Abstract
Even successful aging is associated with regional brain shrinkage and deterioration of the cerebral white matter. Aging also brings about an increase in vascular risk, and vascular impairment may be a potential mechanism behind the observed patterns of aging. The goals of this study were to characterize the normal age-differences in white matter integrity in several brain regions across the adult lifespan, and to assess the modifying effect of vascular risk on the observed pattern of regional white matter integrity. We estimated fractional anisotropy and diffusivity of white matter in nine cerebral regions of interest in 77 healthy adults (19–84 years old). There was a widespread reduction of white matter anisotropy with age, and prefrontal and occipital regions evidenced the greatest age-related differences. Diffusivity increased with age, and the magnitude of age differences increased beginning with the middle of the fifth decade. Vascular risk factors modified age differences in white matter integrity. Clinically diagnosed and treated arterial hypertension was associated with reduced white matter anisotropy and increased diffusivity beyond the effects of age. In the normotensive participants, elevation of arterial pulse pressure (a surrogate of arterial stiffness) was linked to deterioration of the white matter integrity in the frontal regions. Although the causal role of vascular risk in brain aging is unclear, the observed pattern of effects suggests that vascular risk may drive the expansion of age-related white matter damage from anterior to posterior regions.
Keywords: aging, MRI, brain, diffusion tensor imaging, white matter, hypertension, blood pressure
1. Introduction
Differential aging of the brain has been amply demonstrated in multiple postmortem (Kemper, 1994) and in vivo studies (Raz & Kennedy, 2009). Whereas the cerebral cortex and subcortical nuclei exhibit predominantly linear age-related declines in volume during the adult life span, the trajectory of white matter volume maturation and decline fits an inverted U-shaped function. The volume of the cerebral white matter increases from infancy into young adulthood, reaches a plateau in middle age, and declines toward the later part of the lifespan (Allen et al., 2005; Bartzokis et al., 2004; Fotenos et al., 2005; Jernigan et al., 2001; Raz & Kennedy, 2009; Lenroot et al., 2007; Raz et al., 2005). Although multiple factors determine the volume of brain parenchyma, it is widely held that the size of its white-matter fraction depends on the bulk of myelin. Thus, most significant age-related changes in white matter are believed to reflect changes in myelin structure and volume (Bartzokis et al., 2004; Peters, 2002).
Development of diffusion tensor imaging (DTI; Pierpaoli & Basser, 1996) enabled the investigation of age differences in white matter microstructure by quantifying its diffusion properties. The preponderance of the extant DTI studies show widespread age-related declines in fractional anisotropy (FA) and elevations in diffusivity (Ardekani et al., 2007; Benedetti et al., 2006; Charlton et al., 2006; Chen, et al., 2001; Grieve et al., 2007; Lehmbeck et al., 2006; Rovaris et al., 2003; Shenkin et al., 2003). However, white matter vulnerability to aging is not uniform, and many studies reported regional variability in the magnitude of age-related declines in FA and increases in apparent diffusion coefficient (ADC). According to some studies, the effect of age is greater in the anterior than posterior regions of the brain (Ardekani et al., 2007; Head et al., 2004; Hugenschmidt et al., 2008; Kochunov et al., 2007; Madden et al., 2007; O’Sullivan et al., 2001; Pfefferbaum, et al., 2000; 2005; Salat et al., 2005; Sullivan et al., 2001; Sullivan & Pfefferbaum, 2006). Fiber-tracking analysis of DTI data also show that the most prominent age-related deterioration of the white matter is observed in association fibers (Pagani et al., 2008; Stadlbauer et al., 2008; Sullivan et al., 2006), which connect the regions that are the latest to complete myelination in the course of development (Flechsig, 1901). However, age-related declines in the splenium of the corpus callosum have been reported as well (Abe et al., 2002; Bhagat & Beaulieu, 2004; Chepuri et al., 2002; Head et al., 2004; Ota et al., 2006; Pfefferbaum et al., 2000; 2005; Pfefferbaum & Sullivan, 2003; Salat et al., 2005; Sullivan, et al., 2006).
It remains unclear what drives the observed morphological differences in cerebral white matter, and it is possible that they are influenced by cerebrovascular risk factors. White matter is highly vulnerable to major ischemic events (Pantoni & Garcia, 1997), and vascular risk factors contribute significantly to increases in crude indices of white matter integrity such as white matter hyperintensities (WMH) (Artero et al., 2004; de Leeuw, et al., 2002; Goldstein et al., 1998; Gunning-Dixon & Raz, 2000; Gunning-Dixon & Raz, 2003; Marstrand et al., 2002; Pantoni & Garcia, 1997; Raz, 2004; Raz & Rodrigue 2006; Raz, Rodrigue, Kennedy, & Acker, 2007; Söderlund et al., 2003; Swan et al., 1998; Yoshita et al., 2006). It has been suggested that the disorganization of the normal white matter introduced by WMH might play a major role in the genesis of age-related differences in white matter diffusion properties (Vernooij et al., 2008).
Age-related increase in vascular risk most commonly stems from changes in the circulatory system that produce up-regulation of the arterial blood pressure and eventually, chronic hypertension (Hajjar & Kotchen, 2003). Hypertension, even in its milder forms, has been linked to reduction in white matter volume (Raz et al., 2003; Raz et al., 2005; Strassburger et al., 1997) and increase in WMH burden (Breteler et al., 1994; Goldstein et al., 1998; 2005; Murray et al., 2005; Raz et al., 2007). Some studies have noted that, in addition to the effects on white matter volume and WMH burden that are observed in normal aging, in hypertensive adults, there is decline in posterior white matter as well (Artero et al., 2004; Raz et al., 2007; Strassburger et al., 1997). Given the acknowledged importance of hypertension in the genesis of WMH and its role as a threat to white matter integrity, surprisingly little is known about microstructural correlates of elevated blood pressure. In contrast to the detrimental effects of cerebrovascular diseases and untreated hypertension on diffusion-based parameters of the white matter (Hannesdottir et al.2008; Hoptman et al., 2008; Nitkunan et al., 2008; Shenkin et al., 2005), the influence of milder vascular risk factors such as controlled hypertension, or high normal blood pressure is largely unknown (Huang et al., 2006).
Thus, the goals of the current study were to examine regional age-related differences in white matter microstructure in a sample of healthy adults, and to assess whether controlled vascular risk factors act as a negative modifier of those aspects of brain aging. We hypothesized that in healthy aging, the declines would demonstrate predilection for anterior brain regions. In accord with the studies of its effect on white matter volume and WMH studies (Artero et al., 2004; Raz et al., 2007), we also hypothesized that hypertension, even controlled by medication, would be associated with reduced white matter integrity in more posterior regions.
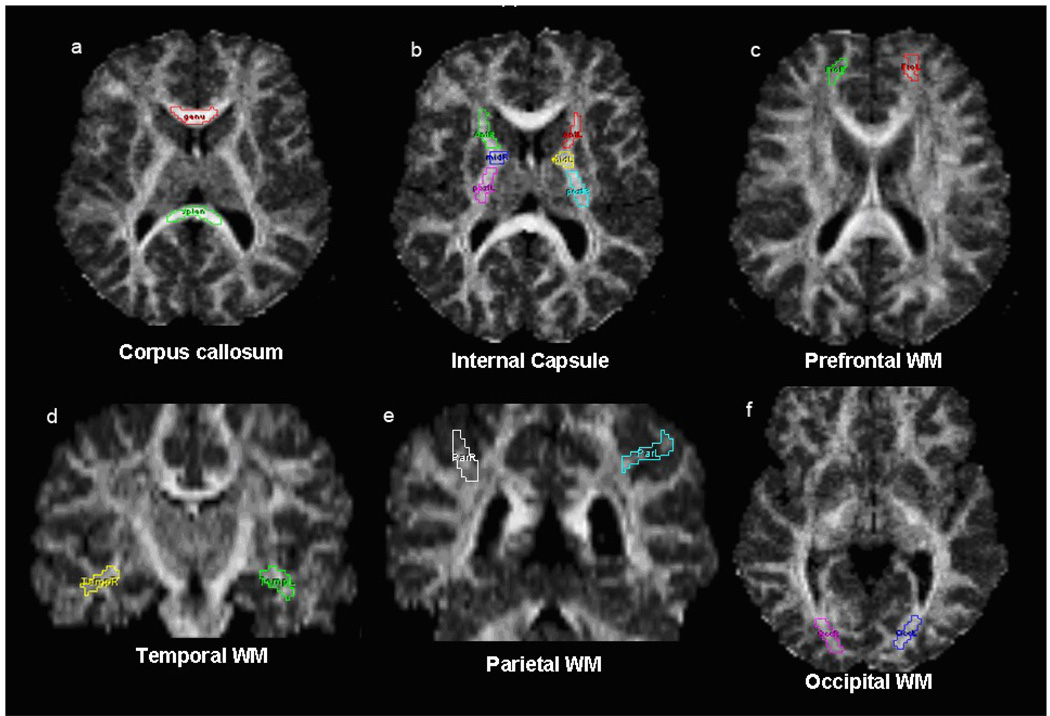
White matter regions of interest (ROIs) are illustrated in Figure 1. They included the corpus callosum (genu and splenium), the internal capsule (anterior, genu, and posterior limbs), and subcortical association white matter samples from prefrontal, parietal, temporal and occipital regions.
