Abstract
There is a need for safe medications that can effectively support recovery by treating symptoms of protracted abstinence in alcoholics that may precipitate relapse e.g., craving and disturbances in sleep and mood. This proof-of-concept study reports on the effectiveness of gabapentin 1200 mg for attenuating these symptoms in a non treatment-seeking sample of cue-reactive, alcohol-dependent individuals. Subjects were 33 paid volunteers with current DSM-IV alcohol dependence and a strength of craving rating 1σ or greater for alcohol than water cues. Subjects were randomly assigned to gabapentin or placebo for 1-week and then participated in a within-subjects trial where each was exposed to standardized sets of pleasant, neutral, and unpleasant visual stimuli followed by alcohol or water cues. We found a significant attenuating effect of gabapentin (vs. placebo) on several measures of subjective craving for alcohol as well as for affectively-evoked craving. Gabapentin was also found to significantly improve several measures of sleep quality. Side effects were minimal, and gabapentin effects were not found to resemble any major classes of abused drugs. Results suggest that gabapentin may be effective for treating the protracted abstinence phase in alcohol dependence and, hence, that a randomized clinical trial would be an appropriate next step. The study also suggests the value of cue reactivity studies as proof-of-concept screens for potential anti relapse drugs.
Keywords: Affective stimuli, alcohol dependence, craving, cue reactivity, gabapentin, proof-of-concept
INTRODUCTION
Neurobiological approaches to alcohol addiction have suggested that it develops in a process of homeostatic adaptation to chronic high doses of alcohol that increases set point for reward (Koob and Le Moal, 1997; Koob et al., 1998). The process is thought to involve several neurotransmitter systems, including GABA (protective inhibitory effects) and glutamate (endogenous stress). These neurotransmitter systems modulate internal states associated with positive and negative affect (Koob and Le Moal, 1997), states implicated in clinical vulnerability to relapse in protracted abstinence (Marlatt, 1985; Baker et al., 1987; Geller, 1991; Addolorato et al., 2005). Medications that normalize imbalance in these systems may protect against drinking relapse during protracted abstinence.
At the present time, only disulfiram (Antabuse), naltrexone (ReVia, Vivitrol) and acamprosate (Campral) have received FDA approval for treatment of alcohol dependence (for review see e.g. Garbutt et al., 1999). Disulfiram and naltrexone interrupt the binge/intoxification phase of alcohol dependence by punishment and reducing the positive reinforcing effects of drinking, respectively. Disulfiram changes the metabolite profile of alcohol, such that drinking produces a swift and an extremely unpleasant reaction (Christensen et al., 1991). Naltrexone blocks alcohol-induced stimulation of the endogenous opioid system thereby hypothetically reducing the pleasurable effects of drinking (Monti et al., 1999). Acamprosate, on the other hand, is thought to be effective in the protracted abstinence phase by reducing the secondary reinforcing effects of drinking by acting on the glutamate system to attenuate CNS excitability (Littleton, 1995 or De Witte et al., 2005).
In this paper we present results from an early Phase II proof-of-concept human laboratory cue reactivity study of the anticonvulsant drug gabapentin (Neurontin). Like acamprosate, it acts on GABA and glutamate systems to normalize CNS activity (Roberto et al., 2008). Published reports have suggested it may have efficacy as an off-label treatment for depression (Ghaemi et al., 1998; Harden et al., 1999; Maurer et al., 1999; Ghaemi and Goodwin, 2001; Yasmin et al., 2001), anxiety (Pollack et al., 1998; Pande et al., 2000), and insomnia (Ehrenberg, 2000; Karam-Hage and Brower, 2000; Placidi et al., 2000), all of which have been identified as symptoms of protracted abstinence and which can increase risk of relapse in abstinent alcoholics (Hershon 1977; Marlatt, 1985; Lowman et al., 1996; Zywiak et al., 1996; Annis et al., 1998; Mason et al., 1999). If gabapentin were found to attenuate such protracted withdrawal-related symptoms, such a finding would tend to confirm the hypothesized significance of GABA and glutamate system dysregulation in alcohol dependence, because gabapentin and acamprosate have these mechanisms in common.
Of equal importance, this study exemplifies a type of early Phase II proof-of-concept study that can be of value in screening such potential pharmacological treatments for relapse prevention in alcohol dependence. A week of double-blind outpatient drug administration permits an initial estimation of drug safety and efficacy and establishes chronic dosing for studying drug effects on cue reactivity in the laboratory. The cue-reactivity paradigm has a long history (e.g. Baker et al., 1987; Rohsenow and Monti, 1999). However our study is designed to elicit responses that may threaten abstinence in the period following acute withdrawal--responses that include craving as well as negative and positive affect--and to compare reactivity to beverage (alcohol vs. water) and affective (positive or negative vs. neutral) cues, alone and in combination, in a group of alcohol dependent subjects treated with gabapentin relative to placebo.
A key aspect of the study design is exposure to alcohol cues (sight and smell of the subject’s favorite alcoholic beverage) without consumption. Alcohol consumption would be expected to mask the effects of any medication that targets symptoms of protracted abstinence, as either or both could attenuate such symptoms; the effects of alcohol and the drug to be tested would therefore be inextricably confounded. Our design is therefore relatively novel in combining elicitation of craving while inducing both positive and negative affect (along with interaction effects) with non-consumptive alcohol cue exposure. As such it may be particularly well-suited for testing the effectiveness of pharmacological treatments intended to normalize the neurobiological imbalances associated with protracted abstinence in alcoholics.
At least one prior study (Rubonis et al., 1994) has found cue reactivity effects to be most clear-cut for those with an inclination to respond strongly to alcohol cues. Therefore, in order to measure medication effects on this outcome, we included only “cue reactive” subjects in the present study.
In view of the foregoing discussion, we hypothesized that gabapentin subjects would report less craving and arousal when exposed to alcohol cues and either positive or negative affective cues. Secondarily, we hypothesized that gabapentin would be found to attenuate general (not cue-specific) craving, negative mood, and sleep disturbance following a week of medication administration.
