Abstract
The best studied mechanisms of B cell tolerance are receptor editing, clonal deletion and anergy. All of these mechanisms of B cell tolerance depend on the induction of signaling downstream of the B cell receptor by self-antigens. However another important and distinct mechanism of B cell tolerance involves the repression of antigen receptor signaling rather than its induction, utilizes the Lyn Src-family kinase, the SHP-1 tyrosine phosphatase, inhibitory members of the Siglec family and a carbohydrate modifying enzyme that is capable of negatively regulating B cell receptor activation known as sialic acid acetylesterase.
One major reason for attempting to view autoimmune diseases through the prism of enzymes that regulate immunological tolerance is that enzymes are particularly suitable targets for therapy. Verdine and colleagues divide all therapeutics into two categories – protein therapeutics and small molecules (1). Protein therapeutics include antibodies, cytokines, and receptor-Ig fusion proteins and these target only extracellular molecules such as growth factor receptors or integrins. Small molecules, on the other hand, can only target a subset of proteins – proteins that posses a surface hydrophobic pocket (1). Targets containing such surface hydrophobic pockets are estimated to constitute only 10–15% of all known proteins, and a large fraction of these are enzymes. Some of the most important drug targets in diseases of immunological interest are enzymes. In this review we will examine the role of a hitherto poorly studied enzyme and the pathway that it regulates to mediate a distinct mechanism of B cell tolerance.
We will initially consider the most widely studied mechanisms of B cell tolerance, which may be categorized as tolerance mechanisms that depend on the induction of B cell receptor (BCR) signaling by self-antigen. The rest of the review will focus on a distinct mechanism of B cell tolerance that involves the repression of BCR signaling, and depends on the activity of sialic acid acetylesterase (SIAE), a negative regulator of B cell signaling, functioning in a pathway that requires the synergistic function of the Lyn tyrosine kinase and the SHP-1 tyrosine phosphatase (2). The function of SIAE is opposed by a yet to be identified sialic acid 9-O-acetyltransferase. This mechanism of peripheral B cell tolerance is one that is not addressed by conventional antigen receptor transgenic and knockin approaches to the study of tolerance, which typically examine tolerance to high affinity model self-antigens. Unlike all the other “conventional” mechanisms of tolerance, this SIAE-linked process depends not on BCR signaling itself but on inhibitory receptors that attenuate BCR signaling. SIAE may also be particularly relevant from a human autoimmunity standpoint.
BCR signal strength and positive and negative selection events during B cell ontogeny
The pre-BCR selects developing B lineage cells that have made in-frame rearrangements of their Ig heavy chain genes and helps mediate allelic exclusion at the Ig heavy chain locus in these selected cells. Pre-BCR selected cells then go on to rearrange their Ig κ light chain genes. Subsequently, many different cell-fate decisions are mediated by BCR signaling. BCR signaling may be antigen-independent, and these signals from the receptor in the absence of a ligand are often referred to as tonic signals. It may also be initiated by self-antigen of relatively high affinity that can contribute to the induction of self-tolerance mechanisms in immature B cells. Activation of B cells by self-antigen, presumably of lower affinity than that of tolerogenic self-antigens, (or possibly by self-antigen that is not available to immature B cells but can be accessed by mature B cells), can influence lineage commitment events in developing B cells, in a manner akin to positive selection in T cells.
An immature B cell that has productively rearranged its κ light chain locus initially generates tonic BCR signals if it does not immediately encounter self-antigen. These tonic BCR signals can contribute to the shut-off of Rag gene expression and thus complete the processes of allelic and isotypic exclusion by terminating rearrangement at all Ig loci. Tonic signals may also contribute to the emigration from the bone marrow to the spleen of the majority of newly generated B cells (3–5). While self-tolerance mechanisms in immature B cells depend on self-antigen mediated BCR signaling that is stronger than tonic signaling, as cells mature these central tolerance mechanisms presumably cease to operate. In more mature B cells the tickling of B cells by self-antigen of differing affinities may contribute to lineage commitment events such as the follicular versus marginal zone B lymphoid cell fate decision (6). There is a continuum of BCR signal strength that contributes to both positive and negative selection events during B cell development.
