Abstract
Symbolic visual cues indicating the location of an upcoming target are believed to invoke endogenous shifts of attention to cued locations. In the present study, we investigated how visual attention is shifted during such cuing paradigms by recording event-related potentials (ERPs). We focused on a component known to index lateralized shifts of perceptual attention during visual search tasks, known as the N2pc component. The ERP data show that attention was shifted to a cued location in anticipation of a target shape when the location is marked by a placeholder-object (Experiments 1 and 2). However, when the possible locations were not marked by placeholder objects we found no evidence for an anticipatory shift of attention to the cued location (Experiment 3). These findings indicate that the perceptual-attention mechanism indexed by the N2pc is deployed to objects and not simply locations in space devoid of object structure.
Keywords: visual attention, attentional cuing, N2pc, event-related potentials, object-based attention
Introduction
Mechanisms of visual selective attention allow us to dedicate our limited-capacity processing mechanisms to the most relevant information available in our complex visual environments. Cognitive scientists often use the visual cuing paradigm to study the characteristics of selective visual processing. This paradigm, pioneered by Eriksen and colleagues (e.g., Eriksen & Collins, 1969; Eriksen & Rohrbaugh, 1970), and popularized by Posner (1980), uses symbols or transient peripheral stimuli to direct covert visual attention to specific locations. In the present study, we used event-related potentials (ERPs) to test hypotheses regarding how visual-spatial attention is deployed during a symbolic-cuing paradigm.
In this study, we used a component related to the deployment of visual-spatial attention, the N2pc (N2-posterior-contralateral – see Luck, Girelli, McDermott, & Ford, 1997; Luck & Hillyard, 1994b; Woodman & Luck, 1999), to measure when attention was shifted to a cued location during a symbolic-cuing paradigm. The first hypothesis we tested was whether visual-spatial attention is shifted to the cued location in advance of the presentation of the target object, rather than being triggered by the actual presentation of the target. It is quite difficult to assess this with behavioral measures, and it is plausible that the behavioral costs and benefits of attention reflect processes that occur after the target is presented (e.g., Mulligan & Shaw, 1981). ERPs, in contrast, provide a continuous measure of processing between a cue and a target, making it possible to measure the exact time at which attention is shifted.
We also examined the role that visible objects play in whether attention is shifted to a cued location. Recent behavioral work suggests that certain signatures of visual-spatial selection might be observed only when visible objects are present as anchors for attention (Mueller & von Muehlenen, 2000). Similarly, research suggests that attention can be directed to entire visual hemifields or quadrants in the absence of any visual stimuli (Hughes & Zimba, 1985) but that it is directed more precisely when location markers are present (Zimba & Hughes, 1997). In the present study, we found that the attention mechanism indexed by the N2pc component shifted to symbolically cued target locations in advance of a target embedded among distractors. However, this anticipatory N2pc was observed only when an object was actually present at the cued location.
Why were we interested in how the N2pc component behaves during a cuing task? Previous work by Luck and colleagues (Luck, Fan, & Hillyard, 1993; Luck, Girelli et al., 1997; Luck & Hillyard, 1995; Luck, Hillyard, Mangun, & Gazzaniga, 1994), among others (e.g., Eimer, 1996; Hickey, Di Lollo, & McDonald, in press) has shown that the negativity known as the N2pc is observed at posterior electrode sites contralateral to where in the visual field attention is focused. For example, Woodman & Luck (1999; 2003b) found that the N2pc switched between hemispheres as attention was shifted between potential target items on opposite sides of a visual search array. However, the N2pc component has been studied almost exclusively in the context of visual search tasks, and it is not clear how it would behave during a cuing study that recruited the N2pc selection mechanism.
Attention researchers frequently use spatial cuing paradigms to study the deployment of perceptual attention in the visual field (for a review see Yantis, 1996). The basic paradigm is simple. A target stimulus is presented at one of two or more possible peripheral locations in the visual field. Preceding this target stimulus, a symbolic or peripheral stimulus indicates the likely location of the upcoming target. Participants can detect or discriminate targets more quickly or accurately when the cue accurately indicates the location of the target. Posner (1980) among others (Egly & Homa, 1984; Eriksen & Yeh, 1985) proposed that improved behavioral performance at the cued location is observed because the cues cause visual-spatial attention to be shifted to the cued location in advance of the target object. A number of ERP studies have been conducted to test this general hypothesis regarding the covert orienting of attention based on the information that cuing stimuli provide.
A number of laboratories have used ERPs to examine how attention operates during cuing paradigms. In a seminal study, Harter, Miller, Price, LaLonde, and Keyes (1989) used a centrally presented arrow stimulus to cue the location where an isolated target object would subsequently appear. They found that the waveform recorded at posterior electrodes sites contralateral to the cued location became more negative than ipsilateral electrode sites at approximately 200 ms after cue onset. Harter and colleagues termed this effect the early directing attention negativity (EDAN), based on the interpretation that the EDAN is an index of the shifting of attention to the cued location. This component ended well before the onset of the target stimulus, but it was followed by a positive potential at posterior contralateral electrodes that remained until target onset, an effect they called the late directing attention positivity (LDAP). Subsequently, other groups have replicated this pattern of findings using similar paradigms (Hopf et al., 2000; Nobre, Sebestyen, & Miniussi, 2000; Yamaguchi, Tsuchiya, & Kobayashi, 1994).
However, a recent study suggests that the previous reports of the EDAN may instead be a consequence of selecting and processing the cuing stimuli themselves and not covertly deploying attention to a yet unoccupied target location. Specifically, van Velzen and Eimer [, 2003 #7410] noted that previous studies used either a central arrow pointing to the left or right or two simultaneously presented arrows to the left and right of fixation pointing to the left and right visual field, respectively. van Velzen and Eimer (2003) proposed that the early-lateralized effect was related to shifting attention to the relevant lateralized cue stimulus itself (i.e., focusing attention on the cue) and not a shift of attention to the location indicated by the cue’s symbolic meaning.
To test the hypothesis that the EDAN component is actually related to the lateralized processing of the cue stimulus, van Valzen and Eimer compared the ERPs recorded in two different cuing conditions. In one condition, two symbolic cue stimuli (i.e., colored arrows) were presented on each trial, with the arrow to the right of fixation pointing into the right visual field and the arrow to the left of fixation point to the left, just as in previous studies (e.g., Nobre et al., 2000). They compared the ERPs recorded in this condition with one in which the spatial locations of the cuing stimuli were reversed. That is, the arrow on the left pointed to the right visual field and the arrow to the right of fixation pointed to the left. This allowed van Valzen and Eimer (2003) to distinguish between contralateral activity related to processing of the cue itself and contralateral activity related to shifting attention into the cued hemifield. They found that the hemisphere of the early contralateral negativity was determined by the hemifield in which the relevant cue stimulus appeared and not the hemifield cued by the relevant arrow. More specifically, these findings suggest that the EDAN component previously reported was actually an N2pc elicited by observers shifting attention to the relevant cue stimulus.
The boundary conditions for eliciting LDAPs are also not completely clear. Specifically, although studies showing a LDAP preceding a target have varied somewhat in using single-item (e.g., Nobre et al., 2000) or multiple-item target discrimination tasks (e.g., Hopf & Mangun, 2000), all of these studies have required observers to process targets presented in a visual field that was devoid of simultaneously presented distractors. This feature of the previous studies is striking because it has been proposed that the N2pc is related to filtering distractors presented near targets (Luck & Hillyard, 1994b). As a result, previous cuing paradigms that used isolated targets would not be expected to recruit the visual selection mechanism indexed by the N2pc component. It is possible that, when a cued target is embedded among distractors, a contralateral negativity (the N2pc), as opposed to a contralateral positivity (the LDAP), would be observed immediately prior to target presentation.
