Abstract
Objectives
Delayed diagnosis of colorectal cancer (CRC) is among the most common reasons for ambulatory diagnostic malpractice claims in the United States. Our objective was to describe missed opportunities to diagnose CRC before endoscopic referral, in terms of patient characteristics, nature of clinical clues, and types of diagnostic-process breakdowns involved.
Methods
We conducted a retrospective cohort study of consecutive, newly diagnosed cases of CRC between February 1999 and June 2007 at a tertiary health-care system in Texas. Two reviewers independently evaluated the electronic record of each patient using a standardized pretested data collection instrument. Missed opportunities were defined as care episodes in which endoscopic evaluation was not initiated despite the presence of one or more clues that warrant a diagnostic workup for CRC. Predictors of missed opportunities were evaluated in logistic regression. The types of breakdowns involved in the diagnostic process were also determined and described.
Results
Of the 513 patients with CRC who met the inclusion criteria, both reviewers agreed on the presence of at least one missed opportunity in 161 patients. Among these patients there was a mean of 4.2 missed opportunities and 5.3 clues. The most common clues were suspected or confirmed iron deficiency anemia, positive fecal occult blood test, and hematochezia. The odds of a missed opportunity were increased in patients older than 75 years (odds ratio (OR) = 2.3; 95% confidence interval (CI) 1.3–4.1) or with iron deficiency anemia (OR = 2.2; 95% CI 1.3–3.6), whereas the odds of a missed opportunity were lower in patients with abnormal flexible sigmoidoscopy (OR = 0.06; 95% CI 0.01–0.51), or imaging suspicious for CRC (OR = 0.3; 95% CI 0.1–0.9). Anemia was the clue associated with the longest time to endoscopic referral (median = 393 days). Most process breakdowns occurred in the provider–patient clinical encounter and in the follow-up of patients or abnormal diagnostic test results.
Conclusions
Missed opportunities to initiate workup for CRC are common despite the presence of many clues suggestive of CRC diagnosis. Future interventions are needed to reduce the process breakdowns identified.
Introduction
Delayed diagnosis of colorectal cancer (CRC) is among the most common reasons for ambulatory malpractice claims related to missed and delayed diagnosis in the United States (1,2). Several randomized controlled trials (3,4) have shown that survival in patients with CRC is significantly longer when the diagnosis is made at a more localized early stage, making a compelling case for early detection through screening programs (5). However, most patients with colorectal cancers are diagnosed after the onset of cancer-related symptoms (6) and it is imperative that frontline providers recognize clues to CRC diagnosis early.
Understanding and subsequently preventing delays in CRC diagnosis is consistent with high quality health care, but few contemporary studies describe the origins and characteristics of missed opportunities that lead to these delays (7). Limited endoscopic capacity (endoscopists, space, equipment) (8), and delays associated with patients completing their scheduled colonoscopy procedures can be responsible for delayed CRC diagnosis after a colonoscopy referral has been made (9,10). However, an important and potentially preventable determinant of delay in CRC diagnosis is a failure to pursue a workup for CRC in the presence of clues to its diagnosis. The breakdowns in the process of detecting and investigating clues represent potential missed opportunities that could lead to an earlier referral for colonoscopy, and an earlier diagnosis of CRC. This knowledge is important in guiding interventions to improve CRC diagnosis in primary care.
In this study, we identified patients diagnosed with a new CRC and evaluated episodes of care where opportunities for an earlier diagnosis of CRC were missed before initiation of an endoscopy consultation. Our objective is to describe missed opportunities to diagnose CRC before endoscopic referral, in terms of patient characteristics, the nature of the clues and the types of breakdowns involved in the diagnostic process.
Methods
Setting
We used a retrospective cohort design to identify and evaluate newly diagnosed cases of primary CRC at a tertiary care health system in Texas from February 1999 through June 2007. The system includes a large referral center with a multispecialty ambulatory care clinic and several satellite clinics. All patients are assigned a primary care provider and most patients continue to obtain their care within the system. Types of practitioners included both academic and non-academic practitioners and resident trainees, who are supervised closely by attending physicians. Using an integrated electronic medical record, we conducted a detailed review of care processes related to patient presentation, test ordering, referral, and follow-up procedures by obtaining data from progress notes, consultations menu, laboratory and radiology menus, discharge summaries, etc. The study was approved by the local Institutional Review Board.
Inclusion and exclusion criteria
We included all pathologically confirmed, newly diagnosed cases of primary CRC diagnosed between February 1999 and June 2007. After review of the electronic health record, we excluded patients who were diagnosed (or received their CRC diagnosis care) outside the institution, were in the system for less than 30 days before diagnosis, and those with recurrent CRC (diagnosed with CRC within past 5 years).
Data collection procedures
Two reviewers (both physicians) independently reviewed each CRC case using a detailed data collection instrument. The purpose of two independent reviews was to evaluate agreement on the presence or absence of missed opportunities. Both reviewers evaluated all the relevant electronic data available (in most cases as far back as 1997) for presence of predefined clinical clues that warrant a diagnostic workup for CRC. Definitive clues were derived from expert opinion and current literature and included anemia (suspected iron deficiency (unexplained hemoglobin/hematocrit below lab norm, MCV below lab norm, absent iron studies) or confirmed iron deficiency), positive fecal occult blood test (FOBT), computerized tomographic (CT) imaging suspicious for CRC, suspected rectal or abdominal mass on physical exam, change in stool caliber, worsening constipation (recent (within 1 year) decrease in stool frequency), abdominal pain, weight loss, melena, intestinal obstruction, abnormal flexible sigmoidoscopy (showing polyp or mass), or abnormal double contrast barium enema (DCBE showing polyp, mass or filling defect) (11,12). Two other clues, positive family history of colon cancer (at least one first degree relative with CRC) and “other suspicious lower gastrointestinal symptoms” were categorized as possible prompts for a diagnostic workup.
Table 1 lists data items collected on chart review and illustrates how the data was used to make assessment on missed opportunities. We defined missed opportunities as episodes of care where endoscopic evaluation was not initiated in the presence of one or more predefined clinical clues. An episode of care was defined by a patient–provider interaction such as a clinic visit or hospitalization. A rigorous criteria was used to define missed opportunities. For example, if the provider was a subspecialist such as an ophthalmologist or dermatologist, he/she was not expected to follow-up on a hemoglobin level ordered by the primary care provider. We collected information on types of providers involved and the setting where missed opportunities occurred. Because our study was focused on understanding suboptimal care processes that cause diagnostic delays rather than their associated adverse outcomes, we collected data on all missed opportunities regardless of delay in diagnosis or potential harm.
