Abstract
Objectives
Patients with postural tachycardia syndrome (POTS) often appear anxious and report inattention. We formally assessed patients with POTS for psychiatric disorders and inattention and compared them to patients with attention deficit hyperactivity disorder (ADHD) and control subjects.
Methods
Patients with POTS (N=21), ADHD (N=18) and normal control subjects (N=20) were assessed for DSM-IV psychiatric disorders and completed a battery of questionnaires that assessed depression, anxiety and ADHD characteristics.
Results
Patients with POTS did not have an increased prevalence of major depression or anxiety disorders, including panic disorder, compared to the general population. Patients with POTS had mild depression. They scored as moderately anxious on the Beck Anxiety Inventory, but did not exhibit a high level of anxiety sensitivity. Patients with POTS scored significantly higher on inattention and ADHD subscales than control subjects. These symptoms were not present during childhood.
Conclusions
Patients with POTS do not have an increased lifetime prevalence of psychiatric disorders. Although they may seem anxious, they do not have excess cognitive anxiety. They do experience significant inattention, which may be an important source of disability.
Search Terms: Postural tachycardia syndrome (POTS), Orthostatic Intolerance, Attention, Depression, Anxiety
INTRODUCTION
Postural tachycardia syndrome (POTS), a disorder that affects an estimated 500,000 people (~0.18%) in the United States alone, is an important source of disability in young adults.[1] It shows a strong predilection for females, typically between the ages 20–50 years.[2] POTS is a form of orthostatic intolerance characterized by an excessive increase in heart rate (>30 bpm) on assuming the upright position associated with orthostatic symptoms, but in the absence of orthostatic hypotension.[2–5] Their symptoms of dizziness, nausea, tremor, chronic fatigue and exercise intolerance make even simple activities of daily living such as walking and bathing exhausting prospects.[6]
Although the role of noradrenergic dysfunction is unclear, there is significant evidence of central dysregulation of the noradrenergic system in POTS and an association between dysregulation of norepinephrine (NE) homeostasis and psychiatric disorders, including depression, panic disorder and attention deficit hyperactivity disorder (ADHD). [7–9] Centrally acting drugs that inhibit the pre-synaptic norepinephrine transporter (NET) (e.g. tricyclic antidepressants, duloxetine, reboxetine, amphetamines and cocaine) produce the clinical features of POTS.[10–13] Peripheral plasma norepinephrine (NE) is frequently raised in patients with POTS, particularly when upright, and many clinical features of POTS such as tachycardia, palpitations, shortness of breath, chest discomfort and tremor mimic the hyperadrenergic features of a panic attack. In one family, the POTS phenotype segregated with a heterozygous missense mutation in NET effecting >98% loss of function and elevated plasma norepinephrine levels. The mutation tracked with POTS characteristics and the probands complained of inattention.[10] While stimulants used to treat ADHD enhance alertness, attention and working memory, very high levels of catecholamines disrupt these higher cognitive functions. This effect can be reversed by α2–adrenoreceptor agonists which reduce NE release.[9] Together, these findings suggest that the correct balance of central NE activity is required to maintain normal attention and this could be impaired in POTS.
This cross-sectional controlled study assessed attention, mood, and anxiety symptoms in patients with POTS and contrasted them with ADHD subjects and psychiatrically normal control subjects. We tested the following hypotheses:
Patients with POTS will report a higher lifetime prevalence of depression and anxiety disorders, and a higher magnitude of current depression and anxiety symptoms, than controls and the prevalence will be comparable to subjects with ADHD.
Patients with POTS will show significant inattention relative to controls, and similar to levels shown by subjects with ADHD.
METHODS
Patients and Study Design
The study was approved by the Vanderbilt University Institutional Review Board and all participants provided written informed consent prior to the study. Patients with POTS (N=23), aged 18–65 years, were admitted as research subjects to the Vanderbilt Clinical Research Center between January 2003 and December 2004. They were referred to Vanderbilt due to a clinical suspicion of POTS or had received a clinical diagnosis. The diagnosis of POTS was confirmed with current criteria, which include (1) symptoms of orthostatic intolerance and an increase in heart rate of ≥30 beats per minute within 10 minutes of standing from a supine position, but in the absence of orthostatic hypotension (fall in blood pressure of ≥20/10 mm Hg);[2–4] (2) symptoms with standing that improved with recumbence; (3) symptoms had occurred for at least 6 months; and (4) symptoms had occurred in the absence of other major medical co-morbidity or another obvious cause of tachycardia (such as acute dehydration). None of the subjects were receiving medications which could affect autonomic function during their admission, including psychotropic drugs. Subjects participated in the seated position and were not acutely symptomatic during the testing.
