Abstract
The current study assessed the influence of excitotoxic lesions of the insular cortex (IC) on taste-potentiated odor aversion (TPOA) learning. Water-deprived rats initially received a single odor-toxicosis or odor/taste-toxicosis pairing and were subsequently tested, in separate trials, with the odor and the taste stimulus. Indicating TPOA, neurologically intact rats conditioned with the odor/taste compound stimulus acquired significantly stronger odor aversions than normal rats conditioned with the odor stimulus. IC lesions disrupted TPOA, conditioned taste aversion and taste neophobia. The finding that taste did not potentiate odor aversion learning in the IC lesioned rats provides support for the “within-compound association” analysis but is inconsistent with the “sensory and-gate” account of TPOA learning.
Keywords: Taste-potentiated odor aversion, within-compound associations, insular cortex, rat
1. Introduction
Overshadowing refers to the finding that a low salience stimulus acquires less associative strength when it is conditioned with a high salience cue relative to when it is conditioned alone (D'Amato and Fazzaro, 1966; Kamin, 1969; Mackintosh, 1971; Miles and Jenkins, 1973; Pavlov, 1927). This phenomenon is not observed in every case, however. An exception has been found when olfactory and taste cues are conditioned together in a conditioned odor aversion (COA) procedure using gastrointestinal malaise as the unconditioned stimulus (US). Contrary to overshadowing, if an odor and taste (i.e., an OT compound) are presented together during conditioning, a stronger odor aversion can be obtained compared to the COA found in the odor-alone (O) control group. This phenomenon is termed taste-potentiated odor aversion (TPOA) learning (e.g., Bouton, Jones, McPhillips and Swartzentruber, 1986; Palmerino, Rusiniak and Garcia, 1980; Rusiniak, Hankins, Garcia and Brett, 1979; Westbrook, Homewood, Horn and Clarke, 1983; for reviews see Batsell and Blankenship, 2002; LoLordo and Droungas, 1989).
Two prominent interpretations of TPOA have emerged. The first, the “sensory and-gate” account, is derived from a proposed dichotomy between the external (or skin) and internal (or gut) defense systems (Garcia, Lasiter, Bermudez-Rattoni and Deems, 1985). Based on the neuroanatomy of the tiger salamander and extrapolated to mammals, it was proposed that convergence between the somatosensory and auditory pathways provides the neural substrate for skin defense whereas convergence of gustatory and viscerosensory pathways underlie gut defense. According to this account, olfactory and visual pathways belong to neither skin defense nor gut defense but have access to each system. A sensory gate between the two defense systems, normally closed to prevent access to gut defense, ensures that odor and visual stimuli are treated as exteroceptive cues and utilized within the skin defense system. However, the gate can be switched open to gut defense by the presence of feeding cues. By this analysis, then, the presence of a taste stimulus “gates” the odor into the gut defense system where it can be processed like a taste. In this circumstance, a stronger odor aversion, the TPOA, is found than otherwise occurs when the odor is not gated into gut defense and is conditioned alone with the aversive US. It is important to note that according to this anatomical account of TPOA the strength of the aversion to the taste stimulus is not a factor that governs the occurrence of TPOA. Rather, the taste stimulus functions to gate the odor into the gut defense system where it can be associated with other feeding stimuli such as gastrointestinal malaise.
The second, “within-compound association” account, based on general principles of learning, explicitly states that the strength of the aversion to the accompanying taste stimulus is the determinant of TPOA learning (Durlach and Rescorla, 1980). Specifically, during compound (OT) conditioning associations develop not only between each element of the compound and the aversive US but also between the two elements (i.e., an odor-taste association is acquired). Thus, the manifest aversion expressed to the odor on the test trial is derived from two sources: the odor-illness association and the taste-illness association, which is activated by the odor-taste association. According to this account, then, the strength of the acquired aversion to the taste stimulus is critical for the occurrence of TPOA: the stronger the taste aversion the larger the potentiated odor aversion. In comparison, the strength of the COA in the O control group is dependent upon a single association, the odor-illness association.
Attempts to evaluate the merits of these two accounts of TPOA learning have tended to focus on the predictions that each make concerning the strength of the aversion to the potentiating taste stimulus. As noted above, the strength of the conditioned taste aversion (CTA) is irrelevant for the sensory and-gate account but it is fundamental for the within-compound association account. The most frequently used experimental manipulation to test this prediction involves postconditioning extinction of the CTA that was acquired during OT conditioning trial. A number of studies report that this manipulation eliminates TPOA thereby providing support for the within-compound association account (Durlach and Rescorla, 1980; Miller, McCoy, Kelly and Bardo, 1986; Trost and Batsell, 2004; von Kluge, Perkey and Peregord, 1996; Westbrook et al., 1983). On the other hand, some studies have found that postconditioning extinction of the taste conditioned stimulus (CS) has little or no influence on the magnitude of the TPOA, results that favor the sensory-and gate account (Droungas and LoLordo, 1991; Lett, 1984).
