Abstract
Ultraviolet radiation (UVR) and exposure to tobacco smoke, a source of polycyclic aromatic hydrocarbons (PAH), have been linked to skin carcinogenesis. UVR-mediated activation of the aryl hydrocarbon receptor (AhR) stimulates the transcription of CYP1A1 and CYP1B1, which encode proteins that convert PAH to genotoxic metabolites. We determined whether UVR exposure sensitized human keratinocytes to PAH-induced DNA adduct formation. UVR exposure induced CYP1A1 and CYP1B1 in HaCaT cells, an effect that was mimicked by photooxidized tryptophan (aTRP) and FICZ, a component of aTRP. UVR exposure or pretreatment with aTRP or FICZ also sensitized cells to benzo[a]pyrene (B[a]P) induced DNA adduct formation. α-Naphthoflavone (αNF), an AhR antagonist, suppressed UVR-, aTRP- and FICZ-mediated induction of CYP1A1 and CYP1B1 and inhibited B[a]P induced DNA adduct formation. Treatment with 17-AAG, a Hsp90 inhibitor, caused a marked decrease in levels of AhR, inhibited UVR-, aTRP- and FICZ-mediated induction of CYP1A1 and CYP1B1 and blocked the sensitization of HaCaT cells to B[a]P induced DNA adduct formation. FICZ has been suggested to be a physiological ligand of the AhR that may have systemic effects. Hence, studies of FICZ were also carried out in MSK-Leuk1 cells, a model of oral leukoplakia. Pretreatment with αNF or 17-AAG blocked FICZ-mediated induction of CYP1A1 and CYP1B1, and suppressed the increased B[a]P-induced DNA adduct formation. Collectively, these results suggest that sunlight may activate AhR signaling and thereby sensitize cells to PAH-mediated DNA adduct formation. Antagonists of AhR signaling may have a role in the chemoprevention of photocarcinogenesis.
Introduction
Ultraviolet radiation (UVR) is a major cause of non-melanoma skin cancer including squamous cell carcinoma (1). Several studies have suggested that tobacco smoke is an independent risk factor for cutaneous squamous cell carcinoma (1–4). Smoking and sun exposure are also important risk factors for squamous cell carcinoma of the lip (5). Polycyclic aromatic hydrocarbons (PAH) represent an important class of carcinogens found in tobacco smoke (6). The effects of UVR and tobacco smoke as individual carcinogens have been extensively studied. Importantly, combined exposure to UVR and tobacco smoke may have synergistic effects that contribute to cancer (7–9).
The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, binds with high affinity to PAH including benzo[a]pyrene (B[a]P). Exposure to UVR generates tryptophan (TRP) photoproducts that also bind to and activate the AhR (10–13). One UV photoproduct that has received substantial attention is FICZ (6-formylindolo[3,2-b]carbazole) (10,12). Recently, exposure of an aqueous solution of TRP to window sunlight has been shown to produce multiple AhR-activating photoproducts in addition to FICZ (14). The diverse biochemical, biological and toxicological responses caused by exposure to PAH and the environmental toxin 2,3,7,8 tetrachlorodibenzo-p-dioxin (dioxin, TCDD) are thought to be mediated by the AhR (15). In the absence of ligand, the AhR is present in the cytosol complexed with a dimer of the chaperone heat shock protein 90 (Hsp90), p23 and XAP2 (16,17). Following ligand binding, the AhR translocates into the nucleus (18) where it dissociates from its chaperone complex and forms a heterodimer with the AhR nuclear transporter (ARNT) (19,20). The AhR–ARNT dimer then binds to the upstream regulatory region of genes containing xenobiotic responsive elements (19,20), resulting in the transcriptional activation of several genes, including those encoding enzymes CYP1A1 and CYPB1. PAH are generally biologically inert and require metabolic activation by inducible enzymes including CYP1A1 and CYP1B1 to exert their genotoxic actions (21,22). B[a]P, a potent ligand of the AhR, induces its own metabolism to noncarcinogenic B[a]P phenols (23) and a toxic metabolite, anti-7,8-dihydroxy-9,10-epoxy-7,8,9,10-tetrahydroB[a]P (BPDE; ref. 24), which covalently binds to DNA, forming bulky DNA adducts that induce mutations (25). The potential significance of the AhR in carcinogenesis is supported by recent studies in engineered mice. Constitutive activation of the AhR rendered mice more susceptible to chemical carcinogens (26). Moreover, AhR deficient mice were protected against B[a]P-induced skin tumors (27).
Exposure to UVB radiation (midrange UVR, 280–320 nm) induces CYP1A1 and CYP1B1 in human skin (28). This inductive effect can be explained by TRP photoproduct-mediated activation of AhR signaling (10–13). UVR-mediated induction of CYP1A1 and CYP1B1 in human skin can potentially stimulate the bioactivation of PAH. In smokers, this could lead, in turn, to increased risk of squamous cell carcinomas. In the present study, we show that exposure to UVR or TRP photoproducts induced CYP1A1 and CYP1B1 in keratinocytes and sensitized cells to B[a]P-mediated DNA adduct formation. These effects were suppressed by inhibiting the activation of AhR signaling. It is possible, therefore, that agents that target the AhR will reduce the risk of skin carcinogenesis in individuals exposed to UVR and PAH.