Figure 1.
ROIs used for DTI analyses. White matter regions of interest (ROIs manually drawn for obtaining FA and ADC values. (a) genu and splenium of corpus callosum, (b) bilateral anterior, genu, and posterior limbs of internal capsule, (c) bilateral superior prefrontal, (d) bilateral temporal stem, (e) bilateral superior posterior parietal, (f) bilateral optic radiations. Demarcation of the ROIs is displayed on the FA map.
2. Results
Descriptive statistics of the sample are presented in Table 1. The data were analyzed in a series of general linear models (GLMs). In each model, age (centered at the sample mean) served as a continuous independent variable; sex was a categorical independent variable, and regional FA and ADC were dependent variables with ROI as a within-subjects factor. To minimize rounding error and to simplify reporting, all ADC values (mm2/sec) were multiplied by 103. Full models including all the interactions were tested, but to maximize statistical power, all nonsignificant interactions with between-subject independent variables (p > .10) were removed from the models. Within-subjects interactions were adjusted by Huynh-Feldt factor to correct for violation of sphericity assumption.
Table 1.
Sample demographic information by sex, and hypertension status: mean ± SD
| Systolic | Diastolic | ||||||
|---|---|---|---|---|---|---|---|
| Group | N | Age | Edu | MMSE | CES-D | ||
| BP | BP | ||||||
| Normotensive | 47.33± | 15.39± | 28.85± | 3.79± | 118.17± | 72.75± | |
| 33 | |||||||
| Women | 18.01 | 2.30 | 1.00 | 3.09 | 10.87 | 6.96 | |
| Hypertensive | 64.50± | 14.94± | 28.13± | 4.06± | 135.27± | 79.92± | |
| 16 | |||||||
| Women | 10.37 | 2.77 | 1.09 | 3.84 | 11.38 | 10.70 | |
| 52.94± | 15.25± | 28.61± | 3.88± | 123.76± | 75.09± | ||
| Women Total | 49 | ||||||
| 17.78 | 2.45 | 1.08 | 3.31 | 13.59 | 8.93 | ||
| Normotensive | 61.21± | 16.37± | 28.53± | 2.95± | 123.67± | 74.18± | |
| 19 | |||||||
| Men | 13.31 | 2.67 | 1.31 | 3.58 | 7.70 | 7.80 | |
| Hypertensive | 65.89± | 17.22± | 28.00± | 7.89± | 139.86± | 78.14± | |
| 9 | |||||||
| Men | 12.47 | 2.59 | 1.50 | 4.59 | 12.62 | 7.80 | |
| 62.71± | 16.64± | 28.36± | 4.54± | 128.88± | 75.46± | ||
| Men Total | 28 | ||||||
| 13.01 | 2.63 | 1.37 | 4.51 | 12.08 | 7.94 | ||
| Normotensive | 52.40± | 15.75± | 28.73± | 3.48± | 120.18± | 73.27± | |
| 52 | |||||||
| Total | 17.65 | 2.46 | 1.12 | 3.27 | 10.11 | 7.24 | |
| Hypertensive | 65.00± | 15.76± | 28.08± | 5.44± | 136.92± | 79.28± | |
| 25 | |||||||
| Total | 10.94 | 2.88 | 1.22 | 4.44 | 11.79 | 9.68 | |
| 56.49± | 15.75± | 28.60± | 4.12± | 125.62± | 74.25± | ||
| Sample Total | 77 | ||||||
| 16.80 | 2.59 | 1.18 | 3.78 | 13.22 | 8.53 | ||
Note. N = sample size; edu = years of education; MMSE = mini-mental status exam; CES-D = center for epidemiological study depression scale; BP = blood pressure (mm Hg).
2.1 Age effects on white matter microstructure
Descriptive Statistics
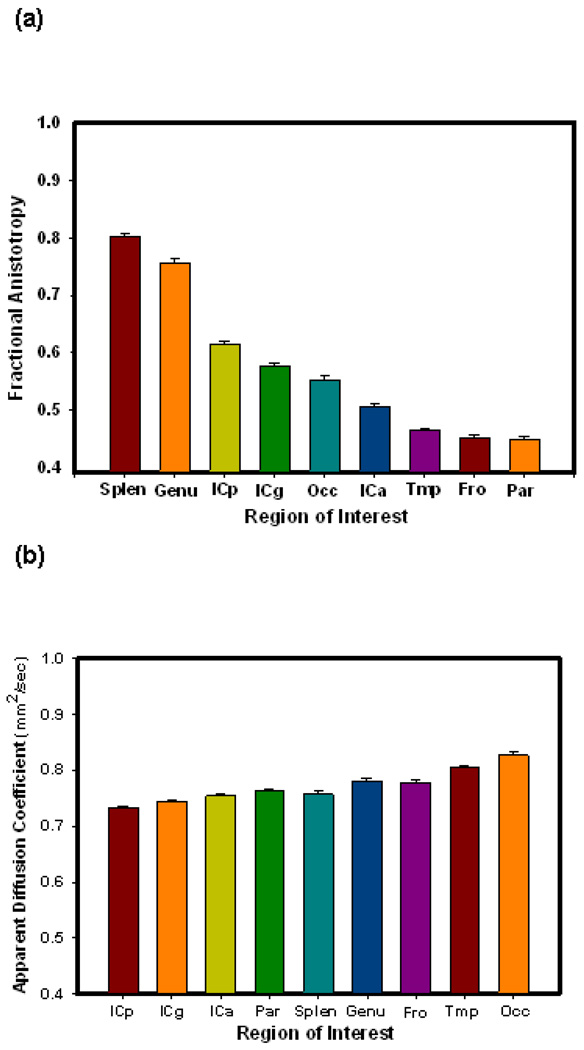
Because all ROIs except corpus callosum were measured bilaterally, left and right hemisphere measures were compared. We observed minor, though significant laterality differences only in parietal FA (.44 on the left vs. 46 on the right, t(76) = 2.66, p = .01), prefrontal FA (.77 on the left and.78 right, t(76) = 2.72, p = .008), and temporal ADC (.82 left vs .81 right, t(76) = 7.65, p < .001). In light of virtual absence of lateral differences and lack of pertinent a priori hypotheses regarding asymmetry the measures were averaged across the hemispheres. Descriptive statistics for regional FA and ADC as well as their zero-order correlations with age are displayed in Table 2. As evident from that table, FA ranged from .45 in the frontal and parietal white matter regions (least directionally constrained) to .80 in the splenium of the corpus callosum (most directionally constrained). ADC had a narrower range of values from .73 in the posterior internal capsule (lesser diffusivity) to .83 in the occipital white matter (most diffusivity). FA displayed a significant negative correlation with age in all measured regions except the anterior limb and genu of the internal capsule. FA and ADC by region are compared graphically in Figure 2.
Table 2.
Descriptive statistics for DTI coefficients by region of interest.
| ROI | FA | ADC | rFA_ADC | ||||
|---|---|---|---|---|---|---|---|
| C | |||||||
| Mean ± SD | r with age | Mean ± SD | CV | r with age | |||
| V | |||||||
| CC genu | .76 ± .05 | .07 | −.47*** | .78 ± .05 | .07 | .35** | −.73*** |
| CC splenium | .80 ± .04 | .05 | −.52*** | .76 ± .05 | .06 | .47*** | −.69*** |
| IC ant limb | .51 ± .04 | .08 | .12 | .75 ± .04 | .05 | .32** | −.18 |
| IC genu | .58 ± .06 | .10 | .15 | .74 ± .03 | .05 | .30** | −.44*** |
| IC post limb | .62 ± .03 | .05 | −.46*** | .73 ± .02 | .03 | .20 | −.48*** |
| Frontal | .45 ± .06 | .12 | −.56*** | .78 ± .04 | .05 | .36** | −.54*** |
| Parietal | .45 ± .05 | .10 | −.38** | .76 ± .04 | .05 | .35** | −.36** |
| Temporal | .47 ± .04 | .08 | −.25* | .81 ± .03 | .04 | .29* | −.49*** |
| Occipital | .55 ± .06 | .10 | −.58*** | .83 ± .05 | .06 | .25* | −.27* |
Note ROI – region of interest; FA – fractional anisotropy; ADC -- apparent diffusion coefficient×103 (mm2/sec); C.V. – coefficient of variation = standard deviation/mean.
p < .05
p < .01
p < .001.