With the same end in mind, an alternative strategy is to prevent the acquisition of the taste aversion on the OT conditioning trial which, according to the within-compound association account, is essential for TPOA learning. This approach usually involves giving nonreinforced exposures to the taste stimulus prior to the TPOA conditioning trial. Nonreinforced preexposures render the taste stimulus familiar and safe which consequently retards the development of a CTA when the taste is subsequently paired with the illness US, a phenomenon known as latent inhibition (e.g., Lubow, 1989, 2009). Using this manipulation, Durlach and Rescorla (1980) found that the magnitude of the TPOA effect was significantly diminished in rats familiarized with the taste prior to the OT-toxicosis pairing. Although this result would seem to favor the within-compound association account, confidence in this conclusion is undermined by the procedures employed during the preexposure phase. That is, the rats preexposed to the taste stimulus were also preexposed to the OT compound stimulus, which, as noted by Lett (1984), would have encouraged the development of within-compound associations. Subsequently, Holder, Leon, Yirmiya and Garcia (1987; Experiment 3) also found that taste preexposure eliminated TPOA learning. But, the pattern of results was not consistent with the within-compound association account. First, it should be noted that a TPOA effect was obtained in rats that were given no taste preexposure. However, in the groups preexposed to the taste stimulus, no TPOA was obtained (the odor alone rats showed comparable COAs as the OT rats). Interestingly, the level of COA in the two taste preexposed groups was as strong as that acquired by the non-preexposure OT group. This strong COA is not consistent with the within-compound association account, which predicts that taste preexposure should significantly diminish if not eliminate TPOA and requires that the odor aversion in both groups of taste preexposed rats should be as weak as the COA acquired by the rats in the non-preexposed odor alone group. In explanation of their results, Holder et al. speculated that taste preexposure enhances the rat's capacity to learn odor aversions. If this analysis is correct, then taste preexposure has limited utility as a method to evaluate the relative merits of the two competing accounts of TPOA learning.
There is, however, an alternative way to manipulate taste familiarity that avoids preconditioning exposures to the taste element of the OT compound stimulus. Building on previous research (e.g., Kiefer and Braun, 1977), we have shown that the retardation of CTA acquisition found in rats with excitotoxic lesions of the insular cortex is a secondary consequence of a lesion-induced disruption in the perception of taste novelty (Roman, Lin and Reilly, 2009; Roman and Reilly, 2007). That is, rats with IC lesions treat novel taste stimuli as if they are familiar (Lin, Roman, St. Andre and Reilly, 2009) and CTA acquisition is delayed because of a latent inhibition-like effect. It might also be noted that the same IC lesion that disrupts CTA acquisition has no influence on COA acquisition (Roman, Nebieridze, Sastre and Reilly, 2006; see also Bertrand, Yannick, Mathilde, Frederic, Nadine and Guillaume, 2009). To investigate whether a taste-illness association is a pre-requisite for the potentiation of an odor aversion, in the present study we examined TPOA in rats with excitotoxic lesions of the IC. If the within-compound association account is correct, we would expect IC lesions to attenuate TPOA. On the other hand, if TPOA is independent of the CTA to the potentiating taste stimulus, as predicted by the sensory and-gate account, we would expect rats with IC lesions to show normal TPOA. Furthermore, irrespective of either theory, we expect that IC lesions will attenuate CTA and taste neophobia while having no influence on COA learning.
Four experimental design issues merit brief discussion. First, neurologically intact rats show significantly stronger neophobic reactions to the OT compound stimulus relative to the odor alone. Moreover, as noted above, rats with IC lesions show significantly weaker neophobic reactions to taste stimuli than do normal rats. Thus, to prevent these effects from confounding interpretation of the results, intake on the single conditioning trial was limited to a maximum of 5 ml. in the present study. Second, TPOA is defined at test by a within-experiment comparison between odor intake of the OT group with the intake of the odor stimulus in the O control group, a fact that has not always been appreciated in studies employing neurological manipulations. Third, the practice of assaying the strength of an acquired association by examining performance over multiple CS-only (i.e., extinction) trials is problematic for the simple reason that extinction does not involve, as is tacitly assumed in such studies, unlearning of the original association. Rather, a growing literature indicates that in extinction a new association is acquired that competes with and masks the original association (e.g., Calton, Mitchell and Schachtman, 1996; Rosas and Bouton, 1997; Schachtman, Threlkeld and Meyer, 2000; for reviews see Bouton, 2004; Bouton and Nelson, 1998; Miller, Kasprow and Schachtman, 1986). Thus, if a lesion influences performance after the first extinction trial it is not clear if the underlying deficit involves the original learning or learning of the new association. Finally, in order to draw confident conclusions about the attribution of function to structure, it is necessary to employ brain manipulations that selectively target intrinsic cell bodies while sparing fibers of passage. Thus, in lesion studies, excitatory neurotoxic agents (e.g., ibotenic acid or NMDA) are nowadays favored over electrolytic or other types of mechanical lesions (for discussion see, for example, Jarrard, 1989, 2002). Given these considerations, neurologically intact (SHAM) subjects and IC lesioned (ICX) rats received either a single (5 ml) odor (O) alone or odor/taste (OT) compound conditioning trial followed, 48 hr later, by a single odor test trial. TPOA is defined as significantly lower odor intake at test in the OT versus the O rats. To verify the expected lesion-induced deficits in CTA and taste neophobia, a taste test trial was administered 48 hr after the odor test trial. Of course, only the rats conditioned with the OT compound stimulus could show a CTA; for the rats conditioned with the O stimulus, the test trial assessed taste neophobia.