Patients with POTS were matched by age, gender and race with 20 psychiatrically healthy control subjects, who formed a comparison group for the self-report questionnaires used in the study. They were free of current or lifetime history of DSM-IV [14] Axis I psychiatric disorders as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID).[15] Age-matched adults with ADHD (n=18) formed a second comparison group with the POTS patients, in particular regarding symptoms of inattention and chronic psychiatric illness. All ADHD patients had been previously diagnosed and treated for ADHD by a psychiatrist or psychologist. To ensure they currently met criteria, we re-evaluated patients using DSM-IV diagnostic criteria as recommended by Barkley.[16] All control and ADHD subjects were free of medical co-morbidities and were not taking psychotropic agents. The control and ADHD subjects did not receive formal autonomic function testing, but none complained of autonomic symptoms on screening by a medical doctor (VR), and ADHD subjects did not meet POTS criteria on testing supine and standing heart rate and blood pressure.
For data on DSM IV axis I diagnoses, subjects with POTS were compared with subjects with ADHD and data from a large population of respondents (n=9282) to the National Comorbidity Survey Replication (NCS population norms).[17] Similarly, data on Anxiety Sensitivity Index score was also compared to published general population normative data.[18]
Measures
Subjects were assessed by a trained research assistant for current and lifetime history of axis I psychiatric disorders using the SCID. Psychiatric diagnoses were made only if not accounted for by the presence of a medical disorder. Any diagnostic questions were resolved based on the consensus opinion of the project clinicians (KLH, RCS). Subjects completed the following self-report questionnaires (further details are in a supplemental file):
The Beck Depression Inventory Second Edition (BDI-II) - a well validated screening questionnaire for depression.[19, 20]
The Beck Anxiety Inventory (BAI) – a commonly used measure of anxiety severity.[21]
The Anxiety Sensitivity Index (ASI) - assesses fear of anxiety-related symptoms.[22]
The Connors Adult ADHD Rating Scale long form (CAARS-L) - rates items in four factor domains including inattention/memory problems and hyperactivity/restlessness.[23]
The ADHD Behavior Checklist for Adults (ABC-A) - measures the specific inattentive and hyperactive-impulsive symptoms that comprise the DSM-IV criteria for ADHD.[24]
Statistical Analysis
Differences among the groups were assessed using Fisher’s exact test for categorical variables and one-way analysis of variance (ANOVA) for continuous variables, with a post-hoc Scheffe multiple comparisons procedure used for pair-wise comparisons. Spearman’s test was performed to analyze for bivariate correlations. A general linear model with inattention as the dependent variable and gender, anxiety and depression as fixed factors was used to test for the influence of potential confounding variables. All tests were two-sided and results were considered significant if P<0.05. Data were analyzed with SPSS for Windows software, version 14.0 (SPSS Inc.), and GraphPad Prism software, version 4 (GraphPad Software, San Diego, Calif.). Results presented are means and standard deviations unless otherwise specified.
RESULTS
Patient Characteristics
One POTS patient declined to participate, reporting she felt too symptomatic. Data collected on 2 of the 23 patients with POTS were not analyzed due to co-morbid ADHD that pre-dated the diagnosis of POTS. One control subject was excluded after meeting criteria for a past diagnosis of major depressive disorder. No ADHD volunteers were excluded. However, 3 of 23 initial subjects failed to participate and 2 subjects failed to complete the study so their data could not be included. ADHD subjects could not be closely gender-matched with the POTS patients due to the male bias of ADHD compared to the strong female predominance of POTS.
POTS patients reported mean length of illness from self-reported onset of symptoms as 6.9±9.2 years. Characterizing data in the POTS subjects is presented in table 1 and baseline demographic data for each patient group is compared in Table 2:
Table 1.
Characterizing Data in Patients with POTS
| Characteristic | Supine | Standing |
|---|---|---|
| Heart rate (bpm*) | 75±12 | 128±19 bpm |
| Blood pressure (mm Hg**) | 108/65±11/8 | 118/73±19/12 mmHg |
| NE (pg/ml***) | 267±98 | 962±720 pg/ml |
POTS – Postural Tachycardia Syndrome. Data are presented as mean ± standard deviation.
bpm = beats per minute
mmHg = millimeters Mercury
pg/ml = picograms per milliliter.
Table 2.