B cell tolerance mechanisms that depend on the induction of BCR signaling
There are three widely studied mechanisms of B cell tolerance: receptor editing, deletion, and anergy. In all of these mechanisms, either enhanced BCR signaling or repetitive BCR signaling by a self-antigen drives the tolerance induction process. Receptor editing and deletion represent central tolerance mechanisms whereas anergy is initiated in a central or peripheral lymphoid organ but is completed in the periphery. Receptor editing involves the reactivation or continued expression of Rag genes when an autoreactive B cell encounters a high affinity, likely multivalent, self-antigen, which results in continued Rag gene expression that induces further light chain gene rearrangement and the deletion of the previously auto-reactive immunoglobulin light chain gene (7, 8). The “new” light chain in an edited B cell may be either a κ light chain or a λ light chain. All λ light chain expressing B cells are believed to represent cells that have gone through the process of receptor editing.
An alternative mechanism of central tolerance is the deletion of self-reactive B cells, and this may occur in immature B cells in the bone marrow or in transitional B cells in the spleen (9–11). Another mechanism of tolerance revealed from studies involving BCR transgenic mice is referred to as anergy (12). Anergic self-reactive B cells are particularly dependent on BAFF (B cell activating factor of the tumor necrosis factor family) for survival in follicles and have a shortened lifespan (13, 14).
The earliest mouse models involving B cell receptor transgenes typically involved the insertion of DNA concatemers into random euchromatin sites which favored expression. Transgenes encoded relatively high affinity B cell receptors for selected model antigens. When such mice were crossed into the appropriate membrane bound antigen transgenic mouse (in which the model self antigen was functionally multivalent), even if editing occurred, as we can now infer it did, the editing process did not have the ability to recombinationally delete the transgenic self-reactive light chain that was integrated at some site other than the κ light chain locus itself (9, 10). Although the earliest transgenic models described deletion-dependent tolerance, deletion perhaps occurred because the editing process in this model system could not eliminate the self-reactive (transgenic) light chain. Once BCR knockin models became available, and the introduced rearranged self-reactive light chain was integrated in the endogenous κ locus, it became apparent that relatively high affinity multivalent self antigens preferentially drive receptor editing, while soluble high affinity antigens appear to be capable of both inducing receptor editing and anergy (15–17). It is possible that the recognition of some self-antigens is largely dependent on the Ig heavy chain, and in these cases deletion may be crucial for tolerance. Most data suggests however that receptor editing is probably the major mechanism of central B cell tolerance.
The majority of developing human B cells are self-reactive (18) and defects in receptor editing have been in described in patients with systemic lupus erythematosus and type I diabetes (19). Mice carrying allelic variants of the SLAM family Sle1 gene are more susceptible to lupus and it is presumed that these mutant mice have a poorly explained defect in BCR signaling that leads to defective receptor edition and/or deletion (20).
Peripheral B cell tolerance mediated by the repression of BCR signaling by the SIAE-Siglec-SHP-1 pathway
Siglecs are sialic acid binding Ig domain containing lectins (reviewed in 21). A prominent and well-studied Siglec in B cells is CD22 or Siglec 2. CD22 binds to N -glycans that contain sialic acid in α2,6 linkage to an N-acetyllactosamine moiety, and is rapidly phosphorylated on cytoplasmic inhibitory tyrosines by Lyn, a Src family tyrosine kinase, and this is followed by the recruitment of SHP-1, an SH2 domain containing tyrosine phosphatase to immunoreceptor tyrosine-based inhibitory motifs (ITIM) on the cytoplasmic tail of CD22 (22–26). Although initial studies on B cell lines and tonsillar B cells demonstrated that in activated B cells CD22 associates with membrane immunoglobulins including both IgM and IgG depending on the B cell involved (27), the “physiologically relevant” ligand for CD22 has variously been reported to be CD45, or CD22 itself and this issue has remained somewhat controversial and will be addressed further below (28, 29).
SIAE is an enzyme that removes O-acetyl moieties from 9-O-acetylated sialic acid (30, 31). We have demonstrated recently that SIAE is a protein that can localize to the cell surface or be secreted following overexpression in vitro, but nonetheless functions in a B cell intrinsic manner in vivo to permit the inhibition of BCR signaling by CD22 and possibly other Siglecs (2). The B cell intrinsic requirement was ascertained by performing BCR-induced calcium flux studies on purified B cells and by reconstitution studies using Siae mutant hematopoietic stem cells of Rag null recipients in which all the developmental phenotypic changes seen in Siae mutant mice are preserved.