METHODS AND MATERIALS
Subjects
This study was conducted at the University of Miami School of Medicine, Miami, FL and approved by that institution’s Institutional Review Board as conforming to the ethical standards of the 1964 Declaration of Helsinki. Subjects provided written informed consent prior to participation. Subjects were non treatment-seeking paid volunteers recruited primarily through advertisements, meeting the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV; American Psychiatric Association, 1994) criteria for current alcohol dependence, abstinent from alcohol on the day of the study, as verified by Breath Alcohol Concentration (BAC), not in acute withdrawal as verified by a Clinical Institute Withdrawal Assessments for Alcohol (CIWA-Ar; Sullivan et al., 1989) score of < 8, and meeting the definition of “cue reactive”. An individual was considered cue reactive if his or her “strength of craving” score under a neutral affect condition (see Study Overview below) was 1 standard deviation (3 VAS rating scale points; see measures discussion) greater for alcohol than for water cues during a mini cue reactivity session. This is similar to the criterion described by Rubonis et al. (1994) to classify cue reactors. Exclusionary criteria consisted of clinically significant medical or psychiatric disorders including depression, anxiety, or dependence on substances other than alcohol and nicotine.
Study Overview and Experimental Design
Subjects meeting admission criteria were randomized to receive gabapentin 1200mg or placebo1. Dosages (matched in the placebo condition) were titrated as follows: 300mg morning on day 1, 300mg morning and also evening on day 2, 300mg morning, midday and evening on day 3, and 300mg morning and midday followed by 600mg evening for days 4–7. On day 7 of double-blind medication, overall measures of mood, sleep, and craving were assessed. This one-week interval of chronic dosing is intended to model and predict the critical first week on medication in a Phase II or III clinical trial, when participants’ experiences with sleep and mood, or side effects, can dramatically affect treatment adherence and risk for dropout.
Subjects were given the cue reactivity protocol on day 7 of study medication. Our cue reactivity methods have been described previously (Mason et al., 2008). The 4-hour cue reactivity protocol included a baseline evaluation, followed by the cue reactivity procedures, and subsequent debriefing. A 3 (affective stimuli: positive, neutral, negative) × 2 (beverage cue: alcohol, water) within-subjects, block-factorial design (6 repeated measures; Kirk, 1995) was employed for the cue reactivity manipulation. Thus, the 6 affect-beverage trial types were: positive-alcohol, neutral-alcohol, negative-alcohol, positive-water, neutral-water, negative-water. All six mood-beverage cue combinations were presented to each subject (with order varying systematically across subjects) during the course of a single afternoon. Since order effects of cue presentation have been observed in previous studies (Monti et al., 1987; Cooney et al., 1997), subjects were systematically assigned one of six cue order combinations, in the order they were enrolled in the study.
MEASURES
Assessments
Standardized measures were collected prior to randomization to characterize the sample and ensure admission criteria were met. Alcohol dependence criteria were ascertained with the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996), which was also used to exclude individuals with depressive, anxiety, or other drug use disorders. A protocol-specific standardized questionnaire was used to obtain significant drinking and substance use history, including age of first use and total years of heavy drinking (5 drinks/day for males, 4/day for females). The Timeline FollowBack Interview (TLFB; Sobell and Sobell, 1992) was used to determine recency, quantity and frequency of drinking in the 90 days prior to testing. The Alcohol Dependence Scale assessed dependence severity (ADS; Skinner and Horn, 1984). Overall subjective sleep quality and 7 components of disturbed sleep were assessed by the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). The Beck Depression Inventory (BDI) assessed severity of sub-syndromal depressive symptomology (Beck et al., 1961). BAC was measured by breathalyzer to confirm abstinence prior to cue exposure.
To verify a safe return to a baseline state following the cue exposure trials, the Alcohol Craving Questionnaire (ACQ; Singleton et al., 1994) was administered both prior to and following the cue reactivity procedure to ensure that the trials had not resulted in increased subjective urge to drink. Similarly, the Positive and Negative Affect Scale (PANAS; Watson et al., 1988) was administered at baseline and again after the experimental session, to ensure that subjects had been adequately debriefed and had returned to their normal emotional state. The SAFTEE-G (Levine and Schooler, 1986) was obtained after the experimental session (following 1 week on drug or placebo) to assess side effects. The 49-item version of the Addiction Research Center Inventory (ARCI; Martin et al., 1971) was used to assess abuse potential of gabapentin in alcoholics by evaluating the similarity of its effects to known drugs of abuse.
Cue Reactivity: Subjective Measures
Alcohol craving in response to each affect-beverage condition was assessed using four individual Visual Analog Scale items (VAS; endpoints were marked with a 0 on the left indicating no craving, and a 20 on the right indicating severe craving) adapted from the ACQ (Singleton et al., 1994)2. The items represented expectancy for positive reinforcement (“Having a drink would make things just perfect”), strength of craving (“How strong is your craving to drink alcohol”), intent (“If I could drink alcohol now, I would drink it”), and lack of control (“It would be hard to turn down a drink right now”).
Emotional reactivity was assessed using a computerized version of the Self-Assessment Manikin (SAM; Bradley and Lang, 1994). SAM is a cartoon figure used to assess three dimensions of affect; valence (how happy or unhappy one is), arousal (excitement, possibly anxiety), and dominance (subjective sense of control). Subjects were instructed to indicate “how you are feeling right now.” Anchors for the valence dimension included “happy, satisfied, contented” versus “unhappy, sad, bored.” Arousal anchors included “stimulated, excited, jittery” versus “relaxed, calm, sluggish.” Dominance anchors included “powerful, strong, controlling” versus “powerless, submissive, controlled.” Potential responses were marked with 0 (least strong) on the left end to 20 (strongest) on the right end. Two additional VAS ratings were used to provide manipulation checks on the experimental conditions. These questions represent beverage preference (“How much did you like the beverage just given to you”) and picture emotiveness (“Watching the pictures made me feel a strong emotion”). These questions also were anchored with extreme values of 0 and 20 (20 indicating strongest emotion).