Table 1. Types of data collected on medical record review.
| Data collection instrument categories | Details of data collected | An example of missed opportunity |
|---|---|---|
| Patient characteristics | Age, race/ethnicity, gender | 68 Years, African American, male, none |
| Medical and psychological comorbidities | ||
| Type of clue | Suspected or confirmed iron deficiency anemia | Confirmed iron deficiency anemia with Hb=10.2 gm/dl |
| Defined as predefined symptom and sign that should prompt an endoscopic evaluation for colorectal cancer | Screen positive for FOBT | |
| Screen positive for flex-sig | ||
| Screen positive for barium enema | ||
| Abnormal imaging suspicious for colon cancer (CT) | ||
| Suspected rectal or abdominal mass | ||
| BRBPR (in the absence of negative colonoscopy within previous 1-year period) | ||
| Change in stool caliber | ||
|
| ||
| Date clue first appeared on medical record review | __/__/___ | 12 March 2004 |
|
| ||
| Presence of missed opportunity? | Did a missed opportunity occur? | Yes, twice |
| Defined as care episodes where endoscopic evaluation was not initiated despite the presence of one or more clues that warrant a diagnostic workup for CRC. | Dates of missed opportunity | No mention of iron deficiency anemia on two subsequent primary care visits on 26 March 2004 and 12 August 2004 |
|
| ||
| Process breakdown (adapted from Gandhi et al. (1)) | Medical history deficient | Diagnostic/laboratory resultsnot followed up |
| Physical examination deficient | ||
| Diagnostic/laboratory tests (delayed/not ordered) | ||
| Consult delayed or not requested | ||
| Diagnostic/laboratory test ordered but not performed | ||
| Diagnostic/laboratory test not performed correctly | ||
| Diagnostic/laboratory tests not interpreted correctly | ||
| Diagnostic/laboratory results not followed up | ||
| Problems with follow-up plans | ||
|
| ||
| Contributory factors (reasons gleaned from chart review about why appropriate diagnostic workup was not performed) | Factors adapted from Gandhi et al. (1) | Inadequate test-result tracking system |
|
| ||
| Type of personnel involved | Codes for personnel adapted from Gandhi et al. (1) | Generalist/primary care physician |
|
| ||
| Setting of care | Codes for settings adapted from Gandhi et al. (1) | Primary care physician's office |
|
| ||
| Date of first colonoscopy referral | __/__/___ | 31 January 2005 |
|
| ||
| Date of colonoscopy performance | __/__/___ | 15 February 2005 |
|
| ||
| Date of CRC diagnosis | __/__/___ | 18 February 2005 |
In all cases with at least one missed opportunity, one of the reviewers was pre-assigned to perform additional chart review to evaluate process breakdowns. Elements from this portion of chart review were adapted from the data collection instrument used to study diagnostic claims in the Malpractice Insurers Medical Error Prevention Study (1) and a taxonomy proposed by Schiff et al. (13) to study diagnostic breakdowns. The data collection instrument allows categorization of identified missed opportunities according to provider encounter (history, physical examination, ordering non-endoscopic tests, or consultation for further workup), diagnostic tests (ordered tests either not performed or performed/interpreted incorrectly) or follow-up (abnormal diagnostic test results or visits). Each missed opportunity could be associated with several process breakdowns. Furthermore, we collected data on contributory factors associated with a specific process breakdown. The instrument was reviewed by multiple clinicians to ensure relevance and comprehensiveness.
The study team supervised and trained the reviewers during pilot testing to ensure reliable and consistent data collection. The reviewers were instructed not to designate opportunities as missed if there was insufficient documentation in the medical record to support them or when documentation supported an informed and intentional decision not to workup a particular clue. To reduce hindsight bias (14), we did not ask reviewers to make assessments of patient outcomes in terms of harm or stage of diagnosis. To ensure quality control of data entry about 10% of cases (n = 50 from each reviewer) were checked by a third investigator and found to be 98% accurate.
Data analysis
We evaluated the agreement between the two reviewers on the presence of at least one missed opportunity. We identified two groups of patients: (1) those for whom both reviewers agreed completely that at least one missed opportunity was present (i.e., endoscopic evaluation not initiated in the presence of at least one predefined clue) and (2) those for whom both reviewers agreed that there was no missed opportunity. We categorized the diagnostic clues according to the overall level of agreement between the two reviews on the presence of that clue into clues with “substantial” agreement (κ = 0.60–0.79) and clues with “fair to moderate” agreement (κ < 0.60).
We evaluated the agreement between the two reviewers on the presence of at least one missed opportunity. We identified two groups of patients: (1) those for whom both reviewers agreed completely that at least one missed opportunity was present (i.e., endoscopic evaluation not initiated in the presence of at least one predefined clue) and (2) those for whom both reviewers agreed that there was no missed opportunity. We categorized the diagnostic clues according to the overall level of agreement between the two reviews on the presence of that clue into clues with “substantial” agreement (κ = 0.60–0.79) and clues with “fair to moderate” agreement (κ < 0.60).
We compared the distribution (as proportions) of each clue as well as distributions of the referral times (from first appearance of a clue to endoscopy referral) between the two study groups with and without missed opportunities. We also compared the two groups with regards to patient related demographic (age, gender, race) and clinical (e.g., comorbid medical or psychologic/mental disorder) features. We used the Fisher's exact test for categorical variables and the nonparametric Wilcoxon's continuity-corrected normal approximation two-sample test for continuous variables. Multivariable logistic regression models were used, in which the outcome variable in these models was whether or not a patient had at least one missed opportunity, and predictor variables included age (< 65, 65–74, >75 years), race (white, black, Hispanic, other), comorbid medical and psychologic/mental conditions, and each of the clinical clues. The models were fit using maximum likelihood estimation, and odds ratios and 95% confidence intervals were calculated. All available covariates (demographic variables, medical and psychiatric comorbidities and clues) were initially entered into the logistic regression model, and only covariates with significant as well as stable risk estimates were kept in the final model. Data were analyzed using the SAS software (SAS Institute Inc., Cary, NC, version 9.1.3).