Demographic Data for Patients with POTS, Normal Controls and ADHD Patients
| Characteristic | Normals (N = 20) |
POTS (N = 21) |
ADHD (N = 18) |
P value |
|---|---|---|---|---|
| Age (years) | 36.6±11.5 | 35.9±10.9 | 41.8±9.1 | 0.117* |
| Female, N (%) | 18 (90%) | 18 (86 %) | 10 (56%) | 0.022** |
| Race/Ethnicity – Caucasian N (%) | 19 (95%) | 20 (95%) | 18 (100%) | 0.634** |
| Education (years) | 16.4±3.4 | 14.3±2.8 | 14.8±2.6 | 0.090* |
POTS – Postural Tachycardia Syndrome; ADHD – Attention Deficit Hyperactivity Disorder. Data are presented as mean ± standard deviation.
P value based on one way ANOVA assessing for difference between the groups.
P value based on Fisher’s exact test for difference between the groups.
Groups did not differ significantly in age and education level and there was no difference in gender distribution between the POTS and control groups. There were more men in the ADHD group compared with the control group (P=0.027) reflecting the male predominance of ADHD. All subjects were Caucasian, with the exception of one African-American patient with POTS who was matched with an African-American control subject.
Prevalence of Mental Disorders
Lifetime prevalence of DSM-IV axis I psychiatric disorders in POTS patients and ADHD subjects are presented in Table 3.
Table 3.
Lifetime Prevalence of Axis I Psychiatric Disorders in POTS Patients and ADHD Subjects
| POTS (N=21) | ADHD (N=18) | Population** (N=9282) | |
|---|---|---|---|
| None | 8 (38.1%) | 5 (25%)* | |
| Major Depressive Disorder | 4 (19.0%) | 10 (50%) | 1541 (16.6%) |
| Anxiety Disorders | 7 (33.3%) | 9 (45%) | 1639 (28.8%, N=5692) |
| - Panic Disorder | 1 (4.8%) | 3 (15%) | 436 (4.7%) |
| Substance abuse/dependence | 2 (9.5%) | 8 (40%) | 1355 (14.6%) |
POTS – Postural Tachycardia Syndrome; ADHD – Attention Deficit Hyperactivity Disorder.
For ADHD subjects, no other psychiatric disorder.
Population data from National Comorbidity Survey replication data on the lifetime prevalence of DSM-IV axis I disorders.[17]
The lifetime prevalence of anxiety disorders in patients with POTS was not significantly greater than NCS population norms (P=0.83) and did not differ significantly from subjects with ADHD (P=0.34). Subjects with ADHD had a non-significant trend toward a higher prevalence of anxiety disorders than NCS population norms (P=0.08), including panic disorder (P=0.066). Amongst anxiety disorders, the prevalence of panic disorder in patients with POTS did not differ from NCS population norms (P=0.99) or subjects with ADHD (P=0.32).
There was no difference in the lifetime prevalence of major depressive disorder in patients with POTS compared to NCS population norms (P=0.99). However, there was a higher prevalence of major depressive disorder in subjects with ADHD compared to patients with POTS (P=0.024) or NCS population norms (P<0.001).
Finally, the prevalence of substance abuse or dependence in patients with POTS did not differ significantly from NCS population norms (P=0.73), but was significantly less than in subjects with ADHD (P=0.025).
Prevalence of Depression Symptoms
The BDI-II score compared the magnitude of current depression symptoms in patients with POTS, subjects with ADHD and control subjects free of DSM-IV axis I disorders. Patients with POTS scored as mildly depressed (4.8±8.5). Overall, BDI-II scores differed significantly among the groups (P< 0.001). Post-hoc analysis showed a significantly higher score in patients with POTS compared to control subjects (14.8±8.5 vs. 1.4±1.5; P<0.001), and in ADHD subjects compared to controls (10.7±9.8 vs. 1.4±1.5; P<0.001), but there was no significant difference between the POTS and ADHD groups (P=0.239). For patients with POTS, there was a significant inverse correlation between length of illness and BDI-II score (rs=−0.47, p=0.04). There was no significant correlation of supine, upright or delta NE with BDI-II score in the POTS group.
