Abstract
Purpose To monitor the metabolic effects of an individual patient-tailored, experimental glioma therapy regimen that included repetitive multiple neurosurgical resections, radiosurgical interventions, and an adjuvant maintenance therapy based on the tyrosine kinase inhibitor imatinib in combination with the chemotherapeutic agent hydroxyurea (HU). Procedures Therapeutic effects were monitored in a 26-year-old male patient with a glioblastoma multiforme by multimodal imaging using sequential l-[methyl-(11)C]-methionine positron emission tomography (MET–PET) and MRI. The normalized MET uptake and volume of the metabolically active tumor were assessed sequentially. Results The individual patient-tailored, experimental glioma therapy caused a continuous decline of metabolically active tumor volume, associated with clinical remission over a period of more than two years. Conclusions MET–PET seems to be useful for monitoring patient-tailored, experimental glioma therapy regimens, especially when patients are treated with a multi-step therapeutic regimen. Monitoring and guidance of those experimental therapy regimens by MET–PET in a larger patient group are needed to confirm its clinical value.
Keywords: Experimental glioma therapy, l-[methyl-(11)C]-methionine PET, Platelet-derived growth factor (PDGF) receptor, Tyrosine kinase inhibitor
Introduction
Since the EORTC 26981 study [1] standard glioblastoma therapy has become multidimensional involving surgery, concomitant radio/chemotherapy, and adjuvant chemotherapy. However, the benefits of this standard regimen are modest, and the problem of primary lack of therapeutic effectiveness or tumor recurrence remains a serious risk for nearly all patients [1]. Because of the lack of effective salvage therapies to date, most GBM patients die within one to two years after diagnosis has been made [2]. Therefore, novel strategies for the treatment of therapy-refractory GBM are desperately needed. Individual patient-tailored, experimental glioma therapy regimens might be one approach to the problem. Evaluating individual therapies has to overcome two main obstacles:
therapy failure needs to be recognized as early as possible to allow for switching the therapy before the clinical status worsens;
multiple therapies may interact, which makes assessment of the individual contribution to the therapeutic effect more difficult thereby making any interference with the treatment of GBM patients, in general, more difficult.
Novel therapeutics targeting activated signal-transduction pathways are currently evaluated in oncology. Imatinib mesylate (Gleevec; Novartis Pharmaceuticals, East Hanover, NJ, USA), a selective tyrosine kinase receptor inhibitor, especially of platelet-derived growth factor (PDGF) receptors, has established effects against both hematologic and solid organ cancers [3–5]. Imatinib has also been evaluated in malignant glioma patients based on overexpression of PDGF and PDGF receptors [6]. Although preclinical studies suggest substantial antiglioma activity [7, 8] the therapeutic effect of imatinib in malignant glioma trials, e.g. the NABTC 99-08 trial, has been modest [9]. In contrast, a marked effect of imatinib has been reported in a small series of GBM patients with tumor recurrence treated with imatinib plus the chemotherapeutic agent hydroxyurea (HU), a ribonucleoside diphosphate reductase inhibitor [10]. Although the precise mechanism of combining imatinib with HU remains to be elucidated, clinical studies suggest that signal transduction inhibitors can enhance the activity of cytotoxic agents [11]; combining a signal transduction inhibitor with a cytotoxic agent was effective and well tolerated among GBM patients. Therefore, the combination of imatinib and HU offers the potential of added value over and above the effects of neurosurgical intervention and radiosurgery as part of an individual patient-tailored, experimental glioblastoma relapse therapy regimen.
Typically, in malignant gliomas during the course of the disease multiple therapy events like surgery, radiotherapy, and chemotherapy take place. Because of multiple interventions, evaluation of the specific effect of one therapeutic intervention is very difficult and, therefore, diagnostic methods which can provide information about short-term therapeutic effects can be helpful. Monitoring metabolic changes in tumors may provide an indicator of tumor response. Tumor resistance may be detected early on and in the case of tumor resistance to the therapy applied, another salvage therapeutic option could be tried.
