Abstract
During spermatogenesis in mammalian testes, junction restructuring takes place at the Sertoli-Sertoli and Sertoli-germ cell interface, which is coupled with the development, such as cell cycle progression, and translocation of the germ cell in the seminiferous epithelium. In the rat testis, the restructuring of the blood-testis barrier (BTB) formed between Sertoli cells near the basal region and the disruption of the apical ectoplasmic specialization (apical ES) between Sertoli cells and fully developed spermatids (spermatozoa) at the luminal edge of the seminiferous epithelium occur concurrently at stage VIII of the seminiferous epithelial cycle of spermatogenesis. These two processes are essential for the translocation of primary spermatocytes from the basal to the apical compartment to prepare for meiosis, and the release of spermatozoa to the lumen of the seminiferous epithelium at spermiation, respectively. Cytokines, such as TNFα and TGFβ3, are present at high level in the microenvironment of the epithelium at this stage of the epithelial cycle. Since these cytokines were shown to disrupt the BTB integrity and germ cell adhesion, it was proposed that some cytokines released from germ cells particularly primary spermatocytes and Sertoli cells, would induce the junction restructuring of the BTB and apical ES at stage VIII of the seminiferous epithelial cycle. In this review, the intricate role of cytokines and testosterone to regulate the transit of primary spermatocytes at the BTB and spermiation will be discussed. Possible regulators that mediate the cytokine-induced junction restructuring, including the gap junction and extracellular matrix, will also be discussed.
Keywords: Testis, spermatogenesis, cytokines, TGF-β3, TNFα, testosterone, blood-testis barrier, primary spermatocytes, seminiferous epithelial cycle
1. The junction restructuring events in the seminiferous epithelium during spermatogenesis
Haploid (1n) spermatids are male gamates developed from diploid (2n) spermatogonia in a multi-step process known as spermatogenesis. Spermatogenesis is divided into four major phases, namely (i) mitosis for the self renewal of spermatogonia, (ii) meiosis for the formation of spermatids, (iii) spermiogenesis for the development of spermatids from step 1 through step 19 in rats, and (iv) spermiation for the detachment of mature spermatids (i.e., spermatozoa) from the seminiferous epithelium with the residual bodies phagocytosed by the Sertoli cell. These testicular spermatozoa can enter the epididymis for their eventual maturation. Spermatogenesis occurs in the seminiferous epithelium of the mammalian testis, where Sertoli cells and germ cells reside [1]. The Sertoli cell is responsible for nurturing and supporting the development of germ cells and is also known as the ‘nursery’ cell in the testis.
Most events of spermatogenesis, namely meiosis, spermiogenesis and spermiation, take place in a unique microenvironment behind the blood-testis barrier (BTB), which is created between adjacent Sertoli cells near the basement membrane of the seminiferous tubule. The BTB thus segregates the seminiferous epithelium into the basal and the apical (or adluminal) compartment, with the spermatogonial renewal (via mitosis) takes place in the basal compartment (Fig. 1). The BTB is responsible for conferring polarity to the Sertoli cell and regulating the paracellular diffusion of water, electrolytes, nutrients and biomolecules from the systemic circulation in the interstitium to the developing germ cells. It also confers an immunological barrier to segregate all the post-meiotic germ cell development from the host immune system because of the appearance, most transiently, of spermatid antigens during spermiogenesis. However, until the release of elongated spermatids into the lumen of the seminiferous tubule at spermiation, germ cells at different stages of their development remain adhered to Sertoli cells. Thus, it is conceivable that junctions formed between Sertoli cells and between Sertoli and germ cells must be restructured continuously to accommodate the translocation and morphological transformation of germ cells during their development.
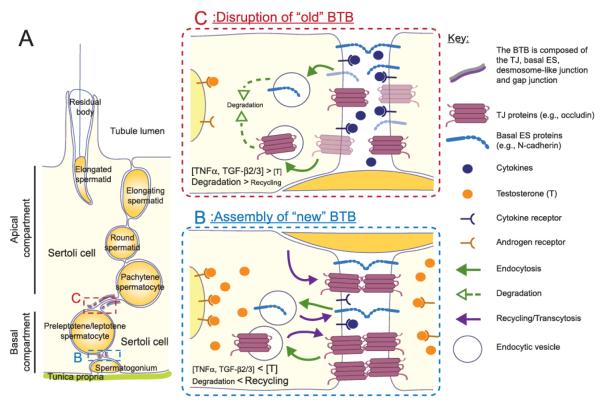
Figure 1. Schematic drawing of the interplay of testosterone and cytokines including TNFα and TGFβs in the regulation of junction dynamics in the seminiferous epithelium during spermatogenesis.
The seminiferous epithelium of the testis is segregated by the BTB into the apical and basal compartment (A). Preleptotene spermatocytes are in transit at the BTB at stages VIII-IX of the seminiferous epithelial cycle to continue the germ cell development in the apical compartment. As discussed in the text, there are reports illustrating the effects of testosterone and cytokines on the trafficking of integral membrane proteins to regulate the junction dynamics at the BTB. Both testosterone and cytokines were shown to accelerate the endocytosis of integral junction proteins at the BTB. But they have opposing effects on the BTB integrity. Testosterone would promote the recycling of the endocytosed protein while cytokines would induce the degradation of the endocytosed protein. Hence, testosterone may promote the junction assembly on the basal side of a spermatocyte in transit (B). The junction complexes on the apical side of the spermatocyte would be disrupted as induced by cytokines such as TNFα and TGFβs to accommodate the spermatocyte movement across the BTB (C). The preleptotene spermatocyte could then pass through the BTB without affecting its integrity concurrently.
During spermatogenesis, the seminiferous epithelial cycle refers to the unique cellular association between Sertoli cells and developing germ cells, most notably spermatids, at different stages of their development (steps 1 to 19 in rats), found in a given area of the seminiferous epithelium [2, 3]. The duration and number of stages in each cycle of spermatogenesis vary in different animals. In adult rats, each cycle consists of 14 stages and completes in ∼12.9 days while only twelve and six stages are found in mice and humans, respectively.
Extensive junction restructuring occurs at stage VIII of the seminiferous epithelial cycle in rats or mice. At this stage, spermiation takes place at the luminal edge of the apical compartment of the epithelium to release mature elongated spermatids (i.e., spermatozoa) into the lumen of the seminiferous tubule [4]. The restructuring of the BTB occurs at the same stage to facilitate the transit of primary preleptotene spermatocytes from the basal to the apical compartment but the BTB integrity has to be maintained at the same time (Fig. 1). During their transit at the BTB, preleptotene spermatocytes have to differentiate into leptotene and zygotene spermatocytes to prepare for the metaphase I of meiosis, such as duplication and condensation of genetic materials. Recent studies have shown that various signaling pathways, including the integrin-laminin network to be discussed below, are probably involved in regulating the concurrent junction restructuring at the BTB and apical ES. Cytokines, especially tumor necrosis factor α (TNFα) and transforming growth factor-β3 (TGF-β3), are postulated to be the signals released by germ cells and to work in concert with androgens, such as testosterone, released from Leydig cells, to trigger these events based on recent studies in the adult rat testis and primary Sertoli cell cultures with established tight junction (TJ)-permeability barrier that mimics the BTB in vivo.