In the present study, we sought to test the hypothesis that a negativity contralateral to a cued-target location is observed when subjects anticipate a target presented simultaneously with nearby distractor objects. Thus, we were primarily interested in when the posterior negativity contralateral to the cued location would be observed. We avoided possible confounds related to processing lateralized cuing stimuli by presenting central words or letters that indicated the location of the upcoming target on each trial. Unlike van Valzen and Eimer (2003), who used a go/no-go detection task, we required subjects to perform a target shape discrimination task. Thus, this study also serves to test the generality of the hypothesis that early contralateral negativities (i.e., 200–300 ms post cuing stimulus) are observed only when the cues are at least partially lateralized. Cognitive psychologists assume that cued targets are processed more efficiently (i.e., more rapidly or accurately) because visual-spatial attention is shifted to the cued location in advance of the presentation of the target (Posner, 1980). Thus, the goal of Experiment 1 was to use the N2pc component to track when attention is deployed to cued locations during a symbolic cuing paradigm.
Experiment 1
In Experiment 1, we used centrally presented color names to cue subjects to the location of a placeholder in which a target shape would subsequently be presented. As illustrated at the bottom of Figure 1, there were four possible target squares, each filled with a different color, and the cue was the name of the color of one square. The target shape was always presented at the location indicated by the cue after a delay of 700 ms. Non-target shapes were presented simultaneously with the target to create perceptual competition, which appears to be important in triggering the attentional mechanism indexed by the N2pc component (Luck & Hillyard, 1994; Luck et al., 1997). We used this somewhat unconventional experimental design as a bridge between conventional cuing methods and the search paradigms that have been used to study the N2pc component. Experiment 2 will show that similar results can be obtained in a more conventional cuing paradigm, in which the cue directly indicates a specific location.
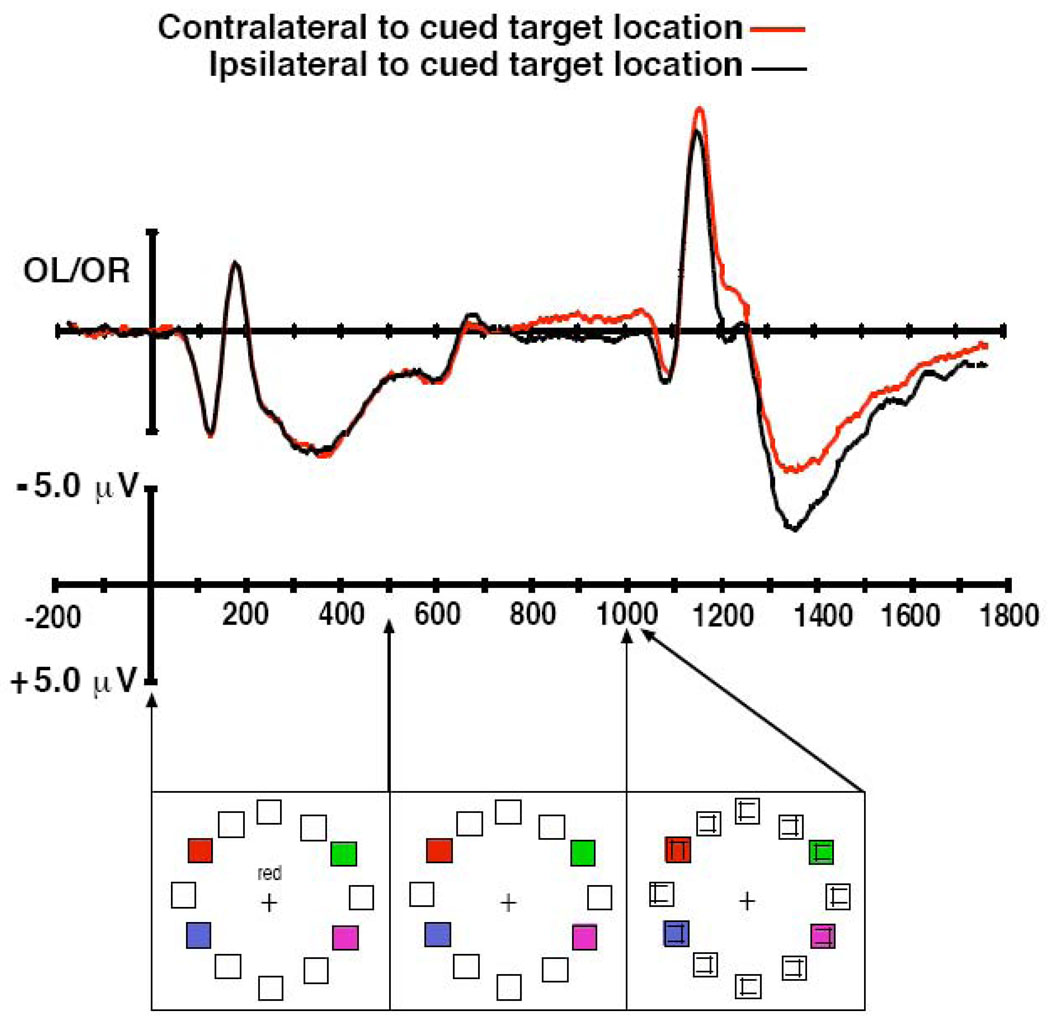
Figure 1.
ERP results and example of stimulus sequence from Experiment 1. Top panel: Grand average ERP waveforms averaged across the observers. The red trace is the waveform from electrode OL or OR that was contralateral to the cued location, and the black trace is the waveform recorded from the ipsilateral site. For presentation only, the ERP waveforms were digitally low-pass filtered by convolving the waveforms with a Gaussian impulse response function (SD = 6 ms, 50% amplitude cutoff at 30 Hz). Bottom panel: Example of the sequence of stimuli presented on each trial. The array of placeholders was presented as a static standing background. The cue word appeared for 500 ms and the visual search array containing the cued target was presented for 33 ms.
There were two objectives of this experiment. The first was to test the hypothesis that attention-directing cues lead to a shift of attention prior to target onset, as suggested by previous studies of attentional cuing (Eriksen & Yeh, 1985; Posner, 1980; Posner & Cohen, 1984). The study of van Valzen and Eimer (2003) suggested that prior ERP evidence for such a shift was insufficient. However, many other sources of evidence indicate that attention is shifted prior to target onset (e.g., Hillyard, Vogel, & Luck, 1998; Hopfinger, Buonocore, & Mangun, 2000; Luck, Chelazzi, Hillyard, & Desimone, 1997; Luck, Woodman, & Vogel, 2000). The second and primary objective was to determine whether the mechanism indexed by the N2pc component is observed during cuing tasks. As described above, previous reports suggest that the lateralized activity observed during cue-to-target intervals typically displays a positive contralateral distribution (i.e., the LDAP). However, as we briefly reviewed, these studies have not required the visual system to suppress information from simultaneously presented distractor objects. Because the N2pc component appears to be related to distractor suppression, previous paradigms might not have used stimuli and tasks well suited to observe the N2pc in the context of a cuing paradigm. Thus, in the present study we required subjects to discriminate the identity of a target item presented at a validly cued location that was presented simultaneous with surrounding distractor items (see Figure 1 bottom). We predicted that this experimental design would recruit the mechanism indexed by the N2pc component in order to suppress the distractor objects and allow the cued target to be accurately processed.
Method
Participants
Six undergraduates (18–28 years of age) from the University of Iowa were paid for their participation. All had normal or corrected to normal vision and reported no history of neurological problems. The participants gave informed consent before the experiment began and were native English speakers.