Results
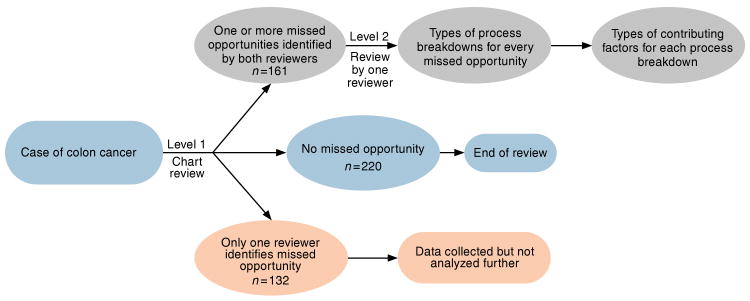
Of 551 patients of CRC identified over the study period, 513 met the inclusion and exclusion criteria for our study. Both reviewers agreed on the presence of at least one missed opportunity in 161 patients and on the absence of any missed opportunities in 220 patients (κ = 0.75; also see Figure 1). The remaining 132 patients where one of the reviewers did not agree on the presence of a missed opportunity were excluded from further analysis on missed opportunities. Among the 161 patients with missed opportunities, there was a mean of 4.2 missed opportunities and 5.3 clues per patient.
Figure 1.
Study flowchart.
We compared demographic and clinical characteristics of patients with and without at least one missed opportunity (see Table 2). Patients with missed opportunitites were significantly older than those without missed opportunities (median age of 71 vs. 67, respectively). Patients over 75 years of age had significantly more missed opportunities (42.9%) compared with < 65 years (29.2%) and 65–74 years (28% respectively; P < 0.001). African-American race, presence of congestive heart failure (CHF), and presence of coronary artery disease were also significantly associated with missed opportunities. Of those with missed opportunities, 12.4% had CHF and 26.7% had coronary artery disease as compared with only 4.1% CHF and 17.3% coronary artery disease among patients without missed opportunities. On the other hand, presence of mental disorders was not associated with missed opportunities.
Table 2. Demographic and clinical characteristics of 381 patients diagnosed with colorectal cancer categorized into two groups: with and without missed opportunities to initiate an earlier endoscopic referral.
| Patients with at least one missed opportunity (n=161) | Patients with no missed opportunities (n=220) | P valuea | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | |||||
|
| |||||
| Median | 71 | 67 | <0.001 | ||
|
| |||||
| <65 | 47 | 29.2 | 96 | 43.6 | <0.001 |
|
| |||||
| 65–74 | 45 | 28.0 | 70 | 31.8 | |
|
| |||||
| ≥75 | 69 | 42.9 | 54 | 24.6 | |
|
| |||||
| Race | |||||
|
| |||||
| White Caucasian | 74 | 52.1 | 118 | 62.4 | 0.05 |
|
| |||||
| Black | 56 | 39.4 | 50 | 26.5 | |
|
| |||||
| Hispanic and other races | 12 | 8.2 | 21 | 11.1 | |
|
| |||||
| Comorbid medical diseases | |||||
|
| |||||
| Congestive heart failure | 20 | 12.4 | 9 | 4.1 | 0.003 |
|
| |||||
| Coronary artery disease | 43 | 26.7 | 38 | 17.3 | 0.03 |
|
| |||||
| Hypertension | 101 | 62.7 | 134 | 60.9 | 0.75 |
|
| |||||
| Diabetes | 44 | 27.3 | 48 | 21.8 | 0.23 |
|
| |||||
| Chronic obstructive pulmonary disease | 21 | 13.0 | 28 | 12.7 | 1.00 |
|
| |||||
| Any of the above medical diseases | 122 | 75.8 | 159 | 72.3 | 0.48 |
|
| |||||
| Comorbid psychological/mental disorders | |||||
|
| |||||
| Depression | 18 | 11.2 | 21 | 9.6 | 0.61 |
|
| |||||
| Anxiety | 4 | 2.5 | 8 | 3.6 | 0.57 |
|
| |||||
| Dementia | 5 | 3.1 | 5 | 2.3 | 0.75 |
|
| |||||
| Post-traumatic stress disorder (PTSD) | 7 | 4.4 | 13 | 5.9 | 0.64 |
|
| |||||
| Schizophrenia | 1 | 0.6 | 1 | 0.5 | 1.00 |
|
| |||||
| Bipolar | 4 | 2.5 | 2 | 0.9 | 0.25 |
|
| |||||
| Alcohol | 21 | 13.0 | 40 | 18.2 | 0.20 |
|
| |||||
| Any of the above psychological/mental disorders | 42 | 26.1 | 74 | 33.6 | 0.12 |
P value based on the nonparametric Wilcoxon's continuity-corrected normal approximation two-sample test for age, and on Fisher's exact test for the rest of the variables.
In Table 3, we compare the presence of predefined clinical clues and the associated referral times in CRC patients with and without missed opportunities. Suspected or confirmed iron deficiency anemia was the most common clue for which there was a high agreement between the two reviewers. It also was strongly associated with missed opportunities and had the longest referral time among clues with more than 10 cases. Hemoglobin values associated with missed opportunities were higher (mean = 11 gm/dl; median = 11.3 gm/dl) compared with those in cases without missed opportunity (mean = 9.9 gm/dl; median = 10.2 gm/dl). Patients with worsening constipation were more likely to have missed opportunities. On the other hand, clues that usually warrant hospitalization and/or urgent colonoscopy or surgery (e.g., obstruction, melena, abnormal flexible sigmoidoscopy or CT scan) were associated with no or few missed opportunities. Among those with a missed opportunity, the median time to referral for the six clues with at least 20 cases ranged from a low of 144 days for those with weight loss to 393 days for those with anemia.
Table 3. A comparison of several clinical clues for colorectal cancer diagnosis and the associated median referral times in colorectal cancer patients with and without missed opportunities.
