Abstract
Dipeptidyl peptidase-IV (DPP-IV) inhibitors decrease degradation of the incretins. DPP-IV inhibitors also decrease degradation of peptides, such as substance P, that may be involved in the pathogenesis of angiotensin-converting enzyme (ACE) inhibitor-associated angioedema. This study tested the hypothesis that DPP-IV inhibition affects risk of clinical angioedema, by comparing the incidence of angioedema in patients treated with the DPP-IV inhibitor vildagliptin versus those treated with comparator in Phase III randomized clinical trials. Prospectively defined angioedema-related events were adjudicated in a blinded fashion by an internal medicine adjudication committee and expert reviewer. Concurrent ACE inhibitor or angiotensin receptor blocker exposure was ascertained from case report forms. Study drug exposure was ascertained from unblinded data from phase III studies. Odds ratios and 95% confidence intervals comparing angioedema risk in vildagliptin-treated and comparator-treated patients were calculated for the overall population and for patients taking ACE inhibitors or angiotensin receptor blockers, using both an analysis of pooled data and a meta-analysis (Peto method). Overall, there was no association between vildagliptin use and angioedema. Among individuals taking an ACE inhibitor, however, vildagliptin use was associated with an increased risk of angioedema (14 confirmed cases among 2754 vildagliptin users versus 1 case among 1819 comparator users: odds ratio 4.57 (95% confidence interval 1.57-13.28) in the meta-analysis. Vildagliptin use may be associated with increased risk of angioedema among patients taking ACE inhibitors, although absolute risk is small. Physicians confronted with angioedema in a patient taking an ACE inhibitor and DPP-IV inhibitor should consider this possible drug-drug interaction.
Keywords: angioedema, angiotensin-converting enzyme inhibitor, hypertension, antihypertensive agents, type 2 diabetes
Diabetes mellitus affects some 195 million people worldwide. Despite the availability of many classes of antidiabetic agents including sulfonylureas, biguanides, thiazolidinediones, meglitinides and insulin and the use of combination therapy, glycemic control is often inadequate. The dipeptidyl peptidase-IV (DPP-IV) inhibitors represent a promising new class of orally active agents for the treatment of type 2 diabetes.1 DPP-IV (CD26; EC 3.4.14.5) is a cell-surface protease that inactivates the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). The incretins play an important role in glucose homeostasis, stimulating insulin secretion, suppressing glucagon release, and slowing gastric emptying. When given as monotherapy, the DPP-IV inhibitors increase circulating concentrations of GLP-1 and GIP and decrease glucose concentrations in type 2 diabetes.2-5 In clinical trials in type 2 diabetic patients with inadequate glucose control despite treatment, the addition of a DPP-IV inhibitor decreases fasting glucose and hemoglobin A1c and improves insulin secretion.6, 7
In addition to degrading the incretins, DPP-IV degrades other peptides with a proline or alanine in the penultimate amino position.8 Theoretically, DDP-IV inhibitors could cause beneficial or adverse effects by preventing the degradation of these other peptides. For example, the vasodilator substance P is normally degraded by angiotensin-converting enzyme (ACE). When ACE is inhibited, however, substance P is inactivated by DPP-IV (Figure 1).9 Substance P contributes to ACE inhibitor-associated tracheal edema in animal models.10 Moreover, plasma DPP-IV activity is decreased in some patients during ACE inhibitor-associated angioedema.11, 12 Taken together, these data suggest the hypothesis that pharmacological inhibition of DPP-IV could increase the risk of angioedema in patients taking ACE inhibitors.
Figure 1.

Schematic diagram showing the role of angiotensin-converting enzyme (ACE) and dipeptidyl peptidase-IV (DPP-IV) in the degradation of bradykinin and substance P. Studies in rodents suggest that DPP-IV is the primary enzyme responsible for the inactivation of substance P when ACE is inhibited.26 The dotted line indicates that bradykinin is already inactivated by aminopeptidase P (APP) before it is degraded further by DPP-IV. CPN indicates carboxypeptidase N; NEP indicates neutral endopeptidase.
The first DPP-IV inhibitor, sitagliptin phosphate, was approved by the FDA for the treatment of type 2 diabetes in October 2006.13 DPP-IV inhibitor use was associated with an increased risk of nasopharyngitis in an early meta-analysis of published 12- to 24-week trials.14 In April 2007, the sitagliptin label and package insert were amended to state that, “There have been postmarketing reports of serious hypersensitivity reactions in patients treated with JANUVIA. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions including Stevens-Johnson syndrome. Onset of these reactions occurred within the first 3 months after initiation of treatment with JANUVIA, with some reports occurring after the first dose.”15 We present here the results of premarketing surveillance for angioedema in clinical trials of the DPP-IV inhibitor vildagliptin.
Methods
Ascertainment of Angioedema Cases from Clinical Studies of Vildagliptin
Beginning in 2005, angioedema was specified as a safety event in clinical trials of vildagliptin, for adjudication by a 5-member, independent Internal Medicine Adjudication Committee (IMAC) and an additional expert reviewer (NJB). IMAC members were not Novartis employees and were affiliated with academic institutions or hospitals other than Vanderbilt. An “Angioedema and Angioedema-Related Events” questionnaire was created to capture information regarding clinical presentation and treatment. Cases were reviewed if a study investigator submitted an adverse event case report form that included any one of seventy-five pre-specified terms. A full list of terms that triggered adjudication for angioedema appears in the appendix. Because cases were to be further reviewed, this list was designed to be sensitive rather than specific.
