Summary
Although it is well known that in vivo radiation depletes immune cells via the Bcl-2 apoptotic pathway, a more nuanced analysis of the changes in the balance of immune cell subsets is needed to understand the impact of radiation on immune function. We show the balance of T cell subsets changes after increasing single doses of total body irradiation(TBI) or after fractionated irradiation of the lymphoid tissues(TLI) of mice due to differences in radioresistance and Bcl-2 expression of the NKT cell and non-NKT subsets to favor CD4+Bcl-2hi NKT cells. Reduction of the Bcl-2lo mature T cell subsets was at least 100 fold greater than that of the Bcl-2hi subsets. CD4+ NKT cells upregulated Bcl-2 after TBI and TLI and developed a Th2 bias after TLI, while non-NKT cells failed to do so. Our previous studies showed TLI protects against graft versus host disease(GVHD) in wild type, but not in NKT cell deficient mice. The present study shows that NKT cells have a protective function even after TBI, and these cells are 10 fold more abundant after an equal dose of TLI. In conclusion differential expression of Bcl-2 contributes to the changes in T cell subsets and immune function after irradiation.
Keywords: Natural Killer T Cells, Transplantation, Irradiation, Apoptosis
Introduction
T lymphocytes remaining in the host after radiation conditioning for hematopoietic cell transplantation can reject allogeneic bone marrow grafts[1, 2], and regulate graft versus host disease (GVHD)[3–9]. Whereas residual conventional T lymphocytes mediate graft rejection, residual host regulatory T cells such as natural killer T (NKT) cells and CD4+CD25+ Treg cells suppress graft rejection and GVHD[3–9]. T cells are highly sensitive to radiation induced cell death that is mediated by the p53/Bcl-2 apoptotic pathway[10, 11]. Early in the pathway pro-apoptotic genes such as Bax and Bad are activated, and the pathway can be blocked by anti-apoptotic genes such as Bcl-2 and Bcl-xL[10–14]. Thymocytes from mice with inactivated Bcl-2 genes are markedly more sensitive to radiation than those from wild type mice[15], and expression of Bcl-2 transgenes induces marked resistance of thymocytes and their early progenitors to TBI or in vitro radiation[16, 17]. However, no previous studies have determined whether differences in Bcl-2 expression among mature T cell subsets in wild type mice results in a changed balance in the subsets after in vivo irradiation.
We have reported that splenic NKT cells become the predominant subset among all T cells in hosts given a conditioning regimen of multiple small doses of irradiation targeted to the spleen, lymph nodes, and thymus (TLI) that facilitates tolerance to organ and bone marrow transplants and reduces GVHD of mice and humans[6, 7, 18–20]. Changes in the balance of NKT and non-NKT cell subsets after conditioning were critical in achieving tolerance and GVHD prevention. TLI administered over 3 weeks (total dose of 4,080 cGy) caused a reduction of about 300 fold in the absolute number of splenic non-NKT cells but splenic NKT cells were reduced only about 10 fold[7]. The results are consistent with other reports that NKT cells constitute a T cell subset that is uniquely resistant to apoptosis due to both high levels of constitutive anti-apoptotic gene expression as well as to the rapid upregulation of anti-apoptotic genes after in vitro exposure to glucocorticoids or after in vitro irradiation[21, 22]. However, these short-term in vitro studies of cell death cannot predict the multiple complex changes in the balance of T cell subsets after in vivo irradiation, including differences in NKT and non-NKT cell renewal in vivo from progenitors that survive radiation. NKT cells have unique features that are not shared with conventional T cells including the TCR recognition of the antigen presenting molecule CD1d instead of class I and Class II MHC antigen presenting molecules[23–28]. NKT cells are considered to be part of the innate immune system, since they have invariant Vα14Jα18 TCRα chain receptors that recognize endogenous glycolipid ligands[25, 27].
In the current study, we examined the mechanisms of the change in the balance of NKT cells and non-NKT cells to graded single doses of TBI and to TLI. The results show that untreated T cells can be divided into Bcl-2hi and Bcl-2lo subsets, and that the minor subset of CD4+ NKT cells upregulated Bcl-2 expression and was resistant to cell death induced by TBI and TLI. The latter subset became the major T cell subset in the spleen as radiation doses increase. The loss of Bcl-2lo NKT and non-NKT cells was at least 100 fold greater than Bcl-2hi cells after high doses of TBI, and there was minimal cell renewal during the changes in the balance of subsets. The Bcl-2hi NKT cells that survived after TBI or TLI attenuated GVHD, and the absolute number in the spleen after TLI was 10 fold higher than after TBI. This is the first report to show that differences in Bcl-2 expression among mature T cell subsets are linked to changes in the balance of subsets after in vivo irradiation.
Results
TBI induces a marked increase in the percentage of NKT cells among all splenic T cells
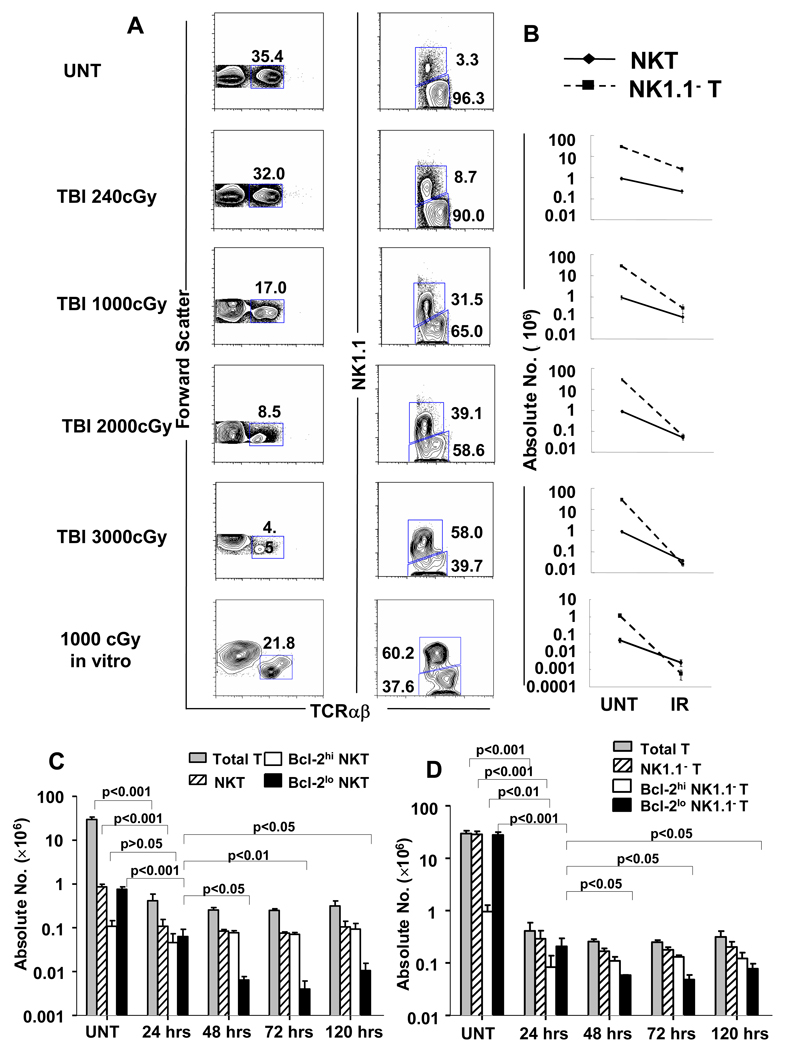
Since our previous reports showed that TLI targeted to the spleen, lymph nodes and thymus of mice altered the balance of T cell subsets in the spleen to favor NKT cells, we determined whether the altered balance is observed also after single doses of TBI. Figure 1A compares the immunofluorescent staining patterns of spleen cells from untreated C57BL/6 mice to those of mice given single doses of 240, 1,000, 2,000 and 3,000 cGy TBI. Cells were stained for TCRαβ and NK1.1 markers 24 hours after irradiation, and the left column of flow cytometry profiles shows the progressive reduction in the percentage of TCRαβ+ cells among cells that were gated by light scatter to contain lymphocytes. Whereas TCRαβ+ T cells represented about 35% (mean ± SD, 34% ± 1%; n=8) of splenic lymphocytes in untreated mice, only 4.5% (mean 6% ±2%) of lymphocytes were T cells after 3,000 cGy TBI (p<0.001) (Figure 1A). Propidium iodide gating was used to analyze only residual live cells.
Figure 1. The NKT cell subset becomes predominant among all T cells after TBI in C57BL/6 mice.