| Clues (κ)a | Patients with at least one missed opportunity (n=161) | Patients with no missed opportunities (n=220) | P valueb | |||
|---|---|---|---|---|---|---|
| Number of missed opportunities | Number of patients | Percentagec | Number of patients | Percentaged | ||
| Median days until first referral | IQRe | Median days until first referral | IQRe | |||
| Clues with high agreement between reviewers (κ > 0.60) | ||||||
|
| ||||||
| Screen positive for barium enema (κ = 0.86) | 6 | 4 | 2.5 | 8 | 3.6 | 0.57 |
| 2,514 | 1,470–3,676 | 0.5 | 0–1.5 | 0.007 | ||
|
| ||||||
| Suspected or confirmed iron deficiency anemia (κ = 0.82) | 357 | 83 | 51.6 | 58 | 26.4 | < 0.0001 |
| 393 | 108–1114 | 2.5 | 0–12 | < 0.0001 | ||
|
| ||||||
| Abnormal imaging suspicious for colon cancer (CT) (κ = 0.81) | 8 | 5 | 3.1 | 22 | 10.0 | 0.01 |
| 71 | 27–274 | 0.5 | 0–1 | 0.001 | ||
|
| ||||||
| BRBPR (in the absence of colonoscopy within previous 1-year period) (κ = 0.80) | 79 | 36 | 22.4 | 48 | 21.8 | 0.90 |
| 161 | 63–321 | 0 | 0–1 | < 0.0001 | ||
|
| ||||||
| Screen positive for flex-sig (κ = 0.78) | 2 | 1 | 0.62 | 23 | 10.5 | < 0.0001 |
| 1,246 | 1,246–1,246 | 0 | 0–0 | < 0.001 | ||
|
| ||||||
| Screen positive for fecal occult blood test (κ = 0.76) | 128 | 64 | 39.8 | 83 | 37.7 | 0.75 |
| 146 | 51–528 | 1 | 0–7 | < 0.0001 | ||
|
| ||||||
| Family history of colon cancer (κ = 0.75) | 12 | 4 | 2.5 | 7 | 3.2 | 0.77 |
| 441 | 210–686 | 0 | 0–0 | 0.003 | ||
|
| ||||||
| Intestinal obstruction (κ = 0.72) | 0 | 0 | 0 | 19 | 8.6 | < 0.0001 |
| n/a | n/a | 1 | 0–2 | —f | ||
|
| ||||||
| Suspected rectal or abdominal mass (κ = 0.69) | 8 | 6 | 3.7 | 7 | 3.2 | 0.78 |
| 14 | 2–44 | 0 | 0–1 | 0.01 | ||
|
| ||||||
| Worsening constipation (κ = 0.66) | 56 | 30 | 18.6 | 19 | 8.6 | 0.01 |
| 281 | 117–487 | 1 | 0–8 | < 0.0001 | ||
|
| ||||||
| Weight loss (κ = 0.64) | 38 | 23 | 14.3 | 29 | 13.2 | 0.76 |
| 144 | 42–395 | 1 | 0–1 | < 0.0001 | ||
|
| ||||||
| Clues with fair or moderate agreement between reviewers (κ between 0.20 and 0.60) | ||||||
|
| ||||||
| History of previous polyps (κ = 0.50) | 61 | 10 | 6.2 | 11 | 5.0 | 0.65 |
| 663 | 277–977 | 0 | 0–8 | < 0.001 | ||
|
| ||||||
| Abdominal pain (κ = 0.49) | 61 | 29 | 18.0 | 34 | 15.5 | 0.58 |
| 140 | 58–333 | 1 | 0–3 | < 0.0001 | ||
|
| ||||||
| Melena (κ = 0.48) | 1 | 1 | 0.62 | 12 | 5.5 | 0.01 |
| 482 | 482–482 | 0 | 0–1 | 0.09 | ||
|
| ||||||
| Other suspicious lower GI symptoms (κ = 0.45) | 38 | 15 | 9.3 | 14 | 6.4 | 0.33 |
| 414 | 64–658 | 1 | 0–31 | 0.002 | ||
|
| ||||||
| Change in stool caliber (κ = 0.39) | 0 | 0 | 0 | 4 | 1.8 | 0.14 |
| n/a | n/a | 0 | 0–1 | —f | ||
Kappa (κ) is calculated using all 381 patients.
Fisher's exact test was used to test for differences in proportions of patients with a given clue. The nonparametric Wilcoxon's continuity-corrected normal approximation two-sample test was used to test for differences in days until first referral.
The percentage of the 161 patients with a missed opportunity who had the given clue.
The percentage of 220 patients without a missed opportunity who had the given clue.
IQR: interquartile range.
Median times could not be compared because there were no patients with missed opportunities.
In the final logistic regression analysis, patients with following characteristics were more likely to experience missed opportunities: age > 75 years (OR = 2.3 compared with those under the age of 65 years; 95% CI of 1.3–4.1) and anemia (OR = 2.2 compared to those without anemia; 95% CI of 1.3–3.6) whereas patients with abnormal flexible sigmoidoscopy (OR = 0.06 compared to those without the procedure; 95% CI of 0.01–0.51), and abnormal CT scan suspicious for CRC (OR = 0.3 compared to those without an abnormal CT scan; 95% CI of 0.1–0.9) were less likely to have missed opportunitites.
Tables 4–6 show the types of process breakdowns and their associated characteristics in terms of settings (such as outpatient clinic, inpatient), personnel (such as primary care physicians vs. other types of providers), diagnostic clues and key contributory factors discernable from record review. Of 937 total missed opportunities we identified, 54 (5.8%) were related to trainee physicians (residents and fellows). In Table 4, we show the characteristics of the diagnostic-process breakdowns related to the provider–patient encounter including history, physical examination, requesting diagnostic tests (non-endocopic tests) and consultations (gastroenterology for an endoscopy). Primary care physicians were most commonly involved. The most common diagnostic-process breakdown was the failure or delay to consult gastroenterology in the presence of a clinical clue most commonly anemia, positive FOBT, and hematochaezia. The other two frequent factors were not recording an adequate physical examination and a delay or failure to order a non-endoscopic diagnostic test including iron studies, barium enema, and complete blood count. The most common aspect of inadequate physical exam was an unrecorded rectal exam. The most common aspect for inadequate medical history was not documenting gastrointestinal bleeding in the presence of other clues (most commonly anemia).
Table 4. Characteristics and number of missed opportunities that arose directly from provider–patient encounters.
| Level of process breakdown | Number of patients | Number of missed opportunities | Most common settings | Most common clinical clues involved | Most common personnel involved | Most common contributory factors |
|---|---|---|---|---|---|---|
| Medical history deficient | 50 | 100 | Provider Office (90) | Anemia (65) | Primary care (58) | Provider: |
| Inpatient (7) | FOBT + (18) | Physician assistant (20) | Deficient recording of critical history data (101) | |||
| Emergency Room (3) | Hematochezia (12) | Patient: | ||||
| None | ||||||
|
| ||||||
| Physical examination deficient | 64 | 125 | Provider office (107) | Anemia (73) | Primary care (67) | Provider: |
| Inpatient (15) | Worsening constipation (20) | Physician assistant (24) | Deficient recording of critical examination data (126) | |||
| Emergency room (3) | Hematochezia (9) | Patient: | ||||
| Declined (8) | ||||||
|
| ||||||
| Diagnostic/laboratory tests (delayed/not ordered) | 59 | 116 | Provider office (90) | Anemia (80) | Primary care (63) | Provider: |
| Inpatient (20) | Hematochezia (12) | Physician assistant (19) | Apparent lack of awareness that subsequent diagnostic testing was required (115) | |||
| Emergency room (4) | FOBT + (8) | Apparent lack of knowledge (35) | ||||
| Radiology (2) | Patient: | |||||
| Patient declined (3) | ||||||
|
| ||||||
| Consult delayed or not requested | 130 | 389 | Provider office (320) | Anemia (187) | Primary Care (203) | Provider: |
| Inpatient (41) | FOBT + (74) | Physician Assistant (77) | Apparent lack of awareness that subsequent referral was required (341) | |||
| Emergency Room (17) | Previous polyps (58) | Patient (23) | Patient: | |||
| Hematochezia (49) | Geriatrics (18) | Declined (68) | ||||
| Worsening constipation (40) | Emergency Medicine (11) | Cancelled appt (39) | ||||
| Missed appt (12) | ||||||
Table 6. Characteristics and number of missed opportunities that arose directly from follow-up-related factors.