Figure 2.
Mean diffusion properties by region. a) fractional anisotropy, b) apparent diffusion coefficient. Error bars represent standard error of the mean. Note the differences in regional variance are greater in FA than in ADC.
Fractional Anisotropy and Age
The Age × Sex interaction was nonsignificant and, therefore, removed from the model. In the reduced model, there was a significant main effect of Age, F(1, 74) = 38.15, p < .001, indicating age-related reduction in FA across the examined brain ROIs. However, the magnitude of the age effect on FA differed across the regions as shown by a significant ROI × Age interaction, F(8, 592) = 9.08, p < .001. Simple effects analyses through univariate regressions (Table 3) revealed significant reduction of FA with age in all regions except the anterior and middle limbs of the internal capsule. The strongest age effects were in the occipital and frontal white matter and the splenium, where age alone accounted for 33%, 31%, and 27% of the variance in fractional anisotropy, respectively in those regions (see Table 3). There was neither main effect of Sex (F < 1) nor ROI × Sex interaction (F = 1.49, ns). A significant main effect of ROI was noted: F(8, 592) = 728.11, p < .001 indicating that FA varied across the assessed regions.
Table 3.
Follow-up univariate analyses for the effects of age on regional FA
| ROI | Slope (units/yr) | t | p | R2 |
|---|---|---|---|---|
| CC genu | −.00153 | 4.66 | < .001 | .22 |
| CC splenium | −.00124 | 5.26 | < .001 | .27 |
| IC ant limb | .00030 | 1.04 | ns | .01 |
| IC genu | .00051 | 1.32 | ns | .02 |
| IC post limb | −.00085 | 4.46 | < .001 | .21 |
| Frontal | −.00185 | 5.86 | < .001 | .31 |
| Parietal | −.00104 | 3.53 | .001 | .14 |
| Temporal | −.00055 | 2.27 | .026 | .06 |
| Occipital | −.00194 | 6.11 | < .001 | .33 |
Note. CC = corpus callosum; IC = internal capsule. Slope is estimate of units of FA loss per year of calendar age. R2 = proportion of variance explained in regional FA by age.
Apparent Diffusion Coefficient and Age
The effects of age on diffusivity were examined in the same manner as above with ADC ROI as a repeated measure. The main effect of Age on ADC was significant: F(1, 73) = 21.65, p < .001, but it was qualified by a significant ROI × Age interaction: F(8, 584) = 2.60, p < .05. The significant effects indicated that ADC increased with age but the magnitude of age differences therein varied across the ROIs. To decompose the interaction we performed univariate tests for each region (see Table 4 for a summary). There was a significant increase in diffusivity with age in each measured location, except for a nonsignificant trend for the posterior limb of the internal capsule (p = .07). The strongest effect of age on diffusivity was observed in the splenium of the corpus callosum, where age accounted for 22% of the variance. See Table 4 for slopes and R2 for ADC for each region. There was a significant main effect of ROI, F(8, 584) = 56.67, p < .001, indicating that diffusivity varied across the examined locations. Neither main effect of Sex (F < 1, ns), nor Sex × Age interaction, F(1, 73) = 2.79, p = .10, nor other within-subjects interactions with Sex (Fs < 1, ns) reached significance.
Table 4.
Follow-up univariate analyses for the effects of age on regional ADC
| ROI | Slope (units/yr) | t | p | R2 |
|---|---|---|---|---|
| CC genu | .00112 | 3.23 | .002 | .12 |
| CC splenium | .00133 | 4.63 | < .001 | .22 |
| IC ant limb | .00068 | 2.95 | .004 | .10 |
| IC genu | .00060 | 2.75 | .008 | .09 |
| IC post limb | .00025 | 1.80 | .076 | .04 |
| Frontal | .00083 | 3.31 | .001 | .13 |
| Parietal | .00078 | 3.24 | .002 | .12 |
| Temporal | .00055 | 2.59 | .011 | .08 |
| Occipital | .00073 | 2.25 | .027 | .06 |
Note. CC = corpus callosum; IC = internal capsule. Slope is estimate of units of ADC increase (mm2/sec ×103) per year of calendar age. R2 = proportion of variance explained in regional ADC by age.
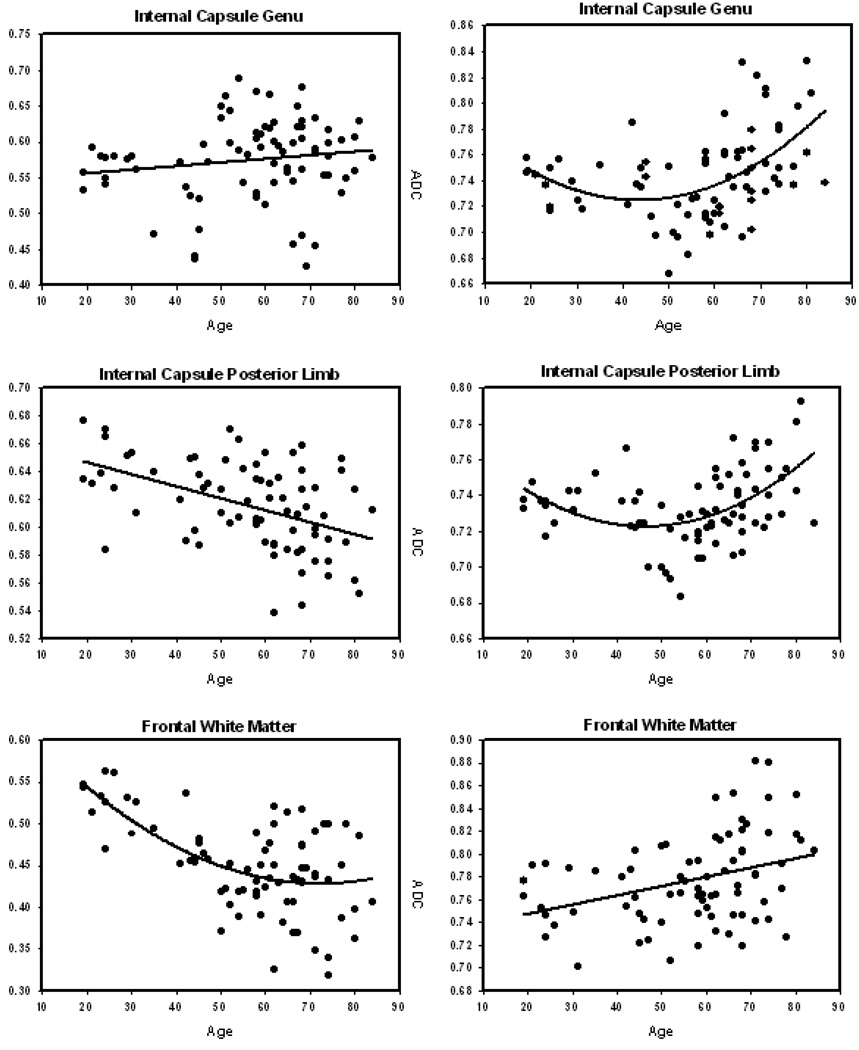
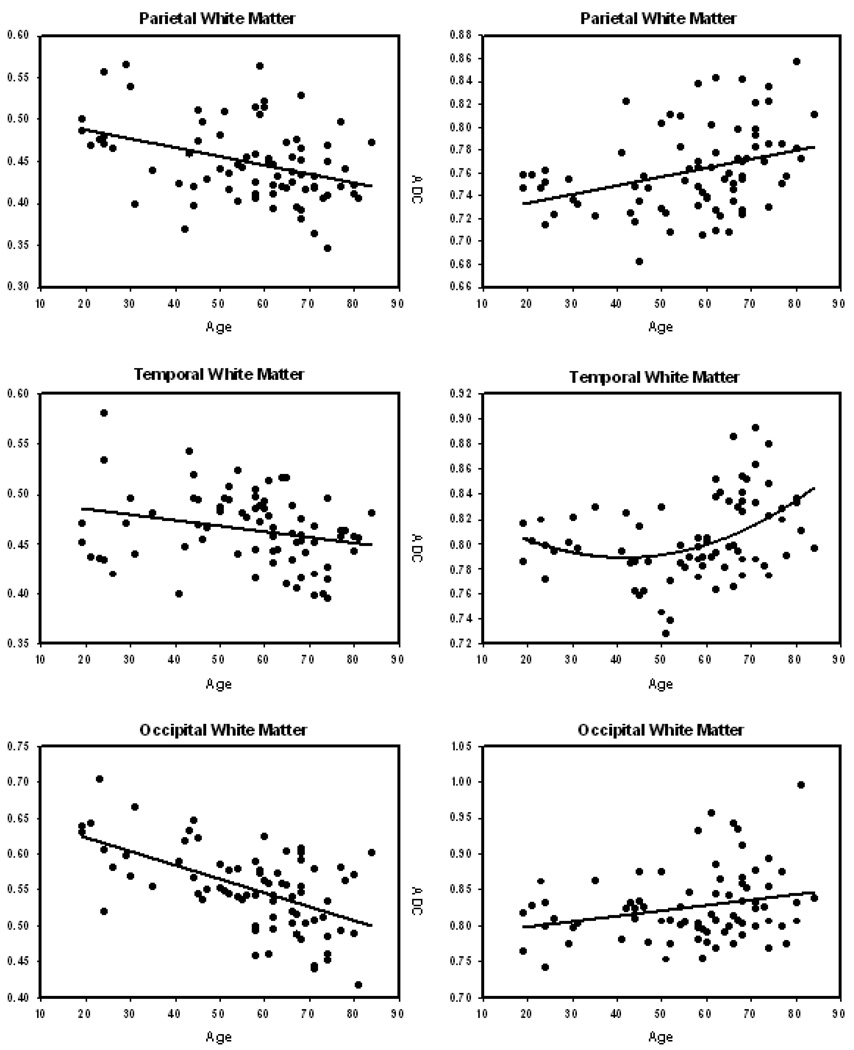
As expected, there was an inverse relation between FA and ADC in every region: Greater diffusivity was associated with reduced fractional anisotropy. Scatter plots of the relation between age and FA and age and ADC by region are displayed in Figure 3.