2. Overview of the junction complexes in the seminiferous epithelium
Various junction complexes can be found between Sertoli cells and between Sertoli and germ cells in the seminiferous epithelium, which include TJ, anchoring junctions and communicating gap junctions (see Table 1). Most of these junction complexes are formed in a tightly regulated spatiotemporal manner. The cell-cell anchoring junctions include the actin-based testis-specific atypical adherens junction named ectoplasmic specialization (ES), the intermediate filament-based desmosome-like junction, and the tubulobulbar complex (TBC). The intermediate filament-based hemidesmosome is the only cell-matrix anchoring junction known in the seminiferous epithelium since the actin-based focal contact has not been described [4, 5].
Table 1.
Different cell junction types, their functions, and structural composition in the testis
| Junction Type | Tight Junction | Anchoring Junction | Gap Junction | ||||
|---|---|---|---|---|---|---|---|
| Adherens Junction |
Ectoplasmic specialization* |
Desmosome-like Junction |
Tubulobulbar Complex |
Hemidesmosome | |||
| Cell-Cell (Sertoli cell- Sertoli cell only) |
Cell-Cell | Cell-Cell | Cell-Cell | Cell-Cell | Cell-matrix | Cell-Cell | |
| Functions | To form polarized barrier, regulate paracellular diffusion and regulate intramembrane diffusion |
For cell adhesion between Sertoli cells and between Sertoli cells and germ cells |
To provide adhesion that can withstand mechanical force |
Proposed to internalize the cytoplasm and junction complexes from the late spermatid and BTB |
For attachment of Sertoli cells and spermatogonia to the basement membrane |
Gap junction channels: to mediate intercellular communication; hemichannels: to release small molecules into the extracellular space |
|
|
Transmembrane protein |
Tetraspan: Occludin, Claudins, Tricellulin; Single span: JAMs (-A and —B), CAR, CLMP, CRB3 |
Cadherins (N-, E-, and P-), Nectins (-2 and -3), JAM-C, Vezatin |
Desmogleins, Desmocollins, Connexins |
/ | Integrins (α6β1) | Connexins | |
|
Peripheral Adaptors |
The ZOs (-1, 2 and -3), MAGIs, Cingulin, ZONAB |
Catenins (α, β, and γ), Afadins, ZO-1 |
Plakophilins, Plakogoblins, Desmoplakins, Plectins |
Dynamins (2 and 3), Cofilin, Arp3, N- WASP, Cortactin, Clathrin |
/ | ZO-1, Drebrin | |
|
Extracellular Matrix |
/ | / | / | / | / | Laminins, Collagens |
/ |
| Cytoskeleton | Actin Filament | Actin Filament |
Actin Filament (hexagonally packed) |
Intermediate filament (Vimentin) |
Microtubule, Actin filament |
Intermediate filament |
Actin Filament |
This table is not intended to be exhaustive. Readers are encouraged to consult the following reviews for further details on the tight junction [86-88], anchoring junction (adherens junction, ectoplasmic specialization [6], desmosome [89], and tubulobulbar complex [90, 91]) [5], and gap junction [51].
Ectoplasmic specialization was listed under the anchoring junction type in this table even though it displayed properties of the tight junction and focal adhesion. Arp, actin-related protein; CAR, coxsackievirus and adenovirus receptor; CLMP, coxsackievirus and adenovirus receptor-like membrane protein; CRB3, crumbs protein homolog 3; JAM, junctional adhesion molecule; N-WASP, neural Wiskott-Aldrich syndrome protein; ZO-1, zonula occludens protein 1; ZONAB, zonula occludens protein 1—associated nucleic acid binding proteins.
Between adjacent Sertoli cells, there is a specialized ultrastructure close to the basement membrane known as the BTB, which is constituted mostly by TJ and the atypical adherens junction complex between Sertoli cells known as the basal ES [4, 6, 7] (Fig. 1). The ES is characterized by the hexagonally packed actin filament bundles sandwiched between the Sertoli cell plasma membrane and the endoplasmic reticulum [8-10]. Besides the TJ and the basal ES, the desmosome-like junction and gap junction are also the integral components of the BTB [11-14]. The BTB is the only site where functional TJ are found in the seminiferous epithelium [4]. However, some proteins known to be restricted to TJ in other epithelia, such as coxsackievirus and adenovirus receptor (CAR) and JAM-C, were also detected in germ cells at the apical ES apart from the BTB [15-17].
The junction complexes at the Sertoli-germ cell interface depend on the stage of development of germ cells. For instance, desmosome-like junctions are formed between primary spermatocytes or round spermatids and Sertoli cells [4, 6]. When round spermatids start to elongate in step 8, all the anchoring junction would be replaced by the apical ES, which forms along the head of elongating or elongated spermatids. The apical ES differs from the basal ES as the typical ES ultrastructure can only be observed on the Sertoli cell side whereas the ES ultrastructure is present on both sides of the Sertoli cells at the basal ES in the BTB [7, 8]. While the ultrastructure of the actin-based cell-matrix junction type known as the focal contact or focal adhesion complex in other epithelia are absent in the testis, components of focal contact were found at the apical ES, including the integrin, laminin, focal adhesion kinase, paxillin, and vinculin [5, 18]. The apical TBC is also detected on the concave side of elongated spermatids a few hours before spermiation at stage VIII of the seminiferous epithelial cycle and it is mutually exclusive with the apical ES [19, 20].
3. The effects of cytokines on the junction dynamics in the seminiferous epithelium
3.1. TNFα and TGFβs
The effects of TNFα and TGF-β3 on the junction dynamics were the most studied in the seminiferous epithelium of rat testes. TNFα is secreted predominantly by germ cells (namely pachytene spermatocytes and round spermatids) and macrophages in the interstitium instead of Sertoli cells in the testes [21-23]. Its receptors, tumor necrosis factor receptor 1 (TNFR1) and TNFR2, however, are mainly expressed by Sertoli cells [21, 23]. TGF-βs are expressed by both Sertoli cells, spermatocytes and round spermatids in the seminiferous epithelium and its receptor can be found on both Sertoli cells and germ cells [24]. Both cytokines were expressed at relatively high level at stage VIII of the seminiferous epithelial cycle and were shown to disrupt the BTB integrity in vivo and in vitro and induce germ cell loss in vivo [21, 22, 24-27]. The restructuring of the BTB and apical ES takes place at the same stage of the epithelial cycle and both cytokines are secreted by germ cells [21, 22]. It was therefore postulated that cytokines, such as TNFα and TGFβ3, are secreted by germ cells, most likely primary spermatocytes, to induce junction restructuring at the BTB and apical ES at stage VIII of the epithelial cycle.