A clinical research scientist (CRS) employed by Novartis reviewed any case meeting one of the pre-specified terms for angioedema. Two cases were designated as possible unexpected severe adverse events and were unblinded to the CRS prior to her review, but not to either the IMAC or the external reviewer. In all other cases, the CRS was blinded to treatment. The CRS completed the “Angioedema and Angioedema-Related Events,” questionnaire based on case report forms, any medical records pertaining to the event (such as physician's office records or emergency department records), and a clinical summary completed by the investigator. The “Angioedema and Angioedema-Related Events,” questionnaire included questions regarding the date of onset of symptoms, the site of any swelling, and treatment (including the withdrawal of study medication or any other medication, administration of H1 or H2 blockers, the use of corticosteroids or epinephrine, hospitalization, and intubation). Information regarding concurrent ACE inhibitor or angiotensin receptor blocker (ARB) use was obtained from concurrent medication and adverse event case report forms. These forms listed the start and stop dates for all medications. Severity and relation of the event to study medication was determined by the examining physician on the adverse event report form.
The questionnaire, any medical records pertaining to the event and a narrative prepared by the CRS were evaluated by the IMAC and separately by the expert reviewer. The IMAC and reviewer were blinded to treatment in all cases. The outside reviewer's classification was forwarded to the IMAC. The IMAC categorized all cases as “Confirmed Angioedema,” “Not Angioedema,” or “Not sufficient information” for diagnosis, by either majority vote or consensus.
Statistical Analysis of Vildagliptin Data
In the summer of 2008, biostatisticians (SB and DC) completed an analysis of a pooled safety dataset for all completed clinical trials of vildagliptin, as well as unblinded data from the 52-week interim analysis of a large clinical trial (CLAF237A 2308). All studies were randomized and double blind, with the exception of study 23119, which was randomized open label. A list of all studies analyzed appears in Appendix II. Although study 2308 was not completed at the time, the rationale for including it in the analysis was that it was a large study and an interim analysis had been completed that had already unblinded the statisticians to most patients, including several known cases of angioedema. Two classifications of angioedema events were defined for analysis. Firstly, only those cases adjudicated as confirmed angioedema were included. Next a sensitivity analysis was performed including both those cases adjudicated as confirmed and those cases adjudicated as having insufficient information available. Events were sub-classified into those occurring during concurrent ACE inhibitor exposure and those occurring during concurrent ARB exposure. The denominator of number of patients (overall and in patients taking an ACE or ARB) was determined from unblinded data from all studies in the database. Using these events and associated denominators, unadjusted odds ratios and 95% confidence intervals comparing angioedema risk in vildagliptin-treated and comparator-treated patients were calculated for the overall patient population and in the subgroups of patients taking ACE inhibitors and patients taking ARBs (pooled analysis).
Because an analysis based on pooled data is vulnerable to Simpson's paradox,16 we also performed meta-analysis, combining odds ratios across studies. We utilized the Peto one-step odds ratio method, a method that is particularly well-suited to analyze studies in which event rates are less than 1 per cent.17 For this analysis, we included those studies with both a vildagliptin arm and a comparator arm and with at least one angioedema event in one of the arms, even if the other arm had no events. We conducted this analysis using both fixed and random effects.
Angioedema in Postmarketing Surveillance of Sitagliptin and Literature Search
On May 25, 2007, one of the authors (NJB) sent a letter to the FDA requesting information about adverse events reported in postmarketing surveillance of sitagliptin. An FDA Adverse Event Reporting System (AERS) Freedom of Information Act Report was generated on July 2, 2007. After reviewing this initial report, the author requested additional information on 32 reports, representing 31 unique cases. This included cases identified as angioedema, anaphylaxis, swelling, hypersensitivity reaction, or rash. This information was provided on November 29, 2007.
Using PubMed, we searched the English language literature for reports of angioedema in patients taking a DPP-IV inhibitor other than vildagliptin. We reviewed the adverse events in published clinical studies of sitagliptin, saxagliptin, and alogliptin. Additional searches using the key words “angioedema”, “edema”, and “swelling” combined with “sitagliptin”, “alogliptin”, “saxagliptin” or “dipeptidyl” did not yield any articles. The latest search was completed in March 2009.