Typically, in malignant gliomas during the course of the disease multiple therapy events like surgery, radiotherapy, and chemotherapy take place. Because of multiple interventions, evaluation of the specific effect of one therapeutic intervention is very difficult and, therefore, diagnostic methods which can provide information about short-term therapeutic effects can be helpful. Monitoring metabolic changes in tumors may provide an indicator of tumor response. Tumor resistance may be detected early on and in the case of tumor resistance to the therapy applied, another salvage therapeutic option could be tried.
Metabolic tracers, for example positron emitter-labeled amino acids, have been proposed as indicators of tumor activity [12]. Positron emission tomography with l-[methyl-(11)C]-methionine (MET–PET) has been used to study the effects of radiotherapy on gliomas [13–16]. Previous studies suggest that monitoring metabolic changes with MET–PET may provide an objective measure of response to temozolomide treatment and moreover, it may enable prediction of clinical outcome in glioma patients [17]. This makes MET–PET a valuable addition to conventional (i.e. morphological) MR imaging. One previous study monitored the effect of chemotherapy with procarbazine, CCNU, and vincristine in an anaplastic oligoastrocytoma by repeated MET–PET [18] and highlighted the potential of this technique to monitor chemotherapy. MET–PET provides a more accurate assessment of extent of the tumor than contrast enhancement of CT or MRI [19–22]. Accumulation of MET is largely because of carrier-mediated transport, which is not altered by dexamethasone [19] and enables distinction between tumor tissue and contrast-enhancing necrosis [23, 24]. MET undergoes a complex metabolism and is incorporated into proteins. Therefore, increased uptake is likely to reflect the metabolic needs of brain tumors [25].
We here explore the potential of MET–PET to monitor an individual patient-tailored, experimental glioma therapy regimen in combination with an adjuvant maintenance therapy with imatinib and HU.
Methods
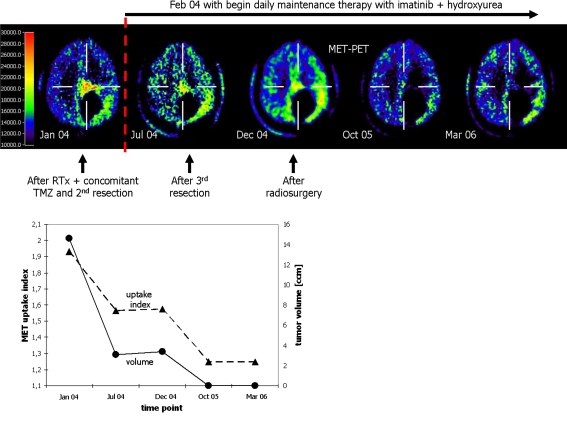
Repetitive PET scans were performed after intravenous slow bolus injection of 20 mCi (740 MBq) MET. MET was synthesized according to the method of Berger et al. [26]. The patient gave written consent to the MET–PET studies. As described previously [19], a circular region of interest 8 mm in diameter was placed in the metabolically most active area of the tumor on images representing activity 20–60 min after injection as measured using a Siemens ECAT EXACT or ECAT EXACT HR (Siemens-CTI, Knoxville, TN, USA). For reference, a circular region was placed in the unaffected contralateral cortex, and an uptake index was calculated from the ratio of tumor to contralateral activity. In addition, the active tumor volume was measured volumetrically by three-dimensional thresholding as the extent of increased MET uptake ≥1.5 in the contiguous tumor region, because this threshold had provided the best separation between tumor and nontumor lesions in a previous study [19]. Longitudinal data of MET uptake index and tumor volume were plotted against time (Fig. 2).
Fig. 2.
