Abstract
During spermatogenesis, fully developed spermatids (i.e., spermatozoa) at the luminal edge of the seminiferous epithelium undergo ‘spermiation’ at stage VIII of the seminiferous epithelial cycle. This is manifested by the disruption of the apical ectoplasmic specialization (apical ES) so that spermatozoa can enter the tubule lumen and to complete their maturation in the epididymis. At the same time, the blood-testis barrier (BTB) located near the basement membrane undergoes extensive restructuring to allow transit of preleptotene spermatocytes so that post-meiotic germ cells complete their development behind the BTB. While spermiation and BTB restructuring take place concurrently at opposite ends of the Sertoli cell epithelium, the biochemical mechanism(s) by which they are coordinated were not known until recently. Studies have shown that fragments of laminin chains are generated from the laminin/integrin protein complex at the apical ES via the action of MMP-2 (matrix metalloprotease-2) at spermiation. These peptides serve as the local autocrine factors to ‘destabilize’ the BTB. These laminin peptides also exert their effects on hemidesmosome which, in turn, further potentiates BTB restructuring. Thus, a novel apical ES-BTB-hemidesmosome regulatory loop is operating in the seminiferous epithelium to coordinate these two crucial cellular events of spermatogenesis. This functional loop is further assisted by the Par3/Par6-based polarity protein complex in coordination with cytokines and testosterone at the BTB. Herein, we provide a critical review based on the latest findings in the field regarding the regulation of these cellular events. These recent findings also open up a new window for investigators studying blood-tissue barriers.
Keywords: Testis, seminiferous epithelial cycle, spermatogenesis, Sertoli cells, germ cells, blood-testis barrier, anchoring junction, ectoplasmic specialization, hemidesmosome
1. INTRODUCTION
In the mammalian testis, spermatogenesis takes place in the seminiferous epithelium of the seminiferous tubule, which is the functional unit that produces spermatozoa (Fig. 1A, B). In rats, spermatozoa (1n, haploid) are formed from more primitive germ cells known as spermatogonia (2n, diploid) (Fig. 1C) in ~58 days via 14 stages of development known as the seminiferous epithelial cycle of spermatogenesis (de Kretser and Kerr, 1988; LeBlond and Clermont, 1952). Spermatogenesis is divided into four phases: mitosis, meiosis, spermiogenesis and spermiation, and these events are concomitant with the movement of developing germ cells from the basal to the adluminal (or apical) compartment (Fig. 1C). These two compartments are created by the blood-testis barrier (BTB) via specialized junctions between Sertoli cells so that post-meiotic germ cell development takes place behind the immunological barrier conferred by the BTB in a specialized microenvironment (Fig. 1C). This thus avoids the production of anti-sperm antibodies against sperm-specific antigens that arise during spermiogenesis in the host body.
Figure 1. The general morphological features of mammalian testes, and a schematic drawing of the seminiferous epithelium illustrating the relative location of different junction types, and the adluminal (apical) and basal compartments of the seminiferous epithelium created by the blood-testis barrier (BTB) in adult rat testes.
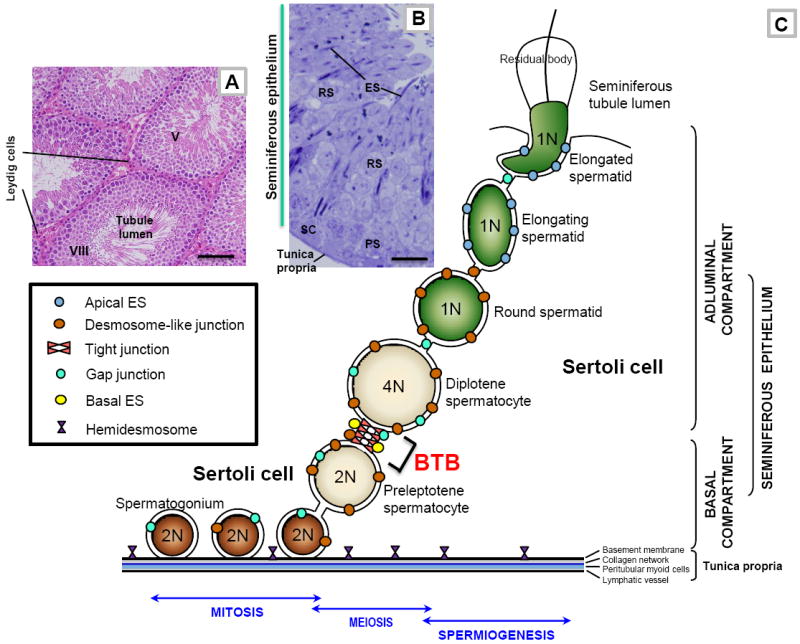
(A) This micrograph is the cross-section of an adult rat testis, showing several seminiferous tubules (e.g., a stage V and a stage VIII seminiferous tubule), which are the functional units that produce spermatozoa. The sperm producing function of the seminiferous tubule during spermatogenesis is also supported by testosterone produced by Leydig cells which are found in the interstitium. Bar = 80 μm. (B) This is the magnified view of a stage V tubule, illustrating the different germ cell types (e.g., PS, pachytene spermatocyte; RS, round spermatid; ES, elongating spermatid) are tightly associated with Sertoli cells (SC, Sertoli cell nucleus) found in the seminiferous epithelium. Bar = 8 μm. (C) This is the schematic drawing illustrating the seminiferous epithelium is composed of Sertoli cells and germ cells at different stages of their development, lying on the tunica propria which is composed of two acellular zones: basement membrane (a modified form of extracellular matrix) and type I collagen network; and two cellular zones: peritubular myoid cells and lymphatic vessel. The BTB, located near the basement membrane, is constituted by coexisting tight junction, gap junction, desmosome-like junction, and basal ectoplasmic specialization (basal ES), between adjacent Sertoli cells. The BTB also physically divides the seminiferous epithelium into the basal and adluminal (apical) compartments and confers cell polarity in the seminiferous epithelium. From step 8 through step 19 spermatids, apical ectoplasmic specialization (apical ES) is the only anchoring device that maintains cell adhesion and orientation of a developing spermatid onto the Sertoli cell in the seminiferous epithelium. Preleptotene spermatocytes differentiated from type B spermatogonia are the germ cells in transit at the BTB at stage VIII of the seminiferous epithelial cycle while differentiating into leptotene and zygotene spermatocytes. Once these primary spermatocytes enter the adluminal compartment, they differentiate into pachytene spermatocytes and diplotene spermatocytes to begin meiosis I to be followed immediately by meiosis II to form haploid round spermatids at stage XIV of the epithelial cycle in rats. Round spermatids undergo spermiogenesis via steps 1 through step 19 spermatids in rats, which is typified by the condensation of the genetic materials. The genetic substances are tightly packed into the spermatid head concomitant with the formation of the acrosome above the head region and the elongation of the spermatid tail. During these morphological changes, developing spermatids are also in transit near the BTB towards the luminal edge of the seminiferous epithelium progressively, then migrate towards the basement membrane (such as at stage IV-V of the epithelial cycle) (see Fig. 1B) and moving towards the luminal edge again (such as stage VI-VII of the epithelial cycle) until the fully developed spermatids (i.e., spermatozoa) are emptied into the seminiferous tubule lumen at spermiation at stage VIII of the epithelial cycle which is associated with disruption of the adhesion protein complexes at the elongated spermatid-Sertoli cell interface, and the shedding of the residual bodies. The Sertoli cell also serves as a scavenger by engulfing the residual bodies via phagocytosis. As a result of this germ cell maturation process and the continuous movement of developing germ cells, in particular elongating spermatids, across the seminiferous epithelium, there are extensive restructuring, namely disassembly and reassembly, of junctions at the Sertoli-Sertoli and Sertoli-germ cell interface during spermatogenesis. Also noted are the three phases of spermatogenesis: mitosis, meiosis, and spermiogenesis (see text for details). 1N, haploid; 2N, diploid; 4N, tetraploid.
Type A spermatogonia composed of A single (As) spermatogonia (i.e., self-renewing germline stem cells that occupy a niche near the basement membrane), A paired (Apr) and A aligned (Aal), and all are undifferentiated germ cells. Type A spermatogonia reproduce via mitosis, some of which, however, transform to differentiated type A spermatogonia (A1, A2, A3, A4). Type A spermatogognia differentiate into intermediate spermatogonia (In) and then type B spermatogonia (B) (Fig. 1C) (de Rooij and Russell, 2000; Hess and de Franca, 2008). At stage VII of the epithelial cycle, type B spermatogonia differentiate into preleptotene, followed by leptotene spermatocytes, which are the primary spermatocytes that cross the BTB while differentiating into zygotene spermatocytes at stages VIII-IX (Parvinen, 1982; Russell, 1977c), illustrating that transit of primary spermatocytes across the BTB associates with cell cycle progression (e.g., condensation of chromosomes) in preparation for metaphase I. Once these cells enter the adluminal compartment (Fig. 1C), pachytene spermatocytes differentiate into diplotene spermatocytes (tetraploid, 4n). These cells undergo diakinesis and enter the first division of meiosis (Div I) to become secondary spermatocytes, followed by the second division of meiosis (Div II) to form spermatids (haploid, 1n) at stage XIV the epithelial cycle in rats. Thereafter, spermatids undergo extensive morphological changes in a process known as spermiogenesis (Fig. 1C). This is typified by: (i) formation of the acrosome, (ii) condensation of chromatin, (iii) packaging of cytosolic substances into the residual body and (iv) elongation. These processes are accompanied by the migration developing spermatids from the basal compartment towards the edge of the tubule lumen so that fully developed spermatids (i.e., spermatozoa) can be emptied into the lumen at spermiation. These events are associated with extensive interactions at the Sertoli-Sertoli cell interface, as well as at the BTB, to allow transit of primary spermatocytes and progression of the germ cell cycle (Fig. 1). Interestingly, restructuring of the BTB, spermiation and cell cycle progression in primary spermatocytes crossing the BTB take place simultaneously at stage VIII of the epithelial cycle, yet these events occur at opposite ends of the seminiferous epithelium (Fig. 1) (Clermont, 1972; Parvinen, 1982). Herein, we provide a biochemical model to explain how these events are coordinated and regulated based on recent findings in the field.
2. THE BLOOD-TESTIS BARRIER (BTB)
2.a. Concept of the BTB
The BTB, also known as the seminiferous epithelium barrier and the Sertoli cell barrier. The concept of the BTB was first described at the beginning of the last century. When dyes were administered to rodents via the jugular vein, all organs were stained except for seminiferous tubules in the testis and the brain [for reviews, see (Miller et al., 2008; Setchell, 2008)]. Subsequent detailed morphological studies have shown that the BTB is different from other blood-tissue barriers, such as the blood-brain barrier (BBB). For instance, the BBB is constituted by TJ of capillary endothelial cells and contributed in part by pericytes (Bernacki et al., 2008), whereas the microvessels in the interstitium of the testes contribute relatively little barrier function to the BTB. However, myoid cells in the tunica propria (Fig. 1) of rodents are known to contribute to barrier function since lanthanum, an electron-opaque marker, failed to penetrate ~85% of seminiferous tubules beyond the myoid cell layer (Dym and Fawcett, 1970). Interestingly, the myoid cell layer is less efficient in blocking dyes and markers from passing through the BTB in primates (Dym, 1973). A reason for this is not known. In short, the BTB in mammals is constituted significantly by specialized junctions between Sertoli cells (Figs. 1 & 2).