Prevalence of Anxiety Symptoms
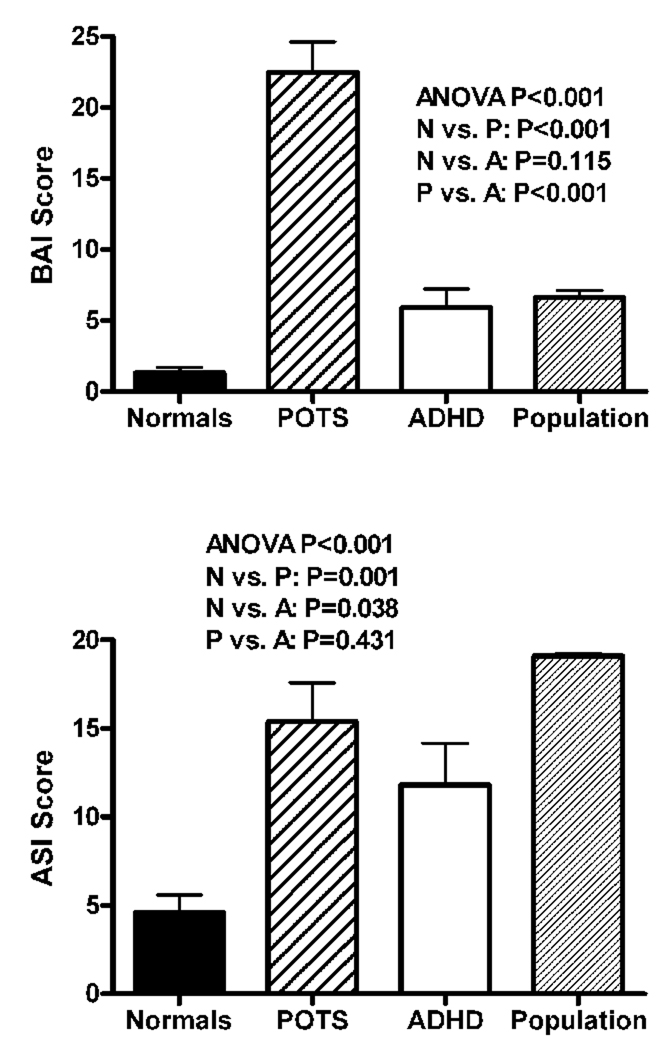
The BAI compared the magnitude of current anxiety symptoms in the three groups (Figure 1 top). Patients with POTS scored as moderately anxious (22.5±9.8). Overall, BAI scores differed significantly among the groups (P<0.001). Post-hoc analysis showed a significantly higher score in patients with POTS compared to control subjects (1.3±1.6; P<0.001), and in patients with POTS compared to subjects with ADHD (5.9±5.7; P<0.001), but there was no significant difference between the ADHD and control groups (P=0.115). In the POTS group, there was no significant correlation between length of illness and BAI score (rs =−0.40, P=0.09). There was also no significant correlation of supine, upright or delta NE with BAI score in the POTS subjects. The 5 BAI items scoring with highest frequency in the POTS group compared to the other groups were heart pounding and racing (2.3±0.6), feeling dizzy and lightheaded (1.8±0.9), numbness and tingling (1.6±0.8), feeling unable to relax (1.5±0.8) and feeling unsteady (1.5±0.9).
Figure 1.
The Beck Anxiety Inventory (BAI) scores (top panel) and the Anxiety Sensitivity Index (ASI) scores (bottom panel) are shown for the healthy control subjects (normals; solid black), patients with postural tachycardia syndrome (POTS; hashed lines), patients with attention deficit hyperactivity disorder (ADHD; open bar), and published population norms (dark gray). The overall P value is measured using an analysis of variance (ANOVA) for the 3 study groups (not including the published population norms) and paired P values were performed using a post-hoc Scheffe test. Data are presented as mean ± standard error. The differences between the 2 anxiety tools may reflect the inclusion of somatic anxiety symptoms in the BAI, whereas the ASI scores only for cognitive anxiety.
The ASI compared the magnitude of current fear of anxiety-related symptoms in the three groups (Figure 1 bottom). Overall, ASI scores differed significantly between the groups (P=0.001). Post-hoc analysis showed a significantly higher ASI score for the POTS patients (15.4±9.8) than the control group (4.6±4.5; P=0.001), and in subjects with ADHD (11.8±9.7) compared to controls (P=0.031), but there was no significant difference between the POTS and ADHD groups (P=0.504). ASI scores for the 3 groups were also compared to the published general population normative data of 19.1±9.11 (obtained from 12 non-clinical samples comprising >4500 participants).[18] Patients with POTS experienced a trend toward lower anxiety sensitivity than the general population (P=0.07), while subjects with ADHD experienced higher anxiety sensitivity than the general population (P=0.001). As expected, our psychiatrically normal control subjects experienced significantly less anxiety sensitivity than the general population (P<0.001).
Among POTS patients, there was a significant inverse correlation between length of illness and ASI score (rs =−0.56, P=0.01).There was no significant correlation of supine, upright or delta NE with ASI score in the POTS subjects.
Prevalence of Inattention Symptoms
Significant group differences were noted on all 8 CAARS-L subscales. Post-hoc analysis showed that patients with POTS did not differ significantly from the controls for hyperactivity (P=0.170), impulsivity (P=0.064), self-concept (P=0.196) and DSM-IV hyperactivity (P=0.060) subscales, but both groups scored significantly lower than the ADHD group.