TPOA can be an elusive, difficult to obtain phenomenon (e.g., Bouton et al., 1986; Bouton and Whiting, 1982; Lashley and Rosellini, 1986; Mikulka, Pitts and Philput, 1982; Reilly, Grigson and Norgren, 1993; Rosellini and Lashley, 1986). Indeed, in our laboratory we have tried many different combinations of odor (almond, isoamyl acetate, orange, and vanilla) and taste (alanine, denatonium saccharide, quinine hydrochloride, sodium chloride, sucrose and sodium saccharin) stimuli with the odor presented either on an “odor disk” (Palmerino et al., 1980) or in the taste solution in order to obtain a TPOA effect. A solution of orange/quinine, the most effective OT combination that we have found, was used in the present study.
2. Results
2.1 Anatomical
Figure 1 presents the results of the histological analysis. The gustatory portion of the IC is located on the dorsal bank of the rhinal fissure and extends anteriorly ∼2 mm from the bregma (Roman et al., 2009; Roman and Reilly, 2007). The presence of gliosis and the absence or shriveling of cell bodies served as evidence of neurotoxic lesions. The histological examination indicated that the lesions were well placed in the IC with minimal encroachment into the somatosensory cortex, claustrum, and piriform cortex. The lesions in the present experiment were comparable in size and location to those in our previous studies (e.g., Kosar, Grill and Norgren, 1986a; Nakashima, Uemura, Yasui, Ozaki, Tabata and Taen, 2000). Rats with unilateral or small lesions were excluded from the behavioral analysis, leaving the final group sizes as follows: 20 SHAM (OT: n = 10, O: n = 10) and 13 ICX (OT: n = 7, O: n = 6).
Fig. 1.
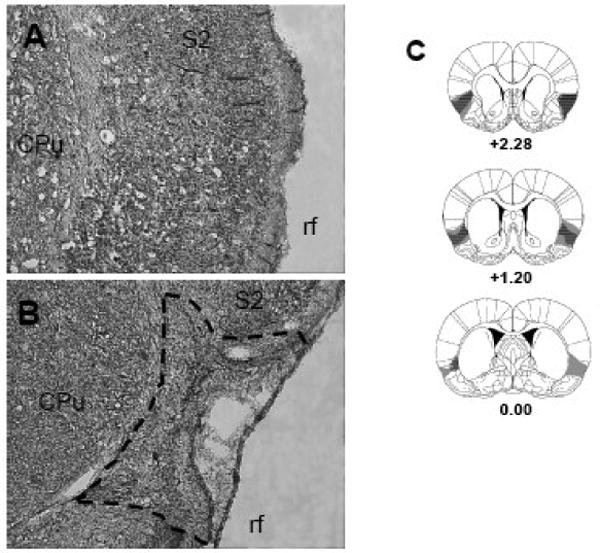
Digitalized photomicrographs of the insular cortex (IC) from a neurologically intact animal (A) and from a rat with a representative NMDA lesion of the IC (B). The extent of the lesion is outlined with a dashed line. Serial reconstructions (C) of the largest (gray) and smallest (diagonal lines) IC lesions at three coronal levels (+2.28, +1.20, 0.00 mm) anterior to bregma. CPu, caudate putamen; rf, rhinal fissure; S2, secondary somatosensory cortex. The diagrams were modified with permission from Paxinos and Watson (2005).
2.2 Behavioral
On the day before the conditioning trial, the mean water intake (±SE) for each group was: SHAM-O, 16.70 ml (±0.90); SHAM-OT, 16.72 ml (±1.12); ICX-O, 18.00 ml (±0.97); ICX-OT, 17.00 ml (±0.89). A two-way analysis of variance conducted on these data revealed no significant main effects or interaction (Fs < 1). During the conditioning trial, each group (SHAM, ICX) drank 5 ml of their respective CS type (O, OT) within the given 15 min period (Fs < 1).
2.2.1. Odor Test
Depicted in Figure 2 are the odor intake results according to CS Condition (O vs. OT) and Lesion (SHAM vs. ICX). Demonstrating a substantial TPOA, SHAM-OT rats drank ∼10 ml less of the aqueous odor than SHAM-O rats. The ICX-OT rats drank nearly 3 times as much aqueous odor than the SHAM-OT rats suggesting that the presence of the taste stimulus during the conditioning trials failed to potentiate the odor aversion in rats with IC lesions. These observations were supported by statistical analyses which found a significant main effects of Condition, F(1,28) = 37.18, p < .05, a significant main effect of Lesion, F(1,28) = 16.14, p < .05, and, more importantly, a significant condition ×lesion interaction, F(1,28) = 8.43, p < .05. Post hoc analysis indicated that while SHAM and ICX rats in the O condition consumed comparable amount of odor solution (p > .05), ICX rats consumed significantly more of the odor solution than SHAM rats in the OT condition, F(1,28) = 24.54, p < .05. These results indicate that IC lesions attenuated the ability of a taste cue to potentiate odor aversion learning.