Cue Reactivity: Psychophysiological Measures
Heart rate (HR), skin conductance (SC), and facial electromyogram (EMG) were monitored throughout each experimental trial as confirmatory measures of the primary subjective measures of craving and emotion. The focus of the present analyses is on the 90-second in vivo beverage cue exposure periods. Following guidelines provided by Fridlund and Cacioppo (1986), electrodes were placed on the corrugator and zygomatic muscle regions on the left side of the face to record negative affect (frowning) and positive affect (smiling) respectively.
Affective Stimuli
Positive, neutral, and negative pictures were selected from the International Affective Picture System (IAPS; CSEA, 1994). Two sets of equivalent images were selected for each affective category (positive, negative, neutral), in order to reduce habituation across the 2 beverage conditions. Thus, 24 pictures from each affective category were used. Prior work in our lab has verified that the selected affective slides are associated with the expected affective category (Mason et al., 2008).
Procedures
The cue reactivity protocol is summarized in Table 1, and details are provided in Appendix 1. Briefly, subjects completed the baseline clinical assessments, and then were escorted to a comfortable chair in an isolated room, and sensors to record psychophysiological responses were attached. For each of the six cue combinations (alcohol/water by positive affect/negative affect/neutral affect) a computer screen displayed the appropriate emotion-evoking IAPS images, followed by placement of the subject’s favorite alcoholic beverage or water on a small table close enough for the subject to see, touch, and smell. Psychophysiological, subjective craving and other ratings were obtained in the course of each such cue combination. After completing all six cue combinations, subjects were debriefed, and reassessed to ensure safe return to baseline.
Table 1.
Schedule of Procedures for Cue Reactivity Session*
| Pre-Test Period | |
| 1:00 p.m. | Subject arrives; vital signs, BAC, urine toxicology screen for illicit drug use, date and time of last drink obtained, and clinical and laboratory assessments completeda |
| 2:00 p.m. | Subject prepped for cue session; electrodes attached, impedance checked |
| 2:20 p.m. | Subject given instructions and cue-reactivity practice trial |
| Cue Reactivity Trials | |
| 2:40 p.m. | Step 1 - Mood Induction: Subject exposed to block of 12 affective images (pleasant, unpleasant or neutral), psychophysiological recording |
| 2:45 p.m. | Step 2 - In Vivo Beverage Cue: Alcohol or water beverage placed in front of subject for 90-sec while recalling picture-induced mood, psychophysiological recording |
| 2:50 p.m. | Step 3 - Ratings: Subjects complete VAS craving, SAM, and manipulation check in presence of beverage, beverage removed from testing area after ratings completed |
| 2:55 p.m. | Repeat Steps 1–3 for remaining affect-beverage trial combinations (6 trials total) |
| Post-Test Period | |
| 3:55 p.m. | Electrodes removed, debriefing and relaxation period, post-cue session assessment of craving and affect to verify return to baseline, subject paid |
| 5:00pm | Subject leaves |
Clinical assessments included: Clinical Institute Withdrawal Assessments for Alcohol (CIWA-Ar), Structured Clinical Interview for DSM-IV (SCID), Alcohol Dependence Scale (ADS), Alcohol Craving Questionnaire (ACQ), Positive and Negative Affect Scale (PANAS), Timeline FollowBack (TLFB)
With kind permission from Springer Science+Business Media: Psychopharmacology, Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics, Epub ahead of print, 2008 Jul 6, page 5, Mason BJ, Light JM, Escher T, Drobes DJ, Table 1.
Statistical Analysis
Mixed-effects modeling was used for statistical analysis. This approach produces results more general than but otherwise similar to repeated measures ANOVA (Laird and Ware, 1982; Gueorguieva and Krystal, 2004). Analyses were conducted using MLwIN software (Rasbash et al., 2000). Beverage (alcohol or water) and affective stimuli (positive, neutral, or negative) were treated as within-subject fixed factors, while drug condition (gabapentin or placebo) was a between-subjects fixed factor. All 33 cue reactive participants provided complete outcome data over all six cue conditions in the laboratory protocol (total observations = 33 × 6 = 198). Models included all possible terms up to two-variable interactions. We decided not to include three-variable interactions because such terms complicate model interpretations without contributing to the study hypotheses. In addition we assumed that there would be no main effect of gabapentin (i.e. an effect that held on average across all cue exposure conditions), because the lab situation and outcome measures (craving, SAM, and cue checks) were designed to focus subjects’ attention on differences between cue conditions and outcome changes occurring over a span of a few minutes, in contrast to more time-stable mood or motivational conditions (e.g. depression or anxiety) that might have been affected by medication. This assumption makes interpreting drug-cue interactions straightforward: coefficients of such terms tell us the extent to which medication modifies cue effects3.
RESULTS
Table 2 shows detailed demographic, substance use, and clinical characteristics of the sample. Most subjects were male and approximately half of the sample was non-white. Mean age was approximately 40, ranging from 19 to 59. Table 2 also provides descriptions of alcohol consumption, which was quite heavy, as intended. However, no subject reported drinking throughout the day, and most drank primarily evenings and weekends, suggesting some ability to function and carry out typical social responsibilities. Only 20% had been in previous alcohol treatment or detox.
Table 2.
