Abstract
The effect of restoring intravascular volume with polyethylene glycol (PEG) conjugated to human serum albumin (PEG-Alb) on systemic parameters and microvascular hemodynamics after hemorrhagic shock resuscitation was studied in the hamster window chamber model. Moderate hemorrhagic shock was induced by controlled arterial bleeding of 50% of blood volume, and hypovolemia was maintained for 1 hour. Fluid resuscitation was accomplished by infusion of 25% of blood volume and recovery was followed over 90 minutes. The PEG-Alb (six chains of maleimide phenyl PEG conjugated human serum albumin at 4%) resuscitation group was compared human serum albumin (HSA) at 5% (HSA5) and 10% (HSA10) protein concentrations. Systemic parameters, microvascular perfusion and capillary perfusion (functional capillary density, FCD) were measured by noninvasive methods. Hyperoncotic solutions provided rapid restoration of blood pressure, blood gas parameters and microvascular perfusion. Systemic and microvascular recovery was best and most rapid with PEG-Alb and followed by HSA10 and HSA5 rapid. Only recovery with PEG-Alb was sustained beyond 90 min. Hemodynamic functional benefits of PEG-Alb and the potential disadvantages associated with HSA, suggest PEG-Alb as better resuscitation solution.
Keywords: Microcirculation, plasma expander, hexaPEGylation, functional capillary density
INTRODUCTION
The efficacy of fluid resuscitation after hemorrhagic shock can be assessed by the recovery of cardiovascular stability, restoration of organ perfusion, and long term hemodynamic effects. Outcome is significantly affected by the nature of the plasma expander used to restore blood volume, an issue for which there is no generalized agreement on the availability of a material with the ideal properties. A principal criterion for rating the efficacy of plasma expanders is the ability to maintain circulatory volume, which is determined by its intravascular retention time. However, prolonged volume expansion produces hemodilution, a condition that is not accompanied in all circumstances by the maintenance of microvascular perfusion. In this context, volume restitution without maintenance of microvascular function can lead to tissue damage resulting from the lack of perfusion and eventually multiorgan failure. Therefore, an optimal plasma expander should provide efficient recovery of intravascular volume and sufficient restoration of blood that insures microvascular perfusion, tissue oxygen delivery and metabolic balance.1,2
Studies focused on the relation between the effectiveness of recovery from hemorrhage and events at the microvascular level show that restoration of perfusion is often as, or more effective than the recovery of oxygen carrying capacity.3 This counterintuitive finding is the result of controlling further injury, during the resuscitation phase, by limiting oxygen delivery, while insuring the recovery of tissue perfusion and taking advantage of the remaining RBCs to provide the needed oxygen. If tissue perfusion is assured, then resuscitation with limited re-oxygenation prevents multiorgan failure from becoming established.3 This approach, based on the recovery of capillary perfusion, focuses on preventing the progression of tissue damage rather than the recovery of endpoints.4
The concept of restoration of perfusion vs. oxygen delivery originates from the observation that in experimental studies in hemorrhagic shock and extreme hemodilution, where we found that maintenance of microvascular perfusion and functional capillary density (FCD) is not necessarily a function of blood oxygen carrying capacity.5-8 These studies show that maintenance of FCD in conditions of prolonged hemorrhagic shock, differentiates between survival and non survival, independent of oxygen carrying capacity and tissue pO2.5,9 FCD is primarily determined by the local capillary pressure, which is the result of central and local hemodynamic factors, vascular regulation and the physical properties of the circulating blood, all factors that are influenced by plasma expanders. Blood viscosity during shock has been shown to be a critical factor in regulating FCD in hemorrhagic shock resuscitation. Therefore, the formulation of a plasma expander must deal with its effect on this blood property.8,10 The viscosity of a plasma expander is determined principally by the size of the colloidal component (molecular volume) and its concentration. The inherent dilution of the material, upon their introduction into the circulation, is a critical factor in setting the attainable final plasma viscosity.11 Concentration of the colloid can often be managed only up to a threshold, beyond which the corresponding increase in colloidal osmotic pressure (COP) limits the increase in concentration by autotransfusion and dilution of the colloid a self-limiting process.
Albumin is considered to be a near optimal resuscitation fluid, because it is naturally occurring in plasma. Its molecular weight is such that between 80 and 100% is retained by the capillary wall (depending on the tissue type), returned by the lymphatic return and endogenous generation, to insure plasma and interstitial fluid concentration gradients. Some of these mechanisms are disrupted in shock, and the restoration of the colloidal composition of plasma with albumin does not result in a steady state plasma composition, since colloid filtration to the interstitium is not compensated. The extravasation of proteins from the circulation is primarily determined by molecular dimension. Therefore, increased albumin molecular radius should, in principle, yield a decreased extravasation and hence, improved volume resuscitation.