After local administration of recombinant cytokines to the testis at a concentration comparable to the steady-state level of either TNFα or TGF-β3 in the testis, the BTB became reversibly disrupted [21, 22]. For instance, after the cytokine treatment, the BTB became permeable to a small fluorescence tracer (e.g., FITC or inulin-FITC) which was administered into the systemic circulation via the jugular vein. The tracer was detected in the apical compartment, illustrating that the BTB has been disrupted. However, in normal testis, the BTB was able to limit the tracer at or near the basement membrane [21, 22]. These findings thus indicate that TNFα and TGFβ3 were capable of disrupting the BTB integrity in vivo. This cytokine-induced junction disruption also led to a loss of germ cell from the epithelium. The disruption of the BTB and loss of germ cell adhesion were caused, at least in part, by a decline in the steady-state levels of integral membrane proteins and their redistribution at the BTB and Sertoli-germ cell interface [21, 25].
Recent in vitro studies using primary Sertoli cell cultures, with the establishment of a functional TJ-barrier that mimics the BTB in vivo, have shown that treatment of the Sertoli cell epithelium with TNFα, TGF-β3 orTGF-β2 caused a rapid disruption of TJ and anchoring junction at the cell-cell interface through an increase in the kinetics of protein endocytosis [22, 28]. The accelerated endocytosis of junction proteins, such as occludin, JAM-A and N-cadherin, is mediated by clathrin and dynamin-2 and -3 as shown in studies with the use of inhibitor and RNAi [22]. The endocytosed proteins from the cell surface would then undergo endosome-mediated degradation as occludin was shown to bind more with the late endosome marker Rab9 after TGF-β2 treatment [28]. These studies indicate that besides lowering the steady-state levels of junction proteins in Sertoli cells, these cytokines are able to induce the BTB restructuring rapidly through reducing the bioavailability of junction proteins on the cell surface.
3.2. Interleukin-1α
Interleukin-1α (IL-1α) is another cytokine that was shown to be involved in the regulation of junction dynamics during spermatogenesis. It was recently shown to increase the BTB permeability after local administration to adult rat testes [29]. IL-1α was expressed by pachytene spermatocytes in immature rats [30] and Sertoli cells [31, 32]. Its secretion by Sertoli cells depends on the presence of germ cells as IL-1α was absent in the testis during busfulan-induced azoospermia or following fetal irradiation to knock-down germ cells [31]. IL-1α was expressed in the seminiferous epithelium at all stages of the seminiferous epithelial cycle except at stage VII when it was expressed at a very low level [31, 33]. This is in contrast to the high level of TNFα and TGFβ3 detected at stages VII-VIII of the seminiferous epithelial cycle. IL-1α exerts its biological effects via its coupling with its receptor interleukin-1 receptor which is localized in both Sertoli and germ cells [34].
3.3. Differential actions of cytokines on junction restructuring in the testis
Besides the expression profile, the mechanisms that mediate the BTB disruption induced by IL-1α appears to be different from that by TNFα and TGF-β3. IL-1α exerted its effect on the actin cytoskeleton network without affecting the steady-state levels of junction proteins [29] whereas TNFα and TGF-β3 were able to lower the steady-state levels and disrupt the localization of TJ and basal ES junction proteins at the BTB besides disrupting the actin filament network [21, 25]. The spermatogenic arrest caused by IL-1α was gradual and did not recover even after 90 days following its administration directly to the testis [29]. On the other hand, the TNFα- and TGF-β3-induced spermatogenic arrest was transient and rapid. Its disruptive effect on the BTB integrity and junction protein localization was reversed after 14 days of the administration [21, 22, 25].
Furthermore, IL-1α and TGF-β2 was shown recently to have opposing effect on the transcriptional control of JAM-B in the mouse Sertoli cell line MSC [35]. The former one activated the expression of JAM-B whilst the later one repressed its transcription. This difference on the transcriptional control of JAM-B was suggested to match the stage-specific expression of JAM-B. A low level of IL-1α together with a high level of TGF-β2 would probably promote a prolonged reduction of JAM-B that is probably needed for the junction restructuring during stage VIII-IX. The difference in the expression profile and effects on the junction dynamics thus imply that IL-1α and TNFα/TGF-βs may have differential roles in the regulation of junction dynamics during spermatogenesis in the testis.
4. Physiological role of cytokines and their interplay with testosterone in the regulation of the junction dynamics during spermatogenesis
4.1. Physiological role of cytokines in junction restructuring during spermatogenesis
Ever since the studies of the putative role of TNFα and TGF-βs on the junction dynamics in Sertoli cells in vitro [27, 36], they have been postulated to have a physiological role in the junction restructuring events at the BTB and apical ES during stage VIII of the seminiferous epithelial cycle (Figs. 1 and 2). The high level of TNFα and TGF-β3 detected at stage VIII [25, 36] coincides with the extensive junction restructuring at the BTB and apical ES. Subsequent studies with the use of recombinant proteins of cytokines at levels that are possibly attainable in the microenvironment of the BTB indicated that both cytokines can induce rapid and reversible disruption of the BTB integrity [21, 25]. These data led to the postulation that some cytokines are secreted by germ cells and/or Sertoli cells as paracrine factors to induce the junction restructuring at the BTB and apical ES.
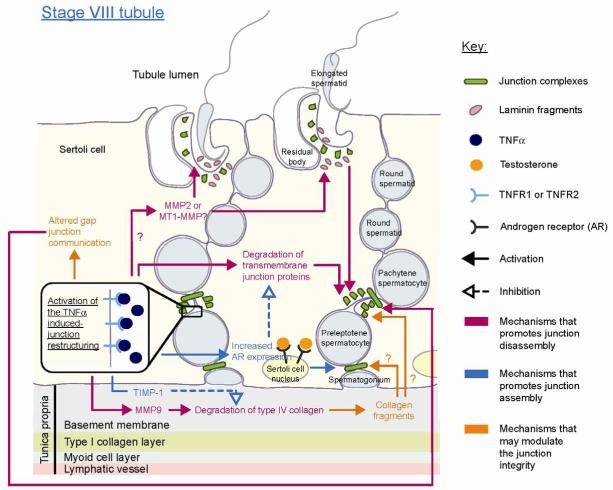
Figure 2. A schematic drawing illustrated cytokine (e.g., TNFα) and basement membrane component-induced junction restructuring of the BTB and apical ES during stage VIII of the seminiferous epithelial cycle.