It had earlier been demonstrated that CD22 binds in vitro only to α2–6 linked sialic acid-containing ligands in which sialic acid is not acetylated at the 9-OH position (32). Murine CD22, in addition, can only bind to α2–6 linked sialic acid containing ligands in which sialic acid, that is not acetylated in the 9-OH position, also contain an N-glycolyl moiety, but not an N-acetyl moiety, at the C5 position (33). This latter modification of sialic acid depends on the cytosolic conversion of CMP-N-acetyl sialic acid to CMP-N-glycolyl sialic by the enzyme CMP Neu5-acetyl hydroxylase (CMAH; 34), an enzyme that is not found in functional form in humans.
In mice harboring a homozygous mutation in Siae, we noted an enhancement of BCR signaling and a predicted reduction in both marginal zone B cells and perisinusoidal bone marrow B cells that are seen in mice lacking negative regulators of the BCR (2, 6, 35, 36). These mutant mice developed high titers of autoantibodies at a relatively young age, and also presented with IgG containing glomerular deposits, typical of an immune-complex disease. We also examined mice that lack Cmah, the hydroxylase required for the cytosolic generation of N-glycolyl sialic acid. These mice lack Neu-5Gc (the N-glycolyl form of sialic acid that is required for binding to murine CD22). Cmah null mice also exhibited enhanced BCR signaling and reductions in marginal zone B cells and bone marrow perisinusoidal B cells (2).
CD22 has been considered an inhibitory enigma (28), and the literature on this particular Siglec is filled with numerous contradictions and unfortunately this review cannot address all the controversies that surround this Siglec. The results from the study of Siae and Cmah mutant mice do not support a model that suggests that α2–6 sialic acid-containing ligands might actually prevent CD22 from inhibiting BCR signaling. A key basis for this latter model, in which CD22 is visualized as requiring its carbohydrate binding activity to prevent it from attenuating BCR signaling, has been the study of double mutant mice lacking both ST6GalI and CD22. ST6GalI null mice lack α2–6 linked sialic acid and exhibit diminished BCR signaling. This was initially not an expected result, since the global loss of α2–6 linked sialic acid might have been expected to result in the attenuation of CD22 function and thus contribute to enhanced BCR signaling. Two groups independently studied cd22−/−/ST6GalI−/− double knockout mice, and reported that BCR signaling was enhanced in a fashion identical to that seen in CD22 null mice (29, 37). This result has been on occasion interpreted as the “restoration” of BCR signaling in double mutant mice (29). In both published reports, the data are impeccable, and the results of the genetic cross are unequivocal: the calcium flux studies demonstrate that the loss of both CD22 and ST6Gal1 results in enhanced BCR signaling in a manner identical to that seen in the cd22−/− mouse. Clearly the cd22 null phenotype is dominant in the double mutant mouse, and the loss of CD22 does not “restore” BCR signaling that is defective in the ST6GalI knockout mouse. A more conventional interpretation of this mouse cross therefore indicates that there is no genetic basis for implying that CD22 can positively regulate BCR signaling.
In contrast to studies in which there is a complete loss of α2–6 linked sialic acid, studies on Siae and Cmah mutant mice each suggest that when a subtle alteration is made to the structure of sialic acid that compromises its ability to bind to CD22, CD22 fails to deliver optimal inhibitory signals and BCR signaling is enhanced. These results lend strong support to the view that in vivo, functional CD22 ligands do contain α2–6 linked sialic acid moieties. These results also indirectly support the original view expressed by Peaker and Neuberger that the BCR may be a physiological ligand for CD22 (36). It may well be that in activated B cells sialic acid moieties linked to the clustered BCR might more readily ligate CD22, bring the latter into the vicinity of active Lyn, and thus contribute to the rapid CD22 ITIM phosphorylation seen after B cell activation. The total absence of α2–6 linked sialic acid in the ST6GalI knockout mouse might therefore represent a relatively drastic alteration that perhaps results in fairly widespread aberrant capping of the N-acetyllactosamine termini of N-glycans; this in turn might contribute in a poorly understood way to the variety of defects seen in ST6GalI knockout mice.