The present study was carried on to test the hypothesis that the plasma expansion properties of human serum albumin (HSA), considered near optimal for some resuscitation scenarios, can be further improved by increasing the molecular dimensions of HSA by conjugation of this protein with polyethylene glycol (PEG) to form PEGylated albumin (PEG-Alb). This hypothesis was tested by comparing the level of restoration of systemic hemodynamics and microhemodynamics during moderate hemorrhagic shock with a moderate volume resuscitation strategy. Hemorrhagic shock was induced by withdrawal of 50% of blood volume, and resuscitation was accomplished by infusion of 25% of blood volume. PEG-Alb formulated at 4% was compared to 5% HSA resuscitation, which approximately matches material concentration, and 10% HSA concentration which matches solution COP (46- 48 mmHg).
METHODS
Animal Preparation
Investigations were performed in 55 - 65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA) fitted with a dorsal window chamber. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the local animal care committee. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique is described in detail elsewhere.12,13 Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and securely attached to the window frame.
Inclusion Criteria
Animals were suitable for the experiments if: 1) systemic parameters were within normal range, namely, heart rate (HR) > 340 beat/min, mean arterial blood pressure (MAP) > 80 mmHg, systemic Hct > 45%, and arterial oxygen partial pressure (PaO2) > 50 mmHg; and 2) microscopic examination of the tissue in the chamber observed under a x650 magnification did not reveal signs of edema or bleeding.
Acute hemorrhage and volume replacement protocol
Acute hemorrhage was induced by withdrawal of 50% of estimated total blood volume via the carotid artery catheter within 5 min. Total blood volume was estimated as 7% of body weight. One hour after hemorrhage induction, animals received a single volume infusion of 50% of shed blood volume (25% of blood volume) of the resuscitation fluid (see experimental groups) within 10 min via the jugular vein catheter. Fifty percent of shed blood volume was used as the resuscitation volume, because autotransfusion restores about half of the shed volume during the shock period. Therefore, restoration of 25% of the blood volume does not cause hypervolemia and reinstates normovolemia in the hamster window model. Animals did not receive any additional fluid during the experiment. Parameters were analyzed before hemorrhage (baseline), after hemorrhage (shock), and up to 90 min after volume replacement (resuscitation).
Resuscitation solutions
HexaPEGylated HSA (PEG-Alb.): Human Serum Albumin (HSA) fatty acid free, ~99% (Product A3782, Batch# 085K7541) lyophilized powder from (Sigma-Aldrich, Inc. MO, USA) was PEGylated by conjugation of PEG to HSA via a thiolation mediated maleimide chemistry based protocol.14 Human serum albumin incubated with 2-Iminothiolane (2-IT) (lot # 10222 from BioAffinity Systems, Inc. Rockford, IL) and free thiols generated on HSA were estimated by 4-PDS method.15 The ratio of concentration of HSA to that of 2-IT was standardized so that 6 free thiols are available on the surface molecular surface of HSA on average. This ratio of HSA and 2-IT reacted with 3 fold molar excess of maleimide phenyl PEG5K over the thiols to get hexaPEGylated HSA (PEG-Alb). In brief, 0.5 mM HSA reacted in one step overnight reaction with 5 mM 2-Iminothiolane and 10mM Maleimide Phenyl PEG5K (MPPEG5K) in cold. The reaction product was dialyzed through 50K MW cutoff membranes against PBS (pH 7.4) to remove excess of iminothiolane and MPPEG5K using a Minim™ Tangential Flow Filtration instrument (Pall Corporation, Ann Arbor, MI, U.S.A.). The retentate was monitored at regular intervals by size exclusion chromatography until complete removal of unreacted PEG in the mixture was confirmed. The final product in the retentate was concentrated further with an Amicon concentrator using pressurized nitrogen. The sample was concentrated to 4 % and sterilized by filtration through 0.22 μM Millipore filters. The concentration of PEGylated HSA was determined using a Bradford protein assay kit (Pierce Biotechnology, Inc. Rockford, IL). The extent of PEGylation of HSA was analyzed by NMR and MALDI-TOF-MS, which confirmed that an average of 6 PEG5K chains was attached to each HSA molecule. Colloidal oncotic pressure was about 48 mmHg and viscosity was about 2.08 cp at 150 s-1. HSA (Baxter Healthcare Corporation, Westlake Village, CA), packed at 25% concentration at physiological pH, normal saline was used to adjust concentration. At 5% protein mass based concentration, HSA has COP of 22 mmHg, and viscosity is 0.9 cp at 150 s-1. At 10% protein mass based concentration, HSA has COP of 46 mmHg, and viscosity is 1.0 cp at 150 s-1.
Experimental Groups
Animals were randomly divided into the following 3 experimental groups before the experiment: 1) Resuscitated with 4% hexaPEGylated human serum albumin, PEG-Alb, 2) Resuscitated with 10% human serum albumin, HSA10; and 3) Resuscitated with 5% human serum albumin, HSA5.