Stimuli
The stimuli were viewed on a homogenous gray background (10 cd/m2) at a distance of 100 cm. Throughout each block of trials, eight white outlined squares (92.5 cd/m2, 1.3° × 1.3°, line thickness of 0.033°) and four filled colored squares of the same size were continuously visible. The colored squares were different colors, including one green (x = 0.648, y = 0.330, 14.54 cd/ m2), one red (x = 0.648, y = 0.330, 14.54 cd/ m2), one blue (x = 0.648, y = 0.330, 14.54 cd/ m2), and one violet (x = 0.648, y = 0.330, 14.54 cd/ m2). Each square was presented 5.2° from the center of the monitor (see Figure 1 for an example). A black fixation point (<0.01 cd/m2, 0.33° × 0.33°) was visible throughout each trial. The cue stimuli were the words ‘green’ (0.9° × 1.8°), ‘red’ (0.9° × 1.1°), ‘blue’ (0.9° × 1.4°), and ‘violet’ (0.9° × 2.2°) presented in white (92.5 cd/m2) 1.3° above the fixation point. The target array of stimuli consisted of 12 black outlined squares (<0.01 cd/m2, 0.7° × 0.7° . composed of line segments 0.1° thick) with one target and 11 non-target objects arranged similar to the number locations on a clock face. Each non-target Landolt-C-like stimulus had a gap (0.2°) on either its left or right side, whereas the target stimulus was a square with a gap on the top or bottom. The target was always presented at the cued location.
Procedure
The beginning of each trial was indicated by the presentation of the fixation point 1200–1600 ms before the onset of the cue stimulus. Next, a word cue was presented for 500 ms. The cue word named the color of the placeholder in which the target object would appear 1000 ms after the onset of the cue stimulus (i.e., a 1000 ms cue-to-target Stimulus Onset Asynchrony or SOA). The target array was then briefly presented for 33 ms. The target shape (i.e., a top gap or bottom gap) was randomly selected on each trial. Participants were instructed to indicate which of the two target shapes was presented at the cued location by pressing a button with the index finger on their right hand for a target with a gap at the top of the square and a button with the middle finger on their right hand for a target with a gap at the bottom of the square on a hand-held game pad. They were instructed to respond as accurately as possible without time pressure (provided they responded within a 4000-ms window following target onset). The cue stimulus was always valid; that is, the target always appeared at the cued location on each trial. This was done to maximize the number of trials contributing to the ERP averages and ensure that participants would not adopt a strategy of directing attention to an uncued location on a subset of trials. The final stimulus event during each trial was the offset of the fixation point following the onset of the target array by a variable interval (randomly jittered from 1200–1600 ms using a rectangular distribution). This event served to indicate to the participants the beginning of an inter-trial interval (2000 ms) in which they were allowed to blink.
Each participant performed 10–20 practice trials prior to the beginning of the first block of experimental trials to ensure they understood the task and could maintain proper fixation. Participants then performed 8 blocks of 64 trials while ERPs were recorded. This large number of trials (i.e., 512) allowed us to obtain low-noise averages from each subject and kept the number of subjects required to a minimum. On each block of trials a different configuration of the four colored placeholders was shown as part of the static background such that all possible configurations of the four colors at the four possible locations appeared. Thus, across all blocks of trials, each cue word referred to a different spatial location and just as often to a left and right visual field location. Participants were allowed to rest between trial blocks.
Recording and Analysis
The electroencephalogram (EEG) was recorded from tin electrodes held on the scalp by an elastic cap (Electrocap International, Eaton, OH). A subset of the International 10/20 System sites were used (F3, F4, C3, C4, P3, P4, T3, T4, T5, T6, O1, and O2) in addition to nonstandard sites OL (halfway between O1 and T5) and OR (halfway between O2 and T6). The right mastoid electrode served as the reference site. The signals were re-referenced offline to the average of the left and the right mastoids (Nunez, 1981). The electrooculogram (EOG) was recorded by placing electrodes 1 cm lateral to the external canthi to measure horizontal eye movements and by placing an electrode beneath the left eye, referenced to the right mastoid, to measure vertical eye movements and blinks. The EEG and EOG were amplified by an SA Instrumentation amplifier with a gain of 20,000 and a bandpass of 0.01–80 Hz. The amplified signals were digitized at 250 Hz by a PC-compatible computer and averaged offline. Trials accompanied by incorrect behavioral responses or ocular or myogenic artifacts were excluded from the averages.
We used a two-step procedure for ocular artifact rejection that has been described previously (Woodman & Luck, 2003b). Briefly, trials with large eye movements were rejected prior to averaging, and averaged horizontal EOG (HEOG) waveforms were used to reject any subjects with significant unrejected eye movements. This procedure led to rejection of an average of 13.3% of trials per participant (with a single participant maximum of 23.8%) and required us to replace 2 participants due to excessive eye movements (either greater than 25% of individual trials rejected or any residual systematic eye movement that resulted in voltage deflections greater than 3.2 µV, corresponding to an ocular deviation of ±0.1°).
N2pc activity was measured as the difference between electrode sites contralateral and ipsilateral to the validly cued target location. On the basis of previous ERP cuing experiments, we focused on three periods of interest. First, we were interested in the lateralized differences 200–400 ms post-cue because this is the time during which the EDAN has been reported (Harter et al., 1989). Second, we focused on the 200 ms prior to the onset of the target stimulus. This period allowed us to determine whether attention was shifted to the cued location in advance of the target, as is assumed to occur in behavioral cuing paradigms. The choice of this window size was guided by our observation that the pre-target activity appeared to qualitatively change beginning 200 ms before the target across subjects (using selection criteria described in Luck, 2005). Our third period of interest was from 200–275 ms post-target stimulus. This is the time range during which the N2pc component is normally observed during visual search tasks (Luck & Hillyard, 1994a, 1994b).
Analyses of variance (ANOVAs) were used for all statistical tests, and p-values were adjusted when appropriate with the Greenhouse-Geisser epsilon correction for non-sphericity (Jennings & Wood, 1976). The data were collapsed across cued color because we did not observe any effects due to sensory differences between which color was cued. We first entered the data into an ANOVA with the following within-subjects factors: time window (post-cue, pre-target, or target), visual field of cue (upper versus lower), hemisphere (left versus right), contralaterality with respect to the cued location (ipsilateral versus contralateral), and electrode site (O1/2, OL/R, versus T5/6). We then performed planned comparisons for each time window.
Results
Subjects correctly discriminated the target identity (i.e., top versus bottom gap) on 95.1% of trials (mean reaction time, RT = 587 ms). The results of a separate pilot experiment verified that a valid cue was necessary for observers to be able to discriminate the identity of the target at this level of speed and accuracy. Specifically, we ran a behavioral experiment with 6 participants in which the cue validly indicated the target location (75% of trials) or the cue was invalid and the target appeared in one of the other three possible target locations (25% of trials). We found that 93.2% of the validly cued targets were correctly discriminated, whereas the accuracy of target discrimination on invalidly cued trials was 58.3% correct, a significant difference (F(1,5) = 51.68, p < 0.001). Significant differences in reaction time accompanied these accuracy effects (Valid RT: 654 ms; Invalid RT: 1249 ms; F(1,5) = 16.37, p < 0.01). The findings of this preliminary behavioral experiment indicate the necessity of a valid spatial cue to perform the target discrimination task at the levels found in this experiment.
The grand-average ERP waveforms are shown in Figure 1. The cue stimulus was followed by a period in which no clear lateralized effects were observed until approximately 200 ms before the presentation of target shape. At that time, the hemisphere contralateral to the cued location became more negative, with the same spatial distribution of voltages as is typically observed for the N2pc during visual search. That is, the effect was maximal at posterior electrode sites OL/OR and decreased across more anterior lateralized pairs of electrodes such that the effect was absent at F3/4. This posterior contralateral negativity with respect to the cued target location continued until the end of the averaged epoch.