| Level of process breakdown | Number of patients | Number of missed opportunities | Most common setting | Most common clues | Most common personnel involved | Most common contributory factors |
|---|---|---|---|---|---|---|
| Diagnostic/laboratory results not followed up | 60 | 97 | Provider office (91) | Anemia (62) | Primary care (38) | Provider: |
| Emergency room (3) | FOBT + (28) | Physician assistant (22) | No evidence of follow-up despite post-test visit (81) | |||
| Inpatient (3) | Failed transmission of result to clinician (16) | |||||
| Inadequate test-result tracking system (14) | ||||||
| Patient: | ||||||
| Missed/cancelled appt (4) | ||||||
|
| ||||||
| Problems with follow-up plans | 27 | 37 | Provider office (26) | Anemia (14) | Patient (11) |
Provider: Provider selected wrong interval for follow-up (13) |
| Patient (9) | FOBT + (8) | Primary Care (10) | Follow-up appt not scheduled (11) | |||
| Emergency room (1) | Abdominal pain (7) | Physician assistant (7) |
Patient: Missed/cancelled appt (18) |
|||
In Table 5, we show diagnostic-process breakdowns associated with performance and/or interpretation of non-endoscopic diagnostic tests. The most common breakdown (68%) occurred when the diagnostic tests were requested but not performed. The reasons were mostly secondary to patients not appearing for appointments and apparent lack of requesting provider awareness about the non-performance of the test. Poor bowel preparation for barium enema accounted for most cases of inadequate performance of diagnostic tests. The types of diagnostic tests not interpreted correctly included complete blood count and iron studies (e.g., not interpreting anemia as iron deficiency). Table 6 shows process breakdowns associated with follow-up. In patients where non-endoscopic diagnostic test results were not acted upon, the most common tests included positive FOBT and complete blood count.
Table 5. Characteristics and number of missed opportunities that arose directly from diagnostic test result–related factors.
| Level of process breakdown | Number of patients | Number of missed opportunities | Most common setting | Most common clues | Most common personnel involved | Most common contributory factors |
|---|---|---|---|---|---|---|
| Diagnostic/laboratory test ordered but not performed | 36 | 53 | Provider office (33) | Anemia (29) | Nurse (17) | Provider: |
| Radiology (14) | Abdominal pain (9) | Primary Care (12) | No tracking mechanism for orders (26) | |||
| Patient's home (3) | Hematochezia (8) | Radiology (11) | Provider unaware test not performed (18) | |||
| Inpatient (2) | FOBT + (7) | Patient (8) | Patient: | |||
| Cancelled appt (5) | ||||||
| Missed appt (4) | ||||||
|
| ||||||
| Diagnostic/laboratory test not performed correctly | 6 | 7 | Radiology (3) | Anemia (2) | Radiology (4) | Provider: |
| Provider office (2) | FOBT + (2) | Incomplete test due to poor bowel prep (11) | ||||
| Inpatient (1) | Rectal mass (2) | Patient: | ||||
| Hematochezia (1) | None | |||||
|
| ||||||
| Diagnostic/laboratory tests not interpreted correctly | 11 | 13 | Provider office (10) | Anemia (8) | Physician assistant (5) | Provider: |
| Inpatient (2) | FOBT + (3) | Primary care (4) | Misinterpretation of test results (10) | |||
| Radiology (1) | Barium enema + (1) | Patient: | ||||
| None | ||||||
We also evaluated 132 patients who were excluded from the analysis due to disagreement on the presence of missed opportunities and explored the reasons for a disagreement between the two reviewers. First, we compared the baseline characteristics of the 132 patients who were excluded from analysis to all the other study patients. As shown in Table A1 in the Appendix, there were no significant differences between the two groups with regards to age, race, and comorbid medical and psychiatric diseases. Second, the mean number of clues per patient in the excluded group of 132 patients was 2.1 compared with 5.3 in the 161 study group patients in whom a missed opportunity was agreed upon. Thus in the 132 excluded patient group, there was less possibility that both reviewers would find at least one clue that they both agreed on that may have led to a missed opportunity. This we believe was the main factor responsible for disagreement. Third, we analyzed the type of clues present in these 132 cases. We found that among the 132 excluded patients, the reviewers' agreement (measured by kappa) was similar to those among the 381 study patients except for three clues (iron deficiency anemia, family history of colon cancer, and weight loss) in which the kappa dropped by more than 0.2 (Table A2 in Appendix). Because iron deficiency was one of the most common clues, it disproportionately affected assessment in the group.
Discussion
Approximately one-third (n = 161) of 513 cases of colorectal cancer (CRC) might have had one or more missed opportunities to initiate an earlier diagnostic endoscopic test. All missed opportunities by definition were associated with the presence of a predefined clinical clue that would ideally warrant a diagnostic evaluation for CRC. There was a mean of 4.2 missed opportunities and 5.3 clues per patient with at least one missed opportunity. Patients who were older than 75 years, African American and those with congestive heart failure or coronary artery disease were more likely to experience missed opportunities. Suspected or confirmed iron deficiency anemia was the most common clue associated with missed opportunities, and had the longest lag time from first appearance to first endoscopy referral. Hemoglobin value in cases associated with missed opportunities was higher compared to those without, suggesting that mild iron deficiency anemia may be an under-recognized clue for CRC diagnosis. Breakdown in several steps of the diagnostic process was identified, most commonly in the provider–patient clinic encounter, but also in ordering, performance and interpretation of diagnostic tests and in follow-up of patients and abnormal diagnostic test results.