At stage VIII of the seminiferous epithelial cycle, the high level of TNFα was proposed to induce the junction restructuring at the BTB and apical ES. This was to facilitate the translocation of preleptotene spermatocytes across the BTB to continue its development in the apical compartment, and spermiation so that spermatozoa can be released into the tubule lumen, respectively. TNFα was shown to disrupt the BTB such as via the endocytosis and degradation of the integral membrane proteins at the Sertoli-Sertoli cell interface. To induce the junction disassembly during spermiation, cytokines (e.g.,TNFα) may activate the MMPs present at the apical ES site, possibly MMP2 or MT1-MMP, to degrade the apical ES adhesion protein complexes (e.g., α6β1-integrin/laminin-333) between the Sertoli cell and elongated spermatid. Laminin would be degraded to produce biologically active laminins fragments, which were shown to signal the disruption of the BTB and apical ES (see text for details). Apart from the junction disruption, TNFα was also reported to promote junction assembly such as by inducing androgen receptor since testosterone is known to promote junction complex assembly and BTB integrity (see text for details). This may serve as a negative feedback mechanism to limit the TNFα-induced junction disruption or to promote the junction assembly on the basal side of the translocating spermatocyte so as to maintain the BTB integrity. Altered gap junction communication and proteolytic fragments of collagen may also serve to mediate the TNFα induced junction restructuring.
Recent studies to examine the effect of cytokines on the kinetics of endocytosis of integral membrane proteins at the BTB using Sertoli cells cultured in vitro that mimics the in vivo barrier further supports the physiological role of these two cytokines. Cytokines, namely TNFα, TGF-β2 and TGF-β3, were shown to enhance the rate of endocytosis of junction proteins, including tight junction proteins occludin and JAM-A, and basal ES protein N-cadherin, from the cell surface [22, 28]. In addition, the germ cell-conditioned medium collected from total germ cells without elongating or elongated spermatids could also enhance the rate of endocytosis of cell surface proteins [22]. These studies thus strengthen the postulate that TNFα and TGF-β2 or -3 are secreted by germ cells into the BTB microenvironment to regulate the junction dynamics during spermatogenesis.
4.2. The interplay of cytokines and testosterone in the regulation of the junction dynamics
As the integrity of the immunological barrier conferred by the BTB cannot be compromised, even transiently, during the transit of preleptotene spermatocytes at the BTB, it was postulated previously that the level of cytokines and their receptors might be tightly regulated to permit a localized disruption of junction integrity [37]. A recent study has suggested that cytokines, such as TNFα, TGF-β2 and TGF-β3, may act in concert with testosterone [28], which has been known to promote the junction integrity at the BTB [38, 39]. Their combined action may be responsible for mediating the junction restructuring at the BTB for the passage of preleptotene spermatocytes at the BTB while maintaining the immunological barrier integrity at the same time (Fig. 1).
In primary Sertoli cell cultures with established TJ-permeability barrier, treatment of these cultures with testosterone were shown to enhance the rate of endocytosis of integral membrane proteins at the BTB similar to TNFα, TGF-β2 or TGF-β3 [28]. While the endocytosed proteins induced by TNFα, TGF-β2 or TGF-β3 were predominantly destined for endosome-mediated degradation, testosterone was shown to promote the recycling of the endocytosed proteins back to the cell surface [28]. It thus indicates that both the cytokines and testosterone could promote the junction restructuring process at the BTB but resulting in the junction disruption and assembly, respectively. It has been postulated that the cytokines would favor the “old” TJ-fibrils’ disruption, likely those at the apical side of the spermatocyte in transit, whereas testosterone would favor the assembly of “new” TJ-fibrils at the basal side of the translocating spermatocyte [28]. The combination of these two actions induced by cytokines and testosterone thus maintains the immunological barrier while permitting the transit of primary spermatocytes at the BTB (see Fig. 1).
The interplay of cytokines and testosterone in regulating the junction integrity in the testis, such as the BTB, is strengthened by the effects of cytokines on the production of testosterone [40-43] and the expression of its receptor, the androgen receptor (AR), in the testis [44]. Cytokines have been reported to modulate the AR expression in the Sertoli cell, and steroidogenesis in the Leydig cell which is the major producer of testosterone in the testis. TNFα and IL-1α were demonstrated to increase the basal level of testosterone production in primary Leydig cell cultures and cultures of dispersed testicular cells from adult rats [40]. Additionally, cytokines were reported to modulate the production of testosterone by Leydig cells with other steroidogenesis regulatory factors, such as cAMP [41, 42] and human chrionic gonadotropin (hCG) [40]. TNFα and IL-1α were capable of stimulating the hCG-induced testosterone secretion by Leydig cells illustrating their additive effects on Leydig cell androgen production. Interestingly, in studies using primary cultures of Leydig cells from 60-70 day-old mice, both cytokines inhibited the cAMP-induced testosterone production [41, 42], illustrating there is a species-dependent and/or age-related response to the cytokine treatment. Nonetheless, these studies demonstrated unequivocally the effects of cytokines on the testosterone production by Leydig cells. Cytokines were shown to regulate steroidogenesis through the expression of steroidogenesis-related enzymes, such as steroidogenic-acute regulatory protein, steroid 17α-hydroxylase/17,20 lyase, 3b-hydroxysteroid dehydrogenase, and P450c17 [41, 42, 45, 46]. On the other hand, the expression of the androgen receptor in primary Sertoli cells was shown to be stimulated following the TNFα treatment [44]. In short, these findings illustrate the stimulatory effects of cytokines on the testosterone induced-junction restructuring in the seminiferous epithelium even though cytokines and androgen alone has opposing effects on the BTB and junction complex integrity in the seminiferous epithelium.
Taking collectively, these data suggest that TNFα is working in concert with testosterone to promote the assembly of TJ-fibrils behind the primary spermatocyte in transit at the BTB prior to the TNFα-induced disruption of established TJ-fibrils overlying the apical end of the migrating spermatocyte. The integrity of the immunological barrier hence can be maintained while allowing the translocation of spermatocytes at the same time (Fig . 1).
5. Mediators of the cytokine-induced junction restructuring
As discussed above, germ cell development in the seminiferous epithelium is synchronous at different stages of the seminiferous epithelial cycle. To synchronize the restructuring of the BTB and apical ES at the stage VIII-IX, it is conceivable that intercellular communication between germ cells and Sertoli cells must exist. The communication between the same stages of germ cells can be mediated through the intercellular bridge, which is possibly resulted from incomplete cytokinesis [47, 48]. Even though cytokines like TNFα secreted by germ cells could serve as paracrine factors for inducing junction restructuring on Sertoli cells, intercellular communication between Sertoli cells may also be required. There are indeed evidence that cytokines, despite capable of eliciting paracrine actions, still require some other signaling pathways for the mediation of its widespread responses. For instance, apoptosis induced by TNFα in the prostate cancer cell line LNCaP is mediated by Cx43, a constituent of gap junction [49]. The overexpression of Cx43 was shown to increase gap junction communication and potentiated the TNFα-induced apoptosis. However, this effect was not observed when Cx43 was co-overexpressed with its dominant-negative form [49], illustrating the importance of the functional gap communication to elicit TNFα-induced apoptosis. The putative roles of gap junction and components of the extracellular matrix in the mediation of the cytokine-induced junction restructuring based on recent findings in the field are to be discussed below.