Materials and Methods
Materials
DMEM and fetal bovine serum were from Invitrogen. Antibodies to β-actin, Lowry protein assay kits, B[a]P, αNF and TRP were obtained from Sigma Chemical. Antibodies to CYP1A1, AhR, XAP2 and Hsp90 were obtained from Santa Cruz Biotechnology, and antibody to p23 was obtained from Affinity Bioreagents. Antiserum to CYP1B1 was a generous gift of Dr. Craig B. Marcus (Oregon State University, Corvallis, OR). Western Lighting Plus ECL was purchased from Perkin Elmer. Nitrocellulose membranes were from Schleicher and Schuell. RNA was prepared using a kit from Qiagen. PCR primers were synthesized by Sigma Genosys. Murine leukemia virus reverse transcriptase, Taq polymerase, and deoxynucleotide triphosphates were purchased from Applied Biosystems. 17-AAG and FICZ were obtained from Biomol International L.P. A BPDE (anti) standard was obtained from the NCI carcinogen repository at the Midwest Research Institute. KBM medium and KGM singlequots were from Lonza.
Cell culture
HaCaT cells are spontaneously immortalized human epithelial keratinocytes (29) and were routinely maintained in DMEM supplemented with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin, grown to 70% confluence and trypsinized with 0.125% trypsin–2 mM EDTA solution. MSK-Leuk1 cells were established from a premalignant dysplastic leukoplakia lesion adjacent to a squamous cell carcinoma of the tongue (30). Cells were routinely maintained in keratinocyte growth medium, grown to 60% confluency, and trypsinized with 0.125% trypsin-2 mmol/L EDTA solution. In all experiments, cells were grown in basal medium for 12 h before treatment. Treatments were carried out in growth factor-free basal medium.
Photoactivation of TRP
Aqueous TRP at 100× [1× is defined as the concentration of TRP in Standard Ham's (14.2 μg per ml or 69 μmol/L] was dissolved in 50 mL of distilled water and placed on the indoor sill of a large east facing window in a polypropylene tube, and exposed to sunlight for 7 days (14). Light-exposed TRP (aTRP) was wrapped in aluminum foil and kept at 4°C before dilution.
UVR
UV irradiation was performed in 10 cm cell culture dishes containing 6 mL of phenol red-free DMEM. For UV irradiation, a bank of six FS-40 sunlamps (Philips) in parallel connection was used. These lamps emit a continuous spectrum from 270 to 390 nm with a peak emission at 313 nm; approximately 65% of the radiation emitted by these lamps is within the UVB range (280–320 nm) (31). These bulbs also emit approximately 0.5% of their energy in the UVC region (200–280 nm) (32). Measured by an IL-1700 UVR meter (International Light, Newburyport, MA), this bank of bulbs deliver an average flux of 0.9 mW per cm2 at the level of exposure of the cells. Control cells were treated identically except that the UV radiation lamps were not turned on.
Western blot analysis
Cell lysates were prepared by treating cells with lysis buffer as described previously (33). Lysates were sonicated for 8 min on ice and centrifuged at 14,000 × g for 10 min at 4° C to sediment the particulate material. The protein concentration of the supernatant was measured by the method of Lowry (34). SDS-PAGE was performed under reducing conditions on 10% polyacrylamide gels. The resolved proteins were transferred onto nitrocellulose sheets and then incubated with antisera to CYP1A1, CYP1B1, AhR, Hsp90, XAP2, p23 and β-actin. Secondary antibody to IgG conjugated to horseradish peroxidase was used. The blots were then reacted with the ECL western blot detection system, according to the manufacturer's instructions.
Quantitative Real-Time PCR
Total RNA from cell lysates was isolated using the RNeasy Mini Kit (QIAGEN Inc.). RNA quantification and quality assessment was performed using a 2100 Bioanalyzer (Agilent Technologies). Samples with a 260/280 ratio of greater than 1.8, RNA Integrity Number (RIN) greater than 8, and no evidence of degradation on electrophoresis were used. RNA (1 μg) was reverse-transcribed using murine leukemia virus reverse transcriptase and oligo d(T)16 primer. The resulting cDNA was then used for amplification. Each PCR reaction volume was 20 μL and contained 5μL cDNA, 2x Syber Green PCR master mix, and forward and reverse primers. Primers used were CYP1A1, forward 5'-CCTGCTAGGGTTAGGAGGTC-3', reverse 5'-GCTCAGCCTAGTTCAAGCAG-3' and CYPIBI, forward 5'-ACGTACCGGCCACTATCACT, reverse 5'-CTCGAGTCTGCACATCAGGA. Experiments were performed using a 7500 real time PCR system (Applied Biosystems). β-actin served as an endogenous normalization control. Relative expression was determined by ddCT (Relative Quantification) analysis.
DNA adducts
DNA was isolated and then hydrolyzed in 0.1 mol/L HCl at 90°C for 2 h. This treatment releases B[a]P tetrols from the N2-BPDE adducts (35,36). After acid hydrolysis, the samples were cooled to room temperature and applied to a Restek Pinnacle® II, 3uM, 150 × 2.1 mm C18 HPLC column. Aliquots of the hydrolysate were eluted in a mobile phase of 33% acetonitrile containing 10 mmol/L ammonium acetate, pH of 6.0 at a flow rate of 0.2 mL/min. The eluate was analyzed using the fluorescence detector set at 344 nm excitation and 400 nm emission. A Shimadzu HPLC system consisting of an LC-20AD solvent delivery system, a SIL-10Ai autoinjector, an SPD-20AV UV-VIS detector and an RF-10AxL fluorescence detector was used for analysis. Quantitation of the adducts was achieved by comparison with standards of the B[a]P tetrol isomers. These were generated by incubating anti-BPDE in water at room temperature for 30 min (37). The major adduct designated BPDE tetrol I-1 (1) was produced in the cultured cells (35). Only trace amounts of the minor adduct, BPDE tetrol I-2 were detected.