Fig. 2.
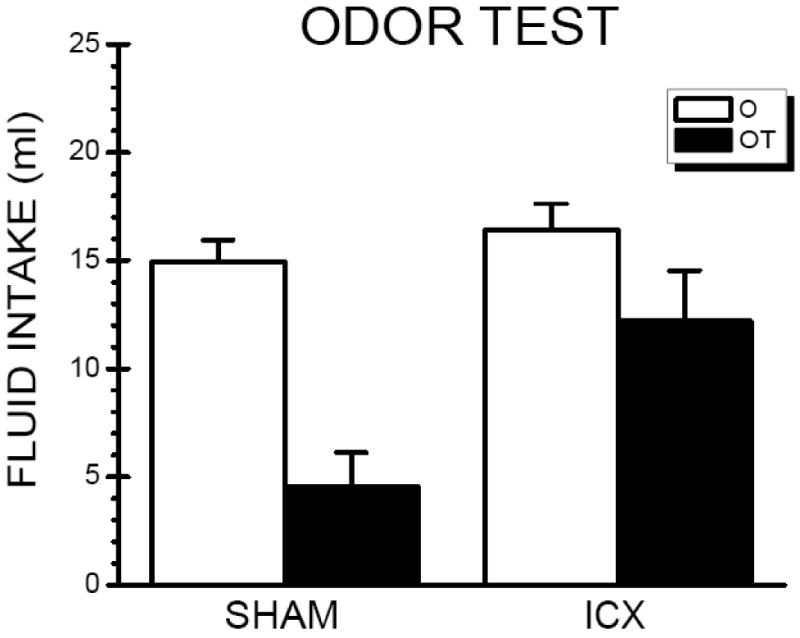
Mean (+SE) intake of the odor stimulus (0.02% orange extract) for the neurologically intact (SHAM) subjects and insular cortex-lesioned (ICX) rats during the odor test trial that followed conditioning with either the odor (O) stimulus or the odor/taste (orange/quinine; OT) compound stimulus.
2.2.2. Taste Test
The data shown in Figure 3 represent a CTA for the rats conditioned with the OT compound stimulus during the acquisition trial. Because the O rats were receiving the taste stimulus for the first time during the taste test, the data shown in Figure 3 for these rats represents taste neophobia to the novel quinine solution. Accordingly, the data from each condition (OT and O) were separately analyzed with student t tests. These analyses confirmed the impressions derived from inspection of the figure. That is, ICX-OT rats acquired significantly weaker CTAs than the SHAM-OT subjects, t(14) = 3.44, p < 0.01. Similarly, the ICX-O rats displayed a significantly less pronounced neophobic reaction to the novel quinine taste solution relative to the SHAM-O subjects, t(14) = 2.49, p < .05.
Fig. 3.
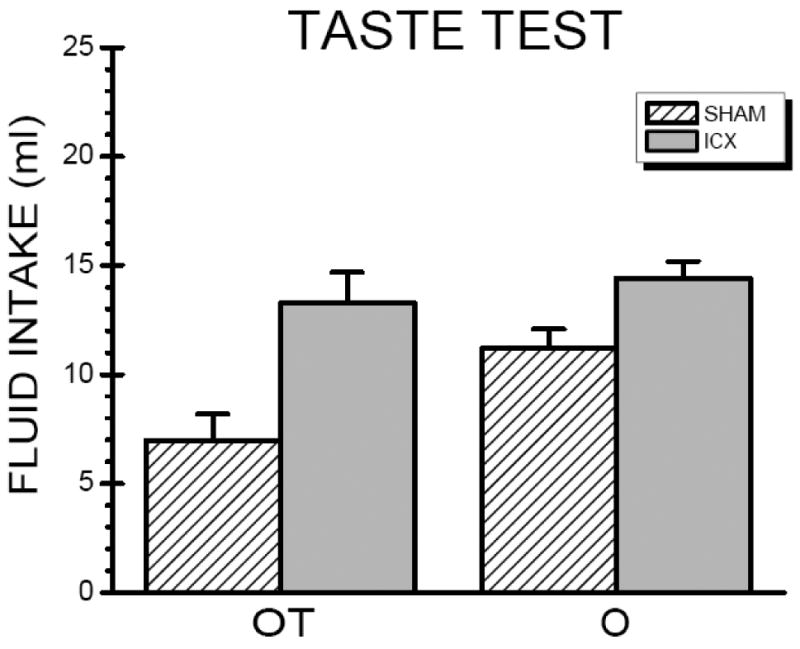
Mean (+SE) intake of the taste stimulus (0.00006 M quinine hydrochloride) for the neurologically intact (SHAM) subjects and insular cortex-lesioned (ICX) rats during the taste test trial conducted after the odor test trial in rats that were conditioned with either the odor (O) stimulus or the odor/taste (orange/quinine; OT) compound stimulus.