Patients with POTS experienced significantly more symptoms than the controls on three CAARS-L subscales: Inattention/Memory problems (P=0.033), the CAARS-L “DSM-IV Inattention” score (P<0.001) and the “DSM-IV ADHD” score (P=0.003) (Table 4). They also scored significantly higher than the controls on the ADHD index (P=0.027). The ADHD group scored significantly higher than either of the other groups on all CAARS-L subscales.
Table 4.
CAARS-L Subscale Scores
| Subscale | Controls (N=20) |
POTS (N=21) |
ADHD (N=18) |
P value* (POTS vs. Controls) |
P value* (POTS vs. ADHD) |
|---|---|---|---|---|---|
| Inattention/Memory | 43.4±5.4 | 52.3±11.1 | 67.5±13.2 | 0.033 | <0.001 |
| DSM-IV Inattention | 41.1±7.4 | 57.7±15.8 | 73.8±14.5 | <0.001 | 0.001 |
| DSM-IV ADHD | 38.1±6.0 | 52.6±16.1 | 70.4±15.5 | 0.003 | 0.001 |
| ADHD Index | 38.9±4.3 | 46.5±10.3 | 62.4±12.0 | 0.027 | <0.001 |
CAARS-L – Conners’ Adult ADHD Rating Scales-Long version; POTS – Postural Tachycardia Syndrome; ADHD – Attention Deficit Hyperactivity Disorder; DSM-IV – Diagnostic and Statistical Manual of Mental Disorders 4th Edition. Data are presented as mean ± standard deviation.
P values based on post-hoc Scheffe multiple comparisons procedure. P values <0.05 are in bolded text.
A general linear model analysis using gender, BDI score and ASI score was used to test for a possible confounding influence by gender, current depression symptoms and current anxiety sensitivity on inattention scores. First, using Connors DSM-IV inattention as the dependent variable, the model showed no statistically significant effect of gender (P=0.307). There was a statistically significant effect of BDI score (P=0.021) and ASI score (P=0.019), but group remained a statistically significant driver of the CAARS-L DSM-IV inattention score (P<0.001) even when BDI and ASI scores were taken into account. Second, using CAARS-L ADHD score as the dependent variable, the model showed no statistically significant effect of gender (P=0.444) or ASI score (P=0.155). There was a statistically significant effect of BDI score (P=0.005), but group remained a statistically significant driver of the CAARS-L ADHD score (P<0.001) even when BDI score was taken into account.
In the POTS group, there were statistically significant inverse correlations between length of illness and the following CAARS-L subscales: inattention/memory score (rs =−0.46, P=0.05), self concept score (rs =−0.47, P=0.04), Connors DSM-IV hyperactivity score (rs =−0.53, P=0.02), DSM-IV ADHD score (rs =−0.49, P=0.04) and ADHD index (rs =−0.52, P=0.02). There was no significant correlation of supine, upright or delta NE with any of the CAARS-L subscale scores in subjects with POTS.
Prevalence of ADHD Symptoms in Childhood and Adulthood
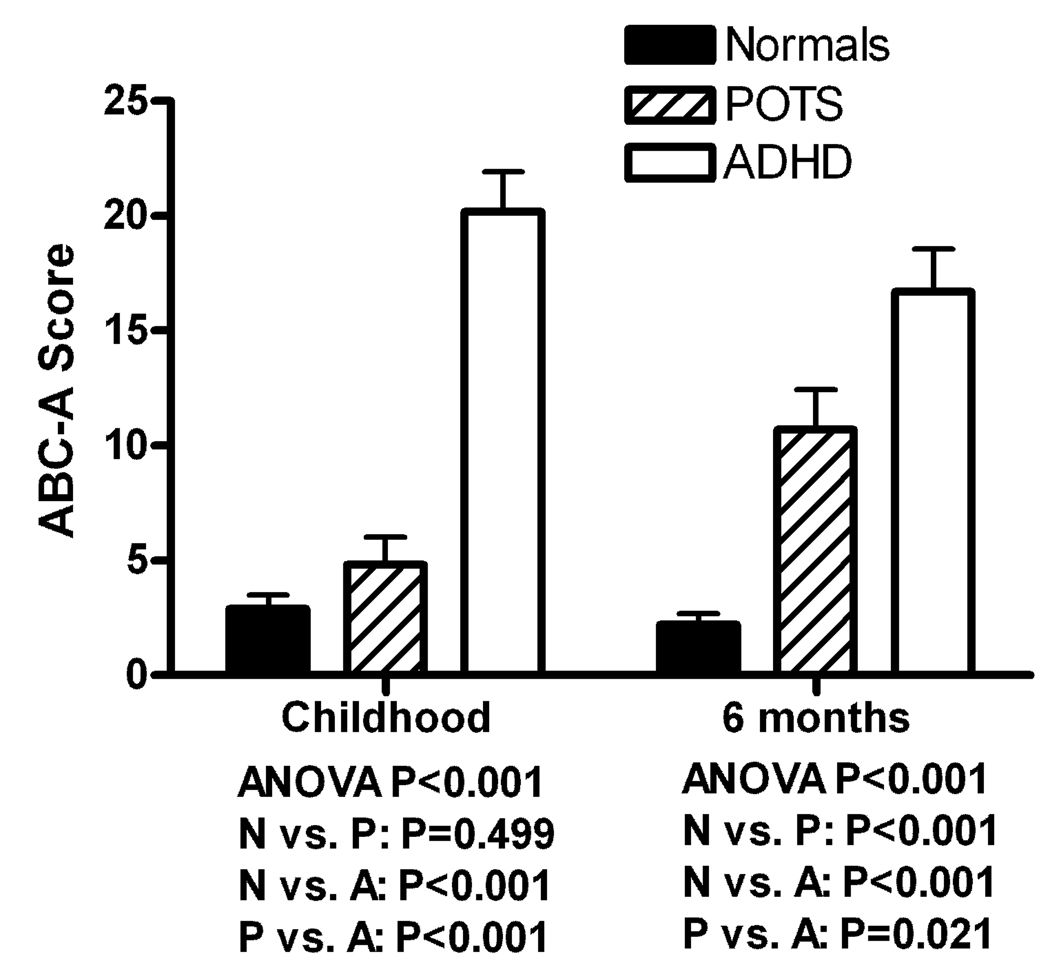