Demographic and Clinical Characteristics of the Sample (n = 33)
| Demographics | n | |
|---|---|---|
| Age, yrs | 39.7 ± 11.9 | 33 |
| Sex, male (%) | 78.8 | 33 |
| Ethnicity (%) | 33 | |
| White | 48.5 | |
| African American | 3.0 | |
| Native American | 18.2 | |
| Latino | 30.3 | |
| Drinking Characteristics | ||
| Drinks per Drinking Daya | 7.2 ± 3.1 | 32 |
| Days Abstinent (%)a | 20.7 ± 18.9 | 33 |
| Years of heavy drinkingb | 12.8 ± 10.3 | 28 |
| Most typical heavy drinking occasion (%) | 28 | |
| Evenings or weekends | 75.0 | |
| Binges | 7.1 | |
| All day long | 0.0 | |
| Other | 17.9 | |
| Prior alcohol detox or treatment (%) | 21.2 | 29 |
| Alcohol Dependence Scale | 16.5 ± 16.7 | 30 |
| Pre and Post Cue Testing: | ||
| Craving and Affect Measures At Baseline Visit | ||
| Alcohol Craving (ACQ) * | ||
| Before cues | 37.2 ± 13.9 | 21 |
| After cues | 36.8 ± 15.5 | 21 |
| PANAS-Positive** | ||
| Before cues | 32.7 ± 8.2 | 27 |
| After cues | 28.9 ± 9.1 | 27 |
| PANAS-Negative* | ||
| Before cues | 13.9 ± 4.9 | 27 |
| After cues | 15.2 ± 5.8 | 27 |
Before-after difference: N.S. (p>.05)
Before-after difference: p<.01
During the 90 days before screening interview (Visit 1)
5+ Drinks per day for males, 4+ drinks per day for females
Safety and Tolerability
Because the control sample was augmented with cue-reactive subjects from studies in which no medication was involved, not all control subjects were assessed for adverse reactions to medication. However, of those subjects who were thus assessed, whether cue-reactive or not (n=30), we did find a significant (t(28) = 2.58, p<.02) difference in total symptom counts over the week on medication (mean 3.3, standard deviation 2.7) or placebo (mean 1.1, standard deviation 1.7). This difference was generated by symptoms of dizziness (χ2(1) = 6.1, p<.02) and fatigue (χ2(1) = 6.1, p<.02); drug and placebo groups did not differ significantly on any other symptoms. Analyses comparing ARCI scores on five drug-of-abuse groups (Morphine-Benzadrine, Pentabarbitol-Clorpromazine-Alcohol, LSD, Benzadrine, and Amphetamine) for gabapentin vs. placebo found no statistically significant differences, suggesting that gabapentin does not produce effects resembling those of drugs in these groups. Like safety and tolerability, ARCI was obtained only for a subsample of subjects (n=28, 17 treatment, 11 placebo).
Mixed Effect Models
Table 3 presents results of mixed-effect analyses for all subjective outcome measures. The models associated with each row contain three within-subject main effect terms, dummy-coded to represent beverage cue (0=water, 1=alcohol), positive affective stimuli (0=neutral, 1=positive), and negative affective stimuli (0=neutral, 1=negative), and two interaction terms, beverage cue by positive affect, and beverage cue by negative affect. Hence within-subject effects are compared to a water, neutral, or water-neutral conditions. In addition, treatment is a dummy-coded (0=placebo, 1=gabapentin) between-subjects effect. Columns in Table 3 contain parameter estimates for all effects specified in each model. Note that each model specification includes the same set of predictor variables.
Table 3.
Mixed-Effect Analysis Summary for Outcome Measures
| Estimated Model Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Medication Effects |
|||||||||
| Outcome | Intercept | Beverage | Induced Affect + | Induced Affect − | Bev x Aff+ | Bev x Aff− | Bev x Gaba | Aff+ x Gaba | Aff− x Gaba |
| Reactivity Check | |||||||||
| 1. Like beverage | 8.27* | 7.52* | −0.38 | −1.75† | 1.09 | 1.46 | −0.25 | −0.04 | 0.98 |
| 2. Feel strong emotion | 7.15* | 1.01 | 7.12* | 7.83* | −0.64 | −0.64 | 1.15 | −1.08 | −1.29 |
| Craving | |||||||||
| 3. Strength | 7.33* | 7.12* | 1.46† | −0.38 | −3.03* | −1.52 | −2.16† | 0.25 | 0.96 |
| 4. Drink Now | 8.88* | 5.86* | 0.43 | −0.49 | −1.27 | −1.21 | −1.96† | 0.16 | 0.58 |
| 5. Difficult to turn down | 7.94* | 3.69* | 0.37 | −1.55† | 0.33 | 0.85 | −1.31†† | −1.34†† | 1.26 |
| 6. Make Things Perfect | 7.42* | 2.96* | 0.27 | −0.65 | −0.46 | 0.15 | 0.23 | 0.44 | 0.20 |
| Subjective Emotional Reaction | |||||||||
| 7. SAM Valence | 12.79* | −1.22 | 0.11 | −2.15* | 1.94† | 0.76 | 3.28* | 0.21 | 1.25 |
| 8. SAM Arousal | 6.24 * | 4.16* | 1.93†† | 3.27* | 1.00 | −3.26 | −1.79 | −3.22† | −4.92* |
| 9. SAM Dominance | 11.27* | −1.66† | 0.80 | −1.50†† | −0.03 | 0.49 | 0.81 | 0.80 | 1.30 |
p < .01 2-tailed
p < .05 2-tailed
p < .05 1-tailed
Manipulation Checks
Validity of beverage and affective cues were investigated in several ways. First, we examined their effects on ratings of beverage-liking and feeling strong emotion (Table 3, rows 1 and 2). We found a statistically significant main effect of beverage cue on beverage-liking, with alcohol preferred to water; and also significant main effects of both positive and negative image exposure relative to neutral images on feeling strong emotion. Since affective stimuli preceded beverage presentation, results suggest that the affective response lasted through the period of beverage presentation. We also calculated within-subject correlations (Snijders and Bosker, 1994) between psychophysiological measures (heart rate, skin conductance, zygomatic, and corrugator averages calculated during in vivo beverage exposure) and all nine subjective measures including the four craving questions, three SAM questions, beverage liking, and feeling strong emotion; thus 4 × 9 = 36 such correlations were examined altogether. While these correlations were all small and most were non-significant, consistent with findings from other studies (Ooteman et al., 2006), four were statistically significant and one was trend-significant, more than would be expected by chance. Specifically, (a) heart rate was positively correlated with SAM Arousal (r=0.181, p<.05), (b) zygomatic response was positively correlated with SAM Valence (r=0.175, p<.05), (c) corrugator response was negatively correlated with beverage liking (r=−0.141, p<.10), and skin conductance and heart rate were positively correlated with feeling strong emotion (r=0.212 and 0.184, respectively, p<.05 for both). Taken together, these results suggest that the experimental conditions affected subjects as expected.
Random Effects
Variances were allowed to differ across cue combinations, and nonzero covariances between model effects were permitted, both within and between subjects. Significant effects were found for some models; however, as they are largely nuisance effects for purposes of the present study, they are not reported.