(A) Left column; representative flow cytometric analyses of staining for TCRαβ versus forward scatter in the spleen of untreated C57BL/6 mice or 24hrs after 240, 1000, 2000 or 3000 cGy of TBI. The bottom panel shows staining of C57BL/6 spleen cells cultured for 24 hours after 1000 cGy in vitro irradiation for comparison. Boxes enclose TCRαβ+ T cells, and percentages within boxes are given. Right column; two color analyses of TCRαβ versus NK1.1 markers on gated TCRαβ+ T cells from the left column. Boxes enclose NK1.1+ TCRαβ+ T cells (upper box) and NK1.1−TCRαβ+ (lower box). The mean percentage ± SD of NKT cells among all T cells in the spleen of untreated mice was 2.93 ± 0.405, and increased to 8.29 ± 0.630; p<0.0001, 26.00 ± 5.237; p<0.0001, 45.60 ± 6.789; p<0.0001, and 58.35 ± 8.581; p<0.0001 in mice given 240, 1,000, 2,000, 3,000 cGy respectively. There are 8 to 10 mice per group. (B) The mean (±SD) absolute numbers of NK T cells and NK1.1− T cells (non-NKT cells) in the spleen of untreated mice (UNT) and in the spleen 24 hours after each dose of irradiation (IR) are shown on a logarithmic scale. (C) Comparison of mean (±SD) absolute numbers of total T cells, total NKT cells, Bcl-2hi and Bcl-2lo NKT cells in the spleen at different time points after irradiation. (D) Comparison of mean (±SD) absolute numbers of total T cells, NK1.1− T, Bcl-2hi and Bcl-2lo NK1.1− T cells at different time points in the spleen. There are 5 to 10 mice per group. The data are representative of three independent experiments.
Associated with the progressive reduction in the percentage of T cells was a progressive increase in the percentage of NK1.1+ TCRαβ+ NKT cells among the gated TCRαβ+ T cells as shown in the right column of cytometry profiles in Figure 1A. The NK1.1+ TCRαβ+ phenotype was used to identify both type I and type II NKT cells whereas CD1d tetramer staining identifies only type I NKT cells with the invariant TCRα chain[27]. In untreated mice, the percentage of NKT cells among all T cells was about 3% (mean 2.9% ± 0.4%) whereas the percentage was 58% (mean 58% ± 9%) in mice given 3,000 cGy (p<0.001). The marked increase in the percentage of NKT cells can be explained by analyzing the mean absolute numbers of NKT cells and NK1.1− T cells (non-NKT cells) in the spleen as shown in the column of graphs in Figure 1B. In untreated mice, the mean number of total T cells was about 30×106 and the mean number of NKT cells was about 1×106. After 1,000 cGy TBI, the mean absolute number of non-NKT cells was reduced about 100 fold (p<0.001) whereas the absolute number of NKT cells was reduced about 10 fold (p<0.01). This accounts for the 10-fold rise in the percentage of NKT cells among all T cells from about 3% to 32%. When the dose of TBI was increased to 3,000 cGy, the number of non-NKT cells fell almost 1,000 fold, and the number of NKT cells fell about 20 fold such that for the first time, the mean number of NKT cells exceeded the number of non-NKT cells in the spleen.
In further experiments, spleen cell suspensions were irradiated with 1,000 cGy in vitro, and the percentages and absolute numbers of NKT cells and non-NKT cells were determined. As shown in Figure 1A, the percentage of NKT cells among all T cells rose to about 60% after irradiation, and the change in the absolute number of non-NKT cells was about 20 fold more than that of NKT cells. After irradiation, the mean absolute number of NKT cells was significantly greater than non-NKT cells (p< 0.01).
The effects of irradiation on T cell subsets were also determined at 48, 72 and 120 hours after 1000 cGy of TBI in addition to 24 hours. The percentage of NKT cells showed similar increases at the three time points (data not shown). Figure 1C shows the comparison of the absolute numbers of total T cells and NKT cells at the different time points. After the marked reduction at 24 hours, there were no significant differences (p>0.05) in the absolute number of total T cells or in the absolute number of NKT or non-NKT cells between 24, 48, 72 and 120 hours. (Figure 1C and 1D).
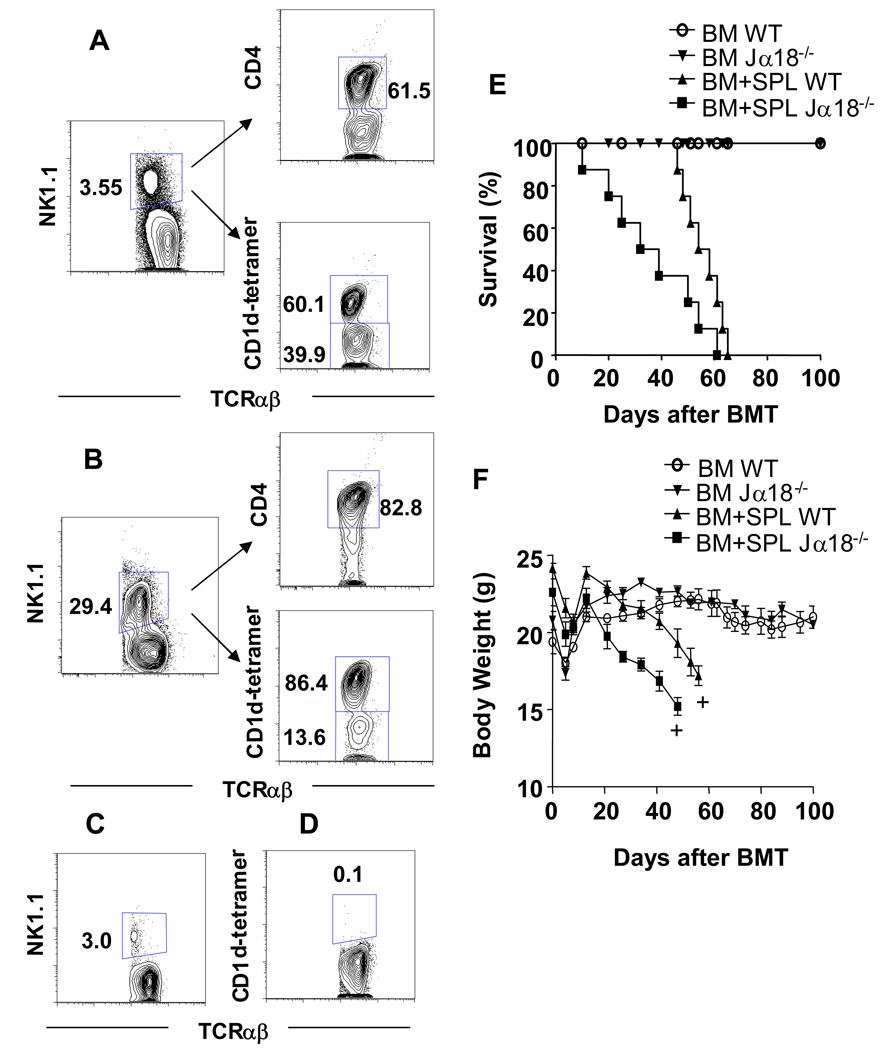
In order to determine the subsets of NK1.1+TCRαβ+ T cells in the spleen before and after 1,000 cGy TBI, gated TCRαβ+ cells were further gated for NK1.1+ cells (Figure 2A), and the percentage of CD4+ and CD1d-tetramer+ cells among the gated cells was determined by multicolor staining and analysis. About 62% of untreated and 83% of irradiated NK1.1+ TCRαβ+ T cells were CD4+ (Figure 2A), and the remaining NKT cells were CD4−CD8− (data not shown).
Figure 2. Host invariant NKT cells that remain after TBI conditioning contribute to protection against GVHD.
(A) Gated TCRαβ+ spleen cells in untreated C57BL/6 mice were stained for NK1.1 versus TCRαβ in the left panel, and gated NK1.1+TCRαβ+ cells were stained for CD4 versus TCRαβ in the right upper panel and CD1d-tetramer versus TCRαβ in the right lower panel. (B) The same analysis was performed using C57BL/6 mice 24 hours after 1,000 cGy TBI. (C, D) Jα18−/− mice were given 1,000 cGy TBI and similar analyses of TCRαβ versus NK1.1 or CD1d tetramer on gated TCRαβ+ cells (as in Fig A, B) are shown for comparison. (E) Lethally irradiated (TBI 900 cGy) wild type C57BL/6 or NKT deficient Jα18−/− C57BL/6 host mice were given 50×106 whole bone marrow (BM) and 60×106 splenocytes (SPL) intravenously from wild type BALB/c donors. Control wild type mice or NKT deficient Jα18−/− C57BL/6 host mice were given 900 cGy TBI followed by 50×106 BM alone. Survival of irradiated hosts after transplantation is shown. (F) Mean body weights (±SE) of host mice given BM plus SPL or BM alone as in panel E. There were 10 hosts in each group. Analysis was stopped for a given group when there were two hosts remaining (+).