Figure 2. The protein complexes and their peripheral regulators and adaptors that constitute the BTB in the rat testis.
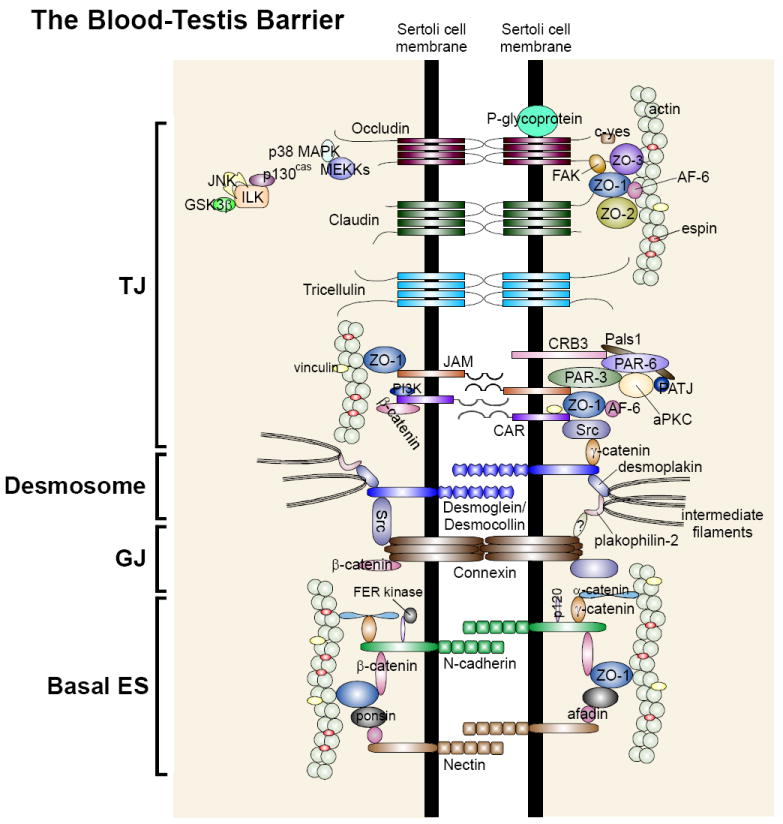
As described in the text that the BTB in the testis is constituted by integral membrane proteins of the: (i) tight junction (TJ): occludins, claudins, tricellulin, JAMs, and CAR; (ii) desmosome-like junction: desmogleins, desmocollins; (iii) gap junction (GJ): connexins; and (iv) basal ES: N-cadherins, nectins. Each integral membrane protein is associated with its corresponding adaptors (e.g., ZOs, plakophilins, catenins, afadins), regulatory proteins (e.g., FAK, MAPKs, ILK, FER kinase), polarity proteins (e.g., Par, Pals1). All these protein complexes, in turn, attach to actin via actin-binding proteins (e.g., vinculin, afadin, espin) except for desmosome-like junction which uses intermediate filaments (e.g., vimentin) for attachment.
2.a. Functions of the BTB
The BTB is a physiologically important structure in the testis, and it confers several functions which are essential for spermatogenesis (Cheng and Mruk, 2002; Mruk and Cheng, 2008a; Pelletier, 2001; Setchell, 2008). First, it functions as a ‘fence’ by selectively restricting the passage of water, electrolytes, ions, food, amino acids, hormones and paracrine factors from both the interstitium and basal compartment into the apical compartment. As such, Sertoli cells can regulate the ‘type’ and ‘amount’ of water, ions, nutrients and hormones needed for haploid spermatid development. Second, the BTB provides an immunological barrier to sequester antigens that are expressed transiently during post-meiotic germ cell development from the host s immune system to prevent the production of anti-sperm antibodies. Third, the BTB also confers cell polarity in the testis. For instance, all Sertoli cell nuclei are located near the basement membrane, and the cytoplasmic processes of each Sertoli cell connects to ~30-50 germ cells at different stages of development (Weber et al., 1983) some of which are connected via intercellular bridges and referred to as germ cell ‘clones’ (Fawcett, 1961; Greenbaum et al., 2006; Kato et al., 2004). A recent study has illustrated that cell polarity proteins such as Par (partitioning-defective) proteins (e.g., Par3, Par6) are crucial proteins that function in the orientation of elongating spermatids, as well as in Sertoli-Sertoli cell adhesion at the BTB (Wong et al., 2008b) (Fig. 2). However, unlike most other blood-tissue barriers, the BTB undergoes extensive restructuring at stages VIII-IX of the seminiferous epithelial cycle to facilitate the transit of preleptotene/leptotene spermatocytes so that meiosis I and II can be completed in the apical compartment at stage XIV of the epithelial cycle (Hess and de Franca, 2008).
2.b. Structural features of the BTB
(i) Integral membrane proteins that constitute the BTB
At present, the BTB is known to be constituted by co-existing TJ, basal ES, gap junctions (GJ) and desmosome-like junctions (Fig. 2). Recent studies have identified the following TJ (e.g., occludin/ZO-1, claudin/ZO-1, JAM-A/ZO-1 and JAM-B/ZO-1), basal ES (e.g., N-cadherin/β-catenin, nectin-2/afadin, CAR/β-catenin), GJ (e.g., connexin43), and desmosome-like (e.g., desmoglein-2/desmocollin-2, connexin43/plakophilin-2) protein complexes at the BTB in the rat testis (Cheng and Mruk, 2002; Mruk and Cheng, 2008a; Pointis et al., 2005; Setchell, 2008; Vogl et al., 2000; Vogl et al., 2008; Wang and Cheng, 2007; Wong et al., 2008a; Yan et al., 2008b; Yan et al., 2007) (Fig. 2). Many of these are integral membrane proteins composed of one or several transmembrane, extracellular and intracellular domains (Fig. 2). However, only intracellular domains bind to adaptor proteins, thereby recruiting other regulatory proteins (e.g., FAK, c-Src), as well as anchoring the multi-protein complex to cytoskeletal proteins (Fig. 2). Thus, the interacting domains between apposing cell adhesion protein complexes residing on two adjacent Sertoli cells that constitute the BTB can be efficiently ‘disassembled’ and ‘reassembled’ via changes in adhesive function, such as changes in the phosphorylation status of integral membrane proteins and/or their adaptors (Gumbiner, 2000; Zhang et al., 2005), affecting the association between the adhesion protein complex and the cytoskeleton.
(ii) Interactions of regulatory proteins with structural protein complexes
The regulation of the BTB, particularly its restructuring which facilitates the transit of preleptotene spermatocytes at stage VIII-IX, has remained largely unknown since the initial discovery of the BTB more than a century ago (Mruk and Cheng, 2008a; Setchell, 2008). This is largely due to the lack of information on the regulatory proteins that are integrated components of the BTB, which ultimately regulate the tight and adhesive function of the BTB. The first report on the likely presence of a regulatory protein at the BTB appeared in 2003 when studies by immunohistochemistry and immunofluorescent microscopy illustrated the localization of focal adhesion kinase (FAK) at the BTB in adult rat testes (Siu et al., 2003b). This observation was difficult for investigators to appreciate at the time because FAK, a non-receptor tyrosine kinase known to regulate diverse cellular functions, is most notably a regulatory protein kinase at the cell-matrix focal adhesion complex which is largely under the control of integrins (Boutros et al., 2008). Subsequent studies by co-immunoprecipitation showed that FAK structurally interacted with occludin and ZO-1 (Siu et al., 2009b; Siu et al., 2009c) and that both proteins localized to the BTB in the adult rat testis, as well as to the Sertoli cell TJ barrier in vitro which mimics the BTB in vivo (Siu et al., 2009a). Since the expression of FAK and its localization of FAK at the BTB was stage-specific, being lowest at stage VIII and coinciding with BTB restructuring (Siu et al., 2009a), it was postulated that FAK may be involved in BTB dynamics via its effects on the occludin/ZO-1 complex. Indeed, when FAK was silenced by FAK-specific siRNA duplexes versus non-targeting (control) siRNA duplexes in Sertoli cells cultured in vitro with an established TJ permeability barrier that mimicked the BTB in vivo (Siu et al., 2005), the knockdown of FAK led to a transient disruption of the Sertoli cell TJ barrier (Siu et al., 2009b). This finding was confirmed when the distribution of occludin in Sertoli cells was examined following FAK RNAi since there was considerable redistribution of occludin from the Sertoli-Sertoli cell interface to the cell cytosol, thereby perturbing Sertoli cell TJ barrier function (Siu et al., 2009b). Additionally, there was a loss of association between occludin and ZO-1 following knockdown of FAK as shown by co-immunoprecipitation (Siu et al., 2009b). These findings are significant because they illustrate for the first time that TJ integral membrane proteins at the BTB are structurally associated with FAK, a regulatory protein normally found at the focal adhesion complex. Furthermore, it was noted that while the steady-state level of occludin in the Sertoli cell epithelium following FAK knockdown did not decrease, a significant decline in occludin phosphorylation at Tyr and Ser, but not Thr, residues was detected, illustrating that changes in occludin redistribution that led to a disruption of the Sertoli cell TJ barrier were mediated by changes in the phosphorylation status of occludin (Siu et al., 2009b). These findings are also consistent with earlier observations in MDCK cells which demonstrated that occludin incorporated into TJ fibrils is phosphorylated (Sakakibara et al., 1997; Tsukamoto and Nigam, 1999), whereas the small pool of occludin at the basolateral region of epithelial cells that are not assembled into TJ fibrils is not phosphorylated (Cordenonsi et al., 1997; Sakakibara et al., 1997). Certainly, additional regulatory protein kinases and phosphatases at the BTB will be identified, and this area of research will continue to be open for future investigation.