6. Is cytokine-induced junction restructuring mediated by gap junctions?
6.1. Overview of gap junction
Gap junction channels allow the chemical communication between neighboring cells [50, 51]. Connexins are the basic building blocks of the gap junction. Six connexins can form a homotypic or heterotypic connexon while connexons on adjacent cells can interact homotypically or heterotypically to form gap junction channels. These gap junction channels allow the passage of molecules smaller than 1.2 kDa, including cAMP and ATP [50, 52, 53], between neighboring cells. These channels have different selectivity on the chemicals that can pass through. The selectivity depends on the connexins comprising the connexons and is named permselectivity [50]. The gap junction channel can be opened or closed through phosphorylation of connexins to regulate gap junction permeability rapidly [54]. Uncoupled connexons are named hemichannels, which can facilitate the release of ATP, NAD+ and glutamate into the extracellular spaces [50, 55, 56]. These molecules possibly serve as paracrine messengers to regulate epithelial cell functions. The release of ATP through hemichannels into the extracellular space was indeed reported to propagate the calcium wave [55, 56].
6.2. Connexins and the junction dynamics in the seminiferous epithelium
In the testis, the expression of various connexins has been reported, including Cx26, Cx32, Cx33, Cx36, Cx37, Cx40, Cx 43, Cx45, Cx46, Cx50 and Cx57 [13, 57, 58]. Gap junction communication has been detected between Sertoli cells as well as between Sertoli and germ cells, excluding steps 8-19 spermatids. Due to the difference in permselectivity, it was shown that the signal that pass from germ cells to Sertoli cells differs from that between Sertoli cells and from Sertoli cells to germ cells [58-60].
Connexins in the testis can be components of the desmosome-like junction (also called desmosome-gap junction), and the gap junction. An ultrastructural study of the desmosome-like junction in the seminiferous epithelium showed that it has the properties of both the desmosome junction and gap junction [11]. A recent study of Cx43, a major connexin in the seminiferous tubule, has shown that Cx43 alone is not essential for the maintenance of the tight junction and anchoring junction in Sertoli cell cultures with an established TJ-permeability barrier [61]. For instance, a knockdown of Cx43 alone in these Sertoli cell cultures by RNAi did not affect the integrity of the TJ-permeability barrier. Interestingly, when the expression of Cx43 and the desmosomal adaptor protein plakophilin-2 (PKP2) were simultaneously knocked down by RNAi, the junction integrity was nonetheless adversely affected. A decline in the integrity of the TJ-permeability barrier and a redistribution of junction proteins from the cell-cell interface to cell cytosol were detected. This thus prompts us to speculate that Cx43 and PKP2 in the desmosome-like junction and/or gap junction may participate in the regulation of the BTB dynamics (Fig. 2). These findings are significant since they illustrate the physiological significance for the coexistence of the desmosome-like junction and/or gap junction with TJ and basal ES at the BTB. It is likely that junction complexes of desmosome-like junctions and gap junctions, such as Cx43-PKP2, could serve as signal and/or regulatory proteins to coordinate the intricate events of BTB restructuring during spermatogenesis (see Fig. 2). It remains to be investigated if cytokines and/or testosterone would impede the expression of Cx43 and/or PKP2 at the BTB, so that these molecules maybe working in concert to regulate BTB restructuring during spermatogenesis.
In addition, a reduction and/or a change in localization of Cx43 in the seminiferous epithelium was often observed in studies when the cell adhesion and junction integrity in the testis was adversely affected, such as by environmental toxicants or drugs [60-64]. When Sertoli cells in vitro were treated with environmental toxicants including cadmium chloride, bisphenol A and DDT, the level of Cx43 was reduced along with tight junction protein occludin and basal ES protein N-cadherin [62, 63]. In the transient loss of germ cells induced by adjudin, a potential male contraceptive known to induce extensive anchoring junction restructuring leading to germ cell loss from the seminiferous epithelium, there was also a drastic decline of Cx43 in the seminiferous epithelium [61]. These studies hence support the notion regarding the possible involvement of Cx43 in the regulation of junction dynamics.
6.3. Possible involvement of connexins in the cytokine-induced junction restructuring
Studies in other epithelia have indicated that gap junctions are sometimes required for the mediation of cytokine actions (see Table 2). For instance, it was demonstrated that Cx43 and gap junction communication are essential for TNFα-induced inflammatory responses [65]. TNFα failed to induce the leukocyte adhesion and transmigration when applied to cell cultures with gap junction blockers or endothelial-specific Cx43 knock-out mice. Nonetheless, the role of gap junction in the cytokine-induced junction restructuring events in the seminiferous epithelium during spermatogenesis remains elusive even though cytokine treatment has been shown to affect the gap junction in Sertoli cells. In the Sertoli cell line 42GPA9, treatment of these cells with IL-1α was shown to reduce the level of Cx43 and increase the level of Cx33 [64], which was earlier shown to be a negative regulator of Cx43 by enhancing its endocytosis [66, 67]. In short, much work is needed to delineate the role of gap junctions, particularly Cx43, in mediating the cytokine-induced junction restructuring.
Table 2.
The mediation of cytokine-induced responses by connexins
| Phenotypes observed after modulation of connexins or gap junction channels |
Cytokine(s) that elicits the responses |
Affected/modulated connexin(s) |
Cell or tissue |
|---|---|---|---|
| A reduction in the transmigration of monocytes across the in vitro blood- brain barrier model; a reduction in the stimulated secretion of MMP2 after blockage of gap junction channels [92] |
TNFα and IFN-γ | Gap junction channels | Human monocytes |
| A reduction in the transmigration and adhesion of leukocytes [65] | TNFα | Connexin 43 and/or gap junction channels |
Venule in the cremaster muscle of endothelium-specific connexin 43 null mice or microcirculation in hamster cheek pouch |
| An increase in the apoptosis of prostate cancer cells induced by TNFα after overexpression of connexin 43 but not for overexpression of the dominant-negative connexin 43 [49] |
TNFα | Connexin 43 | LNCaP (Protstate cell line) |
| The abolishment of mural cell differentiation in Cx43-/- cells in which only latent TGF-β was detected; the differentiation was restored with the overexpression of Cx43 in the Cx43-/- cells or treatment of exogenous TGF-β1 [93] |
TGF-β1 | Connexin 43 | Cultured mesenchymal progenitors from Cx43 knockout mice |
7. Extracellular matrix and metalloproteases in the cytokine-induced junction restructuring in the seminiferous epithelium during spermatogenesis
7.1. Extracellular matrix and its proteolysis
The basement membrane at the periphery of the seminiferous tubule is a modified form of the extracellular matrix (ECM) [2, 68, 69]. The basement membrane, together with the underlying type I collagen layer, encircling the seminiferous epithelium which also constitutes the acellular zone of the tunica propria (Fig. 2). The basement membrane is in direct contact with Sertoli cells and niches of spermatogonia in particular spermatogonial stem cells (e.g., Asingle) and undifferentiated type A spermatogonia. Its major component includes laminin, type IV collagen, heparin sulfate proteoglycan [68] and entactin [70]. The remodeling of ECM is important for tissue transmigration during development, inflammation and cancer metastasis and is involved in the junction restructuring during the spermatogenesis [69, 71, 72]. The ECM remodeling can be achieved through various processes including synthesis, contraction and proteolysis [72]. Proteolytic fragments of the ECM components are produced when ECM is degraded by metalloproteases and other serine proteases. The degradation of ECM is determined by the equilibrium of these proteases and their corresponding inhibitors. Integrins are the best studied receptors for these ECM components and their proteolytic fragments [71-73].