Results
Characteristics of Angioedema Cases in Premarketing Studies of Vildagliptin
Fifty-five cases of potential angioedema, from among 13,921patients, were reviewed by the IMAC and unblinded prior to December 31, 2007. The IMAC confirmed twenty-seven cases as angioedema, judged 19 cases not to be angioedema and determined that there was not sufficient information to diagnose or refute angioedema in 9 cases. The characteristics of the 27 patients with confirmed cases appear in Table 1. The race of the patients who developed angioedema generally mirrored the demographic characteristics of the patients enrolled in clinical trials. A majority were female. Of the 27 cases confirmed as angioedema, 19 were taking vildagliptin. Among vildagliptin users, the most common sites of involvement with angioedema were the face or cheek, the periorbital area, the throat and the tongue. Although urticaria was a pre-specified term, only one patient had urticaria at presentation with angioedema. This subject also had edema of the hands, and was randomized to comparator. In 6 of the 19 patients taking vildagliptin, study drug was stopped; in 3 of these, vildagliptin was restarted without recurrence of angioedema. In 14 out of 19 patients (73 per cent) taking vildagliptin, angioedema was judged to be mild or moderate in severity. Four patients were hospitalized (three taking vildagliptin and 1 taking placebo) for observation. Two patients taking vildagliptin were treated in the emergency department and discharged. The remaining patients were treated as outpatients. No patient was intubated and no patient died.
Table 1.
Characteristics of patients with confirmed angioedema
| Characteristic | Comparators* | Vildagliptin |
|---|---|---|
| Number of patients | 8 | 19 |
| Age (years)† | 58.6 (15.1) | 58.0 (11.2) |
| Race | ||
| Black: White: Asian: Hispanic (N) | 0:5:3:0 | 3:10:4:2 |
| Gender | ||
| Female: Male (N) | 5:3 | 10:9 |
| Study drug exposure prior to angioedema (days)† | 243 (262) | 167 (173) |
| Concurrent drug use | ||
| ACE inhibitor (Yes: No) | 1:7 | 14:5 |
| ACE inhibitor exposure prior to angioedema (days)† | 706 (918) | 911 (964) |
| ARB (Yes: No) | 1:7 | 3:16 |
| ARB exposure prior to angioedema (days) | 2758 | 681 (163) |
| Localization of angioedema | ||
| Face/cheek [N (%)] | 1 (13) | 10 (53) |
| Periorbital or palpebral [N (%)] | 5 (63) | 8 (42) |
| Throat/pharynx [N (%)] | 1 (13) | 7 (37) |
| Lip[N(%)] | 1 (13) | 6 (32) |
| Tongue [N (%)] | 1 (13) | 1 (5) |
| Hands [N (%)] | 2 (25) | 1 (5) |
| Abdomen [N (%)] | 0 (0) | 0 (0) |
| Severity of angioedema | ||
| Mild (N) | 4 (50) | 10 (53) |
| Moderate (N) | 3 (37) | 4 (21) |
| Severe (N) | 1 (13) | 5 (26) |
| Treatment | ||
| Discontinuation study drug (Yes: Interrupt: No) | 2:1:5 | 3:3:13 |
| Discontinuation ACE inhibitor (Yes: No) | 1:0 | 6:7‡ |
| Discontinuation ARB (Yes: No) | 0:1 | 1:2 |
| Corticosteroids (Yes: No) | 1:7 | 7:12 |
| Anti-histamines (Yes: No) | 4:4 | 12:7 |
| Hospitalization (Yes: No) | 1:7 | 3:16 |
| Intubation (Yes: No) | 0:8 | 0:19 |
Comparators included placebo, metformin, pioglitazone, rosiglitazone, glimepiride, and acarbose.
mean (standard deviation)
Information regarding discontinuation of ACE inhibitor use was not available for one patient ACE indicates angiotensin-converting enzyme, ARB indicates angiotensin receptor blocker
Fourteen of the 19 patients who developed angioedema while taking vildagliptin were taking an ACE inhibitor (1 captopril, 4 enalapril, 7 lisinopril and 2 ramipril) concurrently. All of these had been taking the ACE inhibitor prior to starting vildagliptin; eleven had taken an ACE inhibitor for longer than one year prior to starting vildagliptin. In 6 of the 14 patients who developed angioedema while taking vildagliptin and an ACE inhibitor, the ACE inhibitor was discontinued and not restarted. Only one of the 8 patients who developed angioedema while taking a comparator was taking an ACE inhibitor.
Risk of Angioedema in Premarketing Studies of Vildagliptin
Tables 2 and 3 provide rates of angioedema among patients treated with comparators (including placebo and active antidiabetic agents) or vildagliptin. Active antidiabetic agents included metformin, pioglitazone, rosiglitazone, glimepiride, and acarbose. In the overall population, there was no statistically significant association between vildagliptin use and angioedema. The overall risk of confirmed angioedema was 0.22% and 0.15% for vildagliptin-and all comparator-treated patients, respectively (Table 2). Among individuals taking an ACE inhibitor, however, vildagliptin use was associated with an increased rate of angioedema when compared to all comparator use: 0.51% compared with 0.05%, odds ratio 9.29 (95% CI 1.22-70.70) in the pooled analysis. The risk of ACE inhibitor-associated angioedema was significantly increased among patients taking vildagliptin 100mg per day, either in divided doses or as a single dose, but not in those taking vildagliptin 50mg per day. This was true whether rates and odds ratios were computed only for confirmed cases (Table 2) or using a conservative analysis which included confirmed cases and possible cases for which there were not sufficient data to confirm (Table 3). In contrast, there was no association between vildagliptin use and angioedema among individuals taking an ARB.
Table 2.