Figure 3.
Scatter plots and regression line of the association between age and regional fractional anisotropy (left panels) and apparent diffusion coefficient (right panels). The slopes of the regressions and the proportion variance explained for each panel is provided in Table 5.
2.2 Nonlinearity of relation between white matter integrity and age
As evident in the regression plots in Figure 3 and confirmed by analyses summarized in Table 5, some regions exhibited a nonlinear trajectory of aging. Specifically, for most regions ADC evidenced significant age-related acceleration that was the strongest in the limbs of the internal capsule and the corpus callosum genu (p < .001; see Table 5 for all regions). Inspection of the age-ADC scatter plots (Figure 3) indicates that age-related acceleration of diffusivity increase begins approximately at the end of the fifth decade.
Table 5.
Test of nonlinearity of effects of age on regional white matter ROIs across the age span for FA and ADC.
| Slope | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (units/year) | (units/year2) | R2 | F (1, 74) | |||||||
| Linear | Quadratic | Linear | Quadratic | |||||||
| ROI | FA | ADC | FA | ADC | FA | ADC | FA | ADC | FA | ADC |
| CC genu | −.0015 | .0011 | .000020 | .00006 | .22 | .12 | .23 | .25 | < 1 | 12.86*** |
| CC splen | −.0012 | .0013 | .000004 | .00004 | .27 | .22 | .27 | .28 | < 1 | 5.86* |
| ICal | .0003 | .0007 | − .000010 | .00005 | .01 | .10 | .02 | .30 | < 1 | 20.17*** |
| IC genu | .0005 | .0006 | −.000010 | .00004 | .02 | .09 | .03 | .23 | < 1 | 13.70*** |
| ICpl | −.0009 | .0002 | −.000004 | .00003 | .21 | .04 | .21 | .22 | < 1 | 17.42*** |
| Frontal | −.0019 | .0008 | .000040 | .00003 | .31 | .13 | .37 | .16 | 6.17* | 3.32 |
| Parietal | −.0010 | .0008 | .000009 | .00002 | .14 | .12 | .15 | .16 | < 1 | 3.20 |
| Temporal | − .0006 | .0006 | − .000030 | .00003 | .06 | .08 | .11 | .16 | 3.53 | 7.25** |
| Occipital | −.0019 | .0007 | .000009 | .00001 | .33 | .06 | .34 | .07 | < 1 | < 1 |
Note. CC genu = genu of corpus callosum; CC splen = splenium of corpus callosum; ICAL = anterior limb of internal capsule; IC = internal capsule; ICPL = posterior limb of internal capsule. Linear slope indicates estimate of rate of change in FA or ADC (mm2/sec) per year of calendar age; quadratic slope is estimate of speed in change (per year2). At (1, 74) df, F = 3.97 for p = 0.05*; F = 6.98 for p = 0.01**; F = 11.70 for p = .001. ADC data are multiplied by 103.
Fractional anisotropy, in contrast, evidences linear declines with age for all regions, except for the prefrontal white matter, which displayed a quadratic (decelerating) relation with age. Note, however, that a linear decline in anisotropy in that region was quite substantial, with the trend accounting for 31% of the age effect on FA. ADC in prefrontal and parietal white matter evidenced a trend toward nonlinearity that did not reach conventional levels of significance (F (1, 74) = 3.32 and 3.20, p = .07 and p = .08 respectively). Across the ROIs, the proportion of ADC variance explained by the combination of linear and quadratic age components ranged from 16% to 30%.
2.3 Effects of Hypertension on White Matter Microstructure
To examine the effect of hypertension on regional white matter, we analyzed a subsample of 64 adults above age 42 (the age of the youngest participant with hypertension). In the model, the nine FA ROIs served as a dependent repeated measures variable, and age, sex, hypertension status and their interactions as predictors. Main effects of age (F(1,56) = 11.64, p = .001) and ROI (F(8,448) = 407.83, p < .001) were observed. They were modified by a significant interaction: ROI × Age × Hypertension (F(8, 448) = 2.44, p =.029) and a trend for ROI × Age × Sex × Hypertension interaction (F(8, 448) = 2.09, p = .059). Univariate post-hoc analyses (Table 6) revealed that Hypertension × Age interaction was significant only for the occipital and temporal white matter FA: F(1,56) = 6.87, p = .01 and F(1,56) = 5.60, p = .02, with a trend observed for the genu of the internal capsule: F(1,56) = 3.38, p = .07. The ROI × Age × Sex × Hypertension trend was due to stronger differential effects of age and hypertension on FA among women (F(8,264) = 3.18, p = .008) than men (F(8,264) = 1.53, p = .17).
Table 6.
Effects of hypertension on regional fractional anisotropy: Selective effects on posterior white matter (n = 64).
| ROI FA | F(1,56) | p |
|---|---|---|
| CC genu | < 1 | ns |
| CC splen | < 1 | ns |
| ICa | < 1 | ns |
| ICg | 3.38 | .07 |
| ICp | 1.16 | ns |
| Frontal | < 1 | ns |
| Parietal | < 1 | ns |
| Temporal | 5.60 | .021* |
| Occipital | 6.87 | .011* |
Note. CC genu = genu of corpus callosum; CC splen = splenium of corpus callosum; ICa = anterior limb of internal capsule; ICg = genu of internal capsule; ICp = posterior limb of internal capsule.
p < .05.
In the second model, regional ADC was the multivariate dependent variable. Again, we found main effects of age (F(1,57) = 24.50, p < .001), and ROI (F(8,456) = 37.43, p < .001), as well as Age × ROI interaction (F(8,456) = 2.78, p = .012). Neither main effect of hypertension (F(1,57) = 2.28, p = .14), nor ROI × hypertension interaction was found (F < 1). However, a significant Age × Sex × Hypertension interaction was noted: F(1,57) = 4.29, p = .043. Decomposition of that interaction revealed that it was due to the lack of association between ADC and age among hypertensive men (n = 9). Correlations of regional ADC with age ranged between r = −.02 for occipital white matter to r = .35 for the splenium, all ns. In contrast, correlations between ADC (averaged across ROIs) and age were r = .60, p = .015 for hypertensive women, r = .53, p = .014 for normotensive women, and r = .77, p < .001 for normotensive men.
Effects of Duration of Hypertension on FA and ADC
We examined the effects of duration of hypertension on regional FA and ADC in the subsample of 25 hypertensive adults (aged 42–84). Age and duration of hypertensive condition did not correlate (r =.09, ns), and thus both were used along with sex as predictors of regional FA or ADC. We found a trend for main effect of duration (F(1, 20) = 3.92, p =.06) on FA, and a significant age × duration interaction: F(1, 20) = 7.80, p = .001, with no differences among the ROIs. This finding indicates the age-related reduction in FA was exacerbated in persons with longer duration of hypertension. The results for regional ADC were similar: a significant age × duration interaction, F(1, 20) = 4.37, p < .05, suggesting that more prolonged hypertension was linked to a greater age-related increase in diffusivity of the white matter.