Longitudinal data with MET–PET images and a plot of the time course of MET uptake index and tumor volume. In the last two MET–PET scans plotted, all pixels were below the threshold index of 1.5. Thus, there was no detectable tumor volume
Case presentation
In September 2002, a first grand-mal occurred in a 26-year-old patient. MR imaging showed a tumor-suspicious lesion in the left parietal lobe. A stereotaxic biopsy in October 2002 provided no significant finding. Neurosurgical resection and histopathological examination of the lesion in November 2002 revealed an astrocytoma WHO grade II°.
Because of signs of tumor progression with extensive headache, multiple focal epileptic seizures, and gadolinium enhancement in MRI, further neurosurgical resection was necessary in November 2003 (Figs. 1, 3). Histopathological examination of the resected tumor revealed a malignant transformation to a glioblastoma multiforme WHO grade IV°. After resection, external radiation therapy up to a total dose of 60 Gy with concomitant temozolomide chemotherapy (75 mg per square meter of body-surface area per day, seven days per week from the first to the last day of radiotherapy) was performed from December 2003 to January 2004 (Fig. 3).
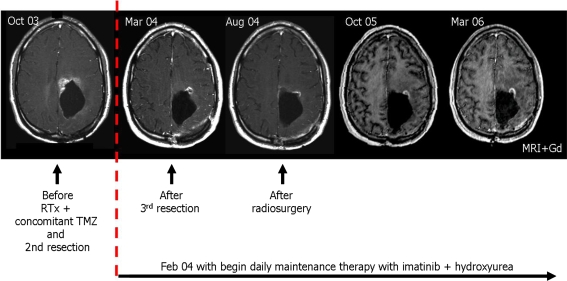
Fig. 1.
MR images in relation to surgery, radiotherapy, and adjuvant maintenance imatinib and HU therapy
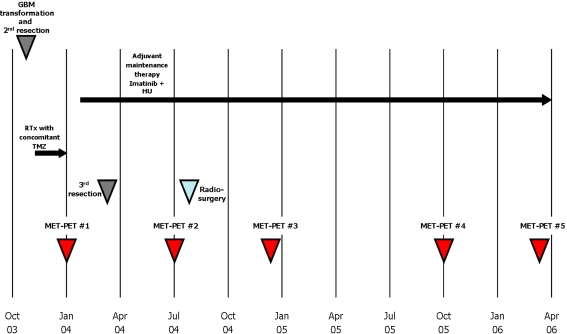
Fig. 3.
Overview of all therapeutic events and MET–PET imaging since diagnosis of GBM was confirmed
Despite further resection and combined radiochemotherapy, an increased metabolic pattern could be observed (Fig. 2). Subsequently, a daily adjuvant experimental maintenance therapy with imatinib (600 mg p.o. daily) and HU (500 mg p.o. daily) was started in February 2004. To minimize residual tumor volume, a third neurosurgical resection was performed in March 2004 and the patient underwent radiosurgery with a gamma knife in August 2004. The adjuvant maintenance therapy with imatinib and hydroxyurea was continued until April 2007 (Fig. 3). Additionally, the patient was treated for symptomatic epilepsy with 600 mg oxcarbazepine, for mood stabilization with 50 mg trimipramine, and for hypothyreoidism with 75 μg levothyroxine per day.
Therapeutic effects of the multiple therapeutic interventions were monitored clinically and by sequential MET–PET scans and MR imaging, respectively, during the observation period from January 2004 until March 2006 (Figs. 1, 2).
Before initiation of imatinib and HU therapy, MET uptake was increased 1.93-fold compared with the unaffected contralateral tissue (January 2004). In July 2004, after a daily therapy of imatinib and HU over six months and neurosurgical resection of residual tumor in March 2004, MET uptake decreased (1.56) and tumor volume shrank from 14.60 to 3.06 cm3. Clinically, signs and symptoms of tumor progression were not observed. In December 2004, after a therapy duration of eleven months and additional radiosurgery in August 2004, MET uptake and the tumor volume were stable (MET uptake 1.57; tumor volume 3.38 cm3). In the follow-up MET–PET examinations after ten months (October 2005), no significant MET uptake (≥1.5) was detectable; this remained unchanged until the end of the observation period in March 2006. The pattern of the gadolinium enhancement also remained unchanged (Figs. 1, 2).