Systemic Parameters
MAP and heart rate (HR) were recorded continuously (MP 150, Biopac System; Santa Barbara, CA). Hct was measured from centrifuged arterial blood samples taken in heparinized capillary tubes. Hb content was determined spectrophotometrically (B-Hemoglobin, Hemocue, Stockholm, Sweden).
Blood Chemistry and Biophysical Properties
Arterial blood was collected in heparinized glass capillaries (0.05 ml) and immediately analyzed for PaO2, PaCO2, base excess (BE) and pH (Blood Chemistry Analyzer 248, Bayer, Norwood, MA). Viscosity was measured at a shear rate of 160/sec (Brookfield Engineering Laboratories, Middleboro, MA). Colloid osmotic pressure was measured using a 4420 Colloid Osmometer (Wescor, Logan, UT).
Microvascular Experimental Setup
The unanesthetized animal was placed in a restraining tube with a longitudinal slit from which the window chamber protruded, then fixed to the microscopic stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY). The animals were given 20 min to adjust to the change in the tube environment before measurements. Measurements were carried out using a 40X (LUMPFL-WIR, numerical aperture 0.8, Olympus) water immersion objective.
Microhemodynamics
Arteriolar and venular blood flow velocities were measured online by using the photodiode cross-correlation method (Photo-Diode/Velocity, Vista Electronics, San Diego, CA).16 The measured centerline velocity (V) was corrected according to vessel size to obtain the mean RBC velocity.17 A video image-shearing method was used to measure vessel diameter (D).18 Blood flow (Q) was calculated from the measured values as Q = π × V (D/2)2. Wall shear stress (WSS) was defined by WSS = WSR × η, where WSR is the wall shear rate given by 8VD-1, and η is the microvascular blood viscosity or plasma viscosity.
Functional Capillary Density (FCD)
Functional capillaries, defined as those capillary segments that have RBC transit of at least one RBC in a 60s period in 10 successive microscopic fields were assessed, totaling a region of 0.46 mm2. Each field had between two and five capillary segments with RBC flow. FCD (cm-1), i.e., total length of RBC perfused capillaries divided by the area of the microscopic field of view, was evaluated by measuring and adding the length of capillaries that had RBC transit in the field of view. The relative change in FCD from baseline levels after each intervention is indicative of the extent of capillary perfusion.19,20
Volume expansion
Changes in volume were calculated as the difference in Hct before infusion (Hct0) and the Hct at a specific time after infusion (Hctt) as, VE = (Hct0-Hctt)/Hctt.
Data analysis
Results are presented as mean ± standard deviation. Data within each group were analyzed using analysis of variance for repeated measurements (ANOVA, Kruskal-Wallis test). When appropriate, post hoc analyses were performed with the Dunns multiple comparison test. Microhemodynamic data are presented as absolute values and ratios relative to baseline values. A ratio of 1.0 signifies no change from baseline while lower and higher ratios are indicative of changes proportionally lower and higher than baseline (i.e., 1.5 would mean a 50% increase from the baseline level). The same vessels and capillary fields were followed so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics for small sample populations. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc., San Diego, CA). Changes were considered statistically significant if P<0.05.
RESULTS
Eighteen animals were entered into this study; all tolerated the entire protocol without visible signs of discomfort. Animals were randomly assigned to the following experimental groups: PEG-Alb (n = 6); HSA10 (n = 6); and HSA5 (n = 6). Systemic data for baseline and shock was obtained by combining all experimental groups.
Systemic parameters
Systemic hemodynamic and blood parameters are presented in detail on Table 1. Hemorrhage reduced Hct and Hb from baseline. At baseline Hct and Hb for PEG-Alb, HSA10 and HSA5 were 47 ± 2% (14.3 ± 1.1 g/dl), 48 ± 2% (14.7 ± 0.9 g/dl) and 48 ± 1% (14.8 ± 0.7 g/dl), respectively. Resuscitation with PEG-Alb and HSA10 decreased Hct and Hb further from shock, where as resuscitation with HSA5 decreased Hct and Hb to less extent. The hamster hemorrhage model does not challenge considerable variability at its responses to shock, as observed by the consistency observed 50 min into the hypovolemic shock.
Table 1.