Metalloproteases, including matrix metalloproteases (MMPs) and a disintegrin and metalloproteases (ADAMs), were reported to be involved in the junction dynamics in the testis [69]. There are currently more than twenty known members of MMPs and they are capable to degrade different ECM components [73]. MMPs are produced in their proactive form. Upon activation, their pro-peptide domain will be cleaved and MMPs are then conferred with the proteolytic activity. There are also membrane-type MMPs as well as the commonly known secretory forms, and they usually bind with MMPs in order to unleash their proteolytic activity. The activity of the MMP can be inhibited by the tissue inhibitors of metalloprotease (TIMP) [73, 74] and all TIMP-1 to -4 are found in the testis [74, 75].
ADAMs, on the other hand, have both metalloprotease domains and adhesion domains. Many of its members are testis-specific or predominantly expressed in the testis [76-78]. Earlier studies of ADAMs were mostly focused on their role in the interaction of spermatozoa and oocytes during the fertilization [78, 79] while their physiological significance in spermatogenesis and junction restructuring events in the seminiferous epithelium remains uncertain. But the ADAM can serve as sheddase, which can cleave the ectodomain of the membrane bound growth factors and cytokines, such as TNFα, TGFβs and IL-1β, to release their biologically active forms from the latent forms [77, 78, 80]. This suggests that ADAMs may have a role in the regulation of the cytokine-mediated junction restructuring during the spermatogenesis via their effects to release the biologically active cytokines in the microenvironment of the BTB and/or apical ES. This possibility must be vigorously investigated in future studies.
7.2. The degradation of ECM components and the junction dynamics in the seminiferous epithelium
A disruption of the basement membrane may disrupt the Sertoli cell adhesion directly by affecting its anchorage [27, 81] as demonstrated in studies by using an anti-collagen antibody [27]. Recent studies have demonstrated that proteolytic fragments of ECM components, such as laminins [82], are also capable of eliciting junction restructuring in the seminiferous epithelium. Apart from being ECM components of the basement membrane, laminins, namely laminins α3β3γ3, are detected at the apical ES site and are restricted to the elongated spermatids that form a bona fide complex with α6β1-integrin restricted to Sertoli cells [18, 82]. It was recently reported that laminin fragments, besides regulating the BTB integrity by modulating the steady-state levels of integral membrane proteins at the BTB, such as occludin, could modulate the BTB integrity indirectly via integrins restricted to hemidesmosome at the Sertoli cell-basement membrane interface. For instance, the overexpression of specific fragments of laminins or the introduction of recombinant laminin fragments was shown to induce a decline of the TJ integrity and disruption of junctions in primary Sertoli cell cultures [82]. It is plausible that the proteolytic fragments generated by ECM degradation could serve as mediators of the cytokine-induced junction restructuring (Fig. 2).
Upon TNFα treatment in primary Sertoli cell cultures, an induction in the level and activation of MMP-9, but not MMP-2, was reported [27]. MMP-9 and MMP-2 are also known as gelatinases [69, 73], whose putative substrates include type IV collagen (one of the major components of the basement membrane) and gelatin. The activation of MMP-9 induced by TNFα would thus induce degradation of the collagen network in the basement membrane and compromise the BTB integrity to facilitate, at least in part, the transmigration of preleptotene spermatocytes across the BTB [27, 69]. When the type IV collagen is cleaved by the activated MMP-9, the active collagen NC1 domain would be released and it could then bind to the integrin receptor. It remains unclear if the collagen NC1 domain or other collagen fragments would function similarly as the laminin fragments to regulate the junction restructuring in the seminiferous epithelium (Fig. 2). There are indeed reports in the literature that fragments of collagens could regulate the anchoring junction function and cell migration. For instance, type I collagen fragments were shown to induce rapid disassembly of focal adhesion complex via the integrin-dependent cleavage of FAK, paxillin, and talin at focal contacts [83]. Peptides from the NC1 domain of type IV collagen were shown to promote the cell adhesion while a peptide from the disrupted helical fragment of type IV collagen promoted the cell migration in the primary culture of rabbit corneal epithelial cells [84].
It is conceivable that the activation of proteases to induce the release of biologically active ECM components can serve as paracrine factors to regulate junction dynamics at the BTB, such as fragments from laminin chains and the NC1 domain of collagens (Fig. 2). The ECM remodeling hence could be partly responsible for the mediation of the cytokine-induced restructuring of the BTB and apical ES at about stage VIII of the seminiferous epithelial cycle. These findings thus demonstrate the presence of a local regulatory functional axis that links the apical ES, the BTB, and the basement membrane with or without the hemidesmosome and designated the apical ES-BTB-basement membrane functional axis.
8. Concluding remarks and future perspectives
As briefly discussed herein, it is obvious that cytokines (e.g., TNFα, TGF-β2, TGF-β3) exert their effects in concert with testosterone, possibility mediated by components of the desmosome-like and gap junction proteins (e.g., the Cx43/PKP-2 protein complex), to facilitate the transit of the primary preleptotene spermatocytes at the BTB at stage VIII of the seminiferous epithelial cycle as shown in Fig. 3. It is through this unique mechanism in which testosterone and TNFα induce the assembly of “new” TJ-fibrils behind the primary spermatocyte in transit whereas other cytokines (e.g., TGF-β2, TGF-β3) promote the disruption of the “old” TJ-fibrils above the migrating primary spermatocyte via their differential effects on the endocytosis, endosome-mediated degradation and/or recycling/transcytosis of integral membrane proteins so that the immunological barrier can be maintained (Fig. 1). These effects are also regulated by other components of the basement membrane and/or hemidesmosome (e.g., biologically active collagen fragments, integrins), and the apical ES (e.g., biologically active laminin chains) (see Fig. 2). While the model depicted in Fig. 2 will be updated as more data are available in the upcoming years, it will serve as a framework for investigators in the field to design functional experiments to understand the regulation and coordination of the junction restructuring that occur in the seminiferous epithelium during different stages of the seminiferous epithelial cycle. A thorough understanding of these events will allow us to better understand the spermatogenesis. This information will help us to understand the mechanism(s) by which environmental toxicants, such as cadmium, induce reproductive dysfunction [85].