Thirteen months after the end of the observation period (April 2007), the clinical status of the patient under discontinued daily therapy with imatinib and HU was stable, signs and symptoms of tumor progression were not observed, and the Karnofsky performance index was 90%. After the observation period, the slight right-sided spastic hemiparesis and the hemianopsy to the right remained unchanged. Further examinations or imaging procedures after April 2007 were not available.
Discussion
This case demonstrates that MET–PET can be used to monitor patient-tailored, experimental therapy with multiple neurosurgical resections and radiosurgical interventions in combination with an adjuvant maintenance therapy based on imatinib and HU. The therapeutic effect could be monitored by using MET–PET and could be followed over the subsequent period of therapy. The observed MET–PET metabolic pattern during therapy may indicate a continuous decline of metabolically active tumor volume below the threshold of significant MET uptake (≥1.5). The metabolically inactive MET–PET pattern of the tumor was sustained until the end of the observation period after almost 2.5 years, and, moreover, clinically after the end of the observation period. Thus, MET–PET meets the clinical need for a method that is sufficiently sensitive to indicate therapeutic efficacy and which enables monitoring of the metabolic tumor pattern during therapy.
Whether the initial decrease of MET uptake and tumor volume observed after the 2nd MET–PET is an effect of neurosurgical resection, radiosurgery, or tyrosine kinase inhibitor-based treatment cannot be differentiated. Likewise, whether the observed stable course of the disease during the observation period is an effect of brachytherapy or tyrosine kinase inhibitor-based experimental treatment cannot be differentiated from our data and therefore requires further study. On the other hand, in a previous study with GBM patients refractory to chemotherapy and radiotherapy treated with imatinib and HU (n = 30) a tumor response (partial or complete response) or a stable disease lasting at least three months was observed in 57% of patients [10]. Additionally, in this study the six-month and two-year progression-free survival rates were 32 and 16%, respectively. Therefore, an adjuvant maintenance therapy with imatinib and HU might be partially effective.
We accordingly suggest that maintenance therapy based on imatinib and HU may be part of the multi-faceted individual glioma therapy. We hypothesize that imaging-guided multi-step therapeutic regimens may help to realize long-term survival in selected subpopulations of glioblastoma patients. Monitoring and guidance of those experimental therapy regimens by MET–PET should be studied in larger patient samples to establish its clinical value.
References
- 1.Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. doi:10.1056/NEJMoa043330 [DOI] [PubMed]
- 2.Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17(8):2572–2578 [DOI] [PubMed]
- 3.Apperley JF, Gardembas M, Melo JV et al (2002) Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 347(7):481–487. doi:10.1056/NEJMoa020150 [DOI] [PubMed]
- 4.Demetri GD, von Mehren M, Blanke CD et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347(7):472–480. doi:10.1056/NEJMoa020461 [DOI] [PubMed]
- 5.Kantarjian H, Sawyers C, Hochhaus A et al (2002) Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 346(9):645–652. doi:10.1056/NEJMoa011573 [DOI] [PubMed]
- 6.Fleming TP, Saxena A, Clark WC et al (1992) Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res 52(16):4550–4553 [PubMed]
- 7.Kilic T, Alberta JA, Zdunek PR et al (2000) Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res 60(18):5143–5150 [PubMed]