Systemic and blood gas parameters.
| Shock 50 min |
Resuscitation 60 min |
Resuscitation 90 min |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | PEG-Alb | HSA10 | HSA5 | PEG-Alb | HSA10 | HSA5 | ||
| Hct, % | 48 ± 1 | 28 ± 1† | 21 ± 1†§ | 23 ± 1† | 24 ± 1† | 21 ± 1†§ | 24 ± 1†§ | 26 ± 1† |
| Hb, g/dl | 14.5 ± 0.7 | 9.4 ± 0.6† | 6.8 ± 0.5†§ | 7.4 ± 0.6† | 7.7 ± 0.4† | 6.3 ± 0.8†‡§ | 8.0 ± 0.4† | 8.1 ± 0.5† |
| MAP, mmHg | 105 ± 7 | 44 ± 4† | 79 ± 5†§ | 78 ± 5†§ | 69 ± 6† | 87 ± 6†§ | 81 ± 7†§ | 73 ± 5† |
| Heart Rate, bpm | 435 ± 32 | 411 ± 24 | 401 ± 27 | 445 ± 21 | 452 ± 23 | 424 ± 28 | 452 ± 32 | 423 ± 30 |
| PaO2, mmHg | 56.2 ± 4.8 | 86.9 ± 8.3† | 65.3 ± 7.2†§ | 69.3 ± 6.1† | 76.2 ± 6.2† | 63.5 ± 6.0§ | 65.8 ± 5.2§ | 80.1 ± 6.4† |
| PaCO2, mmHg | 52.5 ± 4.0 | 37.1 ± 6.7† | 47.3 ± 4.2†§ | 48.2 ± 4.6§ | 39.1 ± 4.8† | 48.4 ± 6.6§ | 46.2 ± 4.5 | 41.7 ± 4.3† |
| Arterial pH | 7.361 ± 0.022 | 7.296 ± 0.035† | 7.344 ± 0.021§ | 7.326 ± 0.016† | 7.310 ± 0.028† | 7.347 ± 0.019‡§ | 7.325 ± 0.023† | 7.321 ± 0.014† |
| BE, mmol/l | 3.6 ± 1.7 | −2.4 ± 1.9† | 0.4 ± 1.0†§ | 0.5 ± 1.3† | −0.7 ± 1.6† | 0.9 ± 1.5†‡§ | −0.6 ± 1.1† | −1.5 ± 1.7† |
PEG-Alb, 4% PEG conjugated albumin; HSA10, 10 g/dl Human serum albumin; HSA5, 5 g/dl Human serum albumin. Values are means ± SD. Baseline and Shock included data fro all the experimental groups in the study. Hct, systemic hematocrit; Hb, hemoglobin content of blood; MAP, mean arterial blood pressure; PaO2, arterial partial O2 pressure; PaCO2, arterial partial pressure of CO2; BE, base excess.
P<0.05 compared to baseline
P<0.05 compared to HSA10
P<0.05 compared to HSA5.
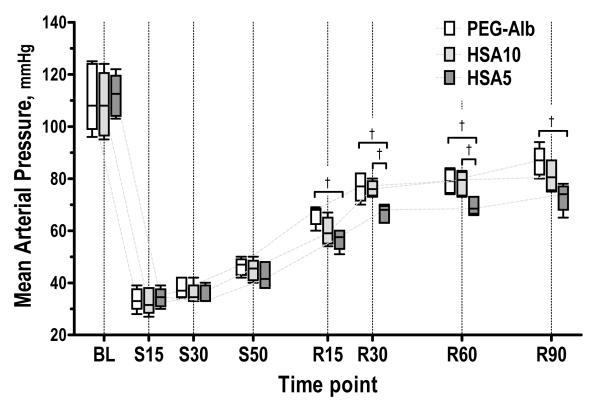
Mean arterial pressure statistically decreased after shock and resuscitation did not restore full baseline MAP for any solution tested, while resuscitation significantly increased MAP from shock for all resuscitation solution. Figure 1 presents changes in MAP for all the experimental groups. Resuscitation with PEG-Alb and HSA10 statistically and significantly restored MAP when compared to HSA5, at all time points after resuscitation. That there were not statistical differences in MAP between PEG-Alb and HSA10 during resuscitation is statistically, non significant. There were no statistically significant changes in the heart rate (neither from baseline nor after resuscitation) among the study groups at any time during the experiment.
Figure 1.
Mean arterial blood pressure (MAP) during hemorrhagic shock resuscitation protocol for PEG-Alb, HSA10 and HSA5. Time period during hypovolemic shock is marked with an S before the respective time (e.g. S15, 15 min under shock), similar nomenclature is used for the time after resuscitation by R before the time. All MAP were significantly lower than baseline during shock and after resuscitation (P < 0.05); †, P<0.05.
Blood gas laboratory parameters and calculated acid base balance paralleled with the restoration of MAP. Blood gas parameters (PaO2 and PaCO2) were statistically and significantly different after shock, when compared to baseline. Resuscitation recovered laboratory parameters from hemorrhagic shock in all groups. Sixty minutes after resuscitation, arterial pO2 decreased, pCO2 increased, and pH was restored.
Blood and plasma viscosities were not statistically different among resuscitation groups (PEG-Alb, 2.3 ± 0.5 cp, 1.1 ± 0.2 cp; HSA10, 2.4 ± 0.6 cp, 1.0 ± 0.1 cp; and HSA5, 2.6 ± 0.6 cp, 1.1 ± 0.1 cp). Blood and plasma viscosities were lower than normal rheological properties for the species. Normal values (blood: 4.2 cp, plasma: 1.2 cp) were obtained from hamsters before undergoing the shock resuscitation protocol.