One advantage of this study is that it outlines a new approach to understand and reduce potentially preventable delays in CRC diagnosis. Previous studies, most of which are from outside the US, described the magnitude of delays and their association with both patient and physician factors (15–19), but did not present a comprehensive assessment of the types of care breakdowns associated with delays. We focused on missed opportunities in diagnostic care rather than delays in diagnosis because these opportunities precede most delays in care and can be targeted for improvement. For instance, if a clinician missed the presence of iron deficiency anemia on a patient visit and a month later the patient was diagnosed with CRC, we would still denote the first visit as a missed opportunity. We also minimized subjectivity by defining missed opportunities beforehand and the types of clinical clues that would warrant a CRC workup. The presence of an integrated electronic medical record provides valuable clinical information about signs and symptoms, diagnostic tests, consultations and follow-up, and is superior and more detailed than administrative data and paper record review.
Previous studies that addressed delays in CRC diagnosis have found 27–62% of these delays to be provider related (20–23). Multiple factors have been implicated in such delays, including misdiagnosis (20), lack of appropriate physical examination (21,24), observation of symptoms suggestive of CRC without subsequent investigation (20,25), lack of awareness of symptoms suggestive of CRC (7,24) and false-negative barium enema (21,26). Our study provides a more comprehensive view of these factors and demonstrates that patients who experience diagnostic delays do so in the presence of multiple definitive clues and likely encounter multiple missed opportunities. In few patients, colonoscopy evaluation was delayed for many years despite the presence of an abnormal test (such as flexible sigmoidoscopy and DCBE) for which colonoscopy was indicated. However, most of these tests were performed before 1996, before the electronic medical record was universally used in the institution and well before notification procedures were put in place to inform providers of abnormal test results. This may explain why providers did not notice the tests on subsequent visits. Other reasons for this unusual delay include change in primary care providers and patients' reduced number of follow-up visits to the health-care system.
Disparities in CRC diagnosis have been described, with African Americans more likely to be diagnosed with more advanced CRC regardless of whether they have health insurance (27,28). Our study further supports this phenomenon in terms of disparities in missed diagnostic opportunities. Although the elderly patients differ in their presentation from younger age groups and perhaps have screening related disparities (29), to our knowledge there is no previous published literature on this finding (22,30–33); in fact one study suggested the opposite (25).
The most common clue as well as the one most significantly associated with missed opportunities was suspected or confirmed iron deficiency anemia. We found failure to document, follow-up, or take an action on suspected or confirmed anemia in about half (52%) of the cases with missed opportunities. Given that most studies show approximately 10% prevalence of CRC among patients with iron deficiency anemia (34–37) and that the majority of patients with new CRC diagnosis are iron deficient at presentation of CRC, this is a potential area of improvement. Previous studies have described delays in CRC diagnosis in patients with anemia (26,31,34,35,38,39), but determinants and duration of such delays have not been comprehensively studied. In our study, suspected or confirmed iron deficiency anemia had the longest lag time to endoscopy referral (median of 393 days) and was the single most common clue across all types of diagnostic-process breakdowns, that is, those related to the clinical encounter, diagnostic test results and follow-up. We also found that milder cases were more likely to be missed, consistent with findings of a previous study from the United Kingdom (35).
Several missed opportunities are likely to have occurred in the clinical encounter. Both systems and cognitive factors have been recently proposed to explain diagnostic breakdowns due to inadequate documentation of history or physical exam or from not requesting certain tests such as for iron deficiency (1,40). Consistent with previous work in other types of diagnostic breakdowns in primary care (41), we found deficiencies in history or physical exam to be common in the medical encounter. However, retrospective reviews such as ours can only obtain limited information about the responsible systems and cognitive factors. Future studies are needed to understand and prevent process breakdowns related to the clinical encounter in CRC diagnosis (42,43).
Our study findings should be interpreted with caution. Although the study was conducted in a single institution, it involved a large number of patients and providers. We did not collect identifiable information on providers and hence were unable to evaluate or adjust for providers' effects. However, patients are allocated to providers non-selectively at the institution (i.e., there is no specific provider assignment pattern for new patients), so it is reasonable to assume that cancer cases are distributed equally among most primary care providers. Subjects in the study do not represent the entire cohort of patients eligible for screening or the cohort of patients who present with signs or symptoms suggestive of CRC but no eventual CRC diagnosis. Hence, we could not account for the impact of false-positive tests, which may also compete for time and resources for diagnostic evaluation. Several clues (signs, symptoms, lab test) that we used to define missed opportunities have unclear and probably low predictive value (and hence high false-positive results). Therefore, chasing these clues in every patient may not be cost-effective in most practice settings. However, the two most common clues (positive-screening FOBT and iron deficiency anemia) are hardly disagreed upon criteria for evaluating the presence of colon lesions (polyps or cancer). For policy makers, most benefit could be garnered by focusing on missed opportunities related to these two clues. We also may have missed clues or follow-up actions that were not documented in the chart. Because of recall bias, we did not interview providers to further analyze why opportunities were missed and what cognitive processes were involved; several were missed many years ago and some providers had since left the institution.
To overcome a methodological limitation of low reliability in studies of diagnostic breakdown (44), we used two independent reviewers and further analyzed cases only where both agreed with high reliability that missed opportunities were present. We could have added more reviews or provided a venue for increasing agreement but we opted to use only cases where complete agreement was achieved in the first round. Despite a low reproducibility agreement for certain clues, we were assured by the high agreement for several others and a substantial (κ = 0.75) agreement on the presence or absence of missed opportunities. This level of agreement is much higher than that achieved in previous studies using the similar methodology (41,44). Lastly, to address hindsight bias (14), we did not ask the reviewers to make judgements on outcomes such as stage at diagnosis and patient harm.
Conclusions
Missed opportunities to initiate an evaluation for CRC diagnosis are common in the ambulatory care setting and occur in the presence of several types of clues. Iron deficiency anemia is the most common clue in missed opportunities. Patients who were older than 75 years, African American, had coronary artery disease or CHF or had certain types of clues such as known or suspected iron deficiency anemia and worsening constipation, were more likely to experience missed opportunities. Future research is needed to address the systems and cognitive breakdowns (40) at the multiple diagnostic processes we identified to reduce delays in CRC diagnosis.
Study Highlights.
What Is Current Knowledge
Delayed diagnosis of colorectal cancer (CRC) is among the most common reasons for ambulatory diagnostic malpractice claims in the United States.
Few contemporary studies describe the frequency, origins, and characteristics of missed opportunities in detecting and investigating CRC-related diagnostic clues.
What Is New Here
Missed opportunities to initiate an earlier diagnostic endoscopic test are fairly common despite the presence of multiple clues to CRC diagnosis.
Patients who are older than 75 years, African Americans, and those with congestive heart failure or coronary artery disease are more likely to experience missed opportunities.