When NK1.1+ TCRαβ+ gated residual cells were stained with a CD1d tetramer that identified only invariant NKT cells[27], about 60% of untreated NKT cells were tetramer+, and 86% of irradiated NKT cells were tetramer+. As shown in Figure 2C, the percentage of splenic NK1.1+TCRαβ+ T cells amongst all T cells in invariant NKT deficient Jα18−/− mice given 1,000 cGy TBI was reduced to about 3% from 29% observed in wild type mice (Figure 2B). The residual cells in Jα18−/− mice are type II non-invariant NKT cells[29, 30]. The percentage of CD1d tetramer+ T cells among all T cells in irradiated Jα18−/− mice was 0.1% (Figure 2D). In summary, the large majority of NKT cells after irradiation are invariant CD4+ NKT cells.
NKT cell deficient mice are more sensitive to GVHD than wild type mice
We have reported that the NKT cells protect against acute GVHD in hosts that have been conditioned with TLI and anti-thymocyte serum (ATS) [6, 9]. Since the percentage of NKT cells is markedly increased among all T cells after a single dose of 1,000 cGy of TBI, we compared the severity of GVHD in wild type and invariant NKT cell deficient Jα18−/− mice after allogeneic bone marrow transplantation using a similar dose of myeloablative conditioning with 900 cGy of TBI. In these experiments, wild type C57BL/6 host mice were injected i.v. with 50×106 bone marrow cells and 60×106 spleen cells from wild type BALB/c donor mice within 24 hours after irradiation. Control wild type or Jα18−/− host mice received wild type bone marrow cells alone or no donor cells.
Figures 2E and 2F show the changes in survival and body weight in the wild type and Jα18−/− host mice given transplants. Control wild type and Jα18−/− mice given only bone marrow cells showed 100% survival over a 100 days observation period. The latter mice gained weight after the transplantation procedure, and weight remained relatively constant thereafter (Figure 2F). There were no significant differences (p>0.05) between wild type and Jα18−/− mice given marrow alone. Host mice given no donor cells all died by 14 days (data not shown). Host wild type mice given both bone marrow and spleen cells showed progressive weight loss after about 15 days associated with features of GVHD such as hair loss, hunched back and swollen face. There was a statistically significant (p<0.01) reduction in mean body weight of wild type hosts given bone marrow and spleen cells as compared to bone marrow cells alone as judged by the student t test by day 50.
All of the wild type hosts given bone marrow and spleen cells died by day 65 and the difference in survival between the latter group and the control group given marrow alone was statistically significant (p<0.001) as judged by the log rank test (Figure 2E). Interestingly, the Jα18−/− hosts given marrow and spleen cells died more rapidly than the wild type hosts given marrow and spleen cells (p<0.05), and their weight loss after day 20 was significantly greater than that of the wild type group (p<0.05). Jα18−/− hosts given marrow and spleen cells had significantly reduced survival (p<0.001) and more severe weight loss (p<0.01) than the Jα18−/− hosts given marrow cells alone. Thus GVHD was more severe in the NKT cell deficient as compared to wild type mice given a single dose of TBI.
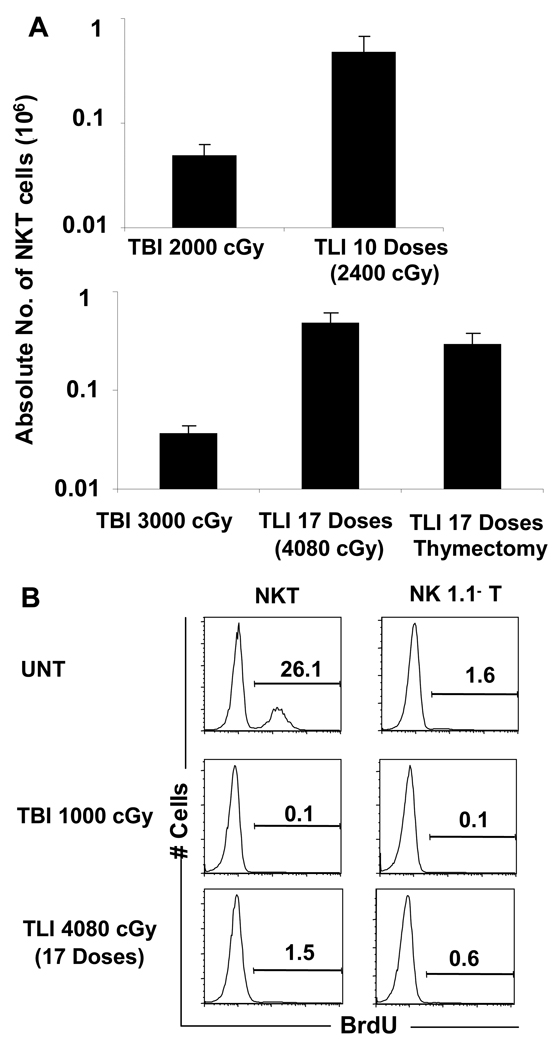
In contrast to the uniform death of C57BL/6 hosts after marrow and spleen transplantation with TBI conditioning, wild type hosts conditioned with TLI and ATS all survived for at least 100 days without GVHD[6, 9]. Protection against GVHD was lost in Jα18−/− hosts[9]. We compared the NKT cell composition in the spleen of wild type C57BL/6 mice given TLI (Figure 3A) to the hosts given TBI (Figure 1A). The percentage of NKT cells among all T cells in the hosts given 10 doses of TLI of 240 cGy each was about 30%, and about 46% in mice given 17 doses of TLI of 240 cGy each (Figure 3A). There was less than a 5-fold reduction (p<0.05) in the absolute number of splenic NKT cells after 10 or 17 doses of TLI (Figure 3A). In contrast the absolute number of non-NKT cells fell more than 50 fold (p<0.001) (Figure 3A). Thus, the increase in the percentage of NKT cells among all T cells was similar after TLI and TBI.
Figure 3. Lack of rapid NKT cell renewal from thymic or extrathymic sources after TLI or TBI.
(A) Yields of NKT cells from the spleen of non-thymectomized or thymectomized (labeled Thymectomy) C57BL/6 mice given 10 doses or 17 doses of TLI (240 cGy each) or single dose of 2,000 cGy or 3,000 cGy TBI are compared. The cells were harvested 24 hours after the last treatment. Spleen cells were stained and analyzed as in Figure 1, and the mean absolute numbers are sown. Bars represent the means of 5 to 10 mice per group, and brackets shows SD. (B) Three doses of BrdU were administered intraperitoneally every 8 hrs and splenocytes were harvested at 24 hrs (8 hrs after third injection) after completion of irradiation of C57BL/6 mice. Mice were either untreated, given TBI (1,000 cGy) or 17 doses of TLI (4080 cGy). Panels show one color staining of intracellular BrdU on gated NK1.1+ TCRαβ+ or NK1.1− TCRαβ+ cells.
Figure 3A shows that after 2,000 cGy TBI, the mean absolute number of splenic NKT cells (0.05×106 cells) was about 10 fold lower than that (0.5×106 cells) after 2,400 cGy TLI (p<0.01). Similarly, the absolute number of splenic NKT cells after 3,000 cGy TBI was about 10 fold lower than after 4,000 cGy TLI (p<0.001). It is possible that rapid renewal of NKT cells from progenitors in the thymus contributed to the residual splenic NKT cells after TLI. However, the mean absolute number of NKT cells in the spleen of TLI treated euthymic (mean 0.5×106 ± 0.1×106) or thymectomized mice (mean 0.3×106 ± 0.1×106) were not significantly different (p>0.05) when measured 24 hours after completion of irradiation (Figure 3A).
We assessed the turnover of NKT cells and non-NKT cells in untreated and irradiated mice by labeling the cells with BrdU. The label was given by three intraperitoneal injections every 8 hours for 24 hours after irradiation, and cells were stained immediately after the 24 hour period for intracellular BrdU incorporation. As shown in Fig. 3B, about 26% (mean 25% ± 4%) of gated NKT cells in the spleen of unirradiated mice were BrdU labeled, and less than 2% (mean 1.6% ± 0.3%) of non-NKT cells were labeled (p<0.001). When labeling was done during the 24-hour period after 1,000 cGy TBI, then staining of both NKT cells and non-NKT cells was about 0.1% (p>0.05). After 17 doses of TLI, the percentage of BrdU labeled splenic NKT cells was reduced to less than 2%, even though considerable shielding of the bone marrow occurs during the procedure.