Calculated volume expansion using Hct changes at 60 and 90 min after resuscitation was significantly higher for PEG-Alb (60min: 39 ± 6%; 90 min: 38 ± 9%) than HSA10 (60min: 21 ± 7%; 90 min: 17 ± 6%) and HSA5 (60min: 17 ± 7%; 90 min: 10 ± 7%). HSA10 had significantly higher volume expansion than HSA5 as expected. PEG-Alb maintained its volume expansion between 60 and 90 min after infusion, HSA5 and HSA10 did not maintain volume expansion.
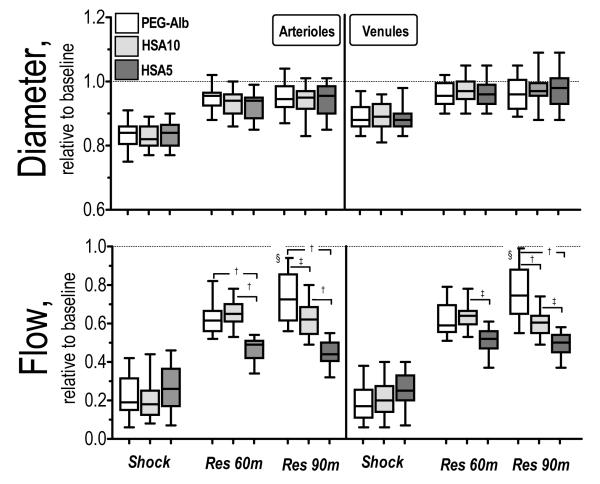
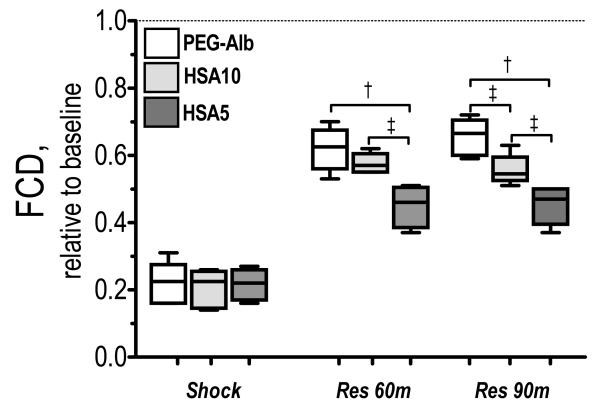
Microvascular hemodynamic measurements are presented in Figure 2. Arteriolar and venular diameters were statistically vasoconstricted during shock for all experimental groups. Arteriolar and venular diameters were not different among experimental groups, and reached baseline 90 min after resuscitation. Arteriolar and venular flow during shock was statistically lower than baseline and all resuscitation solution statistically increased blood flow from shock, although statistically lower than baseline. Arteriolar and venular flows after HSA5 resuscitation were statistically lower than HSA10 and PEG-Alb. Arteriolar and venular flow for PEG-Alb and HSA10 were not different 60 min after resuscitation, and they became statistically different after 90 min (arteriolar P<0.10 and venular P<0.05). Changes in capillaries perfusion are presented in Figure 3. FCD was statistically reduced during shock. Resuscitation statistically increased FCD from shock in all groups. Differences in FCD between HSA5 and HSA10 were significant after 60 and 90 min (P<0.10). PEG-Alb restored statistically higher FCD than HSA5 after resuscitation. Differences in FCD between PEG-Alb and HSA10 were significant 90 min after resuscitation (P<0.10). Calculated arteriolar and venular wall shear rate were not different for PEG-Alb and HSA10, and both were significantly higher than HSA5 (P<0.10).
Figure 2.
Relative changes to baseline in arteriolar and venular hemodynamics for PEG-Alb, HSA10 and HSA5. Broken line represents baseline level. †, P < 0.05 and ‡, P < 0.10, among study groups; and §, P<0.05, between 60 min and 90 min after resuscitation. Diameters (μm, mean ± SD) for each animal group at baseline were as follows: PEG-Alb (arterioles (A): 59.1 ± 9.4, n = 42; venules (V): 59.9 ± 8.0, n = 44); HSA10 (A: 58.1 ± 9.4, n = 44; V: 56.8 ± 9.6, n = 47); HSA5 (A: 57.9 ± 8.6, n = 43, V: 58.6 ± 8.9, n = 44). n = number of vessels studied. RBC velocities (mm/s, mean ± SD) for each animal group at baseline were as follows: PEG-Alb (A: 4.4 ± 1.1, V: 2.4 ± 0.8); HSA10 (A: 4.4 ± 0.9; V: 2.4 ± 0.8); HSA5 (A: 4.5 ± 0.8; V: 2.6 ± 0.6). Calculated flows (nl/s, mean ± SD) for each animal group at baseline were as follows: Baseline: PEG-Alb (A: 11.5 ± 3.6; V: 6.7 ± 2.3); HSA10 (A: 11.5 ± 3.1; V: 6.6 ± 2.0); HSA5 (A: 11.0 ± 2.5; V: 6.5 ± 2.1).