Statistics
Comparisons between groups were made by Student's t test. A difference between groups of P< 0.05 was considered significant.
Results
UVR induces CYP1A1 and CYP1B1 and sensitizes keratinocytes to B[a]P induced DNA adduct formation
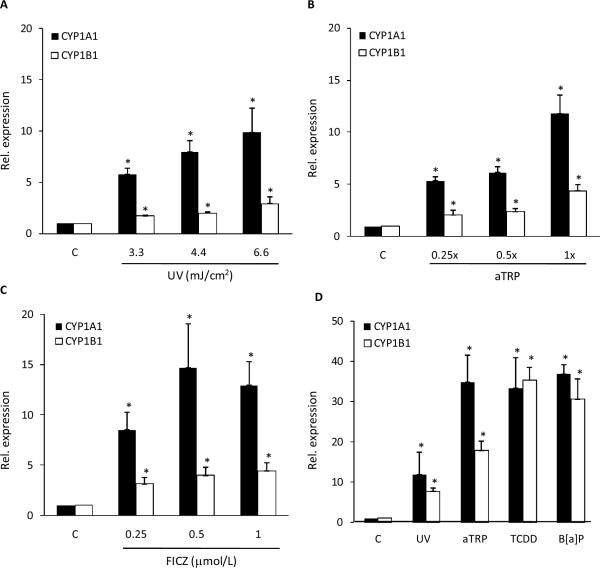
Initially, we determined the effects of UVR exposure on CYP1A1 and CYP1B1 mRNA expression in HaCaT cells. As shown in Fig. 1A, levels of CYP1A1 and CYP1B1 mRNAs were induced by UV treatment. Because sunlight or UVR cause photooxidation of TRP resulting in activation of AhR-dependent gene expression, the effects of aTRP on levels of CYP1A1 and CYP1B1 mRNA were evaluated. Consistent with the findings for UVR, treatment of HaCaT cells with aTRP led to significant induction of both CYP1A1 and CYP1B1 mRNAs (Fig. 1B). FICZ, a known ligand of the AhR, is one of the chemicals found in aTRP. Treatment with FICZ led to a marked increase in amounts of CYP1A1 and CYP1B1 mRNAs (Fig. 1C). To confirm that AhR activation causes induction of CYP1A1 and CYP1B1 in HaCaT cells, we also tested TCDD and B[a]P, prototypic ligands of the AhR. Treatment with either TCDD or B[a]P led to significant induction of CYP1A1 and CYP1B1 mRNAs (Fig. 1D).
Fig. 1.
UVR induces CYP1A1 and CYP1B1 mRNA levels in HaCaT cells. A, Cells were UV irradiated at the indicated doses and then cultured for 6 h prior to cell harvest. B and C, Cells were treated with the indicated concentrations of aTRP (B) or FICZ (C) for 6 h prior to cell harvest. D, Cells were UV irradiated (4.4 mJ/cm2) and then cultured for 6 h or treated with 1X aTRP, 1 nmol/L TCDD or 5 μmol/L B[a]P for 6 h prior to cell harvest. mRNA was isolated and then analyzed by qPCR. Values for CYP1A1 and CYP1B1 were normalized to the expression levels of β-actin. In A–D, control cells (C) were sham irradiated or treated with vehicle. A–D, means ± S.D. are shown, n=3. *, P<0.05.