(iii) Drug transporters
The best studied drug transporters in the testis are P-glycoprotein and multidrug resistance protein 1 (MRP1). Both are important regulatory proteins of the ATP-binding cassette (ABC) superfamily that regulate drug transport across the BTB and into/out of the apical compartment of the seminiferous epithelium. Both P-glycoprotein and MRP1 are efflux pumps that are present in multiple epithelial and endothelial cells (Couture et al., 2006; Miller et al., 2008). P-glycoprotein is the product of multidrug resistance 1 gene: Mdr 1a and 1b in rodents, and Mdr1 in humans, whereas MRP1 is a distant member of the ABC superfamily related to P-glycoprotein (Fromm, 2004; Schaller et al., 1995). P-glycoprotein is found in Sertoli cells, peritubular myoid cells, late spermatids (Bart et al., 2004; Melaine et al., 2002) and spermatogonia (Trezise et al., 1992). MRP1 is also localized to Sertoli cells in mouse and human (Bart et al., 2004; Flens et al., 1996). ABCG2 (breast cancer resistance protein, BCRP, an efflux pump) is another ABC transporter also found at the BTB (Robey et al., 2009). Recent studies have shown that P-glycoprotein is an integrated component of the BTB since it structurally interacts with TJ proteins occludin, claudin-11 and JAM-A, but not with the basal ES protein N-cadherin as demonstrated by co-immunoprecipitation experiments (Su and Mruk, 2009) (Fig. 2). Equally important, in rats treated with adjudin (50 mg/kg b.w., by gavage), a drug that was shown to induce germ cell depletion from the epithelium (Cheng et al., 2005), there was a transient induction in P-glycoprotein and a significant increase in its association with occludin and JAM-A (Su and Mruk, 2009). These findings illustrate that Sertoli cells which constitute the BTB possess the ability to ‘pump’ drugs, such as adjudin, out of the seminiferous epithelium in order to protect post-meiotic germ cell development. This is likely mediated by its interaction with occludin and JAM-A to ‘seal’ the BTB. Besides putative drug transporters, recent studies have also identified multiple organic cation transporters (OCT) and organic cation/carnitine transporters (OCTN) expressed by Sertoli cells, regulating cation transport across the BTB (Maeda et al., 2007). This is an important area that requires additional research since drug transporters in Sertoli cells that constitute the BTB determine which and how much of a therapeutic compound or contraceptive enters the adluminal compartment.
2.c. Regulation of the BTB
(i) Coordinated effects of cytokines and testosterone to maintain BTB integrity during transit of preleptotene/leptotene spermatocytes across the BTB
As discussed above, junction restructuring in different epithelia such as the seminiferous epithelium is regulated by changes in protein phosphorylation. However, unlike other blood-tissue barriers, the BTB cannot ‘open’, even transiently at stages VIII-IX to allow preleptotene/leptotene spermatocytes to cross the BTB, as this would expose post-meiotic germ cell antigens to the host s immune system. Based on earlier morphological studies (Siu et al., 2005), it was postulated that during preleptotene spermatocyte transit, ‘new’ TJ fibrils are first formed below the migrating spermatocyte followed by the dissolution of ‘old’ TJ fibrils above the spermatocyte, thus permitting the migration of spermatocytes across the BTB without eliciting an immune response (Fig. 3). Recent findings have provided the biochemical basis for such a mechanism to operate at the BTB. Cytokines such as TGF-β3 and TNFα are putative products of Sertoli and germ cells, and their receptors are also expressed by these cells (Caussanel et al., 1997; De et al., 1993; Le Magueresse-Battistoni et al., 1995; Skinner, 1993). While these cytokines are known to regulate many other testicular functions (Loveland et al., 2007; Lui et al., 2003a), recent findings have shown that they also play a critical role in the disruption of BTB integrity, possibly at stage VIII of the epithelial cycle (Li et al., 2008; Lui et al., 2003b; Lui et al., 2003c; Siu et al., 2003a). For instance, the integrity of the Sertoli cell TJ permeability barrier in vitro (Lui et al., 2001; Siu et al., 2003a) and in vivo (Li et al., 2006; Xia et al., 2006; Xia et al., 2009) could be reversibly perturbed following inclusion of recombinant TGF-β3 and/or TNFα to Sertoli cell cultures having an established TJ barrier in vitro or administered intratesticularly in vivo. These findings are also consistent with reports from other epithelia regarding the disruptive effects of cytokines on TJ barrier function (Walsh et al., 2000). On the other hand, testosterone was shown to promote BTB integrity in mice in vivo (Meng et al., 2005; Wang et al., 2006), as well as enhance the Sertoli cell TJ barrier in vitro (Chung and Cheng, 2001; Janecki et al., 1992). Using biotinylation in conjunction with endocytosis assays to assess the kinetics of protein internalization (Le et al., 1999) and dual-labeled immunofluorescence analysis to assess changes in protein distribution in Sertoli cells having an assembled TJ permeability barrier, it was shown that TGF-β2/-β3, TNFα and testosterone enhanced clathrin-mediated endocytosis of integral membrane proteins at the BTB (e.g., occludin, N-cadherin) (Xia et al., 2009; Yan et al., 2008c). Interestingly, TGF-β2 promoted endosome-mediated degradation of endocytosed proteins from the BTB, but testosterone promoted endosome-mediated recycling of endocytosed proteins (Yan et al., 2008c). These findings are physiologically significant because they illustrate for the first time that cytokines and testosterone, whose effects on BTB integrity are opposite, may be working synergistically to accelerate protein endocytosis and degradation (i.e., cytokines) above a migrating preleptotene spermatocyte, while promoting protein endocytosis (i.e., testosterone) below this primary spermatocyte so as to assemble ‘new’ TJ fibrils (Fig. 3). This provides an efficient mechanism to regulate the movement of preleptotene spermatocytes across the BTB without compromising the immunological barrier. Nonetheless, additional work is needed to expand the regulatory role of testosterone on protein ‘transcytosis’ at the BTB. For instance, which molecules mediate androgen-induced transcytosis? How is cross talk between cytokines and testosterone mediated in light of the fact that these biomolecules exert different effects on protein endocytosis, recycling, and/or transcytosis? Finally, what are the key proteins that take part in protein recycling, transcytosis, and endosome- or ubiquitin-mediated protein degradation?
Figure 3. A hypothetical model illustrating the likely mechanism utilized by the testis to facilitate the transit of primary preleptotene spermatocytes at the BTB while maintaining the immunological barrier function.
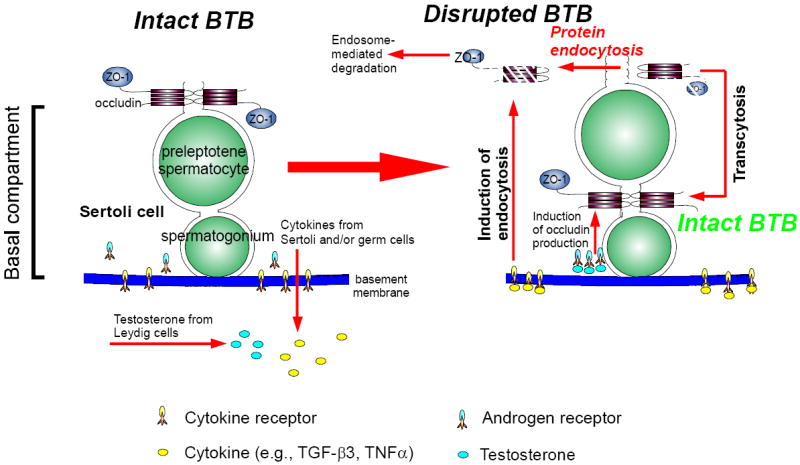
At the time of primary preleptotene spermatocytes in transit at the BTB, such as at stage VIII of the epithelial cycle, testosterone produced by Leydig cells in the interstitium enters the BTB microenvironment, which is known to promote BTB function, binds to androgen receptor to exert is effects or mediates its action via nongenomic pathway, such as c-Src and ERK (Cheng et al., 2007; Fix et al., 2004) (see left panel). This promotes the production of BTB proteins (e.g., claudin-11, occludin) (Kaitu’u-Lino et al., 2007; Yan et al., 2008c) for the assembling of ‘new’ TJ-fibrils behind a migrating primary preleptotene spermatocyte (see right panel). Furthermore, testosterone also appears to promote transcytosis of internalized integral membrane proteins, perhaps from the apical region to the basal region of the migrating spermatocytes to facilitate ‘new’ TJ-fibrils assembly (Yan et al., 2008c). Cytokines (e.g., TGF-β3, TNFα) released from Sertoli and germ cells also bind to their corresponding receptors at the BTB to activate the downstream signaling function, which, in turn, induces protein endocytosis at the ‘old’ BTB site above the migrating spermatocytes but targeted to degradation via the endosome-mediated pathway (Yan et al., 2008c) (left panel). Thus, ‘new’ TJ-fibrils behind the migrating spermatocytes continue to maintain the immunological barrier while the ‘old’ TJ-fibrils above the spermatocytes in transit are disrupted to facilitate cell movement. This thus provides a novel mechanism to maintain the immunological barrier during the transit of primary preleptotene spermatocytes at the BTB.
(ii) Mitogen-activated protein kinases (MAPK)
In studies using cytokines to perturb Sertoli cell TJ barrier function both in vitro and in vivo, an activation of some members of the MAPK family was shown to be associated with these disruptive effects. For instance, TGF-β3 was shown to activate p38 MAPK to impair the Sertoli cell TJ permeability barrier in vitro (Lui et al., 2003b). This cytokine-mediated activation of MAPK appears to be specific since the use of MAPK inhibitors could delay and partially block the adverse effects of TGF-β3 on BTB integrity in vivo (Lui et al., 2003c; Xia et al., 2006) and in vitro (Lui et al., 2003b). These findings reveal that MAPK activation may be associated with the transcriptional regulation of other genes that regulate BTB integrity such as the production of proteases which may facilitate endosome-mediated degradation of integral membrane proteins at the BTB. This may also lead to the activation and/or production of critical proteins needed for endocytosis (Doherty and McMahon, 2009; Galletta and Cooper, 2009), endosome- or ubiquitin-mediated degradation and/or recycling of endocytosed proteins (Chen and Sun, 2009; Mittal and McMahon, 2009; Sorkin and Goh, 2009) and transcytosis (Tuma and Hubbard, 2003). Furthermore, JNK (c-Jun N-terminal protein kinase), a member of the MAPK family, was shown to regulate the production of α2-macroglobulin, a non-specific protease inhibitor, to limit proteolysis during seminiferous epithelial restructuring in the cadmium-induced testicular injury model (Wong et al., 2005). Collectively, these findings illustrate the involvement of MAPK in cytokine-mediated disruptive effects on the Sertoli cell BTB function.