The statistical analyses support the observations that a reliable contralateral negativity was observed in anticipation of the target and that this negativity extended through the activity elicited by the array containing the target. Specifically, the omnibus ANOVA yielded significant main effects of time window (F(1,5) = 16.67, p < .01), contralaterality (F(1,5) = 9.31, p < .05), and a significant interaction of these factors (F(2,10) = 9.84, p < .05). In addition, there were significant interactions of contralaterality X electrode site (F(2,10) = 8.92, p < .01) and time window X contralaterality X electrode site (F(4,20) = 8.36, p < .001), reflecting the fact that the contralaterality effect was largest at the OL/R sites, as in previous N2pc studies (Luck & Hillyard, 1994a, 1994b; Woodman & Luck, 1999, 2003a, 2003b).
Our planned analyses within each time window revealed a significant effect of contralaterality in both the pre-target window and the post-target window (F(1,5) = 9.16, p < .05, and F(1,5) = 9.15, p < .05, respectively) but no such effect in the post-cue window (F < 1.0). There were also significant interactions of contralaterality X electrode site in both the pre-target and target window tests (F(2,10) = 5.93, p < .05, and F(2,10) = 4.89, p < .05, respectively), reflecting the fact that the effect was largest at the OL/R electrode pair. No other main effects or interactions were significant in any of the tests. These analyses support the observations that a significant posterior contralateral negativity was observed in anticipation of the target and this negativity extended through the typical time range of the N2pc following target array presentation until the end of the trial.
To determine whether any N2pc was elicited by the search array, over and above any lateralized activity prior to the onset of the search array, we performed another analysis of the target-evoked activity after baselining the ERP data using the 200-ms interval prior to the onset of the visual search array. This subtracted away any lateralized activity present prior to the onset of the search array, allowing us to determine if additional lateralized activity was elicited by the search array. This analysis confirmed that the electrodes contralateral to the target were more negative than those ipsilateral 200–275 ms after the target array (F(1,5) = 5.96, p < .05).
Several other aspects of these waveforms deserve note. First, the offset of the cue stimulus was at 500 ms, and this led to offset-elicited P1 and N1 waves at approximately 600 and 675 ms, respectively. Second, although this was not the focus of our hypotheses, we observed an extended contralaterality following the typical N2pc time course. A previous study has suggested that this long-latency contralateral negativity can be attributed to post-perceptual processing because it extends into the time range of the P3 component (Woodman & Luck, 2003a), and also is likely to be related to recent studies of lateralizations during visual working memory maintenance (Jolicoeur, Benoit, & Nicolas, 2008; McCollough, Machizawa, & Vogel, 2007; Vogel & Machizawa, 2004; Vogel, McCollough, & Machizawa, 2005). Finally, we did not observe N2pc-like activity contralateral to the cued location (or EDAN) immediately following the presentation of the central cue stimulus.
Discussion
In this experiment, no contralateral negativity was observed immediately following the predictive spatial cue. This is consistent with the results of van Velzen and Eimer (2003), who concluded that early lateralizations following a cue in previous studies (the EDAN) are actually an N2pc reflecting the focusing of attention onto the cue stimulus itself. In the present study, the cues were words centered on the vertical meridian rather than lateralized stimuli, and we could therefore isolate activity contralateral to the cued location without contamination due to processing of a lateralized cue. Despite the lack of an early lateralized effect, we did observed a shift of attention to the cued location approximately 200 ms before the search array was presented. Thus, subjects did not shift attention until just before the target was expected. This negativity continued during and after the time that the target object was presented, with an additional negativity triggered by the search array in the typical N2pc latency range. The voltage distributions of the pre-target and post-target contralateral negativities were similar to the scalp distribution of the N2pc component in previous visual search studies (Luck & Hillyard, 1994b). Consistent with this observation we did not observe anterior potentials (the anterior directing attention negativity, or ADAN) that have previously been described using cuing paradigms without the distractor suppression requirement used presently. We should note that the underlying N2pc may have actually been larger in amplitude than we observed but may have been reduced by an overlapping positive contralaterality related to preparatory shifts of attention (i.e., the LDAP) given the LDAP has been shown to have a similar distribution as the N2pc (e.g., McDonald & Green, 2008). Thus, the findings of Experiment 1 support the proposal that attention is shifted to cued locations in advance of targets embedded among distractors.
It is always difficult to be certain that an ERP component observed in one experiment is the same as an ERP component observed in a different experiment. In the present case, we cannot be certain that the lateralized activity observed in the few hundred ms prior to the target is the same component as the N2pc that is typically observed following a visual search array. However, given that the latency of the P3 wave can vary over hundreds of milliseconds depending on the experiment (see Luck & Hillyard, 1990, for an extreme example), there is certainly precedent for concluding that a component could appear at one time in one experimental paradigm and at a very different time in another paradigm. Indeed, Yeung, Cohen, and Botvinick (2004) have proposed that the error-related negativity elicited by errors, the N200 component elicited by correctly classified incompatible targets in the flankers paradigm, and the N450 component elicited by correctly classified incongruent targets in the Stroop paradigm all reflect the same underlying ERP component. Thus, we provisionally propose that the cue-elicited pre-target contralateral activity observed in the present study is the same as the post-target contralateral N2pc activity observed in previous visual search studies (and in response to the target array in the present study). Previous research that presented targets that were not flanked by distractors reported a more anterior contralateral positivity during the period preceding the target. The distribution of the contralateral activity we observed also appears to be different from that found in a recent attentional cuing study in which targets were preceded by a lateralized negative component. Grent-‘t-Jong and Woldorff (2007) presented a faint target within one of two placeholder objects and found a contralateral negativity that built up preceding the target onset at the cued location. This contralateral effect overlapped with a broader negativity (likely the contingent negative variation) elicited at the same time. However, it is possible that the contralateral negativity observed in Grent-‘t-Jong and Wolforff (2007) may also have been N2pc activity due to the difficulty of detecting the faint target within the cued high contrast placeholder. Thus, these findings support the hypothesis that the mechanism indexed by the N2pc is recruited when the necessity to perform distractor suppression can be anticipated.
Experiment 2
The experimental paradigm used in Experiment 1 was designed to be halfway between a typical N2pc visual search paradigm and a cuing paradigm. The potential problem with this paradigm, in which the cued location was identified by means of its distinctive color, is that the observed N2pc-like activity may have reflected the use of attention to resolve the competition between the color of the cued location marker and the colors of the surrounding location markers. In Experiment 2, we sought to generalize the results of Experiment 1 using a more traditional cuing paradigm, in which central cue directly indicated the to-be-attended location and all location markers were the same color. Specifically, each of the 12 stimulus locations was marked with an outlined white square, and the cue stimuli consisted of two letters indicating the location of the upcoming target (e.g., UL for upper left, LR for lower right). If the N2pc-like effect observed 200 ms prior to the onset of the target array in Experiment 1 reflected advance preparation for processing the target, then this same effect should also be observed in Experiment 2.
Method
The stimuli, procedure, data collection and analysis methods used were identical to those of Experiment 1 with the following exceptions. A new group of 6 observers participated after giving informed consent. As illustrated at the bottom of Figure 2, the static background stimuli were all outlined white squares exactly like those that marked the distractor locations in Experiment 1. The four possible target locations were the same as those used in Experiment 1 (i.e., the stimulus positions just above and below the horizontal meridian in the left and right visual fields). Subjects were instructed that these were the only possible target locations and that distractors would be presented within the other placeholders. The cues were centrally presented two-letter pairs indicating which possible target location would contain the target object. The cue stimuli were the letters UL, UR, LL, and LR, standing for ‘upper left’, ‘upper right’, ‘lower left’, and ‘lower right’, respectively. These letter pairs were presented in a sans serif font, were centered 1.3° above fixation, and subtended 0.9° vertically and 0.7° horizontally. An average of 15.4% of trials were rejected due to artifacts (with a single subject maximum of 21.1%) and three observers were replaced for having either more than 25% of trials rejected or averaged EOG with deflections greater than 3.2 µV.