Primary study hypotheses are tested with results from the last three columns of Table 3, i.e. those involving interactions between medication and, respectively, alcohol, positive affect, and negative affect cues. These parameter estimates indicate the extent to which gabapentin alters subjects’ reaction to these three types of cues, compared to placebo. Negative values imply attenuation, while positive values show augmentation. Figures 1 and 2 summarize the statistically significant findings concerning these effects.
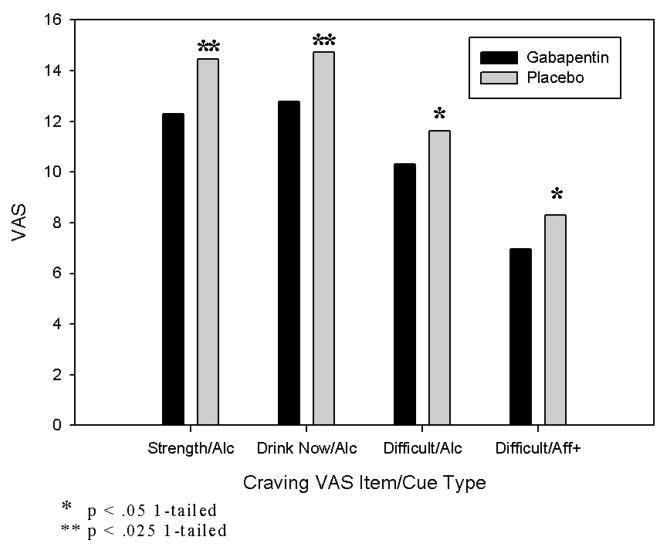
Figure 1. Effects of gabapentin vs. placebo on alcohol and affective cue-induced craving.
Legend. Craving was measured by visual analogue scale (VAS; range: 0–20). Strength = strength of craving (“How strong is your craving to drink alcohol”); Drink = intent (“If I could drink alcohol now, I would drink it”); Difficult = lack of control (“It would be hard to turn down a drink right now”); Alc = alcohol cues; Aff+ = positive affective cues.
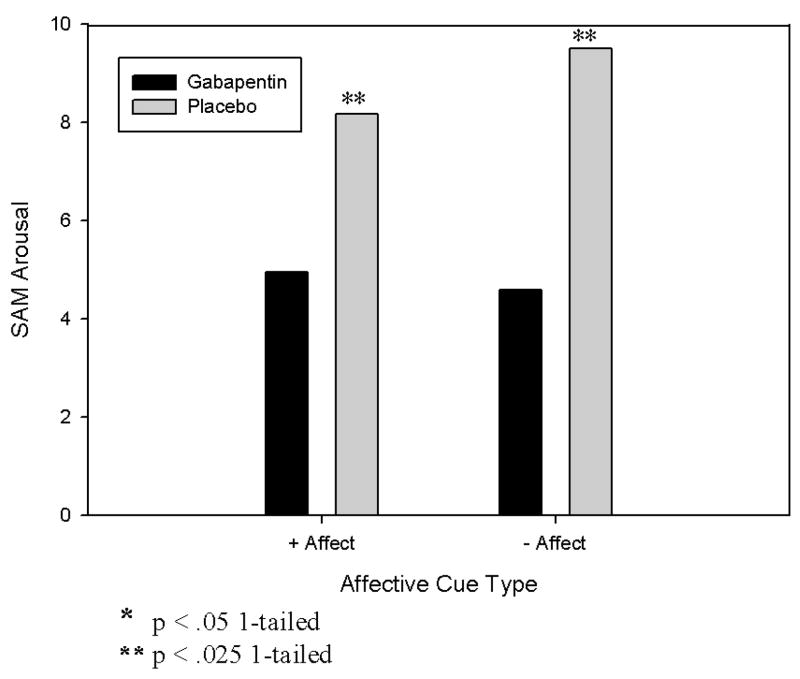
Figure 2. Effects of gabapentin vs. placebo on affective cue-induced SAM arousal.
Legend. Arousal was measured by the Self Assessment Manikin (SAM; Bradley and Lang, 1994) that has a range of 0–20.
We hypothesized that gabapentin would attenuate alcoholic beverage-induced craving compared to placebo. As shown in the first three sets of bars in Figure 1 and rows 3–5 of Table 3, this hypothesis is confirmed for three of the four subjective craving measures (how strong is your urge to drink, I would drink now if I could, it would be difficult to turn down a drink now).
We also hypothesized that gabapentin would attenuate affectively-induced craving significantly more than placebo. As the last set of bars in Figure 1 shows, this hypothesis was supported for the effect of medication on positive-affect-induced craving on one craving measure (difficult to turn down a drink now). No other craving measures showed this effect for positive affect-induced craving, and none showed such an effect for negative affect-induced craving.
We further hypothesized that gabapentin would reduce arousal induced by all three types of cues. The analyses supported this hypothesis directionally for all three cue types, and the level of attenuation was statistically significant for positive and negative affective cues. Figure 2 shows the latter two results; the full model is shown in Table 3, row 8.
Although not a major focus of the study, an additional finding is worthy of mention. Row 7 of Table 3 shows a significant and positive effect of medication on valence (larger values indicate more positive affect) induced by alcohol cue. The main effect of alcoholic beverage exposure (direction only, non-significant) was negative, indicating some negative affect or tension induced by the exposure, plausibly because subjects knew they would not be allowed to consume the alcohol. This negative affect was significantly attenuated for those subjects taking gabapentin compared to placebo, consistent with a beneficial effect of gabapentin.
Gabapentin Effects on Secondary Outcomes
Some technical problems early in the study (corrected as the study proceeded) led to the loss of some secondary outcome data, which is described in the course of the following discussion. Table 4 shows results from a set of between-subject ANOVAs comparing gabapentin and placebo on several secondary outcomes: general (not cue-induced) craving, mood symptoms, and sleep quality. No significant differences were found for either craving (as measured by the Alcohol Craving Questionnaire) or mood symptoms (measured by the Beck Depression Inventory).
Table 4.