In this context, it is of interest to note that at the time of preleptotene/leptotene spermatocyte transit across the BTB, these cells differentiate into zygotene spermatocytes by undergoing chromatin condensation to prepare for metaphase I. It is known that MAPK also plays an essential role in regulating germ cell cycle progression event. For instance, treatment of pachytene spermatocyte cultures with okadaic acid, a Ser/Thr phosphatase inhibitor, was shown to prematurely induce metaphase I by accelerating chromatin condensation. This was accompanied by an increase in intrinsic MAPK activity (Cobb et al., 1999; Wiltshire et al., 1995). This okadaic acid-induced chromosomal condensation could be abolished by preincubating pachytene spermatocytes with an MEK1/2 inhibitor, PD98005 (Di Agostino et al., 2002) or U0126 (Petronczki et al., 2003). These findings also illustrate the involvement of MAPK in germ cell cycle events such as preparation of the chromatin for diakinesis while these cells are migrating across the BTB. This prompts us to speculate whether MAPK regulation of junction restructuring between Sertoli cells at the BTB also plays a role in germ cell cycle progression at stage VIII of the epithelial cycle. It is worth noting that germ cells such as spermatocytes and spermatids develop as a clone, that is, they are connected by intercellular bridges to synchronize differentiation (Dym and Fawcett, 1971; Fawcett et al., 1959; Ren and Russell, 1991). Other studies have shown that spermatogonia undergo apoptosis following exposure to irradiation also occur in clones (Huckins, 1978a; Huckins, 1978b), illustrating factor(s) that regulate programmed cell death can diffuse through the intercellular bridges to coordinate apoptosis within a clone. Intercellular bridges that connect preleptotene spermatocytes during transit at the BTB were also detected in adult rat testes by electron microscopy (Mruk and Cheng, 2008b). While the biology of intercellular bridges is not known, recent studies have shown that TEX14 (Greenbaum et al., 2009; Greenbaum et al., 2006) and δ-tubulin (Kato et al., 2004) are crucial components of intercellular bridges. Furthermore, Sertoli-Sertoli and Sertoli-germ cells are also connected by gap junctions (Pointis and Segretain, 2005) and desmosome-like junctions (also known as desmosome-gap junctions) (Ren and Russell, 1992) for signaling communications to coordinate different events pertinent to spermatogenesis, such as germ cell cycle progression, meiosis, BTB restructuring to facilitate spermatocytes in transit, and others. Thus, it is likely that the ‘signal(s)’ that regulate germ cell cycle progression, such as DNA condensation in preparation of the subsequent diakinese, can also pass between primary spermatocytes in clones via the intercellular bridges to coordinate this event; they can also pass between Sertoli cells and between and germ cells to coordinate BTB restructuring and also germ cell cycle progression simultaneously, such that these events can be mediated by MAPK. This possibility should be vigorously evaluated in future studies.
(iii) Polarity proteins
Two polarity protein complexes are found in adult rat testes. These are the (i) CRB (Crumbs) complex: CRB3/Pals1/PATJ and the (ii) Par (partitioning-defective) complex: Par3/Par6/aPKC (Wong et al., 2008a). Initially identified in Caenorhabditis elegans and Drosophila melanogaster as proteins which confer cell polarity during development, their homologs were subsequently found in mammalian cells, mostly restricted to TJs (Shin et al., 2006; Suzuki and Ohno, 2006). It was shown that Par6 is localized mostly to the BTB and apical ES in the seminiferous epithelium of adult rat testes (Wong et al., 2008b). The apical ES is the only anchoring device present between Sertoli cells and elongating/elongated spermatids (i.e., steps 8-19 in the rat) (Wong et al., 2008a), and the TJ is not found between Sertoli and germ cells. However, developing spermatids must be properly oriented within the seminiferous epithelium, that is, their heads must point towards the basement membrane to maximize the number of elongated spermatids that are packed into the limited space of the seminiferous epithelium. Thus, the apical ES was proposed to function in spermatid polarity since its identification in the 1970s (Mruk and Cheng, 2004b; Russell, 1977b; Russell and Clermont, 1976; Vogl et al., 2008; Wong et al., 2008a). However, the proteins at the apical ES that function in spermatid polarity were not known until recently. Studies by immunohistochemistry, single-/dual-labeled immunofluorescent analysis and co-immunoprecipitation have all demonstrated unequivocally that several polarity proteins such as Par3 and Par6 are integral components of the apical and basal ES at the BTB (Wong et al., 2008b). Equally important, Par6 expression at the apical ES diminished when the polarity of elongated spermatids was lost, followed by their premature depletion from the seminiferous epithelium during adjudin-induced germ cell loss from the testis (Wong et al., 2008b) In addition, the Par6/Pals1 complex was found to structurally interact with JAM-C, an integral membrane protein at the apical ES, in the normal testis, but during adjudin-induced apical ES disruption, Par6 interacted with c-Src (Wong et al., 2008b). These findings are significant because they illustrate that the Par6/Pals1 complex can shift its association to interact with either JAM-C or Src, and this contributes to an intact or disrupted apical ES, respectively. This is also an efficient mechanism to regulate ‘adhesion’ and ‘de-adhesion’ during movement of developing spermatids across the adluminal compartment during spermiogenesis.
These findings prompted us to examine if the Par6 polarity protein complex also regulates cell adhesion at the Sertoli-Sertoli cell interface at the BTB during the transit of preleptotene/leptotene spermatocytes at stage VIII of the epithelial cycle. When Sertoli cells were cultured in vitro with an established TJ barrier that functionally and structurally mimicked the BTB in vivo (except that the apical ES was lacking since elongating/elongated spermatids were absent from this in vitro system), the knockdown of Par3 or Par6 by RNAi was shown to cause redistribution of JAM-A from the cell surface (i.e., it became cytosolic-bound), thereby disrupting cell adhesion (Wong et al., 2008b). Furthermore, the silencing of Par 6, but not Par3, also induced redistribution of N-cadherin from the Sertoli cell surface and into the cytosol (Wong et al., 2008b). These findings suggest that Par proteins may regulate cell adhesion via their effects on protein endocytosis. Indeed, knockdown Par5 (also known as 14-3-3) by RNAi also led to redistribution of N-cadherin from the cell surface and into cytosol (Wong et al., 2009), analogous to results from Par6 knockdown experiments (Wong et al., 2008b). This was accompanied by increased endocytosis of biotinylated N-cadherin and JAM-A (Wong et al., 2009). Taken collectively, Par polarity proteins at the BTB, besides conferring cell polarity, also regulate cell adhesion between Sertoli cells, and this is similar to their function at the apical ES.
(iv) A local regulatory mechanism at the BTB
In most blood-tissue barriers, except the BTB, barrier function is conferred by the TJ, which is functionally and structurally supported by the adherens (AJ) and desmosome junction. These three segregated entities are collectively referred to as the junction complex. The gap junction lies behind the junctional complex (Alberts et al., 2002). In contrast, the BTB is composed of co-existing TJ, basal ES, desmosome-like junction and gap junction (Cheng and Mruk, 2002; Mruk and Cheng, 2008a; Setchell, 2008; Vogl et al., 2008). While these observations were reported almost four decades ago, the physiological significance of this anatomical layout of cell junctions in the seminiferous epithelium has remained unknown until recently. It has been speculated that gap and desmosome-like [also known as desmosome-gap junctions because they have ultrastructural features of both desmosome and gap junctions (Ren and Russell, 1992; Russell, 1977a)] junctions at the BTB may function in ‘relaying’ signals between Sertoli cells for junction restructuring to facilitate transit of preleptotene spermatocytes at stage VIII of the epithelial cycle (Mruk and Cheng, 2004b), but the molecular and biochemical mechanism(s) remain unknown. Recent studies using Sertoli cells cultured in vitro with established TJ-permeability barrier and a ‘loss-of-function’ approach by RNAi, however, have shed new insights on the physiological significance of coexisting junctions at the BTB. For instance, silencing of desmosome-gap junction proteins using specific siRNA duplexes has demonstrated the significance of desmosome-like junctions in the regulation of BTB dynamics. While knockdown of desmoglein-2 (an integral membrane protein of desmosomes) alone by RNAi did not interfere with Sertoli-Sertoli cell adhesive function at the BTB, silencing of both desmoglein-2 and desmocollin-2 (another integral membrane protein of desmosomes) induced redistribution and internalization of CAR and ZO-1, but not occludin and N-cadherin (Lie et al., 2009a). Moreover, silencing of both desmoglein-2 and desmocollin-2 affected TJ barrier function as manifested by a significant decline in transepithelial electrical resistance (TER) across the Sertoli cell epithelium (Lie et al., 2009a). Subsequent studies using protein endocytosis assays demonstrated that loss of CAR from the cell surface was indeed mediated by an increase in its endocytosis (Lie et al., 2009a). Likewise, silencing of connexin 43 (an integral membrane protein at the desmosome-gap junction) alone did not affect Sertoli cell TJ barrier function nor the localization of TJ and/or basal ES proteins (Li et al., 2009). However, simultaneous knockdown of Cx43 and plakophilin-2 (an adaptor at the desmosome-like junction) in Sertoli cells in vitro by specific RNA duplexes perturbed the TJ barrier, concomitant with a redistribution of occludin and ZO-1. Both proteins were internalized, resulting in a disruption of BTB integrity (Li et al., 2009). These findings not only reveal the co-existence of cell junctions at the BTB; they also demonstrate that different junction types function as signaling platforms so that restructuring events can be tightly coordinated. This would avoid an unnecessary ‘opening’ of the BTB during primary spermatocyte transit across the BTB (Yan et al., 2008a), and this would maintain the immunological barrier. As such, the unique morphological layout of co-existing junctions in the seminiferous epithelium has an important physiological function, that is, to maintain BTB integrity during spermatogenesis.
3. THE APICAL ES–BTB–BASEMENT MEMBRANE LOCAL REGULATORY AXIS
(i) Apical ES and laminin chains
Ectoplasmic specialization (ES)
The ES, a testis-specific cell-cell actin-based anchoring junction, was first described in the seminiferous epithelium of rodent testes in the 1970s (Russell, 1977b; Russell and Clermont, 1976; Russell and Peterson, 1985; Vogl et al., 2000; Vogl et al., 1991). It is restricted either to the Sertoli cell-elongating/elongated spermatid (i.e., step 8-19 spermatids) interface known as the apical ES or to the Sertoli-Sertoli cell interface at the BTB known as the basal ES (Mruk and Cheng, 2004a; Mruk and Cheng, 2004b; Mruk and Cheng, 2008a; Toyama et al., 2003; Vogl et al., 2008). Once the apical ES, whose function is to anchor and properly orientate spermatids to Sertoli cells in the seminiferous epithelium (Fig. 1, Fig. 4), it is the only anchoring device present at this site (Russell and Clermont, 1976; Russell and Peterson, 1985; Vogl et al., 1991; Vogl et al., 1993). An in vitro study which quantified the physical force that can disrupt the apical ES versus the desmosome-like junction demonstrated that the apical ES has the strongest adhesive force (Wolski et al., 2005). Ultrastructurally, the apical ES is typified by the presence of actin-filament bundles sandwiched between cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane, and it restricted only to Sertoli cells without any identifiable ultrastructures on spermatids. However, several studies have shown that elongating spermatids express proteins that are putative components of the apical ES such as laminins (Koch et al., 1999; Yan and Cheng, 2006), cadherins (Lee et al., 2003; Wu et al., 1993), Par3, Par6 (Wong et al., 2008b), 14-3-3 (Par5) (Wong et al., 2009) and others (Siu and Cheng, 2004b; Yan et al., 2007) (Fig. 4). Unlike apical ES, basal ES co-exists with TJ, gap junctions (GJ) and desmosome-like junctions (Mruk and Cheng, 2008a; Vogl et al., 2000; Vogl et al., 2008) to constitute the BTB (Fig. 1C, Fig. 2).