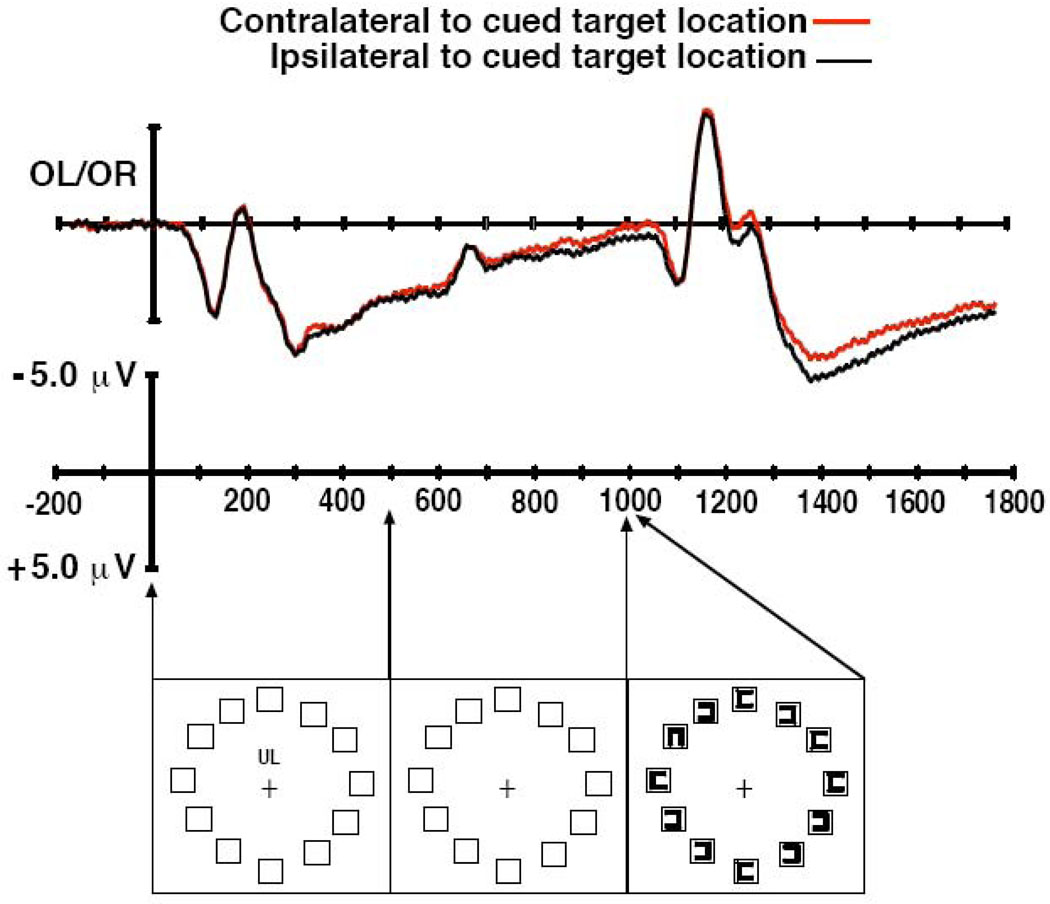
Figure 2.
Grand average ERP waveforms (top) and example of the stimuli (bottom) from Experiment 2.
Results
Subjects accurately (80.2 % correct) discriminated the cued target objects (mean RT = 757 ms). Although this behavioral performance was less accurate and slower than that found in Experiment 1, it is far better than would be expected if attention where not deployed to the target location as shown by the behavioral experiment1.
The grand average ERP waveforms are shown in Figure 2. We observed essentially the same pattern of results as in Experiment 1. Specifically, the ERP waveform contralateral to the cued target location became more negative approximately 200–300 ms before the target object was presented. This posterior contralateral negativity continued throughout the target presentation until the end of the recording epoch.
These observations were supported by the findings of the statistical tests. The omnibus ANOVA yielded a significant main effect of contralaterality (F(1,5) = 10.46, p < .05) and significant interactions of contralaterality X electrode site (F(1,5) = 4.94, p < .05) and time window X contralaterality X electrode site (F(1,5) = 5.07, p < .05). These effects support the observation that N2pc-like activity was present immediately before and after target array onset but not immediately after cue array onset.
The planned follow-up ANOVA for the target window yielded a significant interaction of contralaterality X electrode site (F(2,10) = 6.46, p < .05), reflecting the canonical distribution of the N2pc component with largest amplitude at the OL/R sites. The pre-target window yielded only a significant main effect of contralaterality relative to the cued location (F(1,5) = 10.46, p < .05). The interaction term of contralaterality X electrode site was not significant (F(2,10) = 1.89, p = .20), but the pattern of contralateral activity was consistent with the distribution typically observed in previous studies of the N2pc. That is, the contralateral effect was largest at electrodes OL/R (−0.56 µV), followed by T5/6 (−0.36 µV), and smallest at O1/2 (−0.22 µV). The analysis of the post-cue window yielded a significant main effect of hemisphere (F(1,5) = 27.33, p < .01) due to potentials being more positive over the left hemisphere and particularly for lower visual field cues, leading to a significant interaction of hemisphere X visual field (F(1,5) = 33.73, p < .01). There were different distributions of voltages across hemispheres such that there was also a significant interaction of hemisphere X electrode site (F(1,5) = 10.46, p < .05) during the post-cue window. Note that the pre-target and target windows yielded significant main effects and interactions of contralaterality, indicating N2pc-like activity, whereas no significant contralateral activity was observed in the post-cue window.
As in Experiment 1, we further assessed the target-evoked N2pc activity by rebaselining the ERPs to the 200-ms interval prior to the target array. This baselining procedure reduced the amplitude of the contralateral negativity as it did in Experiment 1. In this case, the main effect of contralaterality and interaction with electrode site were no longer significant after rebaselining (F < 1.0).
To directly compare the findings of Experiment 1 and 2 we entered the ERP data into an ANOVA with the between subjects factor of experiment (1 versus 2). This ANOVA yielded neither a significant effect of experiment nor an interaction of experiment with any other factor (F < 1.0). This supports the observation that the pattern of effects was largely the same in Experiment 1 and 2.
Discussion
In Experiment 2, we observed that the hemisphere contralateral to the cued target location became more negative at posterior sites than the ipsilateral hemisphere approximately 200–300 ms before the target object was presented. This pattern of activity again suggests that attention was shifted to the cued location marked by a placeholder immediately before the target stimulus was expected to appear. These findings replicate the results of Experiment 1 using a new group of observers and demonstrate that the results of Experiment 1 were not simply due to the use of colored location placeholders and word cues.
The distribution of the contralateral effects was similar to that observed in Experiment 1 and in previous studies of the N2pc. This supports our conclusion that the mechanism indexed by the N2pc was recruited in anticipation of the target item in the crowded search array to filter out the features of the distractors allowing accurate processing of the target. Together, the results of Experiment 1 and 2 demonstrate that anticipatory N2pc-like activity is elicited by symbolic spatial cues when the cues indicate the location of a target that will be flanked by distractor objects. Contrasting the present findings with previous studies that did not use multi-element target arrays suggests that the type of selection mechanism deployed to a cued location will depend upon the target processing requirements that are anticipated. This is consistent with previous findings from behavioral (Awh, Sgarlata, & Kliestik, 2005) and neuroimaging experiments (Serences, Yantis, Culberson, & Awh, 2004).
Experiment 3
In Experiments 1 and 2, placeholder objects marked the four possible target locations during the cue-to-target interval. Next we examined whether the anticipatory N2pc-like activity observed in these previous experiments was due to attention selecting the placeholder objects themselves. Previous studies observed the N2pc component in the context of visual search tasks in which attention is deployed to potential target items present in the visual field. In Experiment 1 and 2, the placeholder array contained objects to which attention could be deployed. It is possible that the mechanism indexed by the N2pc component is deployed to objects and that, when the visual field does not contain such structure, the N2pc mechanism has no stimulus on which to focus. That is, this mechanism may not perform purely spatial selection, but may instead operate on object representations. This hypothesis seems likely in light of a recent cuing study of the N2pc in which a blank monitor appeared during the cue-to-target interval and no anticipatory N2pc-like activity was observed (Kiss, van Velzen, & Eimer, 2007).