CD22 and SIAE are both expressed at higher levels in mature as compared to immature B cells. CD22 mutant mice in one study exhibited the presence of anti-DNA antibodies when these mice are about 9 months old (38). Lyn mutant mice and mice with a B cell specific deletion of SHP-1 both develop a lupus like syndrome (39, 40). It is worth keeping in mind that the autoantibody phenotype of CD22 null mice is relatively mild, and that these mice do not develop a lupus like syndrome. Since the phenotype of Siae mutant mice appears to be more severe than that seen in CD22 null mice, we have postulated that SIAE may regulate the availability of ligands for CD22 as well as possibly ligands for additional Siglecs in B cells. A potential candidate Siglec that might be regulated by SIAE in B cells is Siglec G or its human counterpart Siglec 10 (41). A recent study suggests that Siglec G and Siglec 10 may inhibit signals initiated by DAMPs (death associated molecular patterns) via CD24 on innate immune cells and B cells (42).
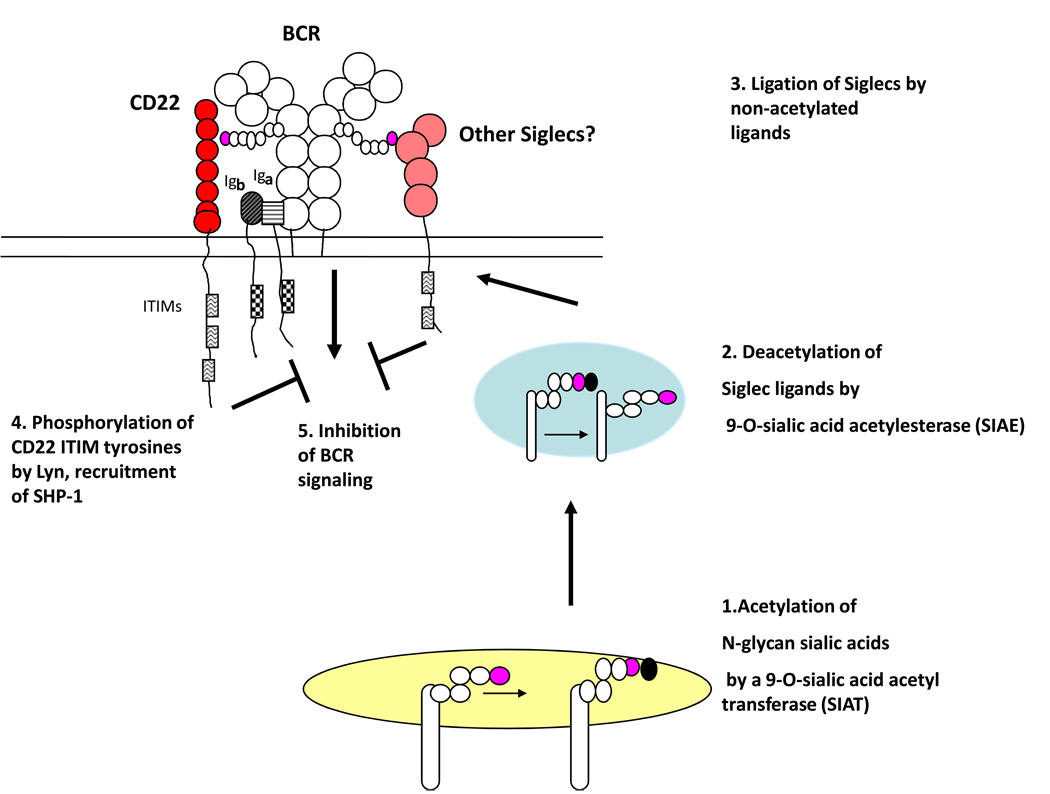
An established biochemical pathway that mediates B cell tolerance involves SIAE, CD22, Lyn, and the phosphatase SHP-1 (2,43–45). It may also involve additional Siglecs (see Figure 1). This pathway dampens BCR signaling, and mutations in it presumably contribute to autoimmunity by resulting in enhanced BCR signal strength. This pathway could be postulated to be biochemically antagonized by SIAT (sialic acid acetyltranferase), the yet to be molecularly characterized enzyme that transfers acetyl moieties on to the 9-OH position of sialic acid (Figure 1).
Figure 1.
The SIAE (sialic acid acetylesterase)-Siglec pathway for inhibitory signaling. [1] The B cell receptor (BCR) and other membrane glycoproteins can have terminal sialic acid decorated N-glycans that are acetylated on the 9-OH position of α2,6 linked sialic acid by SIAT (sialic acid acetyl transferase). [2] These acetyl moieties can be removed (deacetylation) by SIAE either in a vesicle or at the cell surface. [3] Deacetylation of N-glycan sialic acids allows interaction with Siglecs such as CD22 as well as others. This interaction can result in the recruitment of phosphatases such as SHP-1 to Siglec ITIMs (immunoreceptor tyrosine-based inhibitory motifs) [4] and consequently inhibition of BCR signaling [5].