Acknowledgments
This study was supported by grants from the National Institutes of Health (NICHD, R01 HD056034, R03 HD051512, and U54 HD029990 Project 5) to CYC. The authors declare no conflict of interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–74. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 2.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 3.LeBlond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–73. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 4.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 5.Mruk DD, Bruno S, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–80. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cell: new vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 8.Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their associations with germ cells of the rat testis. Tissue Cell. 1977;9:475–98. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 9.Vogl AW, Soucy LJ. Arrangement and possible function of actin filament bundles in ectoplasmic specializations of ground squirrel Sertoli cells. J Cell Biol. 1985;100:814–25. doi: 10.1083/jcb.100.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grove BD, Vogl AW. Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci. 1989;93:309–23. doi: 10.1242/jcs.93.2.309. [DOI] [PubMed] [Google Scholar]
- 11.Russell L. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat. 1977;148:301–12. doi: 10.1002/aja.1001480302. [DOI] [PubMed] [Google Scholar]
- 12.Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–80. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 13.McGinley DM, Posalaky Z, Porvaznik M, Russell L. Gap junctions between Sertoli and germ cells of rat seminiferous tubules. Tissue Cell. 1979;11:741–54. doi: 10.1016/0040-8166(79)90028-4. [DOI] [PubMed] [Google Scholar]
- 14.Risley MS, Tan IP. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J Cell Sci. 1992;103:81–96. doi: 10.1242/jcs.103.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Mirza M, Hreinsson J, Strand M-L, Hovatta O, Soder O, Philipson L, et al. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–30. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoi and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–92. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–4. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 18.Yan HHY, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat tests. J Biol Chem. 2006;281:17286–8303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 19.Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–78. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- 20.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region of late spermatids of the rat. Anat Rec. 1979;194:233–46. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 21.Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHN, Siu MKY, et al. Tumor necrosis factor α reversibly disrupts the blood-testis barrier integrity and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–29. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 22.Xia W, Wong EWP, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De SK, Chen H, Pace JL, Hunt JS, Terranova PF, Enders GC. Expression of tumor necrosis factor-α in mouse spermatogenic cells. Endocrinology. 1993;133:389–96. doi: 10.1210/endo.133.1.8319585. [DOI] [PubMed] [Google Scholar]
- 24.Lui WY, Lee WM, Cheng CY. TGF-βs: their role in testicular function and Sertoli cell tight junction dynamics. Int J Androl. 2003;26:147–60. doi: 10.1046/j.1365-2605.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 25.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 26.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–77. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 27.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumore necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–87. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 28.Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–59. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod. 2008;78:445–54. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haugen TB, Landmark BF, Josefsen GM, Hansson V, Hogset A. The mature form of interleukin-1α is constitutively expressed in immature male germ cells from rat. Mol Cell Endocrinol. 1994;105:R19–R23. doi: 10.1016/0303-7207(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson CK, Zetterstrom RH, Holst M, Parvinen M, Soder O. Constitutive expression of interleukin-1α messenger ribonucleic acid in rat Sertoli cells is dependent upon interaction with germ cells. Endocrinology. 1999;140:3755–61. doi: 10.1210/endo.140.8.6900. [DOI] [PubMed] [Google Scholar]
- 32.Gerard N, Syed V, Bardin W, Genetet N, Jegou B. Sertoli cells are the site of interleukin-1α synthesis in rat testis. Mol Cell Endocrinol. 1991;82:R13–R6. doi: 10.1016/0303-7207(91)90019-o. [DOI] [PubMed] [Google Scholar]
- 33.Wahab-Wahlgren A, Holst M, Ayele D, Sultana T, Parvinen M, Gustafsson K, et al. Constitutive production of interleukin-1a mRNA and protein in the developing rat testis. Int J Androl. 2000;23:360–5. doi: 10.1046/j.1365-2605.2000.t01-1-00253.x. [DOI] [PubMed] [Google Scholar]
- 34.Gomez E, Morel G, Cavalier A, Lienard M-O, Haour F, Courtens J-L, et al. Type I and type II interleukin-1 receptor expression in rat, mouse, and human testes. Biol Reprod. 1997;56:1513–26. doi: 10.1095/biolreprod56.6.1513. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Lui WY. Opposite effects of interleukin-1α and transforming growth factor-β2 induced stage-specific regulation of junctional adhesion molecule-B gene in Sertoli cells. Endocrinology. 2009;150:2404–12. doi: 10.1210/en.2008-1239. [DOI] [PubMed] [Google Scholar]
- 36.Lui WY, Lee WM, Cheng CY. Transforming growth factor β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 37.Li MWM, Mruk DD, Cheng CY. “Unlocking” the blood-testis barrier and the ectoplasmic specialization by cytokines during spermatogenesis: emerging targets for male contraception. Immunology, Endocrine & Metabolic Agents in Medicinal Chemistry. 2008;8:20–7. [Google Scholar]
- 38.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–70. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–33. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 40.Warren DW, Pasupuleti V, Lu Y, Platler BW, Horton R. Tumor necrosis factor and interleukin-1 stimulate testosterone secretion in adult male rat Leydig cells in vitro. J Androl. 1990;11:353–60. [PubMed] [Google Scholar]
- 41.Hales DB. Interleukin-1 inhibits Leydig cell steroidogenesis primarily by decreaseing 17α-hydroxylase/C17-20 lyase cytochrome P450 expresssion. Endocrinology. 1992;131:2165–72. doi: 10.1210/endo.131.5.1425417. [DOI] [PubMed] [Google Scholar]
- 42.Xiong Y, Hales DB. The role of tumor necrosis factor-α in the regulation of mouse Leydig cell steroidogenesis. Endocrinology. 1993;132:2438–44. doi: 10.1210/endo.132.6.8504748. [DOI] [PubMed] [Google Scholar]
- 43.Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–41. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Delfino FJ, Boustead JN, Fix C, Walker WH. NF-κB and TNF-α stimulate androgen receptor expression in Sertoli cells. Mol Cell Endocrinol. 2003;201:1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 45.Morales V, Santana P, Diaz R, Tabraue C, Gallardo G, Blanco FL, et al. Intratesticular delivery of tumor necrosis factor-α and ceramide directly abrogates steroidogenic actue regulatory protein expression and Leydig cell steroidogenesis in adult rats. Endocrinology. 2003;144:4763–72. doi: 10.1210/en.2003-0569. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Youngblood GL, Payne AH, Hales DB. Tumor necrosis factor-alpha inhibition of 17 alpha-hydroxylase/C17-20 lyase gene (Cry17) expression. Endocrinology. 1995;136:3519–26. doi: 10.1210/endo.136.8.7628389. [DOI] [PubMed] [Google Scholar]