The cuing paradigm used in Experiment 3 afforded us the opportunity to test the hypothesis that the N2pc selection mechanism is deployed to objects rather than the locations that the target items would ultimately occupy. To distinguish between these hypotheses, we used a cuing paradigm identical to that of Experiment 2, but removed the static array of placeholder objects that marked the possible target locations. The cued locations were no less ambiguous because the same four possible target locations were used as in Experiments 1 & 2. If the N2pc selection mechanism is deployed to spatial locations in anticipation of a target array, we should still observe the N2pc-like activity shifting contralateral to the cued position during the pre-target interval. In contrast, if the mechanism indexed by the N2pc selects objects and not simply locations in space, then removing the placeholders should eliminate the pre-target N2pc-like activity observed in Experiments 1 and 2.
Method
All methods used here were identical to those of Experiment 2 except as noted below. A new group of six observers were recruited to participate. The stimuli were identical to those of Experiment 2, except that the array of placeholder objects was not shown (see Figure 3, bottom). Observers were shown where the possible target locations were and familiarized with these four locations during the practice trials prior to the experimental trial blocks. Among this group of participants, an average of 10.9% of trials were rejected due to artifacts (with a single subject maximum of 20.9%) and four observers were replaced for having more than 25% of trials rejected or averaged EOG with deflections greater than 3.2 µV.
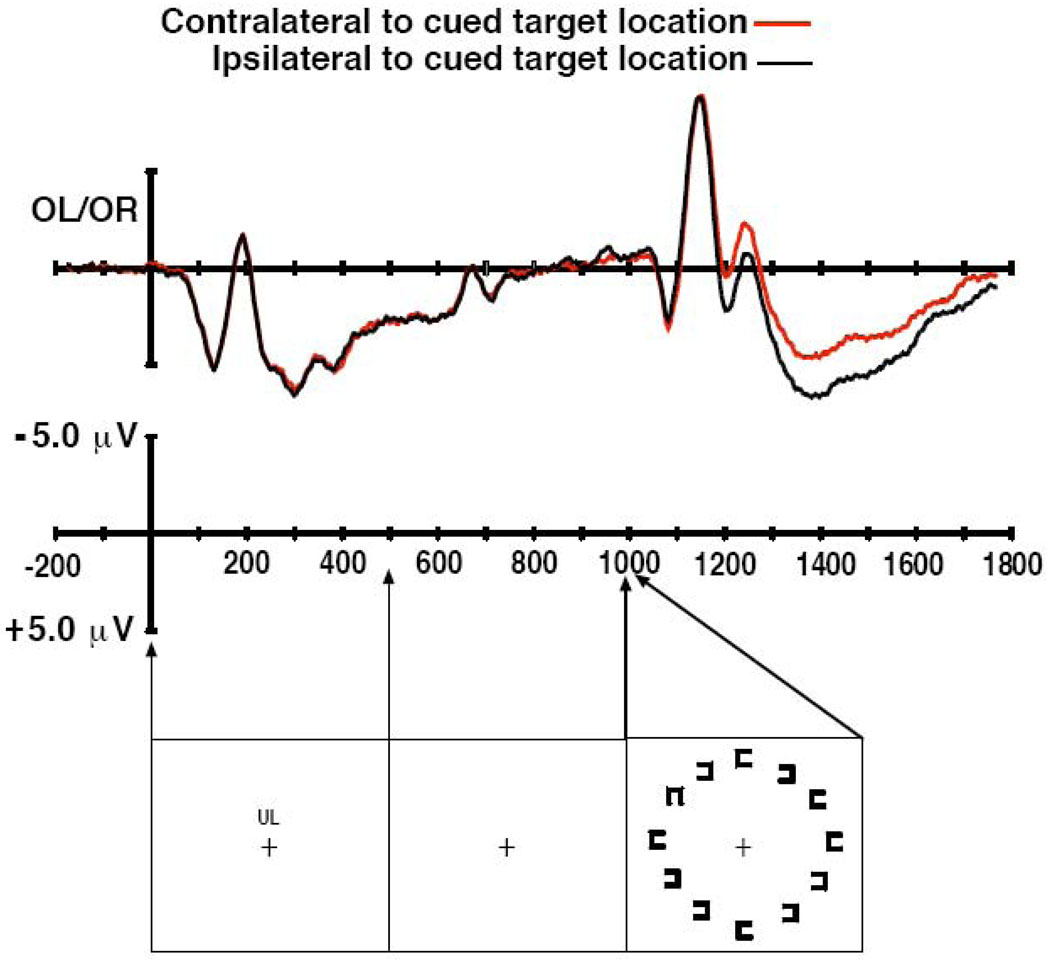
Figure 3.
ERP waveforms and stimulus arrays from Experiment 3. Top panel: Grand average ERP waveforms from Experiment 3. Bottom panel: Stimulus sequence from Experiment 3, which was identical to Experiment 2 except for the absence of the placeholder objects.
Results
Observers correctly discriminated the target item on 93.3% of trials (mean RT = 806 ms). This performance is better than that found in Experiment 2 using a different group of observers, but similar to accuracy in Experiment 1 and to the valid trials of the behavioral pilot experiment.
The grand average ERP waveforms are shown in Figure 3. We observed no sign of a posterior negativity contralateral to the cued target location prior to the presentation of the array containing the target. Indeed, the only N2pc activity was observed following the target within the time range that the N2pc is typically observed during visual search.
The results of the statistical analyses support these observations. The key results in the omnibus ANOVA were a time window X contralaterality interaction (F(2,10) = 21.06, p < .05) and a significant three-way interaction of time window X contralaterality X electrode site (F(4,20) = 5.02, p < .05). These effects reflect the presence of N2pc-like activity following the target array but not immediately preceding the target array or immediately after the cue array. This ANOVA also yielded significant main effects of time window, visual field, and electrode site (ps < .01), along with significant two-way interactions of time window X visual field, visual field X hemisphere, and hemisphere X electrode site (ps < .05).
The planned comparison during the target window yielded a significant effect of contralaterality (F(1,5) = 15.76, p < .05) and a significant contralaterality X electrode site interaction (F(2,10) = 5.19, p < .05), as is typically observed for the N2pc component in visual search experiments. During the pre-target window, we obtained significant main effects of visual field and electrode site and a significant hemisphere X electrode site interaction (ps < .05), but no significant main effects or interactions involving the contralaterality factor. Compared to Experiment 2, the pre-target activity appeared to be reduced by the absence of placeholders. To assess this statistically we performed an analysis of the pre-target interval across Experiment 2 and 3. Consistent with the observation of reduced pre-target activity in Experiment 3 we found a significant interaction of the factor of Experiment (2 versus 3) with contralaterality (F(1,10) = 6.13, p < .05). In analyzing the activity in the post-cue window in Experiment 3, we found a significant main effect of visual field and interactions of visual field X hemisphere and hemisphere X electrode site (ps < .05), but no main effects or interactions involving the contralaterality factor.
As in Experiment 1 and 2, we examined the target-evoked N2pc activity by baselining the ERPs to the period immediately (0–200 ms) before the onset of the target array. The statistical analysis yielded a significant effect of contralaterality (F(1,5) = 23.84, p < .01) and a significant interaction of contralaterality X electrode site (F(2,10) = 6.74, p < .05). This result differs from that of Experiment 2 in which baselining to the pre-target period largely eliminated the contralateral negativity that was observed. To directly compare the N2pc components evoked by the target arrays in Experiment 2 and 3 we entered the ERP data, baselined to the 200 ms period prior to the onset of the search array, into an ANOVA with a between-subjects factor of Experiment (2 versus 3). This analysis yielded a significant effect of contralaterality (F(2,10) = 13.19, p < .01) and, most importantly, a significant interaction of contralaterality X Experiment (F(2,10) = 6.30, p < .05). These findings support the observation that the amplitude of the N2pc to the target was larger in Experiment 3 than Experiment 2.