Marked Increases in the percentage of Bcl-2hi NKT cells and Bcl-2hi non-NKT cells After TBI
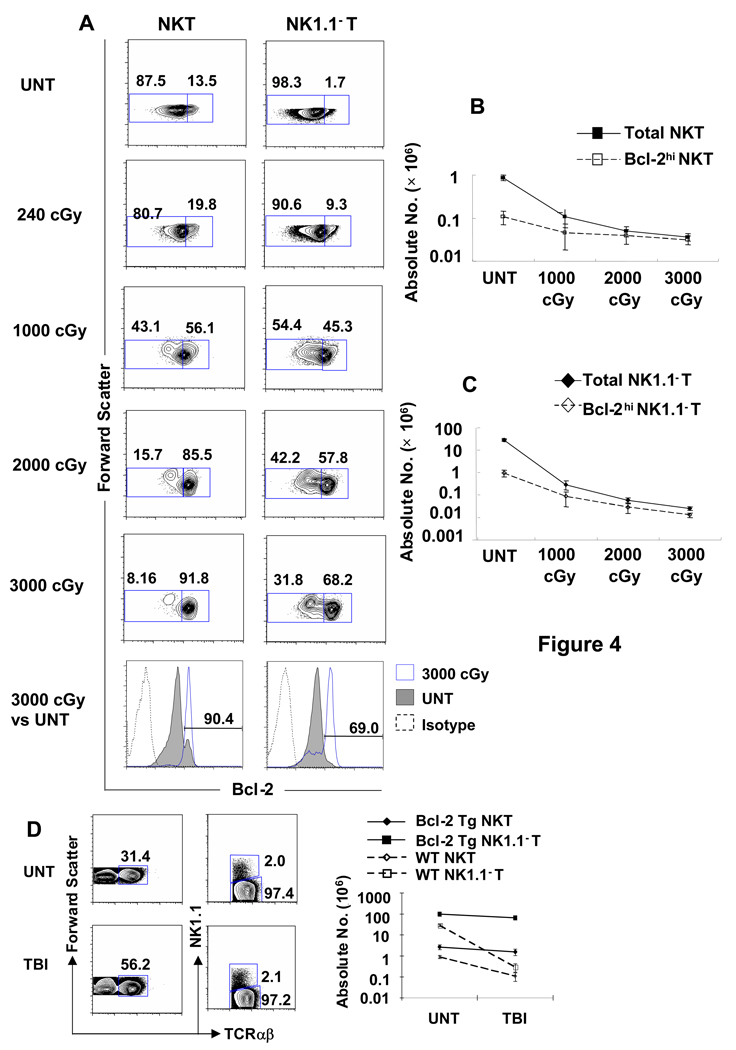
Figure 4A shows that there was a progressive increase in the percentage of gated NKT cells and gated non-NKT cells in the spleen that stained for high levels of intracellular Bcl-2 after progressive increases in the dose of TBI administered to wild type C57BL/6 mice. The left column of cytometry profiles show that about 12.5% (mean 11% ± 3%) of NKT cells in the untreated spleen expressed the Bcl-2hi phenotype, and about 92% (mean 85% ± 8%) expressed the Bcl-2hi phenotype after 3,000 cGy TBI. The thresholds for distinguishing Bcl-2hi and Bcl-2lo cells were determined by single color analysis of Bcl-2 staining of gated NKT cells in untreated mice shown in the shaded profile of the lower left panel. Two clear peaks were observed, and the threshold was set between the two for further analyses of two color staining. The intensity of staining of almost all Bcl-2lo cells was above background as judged by the profile obtained after staining with an irrelevant isotype matched control mAb (Figure 4A). About 1.7% of non-NKT cells were Bcl-2hi in untreated mice and about 68% were Bcl-2hi after 3,000 cGy TBI. Thus the percentage of NKT cells that constitutively expressed the Bcl-2hi phenotype was about 8 to 10 fold higher than that of the non-NKT cells (12.5% versus 1.7%), and the percentage of Bcl-2hi cells rose markedly in both subsets after irradiation (Figure 4A).
Figure 4. Marked increase in intracellular Bcl-2 expression in NKT and NK1.1− T cells that survive after irradiation.
(A) Splenocytes were harvested from untreated C57BL/6 mice or from mice treated with TBI 24 hrs after irradiation, stained for NK1.1 vs TCRαβ, fixed and permeabilized, and stained for intracellular Bcl-2. Dead cells were excluded from the analysis by adding the dye EMA to the staining mixture, and gating on EMA− cells. The left column of panels show the representative analyses of light scatter versus Bcl-2 amongst gated NK1.1+ TCRαβ+ T cells and the right column shows the analyses amongst gated NK1.1− TCRαβ+ T cells. Boxes enclosed either Bcl-2lo (left) or Bcl-2hi (right) cells, and percentages in boxes are shown. The mean percentage of Bcl-2hi NKT cells among all NKT cells in the spleen of untreated mice was 10.9 % ± 3.26%, and increased to 18.7% ± 0.75%; p<0.001, 39.3% ± 14.55%; p<0.001, 77.8% ± 16.57%, p<0.0001, 84.9% ± 8.49%; p<0.0001 in mice given 240, 1,000, 2,000 and 3,000 cGy respectively. There are 8 to 10 mice per group. Thresholds for Bcl-2hi cells were set between the two peaks of Bcl-2 staining after one color analysis of gated NKT cells from untreated mice shown in lower left panel (shaded profile). One color profile of staining with irrelevant isotype matched mAb is shown by dashed line. (B, C) The mean absolute number of total or Bcl-2hi NKT cells and NK1.1− T cells in the spleen of untreated mice (UNT) and in the spleen 24 hours after each dose of TBI (1,000, 2,000 and 3,000 cGy) are shown on a logarithmic scale. There are 10 to 20 mice in each group. (D) The percentage of NKT cells in the spleen of human Bcl-2 transgenic (Tg) C57BL/6 mice fails to increase after irradiation. Bcl-2 transgenic mice were analyzed for the percentage of NKT cells among all T cells in untreated mice or after 1,000 cGy of TBI. Left panels show forward scatter versus TCRαβ; right panels show NK1.1 versus TCRαβ on gated TCRαβ+ cells; graph shows changes in mean absolute numbers of NK1.1− T and NKT cells. Untreated and irradiated wild type mice are shown for comparison. The data are representative of three independent experiments.
Figure 4B shows the changes in the absolute number of total NKT cells and Bcl-2hi NKT cells in the spleen before and after increasing doses of TBI. Whereas the mean absolute number of total NKT cells was about 8 fold higher than that of Bcl-2hi NKT cells before irradiation (0.9×106 versus 0.1×106), almost all NKT cells were Bcl-2hi NKT cells 24 hours after 2,000 or 3,000 cGy TBI. Similarly, the mean absolute number of all non-NKT cells was about 30 fold higher than that of Bcl-2hi non-NKT cells before irradiation, and the majority after 3,000 cGy TBI were Bcl-2hi non-NKT cells (0.036 × 106 total non-NKT cells versus 0.031× 06 Bcl-2hi non-NKT cells) (Figure 4C). The changes after irradiation were the result of a greater reduction in the absolute number of NKT cells and non-NKT cells with the Bcl-2lo phenotype as compared to those with the Bcl-2hi phenotype that were already apparent at 24 hours (Figure 1C and 1D). These changes became more pronounced after 48, 72 and 120 hours because the Bcl-2lo subset had a significant further decline after 24 hours (p<0.05 or 0.01) whereas Bcl-2hi cells increased slightly (Figure 1C and 1D). Conversion from Bcl-2lo to Bcl-2hi cells may contribute to these changes. The 72 hours time point was the nadir of Bcl-2lo NKT and non-NKT cells during the 120 hours period after irradiation.