Rates and odds ratio of angioedema (confirmed cases)
| Treatment group | Events (n) | Exposed (n) | Rate (%) | Odds ratio (95% CI) Versus comparators | P value |
|---|---|---|---|---|---|
| All | |||||
| Comparator | 8 | 5368 | 0.15 | ||
| Vildagliptin 50mg qd | 1 | 1239 | 0.08 | 0.54 (0.07-4.33) | NS |
| Vildagliptin 50mg bid | 12 | 4764 | 0.25 | 1.69 (0.69-4.14) | NS |
| Vildagliptin 100mg qd | 6 | 2550 | 0.24 | 1.58 (0.55-4.56) | NS |
| All Vildagliptin | 19 | 8553 | 0.22 | 1.49 (0.65-3.41) | NS |
| ACE inhibitor-treated | |||||
| Comparator | 1 | 1819 | 0.05 | ||
| Vildagliptin 50mg qd | 1 | 351 | 0.28 | 5.19 (0.32-83.24) | NS |
| Vildagliptin 50mg bid | 8 | 1540 | 0.52 | 9.49 (1.19-75.93) | P<O.05 |
| Vildagliptin 100mg qd | 5 | 863 | 0.58 | 10.59 (1.24-90.81) | P<O.05 |
| All Vildagliptin | 14 | 2754 | 0.51 | 9.29 (1.22-70.70) | P<O.05 |
| Non ACE inhibitor-treated | |||||
| Comparator | 7 | 3549 | 0.20 | ||
| Vildagliptin 50mg qd | 0 | 888 | 0 | 0 | NS |
| Vildagliptin 50mg bid | 4 | 3224 | 0.12 | 0.63 (0.18-2.15) | NS |
| Vildagliptin 100mg qd | 1 | 1687 | 0.06 | 0.30 (0.04-2.44) | NS |
| All Vildagliptin | 5 | 5799 | 0.09 | 0.44 (0.14-1.38) | NS |
| ARB-treated | |||||
| Comparator | 2 | 886 | 0.23 | ||
| Vildagliptin 50mg qd | 0 | 162 | 0 | 0.00 (n.a.) | NS |
| Vildagliptin 50mg bid | 3 | 701 | 0.43 | 1.90 (0.32-11.40) | NS |
| Vildagliptin 100mg qd | 0 | 473 | 0 | 0 (n.a.) | NS |
| All Vildagliptin | 3 | 1336 | 0.22 | 0.99 (0.17-5.97) | NS |
| Non ARB-treated | |||||
| Comparator | 6 | 4482 | 0.13 | ||
| Vildagliptin 50mg qd | 1 | 1077 | 0.09 | 0.69 (0.08-5.76) | NS |
| Vildagliptin 50mg bid | 9 | 4063 | 0.22 | 1.66 (0.59-4.66) | NS |
| Vildagliptin 100mg qd | 6 | 2077 | 0.29 | 2.16 (0.70-6.71) | NS |
| All Vildagliptin | 16 | 7217 | 0.22 | 1.66 (0.65-4.24) | NS |
*Comparators included placebo, metformin, pioglitazone, rosiglitazone, glimepiride, and acarbose. ACE indicates angiotensin-converting enzyme, ARB indicates angiotensin receptor blocker
Table 3.
Rates and odds ratio of angioedema for both confirmed cases and cases with insufficient data to adjudicate
| Treatment group | Events (n) | Exposed (n) | Rate (%) | Odds ratio (95% CI) Versus comparators | P value |
|---|---|---|---|---|---|
| All | |||||
| Comparator | 11 | 5368 | 0.20 | ||
| Vildagliptin 50mg qd | 2 | 1239 | 0.16 | 0.79 (0.17-3.56) | NS |
| Vildagliptin 50mg bid | 16 | 4764 | 0.34 | 1.64 (0.76-3.54) | NS |
| Vildagliptin 100mg qd | 7 | 2550 | 0.27 | 1.34 (0.52-3.46) | NS |
| All Vildagliptin | 25 | 8553 | 0.29 | 1.43 (0.70-2.90) | NS |
| ACE inhibitor-treated | |||||
| Comparator | 1 | 1819 | 0.05 | ||
| Vildagliptin 50mg qd | 1 | 351 | 0.28 | 5.19 (0.32-83.24) | NS |
| Vildagliptin 50mg bid | 10 | 1540 | 0.65 | 11.88 (1.52-92.93) | P<O.05 |
| Vildagliptin 100mg qd | 5 | 863 | 0.58 | 10.59 (1.24-90.81) | P<O.05 |
| All Vildagliptin | 16 | 2754 | 0.58 | 10.62 (1.41-80.18) | P<O.05 |
| Non ACE inhibitor-trated | |||||
| Comparator | 10 | 3549 | 0.28 | ||
| Vildagliptin 50mg qd | 1 | 888 | 0.11 | 0.40 (0.05-3.12) | NS |
| Vildagliptin 50mg bid | 6 | 3224 | 0.19 | 0.66 (0.24-1.82) | NS |
| Vildagliptin 100mg qd | 2 | 1687 | 0.12 | 0.42 (0.09-1.92) | NS |
| All Vildagliptin | 9 | 5799 | 0.16 | 0.55 (0.22-1.36) | NS |
| ARB-treated | |||||
| Comparator | 3 | 886 | 0.34 | ||
| Vildagliptin 50mg qd | 1 | 162 | 0.62 | 1.83 (0.19-17.68) | NS |
| Vildagliptin 50mg bid | 3 | 701 | 0.43 | 1.27 (0.25-6.29) | NS |
| Vildagliptin 100mg qd | 0 | 473 | 0 | 0 (n.a.) | NS |