2.4. Effects of Pulse Pressure on White Matter Microstructure
In a sub-sample of normotensive individuals (N = 52), we examined the effect of a vascular risk factor derived from a normal measured blood pressure on regional white matter microstructure. Pulse pressure (the difference between systolic and diastolic pressure) served as a predictor of FA or ADC in general linear models that included age, sex, and the interactions among them. In this analysis, we observed a main effect of age (F(1,46) = 23.84, p < .001), and also a significant main effect of pulse pressure on FA: F(1,46) = 4.07, p = .05. Higher pulse pressure was associated with lower FA beyond the effects of calendar age. There was no pulse pressure by region interaction, indicating that this effect was global rather than region specific. As in the full-sample analyses, there was a main effect of ROI (F(8,368) = 331.84, p < .001) and a ROI × age interaction (F(8,368) = 5.19, p < .001).
A similar model with ADC as a dependent variable revealed a main effect of age, F(1,45) = 10.85, p = .002, and a sex × age interaction, F(1,45) = 7.33, p < .01. There was also a main effect of ROI, F(8,360) = 31.25, p < .001, and a ROI × pulse pressure interaction, F(8,360) = 2.48, p = .02. These effects, however, were qualified by a ROI × age × pulse pressure interaction, F(8,360) = 2.11, p < .05, which indicated that the effect of pulse pressure on regional ADC depended on age. To decompose this interaction, follow-up correlations between pulse pressure and regional ADC were computed for each age group (young ≤ 35, middle = 36-64, old ≥ 65 years). We found a significant effect of pulse pressure on the prefrontal white matter (r = −.50, p = .05, n = 24) in the older adults, on the anterior limb of the internal capsule for middle aged adults (r = .42, p = .04, n = 16), and no region reached significance for the young, although there was a trend for the splenium (r = −.54, p = .07, n = 12). Of all ROIs, regardless of age, higher pulse pressure was selectively associated with increased ADC in the genu of the corpus callosum: t(50) = 2.30, p = .025.
2.5. The influence of regional WMH burden on diffusion-based measures
In light of recent reports of the influence exerted by leukoaraiosis on diffusion-based indices of white matter integrity, we conducted a subsidiary analysis of WMH effect on FA and ADC. The manually measured regional volumes of WMH were available for the older part of the sample (n = 33, age 52–81, mean age 67.00 ± 7.15 years). In contrast to the full-range sample, in this sub-sample of a restricted age range, we found no age differences in FA in any region. No age differences in ADC were noted in frontal, parietal, or occipital subcortical white matter, or in the anterior limb of the internal capsule.
Lobar volumes of WMH were log-transformed to reduce the skew and were centered at their sample mean. There were also no age differences in WMH volumes in any of the examined lobes: F(1,29) = 1.88, p = .18 for the frontal, F(1,29) = 2.35, p = .14 for the occipital, and F < 1 for the parietal lobes. Temporal WMH were absent in almost half of the sub-sample (n = 15) and therefore were not analyzed.
The lobar WMH volumes were introduced into the models assessing the effects of age, sex, and hypertension on regional diffusion properties. Only WMH volumes from the lobes that corresponded to the ROIs were used in the analyses. The model for anterior regions (frontal subcortical white, anterior limb and genu of IC) contained frontal WMH as a covariate. The parietal WMH volume served a covariate in the models for parietal and posterior IC FA and ADC. Occipital WMH served as a covariate in the analyses of the occipital subcortical and posterior IC measures. No similar analyses were conducted for the corpus callosum as there were no WMH there.
No effects of age on FA were observed in the models that included WMH volumes along with sex and hypertension status. The only instance of a significant influence of WMH volume on FA was in the IC genu, where frontal WMH had a significant effect in the absence of age differences: F(1,28) = 7.77, p = .009. In contrast, several significant effects of WMH on ADC and age differences therein were noted. A significant age difference in ADC was observed in the IC genu (F(1,29) = 4.83, p = .04), but was attenuated to a trend (F(1,28) = 3.04, p = .09) after accounting for the frontal WMH volume. On the other hand, age-related increase in ADC in the posterior limb of IC was significant (F(1,29) = 11.33, p = .002), and was only slightly attenuated by covarying parietal (F(1,28) = 9.82, p = .004) or occipital (F(1,28) = 8.18, p = .008) WMH volumes. Independent effects of WMH burden on ADC were not widespread, although the effect of frontal WMH volume on the ADC of IC genu was marginally significant: F(1,28) = 4.02, p = .054, and higher occipital ADC was associated with larger occipital WMH volume: F(1,28) = 9.67, p = .004.
3. Discussion
The results of this investigation demonstrate that advanced age is associated with differential regional deterioration of the white matter integrity and that vascular risk exacerbates age-related declines. Although we observed a negative effect of age on anisotropy and diffusivity in almost every region examined, the corpus callosum and prefrontal and occipital white matter showed the greatest vulnerability. Furthermore, in most regions, the increase in diffusivity (but not decline in anisotropy) accelerated with age, starting approximately in the fifth decade of life. Others have observed nonlinearity in age-related differences in white matter integrity (Engelter et al., 2000; O’Sullivan et al., 2001), and in this study such trends were replicated at least for diffusivity, though not for FA.
The age-related acceleration of diffusivity increase was observed across almost all examined brain regions. Although the mechanisms underpinning such accelerated declines are unclear, their timing corresponds to the onset of white matter shrinkage (Bartzokis et al., 2001; Bartzokis et al., 2003; Courchesne et al., 2000; Jernigan et al., 2001; Kennedy et al., 2008; Raz et al., 2005; see Raz & Rodrigue, 2006 for a review), and may reflect problems with maintaining adequate myelination (Bartzokis, 2004) or decline in organizational structure of the local connections (Paus, 2009). The observed anterior-posterior gradient of white matter aging is also in accord with the proposed first-in-last-out conceptualization of age associated structural declines (Raz, 2000). The association fibers, which myelinate latest in development (Flechsig, 1901) have the thinnest sheaths, smallest diameter, and greatest length, become the first to succumb to the effects of age (Bartzokis, 2004). Selective deterioration of regional white matter may result in impaired connectivity among cortical brain association regions and contribute to the cognitive decline seen in aging (e.g., Bucur et al., 2007; Charlton et al., 2007; Kennedy & Raz, 2009; Madden et al., 2004; Sullivan et al., 2006).
The observed nonlinearity in age-ADC relationships was reflected in significant age-related increases even in an age-restricted subsample of middle age and older adults examined in the subsidiary analyses. In that group of participants, we found the expected level of leukoaraiosis. However, regional disorganization of the white matter, putatively introduced by WMH, attenuated only some of the effects of age on ADC, mainly in the anterior regions. No such influence was detected in the occipital white matter. We observed no association between FA and WMH burden in any region, except for the genu of the internal capsule. Thus, at least in healthy adults, age differences in diffusion-based indices of white matter integrity are unlikely to represent only the effects of leukoaraiotic changes. It is possible that WMH are especially influential in determining general diffusion properties in a white matter region measured by ADC, rather than organizational aspects reflected by FA. A discrepancy between our findings and those of Vernooij and her colleagues (Vernooij et al., 2008) may stem from the difference in sample selection, which in our case yielded a healthier-than-average group of older adults. In conjunction with the observed lack of age differences in WMH volume, the subsidiary analyses indicate the validity of age differences in ADC and even more so in FA that were revealed by the analyses of the full, adult life-span sample.
As hypothesized, we found that the impact of vascular risk on white matter aging is differential. Although these observations on a relatively small sample should be considered as preliminary, they suggest that the effects of clinically diagnosed hypertension and high–normal pulse pressure in normotensive adults are expressed differentially. Whereas uncomplicated aging is associated with significant decrements to white matter integrity in anterior regions, hypertension is linked to decline in the posterior regions. In other words, vascular risk may underlie the expansion of the age-related deterioration into the posterior areas. The finding that a longer duration of hypertension is related to a greater deterioration in those regions is in accord with that hypothesis. In contrast, in normotensive adults, elevated pulse pressure (and, by inference, increased arterial stiffness) exacerbates the effects of age in the anterior regions. Although it is impossible to determine the order of events in a cross-sectional study, one can speculate that progressive increase in arterial stiffness first affects the age-sensitive prefrontal regions but with development of significant hypertension, the deterioration of posterior (temporal and occipital) regions ensues.
Some of the effects of vascular risk observed in this study are limited to women. The generality of that finding is unclear, as in this study, in accord with others, we found no sex differences in FA and ADC (Abe et al., 2002; Chepuri et al., 2002; Helenius et al., 2002; Ota, et al., 2006; Sullivan et al., 2001; Zhang et al., 2005). Also, in agreement with the extant literature, we observed no significant hemispheric asymmetry (Abe et al., 2002; Furutani et al., 2005; Helenius et al., 2002; Sullivan et al., 2009; Zhang et al., 2005) in regional white matter integrity.