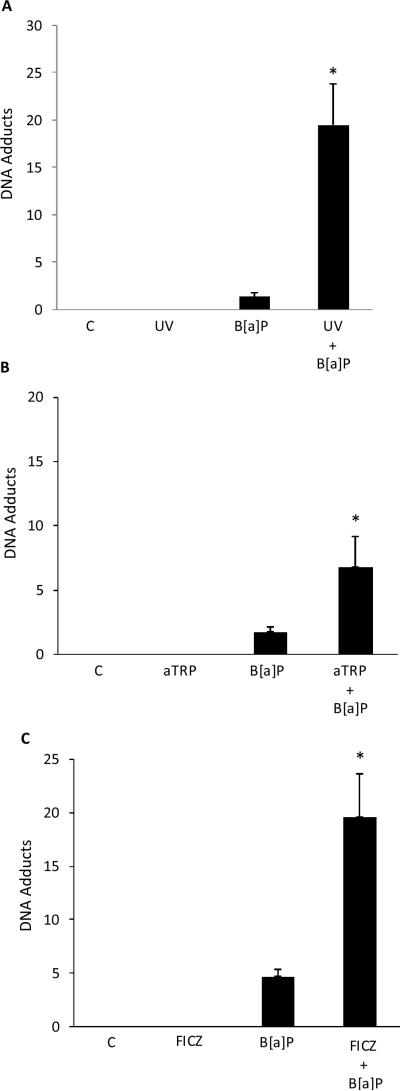
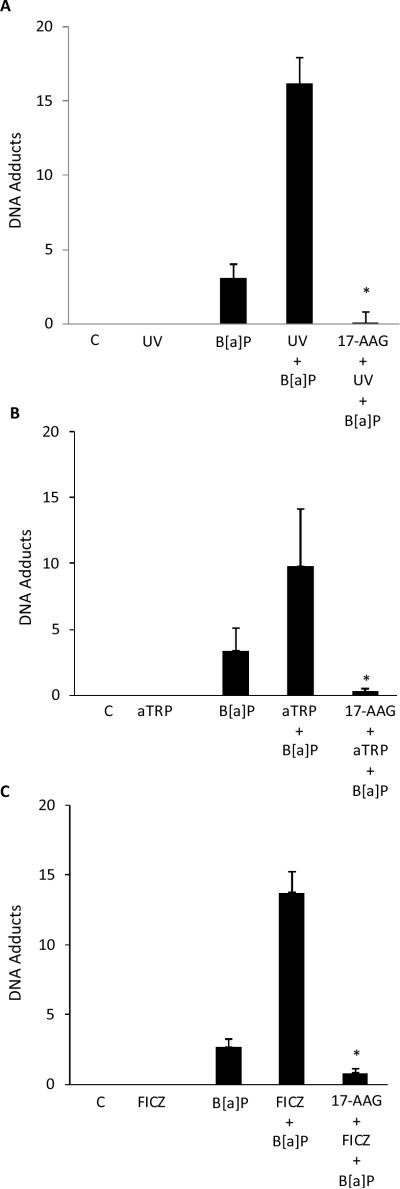
Both CYP1A1 and CYP1B1 can metabolize B[a]P into DNA-reactive species that form adducts. The formation of adducts can, in turn, lead to mutations. It was of interest, therefore, to determine if UVR exposure or treatment with aTRP or FICZ sensitized keratinocytes to B[a]P induced DNA adduct formation. As shown in Fig. 2, exposure to B[a]P alone caused DNA adducts. Notably, pretreatment of cells with UVR, aTRP or FICZ prior to exposure to B[a]P markedly increased the formation of B[a]P adducts.
Fig. 2.
UVR treatment sensitizes keratinocytes to B[a]P-mediated DNA adduct formation. HaCaT cells were UV (4 mJ/cm2) irradiated and then cultured for 12 h (A) or treated with 1X aTRP (B) or 1 μmol/L FICZ (C) for 12 h. Subsequently, 1 μmol/L B[a]P or vehicle was added and cells were incubated for another 6 h. DNA was then isolated for analysis of DNA adducts. In A–C, control cells (C) were sham irradiated or treated with vehicle. A–C, means ± S.D. are shown, n=3. *, P<0.05.
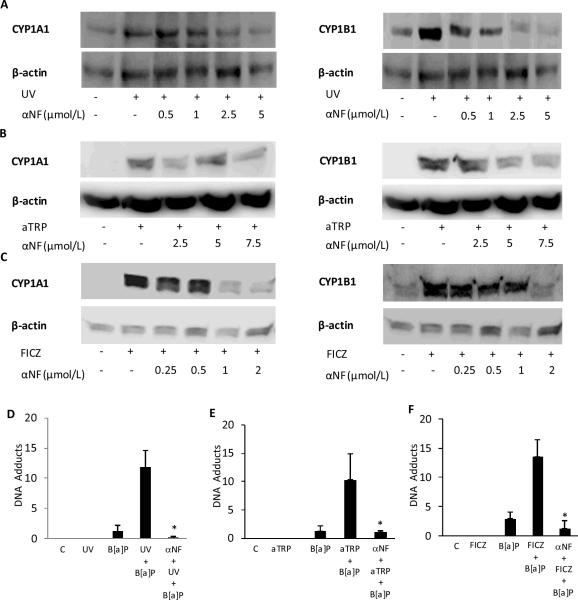
The effects of inhibiting AhR signaling on UVR-mediated induction of CYP1A1 and CYP1B1 and B[a]P induced DNA adduct formation were examined. We determined the effects of αNF, a known AhR antagonist, on UVR-, aTRP- and FICZ-mediated induction of CYP1A1 and CYP1B1 and DNA adduct formation. As shown in Figs. 3A–C, αNF suppressed the induction of CYP1A1 and CYP1B1 mediated by exposure to UVR or treatment with aTRP or FICZ. Next we investigated whether the sensitizing effects of UVR, aTRP and FICZ on B[a]P induced DNA adduct formation could be inhibited by αNF. The increase in B[a]P induced DNA adduct formation mediated by exposure to UVR or treatment with aTRP or FICZ was blocked by pretreatment of HaCaT cells with αNF (Figs 3D–F).
Fig. 3.
α-Naphthoflavone suppresses UVR-mediated induction of CYP1A1 and CYP1B1 and DNA adduct formation. HaCaT cells were pretreated with vehicle or the indicated concentration of αNF for 2 h. Subsequently, cells were UV-irradiated (4.4 mJ/cm2) and then cultured for 12 h (A) or treated with 1X aTRP (B) or 1 μmol/L FICZ (C) for 12 h prior to being harvested for Western blot analysis. Cellular lysate proteins (50 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis and subjected to western blotting with antibodies specific for CYP1A1, CYP1B1 and β-actin. In D–F, HaCaT cells were pretreated with 2 μmol/L αNF or vehicle for 2 h. Cells were then UV-irradiated (4.4 mJ/cm2) and cultured for 12 h (D) or treated with 1X aTRP (E) or 1 μmol/L FICZ (F) for 12 h. Subsequently, 1 μmol/L B[a]P or vehicle was added and cells were incubated for another 6 h. DNA was then isolated for analysis of DNA adducts. In D–F, control cells (C) were sham irradiated or treated with vehicle. D–F, means ± S.D. are shown, n=3. *, P<0.05.