Figure 3.
Effects of resuscitation on capillary perfusion during hemodilution. Functional capillary density (FCD) was drastically reduced after hemorrhage. FCD was lower after resuscitation with HES compared to volume restitution with RBCs. †, P < 0.05 and ‡, P < 0.10, among study groups. FCD (cm-1) at baseline was as follows: PEG-Alb (107 ± 10); HSA10 (112 ± 12); and HSA5 (105 ± 12).
DISCUSSION
The principal finding of this study is that resuscitation with 4% of hexaPEGylated human serum albumin (PEG-Alb) and 10% human serum albumin (HSA10) provide an early similar recovery of systemic and microvascular conditions. These hyperoncotic solutions provided rapid restoration of blood pressure and blood gas parameters. Remarkably, the restoration trend was maintained by PEG-Alb during the entire observation period. Albumin solutions (HSA5 and HSA10) reinstated microvascular perfusion and recovered from metabolic imbalances for the initial hour after infusion however, there was no improvement 90 min after infusion, and some of the parameters tended to revert to pre-resuscitation conditions. These differences in recovery among groups are mostly determined to the combined effects of lack of sustained recovery of arteriolar flow and FCD.
This study compares the effects of volume expansion after resuscitation from hemorrhagic shock. The concentrations of 4% PEG-Alb and 10% HSA were chosen to match COP. The initial similarity of response observed for both materials may be due to the rapid blood volume restoration complementing the autotransfusion that took place during shock. Results 60 and 90 min after resuscitation show a more significant and consistent volume expansion with PEG-Alb than HSA10. 60 min after infusion, HSA10 provided 54% of the volume expansion attained with PEG-Alb. By comparison HSA5 only provided 44% volume expansion attained with PEG-Alb, due to its lower COP. 90 min after resuscitation, volume expansion was less for both HSA5 and HSA10 (44% and 26% of the volume expansion attained with PEG-Alb, respectively).
The volume of the extracellular fluid exchanged, due to the COP of the solutions, is determined primarily by the colloidal concentration of each fluid. However, the dissimilarities in results between PEG-Alb and HSA10 suggest the presence of other mechanisms probably related to the structural modification on the albumin by PEGylation. Albumin solution has a uniform molecular size (monodisperse). Albumin is continuously synthesized in hepatocytes, about 5% of native albumin leaks from the circulation per hour, and 90% of this extravascular pool returns to the circulation. This can increase during hemorrhagic shock and sepsis. 21 PEGylated albumin theoretically remains in the intravascular compartment for a longer time than the unPEGylated albumin, providing larger and long lasting plasma volume expansion for identical infused volumes. HexaPEGylated HSA was generated using extension arm facilitated thiolation mediated maleimide chemistry protocol, which is highly selective and specific to ε-amino groups.14,22 The maleimide phenyl PEG reagent used for this PEGylation reaction was synthesized by functionalization of PEG with paramaleimido phenyl isocyanate using a one step reaction protocol.14,23,24 HexaPEGylation enhances the hydrodynamic volume of HSA as shown in earlier studies with hemoglobin.14 It should be noted that hydrodynamic molecular radius of human serum albumin is 4 nm, and after hexaPEGylation increases to around 7.25 nm. PEG-Alb has 2.5 and 1.5 fold higher COP and viscosity than HSA solution at identical protein concentration. In fact, PEG-Alb and Albumin are two completely different colloids, they have different volumes of distribution (ratio between total amount in the body and in the plasma) and so the magnitude and duration of plasma volume expansion varies is obviously different. Changes in Hct basically present a snapshot of volume expansion (infused colloid plasma concentration) under pseudo-equilibrium condition (distribution of residual amount infused in plasma).