Effects of Gabapentin vs. Placebo on Non Cue-Related Measures of Craving, Mood and Sleep
| Outcome | Gaba | Placebo | G - P | t | p | n |
|---|---|---|---|---|---|---|
| Alcohol Craving Questionnaire | 40.2 | 33.0 | 7.2 | 1.08 | NS | 22 |
| Beck Depression Inventory | 7.4 | 9.3 | −1.9 | 0.63 | NS | 27 |
| Pittsburgh Sleep QualityIndex | ||||||
| Quality | 0.00 | 0.81 | −0.81 | 4.60 | < 0.001 | 29 |
| Latency | 0.50 | 1.00 | −0.50 | 2.05 | < 0.06 | 29 |
| Duration | 1.15 | 1.24 | −0.11 | 0.29 | NS | 29 |
| Efficiency | 0.38 | 1.19 | −0.81 | 2.02 | < 0.06 | 29 |
| Disturbance | 1.12 | 1.05 | 0.08 | 0.41 | NS | 29 |
| Use of sleep meds | 0.25 | 0.43 | −0.18 | 0.51 | NS | 29 |
| Daytime dysfunction | 0.63 | 0.75 | −0.13 | 0.56 | NS | 28 |
| Global index | 4.13 | 6.50 | −2.38 | 2.01 | < 0.05 | 28 |
Note: Larger values imply greater dysfunction across all measures.
However, several of the Pittsburgh Sleep Quality Index component scales showed greater improvement in gabapentin than placebo groups. Subjective quality was significantly (p<.001) better in the gabapentin group than placebo, latency (time required to fall asleep) was shorter, and efficiency (percent of time in bed the respondent actually slept) was greater (trend, p<.06). The global PSQI sleep index was also significantly better in the gabapentin group, vs. placebo (p<.05). Of the 7 PSQI subscales, only one did not show at least some advantage in the gabapentin group compared with placebo.
Table 2 shows that subjects returned to baseline levels on measures of mood and craving following the cue reactivity session.
DISCUSSION
The central objective of this early Phase II proof-of-concept study was to estimate the effects of gabapentin vs. placebo on symptoms of protracted abstinence from alcohol using a laboratory model that comprises many of the conditions found in a clinical trial of such effects. In evaluating gabapentin’s effectiveness for attenuating craving, and affective and sleep-related disturbances known to be associated with protracted abstinence, the study serves as an efficient screen for gabapentin’s potential as a treatment for alcohol dependence. The same general approach may be useful for early clinical tests of other drugs judged to have similar neurobiological potential based on pre-clinical studies.
Alcohol exposure and both positive and negative emotional cues had the expected effects on reactivity check measures (like beverage, feel strong emotion) and on SAM emotional reaction measures. Small but generally consistent correlations with psychophysiological measures were observed, as has been found in previous studies (e.g. Ooteman et al., 2006). Taken together, these findings lend confidence that the human laboratory design employed in this study may provide a model that is useful for identifying outcomes that may be found during a Phase II or III clinical trial (Mason et al., 2008).
Results provide support for the potential efficacy of gabapentin in attenuating craving and other symptoms of protracted abstinence known to predict relapse among alcoholics in treatment. Gabapentin significantly attenuated craving for alcohol vs. water on three of four subjective craving measures. Gabapentin also reduced positive affect cue-based craving significantly on the “difficult to turn down a drink now” rating vs. placebo. Gabapentin was found to attenuate rating of arousal for alcohol (trend), positive affect, and negative affect cues. Finally, gabapentin significantly improved several measures of sleep quality compared to placebo.
These findings stand in contrast to those of Myrick et al. (2007) who found a non-significant trend (p<0.18) for between-subject reduction of craving (measured by Alcohol Urge Questionnaire; Bohn et al., 1995) in a sample of non-treatment-seeking alcoholics. However, this difference could be explained by the fact that Myrick and colleagues employed an alcohol administration paradigm which counters the condition of protracted abstinence and would be expected to reduce the effect of drugs used to treat it. Bisaga and Evans (2006) also found no effect of gabapentin on craving, but their study design involved only a single dose of gabapentin rather than the chronic dosing used in the present study as well as alcohol administration, in a sample of normal, i.e., not alcohol dependent subjects. Pre-clinical studies (Koob, 2000; Roberto et al., 2008) suggest that the neurological imbalances gabapentin is hypothesized to correct would only apply to alcohol dependent individuals. Consistent with this hypothesis, Roberto and colleagues recently reported a dose-dependent reduction in ethanol self-administration with gabapentin in dependent rats, with no effect in non dependent rats (Roberto et al., 2008).
Our results did confirm the conclusions of Bisaga and Evans (2006) and Myrick et al. (2007) regarding the safety and tolerability of gabapentin among alcohol-dependent individuals.
The study has some limitations. While results show that the laboratory design was successful in eliciting craving responses for alcohol (sight and smell of alcohol) vs. water cues, the design did not as consistently produce differential craving in response to either positive or negative affective cues. The effects of IAPS images on self-reported emotions have been extensively validated (e.g. Bradley et al., 1996), but may be less consistently evocative of affect-related alcohol craving than other procedures, particularly in terms of negative affective cues. In the present study, positive cues increased rating of strength of craving, but the effect size was relatively small (d = 0.36). Moreover, gabapentin was not found to attenuate any measure of craving evoked by negative affective cues, whereas one of the craving measures (difficult to turn down) evoked by positive affective cues was attenuated. A plausible explanation is that positive stimuli included images of sporting events and romantic encounters which are commonly associated with alcohol consumption and decreased inhibitory control. Conversely, negative stimuli included images of war scenes or mutilation that were highly unpleasant and arousing but not typically associated with alcohol consumption. Few laboratory studies have included emotionally positive cues, so more remains to be learned about its possible role in craving and relapse. More generally, the role of emotional state, both alone and in contexts where drinking cues are also present, requires additional attention (Niaura, 2000). At the present time, however, it appears that under some circumstances, positive affective cues can increase craving, and gabapentin can attenuate such craving.