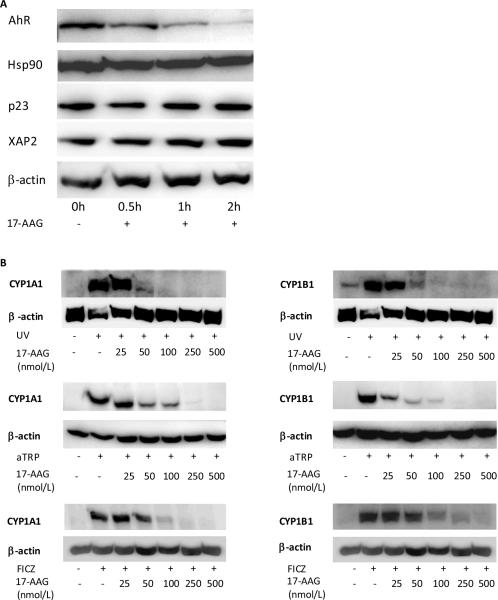
To further evaluate the importance of the AhR in sensitizing HaCaT cells to B[a]P-induced DNA adduct formation, the effects of 17-AAG, an Hsp90 inhibitor, were determined. Consistent with our previous findings (33), treatment with 17-AAG led to a rapid and pronounced decrease in amounts of AhR protein (Fig. 4A). In contrast, 17-AAG did not affect amounts of other proteins including Hsp90, p23, XAP2 that are important for AhR signaling (Fig. 4A). Subsequently, we determined whether 17-AAG blocked UV, aTRP or FICZ-mediated induction of CYP1A1 and CYP1B1. 17-AAG caused a concentration-dependent inhibition of the induction of CYP1A1 and CYP1B1 by each of these inducers (Fig. 4B). Next we investigated whether the sensitizing effects of UVR, aTRP and FICZ on B[a]P induced DNA adduct formation could be inhibited by 17-AAG. As shown in Figs. 5A–C, 17-AAG blocked the sensitizing effects of UV, aTRP and FICZ.
Fig. 4.
17-AAG, a Hsp90 inhibitor, suppresses AhR protein levels and blocks UVR-mediated induction of CYP1A1 and CYP1B1. A, HaCaT cells were treated with vehicle or 500 nmol/L 17-AAG for 0–2 h and were then harvested for Western blot analysis. Cellular lysate proteins (50 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis and subjected to western blotting with antibodies specific for AhR, Hsp90, p23, XAP2 and β-actin. B, HaCaT cells were treated with vehicle or the indicated concentrations of 17-AAG for 2 h. Subsequently, cells were sham or UV-irradiated (4.4 mJ/cm2) and then cultured for 12 h or treated with 1X aTRP or 1 μmol/L FICZ for 12 h prior to being harvested for Western blot analysis. Cellular lysate proteins (50 μg/lane) were loaded onto a 10% SDS-polyacrylamide gel, electrophoresed, and subsequently transferred onto nitrocellulose. Immunoblots were probed with antibodies specific for CYP1A1, CYP1B1 and β-actin.
Fig. 5.
17-AAG suppresses UVR-mediated sensitization of keratinocytes to DNA adduct formation. In A–C, HaCaT cells were pretreated with 500 nmol/L 17-AAG or vehicle for 2 h. Cells were then UV-irradiated (4.4 mJ/cm2) and cultured for 12 h (A) or treated with 1X aTRP (B) or 1 μmol/L FICZ (C) for 12 h. Subsequently, 1 μmol/L B[a]P or vehicle was added and cells were incubated for another 6 h. DNA was then isolated for analysis of DNA adducts. In A–C, control cells (C) were sham irradiated or treated with vehicle. A–C, means ± S.D. are shown, n=3. *, P<0.05.
Antagonizing AhR signaling suppresses DNA adduct formation in FICZ treated MSK-Leuk1 cells
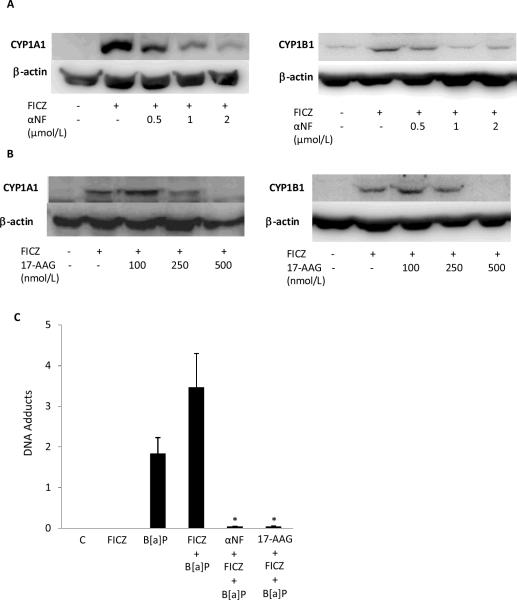
Recently, FICZ, a TRP photoproduct, was suggested to be an endogenous physiological ligand of the AhR (38). The fact that metabolites of FICZ have been detected in human urine raises the possibility that TRP photoproducts will have extracutaneous effects. To extend our findings in HaCaT cells, we investigated whether antagonizing the AhR would attenuate the effects of FICZ in MSK-Leuk1 cells, an in vitro model of oral leukoplakia. Exposure to FICZ induced both CYP1A1 and CYP1B1 in MSK-Leuk1 cells, an effect that was suppressed by pretreatment with either αNF or 17-AAG (Figs. 6A and B). As shown in Fig. 6C, exposure to B[a]P alone caused DNA adducts. Notably, pretreatment of cells with FICZ prior to exposure to B[a]P increased the formation of B[a]P adducts (P<0.05), an effect that was abrogated by exposure to either αNF or 17-AAG (Fig 6C).