Figure 4. The protein complexes and their peripheral regulators and adaptors that constitute the apical ES in the rat testis.
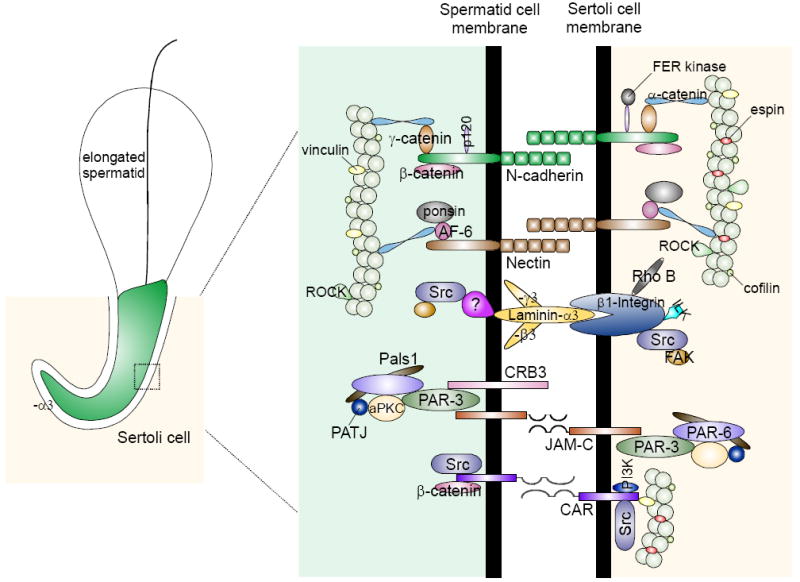
The presently known integral membrane proteins at the apical ES in adult rat testes are: cadherins, nectins, integrins, laminins/receptor protein(s) (note: laminins, such as α3, β3 and γ3 are cell surface proteins without any transmembrane domain, residing on elongating/elongated spermatids that anchor to spermatids at the apical ES via a yet-to-be identified laminin receptor protein), CRB3, JAM-C, and CAR. Each of these protein binds to its corresponding adaptors (e.g., catenins, afadins), polarity proteins (e.g., Par, Pals1), and protein kinases (e.g., c-Src, FAK), using actin for its attachment. Apical ES is an atypical adherens junction (AJ) type (Wong et al., 2008a) since it is composed of proteins usually restricted to cell-matrix focal adhesion complex (or focal contact) (e.g., c-Src, FAK), and TJ (e.g., JAM-C, CAR).
Apical ES
The apical ES is known to date to be constituted by the cadherin/catenin (Johnson and Boekelheide, 2002; Lee et al., 2004; Lee et al., 2003), nectin/afadin (Ozaki-Kuroda et al., 2002), and integrin/laminin(Koch et al., 1999; Mulholland et al., 2001; Palombi et al., 1992; Salanova et al., 1998; Salanova et al., 1995) protein complexes (Fig. 4). Of these, the cadherin-protein complex is detected at the apical ES at some stages of the epithelial cycle, such as stage V-VII (Johnson and Boekelheide, 2002; Lee et al., 2004). One of the most studied protein complex at the apical ES is the α6β1-integrin/laminin-333 (Fig. 4) (Mulholland et al., 2001; Salanova et al., 1998; Salanova et al., 1995; Siu and Cheng, 2004c; Yan and Cheng, 2006). The apical ES differs from other epithelial AJ in many aspects. For instance, apical ES is composed of many proteins that are usually restricted to the anchoring junction at the cell-extracellular matrix (ECM) interface known as the focal contact or focal adhesion complex (e.g., integrins, laminins, FAK, Src, vinculin) which facilitate cell movement (Siu and Cheng, 2004b; Vogl et al., 2000; Yan et al., 2007). Furthermore, proteins restricted to gap junctions (e.g., connexin-43) and TJ (e.g., CAR, JAM-C) (Siu and Cheng, 2004b; Wang and Cheng, 2007; Yan et al., 2007) are known to be integrated components of the apical ES. Thus, the apical ES has been referred to as an atypical AJ type (Wong et al., 2008a).
Laminin-333
Laminins are heterotrimers comprised of an α, β and γ chain to form a functional protein, which serves as the ligand of integrins, and they are usually restricted to the ECM where they serve as ligands for integrins to confer cell adhesion at the focal contact (also known as focal adhesion complex) (Alberts et al., 2002; Cheng and Mruk, 2002). To date, five α, four β, and three γ laminin chains have been reported in mammalian tissues, and different combinations of these laminin chains give rise to at least 17 functional laminin proteins (Aumailley et al., 2005; Hallmann et al., 2005). Laminin γ3 was the first non-basement membrane laminin to be detected at the apical ES in mouse testes (Koch et al., 1999), forming a functional adhesive complex with β1-integrin at the apical ES (Siu and Cheng, 2004c). Subsequent studies have demonstrated that laminin γ3 forms a functional complex with α3 and β3 chains, and it was designated laminin-333 (Yan and Cheng, 2006). Since laminin-333 lacks a transmembrane domain, it is restricted to the cell surface of elongating/elongated spermatids, whereas α6β1-integrin is limited to Sertoli cells at the apical ES, and these two proteins form a bona fide complex that confers spermatid adhesion (Yan and Cheng, 2006). In focal contacts, the laminin-integrin complex can be remodeled by laminin proteolysis via metalloproteases (MMPs) (Carragher and Frame, 2004; Giannelli et al., 1997; Udayakumar et al., 2003). Interestingly, MMP-2 and MT1-MMP [membrane type 1-MMP is a membrane-anchored metalloprotease which forms a functional protein complex with TIMP-2 (tissue inhibitor of metalloproteases-2) to serve as a receptor for pro-MMP-2 and for its activation (McCawley and Matrisian, 2001; Sternlicht and Werb, 2001; Werb, 1997) to MMP-2], which are known to cleave laminins and release biologically-active fragments to affect cell migration and TJ barrier function (Giannelli et al., 1997; Gurney et al., 2006; Udayakumar et al., 2003), were found to co-localize with laminin γ3, β1 integrin and TIMP-2 at the apical ES (Longin et al., 2001; Siu and Cheng, 2004c). Equally important, studies by immunohistochemistry and/or dual-labeled immunofluorescence analysis have illustrated that the expression of MMP-2, MT1-MMP and TIMP-2 was stage-specific, being highest at early stage VIII prior to spermiation, and co-localizing with β1-integrin at the apical ES (Siu and Cheng, 2004c). These findings illustrate that the MMP-2/MT1-MMP/TIMP-2 complex may be used to cleave the laminin-333/α6β1-integrin adhesion complex at the apical ES at spermiation, generating biologically-active fragments of laminin chains to regulate BTB restructuring to facilitate preleptotene spermatocyte transit across the BTB. This is consistent with recent findings regarding the action of laminin fragments derived from laminin-5 on blood-brain barrier function and cell migration (Giannelli et al., 1997; Gurney et al., 2006; Udayakumar et al., 2003). These findings also imply that biologically active laminin fragments generated at the apical ES during spermiation coordinate the events of spermiation and BTB restructuring at stage VIII of the epithelial cycle.
(ii) Basement membrane and hemidesmosome
In adult rat testes, the basement membrane is a modified form of ECM (Dym, 1994), which together with the underlying collagen (type I) layer constitutes the non-cellular zone of the tunica propria, supports the seminiferous epithelium composed of Sertoli and germ cells (Fig. 1). The basement membrane in mammalian testes is composed mostly by type IV collagen, laminin, entactin and heparan sulfate proteoglycans (Dobashi et al., 2003; Dym, 1994; Harvey et al., 2006; Siu and Cheng, 2004a). The hemidesmosome, an intermediate filament-based anchoring junction type restricted to cell-matrix interface, provides adhesion between epithelial cells and the ECM via interactions between integrins (function as receptors on the cell surface) and laminins or collagens (function as ligands in the ECM). Its presence on the basal surface of Sertoli cells was first reported by Connell (Connell, 1977), and hemidesmosomes are observed at the Sertoli cell-basement membrane interface by electron microscopy (Mruk and Cheng, 2004b; Siu and Cheng, 2004b). However, neither its composition nor function in the testis pertinent to spermatogenesis is presently known. Besides its presence at the apical ES, β1-integrin was also shown to be a component of the hemidesmosome, and we have recently co-localized β1-integrin with vimentin (an intermediate filament protein) and laminin α2 (a hemidesmosome protein) at the hemidesmosome in adult rat testes (Yan et al., 2008d). However, the role of hemidesmosome and basement membrane in regulating spermatogenesis and BTB dynamics remains largely unknown, except that earlier studies have shown that antibodies against the basement membrane or its components (e.g., laminins) caused extensive damage to the seminiferous epithelium which mimicked orchitis (Denduchis et al., 1985; Lustig et al., 1978; Lustig et al., 2000).
(iii) An apical ES–BTB–hemidesmosome regulatory axis to coordinate the events of spermiation and BTB restructuring at stage VIII of the seminiferous epithelial cycle
Recent studies using purified recombinant proteins prepared against different domains of the laminin γ3 or β3 chains were shown to transiently perturb the Sertoli cell TJ permeability barrier by reducing the steady-state level of occludin at the Sertoli cell BTB (Yan et al., 2008d). These observations are consistent with findings that fragments of laminin chains released at focal contacts can serve as biologically-active peptides to regulate cell adhesion and TJ barrier function in different epithelia and endothelia. Furthermore, overexpression of laminin γ3 domain IV in Sertoli cells following the establishment of a TJ barrier was shown to perturb barrier integrity associated with a significant decline of occludin and β1-integrin, and an activation of ERK1/2 (Yan et al., 2008d). Collectively these findings illustrate that biologically-active laminin fragments can directly regulate BTB dynamics (Fig. 5), and this is possibly mediated via the ERK MAPK pathway, consistent with earlier findings regarding the role of ERK in regulating BTB dynamics (Xia et al., 2006). Since these Sertoli cell cultures were devoid of any elongating spermatids and no apical ES was present in this in vitro system, the declining β1-integrin steady-state level appears to be the result of hemidesmosome disruption since both β1-integrin and laminin α2 were found to co-localize to the hemidesmosome in rat testes (Yan et al., 2008d). These findings suggest that laminin fragments can also regulate BTB dynamics indirectly via their action on hemidesmosome (Fig. 5). To verify this possibility, β1-integrin was silenced by RNAi in apical ES-absent Sertoli cell cultures with established TJ permeability barrier. The knockdown of β1-integrin led to a redistribution of occludin from the Sertoli cell surface and into the cell cytosol, interacting more with endosome-mediated endocytic vesicles as manifested by an enhanced association with EEA-1 (early endosome antigen-1, an endosome marker) (Yan et al., 2008d). Collectively, these findings demonstrate the presence of a local autocrine-based regulatory axis that coordinates the events of spermiation and BTB restructuring at stage VIII of the epithelial cycle and designated the “apical ES–BTB–hemidesmosome” functional axis (Fig. 5).