An important implication of this study is that aging alone does not account for all of the observed age-related declines in the integrity of the cerebral white matter. A known vascular risk factor, hypertension, emerges as a significant negative modifier of white matter aging, and even in normotensive individuals, higher pulse pressure is associated with decreased anisotropy and increased diffusivity. To date, very few studies have examined the effects of hypertension or other vascular risk factors uncomplicated by significant vascular disease on age-related white matter microstructure in healthy adults, and none of the relevant extant studies was conducted in a carefully screened lifespan sample. In older adults who were not screened for vascular disease, hypertension was linked to increase in white matter diffusivity within frontal regions (Shenkin et al., 2005) or across the cerebral hemispheres (Nitkunin et al., 2008), whereas significant cerebrovascular disease is accompanied by even greater and more widespread increase in diffusivity and decline in FA (Shenkin et al., 2005; Nitkunin et al., 2008). Untreated hypertension was linked to lower global FA than treated hypertension in a small sample of elderly patients (Hannesdottir et al., 2008), and compromised anisotropy in the occipital white matter was noted in hypertensive individuals compared to normotensive controls (Huang et al., 2006). In samples composed of older participants, most of whom were hypertensive, age-related declines in FA were associated with white matter loss and increase in WMH in the same locations (Vernooij et al., 2008). Thus, increase in severity of vascular disease is associated with proportionately greater declines in white matter integrity. However, our findings show that even relatively low doses of vascular risk may be sufficient to exacerbate the impact of age on the cerebral white matter. Together with the findings of Huang and colleagues (2006), our results indicate that hypertension-related microstructural damage to white matter is not only apparent in areas with overt leukoaraiosis (e.g., WMH; Artero et al., 2004; Gunning-Dixon & Raz, 2000; Vernooij et al., 2008); it also occurs (and probably at an earlier stage) at a level undetectable in imaging modalities that are insensitive to diffusion properties of normal-appearing white matter (Taylor et al., 2007).
The damage inflicted by persistent hypertension may result in an expansion of an observed predominantly anterior pattern of normal aging (Davis et al., 2008; Head et al., 2004; Madden et al., 2007; Sullivan & Pfefferbaum, 2006) into more posterior cerebral regions. We report selective effects of hypertension on white matter integrity in temporal and occipital white matter, the same regions that have shown similar effect in volume loss (Strassburger et al., 1997), and WMH accumulation (Artero et al., 2004). Furthermore, we have previously found longitudinal progression of WMH isolated to parietal and occipital regions, and primary visual cortex shrinkage selective to those with vascular risk and we noted an association of increasing blood pressure with increase in deep (but not periventricular) WMH burden (Raz et al., 2007). Notably, after those lesions appear, it is vascular health, not age that affects further accumulation, suggesting a pathological rather than normal aging cause (Raz et al., 2007). The results reported in the current study lend support to the notion of hypertension and vascular risk as the precursor of white matter damage proliferation into the posterior areas of the brain that are relatively spared in healthy aging.
The mechanisms of hypertension-induced white matter damage are unclear, and it is plausible that small artery wall remodeling, which damages the endothelial cells, and leakage of plasma components into the vessel wall and surrounding brain tissue contributes to that association (Sabri et al., 1999). Reduced cerebral blood flow and reactivity in affected white matter may make these areas more prone to transient ischemia-inducing myelin rarefaction (Marstrand et al., 2002) and even high-normal blood pressure is associated with increased WMH burden (Goldstein et al., 1998). Examining the association between indices of arterial and white matter microstructural integrity may shed light on their possible mechanistic relationship.
The findings reported in this study should be considered in the context of its limitations. First, sample size and composition may limit the power and generalizability of the results. Although the current sample size is larger than the median sample size of extant DTI studies of aging, it is still relatively small for the number of comparisons made, especially with regard to the number of hypertensive subjects. Second, this study is limited by its cross-sectional design, which can only estimate true change from the differences across participants, and it provides no means for gauging individual differences in the variance of change. Thus, it is unclear how common the observed mean change is among individuals. Third, the effect of WMH burden on DTI-derived indices of white matter integrity was assessed only in a correlational sense. It is possible that results obtained from studies that examined direct correspondence between DTI and WMH co-registered images may be more sensitive to the effects of WMH on FA and ADC, however, coregistration of older adults’ brains has its own problems (Kennedy et al., 2008; Sullivan & Pfefferbaum, 2006; Tisserand et al., 2002). Finally, the DTI sequence acquired for this study is limited by the parameters that were considered optimal in the early use of the DTI technique.
Conclusions
Age-related reduction in white matter integrity is observed across multiple brain regions and may exhibit an anterior-posterior gradient of severity. However, this pattern is significantly modified by vascular risk, which may, in a dose-dependent fashion, drive the expansion of the damage from anterior to posterior white matter. These findings underscore the importance of taking into account the contribution of vascular risks in conceptualization of “normal” and “successful” aging. Because vascular risk can be ameliorated through treatment and lifestyle changes, our findings suggest that earlier and more aggressive efforts in ameliorating vascular risk may reduce cognitive manifestations of brain aging.
4. Method
4.1 Participants
Participants were paid, healthy volunteers from the Detroit metropolitan community recruited through media advertisements and flyers. All participants were screened with a health questionnaire and augmented by telephone and personal interviews. Persons who reported a history of cardiovascular (except controlled and uncomplicated essential hypertension), neurological or psychiatric conditions, head trauma with loss of consciousness for more than 5 min, thyroid problems, diabetes mellitus, and/or drug and alcohol problems were excluded from participation in the study. Persons with untreated hypertension (established by blood pressure measurements on at least three occasions) were also excluded from participation as were any participants taking anti-seizure medication, anxiolytics, or antidepressants. All participants attained a minimum of high school education (mean 15.75 ± 2.59 years), were native English speakers, and were consistent right-handers (75% on the Edinburgh Handedness Questionnaire; Oldfield, 1971).
Participants were screened for near, far, and color vision (Optec 2000 vision tester; Stereo Optical Co., Inc., Chicago, IL) and speech-range hearing (model MA27; Maico Diagnostics, Eden Prairie, MN) acuity. Participants were also screened for dementia and depression using Mini-Mental Status Examination (MMSE; Folstein et al., 1975) with a cut-off of 26, and a Geriatric Depression Questionnaire (CES-D; Radloff, 1977) with a cut-off of 16. MMSE scores ranged from 26–30, with a mean of 28.52 ± 1.19. None of the participants showed signs of depression on the CES-D, with scores ranging from 0–16 and a mean score of 4.12 ± 3.78. All participants provided written informed consent and were debriefed in accord with university human investigations committee guidelines.
The 77 participants ranged in age from 19-84 years (mean 56.49 ± 16.80) and included 49 (64%) women and 28 (36%) men -- a nonsignificant difference in proportions: χ2 (1) = 5.46, p = .07). Participant demographic information by sex and for the total sample is reported in Table 1. The men were older than the women: 62.71 ± 13.01 years vs. 52.94 ± 17.78, t(75) = 2.54, p = .013, and attained on average a year and a half more education than the women (t(75) = 2.35, p = .02). There were no sex differences, however, in MMSE scores (t < 1, ns), CES-D scores (t < 1, ns), systolic (t = 1.65, ns) or diastolic (t < .01, ns) blood pressure. Twenty five participants (16 women, 9 men, 42 to 84 years of age) reported a diagnosis of hypertension and were taking antihypertensive medication. These medications included beta-blockers (n = 6), angiotensin converting enzyme inhibitors (n = 8), potassium-sparing diuretics (n = 9), and calcium channel blockers (n = 3). Several participants were taking two or three of these medications. The hypertensive participants were significantly older (65 vs. 52 years) than their normotensive peers, t = −2.11, p = .04, but did not differ significantly on education, t(75) = −1.08, ns, CES-D, t < 1, or MMSE score, t < 1, from the rest of the sample.
4.2 Blood Pressure Measures
Each participant had his or her blood pressure measured using an analog mercury sphygmomanometer (Model 12–525; Country Technology, Gays Mills, WI) with a standard brachial cuff (Omron Professional) to obtain systolic and diastolic blood pressure. Participants were seated in a comfortable chair in a climate-controlled office. Blood pressure was sampled twice, once from each arm and averaged for each session. Current and prior hypertensive status and medication information was collected from a comprehensive health questionnaire completed before entrance to the study. Only normotensive participants or those who had diagnosed but controlled hypertension were recruited for the study. Hypertension was operationally defined as systolic blood pressure greater than 140 mm Hg and diastolic pressure greater than 90 mm Hg (Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 1997). For normotensive individuals who were not taking antihypertensive medications, we computed a summary index of blood pressure control: pulse pressure. Pulse pressure, computed as the difference between systolic and diastolic pressure, is a good surrogate measure of arterial stiffness (Franklin et al., 1997; Laurent & Boutouyrie, 2007), and a sensitive correlate of age-related cognitive declines (e.g. Waldstein et al., 2008; Dahle et al., 2009).