3. Discussion
TPOA was found in the neurologically intact subjects in the present study. Specifically, the SHAM-OT subjects drank significantly less odor solution on the odor test trial than did the SHAM-O animals. This difference (∼10 ml) constitutes a substantial TPOA effect that was obtained using a procedure which ensured that the subjects in each of the four groups drank the same amount of fluid on the conditioning trial. In so doing, differences on the test trials can confidently be ascribed to the stimulus processing/learning capacities of the subjects. Excitotoxic lesions of the IC not only impaired TPOA acquisition but, as expected, they also disrupted CTA acquisition and attenuated taste neophobia.
As noted in the Introduction, a number of experimental design issues undermine, to varying degrees, previous attempts to examine TPOA in rats with IC lesions. For instance, Inui, Shimura and Yamamoto (2006) employed a design that involved, for all rats, an OT compound conditioning trial followed by four odor alone test trials interspersed with four taste alone test trials. On the basis of group differences (ICX rats drinking more than SHAM subjects) on odor test trials 3 and 4, Inui et al. claimed that the IC has a role in TPOA learning. However, this experiment did not include an odor-alone control group. As discussed earlier, the occurrence of TPOA is defined by the difference in the strength of the odor aversion in the OT experimental group with that of the odor-alone control group. Furthermore, the relevance of the demonstration in an additional experiment that IC lesions did not influence COA acquisition when the odor was paired with lithium toxicosis is diminished because in this latter experiment the US was administered immediately after the odor presentation but was given 30 min after the OT presentation in the “TPOA” experiment. Thus, it is difficult to draw firm conclusions about the role of the IC in TPOA learning from the Inui et al. study. Lasiter, Deems and Garcia (1985), using electrolytic lesions (a technique that was appropriate for the time but which non-selectively destroys all tissue at the lesion site and therefore constrains the attribution of function to structure) in a design that limited fluid access on all trials to 5 min, reported that IC lesions impaired TPOA and CTA; the short duration of the taste test prevents assessment of the influence of IC lesions on taste neophobia because of a ceiling effect on the amount consumed. Nonetheless, this study does provide the best indication that the IC has a role in TPOA leaning. But, what is the nature of the IC involvement?
Although olfactory information is known to ascend to the IC (Shipley, McLean and Ennis, 1995), IC lesions do not influence COA acquisition as shown by the performance of the ICX-O rats in the odor test of the present study. Indeed, using the same odor stimulus (0.02% aqueous orange) as well as the same US (0.15 M lithium chloride) as employed in the present study, Roman et al. (2006) found normal COA acquisition in ICX rats that showed impaired CTA acquisition (see also Bertrand et al., 2009). Incidentally, this pattern of results also excludes the possibility that delayed CTA acquisition in ICX rats can be attributed to an impairment in the detection of, or responsivity to, the US (lithium-induced toxicosis). Overall, then, the disruption of TPOA acquisition in the ICX-OT rats cannot be reduced to a deficit in odor aversion learning per se.
The IC receives ascending taste information from the thalamus (Cechetto and Saper, 1987; Kosar et al., 1986b; Krettek and Price, 1977; Norgren and Wolf; 1975; Wolf, 1968) as well as the amygdala (Krettek and Price, 1974; Norgren, 1976; Shi and Cassell, 1998) and is the cortical component of the central gustatory system (for reviews see Lundy and Norgren, 2004; Travers, 1993). It has, however, long been recognized that the disruption of CTA acquisition consequent to IC lesions cannot be attributed to a failure to detect or recognize taste stimuli since ICX rats show normal taste detection thresholds and normal concentration-dependent intake curves for the four basic taste types (e.g., Braun, 1999; Braun, Lasiter and Kiefer, 1982). Furthermore, the retardation of CTA acquisition occurs only when the taste is novel; no deficit is found when the taste is familiar (Kiefer and Braun, 1977; Roman et al., 2009; Roman and Reilly, 2007). We have argued that this CTA deficit is a secondary consequence of an IC lesion-induced disruption in the perception of taste novelty (Lin et al., 2009). That is, the ICX rat treats the genuinely novel taste stimulus as if it is familiar and CTA acquisition is retarded because of a latent inhibition-like effect (for a review see Reilly, 2009).
The foregoing pattern of spared and impaired innate/acquired taste functions in rats with excitotoxic lesions of the IC has important implications for the analysis of TPOA learning in neurologically intact subjects. As detailed in the Introduction, the two prominent interpretations of TPOA hold opposite positions concerning the concomitant acquisition of a CTA on the TPOA acquisition trial. For the sensory and-gate account, the aversion acquired by the taste element of the OT compound is irrelevant; the sole function of the taste stimulus is to gate the odor into the gut defense system where it can acquire a stronger aversion than otherwise would develop when it is conditioned via the skin defense system (Garcia et al., 1985). On the other hand, the within-compound association account is predicated on the acquisition of a CTA to the taste element of the compound CS (Durlach and Rescorla, 1980). It will be evident, then, that the IC lesion-induced disruption of CTA and the consequent attenuation of TPOA in the present study supports the within-compound association analysis but are inconsistent with the sensory and-gate account of TPOA learning. Indeed, the present results provide clear evidence of the sequence of events that underlie the disruption of TPOA learning in ICX rats. First, it is important to note that, in the absence of a taste stimulus, COA acquisition was normal in rats with IC lesions. The ICX rats did, however, show attenuated taste neophobia, a deficit which, as noted above, we have argued underlies the retarded acquisition of taste aversions in these rats. The ICX-OT rats showed attenuated CTA acquisition, a deficit that occurs because of the occurrence of a latent inhibition-like effect when the taste stimulus is novel. Finally, the failure of the ICX-OT rats to acquire a CTA to the taste element of the OT compound on the single conditioning trial negates the acquisition of a taste-potentiated odor aversion. More simply stated, IC lesions impair taste neophobia which, in turn, retards taste aversion learning and, consequently, attenuates TPOA acquisition.