An additional limitation of this study is our focus on cue-reactive subjects, rather than alcohol dependent individuals generally. While this choice limits generalizability, it is important to bear in mind that our purpose has been to test the effects of gabapentin on attenuating cue-elicited symptoms that could model those triggered by events in subjects’ lives during a later-phase clinical trial, specifically exposure to alcohol and changes in affective state. Since some individuals do not appear to react strongly to alcohol cues in laboratory settings (Rubonis et al., 1994), including such individuals in a study with these objectives could be quite misleading. Additional studies will be needed to determine the usefulness of our proof-of-concept model (and others as well) as an aid to deciding which pharmacotherapies have promise for treating alcohol dependence and warrant the more extensive and expensive testing of full scale clinical trials.
In summary, we found a significant attenuating effect of gabapentin (vs. placebo) on several measures of subjective craving for alcohol as well as for affectively-evoked craving. Gabapentin was also found to significantly improve several measures of sleep quality. Side effects were minimal, and gabapentin effects were not found to resemble any major classes of abused drugs. Results suggest that gabapentin may be effective for treating the protracted abstinence phase in alcohol dependence and, hence, that a randomized clinical trial would be an appropriate next step. The study also suggests the value of cue reactivity studies as proof-of-concept screens for potential anti relapse drugs.
Acknowledgments
Funding for this project was provided by NIAAA grant number R01AA012602 to BJM.
We are grateful to Dr. Brian Cutler for his assistance with data management and preliminary analyses for this project.
Preparation of this manuscript was supported by NIAAA R01AA012602 to BJM and the Pearson Center for Alcoholism and Addiction Research
APPENDIX 1. Laboratory Procedure Details
Laboratory sessions occurred at approximately 2:00 p.m. to control for effects of circadian rhythm and satiety. Subjects were required to have been abstinent the day of their session, as verified by BAC. Subjects were escorted to a comfortable chair located in a windowless, sound-attenuated testing room, adjacent to the control room and separated by a large one-way mirror.
Subjects were familiarized with laboratory procedures during a practice neutral-water cue reactivity trial, followed by the six experimental trials. Each trial consisted of a set of affective pictures, followed immediately by exposure to alcohol or water beverage cues which they were instructed to not drink. The affective picture viewing procedure consisted of pictures presented on a large screen directly in front of the participant, and included pleasant (e.g. adventure sports, intimate kissing), negative (e.g. traumatic physical injuries, dangerous weapons) or neutral (e.g. household objects, mushrooms) images. For each trial, participants were exposed to a set of 12 pictures within the relevant affective condition, with each picture presented for 10-seconds, and a 4-second interval between pictures. Subjects were instructed to look at each picture for the entire presentation time and remember the mood evoked by the pictures. Immediately following the picture sequence, the computer display went blank, and subjects were then presented with either their preferred alcoholic beverage (e.g. vodka, lager beer) or bottled water on a tray table adjusted to a height that placed the beverage under the subject’s nose. The alcohol or water beverage was presented in the subject’s preferred mode of consumption (e.g. small tumbler for vodka, Pilsner glass for beer) and the alcoholic bottle/can of alcohol or water bottle was placed at the left corner of the tray table for visual reference. Specific alcohol brand preferences were accommodated whenever possible, including choices of mixers (e.g. vodka would be poured into a glass along with orange juice if the favorite drink were a screwdriver). Subjects were instructed to “focus on the sensation you have while smelling the alcohol or water beverage and continue to feel the mood stirred up in your imagination by the pictures you have just viewed.” The beverage cue exposure period lasted for 90-seconds4. Following the beverage exposure period, the beverage was removed. Subjects then completed all subjective ratings by making selections from the computer screen with a computer mouse. Presentation and timing of all affective images and timing of beverage cue exposure, as well as time-locked collection of all psychophysiological measures and ratings, was controlled by a computer running VPM software (Cook et al., 1987). Upon completing all 6 cue reactivity trials, a debriefing period commenced, all electrodes were removed and relevant baseline assessments were repeated to ensure that affect and urge to drink had returned to baseline levels. Subjects received monetary compensation of $100.00 before leaving. Protocol chronology is shown in Table 1.
Footnotes
Draft: Do not cite or quote without permission
The experiments comply with the current laws of the country in which they were performed (USA).
Because image-based affective cue reactivity was not as strong as we had anticipated, to improve statistical power we augmented this control group with untreated individuals from three otherwise-similar lab studies. Criteria for participation were the same in all cases.
In an effort to minimize response burden and attendant fatigue, a subset of the full ACQ was substituted for the entire instrument. The four items comprised the highest-loading item for each of four factors in an analysis presented by Singleton et al. (1994). These items were not specifically validated as a subscale in that study, however.
Interpreting interaction effects in linear models depends upon a variety of possible assumptions concerning the mechanism that generated an observed interaction. For an excellent discussion, see Hargens (unpublished results); some of these issues were also addressed by Allison (1977) and Bobko (1986). In addition to this model specification, we also ran versions of all models with main drug effects added (Table 3), as well as three-way interactions. None of the main effects of medication were significant, and the results from those models (not reported here) were not qualitatively different from those shown in Table 3. Including three-way interactions also did not materially alter reported results.
This somewhat shorter exposure time than employed in some studies (e.g. Monti et al., 1993; Rubonis et al., 1994) was adopted in part to keep the entire protocol as brief as possible, an issue because of the repeated measures design. Pilot testing (not reported) had suggested that 90 seconds was a sufficient length of time for subjects to experience responses to presented beverages similar to what had been found with longer exposures, and subsequent results (reported below) suggest this decision was reasonable.