Mutations contributing to autoimmunity in Shp-1 conditional mutant mice, Lyn mutant mice and Siae mutant mice result in enhanced BCR signaling while the reverse would be believed to be true for potential mutations attenuating receptor editing, anergy, or deletion. The processes of receptor editing, deletion, and anergy occur primarily in immature/transitional B cells, whereas the SIAE checkpoint is likely to be involved in peripheral B cell tolerance only. The view that this biochemical pathway may be relevant only in the periphery is supported by a recent study showing that the Lyn-CD22-SHP-1 inhibitory pathway mediates B cell tolerance only in mature B cells (43).
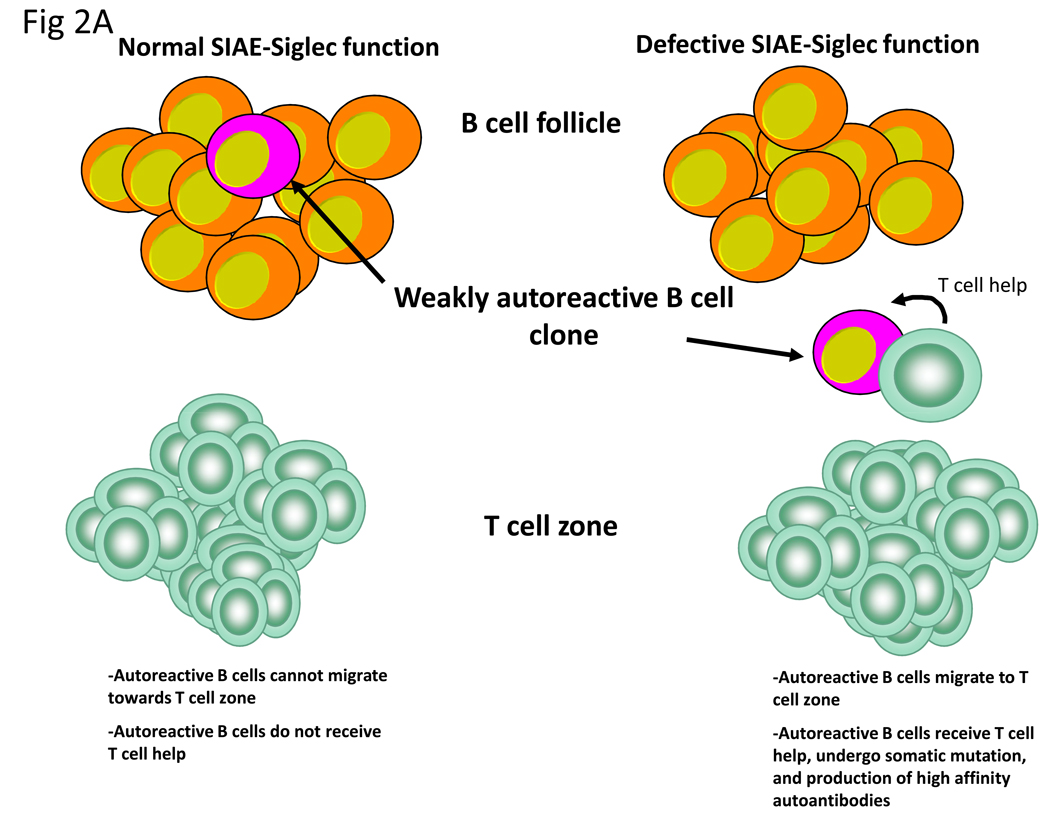
What exactly is the role of SIAE-dependent Siglec inhibitory signaling in a tolerance context? While CD22, SHP-1 and Lyn have been postulated to mediate tolerance by fine tuning BCR signaling (44) and setting a threshold for B cell activation (45), an actual mechanism for tolerance induction by this pathway has not been postulated, and no definitive insights regarding this inhibitory pathway have been revealed from the study of BCR transgenic and BCR knockin mouse models to date. This may well be because the traditionally used BCR transgenes and knockins tend to focus on high affinity BCRs for model self-antigens. We postulate three possible scenarios wherein this biochemical pathway might be critical for peripheral B cell tolerance (Figure 2). Auto-antibodies are almost always class switched IgGs that have undergone somatic mutation. Isotype switching and somatic mutation are generally T-dependent processes although some switching and somatic mutation may also occur in a T-independent manner. Weakly self-reactive B cells may be maintained in a non-activated state by SIAE-Siglec-Lyn-SHP-1signaling and these B cells may thus be unable to effectively internalize cognate self-antigens and/or may be defective in the ability to induce the chemokine receptor CCR7 following encounter with self-antigen. As a result weakly self-reactive B cells may be unable, in the wild type context, to move towards the T cell zone to obtain T cell help. If a component of the SIAE-Siglec-Lyn-SHP-1 pathway is functionally defective, a weakly self-reactive B cell might no longer be constrained and then BCR signaling may be sufficiently strong enough to facilitate CCR7 induction, optimal internalization of self-antigen containing complexes and subsequent migration of the B cell towards the T cell zone (Figure 2A). Either a concurrent break in T cell tolerance or the formation of a transient complex during an infection between a self-antigen and a non-self protein might permit T cell help to be provided to a weakly self-reactive B cell that has a defect in inhibitory signaling. Such a B cell might therefore be at risk for somatic mutation and the consequent generation of high affinity auto-antibodies.