Suspected or confirmed iron deficiency anemia (especially mild) is the most common diagnostic clue associated with missed opportunities, having the longest lag time from first appearance to first endoscopy referral.
Acknowledgments
Financial support: This work was supported by an NIH K23 career development award (K23CA125585) to Dr Singh, and in part by the Houston VA HSR&D Center of Excellence (HFP90-020). These sources had no role in study design or execution, collection of data, the writing of the paper, or the decision to submit the paper for publication.
Appendix
The clinical and demographic characteristics of the colorectal cancer patients are summarized in Table A1. Table A2 shows the clues for which kappa values dropped by > 0.2.
Table A1. Demographic and clinical characteristics of 513 patients diagnosed with colorectal cancer categorized in two groups: those included in analyses and those excluded from subsequent analyses due to lack of consensus on a missed opportunity.
| Patients included in analyses (n=381) | Patients excluded from analyses (n=132) | P valuea | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | |||||
| Median | 69 | 68 | 0.36 | ||
|
| |||||
| < 65 | 143 | 37.5 | 56 | 42.4 | 0.50 |
|
| |||||
| 65–74 | 115 | 30.2 | 40 | 30.3 | |
|
| |||||
| ≥75 | 123 | 32.3 | 36 | 27.3 | |
|
| |||||
| Race | |||||
|
| |||||
| White Caucasian | 192 | 58.0 | 64 | 55.2 | 0.54 |
|
| |||||
| Black | 106 | 32.0 | 43 | 37.1 | |
|
| |||||
| Hispanic and other race | 33 | 10.0 | 9 | 7.8 | |
|
| |||||
| Comorbid medical diseases | |||||
|
| |||||
| Congestive heart failure | 29 | 7.7 | 8 | 6.1 | 0.55 |
|
| |||||
| Coronary artery disease | 81 | 21.3 | 30 | 22.7 | 0.72 |
|
| |||||
| Hypertension | 235 | 61.7 | 87 | 65.9 | 0.39 |
|
| |||||
| Diabetes | 92 | 24.2 | 40 | 30.3 | 0.16 |
|
| |||||
| Chronic obstructive pulmonary disease | 49 | 12.9 | 19 | 14.4 | 0.65 |
|
| |||||
| Any of the above medical diseases | 281 | 73.8 | 102 | 77.3 | 0.42 |
|
| |||||
| Comorbid psychological/mental disorders | |||||
|
| |||||
| Depression | 39 | 10.2 | 20 | 15.2 | 0.13 |
|
| |||||
| Anxiety | 12 | 3.2 | 8 | 3.1 | 0.14 |
|
| |||||
| Dementia | 10 | 2.6 | 4 | 3.0 | 0.81 |
|
| |||||
| Post-traumatic stress disorder (PTSD) | 20 | 5.3 | 4 | 3.0 | 0.30 |
|
| |||||
| Schizophrenia | 2 | 0.5 | 2 | 1.5 | 0.27 |
|
| |||||
| Bipolar | 6 | 1.6 | 1 | 0.8 | 0.49 |
|
| |||||
| Alcohol | 61 | 16.0 | 22 | 16.7 | 0.86 |
|
| |||||
| Any of the above psychological/mental disorders | 116 | 30.5 | 46 | 34.9 | 0.35 |
P value based on the nonparametric median two-sample test for median age, and on Pearson's χ2 test for the rest of the variables.
Table A2. Clues for which kappa values for agreement dropped by more than 0.2 in the 132 patients excluded for analysis compared with study patients.
| Clues | Kappa for presence of clue in 132 patients | Kappa for presence of clue in study patients |
|---|---|---|
| Suspected or confirmed iron deficiency anemia | 0.60 | 0.82 |
| Family history of colon cancer | 0.47 | 0.75 |
| Weight loss | 0.41 | 0.64 |
Footnotes
Specific author contributions: Acquisition of data: Singh, Daci, Collins, and Shethia; analysis and interpretation of data: Singh, El-Serag, N. Petersen, L. Petersen, Daci, Collins, and Shethia; drafting of the paper: Singh El-Serag, N. Petersen, and L. Petersen; critical revision of the paper for important intellectual content: all authors; statistical analysis: Singh, El-Serag, N. Petersen; acquisition of funding: Singh, L. Petersen; administrative, technical, and material support: Singh, Shethia, El-Serag, N. Petersen, and L. Petersen; study supervision: Singh, El-Serag, and L. Petersen.
Conflict Of Interest: Guarantor of the article: Hardeep Singh, MD, MPH.
Potential competing interests: None.
References
- 1.Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145:488–96. doi: 10.7326/0003-4819-145-7-200610030-00006. [DOI] [PubMed] [Google Scholar]
- 2.Phillips RL, Jr, Bartholomew LA, Dovey SM, et al. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13:121–6. doi: 10.1136/qshc.2003.008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selby JV, Friedman GD, Quesenberry CP, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 4.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. The Lancet. 1996;348:1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 5.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton W, Round A, Sharp D, et al. Clinical features of colorectal cancer before diagnosis: A population-based case-control study. Br J Cancer. 2005;93:399–405. doi: 10.1038/sj.bjc.6602714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langenbach MR, Schmidt J, Neumann J, et al. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003;27:304–8. doi: 10.1007/s00268-002-6678-9. [DOI] [PubMed] [Google Scholar]
- 8.Fisher DA, Jeffreys A, Coffman CJ, et al. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15:1232–5. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 9.Yabroff K, Washington KS, Leader A, et al. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60:294–331. doi: 10.1177/1077558703254698. [DOI] [PubMed] [Google Scholar]
- 10.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3:19–30. [PubMed] [Google Scholar]
- 11.Selvachandran SN, Hodder RJ, Ballal MS, et al. Prediction of colorectal cancer by a patient consultation questionnaire and scoring system: a prospective study. The Lancet. 2002;360:278–83. doi: 10.1016/s0140-6736(02)09549-1. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton W, Sharp D. Diagnosis of colorectal cancer in primary care: the evidence base for guidelines. Fam Pract. 2004;21:99–106. doi: 10.1093/fampra/cmh121. [DOI] [PubMed] [Google Scholar]
- 13.Schiff GD, Kim S, Abrams R, et al. Diagnosing diagnosis errors: lessons from a multi-institutional collaborative project. [June 1 2009]; http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=aps.section.2551. [PubMed]