| All Vildagliptin | 4 | 1336 | 0.30 | 0.88 (0.20-3.96) | NS |
| Non ARB-treated | |||||
| Comparator | 8 | 4482 | 0.18 | ||
| Vildagliptin 50mg qd | 1 | 1077 | 0.09 | 0.52 (0.06-4.16) | NS |
| Vildagliptin 50mg bid | 13 | 4063 | 0.32 | 1.80 (0.74-4.34) | NS |
| Vildagliptin 100mg qd | 7 | 2077 | 0.34 | 1.89 (0.68-5.22) | NS |
| All Vildagliptin | 21 | 7217 | 0.29 | 1.63 (0.72-3.69) | NS |
*All comparators included placebo, metformin, pioglitazone, rosiglitazone, glimepiride, and acarbose. ACE indicates angiotensin-converting enzyme, ARB indicates angiotensin receptor blocker
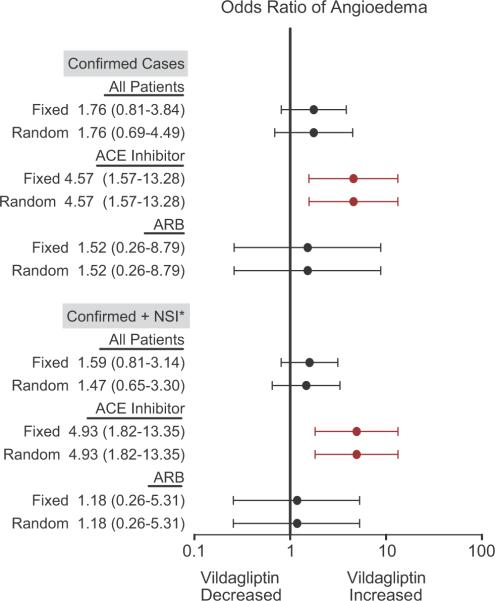
Figure 2 shows the results of the meta-analysis completed using the Peto method. As in the pooled analysis, there was no significant association between vildagliptin and angioedema overall. In ACE inhibitor users, vildagliptin use was associated with a significantly increased risk of angioedema when only confirmed cases were included [OR 4.57 (95% CI 1.57-13.28)] and when both confirmed cases and cases with insufficient information for adjudication were included [OR 4.93 (1.82-13.35)], although the odds ratios were smaller than those calculated in the pooled analysis. Again, there was no association between vildagliptin use and angioedema in ARB users (not shown).
Figure 2.
Meta-analysis showing the effect of vildagliptin on the risk of angioedema, in all patients, those taking an angiotensin-converting enzyme (ACE) inhibitor concurrently, and those taking an angiotensin-receptor blocker (ARB) concurrently. Data are presented as odds ratios and 95% confidence intervals. Odds ratios were estimated using the Peto method, in which all studies with a comparator treatment arm and an event in at least one arm were included. This analysis was conducted using both fixed effect (Fixed) and random effect (Random) models. * NSI indicates not sufficient information.
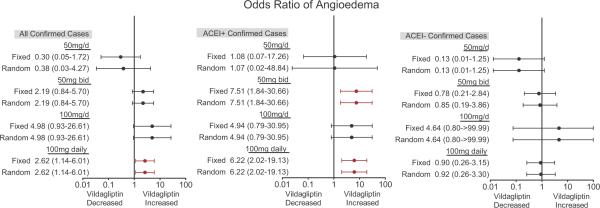
Figure 3 illustrates the relationship between vildagliptin dose and risk of a confirmed case of angioedema in all patients, ACE inhibitor users, and those not taking an ACE inhibitor, determined by meta-analysis using the Peto method. There was an increased risk of angioedema in all patients receiving vildagliptin 100mg daily, either as a divided dose or a single dose but not in those receiving 50mg/d. When data analysis was stratified according to ACE inhibitor use, however, this was attributable to an increased risk of angioedema in ACE inhibitor users taking vildagliptin 100mg daily. There was no increased risk of angioedema in vildagliptin-treated patients not taking an ACE inhibitor concurrently, regardless of vildagliptin dose. The results were similar when both confirmed and cases for which there was not sufficient information were included in the analysis.
Figure 3.
Meta-analysis showing the effect of vildagliptin dose on the risk of confirmed cases of angioedema in all patients, in patients taking an angiotensin-converting enzyme inhibitor concurrently (ACE+), and those not taking an ACE inhibitor (ACE-). Data are presented as odds ratios and 95% confidence intervals. Odds ratios were estimated using the Peto method, in which all studies with a comparator treatment arm and an event in at least one arm were included. This analysis was conducted using both fixed effect (Fixed) and random effect (Random) models. The 100mg daily group includes patients receiving vildagliptin 50mg bid and 100mg/d.