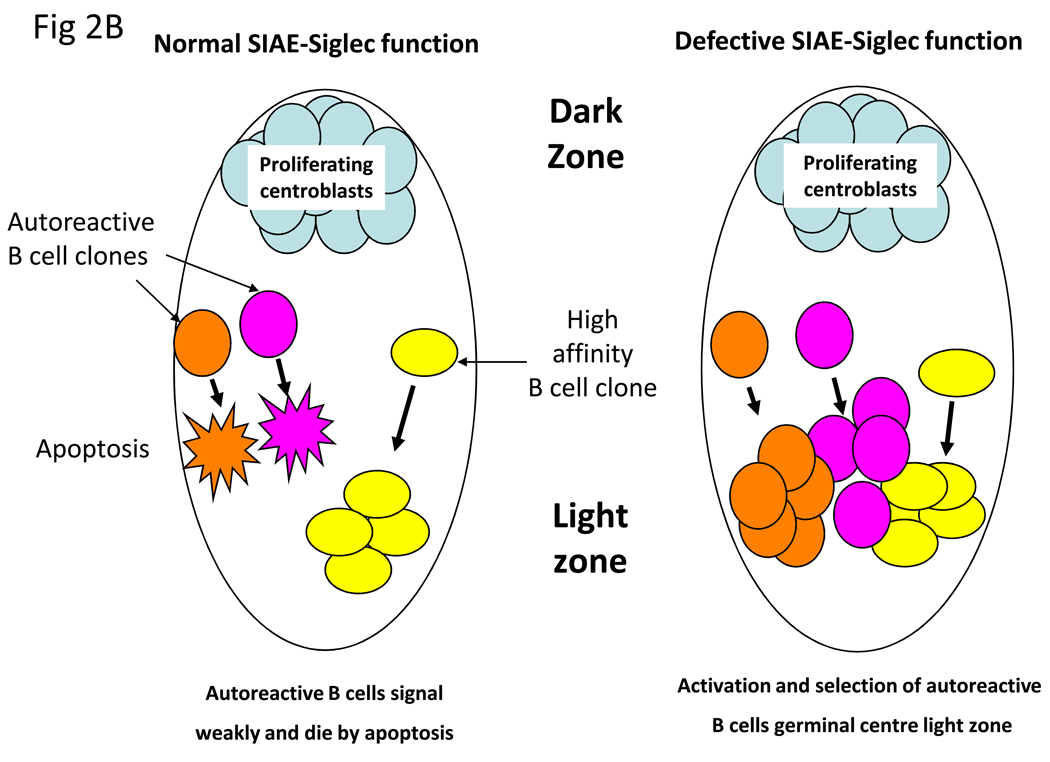
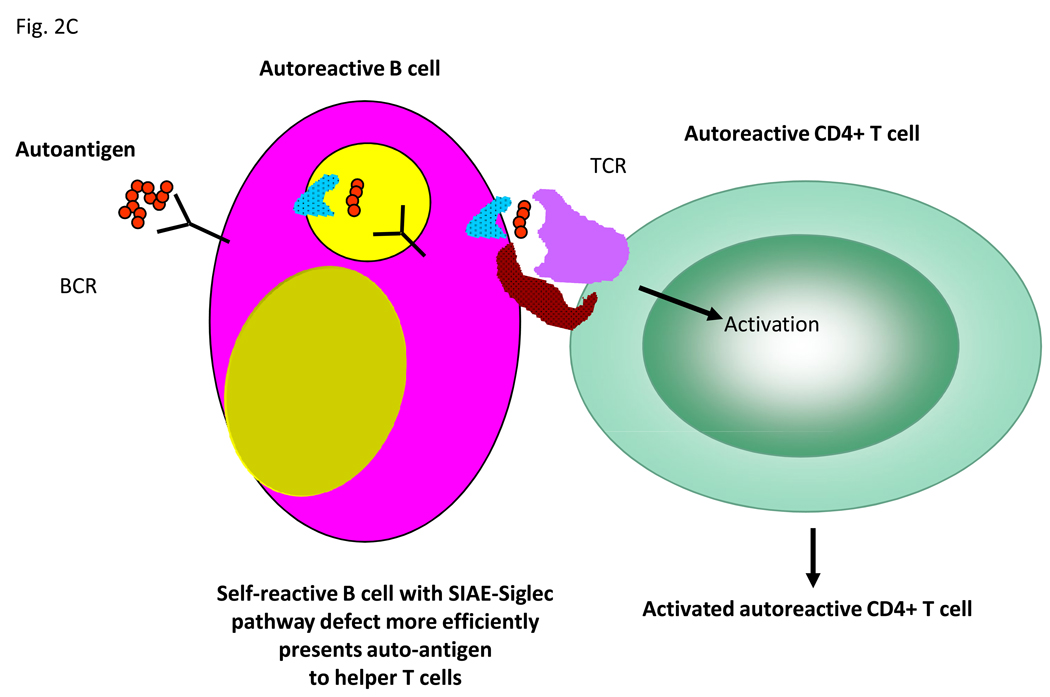
Figure 2.
Possible mechanisms by which the SIAE-Siglec pathway could mediate tolerance at the B cell level. (A) Within the B cell follicles of lymph nodes, inhibitory signals prevent weakly autoreactive B cells from migrating to the T cell zone and receiving T cell help in the form of activating signals. In contrast defective inhibitory signaling via the SIAE-Siglec pathway allows autoreactive B cells to migrate to the T cell zone where they receive T cell help, undergo somatic mutation and secrete high affinity autoantibodies. (B) Within the germinal center, inhibitory signaling prevents autoreactive B cells from being positively selected in the light zone. Attenuation of SIAE-Siglec inhibition increases the probability of selection of low affinity autoreactive B cell clones. (C) Inhibitory signals mediated by the SIAE-Siglec pathway normally prevent B cells from serving as efficient auto-antigen presenting cells however disruption of this pathway allows B cell presentation to autoreactive T cells.
Another possible anatomic site at which the SIAE-Siglec-Lyn-SHP-1 pathway may be crucial for tolerance maintenance is the germinal center itself (Figure 2B). Humans do not express functional CMAH; however in mice Cmah is downregulated in germinal center B cells resulting in B cells that express the GL7 epitope (sialic acid that is exclusively N-acetylated at the C-5 position), but lack physiological ligands for murine CD22 (34). Enhanced BCR signaling is perhaps a prerequisite for proper germinal center formation. It is unclear whether centrocytes in the light zone express functional CD22 ligands. It is conceivable that inhibitory signaling in centrocytes contributes to preventing the selection of self-reactive mutated B cell clones, and that a defect in inhibitory signaling in centrocytes may be permissive for the expansion of autoreactive B cells that may not have otherwise attained the signaling threshold for selection by antigen presented on follicular dendritic cells (Figure 2B).
A final possibility that is worth considering is a possible role for B cells in autoimmunity that is not restricted to autoantibody formation. It is possible that in some autoimmune disorders the primary requirement for auto-reactive B cells in the process of pathogenesis is for the efficient presentation of auto-antigen to autoreactive T cells. A defect in the SIAE-Siglec-Lyn-SHP-1 inhibitory pathway may facilitate the ability of weakly self-reactive B cells to internalize self-antigen and present the auto-antigen in a particularly efficient manner to inflammatory T helper cells that could for instance be of the Th17 or Th1 subsets (Figure 2C). Such a scenario could be relevant in a tertiary lymphoid structure such as a multiple sclerosis plaque.