Whereas, the mean absolute number of Bcl-2hi NKT cells was reduced by less than 2 fold 72 hours after 1,000 cGy TBI as compared to untreated values (0.10×106 to 0.07×106; p> 0.05), the mean absolute number of Bcl-2lo NKT cells was reduced by about 200 fold (0.75×106 to 0.004×106; p<0.001). Similarly, the mean absolute number of Bcl-2hi non-NKT cells was reduced about 7 fold (0.95×106 to 0.13×106; p<0.01), and that of Bcl-2lo non-NKT cells was reduced about 500 fold (27.8×106 to 0.04×106; p<0.001) (Figure 1D). Thus, the increased percentage of NKT cells among all T cells after irradiation represents a changed balance between Bcl-2hi NKT and Bcl-2hi non-NKT cells, since few Bcl-2lo T cells remain after irradiation. The changed balance favors the NKT cells because of the higher percentage of Bcl-2hi cells among NKT cells before irradiation (~13%) as compared to non-NKT cells (~2%), and the less than 2 fold reduction of Bcl-2hi NKT cells at the 72 hours nadir after irradiation as compared to the 7 fold reduction in Bcl-2hi non-NKT cells (Figure 1C). The latter difference is related to the upregulation of Bcl-2 among CD4+ NKT cells after irradiation (see below)
Bcl-2 over-expression prevents alterations of T cell subsets after TBI
In order to determine the effect of Bcl-2 over-expression on the resistance of splenic NKT cells and non-NKT cells to radiation induced loss, we examined the changes in the percentage and absolute numbers of the two T cell subsets before and after 1,000 cGy TBI in C57BL/6 mice that expressed a human Bcl-2 transgene under the control of the mouse class I-MHC gene promoter region[17]. As shown in Figure 4D, about 2% of the total T cells in the untreated transgenic mice were NKT cells before irradiation, and the percentage of NKT cells remained the same after irradiation as judged by staining of spleen cells for the NK1.1 versus TCRαβ markers. Only a minimal change in the absolute number of transgenic NKT cells and transgenic non-NKT cells occurred after irradiation as compared to the 100 fold reduction in non-NKT cells and 10 fold reduction in NKT cells in the wild type mice (Figure 4D).
Bcl-2 but not Bcl-xL, expression is upregulated in CD4+ NKT cells after irradiation
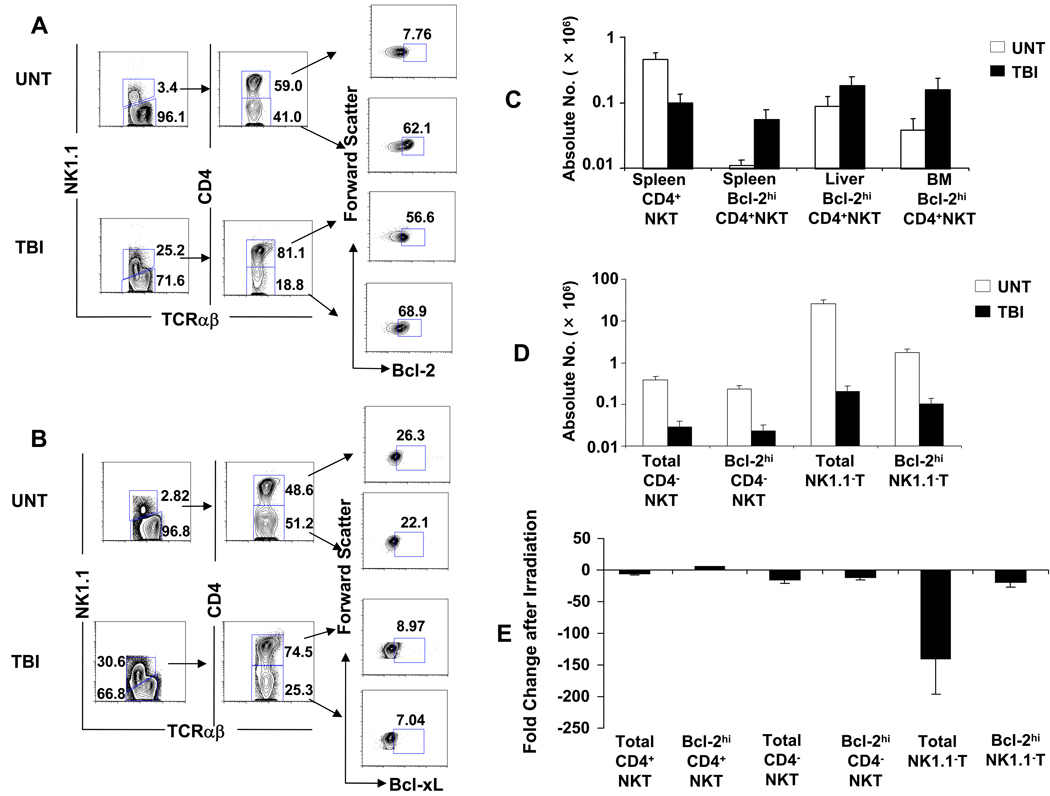
Since NKT cells contain two major subsets that are CD4+ and CD4− CD8−[30], the changes in Bcl-2 expression after 1,000 cGy TBI among these splenic NKT cell subsets were examined separately. Figure 5A shows that in untreated C57BL/6 mice, the gated NK1.1+ TCRαβ+ T cells can be separated into CD4+ cells that constitute about 59% (mean 63% ± 3%) of NKT cells, and CD4− cells that constitute 41% (mean 38% ± 3%). The CD4− subset is double negative (CD4−CD8−) when staining for CD8 receptors was performed (data not shown). Unexpectedly, the constitutive intracellular levels of Bcl-2 in untreated mice was considerably higher in the CD4−CD8− subset than the CD4+ subset using the staining threshold established in Figure 4. Bcl-2hi cells accounted for about 62% (mean 62% ± 6%) of CD4− CD8− NKT cells, the Bcl-2hi cells accounted for only about 8% (mean 7% ± 1%) of CD4+ NKT cells (Figure 5A).
Figure 5. Marked increase in Bcl-2 but not Bcl-xL expression in CD4+ NKT cells that survive after TBI.
(A) Analysis of Bcl-2 expression in CD4+ and CD4−CD8− NKT cell subsets after irradiation. Splenocytes were harvested from untreated C57BL/6 mice or from mice 24 hours after 1,000 cGy TBI. Left panels show NK1.1 versus TCRαβ on gated TCRαβ+ cells; middle panels show CD4 versus TCRαβ on gated NKT cells; right panels show Bcl-2 expression amongst gated CD4+ NKT or CD4−CD8− NKT cells using threshold established in Figure 4. (B) Analysis of Bcl-xL expression in NKT subsets is shown with boxes enclosing cells with staining intensity above background with isotype matched control mAb. (C) Analysis of mean absolute numbers of total or Bcl-2hi CD4+ NKT cell subsets in spleen, liver and bone marrow of untreated mice or after 1,000 cGy TBI. (D) Analysis of mean absolute numbers of total or Bcl-2hi CD4− NKT or NK1.1− T cell subsets. (E) Change in ratio of absolute numbers of different T cell subsets before and 24 hours after 1,000 cGy TBI. There are 10 mice in each group. The data are representative of three independent experiments.
After 1,000 cGy TBI, the CD4+ NKT cells constituted about 81% (mean 80% ± 2%) of all NKT cells, and the percentage of Bcl-2hi cells within this subset rose markedly from about 7% (mean 7% ± 1%) in the untreated mice to about 57% (mean 61% ± 5%) in the irradiated mice (Figure 5A). The increase in Bcl-2hi cells among CD4−CD8− NKT cells was considerably smaller after irradiation (62.1% versus 68.9%). Thus, the rise in the percentage of Bcl-2hi among all NKT cells after irradiation (Figure 4) was mainly due to increases within the CD4+ NKT cell subset.
In order to determine whether irradiation induces an increase in the levels of other intracellular anti-apoptotic proteins, the two subsets of NKT cells in untreated and irradiated mice were examined for the expression of intracellular Bcl-xL. In contrast to the findings with Bcl-2, the constitutive expression of Bcl-xL was similar between the CD4+ and CD4−CD8− NKT cells (26.3% and 22.1% respectively) using isotype matched control mAb to determine positive staining (Figure 5B). Moreover, the levels of Bcl-xL fell after irradiation such that CD4+ NKT cells contained about 9% of Bcl-xLhi cells, and the CD4−CD8− NKT cells contained about 7%.
We also compared the percentage and absolute numbers of CD4+ NKT cells with high levels of Bcl-2 in the spleen, liver, and bone marrow of untreated mice versus mice treated with 1,000 cGy TBI. As shown in Figure 5C, the mean absolute number of total CD4+ NKT cells (0.46×106) was about five times higher in the untreated mouse spleen as compared to the irradiated mouse spleen (0.09×106). Despite this reduction of total CD4+ NKT cells in the spleen after irradiation, the mean absolute number of Bcl-2hi CD4+ NKT cells rose from 0.01 × 106 in the untreated spleen to 0.06×106 in the irradiated spleen (Figure 5C). There was an increase in the percentage of total NKT cells among all T cells in the liver and bone marrow after 1,000 cGy TBI (See S. Figure 1 in Supplementary Material), and an associated increase in the percentage of Bcl-2hi CD4+ NKT cells among all CD4+ NKT cells (See S. Figure 2 in Supplementary Material). This resulted in a significant rise in the mean absolute number of Bcl-2hi CD4+ NKT cells after irradiation in the bone marrow (0.04×106 versus 0.16×106; p<0.05), and to a lesser extent in the liver (0.09×106 versus 0.18×106; p>0.05) (Figure 5C). The rise in the mean absolute number of Bcl-2hi CD4+ NKT cells in all three tissues coupled with the lack of NKT cell turnover (Figure 3) indicates that there was an upregulation of Bcl-2 expression after irradiation, and Bcl-2lo cells converted to Bcl-2hi cells.