A mechanism that could account for FCD recovery in hemorrhagic shock is mechano-transduction resulting from direct interaction of PEG-Alb with the endothelium enhancing shear stress responses, without an increase in plasma viscosity. In extreme hemodilution, PEGylated albumin appears to be effective maintaining flow and microvascular function at significantly lower shear stress than high viscous plasma expanders.26 Shear stress is a mediator of the production of endothelium-derived relaxing factors sensed via the glycocalyx27, triggering the activation of endothelial mechanisms.28,29 Nitric oxide (NO) accounts for most of the biological actions of endothelium-derived relaxing factor and is formed by vascular endothelial cells.30 Therefore, restoring flow should lead to increased NO production, an effect not documented with PEG-Alb although demonstrated with a high viscosity plasma expander.8,10 However it is likely that PEG-Alb activates other mechanisms besides the viscogenic effect.26
Colloid solutions provide rapid restoration of circulating volume with a smaller infused volume than physiologic salt solutions.31 An advantage of albumin solutions is that increases oncotic pressure within the intravascular space; changing Starling’s forces and increasing intravascular volume as a result of water being drawn into the vessels. Albumin has many biological effects unrelated to oncotic pressure, and may have therapeutic and protective effects during shock resuscitation, due to ligand binding and transport properties, antioxidant function, and enzymatic activity.32,33 These include fatty acids, hormones, enzymes, dyes, trace metals, and drugs.32 Furthermore, substances that are toxic in the unbound or free state are generally not toxic when bound to albumin. For example, while unbound plasma free fatty acids are toxic, free fatty acids bound to albumin are not.34 Unfortunately, in the presence of leaky capillary membranes, albumin leaks and exacerbates rather than reduce interstitial edema.35 Currently, the clinical utility of albumin administration to patients is an area of controversy. A Cochrane systematic review to quantify the effect on mortality of human albumin and plasma protein fraction administration in the management of critically ill patients found 31 trials reporting death as an outcome.36 Given the added disadvantages associated with infusion of blood products, the use of albumin in resuscitation is currently not favored, suggesting PEG-Alb as an alternative. Because PEG conjugation traps significant amounts of water within the vicinity of the protein surface and this water layer renders the molecule undetectable by receptors and the immune system.37
In conclusion, this study shows restoration of vascular homeostasis during hemorrhagic shock is improved by increasing the molecular size of albumin. A potential future improvement of present PEG-Alb configuration will be to increase its viscosity to the level shown to be even more beneficial for resuscitation.8 Thus, future development of the technology of PEGylation may lead to enhanced plasma viscosity, while maintaining its oncotic characteristics at moderate protein concentrations. Finally, a yet unexplored area is the potential shear stress mediated NO regulation, by means of mechano-transduction.
ACKNOWLEDGMENTS
This work was supported by Bioengineering Research Partnership grant R24-HL64395, Program project P01 HL071064 and grants R01-HL62354, R01-HL62318 and R01-HL76182. The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
REFERENCES
- 1.Welch HG, Meehan KR, Goodnough LT. Prudent strategies for elective red blood cell transfusion. Ann Intern Med. 1992;116:393–402. doi: 10.7326/0003-4819-116-5-393. [DOI] [PubMed] [Google Scholar]
- 2.Dubick MA, Atkins JL. Small-volume fluid resuscitation for the far-forward combat environment: current concepts. J Trauma. 2003;54:S43–5. doi: 10.1097/01.TA.0000064514.42470.3B. [DOI] [PubMed] [Google Scholar]
- 3.Cabrales P, Tsai AG, Intaglietta M. Deferoxamine Lowers Tissue Damage After 80% Exchange Transfusion with Polymerized Hemoglobin. Antioxid Redox Signal. 2007 doi: 10.1089/ars.2006.1379. [DOI] [PubMed] [Google Scholar]
- 4.Thorup C, Jones CL, Gross SS, et al. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–9. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 5.Kerger H, Saltzman DJ, Menger MD, et al. Systemic and subcutaneous microvascular pO2 dissociation during 4-h hemorrhagic shock in conscious hamsters. Am J Physiol. 1996;270:H827–H36. doi: 10.1152/ajpheart.1996.270.3.H827. [DOI] [PubMed] [Google Scholar]
- 6.Matheson B, Kwansa HE, Bucci E, et al. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93:1479–86. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- 7.Cabrales P, Intaglietta M, Tsai AG. Increase plasma viscosity sustains microcirculation after resuscitation from hemorrhagic shock and continuous bleeding. Shock. 2005;23:549–55. [PubMed] [Google Scholar]
- 8.Cabrales P, Tsai AG, Intaglietta M. Hyperosmotic-hyperoncotic vs. hyperosmotic-hyperviscous small volume resuscitation in hemorrhagic shock. Shock. 2004;22:431–7. doi: 10.1097/01.shk.0000140662.72907.95. [DOI] [PubMed] [Google Scholar]