4.3 MRI protocol
MR images were acquired on a 1.5 T Magnetom Sonata scanner (Siemens Medical Systems, Erlangen, Germany). Diffusion tensor imaging (DTI) data were acquired with a single shot echo-planar imaging (EPI) sequence acquired with whole-head coverage in the axial plane with 6 directions, b = 0 and 1000 mm2/sec, 10 averages, TE = 97 ms, TR = 5400 ms, acquisition matrix = 192 × 192, FOV = 345 mm, voxel size = 1.8 × 1.8 × 3 mm3. Duration of acquisition was 6.25 min.
A fluid attenuated inversion recovery (FLAIR) sequence was used for the measurement of white matter hyperintensities. These were acquired in the coronal plane, with 256 × 256 matrix, voxel size = .8 × .8 × 30 mm, number of slices = 56, slice thickness = 3 mm, flip angle = 180°, TR = 8000, TE = 111, TI = 22.60. Duration of scan was 7.30 min.
4.4 DTI Processing
The DTI data were processed with the DTI module of Analyze software (BIR, Mayo Clinic, Rochester, MN, USA). Each DTI scan was first binned into the baseline (b = 0) and six gradient encoded volumes (each containing 33 slices) using the Dicom Tool module and these seven separated scans were imported into the DTI module. After the diffusion gradient orientation information was entered for each volume, the data were thresholded to reduce extracerebral noise and the tensor was computed. FA (fractional anisotropy) and ADC (apparent diffusion coefficient) maps were computed for each participant.
4.5 Region of Interest (ROI) Measurement
Images for manual tracing of Regions of Interest (ROIs) were displayed on a 21” monitor and on a 21” LCD digitizing tablet (Wacom Cintiq model 21UX; Wacom Inc., Vancouver, WA) and magnified × 2. Each ROI was traced manually with a stylus on the T2-weighted (b = 0) baseline image for each participant in native space and supplemented with simultaneous side-by-side views from the FA and FA color map images in the same native coordinate space to maximize neuroanatomic validity. The saved ROI was applied to the FA and ADC maps and mean and standard deviation FA and ADC were obtained within each ROI, for each participant separately on 3 slices bilaterally (except corpus callosum ROIs), and then averaged across the three slices.
The ROIs were chosen based on the relevant literature with particular attention to long association tracts and the rules for placement were determined by neuroanatomical knowledge of the expert tracer with the aid of neuroanatomical atlases (primarily Duvernoy, 1999 and Mori et al., 2005). The ROIs were specifically drawn well-within the inner portions of the white matter regions to minimize the potential of partial voluming effects that can occur in the border voxels at the interface of gray/white and CSF/white boundaries. Regions measured for this study were the corpus callosum (genu and splenium), the internal capsule (anterior, genu, and posterior limbs), and subcortical association white matter samples from prefrontal, parietal, temporal and occipital regions, and were demarcated as described below. Examples of ROIs traced for DTI analyses are presented in Figure 1.
Corpus callosum (CC)
The FA and ADC of the genu and the splenium were measured with a small ROI drawn on the genu and the splenium, medially-to-laterally on the axial plane, with reference to the sagittal and coronal planes using ortho review function of Analyze ROI module. Care was taken to exclude the major CSF regions (the ventricles), both visually and by monitoring the standard deviations. The genu and the splenium of the corpus callosum were measured on the same three slices in which both were optimally visible, which resulted in an area of the splenium that covered the entire tissue medially to laterally, and was more posterior and more inferior in location.
Internal Capsule (IC)
The FA and ADC of the anterior (ICa), genu (ICg), and posterior (ICp) limbs of the internal capsule were measured separately on the axial plane (bilaterally). Care was taken to remain within the white matter boundary and not include any pixels from the basal ganglia, thalamus or ventricles. The internal capsule was measured on the three slices in which all three limbs were optimally visible.
Prefrontal white matter
The FA and ADC of the prefrontal white matter were measured on the axial plane on three slices. The ROI was drawn on the white matter of the superior frontal gyrus with care taken to exclude any surrounding gray matter. The coronal and sagittal planes were used as references to guide placement. The selected slices were the three slices just ventral to the slice where the body of the corpus callosum became continuous. The white matter sampled was from prefrontal cortex, considerably anterior to the genu of the CC (to ensure prefrontal rather than motor or premotor regions were sampled), more medial than lateral (to capture pericallosal connectivity), and more superior than inferior to capture dorsal rather than orbital/ventral prefrontal cortex. This area reflects prefrontal association areas connecting with parietal association areas ostensibly via the superior fronto-occipital (SFO) and superior longitudinal (SLF) fasciculi.
Parietal white matter
The FA and ADC of the parietal lobe white matter was measured on the coronal plane from three consecutive slices beginning five slices posterior to the splenium. The superior posterior parietal area was located as superior to the posterior/splenial area of the corpus callosum and cingulate gyrus. The ROI was drawn in the widest portion of the posterior parietal white matter excluding somatosensory cortices, angular gyrus, and precuneus white matter to specifically gauge parietal association areas ostensibly connected with prefrontal association areas via the superior fronto-occipital (SFO) and superior longitudinal (SLF) fasciculi.
Temporal white matter
The FA and ADC of the temporal lobe white matter was measured on the coronal plane from three consecutive slices beginning after the anterior commissure and ending before the splenium of the corpus callosum, on the slices where the corticospinal tract descends into the brainstem and appears continuous. The temporal stem white matter was measured ventrally to the superior temporal gyrus and the ROI was drawn down to the widest portion of the white matter before branching into the parahippocampal, fusiform, and inferior temporal branches to maximize temporal association areas (i.e., middle and inferior temporal), ostensibly within the uncinate fasciculus (UNC) which connects to (orbital) prefrontal cortex anteriorly and to the occipital lobes posteriorly via the inferior fronto-occipital (IFO) and inferior longitudinal (ILF) fasciculi.
Occipital white matter
The FA and ADC of the occipital lobe white matter was measured from the axial plane on three consecutive slices approximately between the first slice where the splenium is maximally formed to one slice inferior to the end of the putamen. The ROI was drawn on the white matter adjacent to the occipital horns of the lateral ventricles and below the splenium (the posterior forceps). A narrow rectangle was drawn to avoid inclusion of CSF or adjacent gray matter. This white matter ROI ostensibly includes fibers from the superior longitudinal (SLF) and inferior fronto-occipital (IFO) fasciculi and connects occipital with frontal and parietal and with temporal regions, respectively.
White matter hyperintensities
Hyperintense regions, defined as circumscribed areas of increased signal intensity within the white matter, were identified and manually measured on coronal slices of the FLAIR images using the ROI module of Analyze software. Because of the difficulty in distinguishing WMH from emerging sulci and blood vessels in the superior convexity, the WMH were measured below the vertex. All identifiable WMH – periventricular and deep white matter – were included. The total WMH volume was a sum of the volumes of hyperintensities from all of the ROIs multiplied by the sum of the inter-slice distance and slice thickness. The WMH measurements were divided into frontal, temporal, parietal, and occipital regions of interests (ROIs), as described in our previous publications (Raz, Rodrigue, & Acker, 2003; Raz et al., 2007). White matter hyperintensities were measured on the frontal, parietal, temporal, and occipital lobes separating the deep subcortical white matter and periventricular spaces.
4.6 Reliability of ROI Measurements
In order to ensure reliability of measurement, test-retest reliability was determined by one operator (KMK) who traced each ROI on eight sets of DTI images twice, two weeks apart. Reliability of the ROI measures (mean FA) in this study was assessed by a conservative intraclass correlation formula, ICC(3) (Shrout & Fleiss, 1979). All region reliabilities (ICC 3) equaled or exceeded .90. Reliability of WMH measures exceeded ICC(3) = .90.