Figure 5. A schematic drawing illustrating the presence of a local regulatory loop known as the “apical ES-BTB-hemidesmosome” in the seminiferous epithelium to coordinate the events of spermiation and BTB restructuring that facilitate the transit of primary preleptotene spermatocytes at stage VIII of the seminiferous epithelial cycle.
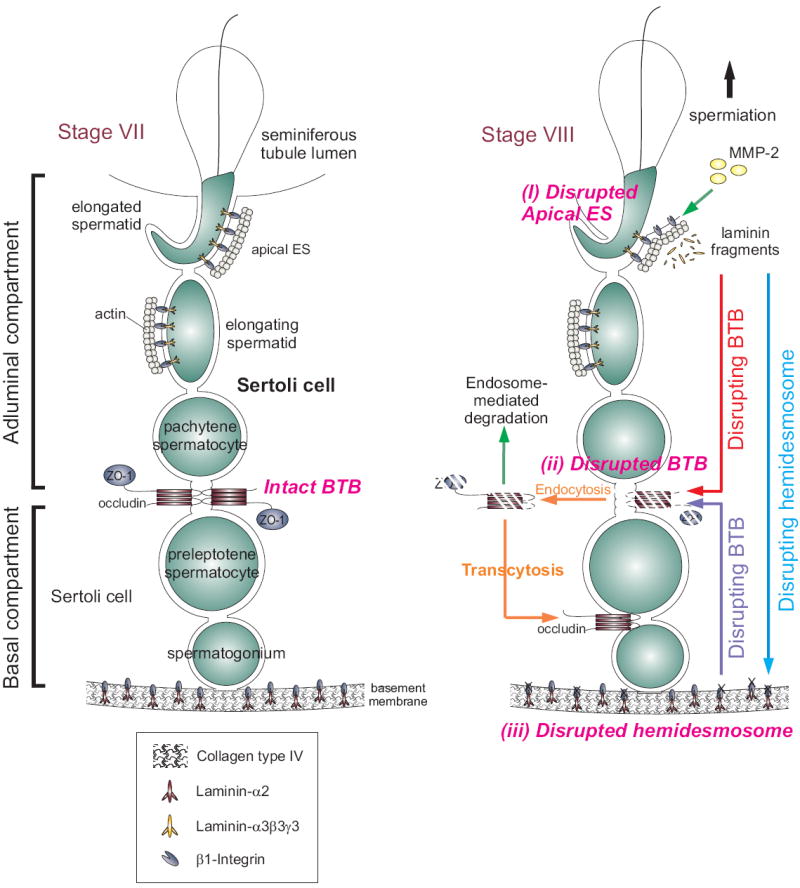
The left panel depicts the seminiferous epithelium of a normal rat testis at stage VII of the epithelial cycle. As described in the text, at the time of spermiation (see right panel), MMP-2 cleaves laminin-333 to release biologically active fragments at the time of apical ES disruption (Step i), which can either destabilize BTB (Step ii) directly by reducing the steady-state level of integral membrane proteins at the site via protein endocytosis and endosome-mediated degradation, or indirectly by perturbing the hemidesmosome function by reducing the steady-state level of β1-integrin. The disruption of the hemidesmosome (Step iii), in turn, perturbs the BTB integrity via a mechanism remains to be investigated. However, it likely involves FAK and/or cSrc. The endocytosed proteins may also be re-used to assemble ‘new ’ TJ-fibrils behind the primary spermatocyte in transit via transcytosis. Using such a mechanism, the events of spermiation and BTB restructuring that take place at the opposite ends of the seminiferous epithelium can be coordinated at stage VIII of the epithelial cycle.
(iv) Collagens and biologically active fragments from collagen chains: a local BTB-basement membrane regulatory axis
In mammalian cells and tissues, four types of collagen namely types I, II, III and IV are found. Types I, II and III collagen are non-basement membrane collagens that confer tensile strength to tissues (Siu and Cheng, 2004a; Timpl and Brown, 1996). In the testis, type I collagen forms a collagen layer located immediately behind the basement membrane (Fig. 1C) (Dym, 1994). It confers tensile strength to the seminiferous tubules. Type IV collagen and laminins are the major building blocks of the basement membrane in adult mammalian testes (Alberts et al., 2002; Dym, 1994; Enders et al., 1995; Siu and Cheng, 2004a; Timpl and Brown, 1996; Tung and Fritz, 1993). In the mammalian testis, collagens are products of Sertoli, germ and myoid cells (Davis et al., 1990; Richardson et al., 1995; Siu et al., 2003a; Skinner et al., 1985). Collagens are also known to facilitate the retention of cytokines (e.g., TNFα and TGF-β3) in the ECM (Huang et al., 1988; O’Connor-McCourt and Wakefield, 1987; Wong et al., 2004), maintaining a pool of cytokines to affect epithelial cell function such as BTB dynamics in the testis and other epithelia (Al-Sadi et al., 2009; Walsh et al., 2000). Structurally, collagens (e.g., Type IV) possess a triple helical structure, consisting of three α chains. This triple helical molecule is also the building block of the collagen network known as the monomer (Siu and Cheng, 2004a). There are six collagen α chains, designated α1 to α6 (Hudson et al., 1993; Timpl et al., 1989). In rat and bovine testes, the basement membrane is composed mostly of collagen α3(IV) and α4(IV) chains instead of the classical α1(IV) and α2(V) chains in non-gonadal basement membranes (Enders et al., 1995; Frojdman et al., 1998; Kahsai et al., 1997). Each collagen monomer is composed of a 7S noncollagenous domain composed of ~15 amino acid residues from the N-terminus, a middle collagenous domain having about 1400 residues of G-X-X repeats (G, glycine) and a carboxyl terminal noncollagenous (NC1) domain about ~230 residues from the C-terminus. It is of interest to note that peptides synthesized based on the NC1 domain of collagens were shown to be biologically active peptides, affecting junction dynamics in different epithelia (Carragher et al., 1999; Davis et al., 1990; Tsilibary et al., 1988; Vogel et al., 1997; Zhang et al., 1994). For instance, the NC1 domain of the collagen α3(IV) chain was shown to inhibit angiogenesis, suppress tumor growth [for a review, see (Hamano and Kalluri, 2005; Siu and Cheng, 2004b)]. Collagen fragments were also shown to disrupt cell adhesion via their effects on FAK, paxillin and talin at the focal adhesion complex (Carragher et al., 1999), likely mediated by an activation of integrins. For instance, α3β1-integrin is one of the putative receptors for the NC1 domain (Borza et al., 2006). Since MMP9, a matrix metalloprotease and product of Sertoli cells, has been localized to the basal compartment consistent with its localization at the BTB (Siu et al., 2003a), and collagens are known to be putative substrates of MMPs (Siu and Cheng, 2004a; Siu and Cheng, 2004b), biologically-active collagen fragments can indeed be formed in vivo to modulate TJ barrier function. This possibility must be carefully evaluated in future studies.
TNFα a cytokine found in the basement membrane, is a product of germ cells (e.g., round and elongating spermatids, and spermatocytes), macrophages, and Sertoli cells (De et al., 1993; Guazzone et al., 2009; Siu et al., 2003a; Skinner, 1993). Two structurally related, but functionally distinct TNFα receptors, p55 and p75, are found in epithelial cells (Tartaglia and Goeddel, 1992), including Sertoli cells (De Cesaris et al., 1999; Li et al., 2006), that mediate the action of TNFα in the testis (Guazzone et al., 2009). A recent study has shown that TNFα was capable of perturbing Sertoli cell TJ permeability barrier function dose-dependently (Siu et al., 2003a), illustrating a putative component of the basement membrane can affect the BTB integrity. Additionally, TNFα stimulated the production and activation of MMP-9 by Sertoli cells. It also stimulated the production of collagen α3(IV) chain and TIMP-1 (tissue inhibitor of metalloproteases I) dose-dependently by Sertoli cells in vitro (Siu et al., 2003a). Collectively, these findings suggest that activated MMP-9 induced by TNFα is used to cleave the existing collagen network in the basement membrane, perturbing the Sertoli cell-TJ barrier. This, in turn, creates a negative feedback that causes TNFα to induce collagen α2(IV) and TIMP-1 production in order to replenish the collagen network in the disrupted TJ barrier and to limit further unwanted proteolytic activity of MMP-9. This postulate is supported by a recent report illustrating that TNFα-mediated disruption of spermatogenesis as a result of Sertoli cell injury is mediated, at least in part, by MMP2 (Yao et al., 2009). These findings also illustrate the presence of a local regulatory loop between the BTB and the basement membrane. Taking these findings together, we propose the presence of a local regulatory loop in the seminiferous epithelium known as the “apical ES–BTB–hemidesmosome/basement membrane” functional axis, which coordinate the events of spermiation and BTB restructuring at stage VIII of the seminiferous epithelial cycle of spermatogenesis. This axis also provides an effective mechanism to regulate the rapid restructuring of the BTB in response to changes in the microenvironment of the seminiferous epithelium during spermatogenesis.
4. CONCLUSION AND FUTURE PERSPECTIVES
There are unprecedented advances in technology in the fields of cell biology, biochemistry, and molecular biology in the last two decades, many of which have been adopted by investigators in the field of male reproductive biology including our laboratory to expand our understanding on testicular biology. These include: (i) the establishment of an in vitro culture system to study Sertoli cell TJ permeability barrier function that mimics the BTB in vivo (Byers et al., 1986; Janecki et al., 1992; Janecki and Steinberger, 1986), (ii) the use of specific siRNA duplexes to knockdown specific target genes in this system (Li et al., 2009; Lie et al., 2009a; Siu et al., 2009b; Wong et al., 2008b; Wong et al., 2009; Yan et al., 2008d), (iii) the use of dual-labeled immunofluorescence analysis (Lie et al., 2009b; Siu et al., 2009c) and confocal microscopy (Xia et al., 2009), and (iv) biochemical techniques to track the ‘fate’ of integral membrane proteins via Sertoli cell surface protein biotinylation, endocytosis and recycling assays (Le et al., 1999; Yan et al., 2008c). Thus, many unresolved questions relating to the biology and regulation of spermatogenesis in the testis have begun to be tackled in recent years. Herein, we have attempted to provide a unified biochemical framework based on recently published findings in the field on the regulation of BTB restructuring which facilitates the transit of preleptotene spermatocytes, and how this event can be coordinated with spermiation (see Figure 5). It is understood that this model will continue to be updated and modified in the future by us and others. Nevertheless, this biochemical model does address several open questions in the field.
Acknowledgments
Studies from the authors laboratory were supported by grants from the National Institutes of Health (NICHD, R01 HD056034; U01 HD045908; R03 HD051512; U54 HD029990 Project 5) to CYC. It is noted that due to the space limits, many important and original references by investigators in the field were not cited. Instead, efforts were made to cite recent reviews for specific topics so that original research articles can be found in these reviews.