In summary, a series of experiments from our laboratory have provided evidence that lesions of the IC disrupt the perception/recognition of taste novelty such that ICX rats treat novel taste stimuli as if they are familiar (e.g., Lin et al., 2009; Roman et al., 2006, 2009; Roman and Reilly, 2007). Building on this foundation, the present study investigated the behavioral mechanisms underpinning TPOA by examining the performance of ICX rats in this task. By showing that the acquisition of a CTA on the OT compound trial is critical for the potentiation of the odor aversion, the results support the within-compound association model of TPOA (Durlach and Rescorla, 1980). The IC modulates the neural representation of one of the sensory inputs into the associative mechanism that governs TPOA acquisition. Whether the same brain structure(s) is/are responsible for the associative learning necessary for COA and TPOA remains an open question, as does the identity of the neural substrate.
4. Experimental procedures
4.1 Subjects
Forty-one male Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN) and housed individually in hanging stainless-steel cages. These rats had previously served as subjects in an experiment concerning morphine-induced suppression of saccharin intake, but they were naïve with respect to all the stimuli (orange odor, quinine, and lithium chloride) used in the present study. The vivarium was set with 12-hr light-dark cycles (light on at 0700) and all the experimental procedures were conducted during the light phase. The rats weighed 280 - 320 g at the time of surgery. Food and water were available ad libitum except during behavioral testing when the rats were water deprived. All the animals were treated according the guidelines established by the National Institutes of Health (1986) and the American Psychological Association (1996) and the experimental methods were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
4.2 Surgery
The rats (n = 21) assigned to Group ICX received bilateral N-methyl-D-aspartate (NMDA) lesions of the IC. In preparation for surgery, they were first anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg) and then fixed into stereotaxic apparatus (ASI, Warren, MI) using blunt ear bars. Body temperature was monitored with a rectal thermometer that provided feedback to a heating pad to maintain 37°C (Harvard Apparatus, Holliston, MA). The skull was exposed by an incision along the midline and the head was leveled between bregma and lambda by adjusting the position of the bite bar. Trephine holes were drilled in the skull above the IC and a glass pipette (tip diameter ∼75 μm) filled with 0.15 M NMDA (Sigma-Aldrich, St. Louis, MO) was lowered into the brain. Using the coordinates of Roman and Reilly (2007), two iontophoretic infusions were made in each hemisphere using a Midgard Precision Current Source (Stoelting, Wood Dale, IL) as follows. Site 1: AP +1.2, ML ±5.2, DV -5.0 (10 min infusion); Site 2: AP +1.2, ML ±5.2, DV -4.3 (6 min infusion). Following the final infusion, the scalp incision was closed with wound clips and the rat was allowed to recover from the anesthesia before being returned to the home cage. Rats in the non-lesion control group (Group SHAM) received either the same surgical procedures as the in ICX rats except the pipette was filled with saline and no infusions were made (n = 10) or they received anesthesia with no additional surgical treatment (n = 10).
4.3 Procedure
Rats in each lesion group (SHAM, ICX) were divided into one of two CS Conditions according to whether they received an odor (O) or an odor-taste (OT) compound stimulus on the conditioning trial. The O stimulus was an aqueous solution of 0.02% (volume/volume) orange extract (Flavorganics, Newark, NJ); OT was 0.02% orange plus 0.00006 M quinine hydrochloride (Sigma-Aldrich) in water. After acclimating to a schedule that permitted 15 min water access each day, all rats were given a single conditioning trial. To prevent lesion- or CS-induced differences in fluid intake on the conditioning trial, all rats were given 15 min to drink 5 ml of the designated CS. Thirty minutes after initial placement of the CS bottle the rats received an intraperitoneal injection of 0.15 M lithium chloride (1.33 ml/100g body weight; Sigma-Aldrich). Two days after the conditioning trial, all rats were given a 10-min test trial with the odor solution and, two days later, a 10-min taste test trial. Fifteen min water access was scheduled on the intervening days. Additionally, to maintain proper hydration, all rats were given 15 min access to water 4 h after the conditioning trial and the odor test trial; the rats were returned to ad libitum water access after the taste test trial.
4.4 Apparatus
All behavioral testing occurred in the home cages. The fluids were presented in inverted graduated cylinders with silicone stoppers and steel drinking tubes. Fluid intake was measured to the nearest 0.5 ml.
4.5 Histology
After all behavioral testing, the rats were given a lethal injection of sodium pentobarbital and then perfused intracardinally with physiological saline followed by 10% formalin. The brain was extracted and stored in 4% formalin at least two days and then in the 20% sucrose for another two days. Subsequently, the brain was blocked and sectioned coronally at 50 μm using a cryostat. Consecutive sections through the IC were mounted and stained for cell bodies with cresyl violet. Reconstructions of the lesions were made with the aid of a microscope (Zeiss Axioskop 40) connected to a computer with Q-capture software (Quantitative Imaging Corporation, Burnaby, British Columbia, Canada).