SIAE might also be defective in patients with autoimmune disease and therefore an understanding of the potential mechanisms by which this esterase and its biochemical partners contribute to tolerance and autoimmunity could eventually prove to be of therapeutic importance. The knowledge that enzymes represent optimal targets for small molecule inhibitors, suggests that this tolerance pathway may well point the way to novel therapeutic approaches in autoimmunity. For instance, small molecule inhibitors of SIAT would theoretically represent a new class of potential therapeutics in autoimmune disorders.
Acknowledgments
This work was supported by grants to SP from the Alliance For Lupus Research and the NIH (AI 064930 and AI 076505)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verdine GL, Walensky LD. The challenge of drugging undruggable targets in cancer: lessons learned from targeting BCL-2 family members. Clin Cancer Res. 2007;13(24):7264–7270. doi: 10.1158/1078-0432.CCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 2.Cariappa A, et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med. 2009;206(1):125–138. doi: 10.1084/jem.20081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam KP, et al. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 4.Kraus M, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Tze LE, et al. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3(3):e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillai S, et al. Marginal Zone B Cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 7.Gay D, et al. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177(4):999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiegs SL, et al. Receptor editing in self-reactive bone-marrow B cells. J.Exp. Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 10.Hartley SB, et al. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 11.Hartley SB, et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72(3):325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 12.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 13.Cyster JG, et al. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371(6496):389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 14.Ekland EH, et al. Requirements for follicular exclusion and competitive elimination of autoantigen-binding B cells. J Immunol. 2004;172(8):4700–4708. doi: 10.4049/jimmunol.172.8.4700. [DOI] [PubMed] [Google Scholar]
- 15.Pelanda R, et al. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7(6):765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 16.Halverson R, et al. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5(6):645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 17.Hippen KL, et al. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol. 2005;175(2):909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 18.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 19.Panigrahi AK, et al. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp Med. 2008;205(13):2985–2994. doi: 10.1084/jem.20082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar KR, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312(5780):1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 21.Crocker PR, et al. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 22.Powell LD, et al. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993;268(10):7019–7027. [PubMed] [Google Scholar]
- 23.Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384(6610):634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 24.O'Keefe TL, et al. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274(5288):798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 25.Sato S, et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5(6):551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 26.Nitschke L, et al. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7(2):133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 27.Peaker CJ, Neuberger MS. Association of CD22 with the B cell antigen receptor. Eur J Immunol. 1993;23(6):1358–1363. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
- 28.Walker JA, Smith KG. CD22: an inhibitory enigma. Immunology. 2008;123(3):314–325. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins BE, et al. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol. 2006;7(2):199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 30.Stoddart A, et al. Molecular cloning of the cDNA encoding a murine sialic acid-specific 9-O-acetylesterase and RNA expression in cells of hematopoietic and non-hematopoietic origin. Nucleic Acids Res. 1996;24(20):4003–4008. doi: 10.1093/nar/24.20.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takematsu H, et al. Lysosomal and cytosolic sialic acid 9-O-acetylesterase activities can be encoded by one gene via differential usage of a signal peptide-encoding exon at the N terminus. J Biol Chem. 1999;274(36):25623–25631. doi: 10.1074/jbc.274.36.25623. [DOI] [PubMed] [Google Scholar]
- 32.Sjoberg ER, et al. Natural ligands of the B cell adhesion molecule CD22 beta can be masked by 9-O-acetylation of sialic acids. J Cell Biol. 1994;126(2):549–562. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blixt O, et al. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278(33):31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 34.Naito Y, et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27(8):3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cariappa A, et al. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14(5):603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 36.Cariappa A, et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23(4):397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands alpha2,6-linked sialic acids. Int Immunol. 2006;18:603–611. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]
- 38.O'Keefe TL, et al. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189(8):1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibbs ML, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83(2):301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 40.Pao LI, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27(1):35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann A, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8(7):695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 42.Chen GY, et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323(5922):1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gross AJ, Lyandres JR, Pangrahi AK, Luning Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1inhibitory pathway promotes B cell tolerance. J. Immunol. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 45.Lanoue A, Batista FD, Stewart M, Neuberger MS. Interaction of CD22 with alpha2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur J Immunol. 2002;32:348–355. doi: 10.1002/1521-4141(200202)32:2<348::AID-IMMU348>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]