Scores on the four ABC-A subscales were compared between the three groups, and data on inattention are presented graphically in Figure 2. There were significant overall group differences on all subscales (attention problems in childhood: P<0.001; hyperactivity in childhood: P<0.001; inattention over the past 6 months: P<0.001; hyperactivity over the past 6 months: P<0.001).
Figure 2.
The ADHD Behavior Checklist for Adults Inattention scores are presented for the 3 groups (as outlined earlier) during their childhood (left) and during the last 6 months (right). The overall P value is measured using an analysis of variance (ANOVA) for the 3 groups and paired P values were performed using a post-hoc Scheffe test. Data are presented as mean ± standard error.
For inattention in childhood, post-hoc analysis showed that patients with POTS (4.8±5.4) had significantly less childhood symptoms than ADHD subjects (20.2±7.0; P<0.001) and did not differ significantly from control subjects (2.9±2.6; P=0.499). However, for inattention over the past six months, patients with POTS (10.7±7.8) had significantly more symptoms than control subjects (2.2±2.1; P<0.001), but less than ADHD subjects (16.7±7.7; P=0.021).
For hyperactivity in childhood, post-hoc analysis showed that patients with POTS (5.7±6.2) had significantly less childhood symptoms than ADHD subjects (18.8±9.3; P<0.001) and did not differ significantly from control subjects (2.5±2.2; P=0.281). For hyperactivity over the past six months, patients with POTS (7.9±6.6) had significantly more symptoms than control subjects (1.8±2.0; P=0.007), but less than ADHD subjects (13.7±7.7; P=0.015).
DISCUSSION
Several studies have reported that patients with POTS have co-morbid psychiatric symptoms, particularly anxiety and panic disorder.[8, 25] Using the Structured Clinical Interview for DSM-IV Axis I Disorders, we found that there was no significant difference in lifetime prevalence of major depressive disorder in patients with POTS compared to a general population sample.[17] Patients with POTS do, however, score as mildly depressed on quantitative measures of current depression symptoms (BDI). This may reflect living with a chronic medical illness rather than a phenomenon specific to POTS, as medical illness is commonly associated with an increased frequency of depressive disorders.[26–28] Certainly, patients with POTS show limitations across multiple domains of quality of life in common with other chronic medical illnesses including chronic obstructive pulmonary disease and heart failure.[6] Interestingly, there is a significant reduction in depressive symptoms (BDI score) as POTS progresses. This could reflect a reduction in symptom burden over time, or indicate the psychological adjustment of patients to living with a chronic illness.