Acknowledgments
This research was supported by grants DC04341 and DC06456 from the National Institute of Deafness and Other Communication Disorders. Portions of the data in this article were presented at the 37th Annual Meeting of the Society for Neuroscience, San Diego, CA, November 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychological Association. Guidelines for ethical conduct in the care and use of animals. Washington, DC: American Psychological Association; 1996. [Google Scholar]
- Batsell WR, Jr, Blankenship AG. Beyond Potentiation: Synergistic Conditioning in Flavor-Aversion Learning. Brain and Mind. 2002;3:383–408. [Google Scholar]
- Bertrand D, Yannick S, Mathilde B, Frederic L, Nadine R, Guillaume F. Critical role of insular cortex in taste but no t odour aversion memory. Eur J Neurosci. 2009;29:1654–1662. doi: 10.1111/j.1460-9568.2009.06711.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Jones DL, McPhillips SA, Swartzentruber D. Potentiation and overshadowing in odor-aversion learning: Role of method of odor presentation, the distal-proximal cue distinction, and the conditionability of odor. Learn Motiv. 1986;17:115–138. [Google Scholar]
- Bouton ME, Nelson JB. The role of context in classical conditioning: Some implications for cognitive behavior therapy. In: O'Donohue WT, editor. Learning and behavior therapy. Needham Heights, MA: Allyn & Bacon; 1998. pp. 59–84. [Google Scholar]
- Bouton ME, Whiting MR. Simultaneous odor-taste and taste-taste compounds in poison-avoidance learning. Learn Motiv. 1982;13:472–494. [Google Scholar]
- Braun JJ. Gustatory cortex: Definition and function. In: Kolb B, Tees RC, editors. The cerebral cortex of the rat. Cambridge, MA: MIT Press; 1990. pp. 407–430. [Google Scholar]
- Braun JJ, Lasiter PS, Kiefer SW. The gustatory neocortex of the rat. Physiol Psychol. 1982;10:13–45. [Google Scholar]
- Calton JL, Mitchell KG, Schachtman TR. Conditioned inhibition produced by extinction of a conditioned stimulus. Learn Motiv. 1996;27:335–361. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representations in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- D'Amato MR, Fazzaro J. Attention ad cue-producing behavior in the monkey. J Exp Anal Behav. 1966;9:469–473. doi: 10.1901/jeab.1966.9-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droungas A, LoLordo VM. Taste-mediated potentiation of odor aversion induced by lithium chloride: Effects of preconditioning exposure to the conditioned stimulus and postconditioning extinction of the taste aversion. Learn Motiv. 1991;22:291–310. [Google Scholar]
- Durlach PJ, Rescorla RA. Potentiation rather than overshadowing in flavor-aversion learning: An analysis in terms of within-compound associations. J Exp Psychol Anim Behav Proc. 1980;6:175–187. [PubMed] [Google Scholar]
- Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Ann N Y Acad Sci. 1985;443:8–21. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- Holder MD, Leon M, Yirmiya R, Garcia J. Effects of taste preexposure on taste and odor aversions. Anim Learn Behav. 1987;15:55–61. [Google Scholar]
- Inui T, Shimura T, Yamamoto T. Effects of brain lesions on taste-potentiated odor aversion in rats. Behav Neurosci. 2006;120:590–599. doi: 10.1037/0735-7044.120.3.590. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Meth. 1989;29:351–359. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Use of excitotoxins to lesion the hippocampus: Update. Hippocampus. 2002;12:405–414. doi: 10.1002/hipo.10054. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. Selective associations and conditioning. In: Mackintosh NJ, Honig WK, editors. Fundamental issues in associative learning. Halifax, Nova Scotia: Dalhousie University Press; 1969. pp. 42–64. [Google Scholar]
- Kiefer SW, Braun JJ. Absence of differential associative responses to novel and familiar taste stimuli in rats lacking gustatory neocortex. J Comp Physiol Psychol. 1977;91:498–507. doi: 10.1037/h0077347. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1986a;379:329–341. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. II. Thalamocortical projections. Brain Res. 1986b;379:342–352. doi: 10.1016/0006-8993(86)90788-2. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A direct input from the amygdala to the thalamus and the cerebral cortex. Brain Res. 1974;67:169–174. doi: 10.1016/0006-8993(74)90309-6. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lashley RL, Rosellini RA. Conditioning of odors in compound with taste is a function other than potentiation. Bull Psychon Soc. 1986;24:159–162. [Google Scholar]
- Lasiter PS, Deems DA, Garcia J. Involvement of the anterior insular gustatory neocortex in taste-potentiated odor aversion learning. Physiol Behav. 1985;34:71–77. doi: 10.1016/0031-9384(85)90080-0. [DOI] [PubMed] [Google Scholar]
- Lett BT. Extinction of taste aversion does not eliminate taste potentiation of odor aversion in rats or color aversion in pigeons. Anim Learn Behav. 1984;12:414–420. [Google Scholar]