Since this analysis, an additional 5 cases were reviewed by the IMAC; three were judged to be angioedema, one was judged to have insufficient data to adjudicate, and one was judged to have peripheral edema and not angioedema. Among the 3 confirmed cases, two of the patients were taking an ACE inhibitor. One patient, who had been taking an ACE inhibitor for 717 days and study drug for 680 days, had stridor and tongue and throat swelling, and died prior to hospitalization. That case was unblinded and the patient was taking the sulfonylurea gliclazide as the study drug. The remaining 2 confirmed cases have not been unblinded. Because the studies from which these cases were ascertained have not yet been unblinded, these cases were not included in the calculation of odds ratios and confidence intervals.
Angioedema in Postmarketing Surveillance of Sitagliptin and Literature Review
Three hundred ninety-five reports to the FDA AERS involving sitagliptin were recorded from 11/02/2006 through 6/29/2007, representing 271 individual cases. In fourteen cases, a diagnosis of angioedema or angioedema-related symptoms, such as tongue swelling, was made. Five of the 14 cases were hospitalized, two were admitted to an intensive care unit and one was intubated. There were two additional cases of Stevens-Johnson syndrome or skin exfoliation. Information regarding concurrent drug use was not available for most cases.
Excluding review articles, we retrieved 116 English-language articles for sitagliptin, 8 for saxagliptin, and 13 for alogliptin. In a 24-week study in which type 2 diabetics treated with pioglitazone were randomized to placebo (N=178) or sitagliptin (N=175), a sitagliptin-treated patient was hospitalized for angioedema. The subject also had urticaria.18 The study investigator graded the adverse event as moderate and possibly related to study drug. Information regarding concurrent medications was not provided.
Discussion
We report the incidence of angioedema in clinical trials of the DPP-IV inhibitor vildagliptin. Overall there was no association between vildagliptin use and angioedema in the pooled analysis. On the other hand, vildagliptin use was associated with a 9-fold increased risk of angioedema in individuals using an ACE inhibitor. This was attributable to an increased risk of angioedema in individuals receiving 100mg per day of vildagliptin, either in a single dose or as divided doses. When meta-analysis was performed using the Peto method, there was an increased risk of angioedema in patients receiving 100mg vildagliptin daily, which was attributable to a 4- to 5- fold increased risk of angioedema in ACE inhibitor users.
Angioedema has also been reported in postmarketing surveillance of sitagliptin, although information regarding concurrent drug use was incomplete and it is not possible to derive incidence data from AERS. Williams-Herman et al. recently reported no association of sitagliptin 100mg/d with “angioedema-like events,” regardless of concurrent ACE inhibitor exposure, in Phase IIB and Phase III trials.19 Unfortunately, events were not adjudicated and the authors included hypersensitivity reactions and urticaria among “angioedema-like events.” The inclusion of allergic events confounds the interpretation of rates of true angioedema and, indeed, the incidence of “angioedema-like events” in Phase IIB and Phase III trials of sitagliptin was approximately 10-fold higher than incidence rates of angioedema reported in epidemiological studies of ACE inhibitors.20, 21 For this reason, it is not possible to determine whether the effect of vildagliptin on the risk of ACE inhibitor-associated angioedema described in the present article represents a class effect.
The finding that pharmacological DPP-IV inhibition increased the risk of angioedema in patients taking an ACE inhibitor, however, corroborates earlier observations that DPP-IV activity is decreased in the sera of some patients during ACE inhibitor-associated angioedema.11, 12 An interactive effect of DPP-IV inhibition and ACE inhibition on risk of angioedema also provides insight into potential mechanism(s) of ACE inhibitor-associated angioedema. Like hereditary angioedema, ACE inhibitor-associated angioedema may remit and recur spontaneously, and it may occur suddenly after prolonged ACE inhibitor exposure.22 In the case of hereditary angioedema, increased production of kinins precipitates symptomatic episodes.23 In the cases of ACE inhibitor-associated angioedema, decreased degradation of kinins may contribute. Bradykinin causes vasodilation and increases vascular permeability via its B2 receptor.24 Bradykinin also stimulates the release of substance P, which increases vascular permeability by acting at the neurokinin 1 (NK1) receptor.25 In patients taking ACE inhibitors, decreased degradation of bradykinin and/or substance P could precipitate angioedema if the inactivation of these vasoactive peptides via other non-ACE pathways is compromised.
During ACE inhibition, DPP-IV inactivates substance P.26 Studies in rodent models suggest that substance P contributes to the pathogenesis of ACE inhibitor-associated angioedema. For example, infusion of bradykinin or substance P causes tracheal edema in rats.27 Likewise, either bradykinin receptor antagonism or substance P (NK1) receptor antagonism decreases plasma extravasation in tracheal and other tissues of ACE inhibitor-treated mice.10 Rats genetically deficient in DPP-IV develop peritracheal edema when treated with an ACE inhibitor, and the formation of edema is decreased by administration of an NK1 receptor antagonist.28 Together with data from the present study, these observations suggest that genetic deficiency or pharmacologic inhibition of DPP-IV predisposes to ACE inhibitor-associated angioedema by decreasing the degradation of substance P.