Whereas there was a significant increase in Bcl-2hi CD4+ NKT cells after irradiation, there was about a 10-fold decrease in Bcl-2hi CD4−CD8− NKT cells (p<0.001) and Bcl-2hi NK1.1− TCRαβ+ T cells (p<0.001) after irradiation (Figure 5D). Figure 5E compares the ratios of the number of the different T cell subsets in the spleen with or without irradiation. The cells most potently affected by irradiation were those in the total non-NKT cell subset that fell almost 150 fold after 1,000 cGy TBI. Among the latter cells the Bcl-2hi non-NKT cells were more resistant to cell death and fell about 20 fold (p<0.001). The relative decrease in the number of total CD4− CD8− NKT cells or Bcl-2hi CD4−CD8− NKT cells was not significantly different from that of the Bcl-2hi non-NKT cells (p>0.05). The unique rise in the Bcl-2hi CD4+ NKT cells was significantly different from the change in all the other subsets (p<0.001) (Figure 5E).
Changes in the levels of the pro-apoptotic Bax and anti-apoptotic Bcl-2 gene expression in NKT cells and non-NKT cells after TBI
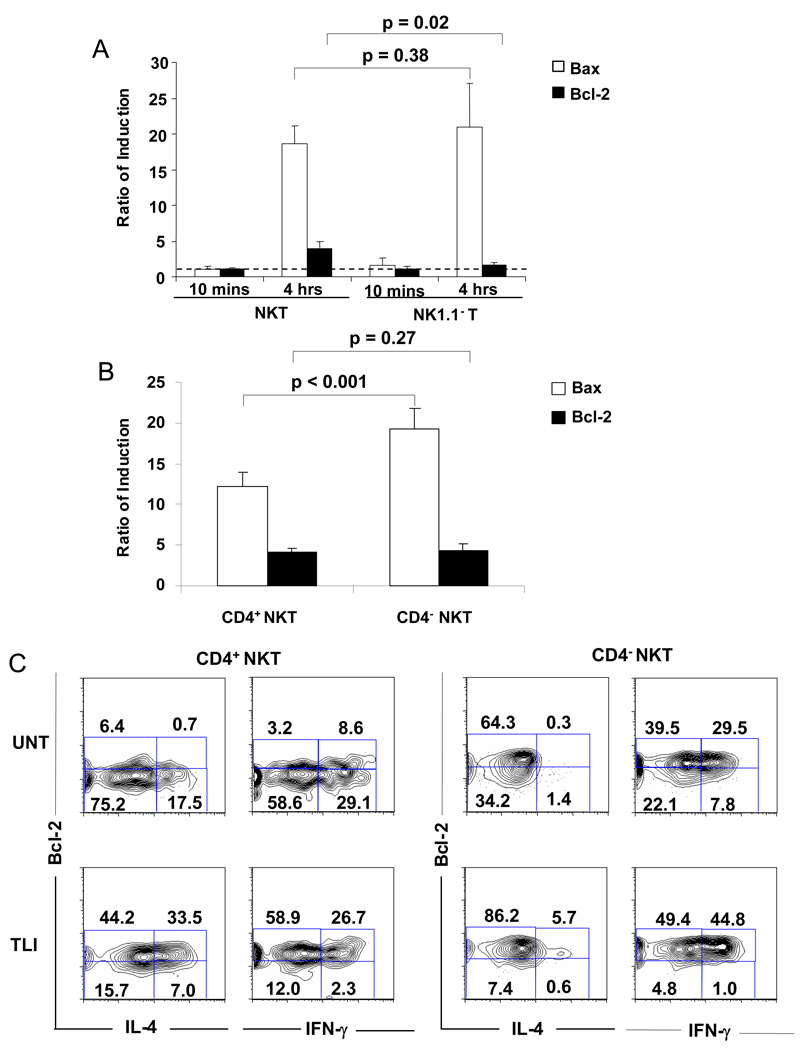
In order to determine whether splenic NKT cells and non-NKT cells differ in the levels of RNA encoding pro-apoptotic Bax and anti-apoptotic Bcl-2 before and after 1,000 cGy TBI, splenic NK1.1+ TCRαβ+ T cells and NK1.1− TCRαβ+ T cells were sorted by flow cytometry from untreated mice, and from mice 20 minutes or 4 hours after a single dose of 1,000 cGy TBI. The yields of live splenic T cells from untreated mice and from irradiated mice at the 4 hour time point were not significantly different (data not shown). Equal numbers of sorted cells were lysed, RNA was extracted, and real time PCR was performed using primers specific for the Bax and Bcl-2 genes. Levels of amplified Bax and Bcl-2 gene products were normalized to β-actin standards. Ratios of normalized amplified gene products were determined by comparing levels after irradiation to those obtained from the untreated mice. Figure 6A shows that the ratios were about 1:1 for both Bax and Bcl-2 obtained from both NKT cells and non-NKT cells 10 minutes after in vivo irradiation. In contrast, ratios of RNA encoding Bax increased to about 17:1 to 21:1 4 hours after TBI in both T cell subsets (p=0.39). Ratios of RNA encoding Bcl-2 rose to about 4:1 at 4 hours time point after irradiation for NKT cells, and to about 1.6:1 for non-NKT cells. (p=0.02). The induced level of Bax as compared to Bcl-2 was significantly greater (p<0.01) at 4 hours in non-NKT cells versus NKT cells. The analysis was repeated comparing sorted CD4+ NKT and CD4− NKT cells 4 hours after irradiation. Figure 6B shows that the increase in Bax expression was significantly higher (p<0.001) in CD4− NKT cells versus CD4+ NKT cells, and the fourfold increase in Bcl-2 was not significantly different (p=0.27). The induced level of Bax as compared to Bcl-2 was significantly higher (p=0.01) in the CD4− versus CD4+ NKT cells.
Figure 6. Real-time PCR analysis of RNA encoding Bcl-2 and Bax in CD4+ NKT, CD4-CD8− NKT and NK1.1− T cells and intracellular staining for IL-4 and Bcl-2 before and after irradiation.
(A) Fresh splenic NK1.1+TCRαβ+ T cells and NK1.1−TCRαβ+ T cells were sorted by flow cytometry from untreated mice, and from mice 10 minutes or 4 hours after a single dose of 1,000 cGy TBI. Total RNA was extracted from the cells and analyzed for expression of Bax and Bcl-2 by quantitative real-time PCR. Induction ratio of PCR products using primers specific for Bax and Bcl-2 were calculated from sorted cells in untreated mice versus irradiated mice at the two time points after irradiation. Mean ratio is shown for three independent experiments. Error bars represent SD. Dotted line shows threshold of changes from untreated mice. (B) The same analysis was performed for expression of Bax and Bcl-2 at 4 hours after irradiation using sorted CD4+NKT1.1+ and CD4− NK1.1+ TCRαβ+ T cells pooled from the spleens of 10 mice. (C) Two color analysis of intracellular staining for IL-4, IFN-γ and Bcl-2 on gated CD4+ and CD4− NKT cells before and 24 hours after irradiation with 10 doses of TLI. Representative flow cytometric patterns are shown.