Patients with POTS did not experience a significantly increased lifetime prevalence of anxiety disorders (including panic disorder) compared to a general population sample [17] or subjects with ADHD. For current anxiety symptoms, patients with POTS scored significantly higher than controls and ADHD subjects on the BAI, and within the moderate anxiety range. However, when using the cognitively focused ASI, patients with POTS did not score higher than the general population (they actually had a trend toward a lower score), although they still scored higher than control subjects free of axis I psychiatric disorders. This is supported by the findings of Masuki et al. who reported that ASI scores in 14 patients with POTS were significantly higher than in control subjects, but within the limits of published general population data.[29]
The contrast between the BAI score (moderate anxiety) and the ASI score (comparable to the general population) in the POTS group is striking. A major difference between the BAI and ASI is that the former measures both somatic symptoms and subjective anxiety and panic symptoms on factor analysis, while the latter measures sensitivity to anxiety-provoking stimuli but not somatic symptoms. Since the orthostatic symptoms experienced by patients with POTS are similar to the somatic criteria required for the diagnosis of panic attacks or other anxiety disorders (e.g. palpitations), it is likely that these symptoms, rather than psychological factors, elevate the BAI scores. Unlike subjects with panic disorder, patients with POTS show several important clinical differences that can be used to distinguish between the two disorders. These include predictable precipitants for the onset of somatic symptoms in subjects with POTS (e.g. dehydration and standing) while patients with panic disorder experience repeated episodes without a clear precipitant. Further, patients with POTS experience a significant worsening of somatic symptoms on adoption of the upright position while subjects with panic disorder typically do not.[30] It is therefore likely that the somatic symptoms in POTS patients do not represent panic disorder, which is appropriately reflected in the lower ASI score. This finding is supported by two recent studies. Masuki et al. showed that excessive increase in heart rate in patients with POTS in response to orthostatic stress is not caused by anxiety.[29] Kharuna collected data on anxiety and somatic symptoms in response to anxiety-provoking stimuli and found that symptoms of POTS were distinguishable from symptoms of panic disorder.[31] Taken together, the evidence suggests that clinically observed anxiety in patients with POTS is caused by biological rather than psychological factors.
As POTS progresses, there is a significant decrease in ASI score but no significant change in BAI score. This suggests that there is a diminution in cognitive anxiety symptoms with time, but not in somatic symptoms.
Our data suggest that patients with POTS experience inattention of a magnitude that is greater than in controls but less severe than in adult ADHD subjects. Unlike ADHD subjects, patients with POTS did not report more inattention during childhood. This could indicate a causal role for POTS in attention difficulties, or suggest that the two problems share common antecedents. Moreover, this might suggest common genetic underpinnings since noradrenergic gene products regulate sympathetic tone, cardiac output, and attention. Interestingly, there is a significant perceived reduction in the magnitude of inattention and ADHD symptoms as POTS progresses. This could reflect patient adaptations or an improvement in POTS symptoms over time.
Study Limitations
First, the sample size in this study is relatively small, although it does compare favorably to other clinical research studies in POTS. It is possible that with a much larger cohort, some of our non-significant trends might have become significant. Second, whereas psychiatric diagnoses were evaluated using the gold standard of a structured interview, data on attention was collected by subjective self-rated questionnaires. While these tools have been well validated, future studies might benefit from using more objective outcome measures.
Conclusions
Patients with POTS do not have a higher than normal prevalence of anxiety disorders. While they register with moderate anxiety using somatic assessment tools like the BAI, they have no more anxiety than the general population using cognitive tools such as the ASI. Similarly, patients with POTS do not have a significantly increased prevalence of major depression, but do exhibit mild depressive symptoms. The main abnormality noted in patients with POTS was difficulty with maintaining attention. These attention difficulties develop later in life, in contrast to ADHD patients. Further investigation is required in order to identify the etiology of these problems, and therefore guide clinicians in the selection of targeted treatments.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the professional assistance of the staff of the Vanderbilt Clinical Research Center and the research subjects whose participation made this work possible.
FUNDING
This work was supported by National Institutes of Health (Bethesda MD, USA) grants 2P01 HL56693, RO1 NS055670, K23 RR020783, M01 RR00095 & UL1 RR024975.
Footnotes
COMPETING INTERESTS
None.