- Lin JY, Roman C, St Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 2009;1251:195–203. doi: 10.1016/j.brainres.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoLordo VM, Droungas A. Selective associations and adaptive specializations: taste aversions and phobias. In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Instrumental conditioning theory and the impact of biological constraints on learning. Hillsdale, NJ: Erlbaum; 1989. pp. 145–179. [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Lubow RE. Conditioned taste aversion and latent inhibition: A review. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 37–57. [Google Scholar]
- Lundy FR, Jr, Norgren R. Gustatory system. In: Paxinos G, Mai J, editors. The rat nervous system. 3rd. San Diego: Academic Press; 2004. pp. 891–921. [Google Scholar]
- Mackintosh NJ. An analysis of overshadowing and blocking. Quart J Exp Psychol. 1971;23:118–125. [Google Scholar]
- Mikulka PJ, Pitts E, Philput C. Overshadowing not potentiation in taste aversion learning. Bull Psychon Soc. 1982;20:101–104. [Google Scholar]
- Miles CG, Jenkins HM. Overshadowing in operant conditioning as a function of discriminability. Learn Motiv. 1973;4:11–27. [Google Scholar]
- Miller JS, McCoy DF, Kelly KS, Bardo MT. A within-event analysis of taste-potentiated odor and contextual aversions. Anim Learn Behav. 1986;14:15–21. [Google Scholar]
- Miller RR, Kasprow WJ, Schachtman TR. Retrieval variability: Sources and consequences. Am J Psychol. 1986;99:145–218. [PubMed] [Google Scholar]
- Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res. 2000;36:297–309. doi: 10.1016/s0168-0102(99)00129-7. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No 86-23) Washington, DC: U.S. Government Printing office; 1986. [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:12–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92:123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- Palmerino CC, Rusiniak KW, Garcia J. Flavor-illness aversions: the peculiar roles of odor and taste in memory for poison. Science. 1980;208:753–755. doi: 10.1126/science.7367891. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex (G V Anrep, translation) London: Oxford University Press; New York: Dover; 1927. reprint. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th. San Diego, CA: Academic Press; 2005. [Google Scholar]
- Reilly S. Central gustatory system lesions and conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. Oxford University Press; New York: 2009. pp. 309–327. [Google Scholar]
- Reilly S, Grigson PS, Norgren R. Parabrachial nucleus lesions and conditioned taste aversion: Evidence supporting an associative deficit. Behav Neurosci. 1993;107:1005–1017. doi: 10.1037//0735-7044.107.6.1005. [DOI] [PubMed] [Google Scholar]
- Roman C, Lin JY, Reilly S. Conditioned taste aversion and latent inhibition following extensive taste preexposure in rats with insular cortex lesions. Brain Res. 2009;1259:68–73. doi: 10.1016/j.brainres.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Nebieridze N, Sastre A, Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav Neurosci. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex on conditioned taste aversion and latent inhibition. Eur J Neurosci. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Rosas JM, Bouton ME. Renewal of conditioned taste aversion upon return to the conditioning context after extinction in another one. Learn Motiv. 1997;28:216–229. [Google Scholar]
- Rosellini RA, Lashley RL. Conditioning of odors in compound with taste: A failure to observe potentiation. Bull Psychon Soc. 1986;24:55–58. [Google Scholar]
- Rusiniak KW, Hankins WG, Garcia J, Brett LP. Flavor–illness versions: Potentiation of odor by taste in rats. Behav Neural Biol. 1979;25:1–17. doi: 10.1016/s0163-1047(79)90688-5. [DOI] [PubMed] [Google Scholar]
- Schachtman TR, Threlkeld R, Meyer K. Retention of conditioned inhibition produced by extinction. Learn Motiv. 2000;31:283–300. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;339:440–468. doi: 10.1002/(sici)1096-9861(19981005)399:4<440::aid-cne2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Shipley MT, McLean JH, Ennis M. Olfactory system. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 899–926. [Google Scholar]
- Travers SP. Orosensory processing in neural systems of the nucleus of the solitary tract. In: Simon SA, Roper SD, editors. Mechanisms of taste transduction. Boca Raton: CRC Press; 1993. pp. 339–394. [Google Scholar]
- Trost CA, Batsell WR., Jr Taste + odor interactions in compound aversion conditioning. Learn Behav. 2004;32:440–453. doi: 10.3758/bf03196040. [DOI] [PubMed] [Google Scholar]
- von Kluge S, Perkey T, Peregord J. An ear for quality: differential associative characteristics of taste-potentiated auditory and odor avoidance. Physiol Behav. 1996;60:331–339. doi: 10.1016/0031-9384(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, Homewood J, Horn K, Clarke JC. Flavour-Odour compound conditioning: Odour-potentiation and flavour-attenuation. Quart J Exp Psychol. 1983;35B:13–33. doi: 10.1080/14640748308400911. [DOI] [PubMed] [Google Scholar]
- Wolf G. Projections of thalamic and cortical gustatory areas in the rat. J Comp Neurol. 1968;132:519–530. doi: 10.1002/cne.901320403. [DOI] [PubMed] [Google Scholar]