Discussion
In Experiment 3, we found that an N2pc component was elicited following the presentation of the target, but unlike in Experiment 1 and 2, we did not observe this component during the interval immediately preceding the target presentation. The only difference between Experiments 2 and 3 was the presentation of an array of placeholder objects in Experiment 2. These findings demonstrate that the selection mechanism indexed by the N2pc is deployed to objects in anticipation of a target embedded in distractors but is not simply deployed to locations that lack the presence of an object to select. An alternative account of the difference in N2pc activity we observed between Experiments 2 and 3 is that no pre-target N2pc was observed because the N2pc is inherently spatial but fails without precise spatial information. However, we can reject this hypothesis because the specificity of the spatial location indicated by the cue was identical between experiments.
We found that the N2pc elicited by the target in Experiment 3 was significantly larger than that found in Experiment 2. This finding is consistent with the explanation that the N2pc-like activity observed in Experiment 2 (and 1) was in fact the N2pc. When the N2pc was already focused on the target location in Experiment 2, less target-elicited N2pc activity was observed because the target information could be processed more quickly when it became available. In other words, a spatial attentional filter could be instantiated prior to the onset of the array such that when the information from the array arrived in the visual system a spatial shift of attention was unnecessary and just the suppression of the distractors indexed by a subset of the N2pc activity in ventral areas occurred (e.g., Hopf et al., 2000). Thus, the findings of Experiment 2 and 3 together support the conclusion that the N2pc-like activity observed in anticipation of the target array in Experiment 1 and 2 was in fact the N2pc.
General Discussion
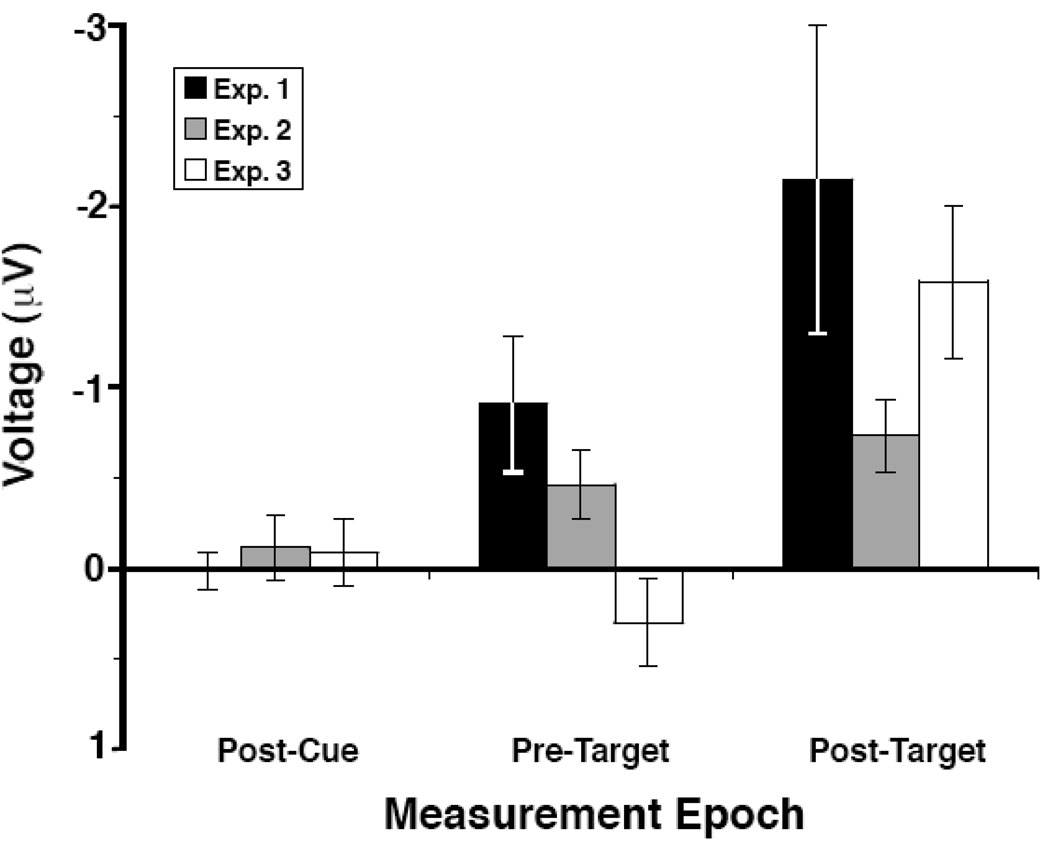
In Experiments 1 and 2, we found that observers shifted attention to the cued placeholder object in anticipation of the presentation of a target flanked by distractors. However, when we removed these placeholder objects in Experiment 3, no such anticipatory shift of attention was found (see Figure 4). These findings not only provide novel insights into how attention is deployed during cuing studies of visual attention but also inform us about the nature of the mechanism that the N2pc component measures.
Figure 4.
N2pc amplitude (contralateral-ipsilateral with respect to the cued-target location) from OL/R for the three measurement windows from Experiment 1 (black bars), 2 (gray bars), and 3 (white bars). The error bars show the standard error of the mean.
The first implication that our findings have is for the debate over whether early lateralized components following the presentation of a cue are related to shifting of attention to the anticipated target location or reflect the perceptual analysis of the cue stimulus itself. As stated before, van Velzen and Eimer (2003) proposed that the EDAN component previously reported was actually an N2pc elicited by attention shifts to the cue stimulus rather than to the cued location. They supported this hypothesis by showing that the early contralateral negativity was relative to the cue stimulus and not the hemifield being cued. In the present study, we further tested this hypothesis by presenting centrally located words or letters as cues. Stimuli presented on the midline between left and right visual fields are known to not elicit N2pc activity (Woodman & Luck, 2003b). Supporting the conclusions of van Velzen and Eimer (2003), we found no early posterior negativity contralateral to the cued hemifield following the presentation of the cue stimulus. The present findings contribute to the view that, when present, early posterior contralateral negativities following the presentation of a spatial cue are related to the processing of the lateralized cue itself.
The present study also demonstrates that the N2pc component can be elicited contralateral to an anticipated imperative stimulus flanked by distractors. Previously the N2pc component was typically observed in the context of visual search tasks (Luck et al., 1993; Luck, Girelli et al., 1997; Luck & Hillyard, 1995; Luck et al., 1994; Woodman, Kang, Rossi, & Schall, 2007; Woodman & Luck, 1999, 2003b). In such tasks, the entire array is presented simultaneously and the N2pc is observed when attention is shifted to a potential target item or the target itself. Here we show that an anticipatory N2pc component can be elicited prior to the actual presentation of the stimulus that requires discrimination. Because previous studies that used isolated targets did not observe N2pc-like activity, our findings support the hypothesis that the N2pc is related, in part, to distractor suppression (with a contribution due to target selection also, i.e., Hickey et al., in press). Finally, the present findings also support an assumption often made in the cognitive literature using cuing paradigms that attention is shifted to the cued location in advance of the actual target presentation (Posner, 1980).
Our final observation was the disappearance of the anticipatory N2pc when the cued locations were not marked by placeholder objects. This finding suggests that the attention mechanism indexed by the N2pc component selects objects and not simply spatial locations. When the visual array contained visible placeholder objects, the anticipatory N2pc was observed. However, when exactly the same task and procedure was used but the placeholder objects were removed, the pre-target N2pc was eliminated. In the previous studies of the N2pc using visual search tasks, it was not possible to dissociate whether objects were being selected or whether attention was selecting the spatial locations occupied by the objects. The present experiments suggest that the N2pc attention mechanism performs object-based selection and not purely location-based selection.