- 9.Cabrales P, Nacharaju P, Manjula BN, et al. Early difference in tissue pH and microvascular hemodynamics in hemorrhagic shock resuscitation using polyethylene glycol-albumin- and hydroxyethyl starch-based plasma expanders. Shock. 2005;24:66–73. doi: 10.1097/01.shk.0000167111.80753.ef. [DOI] [PubMed] [Google Scholar]
- 10.Cabrales P, Tsai AG. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am J Physiol Heart Circ Physiol. 2006;291:H2445–52. doi: 10.1152/ajpheart.00394.2006. [DOI] [PubMed] [Google Scholar]
- 11.Tsai AG, Cabrales P, Manjula BN, et al. Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood. 2006;108:3603–10. doi: 10.1182/blood-2006-02-005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–H17. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 13.Endrich B, Asaishi K, Götz A, Messmer K. Technical report: A new chamber technique for microvascular studies in unanaesthetized hamsters. Res Exp Med. 1980;177:125–34. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 14.Manjula BN, Tsai AG, Intaglietta M, et al. Conjugation of multiple copies of polyethylene glycol to hemoglobin facilitated through thiolation: influence on hemoglobin structure and function. Protein J. 2005;24:133–46. doi: 10.1007/s10930-005-7837-2. [DOI] [PubMed] [Google Scholar]
- 15.Grassetti DR, Murray JF., Jr. Determination of sulfhydryl groups with 2,2′- or 4,4′-dithiodipyridine. Arch Biochem Biophys. 1967;119:41–9. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- 16.Intaglietta M, Silverman NR, Tompkins WR. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res. 1975;10:165–79. doi: 10.1016/0026-2862(75)90004-7. [DOI] [PubMed] [Google Scholar]
- 17.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 18.Intaglietta M, Tompkins WR. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973;5:309–12. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- 19.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287:H363–73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 20.Tsai AG, Cabrales P, Winslow RM, Intaglietta M. Microvascular oxygen distribution in awake hamster window chamber model during hyperoxia. Am J Physiol Heart Circ Physiol. 2003;285:H1537–H45. doi: 10.1152/ajpheart.00176.2003. [DOI] [PubMed] [Google Scholar]
- 21.Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781–4. doi: 10.1016/s0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakaran M, Manjula BN, Acharya SA. Molecular modeling studies of surface decoration of hemoglobin by maleimide PEG. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:381–93. doi: 10.1080/10731190600769164. [DOI] [PubMed] [Google Scholar]
- 23.Manjula BN, Tsai A, Upadhya R, et al. Site-specific PEGylation of hemoglobin at Cys-93(beta): correlation between the colligative properties of the PEGylated protein and the length of the conjugated PEG chain. Bioconjug Chem. 2003;14:464–72. doi: 10.1021/bc0200733. [DOI] [PubMed] [Google Scholar]
- 24.Acharya AS, Manjula BN, Smith PK. Hemoglobin crosslinkers. 5,750,725 U.S. Patent. 1998
- 25.Manjula BN, Tsai AG, Intaglietta M, et al. Thiolation mediated, maleimide-chemistry based pegylation of Hba: design, preparation and characterization of (PEG5K)6-hba, a new non-hypertensive Hb-based oxygen carrier. Bioconjug Chem. 2003 [Google Scholar]
- 26.Cabrales P, Tsai AG, Winslow RM, Intaglietta M. Extreme hemodilution with PEG-hemoglobin vs. PEG-albumin. Am J Physiol. 2005;289:H2392–400. doi: 10.1152/ajpheart.00225.2005. [DOI] [PubMed] [Google Scholar]
- 27.Tsai AG, Acero C, Nance PR, et al. Elevated plasma viscosity in extreme hemodilution increases perivascular nitric oxide concentration and microvascular perfusion. Am J Physiol Heart Circ Physiol. 2005;288:H1730–9. doi: 10.1152/ajpheart.00998.2004. [DOI] [PubMed] [Google Scholar]
- 28.Cooke JP, Rossitch E, Jr., Andon NA, et al. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991;88:1663–71. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lansman JB, Hallam TJ, Rink TJ. Single stretch-activated ion channels in vascular endothelial cells as mechanotransducers? Nature. 1987;325:811–3. doi: 10.1038/325811a0. [DOI] [PubMed] [Google Scholar]
- 30.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Mechanisms of endothelium-dependent vascular smooth muscle relaxation elicited by bradykinin and VIP. Am J Physiol. 1987;253:H1074–H82. doi: 10.1152/ajpheart.1987.253.5.H1074. [DOI] [PubMed] [Google Scholar]
- 31.Advanced Trauma Life Support Course for Physicians Committee on Trauma. American College of Surgeons; Chicago: 1997. pp. 87–107. [Google Scholar]
- 32.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–9. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 33.Evans TW. Review article: albumin as a drug--biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002;16(Suppl 5):6–11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- 34.Broe PJ, Toung TJ, Margolis S, et al. Pulmonary injury caused by free fatty acid: evaluation of steroid and albumin therapy. Surgery. 1981;89:582–7. [PubMed] [Google Scholar]
- 35.Haupt MT, Kaufman BS, Carlson RW. Fluid resuscitation in patients with increased vascular permeability. Crit Care Clin. 1992;8:341–53. [PubMed] [Google Scholar]
- 36.Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Cochrane Injuries Group Albumin Reviewers. Bmj. 1998;317:235–40. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abuchowski A, McCoy JR, Palczuk NC, et al. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–6. [PubMed] [Google Scholar]