The risk of angioedema among patients treated simultaneously with the vildagliptin and ACE inhibitors was similar to the risk of ACE inhibitor-associated angioedema of 0.1% - 0.7% derived from postmarketing surveillance or epidemiologic studies, and lower than the risk of 2.8 - 6% reported in some clinical trials.21, 29-31 This is consistent with previous reports that diabetes is associated with a reduced risk of ACE inhibitor-associated angioedema.12, 21, 32 In addition, ACE inhibitor use was not randomized and typically preceded study enrollment by several years. Because rates of angioedema decrease progressively with time,20, 21 there may have been selection bias for individuals less likely to develop angioedema among patients taking ACE inhibitors prior to study enrollment. This may also explain the low incidence of angioedema in ACE inhibitor-treated patients randomized to comparator.
In most vildagliptin-treated patients, angioedema was judged to be mild or moderate in severity. The relatively modest effect of DPP-IV inhibition on the risk of ACE inhibitor-associated angioedema may reflect the involvement of redundant enzymatic pathways in the degradation of bradykinin and substance P (Figure 1). During ACE inhibition, bradykinin is inactivated primarily by aminopeptidase P (APP, EC 3.4.11.9). Likewise, neutral endopeptidase (NEP-24.11, EC 3.4.24.11) contributes to the metabolism of both bradykinin and substance P. Normally, NEP does not contribute substantially to the inactivation of vasoactive substance P;9, 33 however, diminished degradation of bradykinin or substance P may contribute to the high incidence of angioedema observed when both ACE and NEP are pharmacologically inhibited.31
Even though combined treatment with a DPP-IV inhibitor and ACE inhibitor did not cause life-threatening angioedema in clinical studies of vildagliptin, it is important for clinicians to understand and recognize the potential interactive effect of these two drug classes on the risk of angioedema. Although ACE inhibitors decrease the progression of nephropathy, reduce the risk of myocardial infarction and decrease mortality in diabetic patients,34-36 the death of a comparator-treated patient from ACE inhibitor-associated angioedema highlights the potentially critical nature of this adverse event. A physician confronted with a diabetic patient who presents with angioedema while taking both an ACE inhibitor and a DPP-IV inhibitor needs to consider all possible causes of this adverse event including confounding disease or exposures, the ACE inhibitor, and concurrent treatment with a DPP-IV inhibitor.
Several limitations warrant highlighting. The angioedema event rate was small, in particular in the comparator group on ACE inhibitor. Because most patients had been treated with an ACE inhibitor chronically prior to randomization, there may have been selection for patients at low risk for angioedema. ACE inhibitor use was not randomized, so that it is not possible to compare rates of angioedema in ACE inhibitor users versus non-users within either the vildagliptin group or the comparator group. Angioedema was not a primary endpoint of the Phase III studies and the 24-week to 52-week duration of the studies limits the ability to assess effects of long-term exposure. The study does not address whether the effect of vildagliptin use on the risk of ACE inhibitor-associated angioedema is a class effect.
In summary, the DPP-IV inhibitors increase circulating concentrations of GLP-1 and GIP and decrease glucose concentrations in type 2 diabetes.2-5 In addition to degrading the incretins, DPP-IV degrades vasoactive peptides that have been implicated in rodent models of ACE inhibitor-associated angioedema. We report for the first time that DPP-IV inhibitor use may be associated with an increased risk of angioedema in diabetic patients who take an ACE inhibitor concurrently. This observation provides insight into the mechanism of ACE inhibitor-associated angioedema. Additional studies are needed to replicate these findings. Nevertheless, physicians caring for diabetic patients should be aware of this potential interaction.
Perspectives
Diabetes mellitus affects some 195 million people worldwide. DPP-IV inhibitors are effective new agents in the treatment of type 2 diabetes. Thirty-five to 40 million people take ACE inhibitors worldwide37 and ACE inhibitors are widely prescribed to diabetic patients for renal and cardiac protection. Concurrent DPP-IV inhibitor and ACE inhibitor use may become common, making it important to understand potential drug-drug interactions.
Acknowledgement
The authors wish to thank Kelly Yacuk for her excellent administrative assistance and Dr. Wolfgang Kothny for his review of the manuscript.
Sources of Funding This work was funded by Novartis Pharmaceuticals and Novartis Pharma AG. Dr. Brown also received support from National Institutes of Health grant R01 HL079184.
Footnotes
Conflict of Interest Statement Drs. Stuart Byiers, Mario Maldonado, Barbara Ann Warner are employed by Novartis Pharma or Novartis Pharmaceuticals. David Carr is employed by SDE Services and works at Novartis Pharma. Dr. Brown has received consulting fees from Novartis Pharmaceuticals, and consults for Merck, manufacturer of sitagliptin. Dr. Brown holds a patent for a method of identifying susceptibility to ACE inhibitor-associated angioedema.