Abbreviations used
- BTB
blood-testis barrier
- CAR
coxsackie and adenovirus receptor
- CdCl2
cadmium chloride
- Co-IP
co-immunoprecipitation
- DS
desmosome-like junction, a testis-specific cell-cell intermediate filament-based anchoring junction type sharing the properties of desmosome and GJ
- ECM
extracellular matrix
- EEA-1
early endosome antigen-1
- EM
electron microscopy
- ERK
extracellular signal-regulated kinase
- ES
ectoplasmic specialization, a testis-specific AJ type and is restricted to Sertoli-Sertoli cell interface at the BTB known as basal ES, and Seroli-spermatid interface called apical ES
- FAC
focal adhesion complex also known as focal contact
- FAK
focal adhesion kinase
- FC
cell-cell actin-based cell-matrix focal contacts, an anchoring junction type also known as focal adhesion complex (FAC)
- FITC
fluorescein 5’-isothiocyanate
- GJ
gap junction
- GSK
glycogen synthase kinase, GSK3β is a Ser/Thr protein kinase identical to Tau protein kinase 1 crucial to intracellular signaling function
- HPEC
high performance electrophoresis chromatography
- IFNγ
interferon γ
- ILK
integrin-linked kinase
- JAM
junctional adhesion molecules, a class of TJ-integral membrane proteins consisting of JAM-1, 2, and 3 also called JAM-A, B, and C
- JNK
c-Jun N-terminal protein kinase also known as SAPK
- MAPK
mitogen activated protein kinase
- MEK1
MAP/ERK kinase 1
- MEKKs
MAP/ERK kinase kinases; upstream signaling molecules of p38 MAP kinase
- MESNA
2-mercaptoethanesulfonic acid
- α2-MG
α2-macroglobulin, a non-specific protease inhibitor
- MMP
matrix metalloproteases, 25 MMPs (i.e. MMP1 through MMP25) are found in ECM of multiple epithelia, and eight MMPs are currently found in the testis, including MMP-1, 2, 9, 14, 18, 23, 24 & 28 with MMP3 in Sertoli cells and MMP7 in spermatocytes
- MT1-MMP
membrane type 1-matrix metalloprotease
- p130Cas
a 130 kDa protein encoded by Crkas gene also called Crk-associated protein, a substrate of src family kinases, upon its phosphorylation, it acts as an adaptor for proteins with SH2 domains
- PTK
protein tyrosine kinase
- PTKi
protein tyrosine kinase inhibitor
- SH domains
Src homology domains that are involved in the interaction with phosphorylated Tyr resides on other proteins (SH2 domains) or with Pro-rich regions of other proteins (SH3 domains)
- Src gene
sarcoma-inducing gene of Rous sarcoma virus encoding the PTK Src
- TBC
tubulobulbar complex
- TER
transepithelial electrical resistance
- TGF-β
transforming growth factor-β
- TIMP
tissue inhibitor of metalloproteases 1 - 4 are all found in testes
- TJ
tight junction
- TNF-α
tumor necrosis factor-α
- ZO-1
zonula occludens-1
Footnotes
Studies from the authors laboratory were supported by grants from the National Institutes of Health (NICHD, R01 HD056034; U01 HD045908; R03 HD051512; U54 HD029990 Project 5, all to CYC).
References
- Al-Sadi R, Boivin M, T M. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2002. [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter W, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones J, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Bernacki J, Dobrowolska A, Nierwinska K, Malecki A. Physiology and pharmacological role of the blood-brain barrier. Pharmacol Rep. 2008;60:600–622. [PubMed] [Google Scholar]
- Borza CM, Pozzi A, Borza DB, Pedchenko V, Hellmark T, Hudson BG, Zent R. Integrin α3β1, a novel receptor for α3(IV) noncollagenous domain and a trans-dominant inhibitor for integrin αvβ3. J Biol Chem. 2006;281:20932–20939. doi: 10.1074/jbc.M601147200. [DOI] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Byers S, Hadley M, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Carragher N, Frame M. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14:241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carragher N, Levkau B, Ross R, Raines E. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125FAK, paxillin, and talin. J Cell Biol. 1999;147:619–629. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussanel V, Tabone E, Hendrick J, Dacheux F, Benahmed M. Cellular distribution of transforming growth factor βs 1, 2, and 3 and their types I and II receptors during postnatal development and spermatogenesis in the boar testis. Biol Reprod. 1997;56:357–367. doi: 10.1095/biolreprod56.2.357. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in Sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Cobb J, Cargille B, Handel MA. Acquisition of competence to condense metaphase I chromosomes during spermatogenesis. Dev Biol. 1999;205:49–64. doi: 10.1006/dbio.1998.9101. [DOI] [PubMed] [Google Scholar]
- Connell C. The Sertoli cells of the sexually mature dog. Anat Rec. 1977;185:133. abstract. [Google Scholar]
- Cordenonsi M, Mazzon E, Derigo L, Baraldo S, Meggio F, Citi S. Occludin dephosphorylation in early development of Xenopus laevis. J Cell Sci. 1997;110:3131–3139. doi: 10.1242/jcs.110.24.3131. [DOI] [PubMed] [Google Scholar]
- Couture L, Nash JA, Turgeon J. The ATP-binding cassette transporters and their implication in drug disposition: A special look at the heart. Pharmacol Rev. 2006;58:244–258. doi: 10.1124/pr.58.2.7. [DOI] [PubMed] [Google Scholar]
- Davis C, Papadopoulos V, Sommers C, Kleinman H, Dym M. Differential expression of extracellular matrix components in rat Sertoli cells. Biol Reprod. 1990;43:860–869. doi: 10.1095/biolreprod43.5.860. [DOI] [PubMed] [Google Scholar]
- De Cesaris P, Starace D, Starace G, Filippini A, Stefanini M, Ziparo E. Activation of Jun N-terminal kinase/stress-activated protein kinase pathway by tumor necrosis factor α leads to intercellular adhesion molecule-1 expression. J Biol Chem. 1999;274:28978–28982. doi: 10.1074/jbc.274.41.28978. [DOI] [PubMed] [Google Scholar]
- de Kretser D, Kerr J. The cytology of the testis. In: Knobil E, Neill J, Ewing L, Greenwald G, Markert and C, Pfaff D, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- de Rooij D, Russell L. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- De S, Chen H, Pace J, Hunt J, Terranova P, Enders G. Expression of tumor necrosis factor-α in mouse spermatogenic cells. Endocrinology. 1993;133:389–396. doi: 10.1210/endo.133.1.8319585. [DOI] [PubMed] [Google Scholar]
- Denduchis B, Satz ML, Sztein MB, Puig RP. Multifocal damage to the testis induced in rats by passive transfer or antibodies prepared against non-collagenous fraction of basement membrane. J Reprod Immunol. 1985;7:59–75. doi: 10.1016/0165-0378(85)90021-x. [DOI] [PubMed] [Google Scholar]
- Di Agostino S, Rossi P, Geremia R, Sette C. The MAPK pathway triggers activation of Nek2 during chromosome condensation in mouse spermatocytes. Development. 2002;129:1715–1727. doi: 10.1242/dev.129.7.1715. [DOI] [PubMed] [Google Scholar]
- Dobashi M, Fujisawa M, Naito I, Yamazaki T, Okada H, Kamidono S. Distribution of type IV collagen subtypes in human testes and their association with spermatogenesis. Fertil Steril. 2003;80(Suppl 2):755–760. doi: 10.1016/s0015-0282(03)00775-1. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009 doi: 10.1146/annurev.biochem.78.081307.110540. in press. [DOI] [PubMed] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973;175:639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett D. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett D. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Enders G, Kahsai T, Lian G, Funabiki K, Kille P, BG H. Developmental changes in seminiferous tubule extracellular matrix components of the mouse testis: α3(IV) collagen chain expressed at the initiation of spermatogenesis. Biol Reprod. 1995;53:1489–1499. doi: 10.1095/biolreprod53.6.1489. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. Intercellular bridges. Exp Cell Res. 1961;8:174–187. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Biophys Cytol. 1959;5:453–460. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flens MJ, Zaman GJ, van der Valk P, Izquierdo MA, Schroeijers AB, Scheffer GL, van der Groep P, de Haas M, Meijer CJ, Scheper RJ. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237–1247. [PMC free article] [PubMed] [Google Scholar]
- Frojdman K, Pelliniemi L, Virtanen I. Differential distribution of type IV collagen chains in the development rat testis and ovary. Differentiation. 1998;63:125–130. doi: 10.1046/j.1432-0436.1998.6330125.x. [DOI] [PubMed] [Google Scholar]
- Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004;25:423–429. doi: 10.1016/j.tips.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Galletta BJ, Cooper JA. Actin and endocytosis: mechanisms and phylogeny. Curr Opin Cell Biol. 2009;21:20–27. doi: 10.1016/j.ceb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson E, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Greenbaum MP, Iwamori N, Agno JE, Matzuk MM. Mouse TEX14 is required for embryonic germ cell intercellular bridges but not female fertility. Biol Reprod. 2009;80:449–457. doi: 10.1095/biolreprod.108.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin Y, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci USA. 2006;103:4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzone VA, Jacobo P, Theas M, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Techn. 2009 doi: 10.1002/jemt.20704. in press. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–403. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney K, Estrada E, Rosenberg G. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hallmann R, Hom N, Selg M, Wendler O, Pausch F, Sorokin L. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- Hamano Y, Kalluri R. Tumstatin, the NC1 domain of α3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun. 2005;333:292–298. doi: 10.1016/j.bbrc.2005.05.130. [DOI] [PubMed] [Google Scholar]
- Harvey SJ, Perry J, Zheng K, Chen D, Sado Y, Jefferson B, Ninomiya Y, Jacobs R, Hudson BG, Thorner PS. Sequential expression of type IV collagen networks: testis as a model and relevance to spermatogenesis. Am J Pathol. 2006;168:1587–1597. doi: 10.2353/ajpath.2006.050816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R, de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 1–15. [Google Scholar]
- Huang S, O’Grady P, Huang J. Human transforming growth factor β α2-Macroglobulin complex is a latent form of transforming growth factor β. J Biol Chem. 1988;263:1535–1541. [PubMed] [Google Scholar]
- Huckins C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: An analysis using a simplified classification of the germinal epithelium. Anat Rec. 1978a;190:905–926. doi: 10.1002/ar.1091900410. [DOI] [PubMed] [Google Scholar]
- Huckins C. Spermatogonial intercellular bridges in whole-mounted seminiferous tubules from normal and irradiated rodent testis. Am J Anat. 1978b;153:97–121. doi: 10.1002/aja.1001530107. [DOI] [PubMed] [Google Scholar]
- Hudson B, Reers S, Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures - a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–57. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- Janecki A, Steinberger A. Polarized Sertoli cell functions in a new two-compartment culture system. J Androl. 1986;7:69–71. doi: 10.1002/j.1939-4640.1986.tb00873.x. [DOI] [PubMed] [Google Scholar]
- Johnson K, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherins in rat testis. Biol Reprod. 2002;66:992–1000. doi: 10.1095/biolreprod66.4.992. [DOI] [PubMed] [Google Scholar]
- Kahsai TZ, Enders GC, Gunwar S, Brunmark C, Wieslander J, Kalluri R, Zhou J, Noelken ME, Hudson BG. Seminiferous tubule basement membrane. Composition and organization of type IV collagen chains, and the linkage of α3(IV) and α5(IV) chains. J Biol Chem. 1997;272:17023–17032. doi: 10.1074/jbc.272.27.17023. [DOI] [PubMed] [Google Scholar]
- Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- Kato A, Nagata Y, Todokoro K. δ-Tubulin is a component of intercellular bridges and both the early and mature perinuclear rings during spermatogenesis. Dev Biol. 2004;269:196–205. doi: 10.1016/j.ydbio.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Koch M, Olson P, Albus A, Jin W, Hunter D, Brunken W, Burgeson R, Champliaud M. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse-Battistoni B, Morera A, Goddard I, Benahmed M. Expression of mRNAs for transforming growth factor-β receptors in the rat testis. Endocrinology. 1995;136:2788–2791. doi: 10.1210/endo.136.6.7750505. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: A potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- LeBlond C, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Cheng CY. “Unlocking” the blood-testis barrier and the ectoplasmic specialization by cytokines during spermatogenesis: Emerging targets for male contraception. Immun Endocr Metab Agents Med Chem. 2008;8:20–27. [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a signaling complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0901700106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHY, Siu MKY, Lui WY, Lee WM, Cheng CY. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. Desmosomes are a component of the blood-testis barrier and confer barrier integrity. Proc Natl Acad Sci USA. 2009a in press. [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. The FASEB J. 2009b doi: 10.1096/fj.06-070573. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longin J, Guillaumot P, Chauvin M, Morera A, B LM-B. MT1-MMP in rat testicular development and the control of Sertoli cell proMMP2 activation. J Cell Sci. 2001;114:2125–2134. doi: 10.1242/jcs.114.11.2125. [DOI] [PubMed] [Google Scholar]
- Loveland KL, Dias V, Meachem S, Rajpert-De Meyts E. The transforming growth factor-β superfamily in early spermatogenesis: Potential relevance to testicular dysgenesis. Int J Androl. 2007;30:377–384. doi: 10.1111/j.1365-2605.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- Lui W, Lee W, Cheng C. TGF-βs: their role in testicular function and Sertoli cell tight junction dynamics. Int J Androl. 2003a;26:147–160. doi: 10.1046/j.1365-2605.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003b;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003c;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- Lustig L, Denduchis B, Gonzalez NN, Puig RP. Experimental orchitis induced in rats by passive transfer of an antiserum to seminiferous tubule basement membrane. Arch Androl. 1978;1:333–343. doi: 10.3109/01485017808988354. [DOI] [PubMed] [Google Scholar]
- Lustig L, Denduchis B, Ponzio R, Lauzon M, Pelletier RW. Passive immunization with anti-laminin immunoglobulin G modifies the integrity of the seminiferous epithelium and induces arrest of spermatogenesis in the guinea pig. Biol Reprod. 2000;62:1505–1514. doi: 10.1095/biolreprod62.6.1505. [DOI] [PubMed] [Google Scholar]
- Maeda T, Goto A, Kobayashi D, Tamai I. Transport of organic cations across the blood-testis barrier. Mol Pharm. 2007;4:600–607. doi: 10.1021/mp070023l. [DOI] [PubMed] [Google Scholar]
- McCawley L, Matrisian L. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Melaine N, Lienard MO, Dorval I, Le Goascogne C, Lejeune H, Jegou B. Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod. 2002;67:1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16670. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R, McMahon HT. Arrestins as adaptors for ubiquitination in endocytosis and sorting. EMBO Reports. 2009;10:41–43. doi: 10.1038/embor.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D, Cheng C. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004a;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004b;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008a;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Delivering non-hormonal contraceptives to men: advances and obstacles. Trend Biotechnol. 2008b;26:90–99. doi: 10.1016/j.tibtech.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- O’Connor-McCourt M, Wakefield L. Latent transforming growth factor-β in serum. A specific complex with α2-macroglobulin. J Biol Chem. 1987;262:14090–14099. [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pelletier R. The tight junctions in the testis, epididymis, and vas deferens. In: Cereijido M, Anderson J, editors. Tight Junctions. New York: CRC Press; 2001. pp. 599–628. [Google Scholar]
- Petronczki M, Siomos MF, Nasmyth K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochim Biophys Acta. 2005;1719:102–116. doi: 10.1016/j.bbamem.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Pointis G, Segretain D. Role of connexin-based gap junction channels in testis. Trends Endocrinol Metab. 2005;16:300–306. doi: 10.1016/j.tem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ren H, Russell L. Quantitation of Sertoli cell-germ cell desmosome gap junctions in relation to meiotic divisions in the male rat. Tissue Cell. 1992;24:565–573. doi: 10.1016/0040-8166(92)90072-f. [DOI] [PubMed] [Google Scholar]
- Ren HP, Russell LD. Clonal development of interconnected germ cells in the rat and its relationship to the segmental and subsegmental organization of spermatogenesis. Am J Anat. 1991;192:121–128. doi: 10.1002/aja.1001920203. [DOI] [PubMed] [Google Scholar]
- Richardson L, Kleinman H, Dym M. Basement membrane gene expression by Sertoli and peritubular myoid cells in vitro in the rat. Biol Reprod. 1995;52:320–330. doi: 10.1095/biolreprod52.2.320. [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. ABCG2: a perspective. Adv Drug Deliv Rev. 2009;61:3–13. doi: 10.1016/j.addr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977a;148:301–312. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- Russell L. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977b;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–278. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- Russell L, Peterson R. Sertoli cell junctions: morphological and functional correlates. Int J Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977c;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58:371–378. doi: 10.1095/biolreprod58.2.371. [DOI] [PubMed] [Google Scholar]
- Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- Schaller M, Otey C, Hildebrand J, Parson J. Focal adhesion kinase and paxillin bind to peptides mimicking β integrin cytoplasmic domains. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 212–233. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Applied Pharmacol. 2009a doi: 10.1016/j.taap.2009.01.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009b doi: 10.1073/pnas.0813113106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase (FAK) protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009c doi: 10.1210/en.2008-1741. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu M, Cheng C. Extracellular matrix: Recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod. 2004a;71:375–391. doi: 10.1095/biolreprod.104.028225. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004b;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004c;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003a;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003b;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Skinner M. Secretion of growth factors and other regulatory factors. In: Russell L, Griswold M, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 237–247. [Google Scholar]
- Skinner M, Tung P, Fritz I. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Sternlicht M, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Mruk DD. Drug transporter, p-glycoprotein (MDR1), is an integrated component of the mammalian blood-testis barrier. Endocrinology. 2009 doi: 10.1016/j.biocel.2009.08.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The Par-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Tartaglia L, Goeddel D. Two TNF receptors. Immunol Today. 1992;13:151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown J. Supramolecular assembly of basement membranes. BioEssays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, K K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1989;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cells: new vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Trezise AE, Romano PR, Gill DR, Hyde SC, Sepulveda FV, Buchwald M, Higgins CF. The multidrug resistance and cystic fibrosis genes have complementary patterns of epithelial expression. EMBO J. 1992;11:4291–4303. doi: 10.1002/j.1460-2075.1992.tb05528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilibary E, Cahronis A, Reger L, Wohlhueter R, Furcht T. The effect of nonenzymatic glucosylation on the binding of the main noncollagenous NC1 domain to type IV collagen. J Biol Chem. 1988;263:4302–4308. [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Tung P, Fritz I. Interactions of Sertoli cells with laminin are essential to maintain integrity of the cytoskeleton and barrier functions of cells in culture in the two-chambered assembly. J Cell Physiol. 1993;156:1–11. doi: 10.1002/jcp.1041560102. [DOI] [PubMed] [Google Scholar]
- Udayakumar T, Chen M, Bair E, Von Bredow D, Cress A, Nagle R, Bowden G. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cell cleaves human laminin-5 β3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- Vogel W, Gish G, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Vogl A, Pfeiffer D, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- Vogl A, Pfeiffer D, Redenbach D. Ectoplasmic (“junctional”) specializations in mammalian Sertoli cells: influence on spermatogenic cells. Ann NY Acad Sci. 1991;637:175–202. doi: 10.1111/j.1749-6632.1991.tb27310.x. [DOI] [PubMed] [Google Scholar]
- Vogl A, Pfeiffer D, Redenbach D, Grove B. Sertoli cell cytoskeleton. In: Russell L, Griswold M, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- Vogl A, Vaid K, Guttman J. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 186–211. [Google Scholar]
- Walsh S, Hopkins A, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv Drug Deliv Rev. 2000;41:303–313. doi: 10.1016/s0169-409x(00)00048-x. [DOI] [PubMed] [Google Scholar]
- Wang CQF, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol. 2007;178:549–556. doi: 10.1083/jcb.200704061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant’Agnese PA, deMesy-Bentley KL, Tzeng CR, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junction complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Weber J, Russell L, Wong V, Peterson R. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Wiltshire T, Park C, Caldwell KA, Handel MA. Induced premature G2/M-phase transition in pachytene spermatocytes includes events unique to meiosis. Dev Biol. 1995;169:557–567. doi: 10.1006/dbio.1995.1169. [DOI] [PubMed] [Google Scholar]
- Wolski K, Perrault C, Tran-Son-Tay R, Cameron D. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008a;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008b;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 is a cell adhesion regulator in the seminiferous epithelium of rat testes. Endocrinology. 2009 doi: 10.1210/en.2009-0427. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Gregory C, DePhilip R. Expression of E-cadherin in immature rat and mouse testis and in rat Sertoli cell cultures. Biol Reprod. 1993;49:1353–1361. doi: 10.1095/biolreprod49.6.1353. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- Xia W, Wong EWP, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Cross-talk between tight and anchoring junctions - lesson from the testis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience and Springer Science+Business Media; 2008a. pp. 234–254. [Google Scholar]
- Yan HHY, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- Yan HHY, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: The biology, regulation, and implication in male contraceptive development. Curr Top Dev Biol. 2008b;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- Yan HHY, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHY, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008c;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHY, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008d;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richberg JH. TNFα-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Mruk D, Cheng C. Myotubularin phosphoinositide phosphatases, protein phosphatases, and kinases: their roles in junction dynamics and spermatogenesis. J Cell Physiol. 2005;204:470–483. doi: 10.1002/jcp.20303. [DOI] [PubMed] [Google Scholar]
- Zhang X, Hudson B, Jr, Sarras M. Hydra cell aggregate development is blocked by selective fragments of fibronectin and type IV collagen. Dev Biol. 1994;164:10–23. doi: 10.1006/dbio.1994.1176. [DOI] [PubMed] [Google Scholar]