- 47.Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- 48.Fawcett DW, Ito S, Slautterback D. The occurrence of intercellular bridges in groups of cells exhibiting synchronous differentiation. J Cell Biol. 1959;5:201–6. doi: 10.1083/jcb.5.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M, Berthoud VM, Beyer EC. Connexin43 increases the sensitivity of prostate cancer cells to TNFα-induced apoptosis. J Cell Sci. 2007;120:320–9. doi: 10.1242/jcs.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127:2516–24. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 51.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 52.Herve JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–43. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pahujaa M, Anikin M, Goldberg GS. Phosphorylation of connexin43 induced by Src: regulation of gap junctional communication between transformed cells. Exp Cell Res. 2007;313:4083–90. doi: 10.1016/j.yexcr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol. 2004;16:507. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 57.Risley MS. Connexin gene expression in seminiferous tubules of the Sprague-Dawley rat. Biol Reprod. 2000;62:748–54. doi: 10.1095/biolreprod62.3.748. [DOI] [PubMed] [Google Scholar]
- 58.Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochim Biophys Acta. 2005;1719:102–16. doi: 10.1016/j.bbamem.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Risley MS, Tan IP, Farrell J. Gap junctions with varied permeability properties establish cell-type specific communication pathways in the rat seminiferous epithelium. Biol Reprod. 2002;67:945–52. doi: 10.1095/biolreprod67.3.945. [DOI] [PubMed] [Google Scholar]
- 60.Gilleron J, Carette D, Carpentier F, Segretain D, Pointis G. Three-dimensional analysis of connexin43 gap junction in the ex vivo rat seminiferous tubules: short-term effects of hormonal effectors. Microsc Res Tech. doi: 10.1002/jemt.20731. In press; DOI:10.1002/jemt.20731. [DOI] [PubMed] [Google Scholar]
- 61.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009;106:10213–8. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol. 2004;18:413–21. doi: 10.1016/j.reprotox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009 doi: 10.1016/j.biocel.2009.05.016. doi:10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiorini C, Decrouy X, Defamie N, Segretain D, Pointis G. Opposite regulation of connexin33 and connexin43 by LPS and IL-1α in spermatogenesis. American Journal of Physiology - Cell Physiology. 2006;290:C733–C40. doi: 10.1152/ajpcell.00106.2005. [DOI] [PubMed] [Google Scholar]
- 65.Veliz LP, Gonzalez FG, Duling BR, Saez JC, Boric MP. Functional role of gap junctions in cytokine-induced leukocyte adhesion to endothelium in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H1056–H66. doi: 10.1152/ajpheart.00266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiorini C, Mograbi B, Cronier L, Bourget I, Decrouy X, Nebout M, et al. Dominant negative effect of connexin33 on gap junctional communication is mediated by connexin43 sequestration. J Cell Sci. 2004;117:4665–72. doi: 10.1242/jcs.01335. [DOI] [PubMed] [Google Scholar]
- 67.Carette D, Gilleron J, Decrouy X, Fiorini C, Diry M, Segretain D, et al. Connexin 33 impairs gap junction functionality by accelerating connexin 43 gap junction plaque endocytosis. Traffic. doi: 10.1111/j.1600-0854.2009.00949.x. In press; DOI:10.1111/j.1600-0854.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 68.Hermo L, Dworkin J. Transitional cells at the junction of seminiferous tubules with the rete testis of the rat: their fine structure, endocytic activity and basement membrane. Am J Anat. 1988;181:111–31. doi: 10.1002/aja.1001810202. [DOI] [PubMed] [Google Scholar]
- 69.Siu MKY, Cheng CY. Extracellular matrix and its role in spermatogenesis. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Landes Biosciences; Austin: 2008. pp. 74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lian G, Miller KA, Enders GC. Localization and synthesis of entactin in seminiferous tubules of the mouse. Biol Reprod. 1992;47:316–25. doi: 10.1095/biolreprod47.3.316. [DOI] [PubMed] [Google Scholar]
- 71.Preissner KT, Bronson RA. The role of multifunctional adhesion molecules in spermatogenesis and sperm function: lessons from homeostasis and defense? Semin Thromb Hemost. 2007;33:100–10. doi: 10.1055/s-2006-958468. [DOI] [PubMed] [Google Scholar]
- 72.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–71. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–17. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Le Magueresse-Battistoni B. Proteases and their cognate inhibitors of the serine and metalloprotease subclasses, in testicular physiology. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Landes Bioscience; Austin: 2008. pp. 133–53. [DOI] [PubMed] [Google Scholar]
- 75.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gen families during mouse tissue development. FEBS Lett. 2004;563:129–34. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- 76.Kim T, Oh J, Woo JM, Choi E, Im SH, Yoo YJ, et al. Expression and relationship of male reproductive ADAMs in mouse. Biol Reprod. 2006;74:744–50. doi: 10.1095/biolreprod.105.048892. [DOI] [PubMed] [Google Scholar]
- 77.Primakoff P, Myles DG. The ADAM gene family surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–7. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 78.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ecotdomain shedding. J Cell Sci. 1999;112:3603–17. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 79.Han C, Choi E, Park I, Lee B, Jin S, Kim DH, et al. Comprehensive analysis of reproductive ADAMs: relationship of ADAM4 and ADAM6 with an ADAM complex required for fertilization in mice. Biol Reprod. doi: 10.1095/biolreprod.108.073700. in press; DOI:10.1095/biolreprod.108.073700. [DOI] [PubMed] [Google Scholar]
- 80.Lint PV, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–81. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 81.Messent AJ, Tuckwell DS, Knauper V, Humphries MJ, Murphy G, Gavrilovic J. Effects of collagenase-cleavage of type I collagen on α2β1 integrin-mediated cell adhesion. J Cell Sci. 1998;111:1127–35. doi: 10.1242/jcs.111.8.1127. [DOI] [PubMed] [Google Scholar]
- 82.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125FAK, paxillin, and talin. J Cell Biol. 1999;147:619–29. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cameron JD, Skubitz APN, Furch LT. Type IV collagen and corneal epithelial adhesion and migration. Invest Ophthalmol Vis Sci. 1991;32:2766–76. [PubMed] [Google Scholar]
- 85.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. doi: 10.1016/j.taap.2009.01.028. In press; DOI:10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balda MS, Matter K. Tight junctions at a glance. J Cell Sci. 2008;121:3677–82. doi: 10.1242/jcs.023887. [DOI] [PubMed] [Google Scholar]
- 87.Chiba H, Osanai M, Murata M, Kojima T, Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778:588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 88.Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organizatio of the tight junctions. Biochim Biophys Acta. 2008;1778:646–59. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572–87. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 90.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Landes bioscience; Austin, TX: 2008. pp. 186–211. [DOI] [PubMed] [Google Scholar]
- 91.Young JNS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Tubulobulbar complexes contain cortactin (CTTN), N-WASP (WASL) and clathrin (CLTC) Biol Reprod. doi: 10.1095/biolreprod.108.070615. in press; DOI:10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 92.Eugenin EA, Branes MC, Berman JW, Saez JC. TNF-α plus IFN-γ induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J Immunol. 2003;170:1320–8. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- 93.Hirschi KK, Burt JM, Hirschi KD, Dai C. Gap junction communication mediates transforming growth factor-β activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429–37. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]