- 8.Boruban C, Yavas O, Altundag O, Altundag MB, Altundag K (2005) Imatinib may be useful in the management of patients with glioblastoma. Med Hypotheses 65(6):1205–1206. doi:10.1016/j.mehy.2005.04.004 [DOI] [PubMed]
- 9.Wen PY, Yung WK, Lamborn KR et al (2006) Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American brain tumor consortium study 99–08. Clin Cancer Res 12(16):4899–4907. doi:10.1158/1078-0432.CCR-06-0773 [DOI] [PubMed]
- 10.Dresemann G (2005) Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: a patient series. Ann Oncol 16(10):1702–1708. doi:10.1093/annonc/mdi317 [DOI] [PubMed]
- 11.Reardon DA, Egorin MJ, Quinn JA et al (2005) Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol 23(36):9359–9368. doi:10.1200/JCO.2005.03.2185 [DOI] [PubMed]
- 12.Kaschten B, Stevenaert A, Sadzot B et al (1998) Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 39(5):778–785 [PubMed]
- 13.Nuutinen J, Sonninen P, Lehikoinen P et al (2000) Radiotherapy treatment planning and long-term follow-up with [(11)C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys 48(1):43–52. doi:10.1016/S0360-3016(00)00604-0 [DOI] [PubMed]
- 14.Würker M, Herholz K, Voges J et al (1996) Glucose consumption and methionine uptake in low-grade gliomas after iodine-125 brachytherapy. Eur J Nucl Med 23(5):583–586 [DOI] [PubMed]
- 15.Voges J, Herholz K, Holzer T et al (1997) 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg 69(1–4 Pt 2):129–135. doi:10.1159/000099864 [DOI] [PubMed]
- 16.Shintani S, Tsuruoka S, Shiigai T (2000) Serial positron emission tomography (PET) in gliomatosis cerebri treated with radiotherapy: a case report. J Neurol Sci 173(1):25–31. doi:10.1016/S0022-510X(99)00296-8 [DOI] [PubMed]
- 17.Galldiks N, Kracht LW, Burghaus L et al (2006) Use of (11)C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur J Nucl Med Mol Imaging 33(5):516–524. doi:10.1007/s00259-005-0002-5 [DOI] [PubMed]
- 18.Herholz K, Kracht LW, Heiss WD (2003) Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging 13(3):269–271. doi:10.1177/1051228403013003012 [DOI] [PubMed]
- 19.Herholz K, Holzer T, Bauer B et al (1998) 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 50(5):1316–1322 [DOI] [PubMed]
- 20.Tovi M, Lilja A, Bergstrom M, Ericsson A, Bergstrom K, Hartman M (1990) Delineation of gliomas with magnetic resonance imaging using Gd-DTPA in comparison with computed tomography and positron emission tomography. Acta Radiol 31(5):417–429 [PubMed]
- 21.Kracht LW, Miletic H, Busch S et al (2004) Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 10(21):7163–7170. doi:10.1158/1078-0432.CCR-04-0262 [DOI] [PubMed]
- 22.Pauleit D, Floeth F, Hamacher K et al (2005) O-(2-[18F]fluoroethyl)-l-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128(Pt 3):678–687. doi:10.1093/brain/awh399 [DOI] [PubMed]
- 23.Reinhardt MJ, Kubota K, Yamada S, Iwata R, Yaegashi H (1997) Assessment of cancer recurrence in residual tumors after fractionated radiotherapy: a comparison of fluorodeoxyglucose, l-methionine and thymidine. J Nucl Med 38(2):280–287 [PubMed]
- 24.Sonoda Y, Kumabe T, Takahashi T, Shirane R, Yoshimoto T (1998) Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir (Tokyo) 38(6):342–347. doi:10.2176/nmc.38.342 (discussion 347–348) [DOI] [PubMed]
- 25.Bustany P, Chatel M, Derlon JM et al (1986) Brain tumor protein synthesis and histological grades: a study by positron emission tomography (PET) with C11-l-Methionine. J Neurooncol 3(4):397–404. doi:10.1007/BF00165590 [DOI] [PubMed]
- 26.Berger G, Maziere M, Knipper R, Prenant C, Comar D (1979) Automated synthesis of 11C-labelled radiopharmaceuticals: imipramine, chlorpromazine, nicotine and methionine. Int J Appl Radiat Isot 30(7):393–399. doi:10.1016/0020-708X(79)90049-8 [DOI] [PubMed]