References List
- 1.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am.J.Med.Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Low PA, Opfer-Gehrking TL, Textor SC, et al. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–S25. [PubMed] [Google Scholar]
- 3.Raj SR, Black BK, Biaggioni I, et al. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation. 2005;111:2734–2740. doi: 10.1161/CIRCULATIONAHA.104.497594. [DOI] [PubMed] [Google Scholar]
- 4.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 5.Grubb BP, Kanjwal Y, Kosinski DJ. The postural tachycardia syndrome: a concise guide to diagnosis and management. J Cardiovasc.Electrophysiol. 2006;17:108–112. doi: 10.1111/j.1540-8167.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 6.Benrud-Larson LM, Dewar MS, Sandroni P, et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin.Proc. 2002;77:531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 7.Harro J, Oreland L. Depression as a spreading adjustment disorder of monoaminergic neurons: a case for primary implication of the locus coeruleus. Brain Res.Brain Res.Rev. 2001;38:79–128. doi: 10.1016/s0165-0173(01)00082-0. [DOI] [PubMed] [Google Scholar]
- 8.Esler M, Alvarenga M, Pier C, et al. The neuronal noradrenaline transporter, anxiety and cardiovascular disease. J Psychopharmacol. 2006;20:60–66. doi: 10.1177/1359786806066055. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol.Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 10.Shannon JR, Flattem NL, Jordan J, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N.Engl.J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 11.Robertson D, Flattem N, Tellioglu T, et al. Familial orthostatic tachycardia due to norepinephrine transporter deficiency. Ann.N.Y.Acad.Sci. 2001;940:527–543. doi: 10.1111/j.1749-6632.2001.tb03703.x. [DOI] [PubMed] [Google Scholar]
- 12.Vincent S, Bieck PR, Garland EM, et al. Clinical assessment of norepinephrine transporter blockade through biochemical and pharmacological profiles. Circulation. 2004;109:3202–3207. doi: 10.1161/01.CIR.0000130847.18666.39. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105:347–353. doi: 10.1161/hc0302.102597. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic and Statistical Manual of Mental Disorders DSM-IV. American Psychiatric Association; 1994. [Google Scholar]
- 15.First MB, Gibbon M, Spitzer RL, Williams JBW. User's Guide for the Structured Interview for DSM–IV Axis I Disorders – Research Version (SCID-I, version 2.0, February 1996 final version) New York: Biometrics Research; 1996. [Google Scholar]
- 16.Barkley RA. Attention-Deficit Disorder: A Handbook for Diagnosis and Treatment. New York: Guilford Press; 1998. [Google Scholar]
- 17.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch.Gen.Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 18.Peterson RA, Reiss S. Anxiety Sensitivity Index Revised test manual. Worthington, OH: IDS Publishing Corporation; 1993. [Google Scholar]
- 19.Beck AT, Steer RA, Brown GK. BDI-II manual. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 20.Nezu AM, Ronan GF, Meadows EA, McClure KS. Practitioner's guide to empirically based measures of depression. Springer: 2000. [Google Scholar]
- 21.Beck AT, Epstein N, Brown G, et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 22.Peterson RA, Reiss S. Anxiety Sensitivity Index Manual. Worthington, OH: International Diagnostic Systems; 1992. [Google Scholar]
- 23.Conners CK. Rating scales in attention-deficit/hyperactivity disorder: use in assessment and treatment monitoring. J Clin Psychiatry. 1998;59 Suppl 7:24–30. [PubMed] [Google Scholar]
- 24.Barkley RA. ADHD behavior checklist for adults. The ADHD Report. 1995;3:16.
- 25.Benrud-Larson LM, Sandroni P, Haythornthwaite JA, et al. Correlates of functional disability in patients with postural tachycardia syndrome: preliminary cross-sectional findings. Health Psychol. 2003;22:643–648. doi: 10.1037/0278-6133.22.6.643. [DOI] [PubMed] [Google Scholar]
- 26.Burvill PW, Johnson GA, Jamrozik KD, et al. Prevalence of depression after stroke: the Perth Community Stroke Study. Br.J.Psychiatry. 1995;166:320–327. doi: 10.1192/bjp.166.3.320. [DOI] [PubMed] [Google Scholar]
- 27.Beck DA, Koenig HG. Minor depression: a review of the literature. Int.J.Psychiatry Med. 1996;26:177–209. doi: 10.2190/AC30-P715-Y4TD-J7D2. [DOI] [PubMed] [Google Scholar]
- 28.Lindeman S, Hamalainen J, Isometsa E, et al. The 12-month prevalence and risk factors for major depressive episode in Finland: representative sample of 5993 adults. Acta Psychiatr.Scand. 2000;102:178–184. doi: 10.1034/j.1600-0447.2000.102003178.x. [DOI] [PubMed] [Google Scholar]
- 29.Masuki S, Eisenach JH, Johnson C, et al. Excessive Heart Rate Response to Orthostatic Stress in Postural Tachycardia Syndrome is Not Caused by Anxiety. J Appl Physiol. 2006;102:1136–1142. doi: 10.1152/japplphysiol.00927.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin.Electrophysiol. 2003;26:1747–1757. doi: 10.1046/j.1460-9592.2003.t01-1-00262.x. [DOI] [PubMed] [Google Scholar]
- 31.Khurana RK. Experimental induction of panic-like symptoms in patients with postural tachycardia syndrome. Clin Auton Res. 2006;16:371–377. doi: 10.1007/s10286-006-0365-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.