Fig. 6.
α-Naphthoflavone and 17-AAG suppress FICZ-mediated induction of CYP1A1 and CYP1B1 and DNA adduct formation in MSK-Leuk1 cells. Cells were pretreated with vehicle or the indicated concentration of αNF (A) or 17-AAG (B) for 2 h. Subsequently, cells were treated with 1 μmol/L FICZ for 12 h prior to being harvested for Western blot analysis. Cellular lysate proteins (100 μg/lane) were loaded onto a 10% SDS-polyacrylamide gel, electrophoresed, and subsequently transferred onto nitrocellulose. Immunoblots were probed with antibodies specific for CYP1A1, CYP1B1 and β-actin. C, Cells were pretreated with 2 μmol/L αNF, 500 nmol/L 17-AAG or vehicle for 2 h. Cells were then treated with 1 μmol/L FICZ or vehicle for 12 h. Subsequently, 1 μmol/L B[a]P or vehicle was added and cells were incubated for another 12 h. DNA was then isolated for analysis of DNA adducts. Means ± S.D. are shown, n=3. *, P<0.05.
Discussion
The current results provide new insights into potential mechanisms by which UVR predisposes to non-melanoma skin cancer. We first showed that UVR, aTRP and FICZ induced CYP1A1 and CYP1B1 in HaCaT cells. These findings are consistent with previous reports showing that UVR or TRP photoproducts stimulate the transcription of AhR-dependent genes including CYP1A1 and CYP1B1 (10–14,39). Other investigators have postulated that UVR-mediated induction of CYP1A1 and CYP1B1 would enhance the bioactivation of environmental pollutants including PAH from tobacco smoke and thereby increase the risk for various skin disorders including skin cancer (28). In support of this notion, we show for the first time that exposure to UVR or treatment of cells with aTRP or FICZ prior to exposure to B[a]P enhanced DNA adduct formation.
The AhR represents a potential target for suppressing chemical carcinogenesis (40). It was of interest, therefore, to determine whether inhibiting AhR signaling could block UVR-mediated sensitization of cells to B[a]P induced DNA adduct formation. Interestingly, αNF, an AhR antagonist, blocked UVR-, aTRP- and FICZ-mediated induction of CYP1A1 and CYP1B1. Consistent with these findings, αNF also blocked the increase in B[a]P-induced DNA adduct formation mediated by pretreatment with UVR or exposure to aTRP or FICZ. Hsp90 is a ubiquitously expressed molecular chaperone that modulates client protein folding and prevents the nonspecific aggregation of unfolded or misfolded proteins (41,42). The AhR is among the client proteins of Hsp90 that have been linked to carcinogenesis. Inhibitors of Hsp90 suppress levels of multiple client proteins including the AhR (33,42). Consistent with findings in another recent study (33), treatment with 17-AAG, a synthetic inhibitor of Hsp90, led to a rapid reduction in amounts of the AhR. 17-AAG also suppressed UVR-, aTRP and FICZ-mediated induction of CYP1A1 and CYP1B1 and the sensitization of HaCaT cells to B[a]P induced DNA adduct formation. The TRP photoproduct FICZ has been suggested to be an endogenous physiological ligand of the AhR (38). Metabolites of FICZ have been detected in human urine, raising the possibility that exposure to UVR may have systemic effects on AhR-dependent gene expression and possibly carcinogenesis. Hence, we also carried out experiments with MSK-Leuk1 cells, a cellular model of oral leukoplakia. Exposure to tobacco smoke, a source of PAH, is the major risk factor for oral carcinogenesis. Similar to the findings in HaCaT cells, pretreatment of MSK-Leuk1 cells with αNF or 17-AAG blocked FICZ-mediated induction of CYP1A1 and CYP1B1, and suppressed the increased sensitivity to B[a]P induced DNA adduct formation.