References
- (1).Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- (2).Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–4894. doi: 10.1210/jc.2004-2460. [DOI] [PubMed] [Google Scholar]
- (3).Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–428. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- (4).Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- (5).Herman GA, Bergman A, Stevens C, Kotey P, Yi B, Zhao P, Dietrich B, Golor G, Schrodter A, Keymeulen B, Lasseter KC, Kipnes MS, Snyder K, Hilliard D, Tanen M, Cilissen C, De Smet M, de L I, Van Dyck K, Wang AQ, Zeng W, Davies MJ, Tanaka W, Holst JJ, Deacon CF, Gottesdiener KM, Wagner JA. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–4619. doi: 10.1210/jc.2006-1009. [DOI] [PubMed] [Google Scholar]
- (6).Ahren B, Pacini G, Foley JE, Schweizer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005;28:1936–1940. doi: 10.2337/diacare.28.8.1936. [DOI] [PubMed] [Google Scholar]
- (7).Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- (8).Lambeir AM, Durinx C, Scharpe S, De M I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- (9).Russell JS, Chi H, Lantry LE, Stephens RE, Ward PE. Substance P and neurokinin A metabolism by cultured human skeletal muscle myocytes and fibroblasts. Peptides. 1996;17:1397–1403. doi: 10.1016/s0196-9781(96)00201-x. [DOI] [PubMed] [Google Scholar]
- (10).Emanueli C, Grady EF, Madeddu P, Figini M, Bunnett NW, Parisi D, Regoli, Geppetti P. Acute ACE inhibition causes plasma extravasation in mice that is mediated by bradykinin and substance P. Hypertension. 1998;31:1299–1304. doi: 10.1161/01.hyp.31.6.1299. [DOI] [PubMed] [Google Scholar]
- (11).Lefebvre J, Murphey LJ, Hartert TV, Jiao SR, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension. 2002;39:460–464. doi: 10.1161/hy0202.103054. [DOI] [PubMed] [Google Scholar]
- (12).Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, Adam A, Brown NJ. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).2007. http://www fda gov/cder/previous_news2006 htm.
- (14).Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- (15).JANUVIA™ . (sitagliptin) Prescribing Information. MERCK and CO., Inc.; Whitehouse Station, New Jersey: 2007. [Google Scholar]
- (16).Neutel CI. The potential for Simpson's paradox in drug utilization studies. Ann Epidemiol. 1997;7:517–521. doi: 10.1016/s1047-2797(97)00084-7. [DOI] [PubMed] [Google Scholar]
- (17).Bradburn MJ, Deeks JJ, Berlin JA, Russell LA. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- (18).Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- (19).Williams-Herman D, Round E, Swern AS, Musser B, Davies MJ, Stein PP, Kaufman KD, Amatruda JM. Safety and tolerability of sitagliptin in patients with type 2 diabetes: A pooled analysis. BMC Endocr Disord. 2008;8:14. doi: 10.1186/1472-6823-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Brown NJ, Ray WA, Snowden M, Griffin MR. Black americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60:8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- (21).Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51:1624–1630. doi: 10.1161/HYPERTENSIONAHA.108.110270. [DOI] [PubMed] [Google Scholar]
- (22).Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26:725–737. doi: 10.1016/j.iac.2006.08.001. [DOI] [PubMed] [Google Scholar]
- (23).Schapira M, Silver LD, Scott CF, Schmaier AH, Prograis LJ, Curd JG, Colman RW. Prekallikrein activation and high-molecular-weight kininogen consumption in hereditary angioedema. N Engl J Med. 1983;308:1050–1054. doi: 10.1056/NEJM198305053081802. [DOI] [PubMed] [Google Scholar]
- (24).Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- (25).Campos MM, Calixto JB. Neurokinin mediation of edema and inflammation. Neuropeptides. 2000;34:314–322. doi: 10.1054/npep.2000.0823. [DOI] [PubMed] [Google Scholar]
- (26).Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidas IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther. 1992;260:1257–1261. [PubMed] [Google Scholar]
- (27).Sulpizio AC, Pullen MA, Edwards RM, Brooks DP. The effect of acute angiotensin-converting enzyme and neutral endopeptidase 24.11 inhibition on plasma extravasation in the rat. J Pharmacol Exp Ther. 2004;309:1141–1147. doi: 10.1124/jpet.103.064105. [DOI] [PubMed] [Google Scholar]
- (28).Byrd JB, Shreevatsa A, Putlur P, Foretia D, McAlexander L, Sinha T, Does MD, Brown N. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol. 2007;120:403–408. doi: 10.1016/j.jaci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- (29).Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, Ewan PW. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- (30).Sica DA. The African American Study of Kidney Disease and Hypertension (AASK) trial: what more have we learned? J Clin Hypertens (Greenwich) 2003;5:159–167. doi: 10.1111/j.1524-6175.2003.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- (32).Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, Levy E. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- (33).Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat Med. 1997;3:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- (34).Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- (35).Heart Outcomes Prevention Evaluation Study Investigators Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–259. [PubMed] [Google Scholar]
- (36).Tatti P, Pahor M, Byington RP, Di MP, Guarisco R, Strollo G, Strollo F. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. doi: 10.2337/diacare.21.4.597. [DOI] [PubMed] [Google Scholar]
- (37).Messerli FH, Nussberger J. Vasopeptidase inhibition and angio-oedema. Lancet. 2000;356:608–609. doi: 10.1016/S0140-6736(00)02596-4. [DOI] [PubMed] [Google Scholar]