Acknowledgments
This study was supported in part by grants R37 AG-011230 and T32 HS-013819 and a Dissertation Award from the American Psychological Association, and was conducted in partial fulfillment of requirements for the doctoral degree. Portions of this paper were presented at Society for Neuroscience Annual Meeting in November 2007 and Cognitive Aging Conference in April 2008. We thank Yiqin Yang for her assistance in measuring white matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol. Aging. 2002;23:433–441. doi: 10.1016/s0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Rodrigues E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. NeuroReport. 1996;7:1761–1764. doi: 10.1097/00001756-199607290-00013. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Mag Res Imag. 2007;25:154–167. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Artero S, Tiemeier H, Prins ND, Sabatier R, Breteler MM, Ritchie K. Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J Neurol, Neurosurg, Psychiat. 2004;75:1304–1308. doi: 10.1136/jnnp.2003.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical "disconnection" in aging and Alzheimer's disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66:535–539. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. J Magn Res Imag. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Braak H, del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol. Aging. 2004;25:19–23. doi: 10.1016/j.neurobiolaging.2003.04.001. [Commentary]. [DOI] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol. Aging. 2008;7:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol. Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Chen ZG, Li TQ, Hindmarsh T. Diffusion tensor trace mapping in normal adult brain using single-shot EPI technique. A methodological study of the aging brain. Acta Radiologica. 2001;42:447–458. doi: 10.1080/028418501127347160. [DOI] [PubMed] [Google Scholar]
- Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA. Am J Neuroradiol. 2002;23:803–808. [PMC free article] [PubMed] [Google Scholar]
- Chun T, Filippi CG, Zimmerman RD, Ulug AM. Diffusion changes in the aging human brain. Am J Neuroradiol. 2000;21:1078–1083. [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Dahle CL, Jacobs BS, Raz N. Aging, vascular risk and cognition: Blood glucose, pulse pressure, and cognitive performance in healthy adults. Psychology and Aging. 2009;24:154–162. doi: 10.1037/a0014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MMB. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Deshmukh AB, Rodrigue KM, Kennedy KM, Land S, Jacobs BS, Raz N. Synergistic effects of the MTHFR C677T polymorphism and hypertension on spatial navigation. Biol Psychol. 2009;80:240–245. doi: 10.1016/j.biopsycho.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. 2nd ed. NY: Springer; 1999. [Google Scholar]
- Engelter ST, Provenzale JM, Petrella JR, DeLong DM, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. Am J Roentgen. 2000;175:425–430. doi: 10.2214/ajr.175.2.1750425. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Developmental myelogenetic localisation of the cerebral cortex in the human subject. Lancet. 1901 October 19;:1027–1029. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin W, IV, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure: The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Furutani K, Harada M, Minato M, Morita N, Nishitani H. Regional changes of fractional anisotropy with normal aging using statistical parametric mapping SPM. J Med Invest. 2005;52:186–190. doi: 10.2152/jmi.52.186. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Bartzokis G, Hance DB, Shapiro D. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke. 1998;29:765–772. doi: 10.1161/01.str.29.4.765. [DOI] [PubMed] [Google Scholar]
- Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and the brain: a 5-year follow-up. Neurology. 2005;64:1846–1852. doi: 10.1212/01.WNL.0000164712.24389.BB. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. Am J Neurorad. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988--2000. J Am Med Assoc. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Nitkunan A, Charlton RA, Barrick TR, Macgregor GA, Markus HS. Cognitive impairment and white matter damage in hypertension: a pilot study. Acta Neurol Scand. 2008 Sep 15; doi: 10.1111/j.1600-0404.2008.01098.x. 2008 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Harris P, Alcantara D, Amenta N, Lopez OL, Eiriksdottir G, Sigurethsson S, Gudnason V, Madsen S, Thompson PM, Launer LJ, Carmichael OT. Localized measures of callosal atrophy are associated with late-life hypertension: AGES-Reykjavik Study. NeuroImage. 2008;43:489–496. doi: 10.1016/j.neuroimage.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Helenius J, Soinne L, Perkio J, Salonen O, Kangasmaki A, Kaste M, Carano RA, Aronen HJ, Tatlisumak T. Diffusion-weighted MR imaging in normal human brains in various age groups. Am J Neurorad. 2002;23:194–199. [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Gunning-Dixon FM, Murphy CF, Ardekani BA, Hrabe J, Lim KO, Etwaroo GR, Kanellopoulos D, Alexopoulos GS. Blood pressure and white matter integrity in geriatric depression. J Affect Disord. 2009;115:171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ling XY, Liu SR. Diffusion tensor imaging on white matter in normal adults and elderly patients with hypertension. Chinese Med J. 2006;119:1304–1307. [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; Arch Intern Med; 1997. pp. 2413–2446. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert ML, Knoepfel EJE, editors. Clinical Neurology of Aging. 2nd ed. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2008 Feb 12; doi: 10.1016/j.neurobiolaging.2007.12.020. 2008 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P. Arterial stiffness: A new surrogate end point for cardiovascular disease? Journal of Nephrology. 2007;20:S45–S50. [PubMed] [Google Scholar]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. Neuroreport. 2006;17:467–470. doi: 10.1097/01.wnr.0000209012.24341.7f. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HBW. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33:972–976. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Pestscher LM, van Zijl PCM. MRI atlas of Human White Matter. Amsterdam: Elsevier; 2005. [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Nitkunan A, Charlton RA, McIntyre DJ, Barrick TR, Howe FA, Markus HS. Diffusion tensor imaging and MR spectroscopy in hypertension and presumed cerebral small vessel disease. Magn Reson Med. 2008;59:528–534. doi: 10.1002/mrm.21461. [DOI] [PubMed] [Google Scholar]
- Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW. Regional and global changes in cerebral diffusion with normal aging. Am J Neurorad. 2001;22:136–142. [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical ‘disconnection’ as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–1452. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pagani E, Agosta F, Rocca MA, Caputo D, Filippi M. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage. 2008;41:657–667. doi: 10.1016/j.neuroimage.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain Cogn. 2009 Jul 10; doi: 10.1016/j.bandc.2009.06.002. 2009 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Prog Brain Res. 2002;136:455–465. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. NeuroImage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition - II. Mahwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2004. pp. 17–55. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Kennedy KM. A systems approach to age-related change: Neuroanatomical changes, their modifiers, and cognitive correlates. In: Jagust W, D’Esposito M, editors. Imaging the Aging Brain. New York, NY: Oxford University Press; 2009. [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;212:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: Effects of COMT, BDNF, ApoE and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovaris M, Iannucci G, Cercignani M, Sormani MP, De Stefano N, Gerevini S, Comi G, Filippi M. Age-related changes in conventional, magnetization transfer, and diffusion-tensor MR imaging findings: study with whole-brain tissue histogram analysis. Radiology. 2003;227:731–738. doi: 10.1148/radiol.2273020721. [DOI] [PubMed] [Google Scholar]
- Sabri O, Ringelstein EB, Hellwig D, Schneider R, Schreckenberger M, Kaiser HJ, et al. Neuro-psychological impairment correlates with hypoperfusion and hypometabolism but not with severity of white matter lesions on MRI in patients with cerebral microangiopathy. Stroke. 1999;30:556–566. doi: 10.1161/01.str.30.3.556. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, MacGillivray TJ, Deary IJ, Starr JM, Wardlaw JM. Childhood and current cognitive function in healthy 80-year-olds: a DT-MRI study. Neuroreport. 2003;14:345–349. doi: 10.1097/00001756-200303030-00010. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, Macgillivray TJ, Deary IJ, Starr JM, Rivers CS, Wardlaw JM. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis. 2005;20:310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- Shim YS, Yoon B, Shon YM, Ahn KJ, Yang DW. Difference of the hippocampal and white matter microalterations in MCI patients according to the severity of subcortical vascular changes: neuropsychological correlates of diffusion tensor imaging. Clin Neurol Neurosurg. 2008;110:552–561. doi: 10.1016/j.clineuro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing raters reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Söderlund H, Nyberg L, Adolfsson R, Nilsson LG, Launer LJ. High prevalence of white matter hyperintensities in normal aging: Relation to blood pressure and cognition. Cortex. 2003;39:1093–1105. doi: 10.1016/s0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Age-related degradation in the central nervous system: assessment with diffusion-tensor imaging and quantitative fiber tracking. Radiology. 2008;247:179–188. doi: 10.1148/radiol.2471070707. [DOI] [PubMed] [Google Scholar]
- Strassburger TL, Lee HC, Daly EM, Szczepanik J, Krasuski JS, Mentis MJ, Salerno JA, DeCarli C, Schapiro MB, Alexander GE. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997;28:1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiology of Aging. 2009 May 19; doi: 10.1016/j.neurobiolaging.2008.04.007. 2008 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Bae JN, MacFall JR, Payne ME, Provenzale JM, Steffens DC, Krishnan KR. Widespread effects of hyperintense lesions on cerebral white matter structure. Am J Roentgenol. 2007;188:1695–1704. doi: 10.2214/AJR.06.1163. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, de Groot M, van der Lugt A, Ikram MA, Krestin GP, Hofman A, Niessen WJ, Breteler MM. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43:470–477. doi: 10.1016/j.neuroimage.2008.07.052. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YT, Zhang CY, Zhang J, Li W. Age-related changes of normal adult brain structure: analysed with diffusion tensor imaging. Chinese Med J. 2005;118:1059–1065. [PubMed] [Google Scholar]