Increased expression of both intracellular IL-4 and Bcl-2 in CD4+ NKT cells after irradiation
Since NKT cells have been reported to show a Th2 bias in mice conditioned with TBI or TLI [4, 5, 7, 9], intracellular staining of IL-4, IFN-γ and Bcl-2 was compared for gated CD4+ NKT cells and CD4− NKT cells before and 24 hours after 10 doses of TLI. Spleen cells were stimulated with phorbol myristate acetate (PMA) and calcium ionophore (Ionomycin) in order to induce cytokine production before intracellular staining. Figure 6C shows that the majority of untreated CD4+ NKT cells was Bcl-2lo even amongst the cells that expressed intracellular IL-4. After irradiation, there was a marked increase in the mean percentage of CD4+ NKT cells that expressed intracellular IL-4, from 17% ± 3% to 37% ± 3 % (p<0.05), and most CD4+ NKT cells became Bcl-2hi including those with intracellular IL-4. In contrast, less than 2% of CD4− NKT cells expressed intracellular IL-4 before irradiation, and about 6% after irradiation. Staining for intracellular IFN-γ showed that there was a Th1 bias in untreated CD4+ NKT cells with about a 2:1 ratio of IFN-γ+:IL-4+ cells (mean 2.3±0.5). After irradiation, there was a shift toward a Th2 bias with a ratio of about 1:1 (mean 0.9±0.2). The untreated CD4− NKT cells showed a strong Th1 bias with a ratio of about 20:1 (mean 20±2) (Figure 6C). After irradiation, the Th1 bias persisted with a ratio of about 8:1 (mean 10±3). There was no shift from a Th1 to Th2 bias in non-NKT cells at 24 hours after TLI (supplemental Figure 3)
Discussion
The results of the current studies indicate that NKT cells have a marked resistance to cell death induced by TBI as compared to non-NKT cells, since after irradiation with at least 1,000 cGy, the loss of non-NKT cells was about 10 to 20 fold greater than that of NKT cells in the spleen. Similar changes in these T cells subsets were observed in the bone marrow and liver after in vivo irradiation and in spleen cells after in vitro irradiation. The change in the balance of T cell subsets in the spleen that favored the NKT cells after TBI or TLI in the current studies was not due to rapid regeneration of NKT cells, since incorporation of BrdU during the 24 hours period after irradiation was minimal in both NKT cells and non-NKT cells. The residual NKT cells were mainly CD4+ type I NKT cells, that expressed the invariant Vα14Jα18 TCRα chain[29, 30]. NKT cells have been shown to develop Th2 cytokine bias after TBI or TLI that can ameliorate GVHD by promoting a Th2 bias among donor T cells contained in allogeneic bone marrow transplants[4–7, 9]. The current study shows that the dominant CD4+ NKT cells switched from a Th1 bias to a Th2 bias after TLI, but that the minority CD4−CD8− NKT cells retained their Th1 bias after TLI. Jα18−/− host mice that are deficient only in type I NKT cells had significantly more severe GVHD than wild type host mice after allogeneic bone marrow and spleen cell transplantation after TBI conditioning. The increased amelioration of GVHD by TLI as compared to TBI [6, 7, 9] can be accounted for, at least in part, by the 10 fold greater number of residual NKT cells present in the spleen after similar total doses of TBI and TLI. During TLI, depots of NKT cells in the liver and bone marrow are shielded with lead[31, 32], and can redistribute to the spleen. The current study indicates the Th2 bias and immune regulatory functions of CD4+ NKT cells after in vivo irradiation occurs along with the increased expression of Bcl-2. This association was reported previously after glucocorticoid exposure[21]. However, this linkage was not present before irradiation, since the majority of CD4+ NKT cells that secreted IL-4 were Bcl-2lo.
Staining of NKT cells and non-NKT cells before irradiation for intracellular Bcl-2 identified two subsets of T cells, one with a Bcl-2lo phenotype and one with a Bcl-2hi phenotype. After graded doses of TBI, the fraction of Bcl-2hi cells rose progressively such that more than 90% of all NKT cells were Bcl-2hi 48 and 72 hours after 1,000 cGy TBI. Whereas Bcl-2hi NKT cells decreased less than 2 fold, Bcl-2lo NKT cells decreased about 200 fold. Similarly, Bcl-2hi non-NKT cells decreased less than 10 fold, and Bcl-2lo non-NKT cells decreased about 500 fold. The changed balance of NKT cells and non-NKT cells after radiation reflected a higher constitutive expression among CD4−CD8− NKT cells, and induced upregulation of Bcl-2 among CD4+ NKT cells. Upregulation of Bcl-2 in total NKT cells has been previously reported after in vitro exposure to dexamethasone[21], but not after in vivo irradiation. The mechanism of Bcl-2 upregulation after in vivo irradiation is the subject of our continuing investigation. Ren et al., also reported that the percentage of NKT cells among all T cells in the lymphoid tissues increases after multiple low doses of TBI especially in the blood and liver, but they did not determine whether the increased percentage of NKT cells was among the CD4+ NKT cells or CD4−CD8− NKT cells or was due to increased renewal or cell division, and/or to reduced cell death[33]. The current study shows that the forced expression of Bcl-2 in all T cells using a Bcl-2 transgene protects both NKT cells and non-NKT cells from cell death, and prevents the change in balance of T cell subsets after TBI.
Since proteins other than Bcl-2 can regulate T cell apoptosis after irradiation, the levels of the anti-apoptotic protein, Bcl-xL and the mRNA that encodes the pro-apoptotic protein, Bax were determined before and 4 hours after TBI. Whereas intracellular levels of Bcl-2 markedly increased after TBI, the levels of Bcl-xL did not. In addition, the level of mRNA encoding Bax as compared to Bcl-2 increased significantly 4 hours after irradiation in non-NKT cells versus NKT cells due to greater increase in Bcl-2 in NKT cells. However, increased level of Bax as compared to Bcl-2 gene expression in CD4− as compared CD4+ NKT cells was due to greater increase in Bax levels in CD4− NKT cells at that early time point.
In conclusion, the study shows that differences in Bcl-2 expression among T cell subsets alter their balance after in vivo irradiation. The predominance of the NKT cell subset among all T cells after TBI, TLI and in vitro irradiation is due to the marked radioresistance of the NKT cell subset, and is linked to the increased absolute number of CD4+ NKT cells with high levels of intracellular Bcl-2 observed after radiation. This subset has the highest induced levels of Bcl-2 versus Bax when compared to non-NKT cells and CD4−CD8− NKT cells, and the strongest Th2 bias in response to in vivo irradiation.
Materials and Methods
Animals
Male C57BL/6 wild type mice, 8–10 wk old, were purchased from The Jackson Laboratory (Bar Harbor, ME). Some of the latter mice were thymectomized at the Jackson Laboratory at age 6 weeks. Male C57BL/6 Jα18−/− mice[34] were bred and maintained in the Department of Comparative Medicine, Stanford University (Stanford, CA) according to National Institutes of Health Veterinary guidelines. Male C57BL/6 human Bcl-2 transgenic mice were obtained from the colony of Dr. I. L. Weissman (Stanford University) [17]. All animal protocols were reviewed and approved by the Stanford Administrative Panels on Laboratory Animal Care (APLAC).
Irradiation
TLI was delivered to the abdomen, lymph nodes, thymus, and spleen with shielding of the skull, lungs, pelvis, limbs and tail as described previously[31, 32]. TBI was administered to wild type C57BL/6 or Jα18−/− mice 24 hours before the allogeneic bone marrow and spleen cell infusions. TLI was performed with a Philips x-ray unit (200 kV, 10mA; Philips Electronic Instruments, Rahway, NJ) at a rate of 84 cGy/min with a 0.5 mm Cu filter. TBI was delivered as a single dose from the same x-ray unit.
Bone Marrow Transplantation Technique and Monitoring for GVHD
Single cell suspensions of marrow or spleen cells were obtained after passage through nylon wool meshes[9]. Monitoring for clinical signs of GVHD, loss of body weight, and survival has been described in detail previously[6, 7].
Monoclonal antibodies and chemical reagents
FITC-conjugated anti-Bcl-xL mAb was purchased from Southern Biotechnology Associates (Birmingham, AL). Anti-NK1.1-PE, anti-TCRαβ-APC, and anti-CD4-Cy7APC mAbs, as well as the FITC-conjugated anti-Bcl-2 antibody reagent kit, anti-IL4-Cy7PE, anti-IFN-γ-Cy7PE, BD cytofix/cytoperm kit, BrdU Flow kit, and anti-CD16/32 mAb were purchased from BD PharMingen (San Diego, CA). PE-conjugated PBS57-loaded mouse CD1d tetramers or PE-conjugated unloaded mouse CD1d tetramers were provided by National Institutes of Health Tetramer Facility, Rockville, MD.
Flow cytometry analysis
Single cell suspensions lysed with ammonium chloride buffer were prepared in phosphate-buffered saline (PBS) with 1% calf serum, pre-incubated with anti-CD16/32 mAb to prevent non-specific binding via FcRII/III interactions, and then incubated with the appropriate mAb. Propidium iodide (Sigma, St Louis, MO) was added prior to analysis to exclude any dead cells. To analyze Bcl-2, Bcl-xL, IL-4 and IFN-γ intracellular expression, all cells first were incubated with the appropriate anti-surface receptor mAbs, then fixed and permeabilized with BD cytofix/cytoperm kit for intracellular staining. Ethidium monoazide bromide (EMA) (Invitrogen, Calsbad, CA) was added prior to fixation and permeabilization to exclude any dead cells. Thresholds for Bcl-2 and Bcl-xL staining were determined by using isotype-matched irrelevant mAb. All analyses were performed on a modified dual laser LSRScan (BD Immunocytometry Systems, San Diego, CA) in the Shared FACS Facility (Center for Molecular and Genetic Medicine at Stanford University), using FlowJo software (TreeStar, Ashland, OR) for data analysis.