Based on our results, it seems predictable that suppressing UVR- and FICZ-mediated induction of CYP1A1 and CYP1B1, carcinogen activating enzymes, should reduce the formation of DNA adducts and inhibit carcinogenesis. However, there is evidence that this may not be the case in vivo. Pretreatment of rats with PAH led to reduced tissue levels of orally administered B[a]P because of enhanced metabolism (43). Additionally, studies with CYP1A1 and CYP1B1 knockout mice have suggested that these proteins protect against B[a]P toxicity because in their absence, levels of DNA adducts are increased (44–46). Lastly, some models of PAH-induced carcinogenesis suggest that activation of AhR signaling may protect against the carcinogenic effects of PAH (47,48). The magnitude of tissue-specific expression of the CYP1A1 and CYP1B1 enzymes may regulate sensitivity to B[a]P toxicity and perhaps carcinogenicity (46). Given the potential limitations of our in vitro findings, additional preclinical studies involving animal models are warranted to determine whether UVR sensitizes the skin to PAH-induced DNA adduct formation, mutagenesis, and carcinogenesis. If so, it will be worthwhile to determine whether either AhR antagonists or inhibitors of Hsp90 possess chemopreventive properties. The results of the current study suggest the possibility that topical administration of agents that target the AhR may reduce the risk of carcinogenesis induced by combined exposure to UVR and tobacco smoke.
Acknowledgments
We thank Dr. Silvia Diani-Moore for preparing the aTRP solution used in this paper.
Grant Support: Center for Cancer Prevention Research (A.J.D.), NIH grants ES03606 (A.B.R.) and T32 CA09685 (B.L.J)
References
- 1.Aubry F, MacGibbon B. Risk factors of squamous cell carcinoma of the skin. A case-control study in the Montreal region. Cancer. 1985;55:907–911. doi: 10.1002/1097-0142(19850215)55:4<907::aid-cncr2820550433>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Grodstein F, Speizer FE, Hunter DJ. A prospective study of incident squamous cell carcinoma of the skin in the nurses' health study. J Natl Cancer Inst. 1995;87:1061–1066. doi: 10.1093/jnci/87.14.1061. [DOI] [PubMed] [Google Scholar]
- 3.De Hertog SA, Wensveen CA, Bastiaens MT, et al. Relation between smoking and skin cancer. J Clin Oncol. 2001;19:231–238. doi: 10.1200/JCO.2001.19.1.231. [DOI] [PubMed] [Google Scholar]
- 4.Kanjilal S, Strom SS, Clayman GL, et al. p53 mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604–3609. [PubMed] [Google Scholar]
- 5.Perea-Milla López E, Miñarro-Del Moral RM, Martínez-García C, et al. Lifestyles, environmental and phenotypic factors associated with lip cancer: a case-control study in southern Spain. Br J Cancer. 2003;88:1702–7. doi: 10.1038/sj.bjc.6600975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 7.Balansky RM, Izzotti A, D'Agostini F, et al. Systemic genotoxic effects produced by light, and synergism with cigarette smoke in the respiratory tract of hairless mice. Carcinogenesis. 2003;24:1525–32. doi: 10.1093/carcin/bgg108. [DOI] [PubMed] [Google Scholar]
- 8.Izzotti A, Cartiglia C, Longobardi M, et al. Alterations of gene expression in skin and lung of mice exposed to light and cigarette smoke. FASEB J. 2004;18:1559–1561. doi: 10.1096/fj.04-1877fje. [DOI] [PubMed] [Google Scholar]
- 9.D'Aostini F, Balansky R, Steele VE, Ganchev G, Pesce C, De Flora S. Preneoplastic and neoplastic lesions in the lung, liver and urinary tract of mice exposed to environmental cigarette smoke and UV light since birth. Int J Cancer. 2008;123:2497–2502. doi: 10.1002/ijc.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritsche E, Schäfer C, Calles C, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–6. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helferich WG, Denison MS. Ultraviolet photoproducts of tryptophan can act as dioxin agonists. Mol Pharmacol. 1991;40:674–678. [PubMed] [Google Scholar]
- 12.Wei YD, Bergander L, Rannug U, Rannug A. Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Arch Biochem Biophys. 2000;383:99–107. doi: 10.1006/abbi.2000.2037. [DOI] [PubMed] [Google Scholar]
- 13.Villard PH, Sampol E, Elkaim JL, et al. Increase of CYP1B1 transcription in human keratinocytes and HaCaT cells after UV-B exposure. Toxicol Appl Pharmacol. 2002;178:137–43. doi: 10.1006/taap.2001.9335. [DOI] [PubMed] [Google Scholar]
- 14.Diani-Moore S, Labitzke E, Brown R, Garvin A, Wong L, Rifkind AB. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo. Toxicol Sci. 2006;90:96–110. doi: 10.1093/toxsci/kfj065. [DOI] [PubMed] [Google Scholar]
- 15.Bock KW, Köhle C. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 2006;72:393–404. doi: 10.1016/j.bcp.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Denis M, Cuthill S, Wikström AC, Poellinger L, Gustafsson JA. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988;155:801–7. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- 17.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263:13802–5. [PubMed] [Google Scholar]
- 18.Pollenz RS, Barbour ER. Analysis of the complex relationship between nuclear export and aryl hydrocarbon receptor-mediated gene regulation. Mol Cell Biol. 2000;20:6095–104. doi: 10.1128/mcb.20.16.6095-6104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitelaw M, Pongratz I, Wilhelmsson A, Gustafsson JA, Poellinger L. Ligand-dependent recruitment of the Arnt coregulator determines DNA recognition by the dioxin receptor. Mol Cell Biol. 1993;13:2504–14. doi: 10.1128/mcb.13.4.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Probst MR, Reisz-Porszasz S, Agbunag RV, Ong MS, Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993;44:511–8. [PubMed] [Google Scholar]