References
- Addolorato G, Abenavoli L, Leggio L, Gasbarrini G. How many cravings? Pharmacological aspects of craving treatment in alcohol addiction: a review. Neuropsychobiology. 2005;51:59–66. doi: 10.1159/000084161. [DOI] [PubMed] [Google Scholar]
- Allison PD. Testing for interaction in multiple regression. Am J Sociol. 1977;83:144–153. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Annis HM, Sklar SM, Moser AE. Gender in relation to relapse crisis situations, coping, and outcome among treated alcoholics. Addict Behav. 1998;23:127–131. doi: 10.1016/s0306-4603(97)00024-5. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The Motivation to Use Drugs: A Psychobiological Analysis of Urges. In: Rivers PC, editor. Nebraska Symposium on Motivation, 1986, Volume 34: Alcohol and Addictive Behavior. University of Nebraska Press; Lincoln, NE: 1987. pp. 257–323. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend. 2006;83:25–32. doi: 10.1016/j.drugalcdep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bobko P. A solution to some dilemmas when testing hypotheses about ordinal interactions. J Appl Psychol. 1986;71:323–326. [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: effects of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Christensen JK, Moller IW, Ronstead P, Angelo HR, Johansson B. Dose-effect relationship of disulfiram in human volunteers. I: Clinical studies. Pharmacol Toxicol. 1991;68:163–165. doi: 10.1111/j.1600-0773.1991.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Atkinson L, Lang KG. Stimulus control and data acquisition for IBM PCs and compatibles. Psychophysiology. 1987;24:726–727. [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–250. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- CSEA (Center for the Study of Emotion and Attention) The International Affective Picture System. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1994. [Google Scholar]
- De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- Ehrenberg B. Importance of sleep restoration in co-morbid disease: effect of anticonvulsants. Neurology. 2000;54:S33–S37. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- Geller A. Protracted Abstinence. In: Miller NS, editor. Comprehensive Handbook of Drug and Alcohol Addiction. Marcel Dekker; New York: 1991. pp. 905–913. [Google Scholar]
- Ghaemi SN, Goodwin FK. Gabapentin treatment of the non-refractory bipolar spectrum: an open case series. J Affect Disord. 2001;65:167–171. doi: 10.1016/s0165-0327(00)00218-4. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Katzow JJ, Desai SP, Goodwin FK. Gabapentin treatment of mood disorders: a preliminary study. J Clin Psychiatry. 1998;59:426–429. doi: 10.4088/jcp.v59n0805. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Harden CL, Lazar LM, Pick LH, Nikolov B, Goldstein MA, Carson D, Ravdin LD, Kocsis JH, Labar DR. A beneficial effect on mood in partial epilepsy patients treated with gabapentin. Epilepsia. 1999;40:1129–1134. doi: 10.1111/j.1528-1157.1999.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Hershon HI. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 1977;38:953–971. doi: 10.15288/jsa.1977.38.953. [DOI] [PubMed] [Google Scholar]
- Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 3. Brooks-Cole; Pacific Grove, CA: 1995. [Google Scholar]
- Koob GF. Animal models of craving for ethanol. Addiction. 2000;95:S73–S81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Littleton J. Acamprosate in alcohol dependence: how does it work? Addiction. 1995;90:1179–1188. doi: 10.1046/j.1360-0443.1995.90911793.x. [DOI] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL. Replication and extension of Marlatt’s taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addiction. 1996;91:S51–S71. [PubMed] [Google Scholar]
- Marlatt GA. Situational Determinants of Relapse and Skill-Training Interventions. In: Marlatt GA, Gordon JR, editors. Relapse Prevention. Guilford Press; New York: 1985. pp. 71–127. [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, ephedrine, phemetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology. doi: 10.1007/s00213-008-1192-x. in press. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry. 1999;56:719–724. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- Maurer I, Volz HP, Sauer H. Gabapentin leads to remission of somatoform pain disorder with major depression. Pharmacopsychiatry. 1999;32:255–257. doi: 10.1055/s-1999-7958. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Abrams DB. Alcohol cue reactivity: effects of detoxification and extended exposure. J Stud Alcohol. 1993;54:235–245. doi: 10.15288/jsa.1993.54.235. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31:221–227. doi: 10.1111/j.1530-0277.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- Niaura R. Cognitive social learning and related perspectives on drug craving. Addiction. 2000;95:S155–S163. doi: 10.1080/09652140050111726. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Vserheul R, Schippers GM, van den Brink W. Measuring craving: an attempt to connect subjective craving with cue reactivity. Alcohol Clin Exp Res. 2006;30:57–69. doi: 10.1111/j.1530-0277.2006.00019.x. [DOI] [PubMed] [Google Scholar]
- Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, Taylor CB, Dager SR, Shiovitz T. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol. 2000;20:467–471. doi: 10.1097/00004714-200008000-00011. [DOI] [PubMed] [Google Scholar]
- Placidi F, Diomedi M, Scalise A, Marciani MG, Romigi A, Gigli GL. Effect of anticonvulsants on nocturnal sleep in epilepsy. Neurology. 2000;54:S25–S32. [PubMed] [Google Scholar]
- Pollack MH, Matthews J, Scott EL. Gabapentin as a potential treatment for anxiety disorders. Am J Psychiatry. 1998;155:992–993. doi: 10.1176/ajp.155.7.992. [DOI] [PubMed] [Google Scholar]
- Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healy M, Woodhouse G, Draper D, Langford I, Lewis T. A User’s Guide to MLwiN. Institute of Education; London: 2000. [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM. Does urge to drink predict relapse after treatment? Alcohol Res Health. 1999;23:225–232. [PMC free article] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 1994;55:487–494. doi: 10.15288/jsa.1994.55.487. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Henningfield JE, Tiffany ST. Alcohol Craving Questionnaire: ACQ-Now: Background and Administration Manual. NIDA Addiction Research Center; Baltimore, MD: 1994. [Google Scholar]
- Skinner H, Horn J. Alcohol Dependence Scale: User’s Guide. Addiction Research Foundation; Toronto: 1984. [Google Scholar]
- Snijders TA, Bosker RJ. Modeled variance in two-level models. Sociol Methods Res. 1994;22:342–363. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Yasmin S, Carpenter LL, Leon Z, Siniscalchi JM, Price LH. Adjunctive gabapentin in treatment-resistant depression: a retrospective chart review. J Affect Disord. 2001;63:243–247. doi: 10.1016/s0165-0327(00)00187-7. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Connors GJ, Maisto SA, Westerberg VS. Relapse research and the Reasons for Drinking Questionnaire: a factor analysis of Marlatt’s relapse taxonomy. Addiction. 1996;91:S121–S130. [PubMed] [Google Scholar]