- 14.Fischhoff B. Hindsight not equal to foresight: the effect of outcome knowledge on judgment under uncertainty 1975. Qual Saf Health Care. 2003;12:304–11. doi: 10.1136/qhc.12.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Dominguez E, Trapero-Marugan M, del Pozo AJ, et al. The colorectal carcinoma prognosis factors. Significance of diagnosis delay. Rev Esp Enferm Dig. 2006;98:322–9. doi: 10.4321/s1130-01082006000500002. [DOI] [PubMed] [Google Scholar]
- 16.Korsgaard M, Pedersen L, Sorensen HT, et al. Reported symptoms, diagnostic delay and stage of colorectal cancer: a population-based study in Denmark. Colorectal Dis. 2006;8:688–95. doi: 10.1111/j.1463-1318.2006.01014.x. [DOI] [PubMed] [Google Scholar]
- 17.Potter MA, Wilson RG. Diagnostic delay in colorectal cancer. J R Coll Surg Edinb. 1999;44:313–6. [PubMed] [Google Scholar]
- 18.Ristvedt SL, Birnbaum EH, Dietz DW, et al. Delayed treatment for rectal cancer. Diseases of the Colon & Rectum. 2005;48:1736–41. doi: 10.1007/s10350-005-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young CJ, Sweeney JL, Hunter A. Implications of delayed diagnosis in colorectal cancer. Aust N Z J Surg. 2000;70:635–8. doi: 10.1046/j.1440-1622.2000.01916.x. [DOI] [PubMed] [Google Scholar]
- 20.Funch DP. Diagnostic delay in symptomatic colorectal cancer. Cancer. 1985;56:2120–4. doi: 10.1002/1097-0142(19851015)56:8<2120::aid-cncr2820560840>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Graffner H, Olsson SA. Patient's and doctor's delay in carcinoma of the colon and rectum. J Surg Oncol. 1986;31:188–90. doi: 10.1002/jso.2930310311. [DOI] [PubMed] [Google Scholar]
- 22.Robinson E, Mohilever J, Zidan J, et al. Colorectal cancer: incidence, delay in diagnosis and stage of disease. Eur J Cancer Clin Oncol. 1986;22:157–61. doi: 10.1016/0277-5379(86)90025-8. [DOI] [PubMed] [Google Scholar]
- 23.Rubin M, Zer M, Dintsman M. Factors influencing delay in treatment of cancer of rectum and colon in Israel. Isr J Med Sci. 1980;16:641–5. [PubMed] [Google Scholar]
- 24.Holliday HW, Hardcastle JD. Delay in diagnosis and treatment of symptomatic colorectal cancer. Lancet. 1979;1:309–11. doi: 10.1016/s0140-6736(79)90718-9. [DOI] [PubMed] [Google Scholar]
- 25.Turunen MJ, Peltokallio P. Delay in the diagnosis of colorectal cancer. Ann Chir Gynaecol. 1982;71:277–82. [PubMed] [Google Scholar]
- 26.Harris GJ, Simson JN. Causes of late diagnosis in cases of colorectal cancer seen in a district general hospital over a 2-year period. Ann R Coll Surg Engl. 1998;80:246–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander DD, Waterbor J, Hughes T, et al. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomarkers. 2007;3:301–13. doi: 10.3233/cbm-2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–31. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 29.Koroukian SM, Xu F, Dor A, et al. Colorectal cancer screening in the elderly population: disparities by dual medicare-medicaid enrollment status. Health Serv Res. 2006;41:2136–54. doi: 10.1111/j.1475-6773.2006.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curless R, French JM, Williams GV, et al. Colorectal carcinoma: do elderly patients present differently? Age Ageing. 1994;23:102–7. doi: 10.1093/ageing/23.2.102. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Hermoso F, Perez-Palma J, Marchena-Gomez J, et al. Can early diagnosis of symptomatic colorectal cancer improve the prognosis? World J Surg. 2004;28:716–20. doi: 10.1007/s00268-004-7232-8. [DOI] [PubMed] [Google Scholar]
- 32.Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol. 1999;94:3039–45. doi: 10.1111/j.1572-0241.1999.01454.x. [DOI] [PubMed] [Google Scholar]
- 33.Mulcahy HE, O'Donoghue DP. Duration of colorectal cancer symptoms and survival: the effect of confounding clinical and pathological variables. Eur J Cancer. 1997;33:1461–7. doi: 10.1016/s0959-8049(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 34.Luman W, Ng KL. Audit of investigations in patients with iron deficiency anaemia. Singapore Med J. 2003;44:504–10. [PubMed] [Google Scholar]
- 35.Yates JM, Logan ECM, Stewart RM. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J. 2004;80:405–10. doi: 10.1136/pgmj.2003.015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coban E, Timuragaoglu A, Meric M. Iron deficiency anemia in the elderly: prevalence and endoscopic evaluation of the gastrointestinal tract in outpatients. Acta Haematol. 2003;110:25–8. doi: 10.1159/000072410. [DOI] [PubMed] [Google Scholar]
- 37.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329:1691–5. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 38.Acher PL, Al-Mishlab T, Rahman M, et al. Iron-deficiency anaemia and delay in the diagnosis of colorectal cancer. Colorectal Dis. 2003;5:145–8. doi: 10.1046/j.1463-1318.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 39.Raje D, Mukhtar H, Oshowo A, et al. What proportion of patients referred to secondary care with iron deficiency anemia have colon cancer? Dis Colon Rectum. 2007;50:1211–4. doi: 10.1007/s10350-007-0249-y. [DOI] [PubMed] [Google Scholar]
- 40.Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493–9. doi: 10.1001/archinte.165.13.1493. [DOI] [PubMed] [Google Scholar]
- 41.Singh H, Thomas EJ, Khan M, et al. Identifying diagnostic errors in primary care using an electronic screening algorithm. Arch Intern Med. 2007;167:302–8. doi: 10.1001/archinte.167.3.302. [DOI] [PubMed] [Google Scholar]
- 42.Singh H, Petersen LA, Thomas EJ. Understanding diagnostic errors in medicine: A lesson from aviation. Qual Saf Health Care. 2006;15:159–64. doi: 10.1136/qshc.2005.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh H, Sethi S, Raber M, et al. Errors in cancer diagnosis: Current understanding and future directions. J Clin Oncol. 2007;25:5009–18. doi: 10.1200/JCO.2007.13.2142. [DOI] [PubMed] [Google Scholar]
- 44.Thomas EJ, Lipsitz SR, Studdert DM, et al. The reliability of medical record review for estimating adverse event rates. Ann Intern Med. 2002;136:812–6. doi: 10.7326/0003-4819-136-11-200206040-00009. [DOI] [PubMed] [Google Scholar]