- 21.Bartsch H, Castegnaro M, Rojas M, Camus AM, Alexandrov K, Lang M. Expression of pulmonary cytochrome P4501A1 and carcinogen DNA adduct formation in high risk subjects for tobacco-related lung cancer. Toxicology Lett. 1992;64–65:477–83. doi: 10.1016/0378-4274(92)90222-6. [DOI] [PubMed] [Google Scholar]
- 22.Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers' lung: comparison with aromatic/hydrophobic adduct formation. Carcinogenesis. 2002;23:1969–77. doi: 10.1093/carcin/23.12.1969. [DOI] [PubMed] [Google Scholar]
- 23.Conney AH, Miller EC, Miller JA. Substrate-induced synthesis and other properties of benzopyrene hydroxylase in rat liver. J Biol Chem. 1957;228:753–66. [PubMed] [Google Scholar]
- 24.Harrigan JA, Vezina CM, McGarrigle BP, et al. DNA adduct formation in precision-cut rat liver and lung slices exposed to benzo[a]pyrene. Toxicol Sci. 2004;77:307–14. doi: 10.1093/toxsci/kfh030. [DOI] [PubMed] [Google Scholar]
- 25.Volk DE, Thiviyanathan V, Rice JS, et al. Solution structure of a cis-opened (10R)-N6-deoxyadenosine adduct of (9S,10R)-9,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in a DNA duplex. Biochemistry. 2003;42:1410–20. doi: 10.1021/bi026745u. [DOI] [PubMed] [Google Scholar]
- 26.Moennikes O, Loeppen S, Buchmann A, et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 2004;64:4707–10. doi: 10.1158/0008-5472.CAN-03-0875. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu Y, Nakatsuru Y, Ichinose M, et al. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2000;97:779–82. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114:328–33. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 29.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacks PG. Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metastasis Rev. 1996;15:27–51. doi: 10.1007/BF00049486. [DOI] [PubMed] [Google Scholar]
- 31.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes: an essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 32.Nghiem DX, Walterscheid JP, Kazimi N, Ullrich SE. Ultraviolet radiation-induced immunosuppression of delayed-type hypersensitivity in mice. Methods. 2002;28:25–33. doi: 10.1016/s1046-2023(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 33.Hughes D, Guttenplan JB, Marcus CB, Subbaramaiah K, Dannenberg AJ. Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation. Cancer Prev Res. 2008;1:485–493. doi: 10.1158/1940-6207.CAPR-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randell RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 35.Alexandrov K, Rojas M, Geneste O, et al. An improved fluorometric assay for dosimetry of benzo(a)pyrene diol-epoxide-DNA adducts in smokers' lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Res. 1992;52:6248–6253. [PubMed] [Google Scholar]
- 36.Alexandrov K, Rojas M, Kadlubar FF, Lang NP, Bartsch H. Evidence of anti-benzo[a]pyrene diolepoxide-DNA adduct formation in human colon mucosa. Carcinogenesis. 1996;17:2081–2083. doi: 10.1093/carcin/17.9.2081. [DOI] [PubMed] [Google Scholar]
- 37.Shugart L, Holland JM, Rahn RO. Dosimetry of PAH skin carcinogenesis: covalent binding of benzo[a]pyrene to mouse epidermal DNA. Carcinogenesis. 1983;4:195–198. doi: 10.1093/carcin/4.2.195. [DOI] [PubMed] [Google Scholar]
- 38.Wincent E, Amini N, Luecke S, et al. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P450I substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009;284:2690–2696. doi: 10.1074/jbc.M808321200. [DOI] [PubMed] [Google Scholar]
- 39.Rannug A, Fritsche E. The aryl hydrocarbon receptor and light. Biol Chem. 2006;387:1149–1157. doi: 10.1515/BC.2006.143. [DOI] [PubMed] [Google Scholar]
- 40.Puppala D, Lee H, Kim KB, Swanson HI. Development of an aryl hydrocarbon receptor antagonist using the proteolysis-targeting chimeric molecules approach: a potential tool for chemoprevention. Mol Pharmacol. 2008;73:1064–71. doi: 10.1124/mol.107.040840. [DOI] [PubMed] [Google Scholar]
- 41.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–53. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 42.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 43.Schlede E, Kuntzman R, Haber S, Conney AH. Effect of enzyme induction on the metabolism and tissue distribution of benzo(α)pyrene. Cancer Res. 1970;30:2893–7. [PubMed] [Google Scholar]
- 44.Uno S, Dalton TP, Shertzer HG, et al. Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1 (−/−) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun. 2001;289:1049–56. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- 45.Uno S, Dalton TP, Derkenne S, et al. Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol. 2004;65:1225–37. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- 46.Uno S, Dalton TP, Dragin N, et al. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–14. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- 47.Anderson LM, Jones AB, Riggs CW, Kovatch RM. Modification of transplacental tumorigenesis by 3-methylcholanthrene in mice by genotype at the Ah locus and pretreatment with β-naphthoflavone. Cancer Res. 1989;49:1676–81. [PubMed] [Google Scholar]
- 48.DiGiovanni J, Berry DL, Gleason GL, Kishore GS, Slaga TJ. Time-dependent inhibition by 2,3,7,8-tetrachlorodibenzo-p-dioxin of skin tumorigenesis with polycyclic aromatic hydrocarbons. Cancer Res. 1980;40:1580–87. [PubMed] [Google Scholar]