Cell isolation and sorting
For purposes of T cell staining or sorting, single cell-suspensions from spleens were first enriched using anti-Thy1.2 microBeads on immunomagnetic bead columns (Miltenyi Biotech). Enriched Thy1.2+ cells were stained with PE-conjugated anti-NK1.1 and APC-conjugated anti-TCRαβ mAb before sorting on a FACS Vantage (BD Biosciences, Mountain View, CA) as described previously[35]. Sorted NK1.1+TCRαβ+ and NK1.1−TCRαβ+ cell populations were stored in −80°C freezer before RNA analysis. Liver mononuclear cell preparation was described before[7].
Activation of cells with phorbol myristate acetate (PMA) and calcium ionophore (Ionomycin)
Sorted T cell subsets from the spleen of untreated wild type C57BL/6 mice or from mice 24 hours after the last dose of 10 doses of TLI (240 cGy each) treatment were cultured in vitro with further stimulation in 10% FBS and RPMI complete medium contained 10 ng/ml PMA, 1µM Ionomycin and 2 µM Monensin (Sigma Chemical Co.) in 6-well plates for 4 hours. After that, cells were labeled with surface mAb, fixed, and permeabilized for intracellular IL-4 and IFN-γ staining.
RNA isolation and analysis
Total RNA was isolated from sorted frozen cell pellets using the RNeasy MiniKit (QIAGEN, Valencia, CA). After digestion of genomic DNA (DNA-free; Ambion, Austin, TX), the RNA was reverse-transcribed with TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction (PCR) was then performed on an ABI PRISM 7900 Detection System (Applied Biosystems) using TaqMan Universal PCR Master Mix (Roche, Branchburg, NJ). All cycle threshold values were normalized to β-actin housekeeping gene expression. PCR primer sequences for Bcl-2 and Bax genes and conditions are available on request (Applied Biosystems). Data were expressed as the x-fold induction of gene expression in equal numbers of viable sorted cells from irradiated mice compared with untreated control mice.
In Vitro assay of cell survival after irradiation
The spleen cells prepared from the wild type C57BL/6 mice were irradiated with 1000 cGy by Cesium irradiator (J.L. Shepherd & Associates) and then incubated at 37° C in 6-well tissue culture plates (Falcon) at 4×106 cells/well in a volume of 2 ml/well with complete medium. After 24 hrs, these cells were harvested and stained with both PE-conjugated anti-NK1.1 and APC-conjugated anti-TCRαβ mAb, and then with 2 µg/ml propidium iodide.
BrdU incorporation analysis
Mice were injected i.p. with 100 µl of a 10 mg/ml solution of BrdU (Sigma) in PBS every 8 hours during the 24 hours after irradiation. BrdU-labeled cells were stained with anti-TCRαβ-APC, anti-NK1.1-PE, followed by fixation and permeabilization with BD cytofix/cytoperm buffer. The cells were then stained with anti-BrdU mAb for 20 min at room temperature and analyzed by flow cytometry with the gates of TCRαβ+ NK1.1− and TCRαβ+ NK1.1+ cell subsets. As a negative control, the mice not injected with BrdU were also analyzed.
Statistical analysis
Kaplan-Meier survival curves were made using Prism (GraphPad Software, San Diego, CA). Statistical differences in animal survival were analyzed by log-rank test. Difference in percent and absolute number of immunophenotypic populations of cells were analyzed using the two-tailed Student’s t test. For all tests, p value of 0.05 or less was considered significant.
Supplementary Material
Acknowledgements
We thank Dr. Irving Weissman (Stanford University, Stanford, CA), for the human Bcl-2 transgenic mice, and Dr. M. Taniguchi (Chiba University, Chiba, Japan) for C57BL/6 Jα18−/− mice. We thank the NIH tetramer facility for providing CD1d tetramer. We also thank Glenna Letsinger in preparing the manuscript, and Aditi Mukhopadyahay for technical assistance. This work was supported by grants from the National Institutes of Health NIAID RO1 AI-37683, NCI PO1 CA-49605, NHLBI RO1 HL-58250 and NHLBI PO1 HL-57443.
Nonstandard abbreviations used
- GVHD
Graft Versus Host Disease
- TLI
Total Lymphoid Irradiation
- TBI
Total Body Irradiation
Footnotes
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
- 1.Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986;323:164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BE, McNiff JM, Matte C, Athanasiadis I, Shlomchik WD, Shlomchik MJ. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood. 2004;104:1565–1573. doi: 10.1182/blood-2004-01-0328. [DOI] [PubMed] [Google Scholar]
- 4.Haraguchi K, Takahashi T, Matsumoto A, Asai T, Kanda Y, Kurokawa M, Ogawa S, Oda H, Taniguchi M, Hirai H, Chiba S. Host-residual invariant NK T cells attenuate graft-versus-host immunity. J Immunol. 2005;175:1320–1328. doi: 10.4049/jimmunol.175.2.1320. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto D, Asakura S, Miyake S, Yamamura T, Van Kaer L, Liu C, Tanimoto M, Teshima T. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J Immunol. 2005;174:551–556. doi: 10.4049/jimmunol.174.1.551. [DOI] [PubMed] [Google Scholar]
- 6.Lan F, Zeng D, Higuchi M, Higgins JP, Strober S. Host conditioning with total lymphoid irradiation and antithymocyte globulin prevents graft-versus-host disease: the role of CD1-reactive natural killer T cells. Biol Blood Marrow Transplant. 2003;9:355–363. doi: 10.1016/s1083-8791(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 7.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: "natural suppressor" cells. J Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 8.Morecki S, Panigrahi S, Pizov G, Yacovlev E, Gelfand Y, Eizik O, Slavin S. Effect of KRN7000 on induced graft-vs-host disease. Exp Hematol. 2004;32:630–637. doi: 10.1016/j.exphem.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Pillai AB, George TI, Dutt S, Teo P, Strober S. Host NKT cells can prevent graft-versus-host disease and permit graft antitumor activity after bone marrow transplantation. J Immunol. 2007;178:6242–6251. doi: 10.4049/jimmunol.178.10.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belka C, Budach W. Anti-apoptotic Bcl-2 proteins: structure, function and relevance for radiation biology. Int J Radiat Biol. 2002;78:643–658. doi: 10.1080/09553000210137680. [DOI] [PubMed] [Google Scholar]
- 11.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 12.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok CL, Gil-Gomez G, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady HJ. Bad can act as a key regulator of T cell apoptosis and T cell development. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 15.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 16.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 17.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 18.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, Laport GG, Stockerl-Goldstein KE, Johnston LJ, Hoppe RT, Bloch DA, Blume KG, Negrin RS, Strober S. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 19.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, Strober S. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564–5570. doi: 10.4049/jimmunol.169.10.5564. [DOI] [PubMed] [Google Scholar]
- 21.Tamada K, Harada M, Abe K, Li T, Nomoto K. IL-4-producing NK1.1+ T cells are resistant to glucocorticoid-induced apoptosis: implications for the Th1/Th2 balance. J Immunol. 1998;161:1239–1247. [PubMed] [Google Scholar]
- 22.Seino K, Harada M, Taniguchi M. NKT cells are relatively resistant to apoptosis. Trends Immunol. 2004;25:219–221. doi: 10.1016/j.it.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 24.Yu KO, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi M, Nakayama T. Recognition and function of Valpha14 NKT cells. Semin Immunol. 2000;12:543–550. doi: 10.1006/smim.2000.0270. [DOI] [PubMed] [Google Scholar]
- 27.Kinjo Y, Kronenberg M. Valpha14i NKT cells are innate lymphocytes that participate in the immune response to diverse microbes. J Clin Immunol. 2005;25:522–533. doi: 10.1007/s10875-005-8064-5. [DOI] [PubMed] [Google Scholar]
- 28.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 29.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 31.Slavin S, Fuks Z, Kaplan HS, Strober S. Transplantation of allogeneic bone marrow without graft-versus-host disease using total lymphoid irradiation. J Exp Med. 1978;147:963–972. doi: 10.1084/jem.147.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strober S, Slavin S, Gottlieb M, Zan-Bar I, King DP, Hoppe RT, Fuks Z, Grumet FC, Kaplan HS. Allograft tolerance after total lymphoid irradiation (TLI) Immunol Rev. 1979;46:87–112. doi: 10.1111/j.1600-065x.1979.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 33.Ren H, Shen J, Tomiyama-Miyaji C, Watanabe M, Kainuma E, Inoue M, Kuwano Y, Abo T. Augmentation of innate immunity by low-dose irradiation. Cell Immunol. 2006;244:50–56. doi: 10.1016/j.cellimm.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 35.Dejbakhsh-Jones S, Strober S. Identification of an early T cell progenitor for a pathway of T cell maturation in the bone marrow. Proc Natl Acad Sci U S A. 1999;96:14493–14498. doi: 10.1073/pnas.96.25.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.