These findings may also be related to the role of the N2pc in distractor suppression. In Experiments 1 & 2, the N2pc selection mechanism may have been deployed to inhibit the surrounding placeholders in anticipation of additional distracting information. Thus, these findings build on a large body of evidence suggesting that visual attention mechanisms select entire objects and not simply locations in space (Duncan, 1984; O'Craven, Downing, & Kanwisher, 1999). It should be noted that the present findings are also consistent with hybrid models of object-based selection, such as the grouped array hypothesis (Vecera, 1994; Vecera, 1997; Vecera & Farah, 1994), in which attention mechanisms are essentially location based but that selected locations are determined by structure of the object that is attended. Further research will be needed to distinguish between these competing theoretical explanations.
Acknowledgements
We thank Adam Niese for help with data collection. This study was made possible by a National Research Service Award from the National Institute of Health to G.F.W. (F31 MH12995) and a grant from the National Institute of Mental Health (MH63001) to S.J.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Sgarlata AM, Kliestik J. Resolving visual interference during covert spatial orienting: Online attentional control through static records of prior visual experience. Journal of Experimental Psychology: General. 2005;134:192–206. doi: 10.1037/0096-3445.134.2.192. [DOI] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. Journal of Experimental Psychology: General. 1984;113:501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Egly R, Homa D. Sensitization of the visual field. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:778–793. doi: 10.1037//0096-1523.10.6.778. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalography and clinical Neurophysiology. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Collins J. Temporal course of selective attention. Journal of Experimental Psychology. 1969;80:254–261. doi: 10.1037/h0027268. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Rohrbaugh JW. Some factors determining efficiency of selective attention. American Journal of Psychology. 1970;83:330–342. [Google Scholar]
- Eriksen CW, Yeh Y-y. Allocation of attention in the visual field. Journal of Experimental Psychology: Human Perception & Performance. 1985;11(5):583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Yeh YY. Allocation of attention in the visual field. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Woldorff MG. Timing and sequence of brain activity in top-down control of visual-spatial attention. Public Library of Science: Biology. 2007;5:114–126. doi: 10.1371/journal.pbio.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter MR, Miller SL, Price NJ, LaLonde ME, Keyes AL. Neural processes involved in directing attention. Journal of Cognitive Neuroscience. 1989;1:223–237. doi: 10.1162/jocn.1989.1.3.223. [DOI] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. Electrophysiological indices of target and distractor processing in visual search. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21039. (in press) [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: Electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society: Biological Sciences. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf J-M, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, et al. Neural sources of focused attention in visual search. Cerebral Cortex. 2000;10:1233–1241. doi: 10.1093/cercor/10.12.1233. [DOI] [PubMed] [Google Scholar]
- Hopf J-M, Mangun GR. Shifting visual attention in space: An electrophysiological analysis using high spatial resolution mapping. Clinical Neurophysiology. 2000;111:1241–1257. doi: 10.1016/s1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3(3):284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hughes HC, Zimba LD. Spatial maps of directed visual attention. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:409–430. doi: 10.1037//0096-1523.11.4.409. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Benoit B, Nicolas R. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Research. 2008;1215:160–172. doi: 10.1016/j.brainres.2008.03.059. [DOI] [PubMed] [Google Scholar]
- Kiss M, van Velzen J, Eimer M. The N2pc component and its links to attention shifts and spatially selective visual processing. Psychophysiology. 2007;44 doi: 10.1111/j.1469-8986.2007.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Fan S, Hillyard SA. Attention-related modulation of sensory-evoked brain activity in a visual search task. Journal of Cognitive Neuroscience. 1993;5:188–195. doi: 10.1162/jocn.1993.5.2.188. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiology and human perception: An ambiguity resolution theory of visual selective attention. Cognitive Psychology. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological evidence for parallel and serial processing during visual search. Perception & Psychophysics. 1990;48:603–617. doi: 10.3758/bf03211606. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994a;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994b;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. The role of attention in feature detection and conjunction discrimination: An electrophysiological analysis. International Journal of Neuroscience. 1995;80:281–297. doi: 10.3109/00207459508986105. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mangun GR, Gazzaniga MS. Independent attentional scanning in the separated hemispheres of split-brain patients. Journal of Cognitive Neuroscience. 1994;6:84–91. doi: 10.1162/jocn.1994.6.1.84. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cerebral Cortex. 2007 doi: 10.1016/s0010-9452(08)70447-7. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, Green JJ. Isolating event-related potential components associated with voluntary control of visuo-spatial attention. Brain Research. 2008;1227:96–109. doi: 10.1016/j.brainres.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Mueller HJ, von Muehlenen A. Probing distractor inhibition in visual search: Inhibition of return (IOR) Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1591–1605. doi: 10.1037//0096-1523.26.5.1591. [DOI] [PubMed] [Google Scholar]
- Mulligan RM, Shaw ML. Attending to simple auditory and visual signals. Perception and Psychophysics. 1981;30:447–454. doi: 10.3758/bf03204840. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Miniussi C. The dynamics of shifting visuospatial attention revealed by event-related potentials. Neuropsychologia. 2000;38:964–974. doi: 10.1016/s0028-3932(00)00015-4. [DOI] [PubMed] [Google Scholar]
- Nunez PL. Electric Fields of the Brain. New York: Oxford University Press; 1981. [Google Scholar]
- O'Craven KM, Downing PE, Kanwisher N. fMRI evidence for objects as the units of attentional selection. Nature. 1999;401:584–587. doi: 10.1038/44134. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis DG, editors. Attention and Performance X. Hillsdale, New Jersey: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Serences J, Yantis S, Culberson A, Awh E. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. Journal of Neurophysiology. 2004;92:3538–3545. doi: 10.1152/jn.00435.2004. [DOI] [PubMed] [Google Scholar]
- van Velzen J, Eimer M. Early posterior ERP components do not reflect the control of attentional shifts toward expected peripheral events. Psychophysiology. 2003;40:827–831. doi: 10.1111/1469-8986.00083. [DOI] [PubMed] [Google Scholar]
- Vecera SP. Grouped locations and object-based attention: Comment on Egly, Driver, and Rafal (1994) Journal of Experimental Psychology: General. 1994;123(3):316–320. [Google Scholar]
- Vecera SP. Grouped arrays versus object-based representations: Reply to Kramer et al. (1997) Journal of Experimental Psychology: General. 1997;126:14–18. doi: 10.1037//0096-3445.126.1.3. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Farah MJ. Does visual attention select objects or locations? Journal of Experimental Psychology: General. 1994;123:146–160. doi: 10.1037//0096-3445.123.2.146. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004 April 15;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Kang M-K, Rossi AF, Schall JD. Nonhuman primate event-related potentials indexing covert shifts of attention. Proceedings of the National Academy of Sciences. 2007;104:15111–15116. doi: 10.1073/pnas.0703477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Dissociations among attention, perception, and awareness during object-substitution masking. Psychological Science. 2003a;14:605–611. doi: 10.1046/j.0956-7976.2003.psci_1472.x. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003b;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Tsuchiya H, Kobayashi S. Electroencephalographic activity associated with shifts of visuo-spatial attention. Brain. 1994;117:553–562. doi: 10.1093/brain/117.3.553. [DOI] [PubMed] [Google Scholar]
- Yantis S. Attentional capture in vision. In: Kramer AF, Coles MGH, Logan GD, editors. Converging Operations in the Study of Selective Attention. Washington, DC: American Psychological Association; 1996. pp. 45–76. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- Zimba LD, Hughes HC. Distractor-target interactions during directed visual attention. Spatial Vision. 1997;2:117–149. doi: 10.1163/156856887x00105. [DOI] [PubMed] [Google Scholar]