCG.
Value of growth factor expression from each PCR was normalized to 18s rRNA value from the same RNA extract. By comparing the ratio of normalized growth factor value for a given T-cell culture exposed to GA versus a sister culture that is not, the relative fold change in transcript was thus obtained for that culture.
To verify that protein for growth factors was also elevated in GA-reactive T cells, conditioned media collected for cytokine analyses described earlier were subjected to the measurement of a selected growth factor, IGF-1, using ELISA (Biosource). Furthermore, the cell conditioned medium was analyzed for their capacity to promote OPC in culture as described in SI Text.
Lysolecithin-Induced Demyelination in the Spinal Cord of Mice and Treatment with GA.
C57BL/6 adult 2–3-month-old male mice were anesthetized i.p. with a mixture of ketamine (85 mg/kg) and xylazine (15 mg/kg). A 1.5 μL solution of 1% D-lysophosphatidylcholine (lysolecithin; Sigma) was injected over 1.5 min into the dorsal column of the T3 spinal cord segment using a 32 G needle, attached to a 5 μL Hamilton syringe (Hamilton). The needle was left in the spinal cord for an additional 2 min to prevent backflow of the lysolecithin. Muscles, connective tissue and skin were then sutured above the injection site.
Animals received daily injections of 2 mg per mouse of GA dissolved in saline s.c. for 7 days starting from the day of lysolecithin administration. This dose was used by Aharoni et al. in mice to increase neurogenesis (33) or document OPC numbers (34) in EAE. Control animals were treated with daily s.c. injections of vehicle (PBS) after the lysolecithin insult.
Mice were killed for the evaluation of OPC numbers and cytokine/growth factor content in the spinal cord at day 7 after demyelination, and for the evaluation of myelin reformation at day 28, as described in detail in SI Text.
Supplementary Material
Acknowledgments.
This work was supported by grants from Teva Pharmaceutical Industries, Israel, the Multiple Sclerosis Society of Canada, and NeuroScience Canada. Dr. Skihar was supported by a fellowship from the Multiple Sclerosis Society of Canada.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909607106/DCSupplemental.
References
- 1.Patrikios P, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 2.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 3.Van't Veer ADY, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87:69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25:116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl NM, De Keyser J, De Vries H, Hoekstra D. Insulin-like growth factor binding proteins-1 and -2 differentially inhibit rat oligodendrocyte precursor cell survival and differentiation in vitro. J Neurosci Res. 2002;69:207–216. doi: 10.1002/jnr.10293. [DOI] [PubMed] [Google Scholar]
- 6.Fruttiger M, et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- 7.Kerschensteiner M, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford CC, et al. A prospective open-label study of glatiramer acetate: Over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309–320. doi: 10.1191/135248506ms1318oa. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, et al. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Mult Scler. 2001;7:209–219. doi: 10.1177/135245850100700401. [DOI] [PubMed] [Google Scholar]
- 10.Yong VW. Differential mechanisms of action of interferon-beta and glatiramer aetate in MS. Neurology. 2002;59:802–808. doi: 10.1212/wnl.59.6.802. [DOI] [PubMed] [Google Scholar]
- 11.Aharoni R, et al. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci USA. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber MS, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 14.Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregg C, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27:1812–1823. doi: 10.1523/JNEUROSCI.4441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- 17.Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 18.Zeger M, et al. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinks GL, Franklin RJ. Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci. 1999;14:153–168. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- 20.Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23:7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao DL, Liu X, Hudson LD, Webster HD. Insulin-like growth factor I treatment reduces demyelination and upregulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 1995;92:6190–6194. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang EM, et al. Single-dose application of CNTF and BDNF improves remyelination of regenerating nerve fibers after C7 ventral root avulsion and replantation. J Neurotrauma. 2008;25:384–400. doi: 10.1089/neu.2007.0396. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Hammarberg H, et al. Neuroprotection by encephalomyelitis: Rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moalem G, et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 26.Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- 27.Bieber AJ, Kerr S, Rodriguez M. Efficient central nervous system remyelination requires T cells. Ann Neurol. 2003;53:680–684. doi: 10.1002/ana.10578. [DOI] [PubMed] [Google Scholar]
- 28.Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128:528–539. doi: 10.1093/brain/awh417. [DOI] [PubMed] [Google Scholar]
- 29.Setzu A, et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- 30.Roy A, Liu X, Pahan K. Myelin basic protein-primed T cells induce neurotrophins in glial cells via alphavbeta3 [corrected] integrin. J Biol Chem. 2007;282:32222–32232. doi: 10.1074/jbc.M702899200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnon R, Aharoni R. Neurogenesis and neuroprotection in the CNS–fundamental elements in the effect of Glatiramer acetate on treatment of autoimmune neurological disorders. Mol Neurobiol. 2007;36:245–253. doi: 10.1007/s12035-007-8002-z. [DOI] [PubMed] [Google Scholar]
- 32.Ure DR, Rodriguez M. Polyreactive antibodies to glatiramer acetate promote myelin repair in murine model of demyelinating disease. FASEB J. 2002;16:1260–1262. doi: 10.1096/fj.01-1023fje. [DOI] [PubMed] [Google Scholar]
- 33.Aharoni R, Arnon R, Eilam R. Neurogenesis and neuroprotection induced by peripheral immunomodulatory treatment of experimental autoimmune encephalomyelitis. J Neurosci. 2005;25:8217–8228. doi: 10.1523/JNEUROSCI.1859-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aharoni R, et al. Demyelination arrest and remyelination induced by glatiramer acetate treatment of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:11358–11363. doi: 10.1073/pnas.0804632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.